Abstract
Background
While enumeration of circulating tumor cells (CTCs) has shown some clinical value, the pool of CTCs contains a mixture of cells which contains additional information that can be extracted. Our group sub-classified CTCs by shape features focusing on nuclear size and related this to clinical information.
Methods
A total of 148 blood samples were obtained from 57 PC patients across the spectrum of metastatic states: no metastasis, non-visceral metastasis, and visceral metastasis. CTCs captured and enumerated on NanoVelcro Chips were subjected to pathologic review including nuclear size. The distribution of nuclear sizes was analyzed using a Gaussian Mixture Model. Correlations were made between CTC subpopulations and metastatic status.
Results
Statistical modeling of nuclear size distribution revealed 3 distinct subpopulations: large-nuclear (lnCTC), small-nuclear (snCTC), and very-small-nuclear CTCs (vsnCTCs). snCTC + vsnCTC identified patients with metastatic disease. vsnCTC counts alone, however, were elevated in patients with visceral metastases when compared to those without (0.36 ± 0.69 vs. 1.95 ± 3.77 cells/mL blood, p < 0.001). Serial enumerations suggested the emergence of vsnCTCs occurred prior to the detection of visceral metastases.
Conclusions
There are morphologic subsets of CTCs that can be identified by fundamental pathologic approaches, such as nuclear size measurement. This observational study strongly suggests that they contain relevant information on disease status. In particular, the detection of vsnCTCs correlated with the presence of visceral metastases and should be formally explored as a putative blood-borne biomarker to identify patients at risk for developing this clinical evolution of PC.
Keywords: Prostate cancer, circulating tumor cells, visceral metastasis, NanoVelcro Chips, nuclear size
Introduction
Prostate cancer (PC) remains an important public health problem as almost 30,000 are expected to die of this disease in 2014.1 Within the group of men at risk for death from this disease, there is range of clinical behaviors including progression to visceral metastases, which spread to non-osseous, non-nodal sites including the liver and/or lungs.2–4 It is recognized that men with visceral metastases have significantly shorter survival in comparison to those who do not progress in this fashion5. These progression events often lead to organ failure followed shortly by death. In typical practice, patients are monitored by serum PSA concentration without frequent radiographic assessment. It is recognized, however, that serum PSA changes do not detect6–8 certain disease alterations such as emergence of non-PSA producing neuroendocrine PC (NEPC) or small cell PC. Moreover, PSA monitoring provides little insight into other clinical alterations which have prognostic importance such as the development of visceral metastases. By the time these lesions are detected radiographically or clinically (by altered organ function), the metastatic lesions have been present for some time. Additional highly-sensitive, minimally-invasive, serially-obtainable means of monitoring disease status are still needed, especially for the visceral progression events.
Circulating tumor cells (CTCs) have arisen as contemporary prognostic and predictive biomarkers for PC.9 Their value has been demonstrated mainly in enumeration.10–17 The conventional, FDA-approved CTC assay has not been useful in measuring certain changes in clinical behavior such as the detection of emerging or occult visceral spread.13 CTCs are a heterogeneous population.18, 19 We and others have proposed that subclassification of these cells could augment their clinical utility.20–22 To this end, morphologic classification has always been a fundamental practice in pathology. While Gleason grading has been highly informative, nuclear size and shape in tumor sections have also been shown to correlate with distant metastasis and death in PC.23–25 These morphologic alterations have been associated with changes in signaling pathways that drive various pro-tumorigenic and pro-metastatic processes through a structure-function relationship.26 Since biological alterations, which affect the natural history of disease, cause structural alteration in cells (including the pool of CTCs), we hypothesized that variations in the CTC morphology and nuclear size can be used to detect the changes in disease phenotype such as the detection and possibly prediction of visceral metastases.
In this study (Figure 1), we acquired blood specimens from PC patients across a broad spectrum of metastatic states: no metastasis, non-visceral (osseous and/or nodal) metastasis, and visceral (hepatic and/or pulmonary) metastasis. We isolated and enumerated CTCs using NanoVelcro Chips27, 28, an emerging CTC isolation system that utilizes a combination of a microfluidic chaotic mixer and a nanostructured capture substrate that functions with high sensitivity. By incorporating higher-resolution fluorescence microscopy into the NanoVelcro system, we were able to visualize cellular features of the captured CTCs (DAPI+/CK+/CD45-) and perform pathologic review for cellular morphology and nuclear size. This led to the identification of nuclear size-defined CTC subsets. Our study showed that these subsets correspond to metastatic state.
Figure 1. The study design for identifying very-small-nuclear CTCs (vsnCTCs).
PC patients were recruited for CTC enumeration studies and each sample was placed into one of three cohorts depending on the metastatic status of the patient at the time of collection including: visceral metastasis, non-visceral (osseous/lymph node) metastasis, and non-metastatic disease. CTC enumeration studies were performed using highly sensitive NanoVelcro Chips in conjunction with the use of fluorescence microscopy. The identified CTCs (DAPI+/CK+/CD45-) were then subjected to nuclear size measurements. Through biostatistical analysis and modeling, we identified three subpopulations of CTCs: large-nuclear CTCs (lnCTCs), small-nuclear CTCs (snCTCs) and very-small-nuclear CTCs (vsnCTCs). The emergence of vsnCTCs correlates with the onset or presence of visceral metastases and may serve as the basis for a new diagnostic assay targeting the most aggressive form of PC. (Scale bars in the figures indicate 10 μm.)
Patients and Methods
Patients and samples
Blood specimens for this study were extracted from existing biobanking protocols approved by the Cedars-Sinai Medical Center (CSMC) Institutional Review Board (IRB). The entire pool of patients consisted of men with histologically confirmed PC agreeing to provide blood as part of CSMC biobanking protocols who underwent evaluation and/or treatment at CSMC between January 2013 and December 2014. Use of all biospecimens from the banking studies was also conducted under IRB oversight. As such, this study was conducted as an observational study to determine the range and distributions of CTC nuclear sizes in a single institution cohort of men with PC. Radiographic evaluation within 3 months of sample collection was required for inclusion in this analysis. Multiple blood draws from individual patients were allowed. Patients and their enumeration studies were classified by sites of metastasis into one of three categories: no metastases, non-visceral metastases, or visceral metastases, according to the contemporary radiographic evidence. Samples and patients categorized as “non-visceral metastases” indicated that the subject had osseous lesions and/or lymph node spread on most recent radiographic studies. Samples and patients categorized as “visceral metastases” indicated that the subject had radiographically detected lesions in soft tissue organs such as the liver or lungs with or without bone or lymph node involvement on recent imaging. We conducted a statistical analysis and modeling of CTC nuclear size distribution to sub-classify CTCs. Correlations were made between CTC subpopulations and metastatic status (see Statistical analysis below).
CTC enrichment using NanoVelcro Chips
The general protocol for CTC enrichment by NanoVelcro Chips is summarized in a workflow (Supporting Figure 1). Venous blood was collected in acid-citrate dextrose-containing vacutainers (BD Bioscience, San Jose, CA, USA) and processed within 4 hours of collection. CTCs were isolated using NanoVelcro Chips as previously described.27, 28 In brief, 1.0 mL of venous blood was subjected to red blood cell (RBC) depletion using a standard RBC lysis buffer (containing NH4Cl and Tris, pH 7.2). The remaining cells were incubated with a capture agent (biotinylated goat anti-human EpCAM antibody, R&D Systems). After washing carefully, the sample was loaded into the NanoVelcro Chip, which consists of a streptavidin-coated NanoVelcro substrate and an overlaid polydimethylsiloxane (PDMS) chaotic mixer. In conjunction with the use of an automated fluid handler, the cell suspension was introduced into the chip at a consistent flow rate (0.5 mL/h). After fixation using 2% paraformaldehyde (PFA, Electron Microscopy Science), the cells immobilized on the NanoVelcro substrate were subjected to immunocytochemical (ICC) staining with DAPI (Vector Laboratories, Burlingame, CA, USA), rabbit anti-pan-cytokeratin (Life technologies, Grand Island, NY, USA, and Abcam, Cambridge, MA, USA), mouse anti-CD45 (BD Biosciences, San Jose, CA, USA, and Abcam, Cambridge, MA, USA), Alexa Fluor 488-conjugated anti-rabbit, and Alexa Fluor 555-conjugated anti-mouse (Life technologies, Grand Island, NY, USA). Subsequent microscopic imaging was performed to identify the CTCs. For selected cases, chicken anti-synaptophysin (Abcam, Cambridge, MA, USA) and Alexa Fluor 647-conjugated anti-chicken (Life technologies, Grand Island, NY, USA) were used. (Detailed methodology available in Supporting Methods; see online supporting information)
CTC Imaging followed by nuclear size measurements
NanoVelcro Chips were imaged using an upright fluorescence microscope (Eclipse 90i, Nikon) with NIS-Element imaging software (Nikon). An automatic scan over the NanoVelcro chip was carried out by the imaging system under 40× magnification with DAPI, FITC and TRITC channels corresponding to nuclear, CK, and CD45 staining respectively. The DAPI+/CK+/CD45- events (Figure 1) were selected as candidate CTCs and were further subjected to another scan at either 100× or 400× magnification. All high-resolution images were reviewed by a pathologist to ensure that CTC calls were morphologically consistent with an epithelial cell and not a hematologic cell.
Nuclear size of all identified CTCs was measured along the longest axis and the width perpendicular to that axis (Figure 1).29, 30 The nuclear size for statistical analyses is defined as follows:
To ensure the consistency and accuracy of nuclear size measurements on NanoVelcro Chips, we performed a series of calibration studies using PC cell lines and blood samples from PC patients. (See Supporting Figure 2 for the calibration data).
Statistical analyses
To identify the subpopulations of CTCs, we performed a cluster analysis for the CTC nuclear sizes using the Gaussian Mixture Model (GMM) formed by K Gaussian components with varied variances. The means and variances of K Gaussian components were estimated by the Expectation-Maximization (EM) algorithm 31 and the optimal number K was decided by the Akaike information criterion (AIC) 32 and Bayesian information criterion (BIC) 33. Based on its nuclear size, each CTC was assigned to a cluster with the maximum likelihood of all K Gaussian components. χ2 test was performed to test the independency of our unsupervised clustering of CTCs on metastatic categories.
Since metastatic status was subject to change over the disease course, the correlational analyses in this study were based on enumeration studies instead of the patients, and multiple enumeration studies from serial time points are allowed. Wilcoxon rank-sum tests were used to evaluate the statistical differences of the CTC counts of the enumeration studies between different metastatic categories: 1) metastasis versus no metastasis and 2) non-visceral metastasis versus visceral metastasis. Considering the potentially independent nature of multiple blood samples and enumeration studies from the same patients, we further adopted a Poisson generalized linear mixed model (GLMM) to test the correlation between metastatic status and CTC counts adjusted for the potential effect resulting from repeated measurements.
The statistical tests in this study were performed using the R statistical software version 3.1.1 (http://www.r-project.org/). The GLMM analysis was performed using package glmmML version 1.0.34 All the tests are two-sided and p values less than 0.05 were considered statistically significant.
Results
Patient characteristics and sample collections for CTC enumeration studies
Blood samples from a total of 57 PC patients comprised the full complement of subjects utilized in this analysis. Detailed clinical characteristics of the patients are summarized in Table 1. The classification of the patient and their enumeration studies based on their sites of metastasis is also listed. Each patient had between 1 and 11 enumeration studies performed yielding a total of 148 enumeration studies (enumeration studies from each patient are listed in Supporting Table 1).
Table 1.
Patient characteristics
| Patient classification | Patients (n = 57)* | Enumeration samples (n = 148)* | Category of metastatic status |
|---|---|---|---|
| Patients undergoing local therapy | 14 | 15 | No metastases |
| Patients on surveillance after local therapy | 4 | 5 | No metastases |
| Patient relapsed after local disease | |||
| Castration sensitive | |||
| No metastatic disease (PSA only) | 3 | 3 | No metastases |
| Known metastatic disease | |||
| Bone/lymph node metastases only | 4 | 6 | Non-visceral (osseous/LN) metastases |
| Bone/lymph node and visceral metastases | 0 | 0 | Visceral metastases |
| Castration resistant | |||
| No metastatic disease (PSA only) | 3 | 8 | No metastases |
| Known metastatic disease | |||
| Bone/lymph node metastases only | 17 * | 38 * | Non-visceral (osseous/LN) metastases |
| Bone/lymph node and visceral metastases | 13 * | 61 * | Visceral metastases |
| Visceral metastases only † | 2 † | 12 † | Visceral metastases |
Patients with change in metastatic status during follow up were placed in all the categories to which they belonged or had belonged. However the change in status is reflected in the categorization of enumeration studies.
Statistical analysis and modeling of CTC nuclear size distribution and CTC subpopulations
The CTC data pool was generated by performing 148 enumeration studies (as serial collections were used) that provided a total of 304 CTCs for analyzing the nuclear size distribution. Based on the AIC and BIC, the optimal model was determined to be a 3-cluster GMM with cluster means at 6.82, 10.63, 21.63 μm, and standard deviations of 1.14, 2.51, 5.67 μm (Figure 2A). The AIC and BIC values of different cluster numbers are also shown (Figure 2B). We then assigned CTCs into the cluster with the maximum Gaussian likelihood, and yielded three CTC subpopulations that we labeled as very-small-nuclear CTCs (vsnCTCs, nuclear size < 8.54 μm), small-nuclear CTCs (snCTCs, nuclear size between 8.54 μm and 14.99 μm) and large-nuclear CTCs (lnCTCs, nuclear size > 14.99 μm).
Figure 2. Statistical analysis and modeling of CTC nuclear size distribution and CTC subpopulations.

A) The histogram depicts the distribution of 304 CTCs according to their nuclear sizes. The black solid line shows the density of the optimal Gaussian mixture model (GMM) that best fits the histogram. The red, blue and grey solid lines represent the three cluster-specific Gaussian density curves. The dash lines indicate the cutoffs of our classification rule: assigning a sample to the cluster with the maximum Gaussian likelihood. B) The blue line shows the AIC values and the red line shows the BIC values for K (cluster number) from 1 to 4. The best cluster number is 3 as both AIC and BIC reached the minimum when K = 3.
Relationship between CTC nuclear sizes and metastatic status
To further analyze the relationship between the three CTC subpopulations and metastatic status, histograms were plotted using density of CTC counts versus nuclear sizes for each metastatic status category (Figure 3). The proportions of the three CTC nuclear subtypes (examples in Figure 3A and Supporting Figure 3) varied significantly between the different metastatic categories. In the cohort of non-metastatic patients (Figure 3B), the majority of cells were classified as lnCTCs (62%). In the category of non-visceral metastasis (Figure 3C), the snCTCs constituted the major CTC subpopulation (51%), followed by vsnCTCs (27%). In the category of visceral metastasis (Figure 3D), the vsnCTCs constituted the major CTC subpopulation (65%), followed by snCTCs (20%). This result shows that our unsupervised clustering of CTCs has significant dependency on metastatic categories (two sided χ2 test, p = 2.29×10−13, Supporting Table 2).
Figure 3. Relationship between CTC nuclear sizes and metastatic status.
A) Representative images of a lnCTC, a snCTC and a vsnCTC taken by fluorescence microscopy. The cells are stained with DAPI (blue), Alexa Fluor 488-conjugated anti-CK (green), and Alexa Fluor 555-conjugated anti-CD45 (orange). Histograms and cluster-specific Gaussian density curves were plotted using CTC counts versus nuclear sizes and classified into three metastatic statuses: B) No metastasis, in which lnCTCs account for the largest proportion (62%) among all the CTCs; C) Non-visceral metastasis, in which snCTCs constitute the major subpopulation (51%), followed by vsnCTCs (27%); and D) visceral metastasis, in which vsnCTCs account for the largest proportion of cells (65%), followed by snCTCs (20%). The proportions of the three CTC subpopulations varied significantly between different metastatic statuses as demonstrated by a two-sided χ2 test (p = 2.29×10−13).
snCTC + vsnCTC counts correlate with metastasis
We then performed a statistical analysis on the 148 enumeration studies to measure the correlation between CTC subpopulations and metastatic state. The pool consisted of 31 enumeration studies in the no metastasis category, and 117 in the metastatic PC category (combination of non-visceral metastasis and visceral metastasis categories). When examining the total CTC count (lnCTC + snCTC + vsnCTC), there was a statistically significant difference between metastatic and non-metastatic disease (0.94 ± 1.91 versus 2.42 ± 3.60 cells per mL of blood, p = 0.002, Figure 4A). In an additional interrogation, the lnCTC subpopulation alone failed to distinguish metastatic and non-metastatic disease (0.58 ± 1.88 versus 0.40 ± 1.28 cells per mL of blood, p = 0.893, Figure 4B). snCTC + vsnCTC counts, in contrast, remained significantly different (0.35 ± 0.66 versus 2.02 ± 3.36 cells per mL of blood, p < 0.0001, Figure 4C). To assess the potential effect resulting from repeated measurements from the same patients, analysis using GLMM was conducted to test the correlation between metastatic status and CTC counts. The p values obtained using GLMM were consistent with those from the Wilcoxon tests (indicated in figures). These data were consistent with the preferential distribution of snCTCs and vsnCTCs in Figure 3C and 3D, and demonstrated the benefit of categorization of CTCs by nuclear size (< 14.99 μm). Overall, the snCTC + vsnCTC counts obtained by using these CTC definitions provided more information on metastatic status in PC than total CTC count.
Figure 4. Correlation between snCTC + vsnCTC counts and metastatic PC.
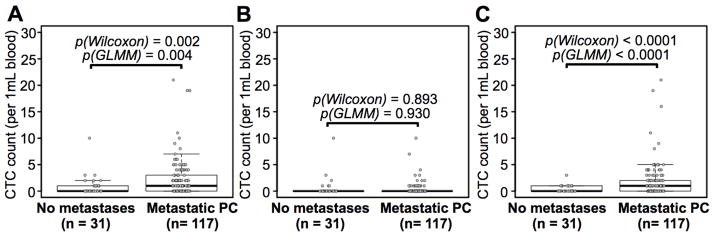
Box plots are shown for A) total CTC counts, B) lnCTC counts, and C) snCTC + vsnCTC counts in 1.0 mL of blood. Boxes represent interquartile range, and the horizontal line across each box indicates median value. The y-axis represents CTC counts per 1.0 mL of blood. There are 31 enumerations from the category of no metastasis and 117 from metastatic PC patients (Supporting Figure 4). The data showed that the snCTC + vsnCTC counts obtained by using these nuclear size-based CTC definitions provide more information on metastatic status in PC than total CTC count. Statistical analyses were performed using two-sided Wilcoxon tests and Wald tests following a Generalized Linear Mixed Model (GLMM). All the p values are indicated in the figures.
vsnCTC counts correlate with the presence of visceral metastasis
We further investigated the correlation between CTC subsets and visceral metastasis. These analyses were performed using 105 enumeration studies from patients who had radiographically detectable osseous and/or nodal lesions, with or without visceral organ involvement. Among these enumeration studies, 44 were in the non-visceral metastases category and 61 were in the visceral metastases category (Supporting Figure 4). We found that snCTC + vsnCTC counts differed significantly between non-visceral metastasis and visceral metastasis categories (1.05 ± 1.26 versus 2.69 ± 4.27 cells per mL of blood, p = 0.030, Figure 5A). Recognizing the preferential distribution of snCTCs and vsnCTCs in Figure 3C and 3D, we pursued further statistical analyses of individual CTC subpopulations. Alone, snCTC counts were unable to distinguish metastatic subpopulations (0.68 ± 1.12 versus 0.74 ± 1.38 cells per mL of blood, p = 0.721, Figure 5B). In contrast, vsnCTC counts successfully distinguished visceral metastatic from non-visceral-metastatic PC patients (0.36 ± 0.69 versus 1.95 ± 3.77 cells per mL of blood, p = 0.002, Figure 5C). The p values obtained using GLMM and were consistent with those from the Wilcoxon tests (indicated in figures). These data combined with the distribution of vsnCTCs in Figure 3D indicated that vsnCTCs rather than snCTCs are correlated with the presence of aggressive PC with visceral involvement.
Figure 5. Correlation between vsnCTC counts and aggressive PC with visceral metastasis.

Box plots are shown for A) snCTC + vsnCTC counts, B) snCTC counts, and C) vsnCTC counts in 1.0 mL of blood. Boxes represent interquartile range, and the horizontal line across each box indicates median value. The y-axis represents CTC counts per 1.0 mL of blood. There are 44 enumerations from the category of non-visceral metastasis and 61 from the category of visceral metastasis (Supporting Figure 4). The results indicated that vsnCTCs rather than snCTCs are correlated with the presence of visceral metastases. Statistical analyses were performed using two-sided Wilcoxon tests and Wald tests following a Generalized Linear Mixed Model (GLMM). All the p values are indicated in the figures.
Summary of clinical cases with visceral metastases
In this study, a total of 15 patients had visceral metastases, including 10 patients with hepatic lesions and 7 with pulmonary nodules. Only 2 patients presented with visceral-only disease, while the other had co-existing osseous involvement. The timeline for each patient is shown in Figure 6 starting from the time of first CTC enumeration in this study to the end of follow-up. We successfully detected vsnCTCs using NanoVelcro Chips in all 12 patients who presented with visceral metastases. The other 3 patients had osseous/nodal metastases initially, but later developed metastatic lesions in visceral organ. vsnCTCs were found in these patients prior to radiographic detection of visceral lesions with the lead time ranging from 104 to 196 days (typical example see patient A in Supporting Figure 5A). These patients received a variety of therapeutic interventions for their visceral disease, including platinum-based and taxane-based chemotherapies, radium-223, bicalutamide, abiraterone, enzalutamide, and an investigational multi-kinase inhibitor. There were no patients in our series that developed or presented with visceral metastases that did not have detectable vsnCTCs. At the time when this study concluded, 47% (7/15) of the patients died from their disease.
Figure 6. Summary of the 15 patients with visceral metastases.

The beginning of each timeline (Day 0) indicates the time of first CTC enumeration by NanoVelcro Chips. The end of each timeline is the time of the end of follow-up. There are 3 patients (1117, 1161, 3701) initially presented with osseous/nodal metastases and later developed visceral lesions. vsnCTCs were found in these patients prior to radiographic detection of visceral metastases with the lead time ranging from 104 to 196 days (typical example see patient A in Supporting Figure 5A). Detailed clinical history of two other example cases (patient B and C) and observations on their vsnCTCs were presented in Supporting Figure 5B and 6.
Discussion
CTC biology has been area of rapid development in several tumor models including PC. Their value has been clearly demonstrated in enumeration.10–17 Newer technologies have further refined our ability to analyze the pool of CTCs creating an opportunity to extract more useful clinical information from this easily obtained source of cancer cells. Our group has pioneered the use of the NanoVelcro Chip which utilizes a precisely engineered nanosubstrate and microfluidics that allows us to take advantage of cell surface marker expression (such as EpCAM) to capture CTCs from whole blood. As demonstrated in this study and in previous publications26, 27, NanoVelcro Chips exhibited higher sensitivity in detecting live CTCs from peripheral blood (also see Supporting Fig 7) than currently used approach in the clinic. Moreover, by incorporating fluorescence microscopy with NanoVelcro Chips, we generated cell images amenable to formal histopathologic review.
Histopathology remains the gold standard for cancer diagnosis and classification. Morphological features have been linked to clinical behavior in a variety of diseases including prostate cancer (e.g. Gleason Scoring). However, this type of histopathologic review has not been typically applied to CTC isolation strategies. Here, we applied this underappreciated and fundamental concept to a highly sensitive and specific CTC enumeration approach based on the NanoVelcro Chip technology. This refined process identified a potential source of false-positive events from leukocytes and other non-epithelial cells. This same approach also enabled us to perform nuclear size measurements on individual CTCs. Among the commonly used morphologic features, we found nuclear sizes to be the most robust feature for the NanoVelcro substrate-immobilized CTCs due to: 1) the high reproducibility of DAPI staining and 2) the minimal attenuation of DAPI fluorescence during the imaging process. Moreover, we acknowledge that in our experience and the reports of other groups, the cellular membrane of CTCs is extremely fragile and susceptible to generation of artifacts and disruption by mechanical forces.35 In contrast, the nuclear morphology was rather well-preserved during the entire CTC enumeration process. As a result, we focused on measuring the size of nuclei in this observational study and revealed three different subpopulations of CTCs using nuclear size, namely lnCTCs, snCTCs and vsnCTCs.
Our study points toward a potential benefit in adding simple morphologic categorization (i.e., nuclear size) to CTC enumeration. Most importantly, this observation lays the foundation for exploring vsnCTCs as a putative biomarker for emerging visceral metastases in PC patients. It has been reported that clinically and/or radiographically detectable visceral metastases are found in less than 20% of patients in reported case series.3 While this is a minority of patients, it is also recognized that patients who develop visceral metastases have poorer outcomes that those patient with bone and/or lymph node only disease.2–5 It has been argued that this clinical behavior identifies an aggressive PC subtype which has not been well studied. Patients with (or those who are at risk of) visceral metastases are an important subcategory of PC patients. Our observation could serve as the basis for a clinical test that could help to identify men at high-risk for visceral metastases and hence mortality from organ failure due to PC. As such, this approach could create an opportunity for early intervention that may impact the negative outcomes related to visceral metastases such as early cytotoxic therapy as demonstrated in E380536. We acknowledge the limitations of this analysis given the observational nature of this report. Based on the information generated on the range and distribution of nuclear sizes in theses CTCs, a formal clinical study is now underway.
Our focus on patients with visceral metastases necessarily limits the sample size achievable in single institution and is a limitation of this exploratory study. It is also important to recognize that metastatic status can change over time – subsets of patients will develop visceral disease while other will have progressive bone lesions only. As such, we and others have hypothesized that the major CTC subsets in circulation may shift as this process occurs.37 It is conceivable that one may detect different CTC subsets at different time points corresponding to different disease status, and the correlation between CTC subsets and radiographic pattern of metastatic spread could still be made using serial specimen collection and enumeration studies for each patient. Therefore, we included serial samples from patients in the correlational analysis considering each enumeration study as an independent event that reflects the current status of the patient’s disease. While this raises concern over bias from repeated sampling from certain patients, the GLMMs tests performed showed that the findings of the Wilcoxon test were still valid. Our results indicated that snCTC + vsnCTC counts were significantly higher in metastatic PC compared to the no metastasis category. Furthermore, vsnCTC counts were significantly higher in aggressive PC with visceral involvement compared to the PC with only osseous and nodal metastases. These findings require further validation, but the observation we report appears to hold true using two independent statistical tests.
Within our patient cohort, we observed that in patients who are undergoing serial CTC enumerations, vsnCTCs were identified prior to the radiographic detection of visceral metastatic lesions (patient A in Supporting Figure 5A). This raises the possibility that vsnCTCs may have predictive value with respect to progression to visceral metastasis. We acknowledge that temporal relationship implied in this observation may potentially be affected by lead time bias due to the limited frequency of clinical imaging in standard clinical practice. We point out that vsnCTCs are not exclusively detected in the setting of existing/detectable) visceral metastases. However, it is possible that patients in whom vsnCTCs are detected could be at increased risk of development of visceral disease. This also requires further formal investigation.
Like dynamic changes in CTC counts measured by CellSearch38–40, changes in vsnCTCs counts may also reflect a positive impact from anti-cancer therapy27, 37–39. Each CTC subset may also exhibit variations in response to therapies. While this report is limited by the sample size and the heterogeneity in the therapeutic interventions, we have observed that visceral metastasis patients who are therapeutic responders have shown trends toward vsnCTC reduction in the face of treatment (typical example see patient B in Supporting Figure 5B).41 A larger prospective study is ongoing to further validate these observations with more longitudinal cases and less heterogeneity in treatment options.
Progression to visceral metastases indicates a change in the underlying molecular mechanisms42 driving the metastatic process in PC. An example of such an alteration would be differentiation toward a more neuroendocrine phenotype of PC (NEPC).7 We report an interesting observation in such a patient (patient C in Supporting Figure 6). This patient underwent a biopsy of a pleural lesion which was found to express CK and synaptophysin. On analysis of his vsnCTCs also reflected this immunophenotype. This is particularly interesting as synaptophysin is a recognized marker for NEPC7 that has not been previously reported in PC CTCs. While this finding is limited to an isolated case report, it not only demonstrated the vsnCTC-tumor relationship in this patient but also supported the idea of including additional cellular marker in CTC enumeration for more clinical information. This observation has driven us to pursue additional interrogations as our collections have proceeded which will be reported at a later time.
In addition to the prognostic utility of CTC count, significant research endeavors have been devoted to characterizing the molecular nature of CTCs using genomic43–45, transcriptomic46, and proteomic approaches47, 48, as well as exploring the functional properties of CTCs based on ex vivo expansion49 and xenograft assays50. Interestingly, recent studies also re-emphasized the significance of morphologic analysis by pointing out that CTC clusters with an increased mesenchymal phenotype have increased metastatic potential51 and correlate with poor prognosis52, which were consistent with early observations53 in histopathology. Our findings echo these studies by showing the importance of CTC nuclear size and its relevance to cancer stage and clinical behaviors. Considering the possible paradigm that it is the combination of variation across all possible omic levels in concert that leads to phenotype54, our observation provides an excellent chance for future studies to comprehensively characterize this aggressive phenotype of PC at different omic levels.
In summary, we report an initial observation that CTC subsets defined by nuclear sizes may have clinical relevance. The NanoVelcro assay we utilized provided a high degree of sensitivity and specificity for CTC identification while making nuclear size assessment possible. While we recognized that this innovative finding has been made in a small patient cohort, the emergence of the CTC subsets and their relationship to clinical metastatic status appeared surprisingly robust. Larger-scale, prospective trials should be conducted to validate this initial observation and investigate its potential application in the detection and perhaps prediction of visceral metastasis. This observation also points towards the importance of understanding the biochemical nature of the vsnCTC to further delineating their relevance in metastatic PC. Given the fundamental nature of morphologic analysis in pathology, we propose that morphologic characterization be incorporated into CTC enumerations and that further studies of the vsnCTCs be conducted to explore its relationship to visceral metastasis.
Supplementary Material
Acknowledgments
Funding Support: The research endeavors at Cedars-Sinai Medical Center were supported by a DoD Idea Award (W81XWH-11-1-0422), the Steven Spielberg Discovery Fund in Prostate Cancer Research, a Young Investigator Award from the Prostate Cancer Foundation (PCF), the St. Anthony Prostate Cancer Research Fund, the CD McKinnon Memorial Fund for Neuroendocrine Prostate Cancer, and the Berns Family Fund. The research endeavors at UCLA were supported by a Creativity Award from PCF, the UCLA Prostate Cancer SPORE Program, and research grants (R21 CA151159 and R33 CA157396) from the NIH/NCI Innovative Molecular Analysis Technologies (IMAT) Program. We were also supported by grants from the National Natural Science Foundation of China (30900650/H1615 and 81372501/H1615).
The authors thank Elizabeth T. Kaufman, Margarit Sievert, Chunyan Liu, and James MacDonald for their efforts in specimen collection and for sharing insights in experimental design and data analysis.
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Pezaro CJ, Omlin A, Lorente D, et al. Visceral disease in castration-resistant prostate cancer. Eur Urol. 2014;65:270–273. doi: 10.1016/j.eururo.2013.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gandaglia G, Karakiewicz PI, Briganti A, et al. Impact of the Site of Metastases on Survival in Patients with Metastatic Prostate Cancer. Eur Urol. 2014 doi: 10.1016/j.eururo.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 4.Pond GR, Sonpavde G, de Wit R, Eisenberger MA, Tannock IF, Armstrong AJ. The prognostic importance of metastatic site in men with metastatic castration-resistant prostate cancer. Eur Urol. 2014;65:3–6. doi: 10.1016/j.eururo.2013.09.024. [DOI] [PubMed] [Google Scholar]
- 5.Halabi S, Kelly WK, Zhou H, et al. The site of visceral metastases (mets) to predict overall survival (OS) in castration-resistant prostate cancer (CRPC) patients (pts): A meta-analysis of five phase III trials. J Clin Oncol. 2014;5s:2014. (suppl; abstr 5002) [Google Scholar]
- 6.Vinjamoori AH, Jagannathan JP, Shinagare AB, et al. Atypical metastases from prostate cancer: 10-year experience at a single institution. AJR Am J Roentgenol. 2012;199:367–372. doi: 10.2214/AJR.11.7533. [DOI] [PubMed] [Google Scholar]
- 7.Beltran H, Tomlins S, Aparicio A, et al. Aggressive Variants of Castration-Resistant Prostate Cancer. Clin Cancer Res. 2014 doi: 10.1158/1078-0432.CCR-13-3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beltran H, Tagawa ST, Park K, et al. Challenges in recognizing treatment-related neuroendocrine prostate cancer. J Clin Oncol. 2012;30:e386–389. doi: 10.1200/JCO.2011.41.5166. [DOI] [PubMed] [Google Scholar]
- 9.Danila DC, Fleisher M, Scher HI. Circulating tumor cells as biomarkers in prostate cancer. Clin Cancer Res. 2011;17:3903–3912. doi: 10.1158/1078-0432.CCR-10-2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldkorn A, Ely B, Quinn DI, et al. Circulating tumor cell counts are prognostic of overall survival in SWOG S0421: a phase III trial of docetaxel with or without atrasentan for metastatic castration-resistant prostate cancer. J Clin Oncol. 2014;32:1136–1142. doi: 10.1200/JCO.2013.51.7417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Resel Folkersma L, San Jose Manso L, Galante Romo I, Moreno Sierra J, Olivier Gomez C. Prognostic significance of circulating tumor cell count in patients with metastatic hormone-sensitive prostate cancer. Urology. 2012;80:1328–1332. doi: 10.1016/j.urology.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Wang FB, Yang XQ, Yang S, Wang BC, Feng MH, Tu JC. A higher number of circulating tumor cells (CTC) in peripheral blood indicates poor prognosis in prostate cancer patients--a meta-analysis. Asian Pac J Cancer Prev. 2011;12:2629–2635. [PubMed] [Google Scholar]
- 13.Scher HI, Jia X, de Bono JS, et al. Circulating tumour cells as prognostic markers in progressive, castration-resistant prostate cancer: a reanalysis of IMMC38 trial data. Lancet Oncol. 2009;10:233–239. doi: 10.1016/S1470-2045(08)70340-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olmos D, Arkenau HT, Ang JE, et al. Circulating tumour cell (CTC) counts as intermediate end points in castration-resistant prostate cancer (CRPC): a single-centre experience. Ann Oncol. 2009;20:27–33. doi: 10.1093/annonc/mdn544. [DOI] [PubMed] [Google Scholar]
- 15.de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14:6302–6309. doi: 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- 16.Moreno JG, Miller MC, Gross S, Allard WJ, Gomella LG, Terstappen LW. Circulating tumor cells predict survival in patients with metastatic prostate cancer. Urology. 2005;65:713–718. doi: 10.1016/j.urology.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Thalgott M, Rack B, Maurer T, et al. Detection of circulating tumor cells in different stages of prostate cancer. J Cancer Res Clin Oncol. 2013;139:755–763. doi: 10.1007/s00432-013-1377-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wicha MS, Hayes DF. Circulating tumor cells: not all detected cells are bad and not all bad cells are detected. J Clin Oncol. 2011;29:1508–1511. doi: 10.1200/JCO.2010.34.0026. [DOI] [PubMed] [Google Scholar]
- 19.Alix-Panabieres C, Pantel K. Challenges in circulating tumour cell research. Nat Rev Cancer. 2014;14:623–631. doi: 10.1038/nrc3820. [DOI] [PubMed] [Google Scholar]
- 20.Ligthart ST, Coumans FA, Bidard FC, et al. Circulating Tumor Cells Count and Morphological Features in Breast, Colorectal and Prostate Cancer. PLoS One. 2013;8:e67148. doi: 10.1371/journal.pone.0067148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marrinucci D, Bethel K, Bruce RH, et al. Case study of the morphologic variation of circulating tumor cells. Hum Pathol. 2007;38:514–519. doi: 10.1016/j.humpath.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 22.Bobek V, Hoffman RM, Kolostova K. Site-specific cytomorphology of disseminated PC-3 prostate cancer cells visualized in vivo with fluorescent proteins. Diagn Cytopathol. 2013;41:413–417. doi: 10.1002/dc.22843. [DOI] [PubMed] [Google Scholar]
- 23.Partin AW, Steinberg GD, Pitcock RV, et al. Use of nuclear morphometry, gleason histologic scoring, clinical stage, and age to predict disease-free survival among patients with prostate cancer. Cancer. 1992;70:161–168. doi: 10.1002/1097-0142(19920701)70:1<161::aid-cncr2820700126>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 24.Mohler JL, Figlesthaler WM, Zhang XZ, Partin AW, Maygarden SJ. Nuclear shape analysis for the assessment of local invasion and metastases in clinically localized prostate carcinoma. Cancer. 1994;74:2996–3001. doi: 10.1002/1097-0142(19941201)74:11<2996::aid-cncr2820741117>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 25.Khan MA, Walsh PC, Miller MC, et al. Quantitative alterations in nuclear structure predict prostate carcinoma distant metastasis and death in men with biochemical recurrence after radical prostatectomy. Cancer. 2003;98:2583–2591. doi: 10.1002/cncr.11852. [DOI] [PubMed] [Google Scholar]
- 26.Zink D, Fischer AH, Nickerson JA. Nuclear structure in cancer cells. Nat Rev Cancer. 2004;4:677–687. doi: 10.1038/nrc1430. [DOI] [PubMed] [Google Scholar]
- 27.Wang S, Liu K, Liu J, et al. Highly efficient capture of circulating tumor cells by using nanostructured silicon substrates with integrated chaotic micromixers. Angew Chem Int Ed Engl. 2011;50:3084–3088. doi: 10.1002/anie.201005853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu YT, Zhao L, Shen Q, et al. NanoVelcro Chip for CTC enumeration in prostate cancer patients. Methods. 2013;64:144–152. doi: 10.1016/j.ymeth.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vesalainen S, Lipponen P, Talja M, Kasurinen J, Syrjanen K. Nuclear morphometry is of independent prognostic value only in T1 prostatic adenocarcinomas. Prostate. 1995;27:110–117. doi: 10.1002/pros.2990270208. [DOI] [PubMed] [Google Scholar]
- 30.Klingauf M, Stanek D, Neugebauer KM. Enhancement of U4/U6 small nuclear ribonucleoprotein particle association in Cajal bodies predicted by mathematical modeling. Mol Biol Cell. 2006;17:4972–4981. doi: 10.1091/mbc.E06-06-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dempster AP, Laird NM, Rubin DB. Maximun Likelihood from Incomplete Data via the EM Algorithm. Journal of the Royal Statistical Society, Series B. 1977;39:1–38. [Google Scholar]
- 32.Akaike H. A new look at the statistical model identification. IEEE Transactions Automatic Control. 1974;19:716–723. [Google Scholar]
- 33.Schwarz GE. Estimating the dimension of a model. Annals of Statistics. 1978;6:461–464. [Google Scholar]
- 34.Broström G. Generalized linear models with clustering. 2009 Available from URL: http://cran.r-project.org/web/packages/glmmML/glmmML.pdf.
- 35.Lin M, Chen JF, Lu YT, et al. Nanostructure embedded microchips for detection, isolation, and characterization of circulating tumor cells. Acc Chem Res. 2014;47:2941–2950. doi: 10.1021/ar5001617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sweeney C, Chen Y-H, Carducci MA, et al. Impact on overall survival (OS) with chemohormonal therapy versus hormonal therapy for hormone-sensitive newly metastatic prostate cancer (mPrCa): An ECOG-led phase III randomized trial. J Clin Oncol. 2014;5s:2014. (suppl; abstr LBA2012) [Google Scholar]
- 37.Krebs MG, Metcalf RL, Carter L, Brady G, Blackhall FH, Dive C. Molecular analysis of circulating tumour cells-biology and biomarkers. Nat Rev Clin Oncol. 2014;11:129–144. doi: 10.1038/nrclinonc.2013.253. [DOI] [PubMed] [Google Scholar]
- 38.Attard G, Reid AH, A’Hern R, et al. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J Clin Oncol. 2009;27:3742–3748. doi: 10.1200/JCO.2008.20.0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reid AH, Attard G, Danila DC, et al. Significant and sustained antitumor activity in post-docetaxel, castration-resistant prostate cancer with the CYP17 inhibitor abiraterone acetate. J Clin Oncol. 2010;28:1489–1495. doi: 10.1200/JCO.2009.24.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Danila DC, Morris MJ, de Bono JS, et al. Phase II multicenter study of abiraterone acetate plus prednisone therapy in patients with docetaxel-treated castration-resistant prostate cancer. J Clin Oncol. 2010;28:1496–1501. doi: 10.1200/JCO.2009.25.9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Posadas EM, Tighiouart M, Chen J-F, et al. A translational phase 2 study of cabozantinib in men with metastatic castration resistant prostate cancer with visceral metastases with characterization of circulating tumor cells and large oncosomes. Annals of Oncology. 2014;25(suppl_4):iv546–iv563. [Google Scholar]
- 42.Akfirat C, Zhang X, Ventura A, et al. Tumour cell survival mechanisms in lethal metastatic prostate cancer differ between bone and soft tissue metastases. J Pathol. 2013;230:291–297. doi: 10.1002/path.4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao L, Lu YT, Li F, et al. High-Purity Prostate Circulating Tumor Cell Isolation by a Polymer Nanofiber-Embedded Microchip for Whole Exome Sequencing. Adv Mater. 2013 doi: 10.1002/adma.201205237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hou S, Zhao L, Shen Q, et al. Polymer nanofiber-embedded microchips for detection, isolation, and molecular analysis of single circulating melanoma cells. Angew Chem Int Ed Engl. 2013;52:3379–3383. doi: 10.1002/anie.201208452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lohr JG, Adalsteinsson VA, Cibulskis K, et al. Whole-exome sequencing of circulating tumor cells provides a window into metastatic prostate cancer. Nat Biotechnol. 2014;32:479–484. doi: 10.1038/nbt.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu M, Bardia A, Wittner BS, et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 2013;339:580–584. doi: 10.1126/science.1228522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chan CM, Au TC, Chan AT, et al. Advanced technologies for studying circulating tumor cells at the protein level. Expert Rev Proteomics. 2013;10:579–589. doi: 10.1586/14789450.2013.858021. [DOI] [PubMed] [Google Scholar]
- 48.Scatena R, Bottoni P, Giardina B. Circulating tumour cells and cancer stem cells: a role for proteomics in defining the interrelationships between function, phenotype and differentiation with potential clinical applications. Biochim Biophys Acta. 2013;1835:129–143. doi: 10.1016/j.bbcan.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 49.Yu M, Bardia A, Aceto N, et al. Cancer therapy. Ex vivo culture of circulating breast tumor cells for individualized testing of drug susceptibility. Science. 2014;345:216–220. doi: 10.1126/science.1253533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baccelli I, Schneeweiss A, Riethdorf S, et al. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat Biotechnol. 2013;31:539–544. doi: 10.1038/nbt.2576. [DOI] [PubMed] [Google Scholar]
- 51.Aceto N, Bardia A, Miyamoto DT, et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell. 2014;158:1110–1122. doi: 10.1016/j.cell.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hou JM, Krebs MG, Lancashire L, et al. Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. J Clin Oncol. 2012;30:525–532. doi: 10.1200/JCO.2010.33.3716. [DOI] [PubMed] [Google Scholar]
- 53.Liotta LA, Saidel MG, Kleinerman J. The significance of hematogenous tumor cell clumps in the metastatic process. Cancer Res. 1976;36:889–894. [PubMed] [Google Scholar]
- 54.Ritchie MD, Holzinger ER, Li R, Pendergrass SA, Kim D. Methods of integrating data to uncover genotype-phenotype interactions. Nat Rev Genet. 2015;16:85–97. doi: 10.1038/nrg3868. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




