Abstract
Adolescence is characterized by heightened risk-taking, including substance misuse. These behavioral patterns are influenced by ontogenic changes in neurotransmitter systems, particularly the dopamine system, which is fundamentally involved in the neural coding of reward and motivated approach behavior. During adolescence, this system evidences a peak in activity. At the same time, the dopamine system is neuroplastically altered by substance abuse, impacting subsequent function. Here, we describe properties of the dopamine system that change with typical adolescent development and that are altered with substance abuse. Much of this work has been gleaned from animal models due to limitations in measuring dopamine in pediatric samples. Structural and functional neuroimaging techniques have been used to examine structures that are heavily DA-innervated; they measure morphological and functional changes with age and with drug exposure. Presenting marijuana abuse as an exemplar, we consider recent findings that support an adolescent peak in DA-driven reward-seeking behavior and related deviations in motivational systems that are associated with marijuana abuse/dependence. Clinicians are advised that (1) chronic adolescent marijuana use may lead to deficiencies in incentive motivation, (2) that this state is due to marijuana’s interactions with the developing DA system, and (3) that treatment strategies should be directed to remediating resultant deficiencies in goal-directed activity.
Introduction
1- Substance use in adolescence
Experimentation with drugs, particularly alcohol, tobacco, and marijuana, is highly common among adolescents. While drug experimentation may be considered virtually normative in the adolescent US culture, regular drug use or the transition into drug problems is not. Some adolescents do use substances frequently, most typically during the week-end, but others use substances daily. These substances are either legally available (alcohol, nicotine, inhalants, prescription drugs, wild plants) or illicit (marijuana, cocaine, narcotics, numerous hallucinogens.)
Epidemiological studies via surveys have been tracking the landscape of adolescent substance use.1–3 Of these studies, we highlight two points, age of onset of initiation and gender distribution. First, although drug use initiation starts at various ages, which is largely dependent on the types of substances used, it overwhelmingly begins in adolescence. For example, initiation prior to the end of 9th grade (~15 year olds) is reported by more than 50% of users of alcohol, tobacco, and inhalants, but by fewer than 30% of users of cocaine or hallucinogens. These rates are probably underestimated because, due to how the survey data were collected, they do not capture heavier users who are school-dropouts. Second, gender distribution seems to vary slightly by drug type. According to the 2009 Youth Risk Behavior Surveillance, among US high school students, 34% of females and 39% of males have used marijuana at least once, 18% of females and 23% of males have used it in the past month.3 Five percent of females and 10% of males report using marijuana for the first time before age 13 years. Inhalant use is reported by 13% of females and 10% of males, and Ecstasy by 5% of females and 7% of males.
In contrast to studies of adolescent substance use, national epidemiologic information on adolescent substance-use disorders is substantially scarcer. Recent findings from the National Survey on Drug Use and Health (NSDUH) reported that about 7% of 12–17 year olds had a diagnosable alcohol or drug disorder (i.e., DSM-IV abuse or dependence on illicit drugs).4 Other epidemiologic data indicate rates of adolescent substance use disorder between 1% and 24%, with a median of 5%, varying in part with age of the sample.5
Finally, youth with a psychiatric disorder are three times more likely to develop a substance use/disorder than those without a psychiatric disorder.6 The most prevalent comorbid disorders are conduct disorder, depression, anxiety, and certain personality disorders. Whereas the directionality of these relationships remains unclear, they suggest common vulnerability factors, as will be discussed below.
Taken together, this brief overview places adolescence as a prime time for the development of substance use problems, which put these youths on a life trajectory fraught with behavioral and mental challenges, potentially jeopardizing a successful transition and integration into the adult world.
2- Behavioral vulnerabilities
The emergence of substance use problems in adolescence coincides with radical transformations at multiple biological and environmental levels. These changes manifest themselves behaviorally and emotionally in ways that have been proposed to facilitate the development of substance use problems. Adolescence is typically associated with higher levels of sensation seeking (e.g., skydiving), risk-taking (e.g., sex without protection) and emotional impulsivity (increased emotional lability and intensity), as well as a social reorientation that shifts the adolescent social world from being family-oriented to becoming peer-oriented.7–12 These behavioral and emotional shifts show large ranges of inter-individual variability, which can reflect unique individual biological predispositions, environmental conditions, and biological-by-environmental interactions. However, the general patterns of accentuated novelty-seeking and difficulty in regulating emotional responses have been described across centuries,e.g., 13,14 and is also observed in other mammalian species.15 Therefore, such a highly conserved behavioral pattern is believed to be evolutionary adaptive and necessary to species survival.15 For the purpose of this review, it is important to note that these behavioral patterns are influenced by ontogenic changes in neurotransmitter systems, including the dopamine system, which is fundamental in the neural coding of reward processes, and, more generally, motivated approach behavior.see reviews 16,17
3- Reward system, dopamine and addiction
Substance use problems represent the prototypical disorders of dysfunction in reward systems. Drug addiction is by definition a disorder of motivated approach behavior, in which the need to approach the addictive object (i.e., drug) is overwhelming and may hijack other approach behaviors necessary to individual survival such as food seeking.18 From a biological perspective, the large body of functional neuroimaging research on addiction has identified key brain regions, which are mainly centered on the frontocortical limbic reward circuitry.for reviews 19–21 Dopamine has been conceptualized as the “reward molecule”,22 and the dopaminergic system has been the object of intensive studies in animals and, more recently, in humans with the advent of neuroimaging. Additional evidence for the central role of dopamine in drug addiction is provided by the fact that the majority of drugs of abuse increase dopamine activity.23,24 However, the precise role of dopamine in the development and maintenance of addiction is still debated. The reasons for the difficulty in clarifying the dopaminergic mechanisms that contribute to addiction are related, on the one hand, to the multiple biological mechanisms implicated in addiction (e.g., serotonin, GABA, and glutamate systems all interact with the dopamine system) as well as the multiple brain regions that are impacted, and, on the other hand, the complexity of the substance addiction phenotype.
By definition, addiction is a chronic relapsing disorder, which manifests itself in different mental/physical states across the addiction cycle (e.g., from craving to drug intake to intoxication to withdrawal to remission). Dopamine may have distinct modulatory roles in these different states. Other basic behavioral and cognitive disruptions, such as impulsivity, or alterations in learning/conditioning, reward processes and executive function, are also proposed to play key roles in the development and maintenance of addiction.
Here, we will concentrate on the core reward-related systems, particularly focusing on subcortical regions, and specifically dopamine function within these regions, which undergoes considerable ontogenic changes during adolescence. We first describe various facets of the dopamine system before delineating the developmental changes that could account for the adolescent vulnerability to substance use problems.see also 15–17,25
Dopamine System
Dopamine function can be examined at many levels, including brain circuitry, receptor units, enzymatic pathways, or cellular firing pattern. We will review broadly the components that can be studied with neuroimaging and for which developmental changes have been described.
1. Dopamine neurocircuitry (see Figures 1 and 2)
Figure 1.
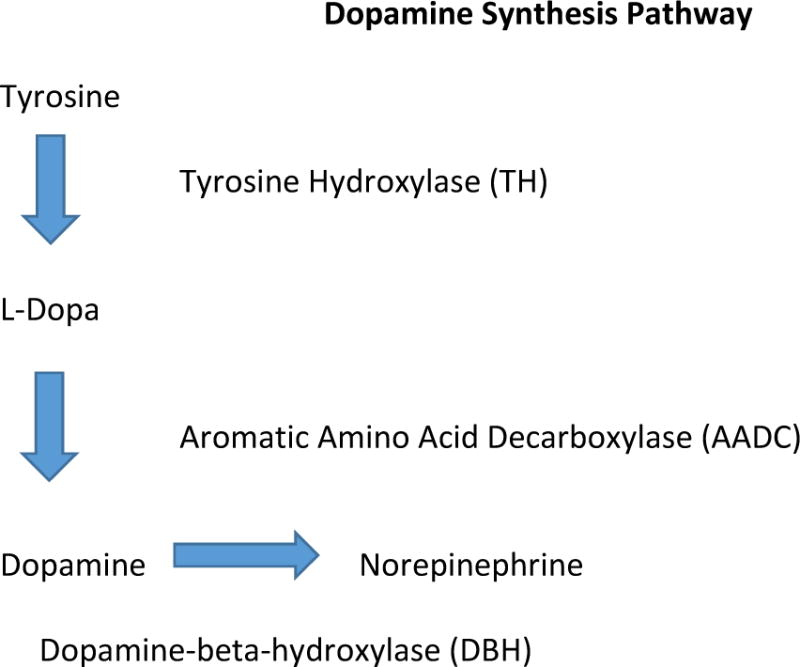
The dopamine synthesis pathway begins with the amino acid tyrosine. Through enzymatic actions, tyrosine is converted to L-Dopa and then to Dopamine.
Figure 2.
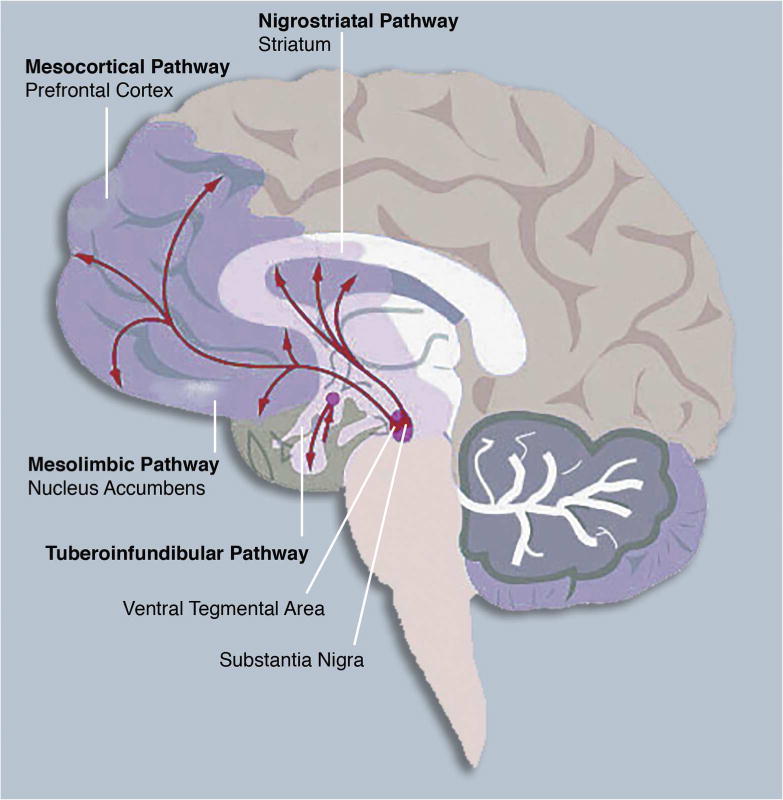
The central nervous system dopamine pathways arising in the ventral tegmental area (VTA) and substantia nigra (SN) of the midbrain. These pathways are implicated in reward processing and are impacted by substance abuse, as described in the text.
As illustrated in Figure 1, dopamine is synthesized from the amino acid, tyrosine through the enzymatic actions of tyrosine hydroxylase (TH) and dopa decarboxlase.26 Within the brain, four major dopamine-rich pathways have been identified, including the mesolimbic, mesocortical, nigrostriatal and tuberoinfundibular tracts (Figure 2). These pathways arise from two regions of the midbrain, the ventral tegmental area (VTA) and the substantia nigra (SN), which contain dopamine cell bodies (i.e., TH-containing neurons). Three of these pathways originate in the ventral tegmental area (VTA) and substantia nigra (SN), and project broadly to facilitate affective, cognitive, and sensorimotor functions. The other pathway, the tuberoinfundibular system, involves hypothalamic-to-pituitary connections that regulate prolactin levels and other hormonal secretions.
More specifically, the VTA projects to the dorsal striatum, prefrontal cortex, thalamus, and parietal regions (the mesocortical DA system) and to core limbic and ventral striatal regions, including, amygdala, anterior cingulate cortex, orbitofrontal cortex, and nucleus accumbens (the mesolimbic DA system).27,28
Dysfunctions in these pathways are linked to psychiatric disorders that are associated with compromised emotion regulation and cognitive function, such as schizophrenia, attention-deficit hyperactivity disorder, impulse-control disorders, and affective pathology.29,30 Most relevant to this review, these pathways are also those that are critically involved in drug addiction.e.g., 19 On the other hand, the SN sends projections to the dorsal striatum, which is implicated more prominently in motor and cognitive processes (nigrostriatal pathway). For example, the degeneration of DA cells in the SN leads to Parkinson’s disease, a prototypical movement disorder pathology.
Dopamine receptors are classified into two broad families (D1-like, consisting of D-1 and D-5 receptors, and D2-like, consisting of D-2, D-3 and D-4 receptors). D-1-like receptors activate adenylyl cyclase through G-protein coupling; they are relatively prominent in mesocortical terminal regions. Mesolimbic terminal regions contain relatively large numbers of both receptor types, but the mesolimbic D-2 system garners most of the research attention in relation to behaviors facilitated by this circuitry.31,32 Receptors in the D-2 family are G-protein coupled, inhibit adenylyl cyclase, and activate potassium channels.33 Mesocortical DA projections and termination sites have been implicated in cognitive control functions such as behavioral flexibility, working memory, and high level decision-making.34 Individual differences in functional activation within mesolimbic structures, particularly the nucleus accumbens/ventral striatum, are associated with positive incentive motivation, the motivation-action interface, and heightened responses to substances of abuse.35 It has been estimated that 75% of the VTA-to-ventral striatal projection is dopaminergic.36
DA models of drug addiction and incentive motivation also posit influences of other chemical systems, given DA’s neuromodulatory regulation of various neuronal populations, its frequent co-release with glutamate and gamma-Aminobutyric acid (GABA), and accordingly, the combination of excitatory and inhibitory effects that it exerts within neural pathways.19,37,38
2. Firing Modes
When depolarized, DA cells display two firing modes, tonic and phasic. While these exert independent influences over behavior, the difference between the levels of tonic and phasic activity also modulates the intensity of the behavioral output.see 39 for discussion Thus, both tonic and phasic tones are important to understand.
The tonic mode is low in frequency, resulting in low concentrations of extracellular DA. It reflects basal or resting DA neuron firing patterns, regardless of behavioral stimulation.40,41 Extracellular DA levels are regulated by presynaptic autoreceptors, the action of the DA transporter (via reuptake) and by catabolic enzymes (i.e., monoamine oxidase, catechol-o-methyltransferase). Tonic firing is controlled by the neuron’s membrane properties and is regulated by GABAergic inhibition.42,43
In contrast, phasic firing is high frequency, characterized by transient bursts of activity.44–46 Relatively high levels of DA are released into neural synapses as a result. Phasic activity is triggered by environmentally salient events, particularly in the context of instrumental learning.46–48 Phasic bursts within the striatal region are influenced by glutaminergic excitatory input from brainstem and midbrain sources.
The nature of the relationship between the two firing modes is debated.43,49 Schultz and otherssee review 48 have demonstrated that phasic DA activity increases in the context of unexpected rewards and in response to reward during initial phases of instrumental reward learning. Furthermore, tonic DA levels transiently decline when anticipated rewards are not delivered or when animals encounter aversive stimuli. For learning to proceed optimally, phasic signals must be detectable. The detection threshold is lowered in the context of decreased background (tonic) firing rates, suggesting that interactions between the two firing modes are important in influencing behavioral output. However, increasing attention is directed to the study of tonic dopamine levels, which could contribute to traits that reflect individual differences in incentive motivation.47,50,51 Accordingly, each mode of firing independently influences behavior, but interactions are also important. Tonic DA may represent the substrate for basic approach or incentive motivation, while phasic DA reflects learning as it unfolds as a function of experience.
3. Dopamine System Changes with Adolescent Development
Given the complexities of DA synthesis, release, enzymatic activity in neural synapses, receptor density, and receptor sensitivity, it is beyond the scope of this review to consider developmental changes in all aspects. We refer the reader to recent reviews of this topic.16,52 We focus here on shifts in DA receptor densities during adolescence as well as changes in tonic and phasic DA activity.
a) DA receptor changes during adolescence
Dopamine receptors are expressed early in development and change in number through the lifespan. Generally, D-1 receptors are more abundant than D-2 receptors regardless of the lifespan period.53,54 However, their developmental trajectories differ. Regarding D1 receptors, rat studies have revealed that striatal D1 receptors increase in number during adolescence and decrease thereafter.53,55,56 This developmental pattern is most marked in the dorsal striatum, caudate and putamen.54–57 Reports on the temporal pattern of developmental changes in D-1 receptor density in the ventral striatum are more inconsistent, with some studies reporting steady increases,58 and others suggesting a periadolescent-limited peak,55 followed by a decline and then another peak in late adulthood.53,56
Similarly, D-2 receptor density peaks in the caudate and putamen in early puberty.54,56,59,60 In addition, D-2 density was found to increase linearly in the medial prefrontal cortex (PFC) up till adulthood.59 In the nucleus accumbens, D-2 receptors do not exhibit the same late adulthood increases in density as do D-1 receptors.53,56
These changes in receptor profiles are important to describe, because tonic and phasic firing modes may be differentially associated with the activation of distinct receptor subtypes.25 Phasic DA release activates D1 receptors to facilitate inputs from the VTA to mesolimbic structures, enabling the learning of behavioral strategies during reinforcement learning.43 Accordingly, the increases in D-1 densities observed in the striatum during adolescence might ultimately facilitate aspects of reinforcement learning that are mediated by phasic DA signaling. In contrast, tonic DA influences the VTA-mesocortical projections through D2 receptor actions. Increases in tonic D-2 stimulation dampen afferent inputs to the PFC, whereas decreases in tonic D-2 stimulation facilitate those inputs.43 These D-2 mediated effects ultimately enable switches to new response strategies when current responses fail to yield anticipated rewards.62 There may be increases in tonic D-2 stimulation during adolescence given the observed increase in striatal D-2 receptor density. Ultimately, this increase would serve to enhance behavioral flexibility.
Recently, it was found that there is an age-dependent function of a subtype of D-2 receptors. Activation of these receptors in adolescence, but not other periods of the lifespan, decreases synaptic spine development, providing evidence that these receptors play a role in synaptic pruning given that a decline in spine density is part of the pruning process.61 Given the evidence for peak D-2 receptor production during adolescence, this finding suggests an important link between neurochemical changes during development and resultant alterations in regional brain morphology.
The role of DA in the pruning process has also been explained in relation to developmental changes in PFC glutamatergic (GLU) activity. Both DA and GLU systems contribute interactively to neuroplasticity, which is prominent during adolescence.63 Particularly, the developmental trajectories of PFC GLU and striatal DA systems may interact across adolescence in ways that increase both the risk for addictive behaviors and the deleterious consequences of exposure to addictive substances on neurodevelopment.64,65 Glutamate receptor (GLUR)-mediated synaptic plasticity, via configurations of the N-methyl-D-aspartate (NMDA) and alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptor systems, generate long-term depression (LTD), which is thought to underlie synaptic pruning, a characteristic of neural changes in adolescence.64 Importantly, D-1 receptor activation promotes LTD through indirect actions on AMPA receptors.66 Thus, the proposed increase in striatal dopamine signaling in adolescence25,52 may, through D-1 and D-2 receptor-mediated mechanisms, contribute to the regulation of synaptic pruning within the striatum, but also in higher cortical regions, through glutamatergic mechanisms. The DA/GLU interactive effects on the progression of the pruning phase may be disrupted by exposure to substances of abuse during the sensitive period of adolescence, and have unique long-term consequences, not seen when drugs are used after this developmental period.67,68 Although changes in other neuromodulatory systems (i.e., GABA) and their maturing interactions with DA function are also important to consider, coverage of these interactions is beyond the scope of this review.
b) Tonic and phasic dopamine activity
We have previously summarized evidence that tonic DA levels underlie adolescent-specific developmental increases in incentive motivation.17,25,39,52 Tonic firing rates in the VTA are observed to be higher in adolescents than in adult animals.69
We have suggested that these increases enhance approach behavior, and serve to bring adolescents into contact with reward contexts (even those that confer risks). The tendency to approach contexts that are high in reward salience is supported by adolescents’ social, sexual, and other risk-taking behaviors,3 by their self-reports of reward-responsivity and sensation-seeking,70–72 and by brain responses as observed in laboratory-based neuroimaging studies of adolescent reward processing.39,73,74 Reward-relevant structures within the mesolimbic DA system are most strongly activated, relative to children and adults, when adolescents experience or anticipate rewards that are highly salient. In real-world contexts, these reward stimuli may include substances of abuse. Thus, adolescent peaks in tonic dopamine levels encourage experimentation with drugs of abuse as well as engagement with other rewarding stimuli (see Figure 3).
Figure 3.
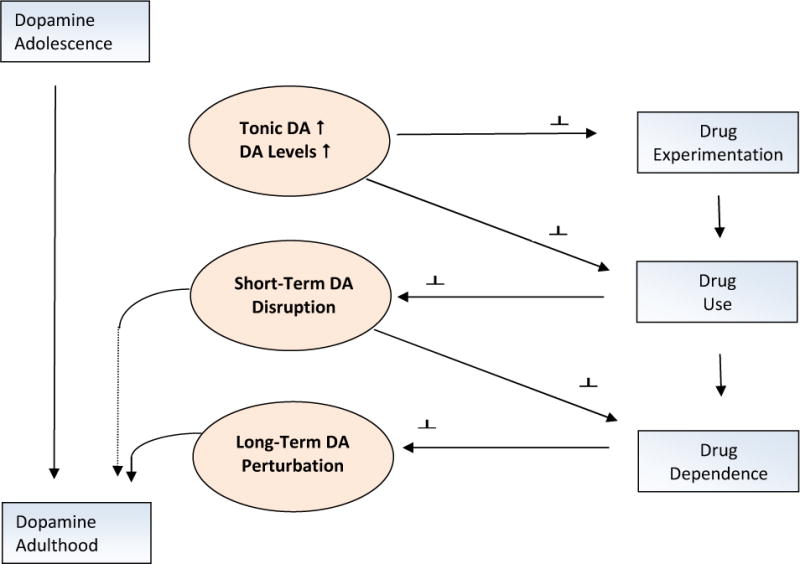
The dopamine system becomes more functionally active during adolescence through increases in tonic DA activity and phasic signaling. These changes are associated with experimentation with drugs of abuse. With increasing drug use, parameters of DA transmission are neuroplastically altered, leading to drug addiction and long-term alterations in the reward system function.
Contexts in which rewards are present bring opportunities for learning. When an individual encounters a potentially rewarding object or situation, he or she must make a decision about whether consumption of that stimulus will lead to a positive versus a negative outcome. The decision to consume is partly determined by individual differences in tonic dopamine, since those with higher levels of tonic dopamine (and accordingly, higher levels of incentive motivation) are more responsive to such cues in the environment. For instance, in animal models, increases in incentive motivation are associated with heightened tonic DA activity in the striatum and with increasing response vigor, as demonstrated through behavioral and computational models.50,75 Importantly, though, outcomes of decisions during reinforcement learning may be particularly uncertain in adolescents, who are not necessarily familiar with social contexts, potential romantic partners, illicit substances, or other sources of positive reinforcement. Feedback or outcome-based learning, when outcomes of behavioral choices are uncertain, is accompanied by phasic bursts of DA in striatal regions. Through repeated experiences, the individual must learn how to make decisions despite this uncertainty in order to promote adaptive future behavior. This decision-making process involves regions of the frontal cortex. Accordingly, during the learning process, prefrontal circuits are called upon in a “bottom up” manner, via subcortical-to-cortical relays, to structure behavior. Notably, there is some evidence of relative increases in these learning signals (attributed to phasic dopamine) during adolescence.76 When individuals use substances of abuse, all of which activate the dopamine system as do natural rewards,77–79 there are bursts of phasic dopamine firing.80
If tonic signals are high in the adolescent as we hypothesize, then these phasic signals must be very strong in magnitude to serve as appropriate learning signals. Accordingly, the adolescents appear to be vulnerable to risky behaviors because of a strong drive (related to high dopamine tone) to seek rewards or rewarding contexts, which are extremely compelling. These contexts, in turn, will be particularly potent sources of learning (under the control of the phasic tone). The value of such a system is apparent when behavior is appropriately regulated. However, in adolescence, such regulation is inconsistent, leaving the adolescent vulnerable (behaviorally and neurobiologically) to the effects of illicit substances, especially when they are used in high amounts.
Over time, our assertion is that regular experience with phasic dopamine, in the context of probabilistic learning in novel contexts, “teaches” (through subcortical to cortical afferent signals) the prefrontal cortex to activate in service of behavioral regulation. Ultimately, prefrontally-mediated behavioral control can be engaged more readily as the transition into adulthood is reached.25,39 Concordantly, Mastwal et al.81 recently compared young adolescent with adult mice on phasic and tonic dopamine activity. Using optogenetic stimulation and in vivo two-photon imaging, they observed in the adolescent animals a potentiation of mesocortical DA circuit activity after a period of induced phasic (but not tonic) DA activation. Moreover, phasic activation led to lasting changes in meso-prefrontal structure and function. Overall, these findings indirectly suggest that the pursuit of motivationally salient events in adolescence is a positive and necessary behavioral change, one that promotes the development of self-regulatory control. This pursuit is facilitated by tonic increases in DA, rendering the adolescent more receptive to, and in fact eager to pursue, positive incentives. This engagement provides critical contexts for learning and enhances the later functional integrity of frontal circuits that are important for behavioral regulation. Changes in tonic and phasic DA levels during reinforcement learning occur very rapidly, at the sub-millisecond level, to promote immediate behavioral responses. Recently, an extended mode of reward-predictive dopamine signaling in the striatum was identified. It enabled animals to approach distant (versus immediate and proximal) goals,82 lending support to the notion that adolescent-limited changes in tonic and phasic dopamine signaling have longer-term behavioral relevance.
In sum, the above-described changes in various elements of the dopamine system during adolescence are thought to underlie the typically adolescent behaviors that promote risk for substance use. While the developing neural system is, on the one hand, plastic and open to sources of positive influence,83 on the other hand, it is also highly sensitive to adverse experiences, and, accordingly, the introduction of toxic compounds bring risks for life-long alterations in neural structure, function, and behavior.84 With the advent of imaging techniques we can now study in vivo the dopamine system in humans and its developmental changes. These techniques, their strengths and their weaknesses are described in the next section and summarized in Table 1.
Table 1.
Neuroimaging Techniques to Measure Central Dopamine Function in Humans
| Technique | Description | Outcome measures | Strengths | Limitations | Used in pediatric samples? |
|---|---|---|---|---|---|
| Positron Emission Tomography (PET) | A radioactive ligand is administered intravenously to index DA release or receptor binding | Metabolic increases or decreases measured in discrete brain regions of interest where the ligand has bound | Responsivity of the DA system is directly measured | Poor spatial resolution as compared to fMRI; poor temporal resolution; complex procedure is ethically challenging when used with vulnerable populations | No |
| MR-spectroscopy | Single or multiple voxels are targeted for neurochemical examination in an MR environment | Levels of neurochemicals are measured; these reflect cellular processes involved in energy expenditure | Relative ease of data collection; non-invasive; impairments at the cellular level can be assessed in the living brain | Difficult to examine whole-brain; resolution limited at low field strengths; only chemicals present in large concentrations can be measured | Yes |
| Structural MRI | Whole-brain or regional brain volumes are measured | White matter volumes, indices of white matter connectivity, and gray matter volumes in areas known to be innervated by DA | Relative ease of data collection; non-invasive | Links between structure and function are indirect and unclear in relation to neurochemical function | Yes |
| Functional MRI | Whole-brain or regional brain activations are measured through blood-oxygenation-level-Dependent (BOLD) signals during a behavioral task or at rest | Activations are measured in areas known to be innervated by DA; these activations can be provoked through the use of well-designed behavioral probes | Ease of data collection; non-invasive; use of behavioral probes in the scanner allows function to be directly linked to neural signals | BOLD signal cannot resolve increases or decreases in activation at the neurochemical level; interpretation is challenging | Yes |
| Pharmacological Challenge During fMRI or PET | Individuals systemically ingest drugs that activate (agonists) or inhibit (antagonists) DA release or receptor activity; can be combined with fMRI or PET | Behavioral functions typically compared pre-versus post-ingestion; some recent studies combine with neuroimaging to index brain function following drug challenge | Probes can be utilized that have selective effects on various aspects of DA synthesis or receptor function; many treatment applications | Most studies are conducted under acute challenge conditions; long-range effects of drug ingestion less well-studied; systemic administration has poor specificity for regional brain effects | No |
Neuroimaging techniques to study the dopamine/reward system in humans (Table 1)
1) Brief review of neuroimaging techniques
The two major types of imaging techniques include the radionuclear and the electromagnetic techniques. Radionuclear techniques consist of single photon emission computed tomography (SPECT) and positron emission tomography (PET), originally used to assay a non-specific index of brain activity, i.e., regional cerebral blood flow (rCBF) or regional cerebral metabolic rates of glucose (rCMRglc), but currently utilized to investigate specific neurochemical systems (e.g., neurotransmitter systems such as D2 receptor density with the [11C]-raclopride ligand). Electromagnetic imaging uses magnetic resonance (MR) properties of the body constituents to assess morphology (structural MRI, sMRI), fiber tracks (diffusion tensor imaging, DTI), function (functional MRI, fMRI) and concentrations of chemicals present in large quantities in the brain (magnetic resonance spectroscopy, MRS).
FMRI has replaced the PET/SPECT non-specific measurements of brain activity (CMRglc, rCBF) for several reasons. FMRI provides indices of rCBF changes with a temporal resolution down to 1 s (vs. 60 s with PET) and spatial resolution of 1 mm (vs. 4 mm with PET). However, its main advantage is the absence of exposure to ionized radiation, which permits investigators to study pediatric subjects and to conduct multiple repeated studies within the same subject. Using repeated scans, fMRI can also provide measures of functional connectivity which have raised a huge interest. These studies examine functional connectivity changes in response to a task (connectivity modulated by cognitive processes), or during resting state.
However, MRI does not permit the study of neurochemical systems in the brain, which is the monopoly of PET/SPECT. Indeed, while MRS provides measures of regional concentrations of brain chemicals, such as amino acids and metabolites (e.g., metabolites of glutamate [Glu], N-acetylaspartate [NAA] and gamma-aminobutyric acid [GABA]), it is limited by its high threshold of detection (millimolar range with MRS vs. nanomolar range with PET or SPECT). In addition, the roles of the various biological compounds measured with MRS are still unclear, thus complicating the interpretation of findings.
The scientific yield of these neuroimaging techniques can be potentiated by pharmacological challenges. For example, fMRI studies can be paired with a dopamine specific drug challenge (e.g., methylphenidate) to examine the effects of dopamine modulation on specific regional functions. In PET/SPECT, such pharmacological challenge can serve to examine the integrity of dopamine receptor binding potential when synaptic dopamine concentration is being manipulated.85,86
Specifically, in PET imaging of the dopamine system, a radioactive ligand (e.g., raclopride, a D2 receptor ligand) is injected intravenously into the bloodstream. Using mathematical models, PET/SPECT allows the quantification of the radioactivity being bound to receptors, which is translated into receptor binding potential. Specific ligands have been developed that cross the blood-brain barrier to label dopamine receptors, transporters, precursors, or enzymes that degrade dopamine.85 Other tracers bind to glucose and can be measured to infer more general changes in brain metabolism (CMRglc). When combined with pharmacological manipulations, these tracers/ligands can be used to assess functional changes within the brain following systemic manipulations of DA.
2) Neuroimaging limitations
It is considered unethical to administer pharmacological probes to pediatric samples, including older adolescents, who are not otherwise being treated for clinical conditions. Furthermore, because PET relies on radioactive ligands, it is not approved for use in pediatric research.but see 87 Accordingly, human pediatric imaging studies have relied on MRI techniques, which offer less direct measures of metabolic activity. Typically, fMRI involves measures of blood-oxygen-level-dependent (BOLD) signal changes, which reflect synaptic activity, but can be affected by rate of blood flow, which may vary as a function of age.
A limitation to the study of the dopamine system with conventional MRI techniques is the difficulty of reliably ascertaining signals in small structures such as midbrain nuclei (i.e., VTA), or brainstem regions that are the sources of DA synthesis and release.but see 88 This is true regardless of age group studied. The use of more sophisticated MRI scanners, with higher field strengths (7-tesla), might permit us to more reliably capture individual differences in small structures.89
Finally, fMRI requires complete stillness of research participants, which is difficult for young participants, and can make certain studies impossible to conduct in youths. Recent studies have also focused on structural attributes of regions that are strongly DA-innervated.
We will show how these various imaging techniques can help in the delineation of dopamine-linked changes associated with drug exposure starting in adolescence. To this aim, we take marijuana studies as exemplars. We focus on structural MRI (sMRI), fMRI, and spectroscopy.
MRI studies of the Dopamine Reward System in Marijuana Users
1- Scope of the adolescent marijuana use problem
Marijuana use is becoming a critical issue in view of the spreading legalization of this drug across the US. The implicit message sent by the legalization of marijuana is that this compound is not harmful. At the same time, marijuana is the most commonly used “illicit” drug among teenagers in the United States. Whereas 17% of 8th graders have tried marijuana, almost half of teens have used marijuana by 12th grade.90,91 Most critically, many youths develop a regular pattern of use after initial exposure, with 20% of 12th graders reporting use in the past month, and 5% of 12th graders reporting daily use.91
Together, these data are alarming, particularly since the deleterious effects of the regular use of marijuana in adolescents might have serious, long-lasting consequences. Indeed, the stakes may be higher for adolescents than for adults, because adolescence qualifies as a sensitive period in brain development, and because of the specific dysfunctions associated with marijuana use, including addictive liability, cognitive deficits, and anhedonia.92
First, regarding addiction, albeit still debated, evidence indicates that long-term marijuana can lead to dependence, but also predispose to the use of other illicit drugs. In addition, a well-described cannabis withdrawal syndrome (including irritability, sleeping difficulties, dysphoria, craving, and anxiety) has been identified93 and can impinge cessation attempts and contribute to relapse. The addictive aspect of marijuana is likely to involve changes within the dopamine system, changes that are shared by all addictive drug actions.94 Second, neurocognitive deficits have been reportedsee review 95 in adult and adolescent marijuana users, affecting spatial and visual learning and memory (particularly short-term memory), executive functioning and psychomotor speed. Although not consistently, studies suggest that these deficits may be long-lasting. Cognitive deficits in adolescence are particularly troublesome because of the academic implications, which can have far-reaching consequences for the optimal future occupational and social insertion of the adolescent. Here again, the dopaminergic system is thought to play a critical role in “tuning” the prefrontal cortex for optimal cognitive function.see review 96
Akin to tobacco smoking for which a huge effort has been made to inform the public on tobacco’s adverse effects, marijuana should become a target for informing the population at large of the potential negative short-term and long-term outcomes of regular marijuana use, particularly in adolescence. Neuroimaging findings could help in this mission. Here, we focus on studies that address the influence of marijuana exposure on the dopamine reward system in adolescents. Among subcortical regions where dopamine receptors are prominent, the active ingredient in marijuana, Delta-9-TetrahydroCannabinol (THC), binds to endogenous cannabinoid receptors in the nucleus accumbens and in the amygdala.97 Synaptic transmission in these regions is altered after marijuana use.see 98 for discussion We emphasize studies that focus on dopamine-innervated structures in the reward system; among these are the nucleus accumbens of the ventral striatum, medial prefrontal cortex (including orbitofrontal and anterior cingulate regions), and amygdala.
2-Structural neuroimaging with sMRI
a) Normative development
Until recently, it has not been possible to reliably measure the volumes or anatomical connectivity of subcortical regions that comprise the dopaminergic reward system. Using automated parcellation techniques, several groups have examined the development of structures such as the nucleus accumbens, amygdala, and medial prefrontal cortex across healthy adolescent development. These findings are important to our understanding of normative developmental trajectories and how substance use might impact those trajectories. For instance, Urosevic et al.72 reported that the nucleus accumbens increased in volume from early to mid-adolescence (a pattern also reported by Dennison et al.),99 and decreased in volume thereafter, demonstrating a quadratic pattern. Self-reported reward responsivity showed a similar pattern with a peak in mid-adolescence and a decline thereafter, suggesting an association between these processes. This quadratic pattern is important given that it could reflect an adolescent-specific peak in dopamine-mediated reward sensitivity. Use of substances of abuse during this time has the potential to alter the development of the system. In contrast to these findings for the nucleus accumbens/ventral striatum, Dennison et al.99 found that the caudate, thalamus and putamen declined in volume. Amygdala volume did not change over time. Ostby et al.100 reported heterogeneity in developmental trajectories among basal ganglia regions and between subcortical and cortical regions. Overall, these studies suggest that early-to-mid-adolescence represents a potentially important time period for the development of subcortical regions that are key nodes of the reward processing network.
Individual differences interact with age-related changes. Schneider et al.101 used voxel-based morphometry (VBM) and found, in 14 year-olds, that greater risk-taking bias was associated with, and partially mediated by, lower gray matter volumes in the ventral striatum. With increased risk-taking bias, there was a decrease in the functional activation of the ventral striatum during reward anticipation. Given that risk-taking propensity is an important predictor of adolescent substance use, these findings are noteworthy in stressing a role for the ventral striatum in this association. Concordantly, Urosevic et al.102 found that substance-naïve adolescents with smaller left nucleus accumbens volumes, as well as those with higher self-reported reward sensitivity, were more likely to initiate substance use in the subsequent two years. Accordingly, it is clear that individual differences in baseline measures of brain structures in children are important in predicting who will go on to engage in problematic substance use. Of note, these baseline indicators are linked to the DA system.
b) Effects of marijuana on brain morphology
With respect to structural brain changes associated with marijuana use, much of the literature is characterized by cross-sectional case-control comparisons through which marijuana-using adolescents or young adults are compared to non-using controls. Baseline assessments of marijuana users, before onset of substance use, are frequently unavailable. Thus, it is unclear whether observed differences from control samples represent baseline differences between groups or consequences of drug use. Nonetheless, alterations in frontal gray matter density have been observed with recreational marijuana use,98 and there are many findings of altered white matter structure in frontal regions in users.103–106 The pattern of abnormalities across studies is consistent, with reports of increased mean diffusivity of white matter as well as relative decreases in the directionality of fibers and fiber coherence (measured with DTI) in users.
Cause-effect links are indirectly suggested by associations between these differences and parameters of drug use. Earlier use of marijuana has been found to be associated with more pronounced deviations as well as with increased behavioral impulsivity.105 These studies generally have not focused on the reward system per se and links to the dopaminergic system are tentative at best. However, a recent study addresses this shortcoming.98 Young (~age 20) recreational users of marijuana were compared to demographically-matched non-using controls. Analysis of whole-brain gray matter volume densities revealed greater densities in marijuana users in the left nucleus accumbens, extending to subcallosal regions, the hypothalamus, the extended amygdala as well as in the left amygdala proper.107 In the left nucleus accumbens, average volume was also higher in users than controls, and its surface topography was altered. Animal studies have indicated that exposure to THC increases dendritic length and branching in the shell region of the nucleus accumbens, but not in other areas of the striatum or cortex,108 which could account for increased volumes in marijuana users.
The finding of increased gray matter density for the amygdala coheres with other reports of increased density in relation to severity of marijuana dependence,109 but suggests that brain structure is altered even before behavior has reached the level of addiction, given that this study focused on recreational users. Generally, these studies suggest that the structural development of the DA-modulated reward system is important in promoting increases in reward sensitivity during adolescence; these same structures are altered in the context of recreational marijuana use.
3- Functional neuroimaging
Most of the functional imaging work relevant to marijuana use in adolescents has actually been conducted in adults presenting with variable levels of marijuana use, and most importantly, variable ages of onset of marijuana use. Only a few imaging studies have been conducted in adolescents, and none focused on the dopamine system.110–112 Both types of functional imaging have been used, PET and fMRI. We present selected studies for each methodology.
Using PET and the D2/D3 receptor ligand [11C]raclopride, 24 non-user adults were compared with 24 marijuana users on the effects of methylphenidate (MP) on dopamine activity. MP blocks dopamine transporters, and, in turn, increases synaptic dopamine concentration.113 Findings revealed no group differences at baseline, in contrast to blunted receptor availability when challenged with MP, in marijuana users compared to non-users. The decreased dopamine reactivity to MP in marijuana abusers vs. non-users was associated with higher self-report of negative emotionality and craving.
On the other hand, the absence of group differences at baseline is consistent with other studies of striatal D2/D3 receptor availability in four studies of chronic cannabis users compared with healthy controls.114–117 However, in the only study where the chronic cannabis users were not abstinent,114 an inverse correlation between recent cannabis consumption and D2/D3 receptor availability was found, suggesting a direct effect of cannabis smoking on the expression of striatal DA receptors in heavy cannabis users.114 Thus, two factors are important to evidence dopamine-related perturbations associated with marijuana exposure, the pharmacologic (e.g., MP) activation of DA function, and the direct action of the drug (non-abstinent users).
The prominent action of marijuana use that started in adolescence seems to be a blunting effect on dopamine activity. This effect is further substantiated by PET studies of young adult users who present a blunting of striatal dopamine release in long-term cannabis users,118 as well as reduced dopamine synthesis capacity.119 These findings may be important to our understanding of the frequently reported observation of anhedonia or lack of motivation in chronic users.
Using fMRI with a decision-making task, Nestor et al.120 and van Hell et al.121 recorded neural activity during reward and anticipation of loss with different versions of a monetary reward task. Both studies failed to reveal behavioral differences between the groups. However, Nestor et al.120 reported a greater right ventral striatal activity in cannabis users than controls during reward anticipation. This measure was significantly correlated with number of years of lifetime cannabis use. In addition, a post-hoc analysis of the contrast loss/win cues vs. no-outcome cues showed greater BOLD response in the right ventral putamen in marijuana users vs. non-users.120 Conversely, comparing cannabis users to non tobacco-smoking controls, van Hell et al.120 demonstrated attenuated activity in the nucleus accumbens and caudate nucleus bilaterally during reward anticipation, as well as left putamen and other prefrontal regions.121 These findings could be seen to be at odds with the PET study of MP challenge of DA function. A blunted dopamine response to a stimulant challenge might suggest lower striatal response to a reward-related behavioral challenge. It is difficult to integrate these two findings as of yet, because of the many factors that differ between these studies, e.g., the methodology, the significance of the D2/D3 receptor measure at the behavioral level, and the nature of the samples.
Finally, a systematic study of the functional connectivity of the dopamine circuitry across development and in response to drug exposure can greatly enhance our understanding of how such exposure impacts the developing brain as well as the behavioral consequences of drug use.
4- Spectroscopy
Very few studies have used MRS to investigate the impact of marijuana on brain chemistry.see review 122 In addition, only a limited number of these studies have focused on the adolescent period; select studies are described here.
Across studies, individuals ages 16-to-42 years have been tested using either single or multiple voxel techniques. Some studies have focused only on males, given higher use rates in males versus females.123,124 In most cases, last marijuana use was reported at 20 or more days per month. Lower levels of Glu, N-acetyl-aspartate (NAA), and myo-inositol (mIns) were observed in marijuana users compared to controls in regions known to be associated with substance use, including the basal ganglia (lower Glu, NAA and choline,125 lower glutamine [Glx] and higher mIns in females),126 thalamus (higher total creatine [tCr]),125 cingulate cortex (lower Glu, NAA, tCr, and mIns),127 dorsolateral prefrontal cortex (lower NAA),124 and the striatum as well as posterior cortical regions (lower mIns).123
Because of the limitations of the single voxel approach, it is unclear whether these findings would generalize to ventral striatal regions. However, the pattern of findings suggests that marijuana users may be vulnerable to gliosis and white matter injury as a consequence of chronic use. Moreover, because DA interacts with both GABA and glutamate systems in the striatum as well as other regions, and because this network of neurotransmitters is adversely impacted as addiction progresses,128 these findings across studies are cumulatively suggestive of a broader range of impairments in the brain’s reward processing and learning networks.
Conclusions
A neurodevelopmental approach to understand the mechanisms underlying the transition from drug experimentation to drug abuse is essential to the formulation of primary and secondary treatment strategies. Adolescence is the critical period of vulnerability because of the unique neuroplasticity of the brain, which significantly affects the morphology and functional dynamics of the dopamine system, the key neural substrate of drug addiction.
A large body of work is being devoted to the understanding of the normative maturation of the dopamine system on the one hand, and the transient and long-lasting effects of addictive drugs on dopamine function on the other hand. The integration of these two lines of research is complex and depends on the formulation of models that can be tested in animals and humans, feeding a continual dialogue between both basic and clinical fields.
The complexity of this work is phenomenal because of the nature of the dynamics of drug addiction, a moving target that is defined in, not one, but three distinct time frames: ontogenic trajectory, severity of drug problem (initiation, use, dependence), and addiction cycle (craving, withdrawal). In addition, polysubstance use is the rule rather than the exception, which complicates research in humans. Many other biological and environmental factors interact with the propensity for developing a substance use problem. Therefore, it is only through a wide variety of research approaches that the development and maintenance of drug addiction can be understood.
Neuroimaging techniques, including PET/SPECT and MRI provide unique tools that can be used in both animals and humans. As illustrated with marijuana use, neuroimaging studies clearly document alterations within the dopamine system that are linked to behavioral changes associated with drug actions. These findings not only provide pieces to the puzzle of addiction, but can serve as persuasive evidence for the profound effects of chronic use of drugs on brain function. These can also be of great benefit to public health programs concerned with preventing drug abuse.
The prevention of drug use starts with reducing drug experimentation. At this stage, three factors can be impacted, drug access, beliefs and attitudes towards drug experimentation, and risk-taking propensity. The issue of drug access is outside the scope of this review. The second factor, beliefs and attitudes can be influenced by information delivered through the media, school, and home. Clear information on the potential short- and long-term deleterious effects of drugs, particularly marijuana, alcohol, tobacco, the most commonly first drugs used in adolescence, needs to be disseminated. Findings from neuroimaging research can be quite eloquent as “pictures are worth a thousand words”. The third factor raises the question of how to redirect risk-taking behavior in adolescence to adaptive and constructive experiences. Can we train the reward system to respond more strongly to certain stimuli than others? This type of intervention is already being used in anxiety with the development of attention modification training, helping anxious individuals to change their attention bias away from negative stimuli.129 The strategy of targeting attention bias is also receiving interest for the treatment of substance use,130 but has not been considered at the experimentation stage of substance use. Lastly, we believe that any intervention in adolescents should consider the influence of the adolescent social milieu, particularly peers, given the tremendous importance of social relationships in adolescence.10 In sum, more work needs to be done to examine behavioral manipulations that could train and protect the reward system against attraction to dangerous stimuli such as “illicit drugs”.
Regarding the next stage of drug use/dependence, the development of treatment strategies is badly needed for adolescents. One promising approach is the Motivational Enhancement Therapy (MET) & Cognitive Behavioral Therapy (CBT) (MET/CBT).131,132 MET/CBT has been tested for the treatment of cannabis in youths,133 and is particularly relevant to drug-related perturbations of the reward system. This treatment has been found to be efficacious in adolescent marijuana abuse. Identifying how this treatment affects striatal and dopamine function is a critical next step.
In sum, the study of the dopamine/reward system for primary and secondary treatment of substance use problems, combined with a clear understanding of the changes within this system during adolescence, should be a focus of intense scrutiny, and neuroimaging can assist us in the rationale, the design, and the testing of novel therapeutic approaches.
Biographies
Monique Ernst is a child and adolescent psychiatrist and holds a PhD in Neurophysiology. Monique Ernst is Head of the Neurodevelopment of Reward Systems Program, Senior Staff Clinician of the National Institute of Mental Health, Bethesda, MD.
Monica Luciana has a Ph.D. from the University of Minnesota. Monica Luciana is Professor and Chair in the Department of Psychology at the University of Minnesota, Minneapolis, MN.
Footnotes
Disclosure of Commercial and Non-Commercial Interests
Monique Ernst does not have anything to disclose
Monica Luciana has the following disclosure:
National Institute on Alcohol Abuse and Alcoholism, Grant recipient, Grant AA020033
Contributor Information
Monique Ernst, Senior Staff Clinician of the National Institute of Mental Health, Bethesda, MD.
Monica Luciana, Professor and Chair in the Department of Psychology, University of Minnesota, Minneapolis, MN.
References
- 1.Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. In: Monitoring the future national survey results on drug use, 1975–2011 Volume 1: Secondary school students. Institute for Social Research TUoM, editor. Ann Arbor: 2012. [Google Scholar]
- 2.Sustance Abuse and Mental Health Services Adminstration CfBHSaQ. The NSDUH Report: Trends in Adolescent Substance Use and Perception of Risk from Substance Use. Rockville, MD: 2013. [PubMed] [Google Scholar]
- 3.Eaton DK, Kann L, Kinchen S, et al. Youth Behavior Surveillance – United States, 2011. Atlanta, GA: CDC; 2012. [PubMed] [Google Scholar]
- 4.Adminstration SAaMHS, editor. Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: HHS; 2011. [Google Scholar]
- 5.Merikangas KR, Nakamura EF, Kessler RC. Epidemiology of mental disorders in children and adolescents. Dialogues in clinical neuroscience. 2009;11(1):7–20. doi: 10.31887/DCNS.2009.11.1/krmerikangas. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armstrong TD, Costello EJ. Community studies on adolescent substance use, abuse, or dependence and psychiatric comorbidity. Journal of consulting and clinical psychology. 2002 Dec;70(6):1224–1239. doi: 10.1037//0022-006x.70.6.1224. [DOI] [PubMed] [Google Scholar]
- 7.Arnett JJ. Adolescent storm and stress, reconsidered. American Psychologist. 1999 May;54(5):317–326. doi: 10.1037//0003-066x.54.5.317. [DOI] [PubMed] [Google Scholar]
- 8.Dahl RE. Adolescent brain development: a period of vulnerabilities and opportunities. Keynote address. Annals Of New York Academy Sciences. 2004 Jun;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- 9.Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychological medicine. 2006 Mar;36(3):299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nelson EE, Leibenluft E, McClure EB, Pine DS. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychological medicine. 2005 Feb;35(2):163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- 11.Steinberg L. Risk taking in adolescence: what changes, and why? Annals of the New York Academy of Sciences. 2004 Jun;1021:51–58. doi: 10.1196/annals.1308.005. [DOI] [PubMed] [Google Scholar]
- 12.Ernst M, Hale E, O’Connell K. Response to commentaries regarding the Triadic Systems Model perspective. Brain and cognition. 2014 Aug;89:122–126. doi: 10.1016/j.bandc.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Arnett JJ. G. Stanley Hall’s Adolescence: Brilliance and nonsense. History of psychology. 2006 Aug;9(3):186–197. doi: 10.1037/1093-4510.9.3.186. [DOI] [PubMed] [Google Scholar]
- 14.Hall G. Adolescence: Its Psychology and Its Relations to Physiology, Anthropology, Sociology, Sex, Crime, Religion and Education. New York: Appleton; 1904. [Google Scholar]
- 15.Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and biobehavioral reviews. 2000 Jun;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 16.Ernst M, Romeo RD, Andersen SL. Neurobiology of the development of motivated behaviors in adolescence: A window into a neural systems model. Pharmacology Biochemistry And Behavior. 2009 Sep;93(3):199–211. doi: 10.1016/j.pbb.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 17.Wahlstrom D, Collins P, White T, Luciana M. Developmental changes in dopamine neurotransmission in adolescence: behavioral implications and issues in assessment. Brain and cognition. 2010 Feb;72(1):146–159. doi: 10.1016/j.bandc.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robbins TW, Everitt BJ. Neurobehavioural mechanisms of reward and motivation. Current opinion in neurobiology. 1996 Apr;6(2):228–236. doi: 10.1016/s0959-4388(96)80077-8. [DOI] [PubMed] [Google Scholar]
- 19.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010 Jan;35(1):217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011 Nov;12(11):652–669. doi: 10.1038/nrn3119. print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garavan H, Weierstall K. The neurobiology of reward and cognitive control systems and their role in incentivizing health behavior. Prev Med. 2012 Nov;55(Suppl):S17–23. doi: 10.1016/j.ypmed.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 22.Wise RA, Rompre PP. Brain dopamine and reward. Annual review of psychology. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- 23.Wise RA. Neurobiology of addiction. Current opinion in neurobiology. 1996 Apr;6(2):243–251. doi: 10.1016/s0959-4388(96)80079-1. [DOI] [PubMed] [Google Scholar]
- 24.Volkow ND, Fowler JS, Wang GJ. The addicted human brain: insights from imaging studies. The Journal of clinical investigation. 2003 May;111(10):1444–1451. doi: 10.1172/JCI18533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luciana M, Wahlstrom D, Porter JN, Collins PF. Dopaminergic modulation of incentive motivation in adolescence: age-related changes in signaling, individual differences, and implications for the development of self-regulation. Developmental psychology. 2012 May;48(3):844–861. doi: 10.1037/a0027432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carlsson A, Lindqvist M, Magnusson T. 3,4-Dihydroxyphenylalanine and 5-hydroxytryptophan as reserpine antagonists. Nature. 1957 Nov 30;180(4596):1200. doi: 10.1038/1801200a0. [DOI] [PubMed] [Google Scholar]
- 27.Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacological reviews. 2011 Mar;63(1):182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- 28.Bjorklund A, Dunnett SB. Dopamine neuron systems in the brain: an update. Trends in neurosciences. 2007 May;30(5):194–202. doi: 10.1016/j.tins.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 29.Moore H, West AR, Grace AA. The regulation of forebrain dopamine transmission: relevance to the pathophysiology and psychopathology of schizophrenia. Biol Psychiatry. 1999 Jul 1;46(1):40–55. doi: 10.1016/s0006-3223(99)00078-5. [DOI] [PubMed] [Google Scholar]
- 30.Nemoda Z, Szekely A, Sasvari-Szekely M. Psychopathological aspects of dopaminergic gene polymorphisms in adolescence and young adulthood. Neuroscience & Biobehavioral Reviews. 2011 Aug;35(8):1665–1686. doi: 10.1016/j.neubiorev.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joyce JN. Multiple dopamine receptors and behavior. Neuroscience and biobehavioral reviews. 1983 Summer;7(2):227–256. doi: 10.1016/0149-7634(83)90017-9. [DOI] [PubMed] [Google Scholar]
- 32.Kohno M, Ghahremani DG, Morales AM, et al. Risk-Taking Behavior: Dopamine D2/D3 Receptors, Feedback, and Frontolimbic Activity. Cerebral cortex (New York, NY: 1991) 2013 Aug 21; doi: 10.1093/cercor/bht218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiological reviews. 1998 Jan;78(1):189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- 34.Floresco SB, Magyar O. Mesocortical dopamine modulation of executive functions: beyond working memory. Psychopharmacology. 2006 Nov;188(4):567–585. doi: 10.1007/s00213-006-0404-5. [DOI] [PubMed] [Google Scholar]
- 35.Depue RA, Collins PF. Neurobiology of the structure of personality: dopamine, facilitation of incentive motivation, and extraversion. The Behavioral and brain sciences. 1999 Jun;22(3):491–517. doi: 10.1017/s0140525x99002046. discussion 518–469. [DOI] [PubMed] [Google Scholar]
- 36.Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain research bulletin. 1982 Jul-Dec;9(1–6):321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- 37.Kaczmarek LK, Levitan I. Neuromodulation: The biochemical control of neuronal excitability. Oxford, England: Oxford University Press; 1987. [Google Scholar]
- 38.Trudeau LE. Glutamate co-transmission as an emerging concept in monoamine neuron function. Journal of psychiatry & neuroscience: JPN. 2004 Jul;29(4):296–310. [PMC free article] [PubMed] [Google Scholar]
- 39.Luciana M, Collins PF. Incentive Motivation, Cognitive Control, and the Adolescent Brain: Is It Time for a Paradigm Shift? Child development perspectives. 2012 Dec 1;6(4):392–399. doi: 10.1111/j.1750-8606.2012.00252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garris PA, Rebec GV. Modeling fast dopamine neurotransmission in the nucleus accumbens during behavior. Behavioural brain research. 2002 Dec 2;137(1–2):47–63. doi: 10.1016/s0166-4328(02)00284-x. [DOI] [PubMed] [Google Scholar]
- 41.Rice ME, Cragg SJ. Dopamine spillover after quantal release: rethinking dopamine transmission in the nigrostriatal pathway. Brain research reviews. 2008 Aug;58(2):303–313. doi: 10.1016/j.brainresrev.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nature neuroscience. 2003 Sep;6(9):968–973. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- 43.Goto Y, Otani S, Grace AA. The Yin and Yang of dopamine release: a new perspective. Neuropharmacology. 2007 Oct;53(5):583–587. doi: 10.1016/j.neuropharm.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: burst firing. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1984 Nov;4(11):2877–2890. doi: 10.1523/JNEUROSCI.04-11-02877.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: single spike firing. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1984 Nov;4(11):2866–2876. doi: 10.1523/JNEUROSCI.04-11-02866.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wanat MJ, Willuhn I, Clark JJ, Phillips PE. Phasic dopamine release in appetitive behaviors and drug addiction. Current drug abuse reviews. 2009 May;2(2):195–213. doi: 10.2174/1874473710902020195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Willuhn I, Wanat MJ, Clark JJ, Phillips PE. Dopamine signaling in the nucleus accumbens of animals self-administering drugs of abuse. Current topics in behavioral neurosciences. 2010;3:29–71. doi: 10.1007/7854_2009_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schultz W. Multiple reward signals in the brain. Nat Rev Neurosci. 2000 Dec;1(3):199–207. doi: 10.1038/35044563. [DOI] [PubMed] [Google Scholar]
- 49.Niv Y, Joel D, Dayan P. A normative perspective on motivation. Trends Cogn Sci. 2006 Aug;10(8):375–381. doi: 10.1016/j.tics.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 50.Niv Y, Daw ND, Joel D, Dayan P. Tonic dopamine: opportunity costs and the control of response vigor. Psychopharmacology. 2007 Apr;191(3):507–520. doi: 10.1007/s00213-006-0502-4. [DOI] [PubMed] [Google Scholar]
- 51.Ostlund SB, Wassum KM, Murphy NP, Balleine BW, Maidment NT. Extracellular dopamine levels in striatal subregions track shifts in motivation and response cost during instrumental conditioning. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011 Jan 5;31(1):200–207. doi: 10.1523/JNEUROSCI.4759-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wahlstrom D, White T, Luciana M. Neurobehavioral evidence for changes in dopamine system activity during adolescence. Neuroscience and biobehavioral reviews. 2010 Apr;34(5):631–648. doi: 10.1016/j.neubiorev.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Andersen SL, Dumont NL, Teicher MH. Developmental differences in dopamine synthesis inhibition by (+/−)-7-OH-DPAT. Naunyn-Schmiedeberg’s archives of pharmacology. 1997 Aug;356(2):173–181. doi: 10.1007/pl00005038. [DOI] [PubMed] [Google Scholar]
- 54.Gelbard HA, Teicher MH, Faedda G, Baldessarini RJ. Postnatal development of dopamine D1 and D2 receptor sites in rat striatum. Brain research. Developmental brain research. 1989 Sep 1;49(1):123–130. doi: 10.1016/0165-3806(89)90065-5. [DOI] [PubMed] [Google Scholar]
- 55.Tarazi FI, Tomasini EC, Baldessarini RJ. Postnatal development of dopamine D1-like receptors in rat cortical and striatolimbic brain regions: An autoradiographic study. Developmental neuroscience. 1999;21(1):43–49. doi: 10.1159/000017365. [DOI] [PubMed] [Google Scholar]
- 56.Teicher MH, Andersen SL, Hostetter JC., Jr Evidence for dopamine receptor pruning between adolescence and adulthood in striatum but not nucleus accumbens. Brain research. Developmental brain research. 1995 Nov 21;89(2):167–172. doi: 10.1016/0165-3806(95)00109-q. [DOI] [PubMed] [Google Scholar]
- 57.Giorgi O, De Montis G, Porceddu ML, et al. Developmental and age-related changes in D1-dopamine receptors and dopamine content in the rat striatum. Brain research. 1987 Oct;432(2):283–290. doi: 10.1016/0165-3806(87)90053-8. [DOI] [PubMed] [Google Scholar]
- 58.Leslie CA, Robertson MW, Cutler AJ, Bennett JP., Jr Postnatal development of D1 dopamine receptors in the medial prefrontal cortex, striatum and nucleus accumbens of normal and neonatal 6-hydroxydopamine treated rats: a quantitative autoradiographic analysis. Brain research. Developmental brain research. 1991 Sep 19;62(1):109–114. doi: 10.1016/0165-3806(91)90195-o. [DOI] [PubMed] [Google Scholar]
- 59.Tarazi FI, Tomasini EC, Baldessarini RJ. Postnatal development of dopamine D4-like receptors in rat forebrain regions: comparison with D2-like receptors. Brain research. Developmental brain research. 1998 Oct 1;110(2):227–233. doi: 10.1016/s0165-3806(98)00111-4. [DOI] [PubMed] [Google Scholar]
- 60.Rao PA, Molinoff PB, Joyce JN. Ontogeny of dopamine D1 and D2 receptor subtypes in rat basal ganglia: a quantitative autoradiographic study. Brain research. Developmental brain research. 1991 Jun 21;60(2):161–177. doi: 10.1016/0165-3806(91)90045-k. [DOI] [PubMed] [Google Scholar]
- 61.Jia JM, Zhao J, Hu Z, Lindberg D, Li Z. Age-dependent regulation of synaptic connections by dopamine D2 receptors. Nature neuroscience. 2013 Nov;16(11):1627–1636. doi: 10.1038/nn.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goto Y, Grace AA. Dopaminergic modulation of limbic and cortical drive of nucleus accumbens in goal-directed behavior. Nature neuroscience. 2005 Jun;8(6):805–812. doi: 10.1038/nn1471. [DOI] [PubMed] [Google Scholar]
- 63.Crews F, He J, Hodge C. Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacology, biochemistry, and behavior. 2007 Feb;86(2):189–199. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Selemon LD. A role for synaptic plasticity in the adolescent development of executive function. Translational psychiatry. 2013;3:e238. doi: 10.1038/tp.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Selemon LD. Frontal lobe synaptic plasticity in development and disease: modulation by the dopamine d1 receptor. Current pharmaceutical design. 2014;20(32):5194–5201. doi: 10.2174/1381612819666140110122307. [DOI] [PubMed] [Google Scholar]
- 66.Sun X, Zhao Y, Wolf ME. Dopamine receptor stimulation modulates AMPA receptor synaptic insertion in prefrontal cortex neurons. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2005 Aug 10;25(32):7342–7351. doi: 10.1523/JNEUROSCI.4603-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Auclair N, Otani S, Soubrie P, Crepel F. Cannabinoids modulate synaptic strength and plasticity at glutamatergic synapses of rat prefrontal cortex pyramidal neurons. Journal of neurophysiology. 2000 Jun;83(6):3287–3293. doi: 10.1152/jn.2000.83.6.3287. [DOI] [PubMed] [Google Scholar]
- 68.Pascual M, Boix J, Felipo V, Guerri C. Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. Journal of neurochemistry. 2009 Feb;108(4):920–931. doi: 10.1111/j.1471-4159.2008.05835.x. [DOI] [PubMed] [Google Scholar]
- 69.McCutcheon JE, Conrad KL, Carr SB, Ford KA, McGehee DS, Marinelli M. Dopamine neurons in the ventral tegmental area fire faster in adolescent rats than in adults. Journal of neurophysiology. 2012 Sep;108(6):1620–1630. doi: 10.1152/jn.00077.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Harden KP, Tucker-Drob EM. Individual differences in the development of sensation seeking and impulsivity during adolescence: further evidence for a dual systems model. Developmental psychology. 2011 May;47(3):739–746. doi: 10.1037/a0023279. [DOI] [PubMed] [Google Scholar]
- 71.Steinberg L, Albert D, Cauffman E, Banich M, Graham S, Woolard J. Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: evidence for a dual systems model. Developmental psychology. 2008 Nov;44(6):1764–1778. doi: 10.1037/a0012955. [DOI] [PubMed] [Google Scholar]
- 72.Urosevic S, Collins P, Muetzel R, Lim K, Luciana M. Longitudinal changes in behavioral approach system sensitivity and brain structures involved in reward processing during adolescence. Developmental psychology. 2012 Sep;48(5):1488–1500. doi: 10.1037/a0027502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bjork JM, Pardini DA. Who are those “risk-taking adolescents”? Individual differences in developmental neuroimaging research. Developmental cognitive neuroscience. 2014 Aug 12; doi: 10.1016/j.dcn.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Richards JM, Plate RC, Ernst M. A systematic review of fMRI reward paradigms used in studies of adolescents vs. adults: the impact of task design and implications for understanding neurodevelopment. Neuroscience and biobehavioral reviews. 2013 Jun;37(5):976–991. doi: 10.1016/j.neubiorev.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Joel D, Weiner I. The connections of the dopaminergic system with the striatum in rats and primates: an analysis with respect to the functional and compartmental organization of the striatum. Neuroscience. 2000;96(3):451–474. doi: 10.1016/s0306-4522(99)00575-8. [DOI] [PubMed] [Google Scholar]
- 76.Cohen JR, Asarnow RF, Sabb FW, et al. A unique adolescent response to reward prediction errors. Nature neuroscience. 2010;13(6):669–671. doi: 10.1038/nn.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Di Chiara G. Drug addiction as dopamine-dependent associative learning disorder. European journal of pharmacology. 1999 Jun 30;375(1–3):13–30. doi: 10.1016/s0014-2999(99)00372-6. [DOI] [PubMed] [Google Scholar]
- 78.Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proceedings of the National Academy of Sciences of the United States of America. 1988 Jul;85(14):5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fiorino DF, Coury A, Phillips AG. Dynamic changes in nucleus accumbens dopamine efflux during the Coolidge effect in male rats. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1997 Jun 15;17(12):4849–4855. doi: 10.1523/JNEUROSCI.17-12-04849.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Grace AA. The tonic/phasic model of dopamine system regulation and its implications for understanding alcohol and psychostimulant craving. Addiction. 2000 Aug;95(Suppl 2):S119–128. doi: 10.1080/09652140050111690. [DOI] [PubMed] [Google Scholar]
- 81.Mastwal S, Ye Y, Ren M, et al. Phasic dopamine neuron activity elicits unique mesofrontal plasticity in adolescence. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2014 Jul 16;34(29):9484–9496. doi: 10.1523/JNEUROSCI.1114-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Howe MW, Tierney PL, Sandberg SG, Phillips PE, Graybiel AM. Prolonged dopamine signalling in striatum signals proximity and value of distant rewards. Nature. 2013 Aug 29;500(7464):575–579. doi: 10.1038/nature12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kolb B. Brain plasticity and behavioral change. In: Craik F, Robert M, Sabourin M, editors. Advances in Psychological Science Volume 2: Biological and Cognitive Aspects. Psychology Press; 1998. [Google Scholar]
- 84.Robinson TE, Kolb B. Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1997 Nov 1;17(21):8491–8497. doi: 10.1523/JNEUROSCI.17-21-08491.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Volkow ND, Fowler JS, Gatley SJ, et al. PET evaluation of the dopamine system of the human brain. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 1996 Jul;37(7):1242–1256. [PubMed] [Google Scholar]
- 86.Mizrahi R. Advances in PET analyses of stress and dopamine. Neuropsychopharmacology. 2010 Jan;35(1):348–349. doi: 10.1038/npp.2009.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jucaite A, Fernell E, Halldin C, Forssberg H, Farde L. Reduced midbrain dopamine transporter binding in male adolescents with attention-deficit/hyperactivity disorder: association between striatal dopamine markers and motor hyperactivity. Biol Psychiatry. 2005 Feb 1;57(3):229–238. doi: 10.1016/j.biopsych.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 88.D’Ardenne K, McClure SM, Nystrom LE, Cohen JD. BOLD responses reflecting dopaminergic signals in the human ventral tegmental area. Science (New York, NY) 2008 Feb 29;319(5867):1264–1267. doi: 10.1126/science.1150605. [DOI] [PubMed] [Google Scholar]
- 89.Eapen M, Zald DH, Gatenby JC, Ding Z, Gore JC. Using high-resolution MR imaging at 7T to evaluate the anatomy of the midbrain dopaminergic system. AJNR. American journal of neuroradiology. 2011 Apr;32(4):688–694. doi: 10.3174/ajnr.A2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.SAMHSA. Results from the 2005 National Survey on Drug Use and Health: National Findings. Rockville, MD: Office of Applied Studies; 2006. (DHHS Publication No. SMA 06–4194). [Google Scholar]
- 91.Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. In: The Monitoring the Future national survey results on adolescent drug use: Overview of key findings, 2005. Abuse NIoD, editor. Bethesda, MD: 2006. [Google Scholar]
- 92.Volkow ND, Baler RD, Compton WM, Weiss SR. Adverse health effects of marijuana use. The New England journal of medicine. 2014 Jun 5;370(23):2219–2227. doi: 10.1056/NEJMra1402309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gorelick DA, Levin KH, Copersino ML, et al. Diagnostic criteria for cannabis withdrawal syndrome. Drug and alcohol dependence. 2012 Jun 1;123(1–3):141–147. doi: 10.1016/j.drugalcdep.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Oleson EB, Cheer JF. A brain on cannabinoids: the role of dopamine release in reward seeking. Cold Spring Harbor perspectives in medicine. 2012;2(8) doi: 10.1101/cshperspect.a012229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schweinsburg AD, Brown SA, Tapert SF. The influence of marijuana use on neurocognitive functioning in adolescents. Current drug abuse reviews. 2008 Jan;1(1):99–111. doi: 10.2174/1874473710801010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Puig MV, Rose J, Schmidt R, Freund N. Dopamine modulation of learning and memory in the prefrontal cortex: insights from studies in primates, rodents, and birds. Frontiers in neural circuits. 2014;8:93. doi: 10.3389/fncir.2014.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Burns HD, Van Laere K, Sanabria-Bohorquez S, et al. [18F]MK-9470, a positron emission tomography (PET) tracer for in vivo human PET brain imaging of the cannabinoid-1 receptor. Proceedings of the National Academy of Sciences of the United States of America. 2007 Jun 5;104(23):9800–9805. doi: 10.1073/pnas.0703472104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gilman JM, Kuster JK, Lee S, et al. Cannabis use is quantitatively associated with nucleus accumbens and amygdala abnormalities in young adult recreational users. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2014 Apr 16;34(16):5529–5538. doi: 10.1523/JNEUROSCI.4745-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dennison M, Whittle S, Yucel M, et al. Mapping subcortical brain maturation during adolescence: evidence of hemisphere- and sex-specific longitudinal changes. Developmental science. 2013 Sep;16(5):772–791. doi: 10.1111/desc.12057. [DOI] [PubMed] [Google Scholar]
- 100.Ostby Y, Tamnes CK, Fjell AM, Westlye LT, Due-Tonnessen P, Walhovd KB. Heterogeneity in subcortical brain development: A structural magnetic resonance imaging study of brain maturation from 8 to 30 years. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009 Sep 23;29(38):11772–11782. doi: 10.1523/JNEUROSCI.1242-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schneider S, Peters J, Bromberg U, et al. Risk taking and the adolescent reward system: a potential common link to substance abuse. The American journal of psychiatry. 2012 Jan;169(1):39–46. doi: 10.1176/appi.ajp.2011.11030489. [DOI] [PubMed] [Google Scholar]
- 102.Urosevic S, Collins P, Muetzel R, Lim KO, Luciana M. Pubertal status associations with reward and threat sensitivities and subcortical brain volumes during adolescence. Brain and cognition. 2014 Feb 7; doi: 10.1016/j.bandc.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Arnone D, Barrick TR, Chengappa S, Mackay CE, Clark CA, Abou-Saleh MT. Corpus callosum damage in heavy marijuana use: preliminary evidence from diffusion tensor tractography and tract-based spatial statistics. NeuroImage. 2008 Jul 1;41(3):1067–1074. doi: 10.1016/j.neuroimage.2008.02.064. [DOI] [PubMed] [Google Scholar]
- 104.Ashtari M, Cervellione K, Cottone J, Ardekani BA, Sevy S, Kumra S. Diffusion abnormalities in adolescents and young adults with a history of heavy cannabis use. Journal of psychiatric research. 2009 Jan;43(3):189–204. doi: 10.1016/j.jpsychires.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gruber SA, Dahlgren MK, Sagar KA, Gonenc A, Lukas SE. Worth the wait: effects of age of onset of marijuana use on white matter and impulsivity. Psychopharmacology. 2014 Apr;231(8):1455–1465. doi: 10.1007/s00213-013-3326-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gruber SA, Silveri MM, Dahlgren MK, Yurgelun-Todd D. Why so impulsive? White matter alterations are associated with impulsivity in chronic marijuana smokers. Experimental and clinical psychopharmacology. 2011 Jun;19(3):231–242. doi: 10.1037/a0023034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Heimer L, Alheid GF. Piecing together the puzzle of basal forebrain anatomy. Advances in experimental medicine and biology. 1991;295:1–42. doi: 10.1007/978-1-4757-0145-6_1. [DOI] [PubMed] [Google Scholar]
- 108.Kolb B, Gorny G, Limebeer CL, Parker LA. Chronic treatment with Delta-9-tetrahydrocannabinol alters the structure of neurons in the nucleus accumbens shell and medial prefrontal cortex of rats. Synapse (New York, NY) 2006 Nov;60(6):429–436. doi: 10.1002/syn.20313. [DOI] [PubMed] [Google Scholar]
- 109.Cousijn J, Wiers RW, Ridderinkhof KR, van den Brink W, Veltman DJ, Goudriaan AE. Grey matter alterations associated with cannabis use: results of a VBM study in heavy cannabis users and healthy controls. NeuroImage. 2012 Feb 15;59(4):3845–3851. doi: 10.1016/j.neuroimage.2011.09.046. [DOI] [PubMed] [Google Scholar]
- 110.Lorenzetti V, Lubman DI, Whittle S, Solowij N, Yucel M. Structural MRI findings in long-term cannabis users: what do we know? Substance use & misuse. 2010 Sep;45(11):1787–1808. doi: 10.3109/10826084.2010.482443. [DOI] [PubMed] [Google Scholar]
- 111.Jager G, Ramsey NF. Long-term consequences of adolescent cannabis exposure on the development of cognition, brain structure and function: an overview of animal and human research. Current drug abuse reviews. 2008 Jun;1(2):114–123. doi: 10.2174/1874473710801020114. [DOI] [PubMed] [Google Scholar]
- 112.Batalla A, Bhattacharyya S, Yucel M, et al. Structural and functional imaging studies in chronic cannabis users: a systematic review of adolescent and adult findings. PloS one. 2013;8(2):e55821. doi: 10.1371/journal.pone.0055821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Volkow ND, Wang GJ, Telang F, et al. Decreased dopamine brain reactivity in marijuana abusers is associated with negative emotionality and addiction severity. Proceedings of the National Academy of Sciences of the United States of America. 2014 Jul 29;111(30):E3149–3156. doi: 10.1073/pnas.1411228111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Albrecht DS, Skosnik PD, Vollmer JM, et al. Striatal D(2)/D(3) receptor availability is inversely correlated with cannabis consumption in chronic marijuana users. Drug and alcohol dependence. 2013 Feb 1;128(1–2):52–57. doi: 10.1016/j.drugalcdep.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sevy S, Smith GS, Ma Y, et al. Cerebral glucose metabolism and D2/D3 receptor availability in young adults with cannabis dependence measured with positron emission tomography. Psychopharmacology. 2008 May;197(4):549–556. doi: 10.1007/s00213-008-1075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Stokes PR, Egerton A, Watson B, et al. History of cannabis use is not associated with alterations in striatal dopamine D2/D3 receptor availability. Journal of psychopharmacology (Oxford, England) 2012 Jan;26(1):144–149. doi: 10.1177/0269881111414090. [DOI] [PubMed] [Google Scholar]
- 117.Urban NB, Slifstein M, Thompson JL, et al. Dopamine release in chronic cannabis users: a [11c]raclopride positron emission tomography study. Biol Psychiatry. 2012 Apr 15;71(8):677–683. doi: 10.1016/j.biopsych.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bloomfield MA, Morgan CJ, Kapur S, Curran HV, Howes OD. The link between dopamine function and apathy in cannabis users: an [18F]-DOPA PET imaging study. Psychopharmacology. 2014 Jun;231(11):2251–2259. doi: 10.1007/s00213-014-3523-4. [DOI] [PubMed] [Google Scholar]
- 119.Murray JE, Dilleen R, Pelloux Y, et al. Increased impulsivity retards the transition to dorsolateral striatal dopamine control of cocaine seeking. Biol Psychiatry. 2014 Jul 1;76(1):15–22. doi: 10.1016/j.biopsych.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nestor L, Hester R, Garavan H. Increased ventral striatal BOLD activity during non-drug reward anticipation in cannabis users. NeuroImage. 2010 Jan 1;49(1):1133–1143. doi: 10.1016/j.neuroimage.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.van Hell HH, Vink M, Ossewaarde L, Jager G, Kahn RS, Ramsey NF. Chronic effects of cannabis use on the human reward system: an fMRI study. European neuropsychopharmacology: the journal of the European College of Neuropsychopharmacology. 2010 Mar;20(3):153–163. doi: 10.1016/j.euroneuro.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 122.Sneider JT, Mashhoon Y, Silveri MM. A Review of Magnetic Resonance Spectroscopy Studies in Marijuana using Adolescents and Adults. Journal of addiction research & therapy. 2013 Apr 24;(Suppl 4) doi: 10.4172/2155-6105.S4-010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Silveri MM, Jensen JE, Rosso IM, Sneider JT, Yurgelun-Todd DA. Preliminary evidence for white matter metabolite differences in marijuana-dependent young men using 2D J-resolved magnetic resonance spectroscopic imaging at 4 Tesla. Psychiatry research. 2011 Mar 31;191(3):201–211. doi: 10.1016/j.pscychresns.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hermann D, Sartorius A, Welzel H, et al. Dorsolateral prefrontal cortex N-acetylaspartate/total creatine (NAA/tCr) loss in male recreational cannabis users. Biol Psychiatry. 2007 Jun 1;61(11):1281–1289. doi: 10.1016/j.biopsych.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 125.Chang L, Cloak C, Yakupov R, Ernst T. Combined and independent effects of chronic marijuana use and HIV on brain metabolites. Journal of neuroimmune pharmacology: the official journal of the Society on NeuroImmune Pharmacology. 2006 Mar;1(1):65–76. doi: 10.1007/s11481-005-9005-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Muetzel RL, Marjanska M, Collins PF, et al. In vivo H magnetic resonance spectroscopy in young-adult daily marijuana users. NeuroImage. Clinical. 2013 Jan 1;2:581–589. doi: 10.1016/j.nicl.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Prescot AP, Locatelli AE, Renshaw PF, Yurgelun-Todd DA. Neurochemical alterations in adolescent chronic marijuana smokers: a proton MRS study. NeuroImage. 2011 Jul 1;57(1):69–75. doi: 10.1016/j.neuroimage.2011.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. The American journal of psychiatry. 2005 Aug;162(8):1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 129.Hakamata Y, Lissek S, Bar-Haim Y, et al. Attention bias modification treatment: a meta-analysis toward the establishment of novel treatment for anxiety. Biol Psychiatry. 2010 Dec 1;68(11):982–990. doi: 10.1016/j.biopsych.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Field M, Marhe R, Franken IH. The clinical relevance of attentional bias in substance use disorders. CNS spectrums. 2014 Jun;19(3):225–230. doi: 10.1017/S1092852913000321. [DOI] [PubMed] [Google Scholar]
- 131.Sample S, Kadden R. In: Motivational Enhancement Therapy and Cognitive Behavioral Therapy for Adolescent Cannabis Users: 5 sessions. Administration DSAaMHS, editor. Rockville, MD: Center for Substance Abuse Treatment; 2001. [Google Scholar]
- 132.Dennis M, Titus JC, Diamond G, et al. The Cannabis Youth Treatment (CYT) experiment: rationale, study design and analysis plans. Addiction. 2002 Dec;97(Suppl 1):16–34. doi: 10.1046/j.1360-0443.97.s01.2.x. [DOI] [PubMed] [Google Scholar]
- 133.Dennis M, Godley SH, Diamond G, et al. The Cannabis Youth Treatment (CYT) Study: main findings from two randomized trials. Journal of substance abuse treatment. 2004 Oct;27(3):197–213. doi: 10.1016/j.jsat.2003.09.005. [DOI] [PubMed] [Google Scholar]


