Abstract
Several intracellular pathogens display the ability to propagate within host tissues by displaying actin-based motility in the cytosol of infected cells. As motile bacteria reach cell-cell contacts, they form plasma membrane protrusions that project into adjacent cells and resolve into vacuoles from which the pathogen escape, thereby achieving spread from cell to cell. Seminal studies have defined the bacterial and cellular factors that support actin-based motility. By contrast, the mechanisms supporting the formation of protrusions and their resolution into vacuoles have remained elusive. Here we review recent advances in the field showing that Listeria monocytogenes and Shigella flexneri have evolved pathogen-specific mechanisms of bacterial spread from cell to cell.
Keywords: Shigella flexneri, Listeria monocytogenes, spread from cell to cell, membrane protrusion, double membrane vacuole
What is bacterial spread from cell to cell?
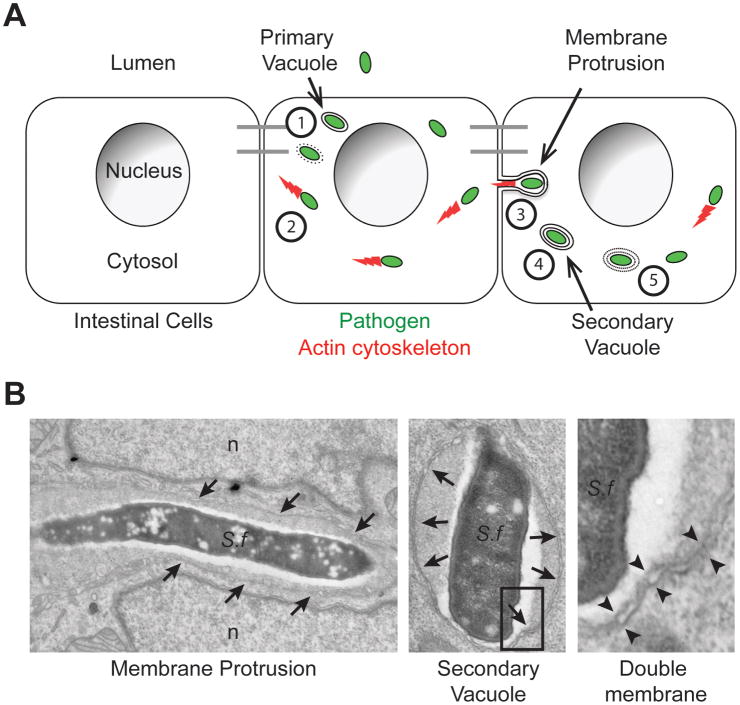
Bacterial spread from cell to cell is the ability of intracellular bacteria that reside in the cytosol of infected cells to access the cytosol of adjacent cells without transiting through the extracellular medium (Figure 1). The sequence of events occurring during bacterial spread from cell to cell through formation of membrane protrusions (see Glossary) and double membrane vacuoles were defined in seminal electron microscopy studies using macrophages infected with Listeria monocytogenes as a model system [1]. Further electron microscopy studies confirmed membrane protrusions and double membrane vacuoles as central features of the spreading process in epithelial cells infected with L. monocytogenes and Shigella flexneri [2,3]. This dissemination process relies on acquisition of actin-based motility in the cytosol of infected cells (Box 1). As bacteria displaying actin-based motility in the cytosol encounter cell-cell contacts, they form plasma membrane protrusions that project into adjacent cells (Figure 1). The formed protrusions resolve into double membrane vacuoles composed of an inner membrane, originating from the primary infected cell, and an outer membrane deriving from the adjacent cell (Figure 1). By escaping the double membrane vacuoles, the pathogen gains access to the cytosol of adjacent cells and achieves spread from cell to cell (Figure 1).
Figure 1. Sequence of events in bacterial spread from cell to cell.
(A) Cytosolic bacteria (green) spread from cell to cell within a monolayer of intestinal cells through the following sequence of events: (1) Escape from the primary vacuole, (2) Actin (red)-based motility, (3) Membrane protrusion formation into adjacent cells, (4) Resolution of membrane protrusions into (double-membrane) secondary vacuoles and (5) Escape from secondary vacuoles into the cytosol of the adjacent cell. Adapted from reference [1].
(B) Electron micrographs of the two main features of bacterial cell-to-cell spread, membrane protrusions and double membrane vacuoles. Left panel: S. flexneri (S.f) within a membrane protrusion in between two lobes of the adjacent cell nucleus (n). Membranes surrounding the protrusion are marked with arrows. Middle panel: S. flexneri within a secondary vacuole. Membranes surrounding the secondary vacuoles are marked with arrows. Right panel: high magnification showing the double membranes of a secondary vacuole corresponding to the boxed area in the middle panel. Double membranes are marked with opposing arrowheads.
Box 1. Mechanisms of actin-based motility in the cytosol of cells infected with the intestinal pathogens L. monocytogenes and S. flexneri.
The mechanisms supporting L. monocytogenes and S. flexneri cytosolic motility have been reviewed recently [48]. In brief, both L. monocytogenes and S. flexneri achieve actin-based motility by recruiting to their surface a major nucleator of actin polymerization in eukaryotic cells, the ARP2/3 complex (Figure I) [49,50]. S. flexneri engages the ARP2/3 complex through expression of IcsA [51,52], a bacterial adaptor that recruits and activates the ARP2/3 nucleation-promoting factor N-WASP [53,54]. L. monocytogenes does not engage the ARP2/3 complex through N-WASP recruitment, but through expression of ActA [11,12], a bacterial factor that displays structural and regulatory mimicry with N-WASP [55,56,57]. The expansion of the actin network formed by the ARP2/3 complex at the bacterial surface generates forces that propel the bacterium throughout the cytosolic compartment [58,59].
Figure I.
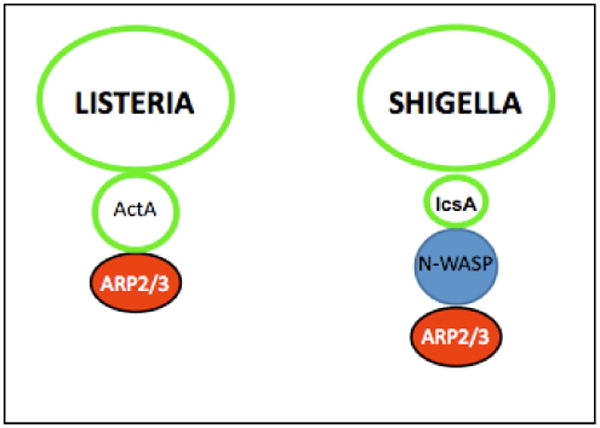
Bacterial and cellular factors supporting Listeria monocytogenes and Shigella flexneri actin-based motility.
Compared to the mechanisms supporting actin-based motility, the mechanisms supporting cell-to-cell spread through formation and resolution of membrane protrusions into vacuoles from which the pathogen escapes, have received little attention. This situation is partly due to the widespread assumption that the forces generated by actin-based motility are necessary and sufficient to deform the plasma membrane, and form membrane protrusions that undergo non-specific scission into vacuoles. Although experimental evidence has been presented in support of this model [4], a growing body of evidence suggests the existence of alternative and pathogen-specific mechanisms. Here, we review recent advances in the field supporting the notion that, although employing similar strategy of cytosolic motility based on the actin cytoskeleton, the intestinal pathogens L. monocytogenes and S. flexneri have evolved pathogen-specific mechanisms of cell-to-cell spread.
Methods for studying bacterial spread from cell to cell
The formation of essential features of bacterial spread from cell to cell, including membrane protrusions and double membrane vacuoles, has been documented in animal models of human infection, such as rhesus monkeys [5]. As the cost of extensive studies of bacterial spread from cell to cell in relevant models of intestinal infection is prohibitive, in vitro tissue culture systems are commonly used to investigate the ability of intestinal pathogens to disseminate within monolayers of cells [6,7]. S. flexneri and L. monocytogenes readily spread from cell to cell in human intestinal cell lines [8,9]. In addition to the intestine, L. monocytogenes also infects macrophages in vivo, and spreads to distant organs, including the liver, the spleen and the brain [10]. Accordingly, various phagocytic and non-phagocytic cell types, support L. monocytogenes spread from cell to cell in vitro [1,7,11,12,13,14].
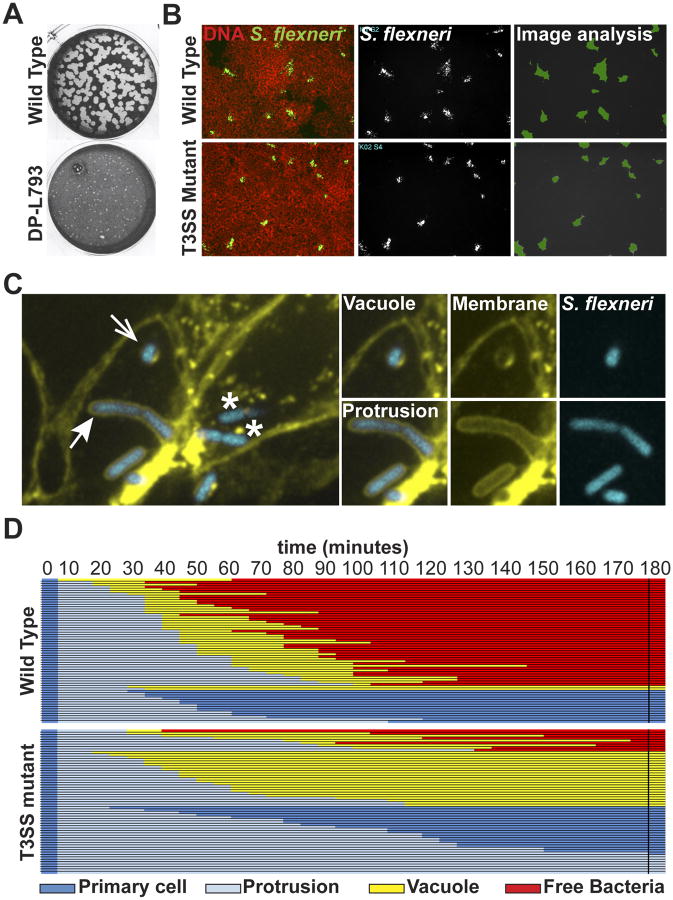
The plaque assay constitutes a standard readout of cell-to-cell spread in tissue culture systems [6,7]. The assay relies on confluent epithelial cells or fibroblasts, which are infected at a low multiplicity of infection and then overlaid with an agar-tissue culture medium mixture to prevent long-range dissemination through the extracellular medium. Pathogens multiply and spread to adjacent cells leading to the formation of infection foci. After a few days of infection, primary infected cells succumb to the infection process leading to the formation of patches of dead cells or plaques in the monolayer of non-infected cells. The size of the plaques is used as a measure of the efficiency of cell-to-cell spread (Figure 2A). In addition to the plaque assay, cellular imaging offers the possibility of quantifying early events in foci formation, when the death of primary infected cells cannot be used as readout. Automated microscopy followed by computer-assisted image analysis is used to quantify the size of infection foci and identify spreading defects (Figure 2B) [15,16,17,18,19]. The use of tissue culture cells expressing fluorescent membrane markers allows for visualization of protrusions and double membrane vacuoles by high-magnification confocal microscopy Figure 2C) [15,16,17,18,19]. Finally, time-lapse confocal microscopy provides unprecedented dissection of the dynamics of protrusion and vacuole formation (Figure 2D), which in combination with powerful genetic approaches has helped elucidate mechanisms of bacterial cell-to-cell spread for S. flexneri and L. monocytogenes [15,16,17,18,19].
Figure 2. Readouts of bacterial spread from cell to cell.
(A) L. monocytogenes plaque assay depicting large (wild type) plaques, and a small plaque phenotype (DP-L793). Adapted from reference [7].
(B) Automated microscopy of S. flexneri infection in HT-29 cell monolayer. Left panels, overlay of DNA staining (red) and GFP (green)-expressing wild type and type III secretion system (T3SS) mutant strains. Middle panels: bacterial infection foci. Right panels: computer-assisted image analysis of infection foci size (green).
(C) High-magnification confocal microscopy showing features of bacterial spread form cell to cell in HT-29 cells expressing a fluorescent membrane marker (yellow) infected with S. flexneri (blue). Cytosolic bacteria in a primary infected cell (*) form membrane protrusions (closed arrow) that project into adjacent cells and resolve into secondary vacuoles (open arrow).
(D) Tracking analysis of 60 wild type (top) and T3SS mutant (bottom) strains. Each line represents one bacterium that was tracked for 180 minutes and the progression of the dissemination process is depicted using the color key shown at the bottom. Primary cell, dark blue; Protrusion, light blue; Vacuole, yellow; Free bacteria in adjacent cell, red. The data shows that 75% of the wild type bacteria succeed in forming protrusions that resolve into vacuoles from which the bacteria escape (free). By contrast, the majority of the T3SS mutant bacteria either form protrusions that fail to resolve into vacuoles and retract to the primary infected cell, or fails to escape the formed vacuole. Adapted from reference [18].
What is the role of cell-cell contact in bacterial spread?
Bacterial spread from cell to cell is supported by the formation of membrane protrusions that project into the cytosol of adjacent cells. In epithelial structures, this process is facilitated by cell-cell contacts where the plasma membranes of the primary infected cells and adjacent cells are in close apposition. In addition to providing physical proximity, several studies have suggested that cell-cell contacts may support specific functions in bacterial spread from cell to cell.
Bacterial spread in epithelial structures
The formation of cell-cell contact in epithelial structures is critical to support the formation of membrane protrusions that project into adjacent cells upon bacterial spread from cell to cell. Accordingly, S. flexneri spreads poorly in the cell-cell contact deficient cell line S180, a phenotype that can be rescued by expression of the cell adhesion molecule E-cadherin [20]. Similarly, depletion of E-cadherin expression abrogates cell-cell contacts in HT-29 cells, which prevents S. flexneri spread from cell to cell [16]. In addition to their establishment, cell-cell contacts must be maintained during the infection process, as intracellular pathogen infection may affect the integrity of epithelial structures. For instance, the bacterial factor OspE2 contributes to the maintenance of integrin-dependent cell adhesion during S. flexneri infection [21,22]. Conversely, cell-cell contacts may represent a physical barrier that needs to be manipulated in order to facilitate protrusion formation, as exemplified by the role of the bacterial factors internalin C (InlC) in relieving Tuba/N-WASP-dependent cortical tension in polarized epithelial cells infected with L. monocytogenes [23,24,25,26].
Remarkably, the role of InlC in bacterial spread from cell to cell was confirmed in vivo in a mouse model of L. monocyogenes infection [27]. Finally, the cellular identity and composition of the plasma membrane are important, as suggested by the rescue of the poor efficiency of S. flexneri spread in HeLa cells by ectopic expression of cellular factors such as the gap junction protein connexin 26 and the cell polarity kinase STK11 [16,28]. It is thus critical to study cell-to-cell spread of intestinal pathogens such as L. monocytogenes and S. flexneri in tissue culture systems that model the in vivo properties of the intestinal epithelium [16,25].
Bacterial spread in macrophages
In addition to epithelial structures, intestinal pathogens such as L. monocytogenes can spread from cell to cell in macrophages. A recent study suggested a novel mechanism of L. monocytogenes dissemination supported by efferocytosis, the process by which macrophages remove phosphatydilserine (PS)-positive cellular debris [29]. This cell-to-cell spreading process is associated with plasma membrane damage due to the expression of the pore-forming toxin listeriolysin O (LLO). LLO promotes the release of bacteria-containing protrusions from primary infected macrophages, generating membrane-derived vesicles with exofacial PS. As macrophages derived from mice deficient for the PS-binding receptor TIM-4 did not support L. monocytogenes spread as efficiently as macrophages derived from C57BL/6 mice, a model of cell-to-cell spread was thus proposed in which TIM-4 mediates L. monocytogenes spread to recipient macrophages through efferocytosis. It remains to be determined whether this mode of pathogen dissemination from infected cells delivering pathogen-containing vesicles to phagocytic cells is restricted to macrophages or may contribute to pathogen spread in other cell types as well.
What are the mechanisms of membrane protrusions formation?
A central question with respect to the mechanisms supporting protrusion formation is whether the formation of actin tails in the cytosol and actin networks in protrusions are governed by the same mechanisms. Similar to the situation observed in the cytosol, L. monocytogenes and S. flexneri appear to utilize the core components of the ARP2/3-dependent machinery to assemble the network of actin filaments in protrusions. However, recent studies have revealed protrusion-specific features that probably relate to structural and functional properties of the plasma membrane surrounding protrusions.
Mechanisms of actin network assembly in protrusions
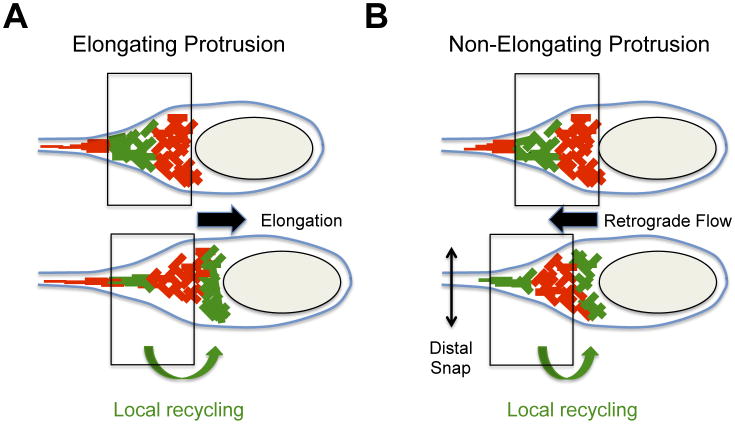
The first indication that the mechanisms supporting the formation of actin networks may differ in the cytosol and in protrusions came from an ultra-structural study of cells infected with L. monocytogenes [30]. Unlike the fully branched network generated by the ARP2/3 complex, typical of cytosolic tails, the actin filaments found in protrusions appeared short and branched proximal to the bacteria, but long and bundled in the distal region [19,30]. In full agreement with these observations, actin was detected along protrusions, but the ARP2/3 complex was only detected proximal to the bacterial pole [19,31]. Studies on the dynamics of actin and the ARP2/3 complex revealed that the elongation of protrusions requires the local recycling of the ARP2/3 complex in protrusions (Figure 3A) [19]. This local recycling is supported by the AIP1/CFL1/GMF/TWF2 machinery that disassembles the distal network to fuel continuous ARP2/3-mediated assembly of the actin network formed at the bacterial pole in protrusions [19]. Importantly, this local recycling process does not take place in the cytosol where interfering with the activity of the disassembly machinery does not critically impair bacterial velocity. This is in contrast with the situation observed in protrusions where interfering with the actin disassembly machinery severely impairs protrusion elongation and protrusion resolution into vacuoles [19]. Thus, local recycling appears as a specific feature of the dynamics of ARP2/3 complex-mediated network in protrusions.
Figure 3. Dynamics of the actin cytoskeleton in protrusions.
Dynamics of the actin cytoskeleton were determined by photo-activation experiments with photo-activatable GFP-actin fusion protein. Photo-activated GFP-actin (green network) demonstrating local recycling (green arrow at the bottom) from the distal network (top panel) to the bacterial pole (bottom panel). The vertical boxes indicate the position of the actin network at the bacterial pole at the instant of photo-activation (top panel) and shortly after photo-activation (bottom panel).
(A) Cytoskeleton dynamics in elongating protrusions. The disassembly of the distal network (top panel, photo-activated actin-GFP, green) fuels the assembly of the proximal network at the bacterial pole (bottom panel, photo-activated actin-GFP, green). Note that the network at the bacterial pole did not move with reference to the plasma membrane (vertical boxes). The assembly of the network at the bacterial pole provides the forces leading to protrusion elongation (black arrow).
(B) Cytoskeleton dynamics in non-elongating protrusions. The disassembly of the distal network (top panel, photo-activated actin-GFP, green) fuels the assembly of the proximal network at the bacterial pole (bottom panel, photo-activated actin-GFP, green). Note that the network at the bacterial pole moves backwards (retrograde flow) with reference to the plasma membrane (vertical boxes). The assembly of the network at the bacterial pole provides the forces leading to retrograde flow (black arrow) and membrane scission (distal snap).
In addition to the ARP2/3 complex, recent research has unveiled a possible role for ARP2/3-independent components in actin network assembly in protrusions. Diaphanous-related formins have been established as important determinants of protrusion formation in S. flexneri infected cells [32]. Diaphanous-related formins have also been localized to L. monocytogenes protrusions and identified as important factors in protrusion formation and cell-to-cell spread [31]. The importance of these formins and the existence of bundled actin network in protrusion [19,30] are strongly suggestive of multi-factorial mechanisms of actin assembly in protrusions. Future research should determine the exact role of formins and whether pathogen-specific features regulate their activity in protrusions.
Additional cytoskeleton factors in protrusions
Aside from factors directly participating in actin network assembly, accessory cytoskeleton factors have also been found in membrane protrusions (Table 1). For instance, the ERM family protein ezrin is present specifically in L. monocytogenes protrusions where it is required for normal protrusion shape and length [33]. Ezrin harbors an actin-binding domain and a membrane-binding domain and provides structural support to protrusion formation by linking the actin cytoskeleton to the plasma membrane [33]. Similarly, myosin X has recently been found to localize to the plasma membrane surrounding S. flexneri protrusions [34]. Myosin X depletion results in shorter and wider protrusions, indicating a role for this protein in proper protrusion structure [34]. Myosin X also localizes to L. monocytogenes protrusions and is important for efficient cell-to-cell spread [34]. S. flexneri dissemination relies on myosin II as well, and its regulator, the myosin light chain kinase MLCK [35,36]. The specific role of myosin II in S. flexneri spread from cell to cell is unclear. However, MLCK inhibition had apparently no effect on L. monocyotgenes dissemination [36], which constituted the first observation suggesting the existence of pathogen-specific mechanisms of cell-to-cell spread.
Table 1. Cytoskeleton factors in the actin networks formed by Shigella flexneri and Listeria monocytogenes in the cytosol and in protrusionsa.
| Cytoskeleton factors | Shigella flexneri | Listeria monocytogenes | ||||
|---|---|---|---|---|---|---|
| Cytosol | Protrusion | Refs | Cytosol | Protrusion | Refs | |
| ARP2/3 complex | + | + | [53] | + | + | [50] |
| N-WASP | + | + | [60] | - | - | [60] |
| Profilin | + | + | [61] | + | + | [62] |
| Cofilin | + | + | [2] | + | + | [2,63] |
| VASP | + | + | [2] | + | + | [2,63,64] |
| Coronin 1 | ND | ND | + | ND | [63] [65] | |
| CapZB | + | ND | [2] | + | + | [2,63] |
| Ezrin | + | + | [2,34] | - | + | [33] |
| α -actinin | + | ND | [2] | + | - | [2,30] |
| Fimbrin | + | ND | [66] | ND | ND | |
| Dynamin 1-2 | ND | ND | + | ND | [67] | |
| Fascin | ND | ND | + | ND | [68] | |
| mDia1 | + | + | [34] | - | + | [31] |
| mDia2 | ND | ND | - | + | [31] | |
| mDia3 | ND | ND | - | + | [31] | |
| AIP1 | ND | ND | + | + | [19,65] | |
| Myosin X | - | + | [34] | ND | ND | |
| Myosin IIA | + | ND | [35] | ND | ND | |
| Caldesmon | ND | ND | + | ND | [65] | |
| 14-3-3 protein | ND | ND | + | ND | [65] | |
| Cortactin | ND | ND | + | ND | [65] | |
Symbols: +, present; -, absent; ND, not determined.
What are the mechanisms of protrusion resolution into vacuoles?
The resolution of protrusions into vacuoles is probably the least understood aspect of the dissemination process. Protrusion elongation and vacuole formation require seemingly antagonistic events, as elongation relies on actin network assembly and vacuole formation necessitates the disassembly of the actin network, a mandatory step toward membrane scission. Recent research has revealed that L. monocytogenes and S. flexneri have evolved strikingly different strategies to solve this conundrum.
L. monocytogenes relies on local network recycling
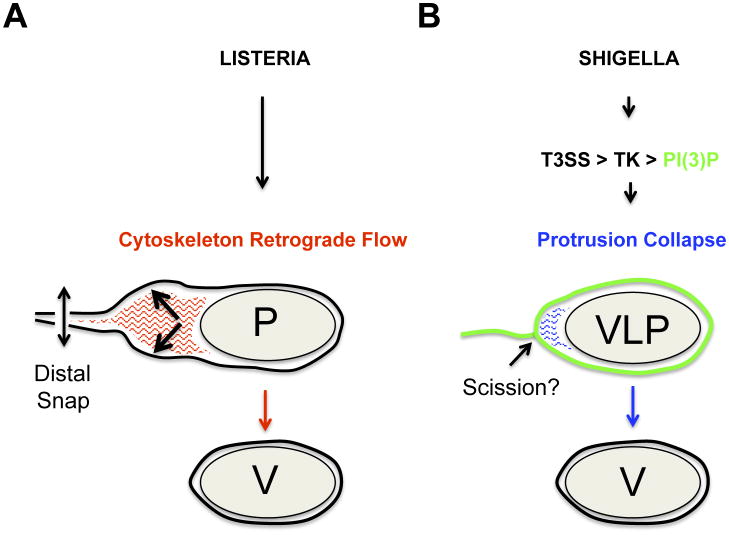
Time-lapse microscopy studies of cells infected with L. monocytogenes revealed that the resolution of protrusions into secondary vacuoles does not occur during the elongation phase of protrusion formation [19,37]. Instead, the elongation phase is followed by a non-elongation phase during which protrusions display ‘fitful’ movement characterized by a period of slow and erratic motility [37]. The resolution of L. monocytogenes protrusions occurs by scission of the distal region of protrusions in a snapping motion that suggest a sudden release of tension [37]. These observations first suggested the existence of a mechanism that creates the tension forces required for membrane rupture, and concomitantly, mediates clearance of the distal network that would otherwise hinder protrusion scission. Accordingly, a model of L. monocytogenes protrusion resolution was recently proposed [19] in which the activity of the disassembly machinery is critical for exhausting the actin network from the distal region in protrusions (Figure 3B). The disassembled network fuels the continuous activity of the assembly machinery at the bacterial pole (Figure 3B). As the protrusions do not elongate any longer, actin assembly creates a massive retrograde flow of the actin network (Figure 3B, retrograde flow), which was proposed to generate tension forces against the plasma membrane in the distal region, where scission occurs (Figure 3B, distal snap). To date, all evidence thus point to a model of resolution of L. monocytogenes protrusions that relies on the physical disruption of the membrane as a consequence of the tension forces generated by the actin cytoskeleton (Figure 4A). It is however unclear whether, in addition to the actin network assembly and disassembly machineries, additional cellular or bacterial factors contribute to the efficiency of this resolution process.
Figure 4. Mechanisms of L. monocytogenes and S. flexneri protrusion resolution.
(A) Mechanism of L. monocytogenes protrusion resolution. After elongation, the actin network (red lines) in protrusions (P) undergoes retrograde flow (black arrow), which generates the forces leading to membrane scission (vertical arrow, distal snap) and vacuole formation (V).
(B) Mechanism of S. flexneri protrusion resolution. After elongation, the actin network (blue lines) collapses in response to PI(3)P production in the protrusion membrane (green line). PI(3)P production is mediated by a signaling cascade involving the type 3 secretion system (T3SS), host cell tyrosine kinase (TK) and PI3KC2A-dependent production of PI(3)P. Cytoskeleton collapse leads to the formation of vacuole-like protrusions (VLP) and the resolution of the membrane tether (scission?) connecting VLP to the primary infected cells leads to the formation of a genuine vacuole (V).
S. flexneri manipulates cellular signaling at the plasma membrane
Similar to L. monocytogenes, S. flexneri forms protrusions that first undergo elongation and then stop elongating [16,17]. In stark contrast to the situation observed with L. monocytogenes, however, non-elongating S. flexneri protrusions transition into a membrane compartment termed vacuole-like protrusions (Figure 4B, VLP) [17]. Similar to vacuoles, VLPs display a seemingly continuous lining of the plasma membrane around the bacterium. Unlike vacuoles, however, VLPs remain connected to the primary infected cells through a membranous tether that apparently results from the complete collapse of the protrusion neck and underlying cytoskeleton (Figure 4B). The progressive disappearance of the membranous tether leads to the formation of genuine vacuoles (Figure 4B), without the brutal tension release observed during the resolution of L. monocytogenes protrusions. Genetic investigations revealed that the class II phosphatidyl-inositol 3-phosphate [PI(3)P] kinase PIK3C2A is specifically required for S. flexneri dissemination through production of PI(3)P in the protrusion membrane of primary infected cells [17]. PIK3C2A-dependent production of PI(3)P relied on the activation of host cell tyrosine kinase (TK) signaling by the bacterial type III secretion system (T3SS) through an unknown mechanism [Figure 4B, T3SS>TK>PI(3)P] [16,17,18]. Thus, S. flexneri manipulates phosphoinositide signaling at the plasma membrane of primary infected cells to resolve protrusions into vacuoles through VLP formation, a process that most likely involves the collapse of the actin cytoskeleton in protrusions and remains to be elucidated. Thus, the resolution of S. flexneri and L. monocytogenes protrusions into vacuoles relies on radically different mechanisms (Figure 4A and 4B).
Role of the adjacent cells in bacterial spread from cell to cell
In addition to the signaling events occurring in the plasma membrane of the primary infected cells, recent studies have revealed a role for signaling events taking place in adjacent cells. On the basis of genetic depletion and pharmacological inhibition, it was proposed that the engulfment of the protrusion formed by S. flexneri relies on a non-canonical form of endocytosis executed by the adjacent cells [38]. This pathway involves class I PI3K, clathrin, epsin-1 and dynamin-2, but not AP-2, Dab2 and Eps15 [38]. Time-lapse microscopy indicated the recruitment of clathrin- and Eps-1-positive materials in the vicinity of protrusions, which led the authors to draw a functional analogy between the engulfment of protrusions and the endocytosis of clathrin-coated pits [38]. Importantly, the requirement for dynamin-2 strongly suggests a role for the adjacent cells in the scission of the protrusion membrane. Together with the observation that S. flexneri protrusions resolve into vacuoles through VLP formation [17], this may indicate a role for dynamin-2 in the scission of the membranous tether connecting VLPs to primary infected cells. Although the scission machinery most likely acts on the membrane of the adjacent cells, the mechanisms supporting the resolution of the primary infected cell membrane in protrusions remains obscure. Future research will be required to address these questions and determine whether S. flexneri plays an active role in this process, potentially through its T3SS [18,29].
What are the mechanisms of vacuole escape?
Cell-to-cell spread through formation and resolution of membrane protrusions leads to the formation of double membrane vacuoles composed of an inner membrane, originating from the primary infected cell, and an outer membrane deriving from the adjacent cell. Access to the cytosol requires the disruption of both inner and outer membrane through production of bacterial factors that challenge the integrity of host cell membranes.
Role of pore-forming toxin and phospholipases in L. monocytogenes vacuole escape
L. monocytogenes displays several activities that challenge the integrity of host cell membranes. After the initial invasion step, bacteria promote their escape from the primary vacuole through production of the pore-forming toxin LLO [39]. In absence of LLO, L. monocytogenes can escape from primary vacuoles during infection of human epithelial cells through production of the broad-range phospholipase C, PC-PLC [40,41]. Interestingly, the use of inducible systems demonstrated that, in absence of LLO, PC-PLC is not only required for escape from primary vacuoles, but also from double membrane vacuoles formed upon cell-to-cell spread [40]. Moreover, upon L. monocytogenes spread from cell to cell in macrophages, the use of a strain conditionally deficient for LLO expression showed that the bacterial phospholipases PI-PLC (PlcA) and PC-PLC (PlcB) first facilitate the destruction of the inner membrane of the double membrane vacuole, but remain trapped in single-membrane compartments [42]. Thus, the pore-forming toxin LLO is essential for degradation of the outer membrane in macrophages, perhaps because of the phospholipid components of the receiving cell membrane. However, when epithelial cells were used as the secondary infected cell, the LLO deficient strain was able to fully escape the double membrane vacuole using only the bacterial phospholipases, highlighting the differences in membrane phospholipid composition present in different cell types [42]. These studies point to PC-PLC as a bacterial factor, along with LLO that distinctly contributes to L. monocytogenes spread from epithelial cells to epithelial cells within the intestine, and from macrophages to distant organs.
Post-invasion role of the T3SS in S. flexneri vacuole escape
After the initial invasion step, S. flexneri escapes from primary vacuoles in a process dependent on the T3SS and the pore-forming translocases IpaB and IpaC [43,44]. The secreted effector protein IpgD functions just prior to primary vacuole escape [45]. The phosphoinositide phosphatase, IpgD depletes the phosphatidylinositol 4.5 biphosphate PI(4,5)P2 from the primary vacuolar membrane resulting in recruitment of Rab11-positive vesicles and subsequent disruption of the vacuole through an unknown mechanism [45]. The use of inducible systems demonstrated that, in addition to its role in primary vacuole escape, the T3SS is also necessary for S. flexneri escape from the double membrane vacuoles formed upon cell-to-cell spread, as demonstrated by electron micrographs revealing the accumulation of bacteria in double membrane vacuoles in adjacent cells [18,46,47]. As opposed to the demonstrated role for effector proteins such as IpgD in primary vacuole escape, it is unclear whether secreted effector proteins in addition to the core components of the T3SS and associated translocases, are required for escape from secondary vacuoles. Similarly, the potential role for cellular processes, such as Rab GTPase-mediated vesicular trafficking, in secondary vacuole escape remains to be explored.
Concluding remarks
The recent research reviewed here support the notion that pathogens displaying actin-based motility have evolved specific mechanisms of cell-to-cell spread. As we realize the existence of these mechanisms, future work will be required to identify and characterize the bacterial and cellular factors that support this important aspect of bacterial pathogenesis. Powerful genetic approaches in relevant model systems are now available to identify and characterize the bacterial and cellular factors supporting bacterial spread from cell to cell (Box 2). In addressing questions related to the mechanisms supporting the resolution of protrusions into vacuoles and vacuole escape, the field is likely to depart from previous studies that focused on the mechanisms supporting actin assembly, and begin to tackle questions related to the dynamics of actin networks in membrane protrusions, and the remodeling of the plasma membrane supporting vacuole formation. It is also noteworthy that the exact mechanisms supporting vacuole escape are still unknown. Finally, it is tempting to extend the notion of specificity discussed here to additional pathogens displaying actin-based motility, and speculate that similar to S. flexneri and L. monocytogenes, Rickettsia spp. and Burkholderia spp. may have evolved specific mechanisms of spread from cell to cell as well. We suggest that, beyond actin-based motility, studies on bacterial spread from cell to cell will reveal a fascinating diversity of strategies deployed by pathogens to achieve dissemination.
Box 2. Outstanding questions.
What are the bacterial and cellular factors supporting L. monocytogenes spread from cell to cell?
What are the role(s) of the bacterial T3SS and cellular signaling events in S. flexneri spread from cell to cell?
What are the mechanisms supporting scission of protrusion membranes in primary infected and adjacent cells?
What are the mechanisms supporting Rickettsia spp. and Burkholderia spp. dissemination?
Highlights.
Intracellular bacteria displaying actin-based motility have evolved specific mechanisms of spread from cell to cell.
The resolution of Listeria monocytogenes protrusions relies on the dynamics of the actin cytoskeleton.
The resolution of Shigella flexneri protrusions relies on the type III secretion system-dependent manipulation of host cell tyrosine kinase and phosphoinositide signaling.
Acknowledgments
This work was supported by the National Institutes of Health grant R01AI073904 (H.A).
Glossary
- Membrane protrusion
a plasma membrane extension containing one or more bacteria at the distal end, originating from the primary infected cell and protruding into an adjacent cell as a result of motile bacteria encountering the plasma membrane
- Primary infected cell
the first infected cell at a site of infection. Pathogens may spread from this cell to adjacent cells creating a focus of infection
- Primary vacuole
a single-membrane vacuole surrounding invading bacteria in the primary infected cell. The single membrane is derived from the plasma membrane of the primary infected cell
- Protrusion resolution
the process of transition from a membrane protrusion connected to the primary infected cell to a secondary vacuole, no longer connected to the primary infected cell
- Secondary vacuole
a double-membrane vacuole that derives from a protrusion by scission of the protrusion neck and contains one or more bacteria within the cytosol of a cell adjacent to the primary infected cell. The inner membrane is derived from the plasma membrane of the primary infected cell, and the outer membrane is derived from the plasma membrane of the adjacent cell
- Vacuole-like protrusion (VLP)
an intermediate membrane-bound compartment that derives from a protrusion and is delineated by a seemingly continuous lining of the plasma membrane surrounding a bacterium yet connected to the primary infected cell by a thin, membranous tether
- Vacuole escape
the process of membrane disruption in the secondary vacuole leading to one or more bacteria gaining access to the cytosol of the adjacent cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tilney LG, Portnoy DA. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol. 1989;109:1597–1608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gouin E, Gantelet H, Egile C, Lasa I, Ohayon H, et al. A comparative study of the actin-based motilities of the pathogenic bacteria Listeria monocytogenes, Shigella flexneri and Rickettsia conorii. J Cell Sci. 1999;112(Pt 11):1697–1708. doi: 10.1242/jcs.112.11.1697. [DOI] [PubMed] [Google Scholar]
- 3.Kadurugamuwa JL, Rohde M, Wehland J, Timmis KN. Intercellular spread of Shigella flexneri through a monolayer mediated by membranous protrusions and associated with reorganization of the cytoskeletal protein vinculin. Infect Immun. 1991;59:3463–3471. doi: 10.1128/iai.59.10.3463-3471.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monack DM, Theriot JA. Actin-based motility is sufficient for bacterial membrane protrusion formation and host cell uptake. Cell Microbiol. 2001;3:633–647. doi: 10.1046/j.1462-5822.2001.00143.x. [DOI] [PubMed] [Google Scholar]
- 5.Takeuchi A, Formal SB, Sprinz H. Exerimental acute colitis in the rhesus monkey following peroral infection with Shigella flexneri. An electron microscope study. Am J Pathol. 1968;52:503–529. [PMC free article] [PubMed] [Google Scholar]
- 6.Oaks EV, Wingfield ME, Formal SB. Plaque formation by virulent Shigella flexneri. Infect Immun. 1985;48:124–129. doi: 10.1128/iai.48.1.124-129.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun AN, Camilli A, Portnoy DA. Isolation of Listeria monocytogenes small-plaque mutants defective for intracellular growth and cell-to-cell spread. Infect Immun. 1990;58:3770–3778. doi: 10.1128/iai.58.11.3770-3778.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mounier J, Ryter A, Coquis-Rondon M, Sansonetti PJ. Intracellular and cell-to-cell spread of Listeria monocytogenes involves interaction with F-actin in the enterocytelike cell line Caco-2. Infect Immun. 1990;58:1048–1058. doi: 10.1128/iai.58.4.1048-1058.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vasselon T, Mounier J, Hellio R, Sansonetti PJ. Movement along actin filaments of the perijunctional area and de novo polymerization of cellular actin are required for Shigella flexneri colonization of epithelial Caco-2 cell monolayers. Infect Immun. 1992;60:1031–1040. doi: 10.1128/iai.60.3.1031-1040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Disson O, Lecuit M. In vitro and in vivo models to study human listeriosis: mind the gap. Microbes Infect. 2013;15:971–980. doi: 10.1016/j.micinf.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 11.Domann E, Wehland J, Rohde M, Pistor S, Hartl M, et al. A novel bacterial virulence gene in Listeria monocytogenes required for host cell microfilament interaction with homology to the proline-rich region of vinculin. EMBO J. 1992;11:1981–1990. doi: 10.1002/j.1460-2075.1992.tb05252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kocks C, Gouin E, Tabouret M, Berche P, Ohayon H, et al. L. monocytogenes-induced actin assembly requires the actA gene product, a surface protein. Cell. 1992;68:521–531. doi: 10.1016/0092-8674(92)90188-i. [DOI] [PubMed] [Google Scholar]
- 13.Dramsi S, Levi S, Triller A, Cossart P. Entry of Listeria monocytogenes into neurons occurs by cell-to-cell spread: an in vitro study. Infect Immun. 1998;66:4461–4468. doi: 10.1128/iai.66.9.4461-4468.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bakardjiev AI, Stacy BA, Fisher SJ, Portnoy DA. Listeriosis in the pregnant guinea pig: a model of vertical transmission. Infect Immun. 2004;72:489–497. doi: 10.1128/IAI.72.1.489-497.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chong R, Squires R, Swiss R, Agaisse H. RNAi screen reveals host cell kinases specifically involved in Listeria monocytogenes spread from cell to cell. PLoS One. 2011;6:e23399. doi: 10.1371/journal.pone.0023399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dragoi AM, Agaisse H. The Serine/Threonine Kinase STK11 Promotes Shigella flexneri Dissemination through Establishment of Cell-Cell Contacts Competent for Tyrosine Kinase Signaling. Infect Immun. 2014;82:4447–4457. doi: 10.1128/IAI.02078-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dragoi AM, Agaisse H. The phosphatidylinositol-3 phosphate kinase PIK3C2A promotes Shigella flexneri dissemination through formation of vacuole-like protrusions. Infection and Immunity. 2015;83:1695–1704. doi: 10.1128/IAI.03138-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuehl CJ, Dragoi AM, Agaisse H. The Shigella flexneri Type 3 Secretion System Is Required for Tyrosine Kinase-Dependent Protrusion Resolution, and Vacuole Escape during Bacterial Dissemination. PLoS One. 2014;9:e112738. doi: 10.1371/journal.pone.0112738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Talman AM, Chong R, Chia J, Svitkina T, Agaisse H. Actin network disassembly powers dissemination of Listeria monocytogenes. J Cell Sci. 2014;127:240–249. doi: 10.1242/jcs.140038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sansonetti PJ, Mounier J, Prevost MC, Mege RM. Cadherin expression is required for the spread of Shigella flexneri between epithelial cells. Cell. 1994;76:829–839. doi: 10.1016/0092-8674(94)90358-1. [DOI] [PubMed] [Google Scholar]
- 21.Kim M, Ogawa M, Fujita Y, Yoshikawa Y, Nagai T, et al. Bacteria hijack integrin-linked kinase to stabilize focal adhesions and block cell detachment. Nature. 2009;459:578–582. doi: 10.1038/nature07952. [DOI] [PubMed] [Google Scholar]
- 22.Miura M, Terajima J, Izumiya H, Mitobe J, Komano T, et al. OspE2 of Shigella sonnei is required for the maintenance of cell architecture of bacterium-infected cells. Infect Immun. 2006;74:2587–2595. doi: 10.1128/IAI.74.5.2587-2595.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gianfelice A, Le PH, Rigano LA, Saila S, Dowd GC, et al. Host endoplasmic reticulum COPII proteins control cell-to-cell spread of the bacterial pathogen Listeria monocytogenes. Cell Microbiol. 2014 doi: 10.1111/cmi.12409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Polle L, Rigano LA, Julian R, Ireton K, Schubert WD. Structural details of human tuba recruitment by InlC of Listeria monocytogenes elucidate bacterial cell-cell spreading. Structure. 2014;22:304–314. doi: 10.1016/j.str.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 25.Rajabian T, Gavicherla B, Heisig M, Muller-Altrock S, Goebel W, et al. The bacterial virulence factor InlC perturbs apical cell junctions and promotes cell-to-cell spread of Listeria. Nat Cell Biol. 2009;11:1212–1218. doi: 10.1038/ncb1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rigano LA, Dowd GC, Wang Y, Ireton K. Listeria monocytogenes antagonizes the human GTPase Cdc42 to promote bacterial spread. Cell Microbiol. 2014;16:1068–1079. doi: 10.1111/cmi.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leung N, Gianfelice A, Gray-Owen SD, Ireton K. Impact of the Listeria monocytogenes protein InlC on infection in mice. Infect Immun. 81:1334–1340. doi: 10.1128/IAI.01377-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tran Van Nhieu G, Clair C, Bruzzone R, Mesnil M, Sansonetti P, et al. Connexin-dependent inter-cellular communication increases invasion and dissemination of Shigella in epithelial cells. Nat Cell Biol. 2003;5:720–726. doi: 10.1038/ncb1021. [DOI] [PubMed] [Google Scholar]
- 29.Czuczman MA, Fattouh R, van Rijn JM, Canadien V, Osborne S, et al. Listeria monocytogenes exploits efferocytosis to promote cell-to-cell spread. Nature. 2014;509:230–234. doi: 10.1038/nature13168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sechi AS, Wehland J, Small JV. The isolated comet tail pseudopodium of Listeria monocytogenes: a tail of two actin filament populations, long and axial and short and random. J Cell Biol. 1997;137:155–167. doi: 10.1083/jcb.137.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fattouh R, Czuczman MA, Kwon H, Copeland JW, Pelletier L, et al. The Diaphanous-related Formins Promote Protrusion Formation and Cell-to-Cell Spread of Listeria monocytogenes. J Infect Dis. 2015;211:1185–1195. doi: 10.1093/infdis/jiu546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heindl JE, Saran I, Yi CR, Lesser CF, Goldberg MB. Requirement for formin-induced actin polymerization during spread of Shigella flexneri. Infect Immun. 2010;78:193–203. doi: 10.1128/IAI.00252-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pust S, Morrison H, Wehland J, Sechi AS, Herrlich P. Listeria monocytogenes exploits ERM protein functions to efficiently spread from cell to cell. EMBO J. 2005;24:1287–1300. doi: 10.1038/sj.emboj.7600595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bishai EA, Sidhu GS, Li W, Dhillon J, Bohil AB, et al. Myosin-X facilitates Shigella-induced membrane protrusions and cell-to-cell spread. Cell Microbiol. 2013;15:353–367. doi: 10.1111/cmi.12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lum M, Morona R. Myosin IIA is essential for Shigella flexneri cell-to-cell spread. Pathog Dis. 2014;72(3):174–87. doi: 10.1111/2049-632X.12202. [DOI] [PubMed] [Google Scholar]
- 36.Rathman M, de Lanerolle P, Ohayon H, Gounon P, Sansonetti P. Myosin light chain kinase plays an essential role in S. flexneri dissemination. J Cell Sci. 2000;113(Pt 19):3375–3386. doi: 10.1242/jcs.113.19.3375. [DOI] [PubMed] [Google Scholar]
- 37.Robbins JR, Barth AI, Marquis H, de Hostos EL, Nelson WJ, et al. Listeria monocytogenes exploits normal host cell processes to spread from cell to cell. J Cell Biol. 1999;146:1333–1350. doi: 10.1083/jcb.146.6.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fukumatsu M, Ogawa M, Arakawa S, Suzuki M, Nakayama K, et al. Shigella targets epithelial tricellular junctions and uses a noncanonical clathrin-dependent endocytic pathway to spread between cells. Cell Host Microbe. 2012;11:325–336. doi: 10.1016/j.chom.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 39.Gaillard JL, Berche P, Mounier J, Richard S, Sansonetti P. In vitro model of penetration and intracellular growth of Listeria monocytogenes in the human enterocyte-like cell line Caco-2. Infect Immun. 1987;55:2822–2829. doi: 10.1128/iai.55.11.2822-2829.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grundling A, Gonzalez MD, Higgins DE. Requirement of the Listeria monocytogenes broad-range phospholipase PC-PLC during infection of human epithelial cells. J Bacteriol. 2003;185:6295–6307. doi: 10.1128/JB.185.21.6295-6307.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marquis H, Doshi V, Portnoy DA. The broad-range phospholipase C and a metalloprotease mediate listeriolysin O-independent escape of Listeria monocytogenes from a primary vacuole in human epithelial cells. Infect Immun. 1995;63:4531–4534. doi: 10.1128/iai.63.11.4531-4534.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alberti-Segui C, Goeden KR, Higgins DE. Differential function of Listeria monocytogenes listeriolysin O and phospholipases C in vacuolar dissolution following cell-to-cell spread. Cell Microbiol. 2007;9:179–195. doi: 10.1111/j.1462-5822.2006.00780.x. [DOI] [PubMed] [Google Scholar]
- 43.High N, Mounier J, Prevost MC, Sansonetti PJ. IpaB of Shigella flexneri causes entry into epithelial cells and escape from the phagocytic vacuole. EMBO J. 1992;11:1991–1999. doi: 10.1002/j.1460-2075.1992.tb05253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Osiecki JC, Barker J, Picking WL, Serfis AB, Berring E, et al. IpaC from Shigella and SipC from Salmonella possess similar biochemical properties but are functionally distinct. Mol Microbiol. 2001;42:469–481. doi: 10.1046/j.1365-2958.2001.02654.x. [DOI] [PubMed] [Google Scholar]
- 45.Mellouk N, Weiner A, Aulner N, Schmitt C, Elbaum M, et al. Shigella subverts the host recycling compartment to rupture its vacuole. Cell Host Microbe. 2014;16:517–530. doi: 10.1016/j.chom.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 46.Page AL, Ohayon H, Sansonetti PJ, Parsot C. The secreted IpaB and IpaC invasins and their cytoplasmic chaperone IpgC are required for intercellular dissemination of Shigella flexneri. Cell Microbiol. 1999;1:183–193. doi: 10.1046/j.1462-5822.1999.00019.x. [DOI] [PubMed] [Google Scholar]
- 47.Schuch R, Sandlin RC, Maurelli AT. A system for identifying post-invasion functions of invasion genes: requirements for the Mxi-Spa type III secretion pathway of Shigella flexneri in intercellular dissemination. Mol Microbiol. 1999;34:675–689. doi: 10.1046/j.1365-2958.1999.01627.x. [DOI] [PubMed] [Google Scholar]
- 48.Welch MD, Way M. Arp2/3-mediated actin-based motility: a tail of pathogen abuse. Cell Host Microbe. 2013;14:242–255. doi: 10.1016/j.chom.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Loisel TP, Boujemaa R, Pantaloni D, Carlier MF. Reconstitution of actin-based motility of Listeria and Shigella using pure proteins. Nature. 1999;401:613–616. doi: 10.1038/44183. [DOI] [PubMed] [Google Scholar]
- 50.Welch MD, Iwamatsu A, Mitchison TJ. Actin polymerization is induced by Arp2/3 protein complex at the surface of Listeria monocytogenes. Nature. 1997;385:265–269. doi: 10.1038/385265a0. [DOI] [PubMed] [Google Scholar]
- 51.Bernardini ML, Mounier J, d'Hauteville H, Coquis-Rondon M, Sansonetti PJ. Identification of icsA, a plasmid locus of Shigella flexneri that governs bacterial intra- and intercellular spread through interaction with F-actin. Proc Natl Acad Sci U S A. 1989;86:3867–3871. doi: 10.1073/pnas.86.10.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Makino S, Sasakawa C, Kamata K, Kurata T, Yoshikawa M. A genetic determinant required for continuous reinfection of adjacent cells on large plasmid in S. flexneri 2a. Cell. 1986;46:551–555. doi: 10.1016/0092-8674(86)90880-9. [DOI] [PubMed] [Google Scholar]
- 53.Egile C, Loisel TP, Laurent V, Li R, Pantaloni D, et al. Activation of the CDC42 effector N-WASP by the Shigella flexneri IcsA protein promotes actin nucleation by Arp2/3 complex and bacterial actin-based motility. J Cell Biol. 1999;146:1319–1332. doi: 10.1083/jcb.146.6.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suzuki T, Mimuro H, Suetsugu S, Miki H, Takenawa T, et al. Neural Wiskott-Aldrich syndrome protein (N-WASP) is the specific ligand for Shigella VirG among the WASP family and determines the host cell type allowing actin-based spreading. Cell Microbiol. 2002;4:223–233. doi: 10.1046/j.1462-5822.2002.00185.x. [DOI] [PubMed] [Google Scholar]
- 55.Chong R, Swiss R, Briones G, Stone KL, Gulcicek EE, et al. Regulatory mimicry in Listeria monocytogenes actin-based motility. Cell Host Microbe. 2009;6:268–278. doi: 10.1016/j.chom.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lasa I, Gouin E, Goethals M, Vancompernolle K, David V, et al. Identification of two regions in the N-terminal domain of ActA involved in the actin comet tail formation by Listeria monocytogenes. EMBO J. 1997;16:1531–1540. doi: 10.1093/emboj/16.7.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Skoble J, Portnoy DA, Welch MD. Three regions within ActA promote Arp2/3 complex-mediated actin nucleation and Listeria monocytogenes motility. J Cell Biol. 2000;150:527–538. doi: 10.1083/jcb.150.3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dabiri GA, Sanger JM, Portnoy DA, Southwick FS. Listeria monocytogenes moves rapidly through the host-cell cytoplasm by inducing directional actin assembly. Proc Natl Acad Sci U S A. 1990;87:6068–6072. doi: 10.1073/pnas.87.16.6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Theriot JA, Mitchison TJ, Tilney LG, Portnoy DA. The rate of actin-based motility of intracellular Listeria monocytogenes equals the rate of actin polymerization. Nature. 1992;357:257–260. doi: 10.1038/357257a0. [DOI] [PubMed] [Google Scholar]
- 60.Suzuki T, Miki H, Takenawa T, Sasakawa C. Neural Wiskott-Aldrich syndrome protein is implicated in the actin-based motility of Shigella flexneri. EMBO J. 1998;17:2767–2776. doi: 10.1093/emboj/17.10.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mimuro H, Suzuki T, Suetsugu S, Miki H, Takenawa T, et al. Profilin is required for sustaining efficient intra- and intercellular spreading of Shigella flexneri. J Biol Chem. 2000;275:28893–28901. doi: 10.1074/jbc.M003882200. [DOI] [PubMed] [Google Scholar]
- 62.Smith GA, Theriot JA, Portnoy DA. The tandem repeat domain in the Listeria monocytogenes ActA protein controls the rate of actin-based motility, the percentage of moving bacteria, and the localization of vasodilator-stimulated phosphoprotein and profilin. J Cell Biol. 1996;135:647–660. doi: 10.1083/jcb.135.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.David V, Gouin E, Troys MV, Grogan A, Segal AW, et al. Identification of cofilin, coronin, Rac and capZ in actin tails using a Listeria affinity approach. J Cell Sci. 1998;111(Pt 19):2877–2884. doi: 10.1242/jcs.111.19.2877. [DOI] [PubMed] [Google Scholar]
- 64.Laurent V, Loisel TP, Harbeck B, Wehman A, Grobe L, et al. Role of proteins of the Ena/VASP family in actin-based motility of Listeria monocytogenes. J Cell Biol. 1999;144:1245–1258. doi: 10.1083/jcb.144.6.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Van Troys M, Lambrechts A, David V, Demol H, Puype M, et al. The actin propulsive machinery: the proteome of Listeria monocytogenes tails. Biochem Biophys Res Commun. 2008;375:194–199. doi: 10.1016/j.bbrc.2008.07.152. [DOI] [PubMed] [Google Scholar]
- 66.Prevost MC, Lesourd M, Arpin M, Vernel F, Mounier J, et al. Unipolar reorganization of F-actin layer at bacterial division and bundling of actin filaments by plastin correlate with movement of Shigella flexneri within HeLa cells. Infect Immun. 1992;60:4088–4099. doi: 10.1128/iai.60.10.4088-4099.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee E, De Camilli P. Dynamin at actin tails. Proc Natl Acad Sci U S A. 2002;99:161–166. doi: 10.1073/pnas.012607799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brieher WM, Coughlin M, Mitchison TJ. Fascin-mediated propulsion of Listeria monocytogenes independent of frequent nucleation by the Arp2/3 complex. J Cell Biol. 2004;165:233–242. doi: 10.1083/jcb.200311040. [DOI] [PMC free article] [PubMed] [Google Scholar]