Abstract
For years, strategies have been proposed to improve translational success in stroke research by improving the quality of animal studies. However, articles that report preclinical intracerebral hemorrhage (ICH) studies continue to lack adequate qualitative and quantitative descriptions of fresh brain tissue collection. They also tend to lack transparency about animal model quality. We conducted a systematic review of 82 ICH research articles to determine the level of detail reported for brain tissue collection. We found that only 24 (29%) reported the volume, weight, or thickness of tissue collected and a specific description of the anatomical location. Thus, up to 71% of preclinical ICH research articles did not properly define how fresh specimens were collected for biochemical measurements. Such omissions may impede reproducibility of results between laboratories. Although existing criteria have improved the quality of preclinical stroke studies, ICH researchers need to identify specific guidelines and strategies to avoid pitfalls, minimize bias, and increase reproducibility in this field.
Keywords: preclinical criteria, translational medicine, intracerebral hemorrhage, hematoma, multidisciplinary collaboration, basic science
Current Progress and Bottlenecks in Preclinical Intracerebral Hemorrhage Research
The pathological mechanism of ischemic stroke is relatively well established owing to decades of exhaustive preclinical and clinical research efforts. This work has led to a decrease in the incidence and mortality of ischemic stroke in the United States. In contrast, intracerebral hemorrhage (ICH) has no approved therapies, and, despite ongoing research, its morbidity and mortality have not declined in the last two decades [1-3]. The fact is that ischemic stroke and hemorrhagic stroke differ in major pathology. Lack of blood supply to a particular brain region causes ischemic stroke damage, whereas accumulation of blood within the brain parenchyma triggers specific pathologic cascades after ICH [4-8]. Thus, additional ICH research is urgently needed that will provide data with more predictive value [9, 10]. Compared with preclinical ischemic stroke research, which traces its roots to the 1970s, preclinical ICH research is still in its infancy. The two most common ICH models induced by collagenase or blood were not refined in rats until the 1990s [11, 12] and were not developed and characterized for mice until the late 1990s and early 2000s [13-15]. Although ischemic stroke research has a longer history, its progress has been fraught with setbacks, as promising therapies that have worked in the laboratory have failed to succeed in clinical trials [16], Therefore, ICH researchers should try not to repeat the history of the ischemic stroke [17], but rather contemplate specific strategies and procedures to improve the quality of preclinical ICH research itself.
Recently, published guidelines have emphasized the need to improve preclinical research quality in translational stroke research [18-25]. Improved and more consistent randomization, blinding, sample size calculation, inclusion/exclusion criteria, allocation concealment, and data handling will reduce bias and increase reproducibility in preclinical stroke research (NINDS, 2011). However, a clear description of the process by which fresh brain tissue specimens are collected for biochemical measurements after ICH has seldom been discussed. In particular, the origin of brain tissue can greatly affect the results of biomedical assays, which are essential for identifying novel molecular targets, testing drug efficacy, and detecting adverse effects of drugs in translational medicine [26].
Hemorrhagic brain is challenging to study because intraparenchymal hematoma, along with the presence of blood degradation products, increases the complexity of tissue architecture. The location of ICH also significantly influences the development of strategies and procedures to treat and manage patients [27]. Therefore, ICH researchers should consider how to define and collect specific brain regions from animals before performing biochemical or molecular biological studies. Furthermore, it has been suggested that collaboration between basic and clinical research teams improves the ease with which basic science results can be translated into clinical application [28, 29]. Having a qualitative, quantitative, transparent, and standardized description of the method for collecting fresh brain tissue, as well as clear measures of success, can help researchers communicate strategies, reduce experimental variation, and assess reproducibility of experimental results within and among individuals or teams.
Currently, little is known about how sampling of fresh specimens is reported in different articles. Therefore, we conducted a systematic literature review to assess how sampling methods for fresh brain specimens are reported, specifically in ICH research. We included articles in which rodent brain tissues were collected and processed primarily for protein extraction, nucleotide purification, and common biochemical endpoints, such as Western blots, enzyme-linked immunosorbent assay, and gel zymography. In addition, we provide an example of how to present preclinical model quality and how to collect standardized fresh brain tissue specimens.
Search Strategy, Selection Criteria, and Data Extraction
We searched articles published between Jan 1, 2009, and Aug 8, 2014, in the PubMed database, with the following search strategy: (“intracerebral hemorrhage” OR “intracerebral haemorrhage” OR “intracranial hemorrhage” OR “intracranial haemorrhage” OR “intracerebral hematoma” OR “intracranial hematoma” OR “hemorrhagic stroke”) AND (rat OR rats OR mice OR mouse OR rodent) AND (“western” OR “protein extraction” OR “cytosolic protein” OR “nuclear protein” OR elisa OR zymography OR PCR OR “RNA extraction” OR “gene expression” OR “DNA extraction”). Studies were included if they met all of the following criteria: 1) they were ICH preclinical studies; 2) they used a rodent collagenase or blood injection model; 3) the investigators conducted biochemical measurements in fresh brain specimens; 4) they were published in English. Two authors (C-F.C. and L.C.) conducted independent title scans and abstract reviews and reviewed the full articles to assess eligibility for inclusion (see supplemental references for inclusive articles). Standardized forms were created for data extraction (see details in supplemental table). Any disagreements between the two authors were resolved through discussion with the third author (J.W.).
Potential Bias in Recent Preclinical ICH Studies
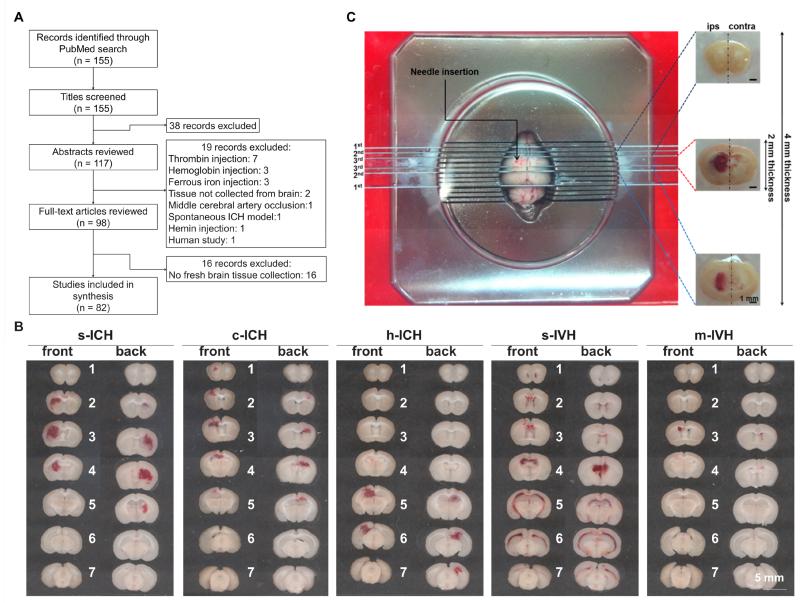
We identified 155 potentially relevant articles, retrieved 98 full articles, and included 82 articles for review (Fig. 1A). As shown in Table 1, in the past few years, more and more studies have used biochemical analyses in preclinical ICH research. Fresh brain tissue collected from euthanized animals was used most frequently for these analyses. However, only 24 of 82 articles (29%) reported qualitative and quantitative details regarding how fresh specimens were collected. These 24 articles provided specific descriptions of tissue region based on a referable stereotaxic brain origin, as well as exact volume, weight, or thickness. Only three studies described the tools used to prepare tissue samples with fixed sizes from referable brain anatomical locations [30-32]. Twenty-one articles (27%) provided an approximate description of what brain areas were collected but offered no quantitative details, and the remaining 36 articles (44%) provided no information regarding a standardized process or the specific brain region collected in relation to hemorrhage location (see supplemental table for original descriptions).
Figure 1.
(A) Flow chart of literature search and selection. (B) Representative coronal brain sections show the difference in hemorrhagic patterns among striatal (s-), cortical (c-), and hippocampal (h-) intracerebral hemorrhage (ICH) and between severe (s-) and mild (m-) intraventricular hemorrhage (IVH). All hemorrhages were induced in the left hemisphere. (C) Example of a strategic method to obtain uniform and reproducible brain tissue samples for biomedical analysis after s-ICH; ips, ipsilateral; contra, contralateral.
Table 1.
Number of studies by tissue collection strategy
| Year | Number of studies | Did not define strategy (n) |
Defined strategy qualitatively (n) |
Defined strategy qualitatively and quantitatively (n) |
|---|---|---|---|---|
| 2009 | 6 | 2 | 2 | 2 |
| 2010 | 7 | 3 | 3 | 1 |
| 2011 | 15 | 7 | 5 | 3 |
| 2012 | 17 | 9 | 2 | 6 |
| 2013 | 24 | 10 | 8 | 6 |
| 2014 | 13 | 5 | 2 | 6 |
| Total | 82 | 36 | 22 | 24 |
Articles published between Jan 1, 2009, and Aug 8, 2014, were included based on the PubMed database search.
Simply stated, the location from which tissue samples are collected can directly influence biochemical analytic results because the biochemical activities in specialized brain areas can differ substantially and the hemorrhagic territory directly affects these activities. If the description of tissue collection is unclear, the results from biochemical experiments may not be reproducible when the same experiment is undertaken by other laboratories, greatly hindering the development of novel interventions [33]. Moreover, few of the articles that we reviewed contained images of the brain hemorrhagic territory to demonstrate the quality of the experimental model used. Therefore, how the tissue samples are collected should be defined both qualitatively and quantitatively to enhance intra- and inter-laboratory reproducibility and to increase translational success rate in the ICH field. More than 10 years ago, in a preclinical ICH study, Wu et al. [34] qualitatively and quantitatively detailed their method for obtaining brain tissue specimens for biochemical measurements. Their description is exemplary of the information that should be provided in a research article. However, today, the practice of defining what type of tissue specimen is collected for biochemical analysis seems to be ignored. The absence of such basic methodological details should be a concern for researchers in the field of translational ICH research.
A Practicable Approach for Fresh Brain Tissue Sampling
In our systematic review, the articles that provided information about tools used to prepare brain tissue samples all mentioned the brain matrix [30-32]. The brain matrix enables researchers to obtain coronal sections with a fixed distance according to the experimental design. By using a delineated brain matrix, one can cut fresh brain into uniform, 1-mm sections at specific times after ICH induction and display images of these sections as shown in Fig. 1B. Defining hemorrhagic territory in an ICH research paper is important because researchers can further detail tissue sample thickness, volume, weight, and characteristics, as well as the specific brain region(s) collected.
Fig. 1B shows images of brain slices prepared from mice that have undergone striatial, cortical, hippocampal, or intraventricular hemorrhage. Here, we will use striatal hemorrhage as an example to describe the strategy of standardized hemorrhagic tissue processing (Fig. 1C; adapted from Chang et al., Ann Clin Transl Neurol, 2014) because putaminal-capsular hemorrhage is the most common type of ICH in humans, and striatal ICH is the most widely used preclinical ICH model. Initially, two blades are used to separate cerebellum and olfactory bulbs. In this case, the major hemorrhagic territory is located between sections 1 and 4 and affects a coronal area approximately 4 mm in thickness (Fig. 1B; s-ICH panel; hemorrhagic territory may vary depending on the conditions of ICH induction). Therefore, the second blades are used to separate this 4-mm region from the whole brain compartment. Then this region is cut into ipsilateral and contralateral hemispheres. All investigators can obtain a uniform tissue sample regardless of experience level. If hemorrhagic or perihemorrhagic core tissue is needed for specific assays, the third blades can be used to divide the core area from the original 4-mm-thick coronal region (the needle track is within the center of this 2-mm-thick coronal tissue). Hematoma can be either delicately removed from the coronal brain tissue or completely preserved according to the specific experimental objective. Use of this procedure can ensure that the tissue collected by different researchers in one lab is of similar quality. One research team can also ensure uniformity between their samples and those of their collaborators, thereby reducing variation and increasing reproducibility.
Recommendations
Here, we make a few recommendations for future preclinical research and specifically focus on ICH. First, we recommend using methodology for fresh brain tissue collection that is described above and in Fig. 1C to increase inter-laboratory reproducibility in preclinical studies. Second, because hematoma is the ictus of this disease, we suggest that a complete image set of the hematoma territory be provided as supplementary information in a research paper (as shown in Fig. 1B). This strategy can help researchers to understand the quality of the preclinical model(s) that was established, regardless of whether the collagenase or blood model was used. Third, the location from which tissue was collected for biomedical studies should be well-defined according to specific brain orientation or origin. We suggest that researchers use the location of hematoma core or specific brain area as a reference origin to describe the orientation of tissue sample and use a gauged tool to standardize the tissue collection procedure.
Conclusion
In the past, the common endpoints used in preclinical ICH studies relied largely on brain lesion volume, edema, cell death, histological evaluation, and functional tests; only 10% of ICH studies used biochemical endpoints [35]. Now, more and more studies are using biochemical assays in preclinical ICH research (Table 1), emphasizing the importance of the molecular and mechanistic studies. It is widely accepted that the preclinical results should be verified by multiple investigators before clinical trials are conducted [36] and that clinical and basic research scientists require extensive collaboration to complete translational research projects [28, 29]. Reducing research bias and establishing effective communication platforms for lab–lab collaboration will be crucial for accelerating preclinical progress in translational medicine. However, the bias produced when the tissue sampling procedure lacks transparency and standardization may hinder the progress of preclinical ICH studies. Although the strategy suggested here may not meet all experimental objectives, we believe that reporting a standardized and well-defined methodological procedure and the quality of the animal model used could enable researchers to replicate experiments and confirm important preclinical results. Efforts have been increasing in the preclinical ICH field, but current treatment options for ICH still lag far behind those for ischemic stroke; consequently mortality for ICH has failed to decline. Therefore, researchers should start to delineate specific strategies that can minimize inter-laboratory variability and bias for translational ICH studies. We also appeal to researchers to start discussing bottlenecks and communicating new strategies that will reduce pitfalls in translational ICH research.
Supplementary Material
Acknowledgement
This work was supported by grants from the American Heart Association (13GRNT15730001) and the National Institutes of Health (K01AG031926, R01AT007317, and R01NS078026). We thank Claire Levine, MS, ELS, for assistance with manuscript preparation.
Footnotes
Author contributions
C-F.C. and J.W. conceived and wrote the paper, C-F.C. and L.C. designed the search strategy and selection criteria, C-F.C., L.C., and J.W. performed article identification and evaluation, C-F.C. performed hemorrhage models for the representative images. All authors revised the manuscript and read and approved the final version before submission.
Compliance with Ethics Requirements
C-F.C. declares that he has no conflict of interest; C.C. declares that she has no conflict of interest; J. W. declares that he has no conflict of interest.
All institutional and national guidelines for the care and use of laboratory animals were followed.
Contributor Information
Che-Feng Chang, Department of Anesthesiology and Critical Care Medicine, Johns Hopkins University School of Medicine, Baltimore, MD 21205.
Li Cai, Department of Maternal and Child Health, School of Public Health, Sun Yat-Sen University, Guangzhou, Guangdong, China and Department of International Health, Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD 21205.
Jian Wang, Department of Anesthesiology/Critical Care Medicine, Johns Hopkins University, School of Medicine, 720 Rutland Ave, Ross Bldg 370B, Baltimore, MD 21205.
References
- 1.Manno EM. Update on intracerebral hemorrhage. Continuum. 2012;18(3):598–610. doi: 10.1212/01.CON.0000415430.99394.3e. doi:10.1212/01.CON.0000415430.99394.3e. [DOI] [PubMed] [Google Scholar]
- 2.Rutledge WC, Ko NU, Lawton MT, Kim H. Hemorrhage rates and risk factors in the natural history course of brain arteriovenous malformations. Translational stroke research. 2014;5(5):538–42. doi: 10.1007/s12975-014-0351-0. doi:10.1007/s12975-014-0351-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen S, Yang Q, Chen G, Zhang JH. An update on inflammation in the acute phase of intracerebral hemorrhage. Translational stroke research. 2015;6(1):4–8. doi: 10.1007/s12975-014-0384-4. doi:10.1007/s12975-014-0384-4. [DOI] [PubMed] [Google Scholar]
- 4.Xiong XY, Wang J, Qian ZM, Yang QW. Iron and Intracerebral Hemorrhage: From Mechanism to Translation. Translational stroke research. 2013 doi: 10.1007/s12975-013-0317-7. doi:10.1007/s12975-013-0317-7. [DOI] [PubMed] [Google Scholar]
- 5.Dezfulian C, Garrett M, Gonzalez NR. Clinical application of preconditioning and postconditioning to achieve neuroprotection. Translational stroke research. 2013;4(1):19–24. doi: 10.1007/s12975-012-0224-3. doi:10.1007/s12975-012-0224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pandey AS, Xi G. Intracerebral hemorrhage: a multimodality approach to improving outcome. Translational stroke research. 2014;5(3):313–5. doi: 10.1007/s12975-014-0344-z. doi:10.1007/s12975-014-0344-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang J. Preclinical and clinical research on inflammation after intracerebral hemorrhage. Progress in neurobiology. 2010;92(4):463–77. doi: 10.1016/j.pneurobio.2010.08.001. doi:10.1016/j.pneurobio.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hafez S, Coucha M, Bruno A, Fagan SC, Ergul A. Hyperglycemia, acute ischemic stroke, and thrombolytic therapy. Translational stroke research. 2014;5(4):442–53. doi: 10.1007/s12975-014-0336-z. doi:10.1007/s12975-014-0336-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donnan GA, Hankey GJ, Davis SM. Intracerebral haemorrhage: a need for more data and new research directions. Lancet neurology. 2010;9(2):133–4. doi: 10.1016/S1474-4422(10)70001-6. doi:10.1016/S1474-4422(10)70001-6. [DOI] [PubMed] [Google Scholar]
- 10.Selim M, Sheth KN. Perihematoma edema: a potential translational target in intracerebral hemorrhage? Translational stroke research. 2015;6(2):104–6. doi: 10.1007/s12975-015-0389-7. doi:10.1007/s12975-015-0389-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenberg GA, Mun-Bryce S, Wesley M, Kornfeld M. Collagenase-induced intracerebral hemorrhage in rats. Stroke; a journal of cerebral circulation. 1990;21(5):801–7. doi: 10.1161/01.str.21.5.801. [DOI] [PubMed] [Google Scholar]
- 12.Yang GY, Betz AL, Chenevert TL, Brunberg JA, Hoff JT. Experimental intracerebral hemorrhage: relationship between brain edema, blood flow, and blood-brain barrier permeability in rats. Journal of neurosurgery. 1994;81(1):93–102. doi: 10.3171/jns.1994.81.1.0093. doi:10.3171/jns.1994.81.1.0093. [DOI] [PubMed] [Google Scholar]
- 13.Clark W, Gunion-Rinker L, Lessov N, Hazel K. Citicoline treatment for experimental intracerebral hemorrhage in mice. Stroke; a journal of cerebral circulation. 1998;29(10):2136–40. doi: 10.1161/01.str.29.10.2136. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Rogove AD, Tsirka AE, Tsirka SE. Protective role of tuftsin fragment 1-3 in an animal model of intracerebral hemorrhage. Annals of neurology. 2003;54(5):655–64. doi: 10.1002/ana.10750. doi:10.1002/ana.10750. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura T, Xi G, Hua Y, Schallert T, Hoff JT, Keep RF. Intracerebral hemorrhage in mice: model characterization and application for genetically modified mice. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2004;24(5):487–94. doi: 10.1097/00004647-200405000-00002. doi:10.1097/00004647-200405000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Seifert HA, Pennypacker KR. Molecular and cellular immune responses to ischemic brain injury. Translational stroke research. 2014;5(5):543–53. doi: 10.1007/s12975-014-0349-7. doi:10.1007/s12975-014-0349-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirkman MA, Allan SM, Parry-Jones AR. Experimental intracerebral hemorrhage: avoiding pitfalls in translational research. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2011;31(11):2135–51. doi: 10.1038/jcbfm.2011.124. doi:10.1038/jcbfm.2011.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fisher M. The spectrum of translational stroke research. Neurological research. 2013;35(5):443–7. doi: 10.1179/1743132813Y.0000000214. doi:10.1179/1743132813Y.0000000214. [DOI] [PubMed] [Google Scholar]
- 19.Landis SC, Amara SG, Asadullah K, Austin CP, Blumenstein R, Bradley EW, et al. A call for transparent reporting to optimize the predictive value of preclinical research. Nature. 2012;490(7419):187–91. doi: 10.1038/nature11556. doi:10.1038/nature11556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. Journal of pharmacology & pharmacotherapeutics. 2010;1(2):100–7. doi: 10.4103/0976-500X.72352. doi:10.4103/0976-500X.72352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Macleod MR, Fisher M, O’Collins V, Sena ES, Dirnagl U, Bath PM, et al. Good laboratory practice: preventing introduction of bias at the bench. Stroke; a journal of cerebral circulation. 2009;40(3):e50–2. doi: 10.1161/STROKEAHA.108.525386. doi:10.1161/STROKEAHA.108.525386. [DOI] [PubMed] [Google Scholar]
- 22.Lapchak PA, Zhang JH, Noble-Haeusslein LJ. RIGOR guidelines: escalating STAIR and STEPS for effective translational research. Translational stroke research. 2013;4(3):279–85. doi: 10.1007/s12975-012-0209-2. doi:10.1007/s12975-012-0209-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang MM, Xi G, Keep RF. Should the STAIR criteria be modified for preconditioning studies? Translational stroke research. 2013;4(1):3–14. doi: 10.1007/s12975-012-0219-0. doi:10.1007/s12975-012-0219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bahjat FR, Gesuete R, Stenzel-Poore MP. Steps to translate preconditioning from basic research to the clinic. Translational stroke research. 2013;4(1):89–103. doi: 10.1007/s12975-012-0223-4. doi:10.1007/s12975-012-0223-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turner RJ, Jickling GC, Sharp FR. Are Underlying Assumptions of Current Animal Models of Human Stroke Correct: from STAIRs to High Hurdles? Translational stroke research. 2011;2(2):138–43. doi: 10.1007/s12975-011-0067-3. doi:10.1007/s12975-011-0067-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harrold JM, Ramanathan M, Mager DE. Network-based approaches in drug discovery and early development. Clinical pharmacology and therapeutics. 2013;94(6):651–8. doi: 10.1038/clpt.2013.176. doi:10.1038/clpt.2013.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. The New England journal of medicine. 2001;344(19):1450–60. doi: 10.1056/NEJM200105103441907. doi:10.1056/NEJM200105103441907. [DOI] [PubMed] [Google Scholar]
- 28.Nagarajan R, Kalinka AT, Hogan WR. Evidence of community structure in biomedical research grant collaborations. Journal of biomedical informatics. 2013;46(1):40–6. doi: 10.1016/j.jbi.2012.08.002. doi:10.1016/j.jbi.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lo EH. Thomas Willis Award Lecture: Causation and collaboration for stroke research. Stroke; a journal of cerebral circulation. 2013;2014;45(1):305–8. doi: 10.1161/STROKEAHA.113.001269. doi:10.1161/STROKEAHA.113.001269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sukumari-Ramesh S, Alleyne CH, Jr., Dhandapani KM. Astrocyte-specific expression of survivin after intracerebral hemorrhage in mice: a possible role in reactive gliosis? Journal of neurotrauma. 2012;29(18):2798–804. doi: 10.1089/neu.2011.2243. doi:10.1089/neu.2011.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu X, Zhang J, Chen X, Liu J, Lu H, Yang P, et al. The increased expression of metabotropic glutamate receptor 5 in subventricular zone neural progenitor cells and enhanced neurogenesis in a rat model of intracerebral hemorrhage. Neuroscience. 2012;202:474–83. doi: 10.1016/j.neuroscience.2011.12.008. doi:10.1016/j.neuroscience.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 32.Chang CF, Cho S, Wang J. (−)-Epicatechin protects hemorrhagic brain via synergistic Nrf2 pathways. Annals of clinical and translational neurology. 2014;1(4):258–71. doi: 10.1002/acn3.54. doi:10.1002/acn3.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sena E, van der Worp HB, Howells D, Macleod M. How can we improve the pre-clinical development of drugs for stroke? Trends in neurosciences. 2007;30(9):433–9. doi: 10.1016/j.tins.2007.06.009. doi:10.1016/j.tins.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 34.Wu J, Hua Y, Keep RF, Nakamura T, Hoff JT, Xi G. Iron and iron-handling proteins in the brain after intracerebral hemorrhage. Stroke; a journal of cerebral circulation. 2003;34(12):2964–9. doi: 10.1161/01.STR.0000103140.52838.45. doi:10.1161/01.STR.0000103140.52838.45. [DOI] [PubMed] [Google Scholar]
- 35.MacLellan CL, Paquette R, Colbourne F. A critical appraisal of experimental intracerebral hemorrhage research. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2012;32(4):612–27. doi: 10.1038/jcbfm.2012.8. doi:10.1038/jcbfm.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dirnagl U. Bench to bedside: the quest for quality in experimental stroke research. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2006;26(12):1465–78. doi: 10.1038/sj.jcbfm.9600298. doi:10.1038/sj.jcbfm.9600298. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.