Abstract
Current influenza vaccines are less efficacious against antigenically different influenza A viruses. This study presents an approach to overcome strain-specific protection, using a strategy of co-immunization with seasonal H3N2 split vaccine and yeast-expressed soluble proteins of a tandem repeat containing heterologous influenza M2 ectodomains (M2e5x). Co-immunization with both vaccines in mice was superior to either vaccine alone in inducing cross protection against heterologous H3N2 virus by raising M2e-specific humoral and cellular immune responses toward a T-helper type 1 profile inducing IgG2a isotype antibodies as well as interferon-γ-producing cells in systemic and mucosal sites. In addition, co-immunization sera were found to confer cross-protection against different subtypes of H1N1 and H5N1 influenza A viruses in naïve mice. A mechanistic study provides evidence that activation of dendritic cells by co-stimulation with M2e5x and split vaccine was associated with the proliferation of CD4+ T cells. Our results suggest that a strategy of co-immunization with seasonal split and M2e5x protein vaccines could be a promising approach for overcoming the limitation of strain-specific protection by current influenza vaccination.
Keywords: Influenza virus, split vaccine, M2e5x, protein, AS04
1. Introduction
Most currently available inactivated influenza vaccines are formulated as a trivalent or quadrivalent blend in a split form to include the two major viral surface proteins, hemagglutinin (HA) and neuraminidase (NA) although there is no regulation on the latter (Grohskopf et al., 2014). Neutralizing antibodies induced by inactivated split vaccines are known to protect against antigenically matched or closely related viruses but provide little protection against infection with other subtypes or with antigenically drifted viruses (Couch et al., 2013; Tumpey et al., 2001). Therefore, new vaccination strategies that will induce broadly cross-protective immunity to antigenically drifted strains within a subtype (heterologous) and different subtypes (heterosubtypic) need to be developed.
The extracellular domain of M2 (M2e) is considered to be a promising target for inducing cross protection against different subtypes of influenza A virus due to the fact that it is well conserved among human influenza subtypes (Ito et al., 1991). Because the immunogenicity of native M2e is very poor, various strategies have been used to overcome the low immunogenicity of M2e, such as fusing M2e to highly immunogenic carriers, using multimeric forms of M2e, formulating with experimental adjuvants, and supplementing inactivated whole viral vaccines with M2e (Ernst et al., 2006; Neirynck et al., 1999; Song et al., 2011a; Wang et al., 2012).
The main goal of this study was to test an effective influenza vaccination strategy by combining the H3 HA-based seasonal split vaccine and conserved M2e epitope-based proteins expressed in yeast cells to induce cross protection against heterologous and heterosubtypic influenza viruses. This study demonstrates that co-immunization with both seasonal H3 split vaccine and M2e tandem repeat (M2e5x) soluble proteins elicited robust humoral and cellular immune responses to M2e and significantly improved cross protection against lethal challenge with heterologous H3N2 influenza virus.
2. Materials and methods
2.1 Viruses, vaccine, and reagents
Influenza A viruses, A/Philippines/2/1982 (A/Phil, H3N2) and A/PR/8/1934 (A/PR8, H1N1) kindly provided by Dr. Huan Nguyen, A/California/04/2009 (A/CA04, H1N1) generously provided by Dr. Richard Webby and reassortant A/Vietnam/1203/2004 (A/VN1203, rgH5N1 containing HA with polybasic residues removed and NA from A/VN1203 and 6 internal genes from A/PR/8/1934) (Song et al., 2010), were propagated in 10-day-old embryonated eggs as previously described (Song et al., 2010). The influenza virus was inactivated by mixing the virus with formalin at a final concentration of 1:4,000 (v/v) as described previously (Lee et al., 2014b). Monovalent seasonal influenza split vaccine (Green Cross, South Korea) used in this study was derived from NYMC X-187 (X-187, HA and NA were derived from A/Victoria/210/2009 (H3N2) and the backbone genes from A/PR8 virus). P. pastoris strain GS115 and the pPIC9 vector were purchased from Life Technologies (Grand Island, NY). Adjuvant System 04 (AS04) is consisted of MPL (3-O-desacyl-4’-monophosphoryl lipid A, Sigma-Aldrich, St Louis, MO) and aluminum hydroxide (Alum, Sigma–Aldrich).
2.2 Preparation of M2e5x protein
The gene construct for encoding M2e5x was genetically designed to contain five copies of influenza virus M2e sequences (a2-20) from human influenza virus-SLLTEVETPIRNEWGSRSN (2x), swine influenza virus -SLLTEVETPTRSEWESRSS (1x), avian influenza virus type I-SLLTEVETPTRNEWESRSS (1x), and avian influenza virus type II -SLLTEVETLTRNGWGCRCS (1x), and a tetramerizing leucine zipper domain of GCN4 (De Filette et al., 2008; Kim et al., 2013b). The M2e5x gene was cloned into yeast-expression shuttle vector pPIC9 and expressed in a secreted form through the use of the α-factor mating secretion signal using the yeast P. pastoris system by following the manufacturer’s instructions. Using ion exchange chromatography on Q-Sepharose FF (GE Healthcare, Pittsburgh, PA) followed by hydrophobic interaction chromatography on phenyl-Sepharose 6FF column (GE Healthcare), M2e5x proteins secreted into culture supernatants were purified. The yield of soluble M2e5x proteins purified from 1 L of yeast culture supernatant was approximately 15 mg.
2.3 Immunization and challenge
Groups of eight female BALB/c mice (6- to 8-week-old, Harlan Laboratories) were intramuscularly immunized with 20 µg (total protein) of M2e5x protein or 0.6 µg of seasonal H3N2 split vaccine or M2e5x protein plus split vaccine at week 0, 4, and 8. All vaccines were formulated with AS04 adjuvant (5 µg MPL plus 50 µg Alum) approved for human use. Mock control mice were immunized with AS04 adjuvant only. Immunized mice were then challenged with a lethal dose (7xLD50) of A/Philippines/2/1982 (A/Phil, H3N2) influenza virus at 4 weeks after second boost and weight loss and survival rates were daily monitored for 14 days post-infection (p.i.). One LD50 of A/Phil virus for BALB/c mice is approximately 50 to 100 plaque forming units. Results were reproducible out of two independent experiments. All animal experiments were reviewed and approved by the Georgia State University IACUC review boards.
2.4 Preparation of BALF and lung extracts
Challenged mice were euthanized at 5 days p.i.. For bronchoalveolar lavage fluids (BALF), the diaphragms were dissected to allow free lung expansion and the lungs were lavaged three times by slowly instilling 1 ml of PBS then gently aspirating. The supernatants were inoculated into the allantoic cavity of 9–11-day-old embryonated eggs to determine lung viral titers as described in detail previously (Lee et al., 2011). The infectivity of the virus in the supernatant was determined from the 50% egg infectious dose (EID50). Cytokine levels in BALF were determined using enzyme-linked immunosorbent assay (ELISA) kits for IFN-γ and IL-6 according to the manufacturers’ instructions (eBioscience, SanDiego, CA).
2.5 Determination of antibody levels, M2e-specific antibody secreting cell and T cell responses
M2e or vaccine-specific antibody titers were determined by ELISA as previously described (Song et al., 2011b). To determine hemagglutination inhibition (HI) titers, serum samples were incubated with receptor destroying enzyme (RDE, Denka Seiken, Japan) and heated at 56 °C. In brief, HI titers were determined using inactivated X-187 virus and 1% chicken erythrocyte suspension with two-fold diluted serum samples after RDE treatment.
For antibody-secreting cell (ASC) assays, 96-well culture plates were coated with M2e5x protein, and bone marrow cells and splenocytes were added after blocking. IgG antibody levels specific to M2e peptide in the culture supernatants were determined by ELISA as described (Lee et al., 2014b; Song et al., 2010). Interferon (IFN)-γ-producing cell spots were determined on Multi-screen 96 well plates (Millipore, Billerica, MA) coated with IFN-γ cytokine specific capture antibodies (BD Biosciences, San Diego, CA) as previously described (Quan et al., 2009).
2.6 In vivo protection assay of immune sera
To determine protective efficacy of immune sera in naïve mice, immune or naïve sera after heat inactivation (56°C, 30 min) were mixed with a lethal dose (6 × LD50) of A/PR8, A/VN1203, and A/CA04 and incubated at room temperature for 30 min prior to administration as described (Kim et al., 2013a; Kim et al., 2014). Briefly, naive BALB/c mice were infected with a mixture of sera and virus, and were monitored for survival rates and weight loss for 14 days p.i..
2.7 Preparation and in vitro stimulation of bone marrow derived dendritic cells (BMDCs)
BMDCs were prepared from bone marrow cells of BALB/c mice as previously described (Lee et al., 2014a). BMDCs were stimulated with 10 µg/ml of M2e5x protein alone or seasonal influenza split vaccines or M2e5x protein plus split vaccine at 2 × 105 cells/ml in 96-well plates for 2 days. To balance the protein concentration in each well, bovine serum albumin (BSA) was included. IL-6 and TNF-α cytokines were determined in the BMDC culture supernatants using ELISA. For mixed lymphocyte reactions, BMDCs were first treated with M2e5x protein alone or seasonal influenza split vaccine or M2e5x protein plus split vaccine, and then cocultured with CFSE-labeled allogeneic C57BL/6 splenocytes. The protein concentrations were also balanced with BSA at the first phase. After 5 days, the cells were stained for anti-CD4-allophycocyanin (APC), anti-CD8α-r-phycoerythrin (PE), and anti-CD11c-PE-Cy7 antibodies. Dead cells were excluded according to low forward scatter and high side scatter characteristics. After gating out CD11cHi cells, T cell proliferation was evaluated by measuring the decrease in CFSE labeling in proliferating cells. Stained cells were acquired and analyzed using LSRFortessa (BD Biosciences) and FlowJo software (Tree Star Inc.) respectively.
2.8 Statistical analysis
Statistical analyses were done using GraphPad Prism software. Data are presented as means ± error of the mean (SEM). Differences between groups were analyzed by 1-way analysis of variance (ANOVA) or 2-way ANOVA where appropriate. P-values less than 0.05 were regarded as significant. Kinetics of mortality was analyzed by Kaplan–Meier curves and log-rank test with Bonferroni adjustment.
3. Results
3.1 Co-immunization with M2e5x protein and split vaccine induces M2e- and virus-specific antibody responses
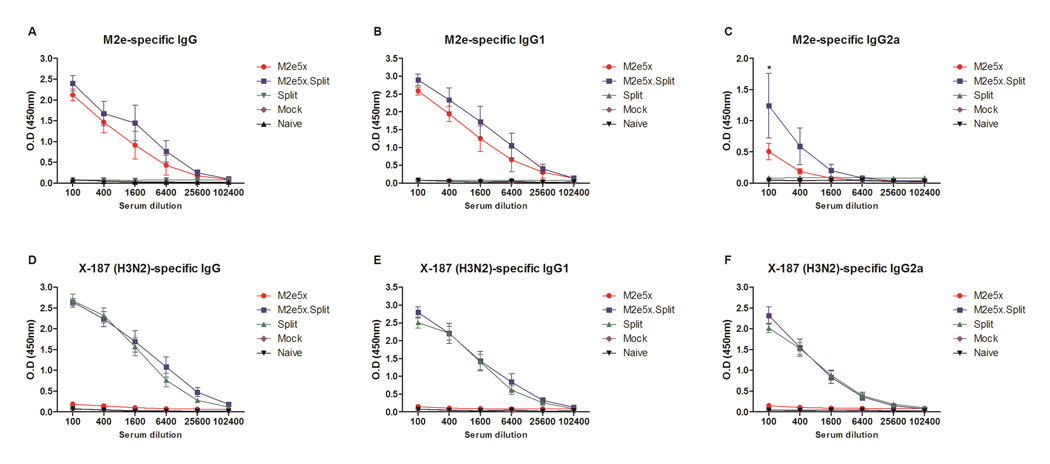
M2e5x soluble proteins were produced using the yeast expression system. M2e5x proteins expressed in yeast cells in a secreted form were purified to approximately 60% as determined by Coomassie Brilliant blue staining (Supplementary Fig. S1A) and confirmed by western blot probed using M2e monoclonal antibody 14C2 (Fig. S1B). We investigated whether coimmunization of mice with seasonal influenza split vaccine and M2e5x proteins would improve the efficacy of cross-protection against influenza A viruses compared to separate M2e5x protein and split standalone vaccination. To evaluate the immunogenicity of M2e5x protein vaccines (M2e5x) or seasonal influenza split vaccines (Split) or M2e5x protein plus split vaccines (M2e5x.Split) formulated with AS04 adjuvant, groups of mice were intramuscularly immunized and antibody responses in sera were determined by ELISA using a human type M2e peptide antigen or H3N2 split vaccine. At 21 days after first and second boost, M2e-specific antibodies were observed in the M2e5x.Split and the M2e5x group at substantially high levels but not in the Split group (Fig. S2 and 1A–C). Interestingly, IgG2a isotype antibody responses specific for M2e were significantly higher in the M2e5x.Split group than those in the M2e5x group after second boost (p < 0.05). High levels of antibody responses specific for X-187 H3N2 vaccine were induced by either split or combined vaccination (Fig. 1D–F). Immune sera from the M2e5x.Split showed high titers of HI activity up to 10.5 ± 0.2 log2 (Fig. S3A). Moreover, sera from split only-immunized mice also had a high level of HI titers (Fig S3A), yet not cross reactive HI activities were detected against A/PR8, A/VN1203 and A/CA04 (data not shown). Substantial reactivity to A/PR8 (H1N1, Fig S3b), A/VN1203 (rgH5N1, Fig S3C), and A/CA04 (H1N1, Fig S3D) were detected by ELISA in mice immunized with Split or M2e5x.Split vaccines. Importantly, antibody levels to A/VN1203 in M2e5x.Split immune sera showed significantly a higher titer than that in sera from split alone immune mice by ELISA (p < 0.05, Fig. S3C).
Fig. 1. Co-immunization with M2e5x protein and split vaccines induces M2e- and virus-specific IgG and IgG isotype antibody responses.
BALB/c mice (n = 8) were immunized with M2e5x protein (M2e5x) or H3N2 influenza split vaccine (Split) alone or M2e5x protein plus split vaccine (M2e5x.Split). All groups of vaccines were immunized with AS04 adjuvant. Blood samples were collected at 3 weeks after second boost. (A–C) M2e-specific total IgG (A), IgG1 (B), and IgG2a (C) antibody. (D–F) Vaccine-specific total IgG (D), IgG1 (E), and IgG2a (F) antibody. ELISA was performed with serially diluted serum samples for antibodies specific for M2e peptide (A–C) or H3N2 influenza split vaccine (D-F) (NYMC X-187). Error bars indicates mean ± SEM. Statistical significance was determined by 2-way ANOVA. Asterisks indicate significant differences (*p < 0.05) compared with the results in the M2e5x group.
3.2 Immunization with mixed M2e5x protein and split vaccines provides enhanced protection against heterologous influenza virus
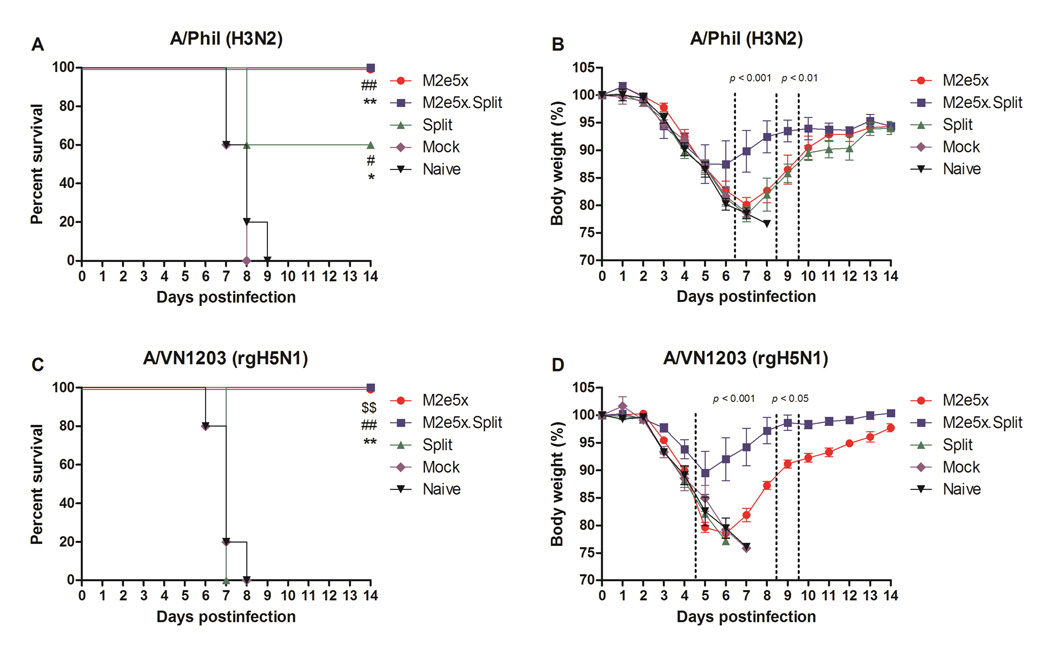
To compare the efficacy of protection, groups of mice were challenged with a lethal dose of A/Phil H3N2 virus (7xLD50) or A/VN1203 rgH5N1 virus (4xLD50) at 4 weeks after second boost (Fig. 2). A/Phil virus is antigenically distinct from NYMC X-187 virus. Identity of nucleotide and amino acid sequences of HA between A/Phil and A/Victoria/210/2009 are 90.8% and 84.6%. All naive and mock control mice lost over 25% in body weight and died or had to be euthanized. In experiment challenge with A/Phil virus, the survival rates of the mice from the split vaccine group were 60% (Fig. 2A). In contrast, the M2e5x.Split and the M2e5x group showed 100% survival protection against lethal challenge with heterologous A/Phil virus. Mice in the Split or M2e5x group showed a substantial weight loss of approximately 22% and 20%, respectively (Fig. 2B). However, mice in the M2e5x.Split group showed a moderate weight loss (~14%). In challenge experiment with A/VN1203, split alone immune mice did not show any protection (Fig. 2C). The survival rates of the mice vaccinated with M2e5x.Split and M2e5x alone were 100%. Mice in the M2e5x group also showed a substantial weight loss of 22% (Fig. 2D). By contrast, the M2e5x.Split group showed a moderate loss of 11%.
Fig. 2. M2e5x.Split co-immunization improves cross protection.
Groups of BALB/c mice (n = 5) were challenged with a lethal dose of A/Philippines/2/82 (H3N2) virus (A, B) or reassortants A/Vietnam/1203/2004 (A/VN1203, rgH5N1) virus (C, D) 4 weeks after second boost. Survival rates (A, C) and body weight (B, D) were monitored for 14 days. Error bars indicates mean ± SEM. Error bars indicates mean ± SEM. Statistical significance was determined using Kaplan–Meier survival curve and log-rank test with Bonferroni adjustment or by 2-way ANOVA. Dollar signs indicate significant differences ($$p < 0.01) compared with the results in the Split group. Pounds indicate significant differences (#p < 0.05, ##p < 0.01) compared with the results in the Mock (AS04 adjuvant only) group. Asterisks indicate significant differences (*p < 0.05, **p < 0.01) compared with the results in the Naive (unimmunized) group.
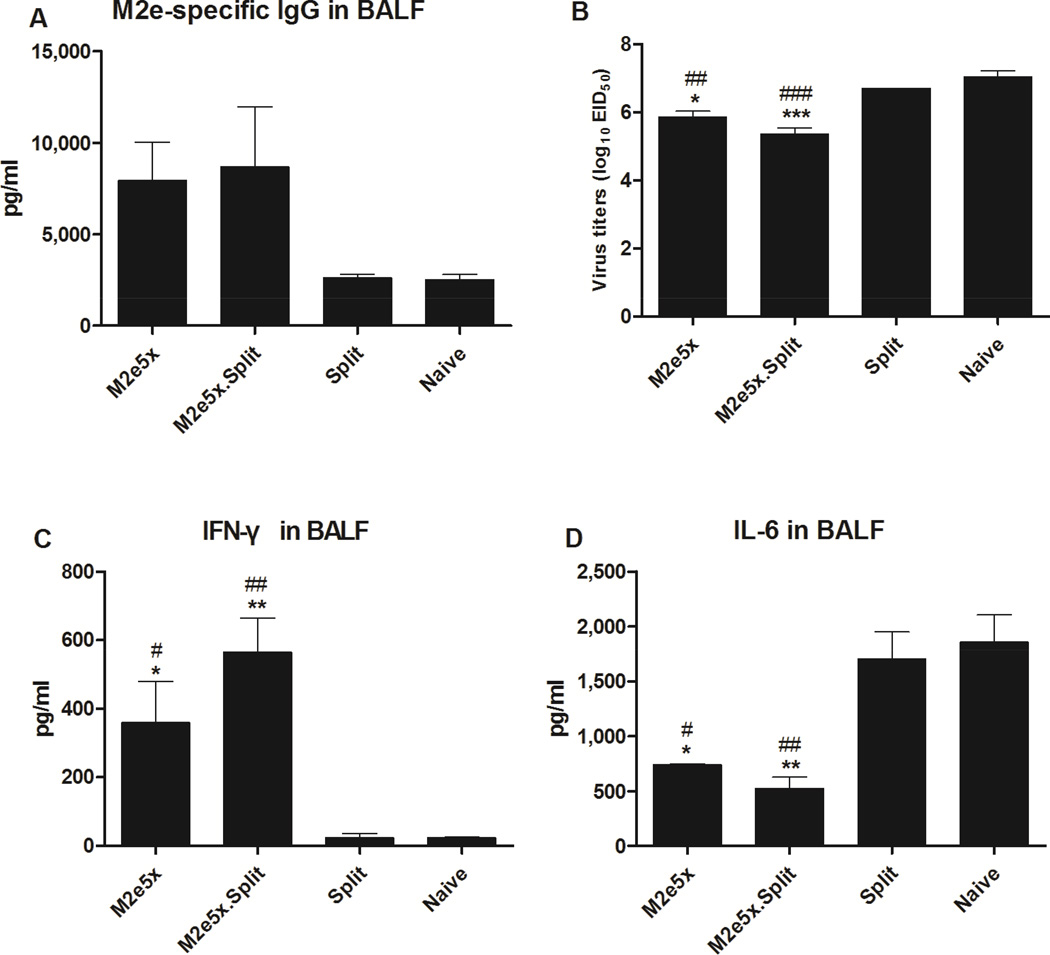
M2e-specific IgG or IgA antibody responses in BALF were determined at day 5 after challenge with A/Phil virus. M2e-specific IgG concentrations in BALF from the M2e5x.Split and M2e5x group were approximately 3.3- and 3.0-fold higher than those from the Split group, respectively, but there was no significant difference (Fig. 3A). M2e-specific IgA antibody concentrations in BALF from the M2e5x and the M2e5x.Split group were approximately 1.5-fold higher than those from other groups (Fig. S4A). The M2e5x.Split and the M2e5x group showed significantly lower lung viral titers compared with those in the Split and Naive group (Fig. 3B). Moreover, the lung viral titers of the M2e5x.Split group (5.9 ± 0.1 Log10EID50/ml) were 12.6-fold lower than those in the Naïve group (7.2 ± 0.3 Log10EID50/ml) even at an earlier time point day 3 p.i., which is statistically significant (p < 0.05, Fig. S4B). The difference between the M2e5x.Split group and other groups was found to be bigger at the later time point day 5 p.i..
Fig. 3. Combined M2e5x.Split vaccine is effective in lowering lung viral titers and inflammatory cytokine levels after heterologous challenge.
Levels of IgG antibodies were determined in bronchoalveolar lavage fluids (BALF) samples collected from mice at day 5 p.i. with A/Philippines/2/82 (H3N2) virus (n = 3). (A) M2e-specific IgG antibody levels in BALF. Lung viral titers (B) were determined by an egg infection assay. IFN-γ (C), and IL-6 (D) cytokine in BALF were determined by a cytokine ELISA at day 5 p.i. Data represent mean ± SEM. Statistical significance was determined by 1-way ANOVA. Pounds indicate significant differences (#p < 0.05, ##p < 0.01, and ###p < 0.001) compared with the results in the Split group. Asterisks indicate significant differences (*p < 0.05, **p < 0.01, and ***p < 0.001) compared with the results in the Naive group.
The production of IFN-γ is known to be important in decreasing viral load of influenza virus (Baumgarth and Kelso, 1996). The levels of IFN-γ in BALF from the M2e5x and M2e5x.Split group were significantly higher than those in BALF from the Split and Naive group on day 5 after infection (Fig. 3C). During highly pathogenic H5N1 infection, cytokine storm was hypothesized to be a cause of tissue damage, ultimately contributing to death. Especially, increased levels of proinflammatory cytokines including TNF-γ and IL-6, have been observed in human and mice infected with highly pathogenic H5N1 influenza virus (Chan et al., 2005; Cheung et al., 2002; de Jong et al., 2005; Lee et al., 2005; Szretter et al., 2007; Xu et al., 2006). The levels of IL-6 inflammatory cytokine were observed at significantly lower in BALF from the M2e5x.Split and M2e5x groups than those of other groups, respectively (Fig. 3D). Thus, these results indicate that co-immunization of split and M2e5x protein vaccines can improve cross protection against heterologous H3N2 viruses by effectively controlling viral replication, increasing antiviral IFN-γ cytokine, and modulating IL-6 inflammatory cytokine after challenge.
3.3 Co-immunization with M2e5x protein and seasonal split vaccines enhances IFN-γ-secreting T cell and antibody-secreting cell responses
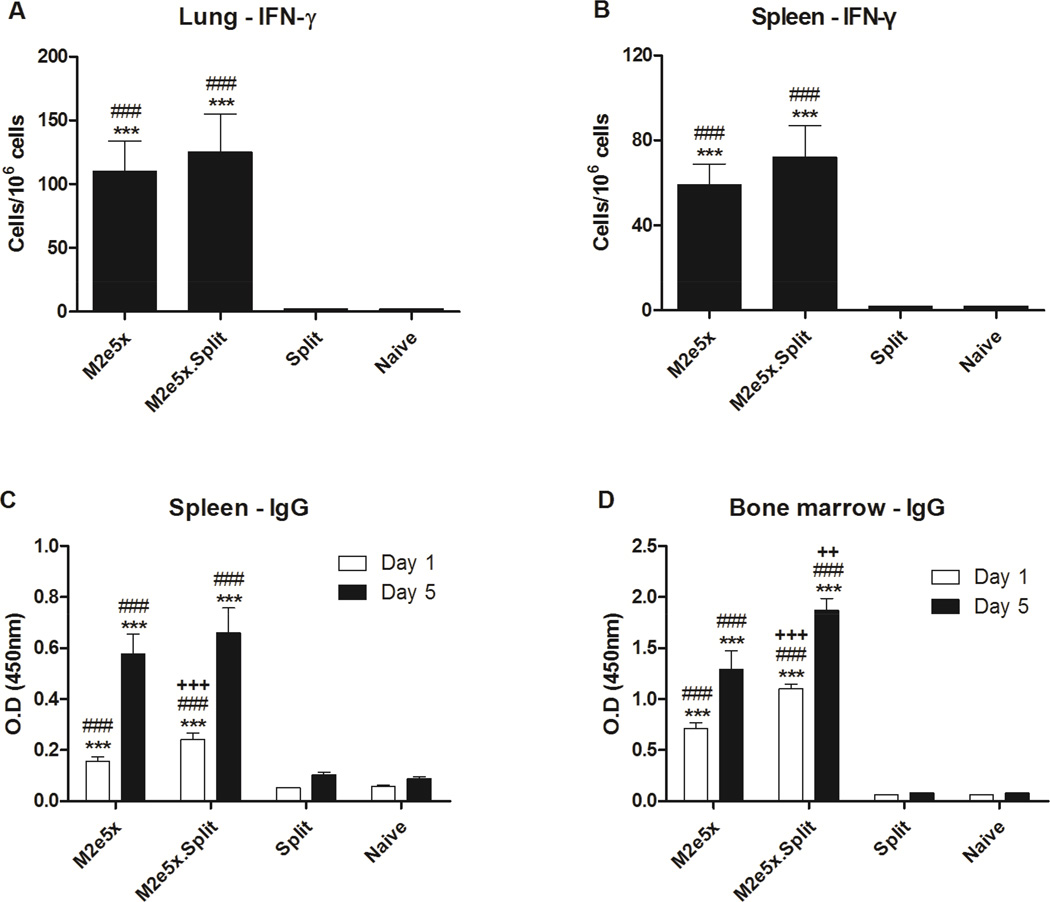
To better understand improved cross protection by dual vaccine components, we determined the induction of IFN-γ-producing M2e-specific T cells in lungs and spleens. After in vitro stimulation of whole lung and spleen cells with M2e peptide, we measured IFN-γ-producing cell spots (Fig. 4A and 4B). The spot numbers of IFN-γ–secreting cells were detected at a significantly higher level in the lungs from mice in the M2e5x.Split and the M2e5x group than those from other groups, respectively (Fig. 4A). Moreover, significantly higher levels of IFN-γ–secreting cells were observed in the spleens from the M2e5x.Split and the M2e5x groups compared with those from other groups, respectively (Fig. 4B).
Fig. 4. M2e5x.Split vaccine enhances M2e-specific cytokine and antibody secreting cellular immune responses.
(A) IFN-γ-secreting cells in lungs (n = 3). (B) IFN-γ-secreting cells in spleens (n = 3). Lung cells and splenocytes were isolated from mice at day 5 p.i. Cytokine-producing cell spots were counted by ELISPOT reader. Spleen (C) or bone marrow (D) cells were isolated from mice at day 5 p.i. and were incubated with M2e5x protein for in vitro stimulation. Culture supernatants were harvested after 1 or 5 days of culture. M2e-specific IgG levels were determined by ELISA. Data represent mean ± SEM. Statistical significance was determined by 1-way ANOVA. Plus sings indicate significant difference (++p < 0.01, and +++p < 0.001) compared with the results in the M2e5x group. Pounds indicate significant differences (#p < 0.05, ##p < 0.01, and ###p < 0.001) compared with the results in the Split group. Asterisks indicate significant differences (*p < 0.05, **p < 0.01, and ***p < 0.001) compared with the results in the Naive group.
There was a correlation between in vitro antibody production and antibody-secreting cell (ASC) responses (Kang et al., 2011; Song et al., 2010). To investigate M2e-specific ASC responses, spleen and bone marrow cells were collected at day 5 p.i. and cultured in vitro for 1 and 5 days. The levels of anti-M2e IgG antibodies were significantly higher in the culture supernatants of splenocytes (Fig. 4C) and bone marrow cells (Fig. 4D) from the M2e5x.Split and M2e5x groups than those from other groups. Furthermore, higher levels of anti-M2e IgG antibodies secreted into the culture supernatants were detected in splenocytes from the M2e5x.Split group compared with those from the M2e5x group after 1 day in vitro culture (p < 0.01). Interestingly, the levels of anti-M2e IgG were detected at a significantly higher level in the supernatants of bone marrow cells from the M2e5x.Split group than those from the M2e5x group after 1 day (p < 0.001) and 5 day (p < 0.01), respectively. Importantly, significant increases in M2e-specific IgG antibody levels at day 5 cultures compared to those at day 1 cultures suggest the effective induction of memory B cells that are differentiating to antibody-secreting plasma cells upon antigenic stimulation. These results indicate that the generation of plasma cells in bone marrow and memory B cells in spleens can be induced more effectively by co-immunization with M2e5x proteins and seasonal split vaccine than vaccination with M2e5x protein alone.
3.4 Immune sera of M2e5x protein with split vaccination confer improved cross-protection
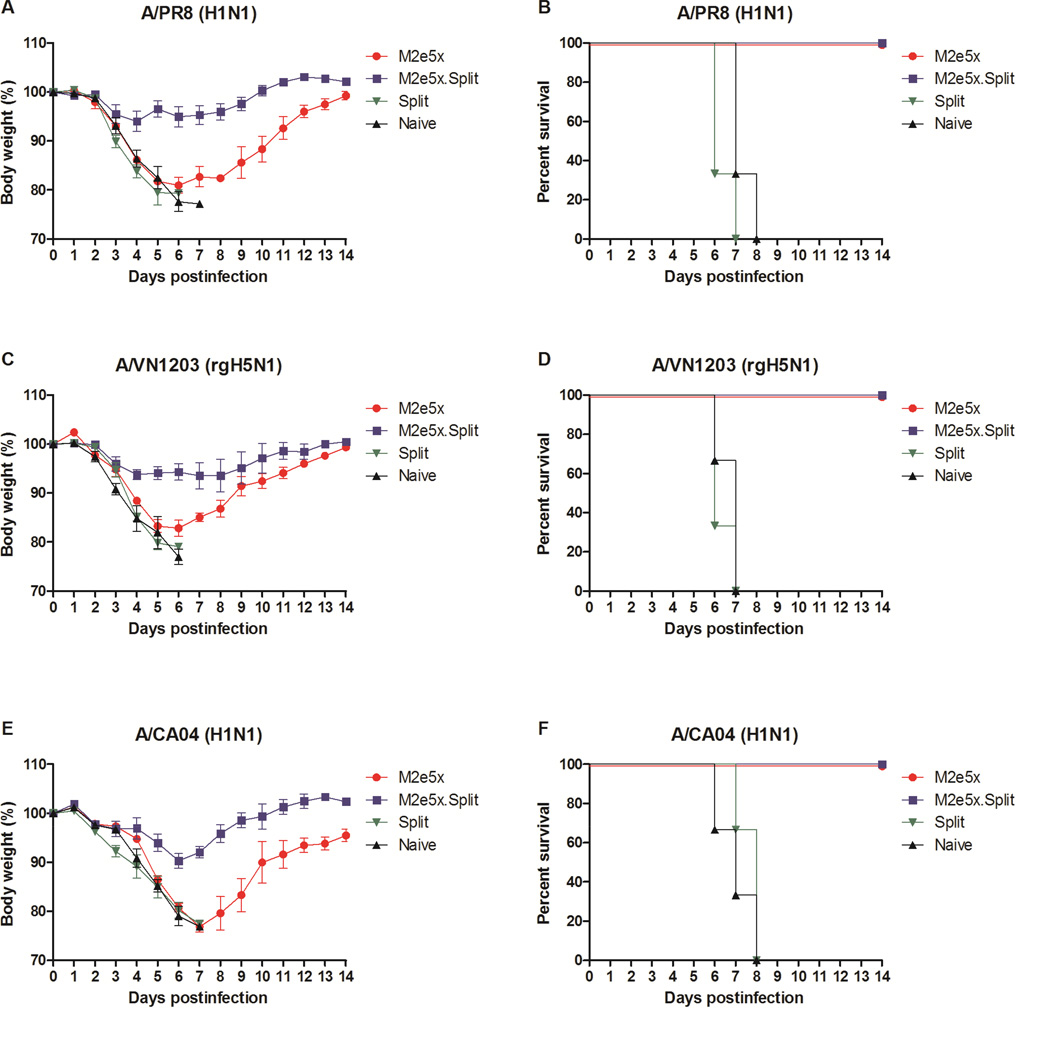
We further evaluated whether immune sera would confer broad cross-protection against heterosubtypic influenza viruses. Naive mice were infected with a mixture of immune sera and different strains of influenza A virus. Sera from naïve or split alone immune mice did not provide any protection to naïve mice (Fig. 5). In contrast, M2e5x.Split and M2e5x only immune sera showed 100% protection to naïve mice that were infected with A/PR/8/1934 (A/PR8, H1N1) (Fig. 5A–B) or A/Vietnam/1203/2004 (A/VN1203, H5N1) (Fig. 5C–D) or A/California/04/2009 with swine M2e (A/CA04, H1N1), respectively (Fig. 5E–F). In addition, only a slight loss (6–10%) in body weight depending on the virus strain used for infection was observed in protected mice from the M2e5x.Split group. Substantial levels of morbidity (18–22% weight loss) depending on the virus strain used for infection were observed in mice from the M2e5x group. Therefore, these results support evidence that combined M2e5x.Split vaccination can induce antibody responses which are superior to seasonal split vaccination in conferring protective immunity against heterosubtypic influenza viruses.
Fig. 5. Immune sera of M2e5x.Split co-immunization are superior to split vaccine immune sera in conferring cross-protection.
Mice (n = 3) were intranasally infected with a lethal dose of influenza virus mixed with 2-fold diluted immune sera or naive sera. Immune sera collected from vaccinated mice after 3rd immunization were incubated with influenza viruses, A/PR/8/34 (A/PR8, H1N1) (A,B), reassortants A/Vietnam/1203/2004 (A/VN1203, rgH5N1) (C, D), and A/California/04/2009 (A/CA04, H1N1) (E, F). Body weight (A, C, E) and survival rates (B, D, F) were monitored for 14 days. The results were reproducible from two independent experiments.
3.5 M2e5x proteins and influenza split vaccines stimulate BMDCs activating CD4+ T cells
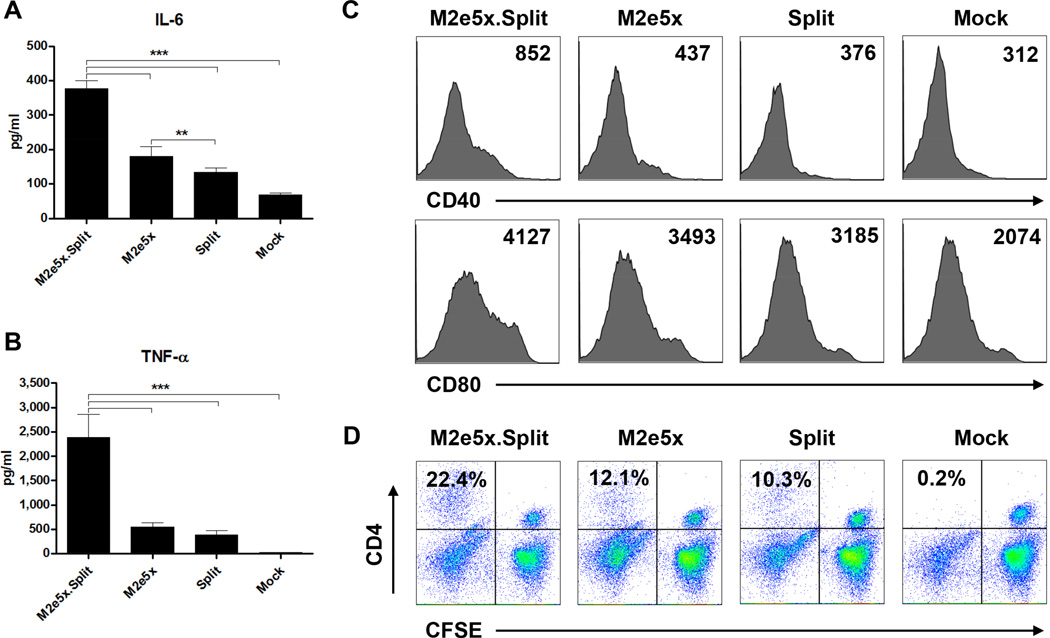
To understand the possible underlying mechanisms by which dual vaccine components confer better cross protection, we investigated the effects of M2e5x proteins and/or influenza split vaccines on stimulating dendritic cells (DCs) in vitro. The levels of IL-6 (Fig. 6A) and TNF-γ (Fig. 6B) in the supernatants from BMDCs treated with both M2e5x proteins and split vaccines for 2 days were significantly higher than those from BMDCs treated with M2e5x protein, split vaccine alone or mock control (p < 0.001). Induction of co-stimulatory molecules CD40 and CD80 is an essential control point for T cell activation. BMDCs treated with both M2e5x protein and split vaccines induced higher levels of CD40 and CD80 activation markers as compared with BMDCs treated with M2e5x proteins, split vaccines alone, or mock control (Fig. 6C).
Fig. 6. M2e5x.Split mixed vaccine activates dendritic cells subsequently promoting CD4+ T cell proliferation.
(A–C) Expression of cytokines and markers by activated dendritic cells. BALB/c BMDCs were stimulated with M2e5x protein alone (M2e5x) or seasonal influenza split vaccine alone (Split) or M2e5x protein plus split vaccine (M2e5x.Spit). TNF-γ (A) and IL-6 (B) cytokines were determined by a cytokine ELISA. Data represent mean ± SEM. (C) Representative histograms of CD40 or CD80 expression of BMDCs. Numbers in the histograms indicate mean fluorescence intensity (MFI) of each marker. (D) BALB/c BMDCs were stimulated with M2e5x protein and/or seasonal influenza split vaccine. After wash, BMDCs were cocultured for 5 days with allogeneic C57BL/6 splenocytes with the ratio of 1:10 for BMDC to splenocytes. CFSE profiles of CD4+ T cells are representative of two independent experiments. The numbers indicate percentages of CFSE− CD4+ T cell populations.
To investigate whether activated BMDCs expressing co-stimulatory molecules by a mixture of M2e5x proteins and split vaccines would translate into activating T cells, CFSE-labeled-allogeneic splenocytes were incubated for 5 days with BMDCs that had been pretreated with M2e5x proteins and/or influenza split vaccines. T cell proliferation was evaluated by measuring the decrease in the CFSE fluorescent intensity of replicating cells. BMDCs that were stimulated with mixed M2e5x protein and split vaccines induced higher levels of proliferated CD4+ T cells compared to BMDCs that were stimulated with M2e5x proteins or split vaccines alone (Fig. 6D). Therefore, these results suggest that M2e5x proteins and split vaccines stimulate DCs to secrete more cytokines and to induce increased levels of CD4+ T cell proliferation.
4. Discussion
Current inactivated influenza vaccines are less efficacious when vaccines are mismatched with a circulating strain or during a new pandemic outbreak. M2e, being highly conserved among different influenza virus subtypes, has been considered a promising universal influenza vaccine antigen. However, M2e immunity alone provides relatively weak protection (Jegerlehner et al., 2004). Since M2e antibodies do not neutralize viruses, the protective efficacy by M2e antibodies is lower than that of HA-based inactivated vaccines that induce neutralizing antibodies against homologous strains. Therefore, it is unlikely that M2e-based vaccines would be developed as a standalone universal vaccine which would completely replace current HA based influenza vaccines.
It has been reported that whole inactivated influenza vaccines supplemented with M2e-based vaccines could improve their cross protective efficacies compared to inactivated vaccine alone (Park et al., 2014; Song et al., 2011a). Also, H1N1 split vaccines supplemented with M2e5x expressed on virus-like particles (VLPs) were shown to partially overcome strain-specificity of protection by split vaccines (Kim et al., 2014). In this study, we demonstrated that co-immunization of H3N2 seasonal split vaccine and M2e5x protein effectively induced M2e-specific humoral and cellular immune responses. To the best of our knowledge, this is the first study to provide evidence that immunization with a combined vaccine of inactivated split H3 vaccine and yeast cell-expressed M2e5x proteins could be significantly effective in conferring cross-protection against heterologous influenza viruses compared to M2e5x proteins and split vaccines alone. In particular, yeast-based expression of recombinant vaccines against human hepatitis B virus and human papilloma virus was licensed for human use (Hilleman, 2000; Shi et al., 2007). Thus, production of M2e5x proteins in yeast cells could present a viable option for human application. In this study to assess immune effects by immunization with yeast-expressed M2e5x proteins, all vaccines were formulated with AS04 adjuvant that was approved in conjunction with human vaccines (Giannini et al., 2006). Previously it was reported that clinical studies of M2e vaccines were performed with bacterial flagellin adjuvant-conjugated forms, resulting in some side effects at high doses (Turley et al., 2011). It is highly significant to find that M2e-based immunity could be better than H3 HA-based split vaccine immunity in inducing heterologous protection against H3N2 virus. Moreover, we found that M2e5x.Split immune sera can confer broader and improved cross protection against diverse subtypes (H1N1, H5N1) of influenza A viruses, which was superior to split vaccine immune sera (Fig. 5). Recently, it has been reported that HA stalk neutralization was enhanced by IgA antibodies compared to IgG antibodies (He et al., 2015). Therefore, M2e-specific antibodies as a polyclonal context might be more effective than those in sera. Thus, results in this study provide a proof-of-concept that the presence of M2e immunity in addition to HA strain specific immunity could help in broadening cross protection against emergence of antigenically different viruses.
It is important to note that combined immunization was significantly more effective than M2e5x vaccination in inducing anti-M2e IgG2a antibodies and heterologous cross protection. The IgG2a isotype antibody is known to be involved in binding to Fc receptors more effectively than IgG1 isotype (Gerhard et al., 1997; Huber et al., 2001). It was reported that Fc receptors play a role in conferring M2e-specific antibody-mediated protection (El Bakkouri et al., 2011; Lee et al., 2014b). Thus, induction of M2e-specific IgG2a isotype antibodies by co-immunization of M2e5x and split vaccines might contribute to viral clearance, possibly via Fc receptor-mediated mechanisms involving antibody-dependent cell-mediated cytotoxicity or antibody-mediated phagocytosis.
A major purpose of vaccination is to induce memory immune responses that can rapidly respond upon subsequent antigen exposure or infection. In the present study, compared with M2e5x proteins or split vaccine alone, immunization with combined M2e5x and split vaccines induced significant levels of antibody-secreting plasma cell responses in bone marrow, which can produce M2e-specific antibodies. Also, functional memory B cells in spleens that can differentiate into ASCs upon antigen stimulation were observed in co-immunized mice. In addition, IFN-γ-secreting cell spots were also highly induced by co-immunization. It has been reported that HA protein has adjuvant-like effects (Cox et al., 2004; Kang et al., 2004). Interestingly, we found that BMDCs treated with both M2e5x protein and HA split vaccines produced higher levels of cytokine and costimulatory molecules as compared with BMDCs treated with M2e5x protein or split vaccine alone. Furthermore, BMDCs stimulated with both M2e5x protein and split vaccine induced the proliferation of CD4+ T cells at higher levels compared to either vaccine alone. It is likely that the induction of IgG2a antibody- and IFN-γ-secreting cells by co-immunization with both M2e5x and split vaccines might have largely resulted from antigen presenting DC-mediated activation of CD4+ T cells. Therefore, co-immunization of M2e5x protein and split vaccines appears to be a promising strategy for developing a vaccine that effectively generates IFN-γ-secreting T cells as well as memory B cells that rapidly differentiate into antibody secreting plasma cells upon antigenic stimulation.
In conclusion, co-immunization with M2e5x protein and seasonal split vaccine was found to induce significantly improved cross-protection against heterologous influenza A virus compared to either vaccine alone. In addition, co-immunization with seasonal split and M2e5x protein vaccines was able to efficiently generate immunologic memory for rapid recall responses of humoral and cellular immune components upon lethal influenza virus infection. Antigen presenting DC-mediated activation of CD4+ T cells by co-stimulation with mixed M2e5x and split vaccines might be involved in a mechanism by which combined vaccination of split and M2e5x proteins works for improved cross protection. Therefore, results in the present study provide evidence that a strategy of co-immunization with seasonal split and M2e5x protein vaccines could be a promising and translatable approach for overcoming the limitation of strain-specific protection by current influenza vaccination as well as strengthening the M2e-mediated cross protection.
Supplementary Material
Research highlights.
Co-immunization with split and M2e5x vaccine improves cross protection.
Co-immunization with both vaccines was superior to either vaccine alone.
Co-immunization sera confer broad-heterosubtypic protection.
DC-mediated activation of CD4 T cells could be involved in a protective mechanism.
Acknowledgments
This work was supported by NIH/NIAID grants AI105170 (S.M.K.), AI093772 (S.M.K.), AI119366 (S.M.K), and Animal and Plant Quarantine Agency grant (Republic of Korea).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baumgarth N, Kelso A. In vivo blockade of gamma interferon affects the influenza virus-induced humoral and the local cellular immune response in lung tissue. J Virol. 1996;70:4411–4418. doi: 10.1128/jvi.70.7.4411-4418.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan MC, Cheung CY, Chui WH, Tsao SW, Nicholls JM, Chan YO, Chan RW, Long HT, Poon LL, Guan Y, Peiris JS. Proinflammatory cytokine responses induced by influenza A (H5N1) viruses in primary human alveolar and bronchial epithelial cells. Respir Res. 2005;6:135. doi: 10.1186/1465-9921-6-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung CY, Poon LL, Lau AS, Luk W, Lau YL, Shortridge KF, Gordon S, Guan Y, Peiris JS. Induction of proinflammatory cytokines in human macrophages by influenza A (H5N1) viruses: a mechanism for the unusual severity of human disease? Lancet. 2002;360:1831–1837. doi: 10.1016/s0140-6736(02)11772-7. [DOI] [PubMed] [Google Scholar]
- Couch RB, Atmar RL, Franco LM, Quarles JM, Wells J, Arden N, Nino D, Belmont JW. Antibody correlates and predictors of immunity to naturally occurring influenza in humans and the importance of antibody to the neuraminidase. J Infect Dis. 2013;207:974–981. doi: 10.1093/infdis/jis935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RJ, Brokstad KA, Ogra P. Influenza virus: immunity and vaccination strategies. Comparison of the immune response to inactivated and live, attenuated influenza vaccines. Scand J Immunol. 2004;59:1–15. doi: 10.1111/j.0300-9475.2004.01382.x. [DOI] [PubMed] [Google Scholar]
- De Filette M, Martens W, Roose K, Deroo T, Vervalle F, Bentahir M, Vandekerckhove J, Fiers W, Saelens X. An influenza A vaccine based on tetrameric ectodomain of matrix protein 2. J Biol Chem. 2008;283:11382–11387. doi: 10.1074/jbc.M800650200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong MD, Bach VC, Phan TQ, Vo MH, Tran TT, Nguyen BH, Beld M, Le TP, Truong HK, Nguyen VV, Tran TH, Do QH, Farrar J. Fatal avian influenza A (H5N1) in a child presenting with diarrhea followed by coma. N Engl J Med. 2005;352:686–691. doi: 10.1056/NEJMoa044307. [DOI] [PubMed] [Google Scholar]
- El Bakkouri K, Descamps F, De Filette M, Smet A, Festjens E, Birkett A, Van Rooijen N, Verbeek S, Fiers W, Saelens X. Universal vaccine based on ectodomain of matrix protein 2 of influenza A: Fc receptors and alveolar macrophages mediate protection. J Immunol. 2011;186:1022–1031. doi: 10.4049/jimmunol.0902147. [DOI] [PubMed] [Google Scholar]
- Ernst WA, Kim HJ, Tumpey TM, Jansen AD, Tai W, Cramer DV, Adler-Moore JP, Fujii G. Protection against H1, H5, H6 and H9 influenza A infection with liposomal matrix 2 epitope vaccines. Vaccine. 2006;24:5158–5168. doi: 10.1016/j.vaccine.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Gerhard W, Mozdzanowska K, Furchner M, Washko G, Maiese K. Role of the B-cell response in recovery of mice from primary influenza virus infection. Immunol Rev. 1997;159:95–103. doi: 10.1111/j.1600-065x.1997.tb01009.x. [DOI] [PubMed] [Google Scholar]
- Giannini SL, Hanon E, Moris P, Van Mechelen M, Morel S, Dessy F, Fourneau MA, Colau B, Suzich J, Losonksy G, Martin MT, Dubin G, Wettendorff MA. Enhanced humoral and memory B cellular immunity using HPV16/18 L1 VLP vaccine formulated with the MPL/aluminium salt combination (AS04) compared to aluminium salt only. Vaccine. 2006;24:5937–5949. doi: 10.1016/j.vaccine.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Grohskopf LA, Olsen SJ, Sokolow LZ, Bresee JS, Cox NJ, Broder KR, Karron RA, Walter EB. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP) -- United States, 2014–15 influenza season. MMWR Morb Mortal Wkly Rep. 2014;63:691–697. [PMC free article] [PubMed] [Google Scholar]
- He W, Mullarkey CE, Duty JA, Moran TM, Palese P, Miller MS. Broadly neutralizing anti-influenza virus antibodies: enhancement of neutralizing potency in polyclonal mixtures and IgA backbones. J Virol. 2015;89:3610–3618. doi: 10.1128/JVI.03099-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilleman MR. Vaccines in historic evolution and perspective: a narrative of vaccine discoveries. Vaccine. 2000;18:1436–1447. doi: 10.1016/s0264-410x(99)00434-x. [DOI] [PubMed] [Google Scholar]
- Huber VC, Lynch JM, Bucher DJ, Le J, Metzger DW. Fc receptor-mediated phagocytosis makes a significant contribution to clearance of influenza virus infections. J Immunol. 2001;166:7381–7388. doi: 10.4049/jimmunol.166.12.7381. [DOI] [PubMed] [Google Scholar]
- Ito T, Gorman OT, Kawaoka Y, Bean WJ, Webster RG. Evolutionary analysis of the influenza A virus M gene with comparison of the M1 and M2 proteins. J Virol. 1991;65:5491–5498. doi: 10.1128/jvi.65.10.5491-5498.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jegerlehner A, Schmitz N, Storni T, Bachmann MF. Influenza A vaccine based on the extracellular domain of M2: weak protection mediated via antibody-dependent NK cell activity. J Immunol. 2004;172:5598–5605. doi: 10.4049/jimmunol.172.9.5598. [DOI] [PubMed] [Google Scholar]
- Kang SM, Guo L, Yao Q, Skountzou I, Compans RW. Intranasal immunization with inactivated influenza virus enhances immune responses to coadministered simian-human immunodeficiency virus-like particle antigens. J Virol. 2004;78:9624–9632. doi: 10.1128/JVI.78.18.9624-9632.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SM, Yoo DG, Kim MC, Song JM, Park MK, O E, Quan FS, Akira S, Compans RW. MyD88 plays an essential role in inducing B cells capable of differentiating into antibody-secreting cells after vaccination. J Virol. 2011;85:11391–11400. doi: 10.1128/JVI.00080-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MC, Lee JS, Kwon YM, O E, Lee YJ, Choi JG, Wang BZ, Compans RW, Kang SM. Multiple heterologous M2 extracellular domains presented on virus-like particles confer broader and stronger M2 immunity than live influenza A virus infection. Antiviral Res. 2013a;99:328–335. doi: 10.1016/j.antiviral.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MC, Lee YN, Ko EJ, Lee JS, Kwon YM, Hwang HS, Song JM, Song BM, Lee YJ, Choi JG, Kang HM, Quan FS, Compans RW, Kang SM. Supplementation of influenza split vaccines with conserved M2 ectodomains overcomes strain specificity and provides long-term cross protection. Mol Ther. 2014;22:1364–1374. doi: 10.1038/mt.2014.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MC, Song JM, O E, Kwon YM, Lee YJ, Compans RW, Kang SM. Virus-like particles containing multiple M2 extracellular domains confer improved cross-protection against various subtypes of influenza virus. Mol Ther. 2013b;21:485–492. doi: 10.1038/mt.2012.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DC, Cheung CY, Law AH, Mok CK, Peiris M, Lau AS. p38 mitogen-activated protein kinase-dependent hyperinduction of tumor necrosis factor alpha expression in response to avian influenza virus H5N1. J Virol. 2005;79:10147–10154. doi: 10.1128/JVI.79.16.10147-10154.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YN, Kim MC, Lee YT, Hwang HS, Cho MK, Lee JS, Ko EJ, Kwon YM, Kang SM. AS04-adjuvanted virus-like particles containing multiple M2 extracellular domains of influenza virus confer improved protection. Vaccine. 2014a;32:4578–4585. doi: 10.1016/j.vaccine.2014.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YN, Lee HJ, Lee DH, Kim JH, Park HM, Nahm SS, Lee JB, Park SY, Choi IS, Song CS. Severe canine influenza in dogs correlates with hyperchemokinemia and high viral load. Virology. 2011;417:57–63. doi: 10.1016/j.virol.2011.05.005. [DOI] [PubMed] [Google Scholar]
- Lee YN, Lee YT, Kim MC, Hwang HS, Lee JS, Kim KH, Kang SM. Fc receptor is not required for inducing antibodies but plays a critical role in conferring protection after influenza M2 vaccination. Immunology. 2014b;143:300–309. doi: 10.1111/imm.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neirynck S, Deroo T, Saelens X, Vanlandschoot P, Jou WM, Fiers W. A universal influenza A vaccine based on the extracellular domain of the M2 protein. Nat Med. 1999;5:1157–1163. doi: 10.1038/13484. [DOI] [PubMed] [Google Scholar]
- Park JK, Lee DH, Cho CH, Yuk SS, To EO, Kwon JH, Noh JY, Kim BY, Choi SW, Shim BS, Song MK, Lee JB, Park SY, Choi IS, Song CS. Supplementation of oil-based inactivated H9N2 vaccine with M2e antigen enhances resistance against heterologous H9N2 avian influenza virus infection. Vet Microbiol. 2014;169:211–217. doi: 10.1016/j.vetmic.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Quan FS, Kim YC, Yoo DG, Compans RW, Prausnitz MR, Kang SM. Stabilization of influenza vaccine enhances protection by microneedle delivery in the mouse skin. PLoS One. 2009;4:e7152. doi: 10.1371/journal.pone.0007152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Sings HL, Bryan JT, Wang B, Wang Y, Mach H, Kosinski M, Washabaugh MW, Sitrin R, Barr E. GARDASIL: prophylactic human papillomavirus vaccine development--from bench top to bed-side. Clin Pharmacol Ther. 2007;81:259–264. doi: 10.1038/sj.clpt.6100055. [DOI] [PubMed] [Google Scholar]
- Song JM, Hossain J, Yoo DG, Lipatov AS, Davis CT, Quan FS, Chen LM, Hogan RJ, Donis RO, Compans RW, Kang SM. Protective immunity against H5N1 influenza virus by a single dose vaccination with virus-like particles. Virology. 2010;405:165–175. doi: 10.1016/j.virol.2010.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JM, Van Rooijen N, Bozja J, Compans RW, Kang SM. Vaccination inducing broad and improved cross protection against multiple subtypes of influenza A virus. Proc Natl Acad Sci U S A. 2011a;108:757–761. doi: 10.1073/pnas.1012199108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JM, Wang BZ, Park KM, Van Rooijen N, Quan FS, Kim MC, Jin HT, Pekosz A, Compans RW, Kang SM. Influenza virus-like particles containing M2 induce broadly cross protective immunity. PLoS One. 2011b;6:e14538. doi: 10.1371/journal.pone.0014538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szretter KJ, Gangappa S, Lu X, Smith C, Shieh WJ, Zaki SR, Sambhara S, Tumpey TM, Katz JM. Role of host cytokine responses in the pathogenesis of avian H5N1 influenza viruses in mice. J Virol. 2007;81:2736–2744. doi: 10.1128/JVI.02336-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumpey TM, Renshaw M, Clements JD, Katz JM. Mucosal delivery of inactivated influenza vaccine induces B-cell-dependent heterosubtypic cross-protection against lethal influenza A H5N1 virus infection. J Virol. 2001;75:5141–5150. doi: 10.1128/JVI.75.11.5141-5150.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turley CB, Rupp RE, Johnson C, Taylor DN, Wolfson J, Tussey L, Kavita U, Stanberry L, Shaw A. Safety and immunogenicity of a recombinant M2e-flagellin influenza vaccine (STF2.4xM2e) in healthy adults. Vaccine. 2011;29:5145–5152. doi: 10.1016/j.vaccine.2011.05.041. [DOI] [PubMed] [Google Scholar]
- Wang BZ, Gill HS, Kang SM, Wang L, Wang YC, Vassilieva EV, Compans RW. Enhanced influenza virus-like particle vaccines containing the extracellular domain of matrix protein 2 and a Toll-like receptor ligand. Clin Vaccine Immunol. 2012;19:1119–1125. doi: 10.1128/CVI.00153-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Qiao J, Zhao L, Wang G, He G, Li K, Tian Y, Gao M, Wang J, Wang H, Dong C. Acute respiratory distress syndrome induced by avian influenza A (H5N1) virus in mice. Am J Respir Crit Care Med. 2006;174:1011–1017. doi: 10.1164/rccm.200511-1751OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.