Abstract
BACKGROUND
Universal tumor screening for Lynch syndrome, the most common form of hereditary colorectal cancer (CRC), has been recommended among all patients newly diagnosed with CRC. However, there is limited literature regarding patient perspectives of tumor screening for Lynch syndrome among patients with CRC who are not selected for screening based on family history criteria.
METHODS
A total of 145 patients aged 39 to 87 years were administered surveys assessing perceived risk, patient perspectives, and potential benefits of and barriers to tumor screening for Lynch syndrome. Associations between patient‐specific and cancer‐specific factors and survey responses were analyzed.
RESULTS
The majority of participants perceived their risk of developing Lynch syndrome as being low, with 9 participants (6.2%) anticipating an abnormal screening result. However, most participants endorsed the potential benefits of screening for themselves and their families, with 84.8% endorsing ≥6 benefits and 50.3% endorsing all 8 benefits. Participants also endorsed few potential barriers to screening, with 89.4% endorsing ≤4 of 9 potential barriers. A common barrier was worry about the cost of additional testing and surveillance, which was endorsed by 54.5% of participants. The level of distress associated with tumor screening for Lynch syndrome, which was very low, was not associated with age or CRC stage.
CONCLUSIONS
The results of the current study indicate that patients with CRC overall have a positive attitude toward tumor screening for Lynch syndrome, endorse the benefits of screening, and experience low levels of distress. These findings provide insight into patient attitudes toward tumor screening for Lynch syndrome among unselected patients with CRC to inform educational approaches that assist in patient decision‐making and guide the successful implementation of screening programs. Cancer 2015;121:3281–3289. © 2015 American Cancer Society.
Keywords: hereditary nonpolyposis colorectal cancer (HNPCC), Lynch syndrome, genetic screening, genetic counseling, colorectal neoplasms
Short abstract
In the current study, perspectives among patients newly diagnosed with colorectal cancer are assessed regarding universal tumor screening for Lynch syndrome. The majority of patients appear to have a positive attitude toward screening and endorse the benefits for themselves and their families, whereas potential barriers include concerns over the cost of additional genetic counseling and testing.
INTRODUCTION
Lynch syndrome is an autosomal dominant cancer syndrome associated with an increased risk of colorectal cancer (CRC) and other extracolonic malignancies, including endometrial, stomach, small bowel, and ovarian cancers. It accounts for approximately 3% of all CRC cases, making it the most common form of hereditary CRC.1, 2, 3, 4 Lynch syndrome most commonly occurs due to the inactivation of 1 of 4 mismatch repair (MMR) genes (MLH1, MSH2, MSH6, and PMS2), either due to germline mutation or epigenetic silencing.5, 6 This MMR defect leads to microsatellite instability (MSI), a hallmark feature of Lynch syndrome tumors.
Amsterdam criteria and Bethesda guidelines have been developed to identify patients with CRC who should undergo evaluation for Lynch syndrome using criteria mainly based on family cancer history and age at onset.7, 8, 9, 10 However, these methods are not sensitive enough to detect all patients with Lynch syndrome given that not all meet these criteria, family history is not always reliable or available, and many patients who do meet these criteria remain undiagnosed.2, 11, 12, 13, 14, 15, 16, 17, 18, 19 In 2005, Hampel et al performed tumor testing on 1066 patients newly diagnosed with CRC and identified 23 patients with Lynch syndrome, 5 of whom (21.7%) did not meet Amsterdam criteria or Bethesda guidelines.16 More recently, Cross et al reported that only 11% and 25%, respectively, of patients who met the Bethesda and Amsterdam criteria had undergone evaluation for Lynch syndrome.14
Genetic testing for germline mutations in MMR genes is costly, and therefore a stepwise process for evaluating patients for Lynch syndrome is typically implemented. As a first step, 1 of 2 molecular tests can be used to test tumor tissue for the presence of an MMR deficiency: immunohistochemistry (IHC) and MSI tests.20, 21 IHC assesses the levels of MMR proteins detected in the tumor tissue whereas MSI testing assesses the level of MSI in the tumor tissue. Tumor samples that are positive for an MMR deficiency based on one or both of these tests typically undergo additional testing to assess hypermethylation of the MLH1 promoter region and BRAF mutations to rule out sporadic mutations. Patients who are negative for BRAF and hypermethylation are then generally referred to genetic counseling, during which the patient is counseled concerning his or her risk of developing Lynch syndrome and provided the opportunity to undergo germline DNA analysis of MMR genes to confirm a diagnosis of Lynch syndrome.
In 2009, the Evaluation of Genomic Applications in Practice and Prevention (EGAPP) working group at the Centers for Disease Control and Prevention recommended a universal CRC tumor screening approach for Lynch syndrome.21, 22 Specifically, all newly diagnosed patients with CRC, regardless of family history or age, would undergo CRC tumor screening for an MMR deficiency (via MSI or IHC testing), and those who tested positive would be offered genetic counseling and germline testing for MMR mutations. This recommendation was based on evidence that universal tumor screening for Lynch syndrome, and subsequent genetic counseling and testing in at‐risk relatives, would reduce the morbidity and mortality associated with CRC.21, 22 More recently, universal tumor screening for Lynch syndrome was endorsed by guidelines published by the National Comprehensive Cancer Network23 and the US Multi‐Society Task Force on Colorectal Cancer.24
Despite this support for universal tumor screening for Lynch syndrome, potential patient‐specific barriers to this screening approach remain, including concerns about psychosocial burdens, cost issues with screening and genetic counseling, adherence to cancer surveillance recommendations, and communication of the risk of Lynch syndrome to relatives.25, 26 These last 2 concerns are significant given that the impact and cost‐effectiveness of universal tumor screening will only be realized if patients and their at‐risk relatives have access to and take advantage of genetic counseling and testing as well as recommendations for long‐term cancer surveillance and risk‐reducing surgery.27, 28 An additional concern is that screening would occur directly after a CRC diagnosis, which is a stressful and overwhelming time. Thus, patients may not want the added burden of genetic counseling and testing while preparing for surgery and treatment, despite the potential benefits.
Therefore, it is important to understand the perspectives of patients newly diagnosed with CRC who will be screened as part of universal tumor screening programs. However, to the best of our knowledge, few studies to date have assessed patient attitudes and perceived benefits of and barriers to genetic testing for Lynch syndrome among “unselected” patients with CRC (ie, those patients who are not selected based on high‐risk factors such as family history or those already seeking genetic testing).29, 30, 31, 32 In addition, the majority of these studies were performed in a focus group setting rather than with individual assessments, and participants were not always surveyed right after the diagnosis of CRC, the timeframe for universal tumor screening.
The overarching goal of the current study was to identify variables that would guide the successful implementation of universal tumor screening for Lynch syndrome. The success of this screening program ultimately lies in patient uptake of subsequent evaluation for Lynch syndrome (ie, genetic counseling and testing) for those who are identified as being at high risk. Thus, understanding patient attitudes and perceived benefits and barriers surrounding tumor screening will guide health care facilities in implementing a successful screening program. As part of this effort, among unselected patients newly diagnosed with CRC, we investigated patient perspectives regarding tumor screening for Lynch syndrome, including perceived susceptibility, benefits and barriers, and distress associated with screening. We explored whether participant attitudes differed by demographic characteristics, clinical factors, and personal and family cancer histories.
MATERIALS AND METHODS
Recruitment
Kaiser Permanente Northwest (KPNW) is an integrated health care system that serves approximately 500,000 members in northwest Oregon and southwest Washington. Study participants were recruited as part of a randomized controlled trial among KPNW members to determine the effectiveness of a universal CRC tumor screening program for Lynch syndrome compared with the current practice of physician referral and self‐referral for Lynch syndrome evaluation. Recruitment identified all individuals newly diagnosed with CRC who were aged >18 years and were scheduled for surgical treatment between January 2012 and November 2014. Patients were randomly allocated to the intervention arm (universal tumor screening) or the control arm (physician referral or self‐referral for screening). Patients were eligible for enrollment if they spoke English, had no known cognitive impairment (eg, Alzheimer disease), had no previous contact with genetics services for CRC, had no previous screening for Lynch syndrome, had no previous diagnosis of Lynch syndrome or another CRC‐related hereditary cancer syndrome, and were not in hospice. For each patient in the intervention arm, a tumor sample was submitted for MSI testing and a set of surveys was administered to assess patient attitudes regarding screening for Lynch syndrome. The current study focused on these survey responses, and therefore the participants included in the current study were limited to those in the intervention arm only. The protocols and consent forms for recruitment were approved by the Institutional Review Board at KPNW.
Measures
Within 1 month of enrollment, and before the return of MSI test results, participants were administered surveys over the telephone by a project team member. The first survey consisted of items designed to assess patient domains associated with patient attitudes toward screening for Lynch syndrome and genetic counseling. This survey was developed by the project team using the Health Belief Model as the underlying foundation for the survey design.33 Participants were asked what they expected their MSI screening result to be and to choose between 4 potential responses: “at high risk of having a Lynch syndrome gene mutation,” “at low risk of having a Lynch syndrome gene mutation,” “inconclusive,” and “no expectation.” Participants were then asked to respond with how much they agreed with statements regarding perceived susceptibility for Lynch syndrome and perceived potential benefits of and barriers to screening for Lynch syndrome. For these items, responses were coded using a 5‐point Likert‐type scale ranging from “strongly disagree” to “strongly agree.” For analyses, the item responses were consolidated into a 3‐point scale: “strongly disagree/disagree,” “neither,” and “strongly agree/agree.” Participants were also asked whether they planned to share their results with certain health care providers, with answers coded as “yes” or “no.”
Participants were administered the Impact of Event Scale‐Revised (IES‐R) to assess the severity of distress that might be associated with screening for Lynch syndrome in addition to any distress associated with the CRC diagnosis and treatment.34 An IES‐R score of 0 indicated a lack of distress, and a total score of ≥22 was used to indicate a high level of distress.
Lastly participants were administered the Genetic Risk Easy Assessment Tool (GREAT) to obtain participant and family history information regarding cancer type and age at the time of diagnosis.35 Survey data from the GREAT were missing for 1 participant who died before completing the survey.
Statistical Analysis
On primary analyses, the goal was to determine whether demographic characteristics were associated with patient perspectives regarding screening for Lynch syndrome. Multivariate logistic regression was used to analyze all dichotomous and multilevel survey outcomes. Given the 3‐point scale, survey responses associated with perceived susceptibility and patient attitudes were first modeled using ordered logistic regression. However, in the event that the odds were not proportional across all 3 levels of responses, multinomial logistic regression was applied. Multinomial logistic regression was also used to analyze responses regarding expected MSI screening results. Predictors included age at survey, sex, annual household income, level of education, and race/ethnicity. Due to low diversity, racial/ethnic groups were consolidated and recoded as 1 (non‐Hispanic white) or 2 (all others). Results are presented as the odds ratio (OR) and 95% confidence interval (95% CI). The numbers of potential benefits and barriers endorsed by each participant were calculated based on the number of “strongly agree/agree” responses to the associated items. Multivariate linear regression models were used to analyze the number of benefits and barriers endorsed as well as IES‐R scores. The results of these models are presented as the regression coefficient (β) and P value.
On secondary analyses, the goal was to explore whether cancer‐related factors were predictors of patient perspectives concerning screening for Lynch syndrome. These predictors were added individually to the models described above. Patient‐specific predictors included the American Joint Committee on Cancer stage of the CRC, patient history of CRC, and patient cancer history (not including a diagnosis of nonmelanoma skin cancer). Self‐reported cancer histories were not confirmed with medical records because a participant's perceived cancer history would more likely influence their survey responses than actual cancer history. A family history of CRC was also considered as a potential predictor, and was defined as the number of first‐degree, second‐degree, and third‐degree relatives who had been diagnosed with CRC at any age, as reported by the participant.
For all models described above, a P value of .05 was used as the threshold to indicate statistical significance. SAS statistical software (version 9.1; SAS Institute Inc, Cary, NC) was used for all statistical analyses.
RESULTS
Study Population
At the time of the current analysis, 145 participants (mean age ± standard deviation [SD], 66.6 ± 11.3 years [range, 39‐87 years]) had completed surveys (Table 1). A slight majority of participants were male (58.6%), whereas most were non‐Hispanic white (81.3%), had an annual household income of >$40,000 (67.4%), and had more than a high school education (75.8%). Approximately 60% of participants were diagnosed with CRC of stage I or II, 20.8% of participants had received a prior cancer diagnosis, and 23.6% of participants reported at least 1 relative with CRC. Due to the low number of participants with a prior CRC diagnosis (2 participants; 1.4%), this variable was not used as a predictor in subsequent statistical models.
Table 1.
Demographic, Clinical, and Family History of Participants (N = 145)
| Variable | Value |
|---|---|
| Sex | |
| Male | 85 (58.6%) |
| Female | 60 (41.4%) |
| Age at survey, mean ± SD (range), y | 66.6 ± 11.3 (39–87) |
| Race/ethnicitya | |
| White, non‐Hispanic | 117 (81.3%) |
| Hispanic | 8 (5.6%) |
| Multiple races or other | 19 (13.2%) |
| Household incomeb | |
| <$40,000 | 45 (32.6%) |
| $40,000‐$59,999 | 32 (23.2%) |
| $60,000‐$79,999 | 19 (13.8%) |
| >$80,000 | 42 (30.4%) |
| Level of educationa | |
| <High school | 5 (3.5%) |
| High school degree or GED | 30 (20.8%) |
| Some college | 57 (39.6%) |
| College degree | 25 (17.4%) |
| Master's or doctorate degree | 27 (18.8%) |
| CRC stagec | |
| I | 44 (34.1%) |
| II | 36 (27.9%) |
| III | 39 (30.2%) |
| IV | 10 (7.8%) |
| Previous CRC diagnosisd | |
| No | 142 (98.6%) |
| Yes | 2 (1.4%) |
| Previous diagnosis of any cancerd | |
| No | 114 (79.2%) |
| Yes | 30 (20.8%) |
| First‐degree relative with CRCe | |
| No | 124 (86.1%) |
| Yes | 20 (13.8%) |
| No. of relatives with CRCf | |
| 0 | 110 (76.4%) |
| 1 | 24 (16.7%) |
| 2 | 9 (6.3%) |
| 3 | 1 (0.7%) |
Abbreviations: CRC, colorectal cancer; GED, General Educational Development test; SD, standard deviation.
Information regarding race/ethnicity and education was missing for 1 participant.
Information regarding household income was missing for 7 participants: 3 who did not know their income and 4 who refused to respond.
CRC stage was based on the current CRC diagnosis and was coded using the American Joint Committee on Cancer classification system; information regarding stage was missing for 16 participants.
Participant history of cancer (CRC and/or any cancer) was based on self‐report and was missing for 1 participant.
Defined as the presence of a first‐degree relative with a prior CRC diagnosis; information was missing for 1 participant.
Defined as the number of first‐degree, second‐degree, and third‐degree relatives with a prior CRC diagnosis; information was missing for 1 participant.
Expected MSI Screening Results
When asked about their expected MSI screening result, the majority of participants (88 participants; 60.7%) had no expectations. A few participants (9 participants; 6.2%) expected their MSI screening test results to indicate a high risk of MSI. Those participants who expected to have a result indicating high MSI were more likely to have more family members with CRC (OR, 3.10; 95% CI, 1.22‐7.92). The participants' expected MSI screening result was not found to be associated with age at onset, previous cancer diagnosis, or cancer stage (results not shown).
Perceived Susceptibility to Lynch Syndrome
A total of 51 participants (35.2%) indicated they were worried that they may carry an altered gene for hereditary CRC (Fig. 1). Those participants were more likely to be younger (OR, 1.04; 95% CI, 1.01‐1.13) or have a lower educational level (OR, 1.70; 95% CI, 1.23‐2.34). In addition, 28 participants (19.3%) suspected that they were a gene carrier (Fig. 1). These participants were more likely to have more relatives with CRC (OR, 2.74; 95% CI, 1.57‐4.80).
Figure 1.
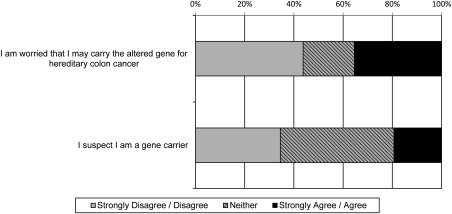
Participants' perceived susceptibility to Lynch syndrome.
Attitudes Regarding Screening for Lynch Syndrome
Greater than 90% of study participants agreed that they would be able to cope with their MSI screening result, that the test should be available to anyone who wants the information, and that they understood the reason for the test (Fig. 2). Participants who agreed that they understood the reason for the MSI screening test were more likely to have a higher educational level (OR, 2.51; 95% CI, 1.24‐5.07).
Figure 2.
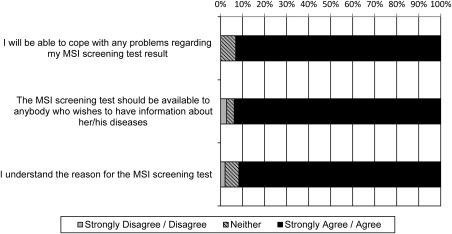
Attitudes of patients with colorectal cancer regarding Lynch syndrome screening. MSI indicates microsatellite instability.
Potential Benefits of and Barriers to Screening for Lynch Syndrome
A total of 73 participants (50.3%) endorsed all 8 benefits whereas 123 participants (84.8%) endorsed ≥6 benefits (Fig. 3). Demographic and cancer‐related variables were not found to be associated with endorsement of benefits (results not shown). Participants endorsed more benefits if they worried that they may carry the altered gene for hereditary CRC (β = 0.45; P = .0014) or suspected they were a gene carrier (β = 0.51; P = .0019). All participants agreed that doctors should have access to the most updated technology.
Figure 3.
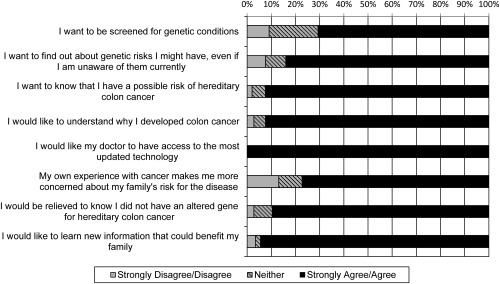
Participants' endorsement of the potential benefits of screening for Lynch syndrome.
In contrast, endorsement of barriers was varied, although the majority of participants (130 participants; 89.7%) endorsed ≤4 of the 9 barriers (Fig. 4). Participants who endorsed fewer barriers were more likely to have more relatives with CRC (β = ‐0.68; P = .0004) and suspected they were a gene carrier (β = ‐0.56; P = .0008). The most common barriers were the absence of a family history of CRC (94 participants; 64.8%) and concern about the cost of additional genetic testing or counseling (79 participants; 54.5%) (Fig. 4). It is interesting to note that only a relatively few participants (18 participants; 12.4%) were concerned about losing their health insurance.
Figure 4.
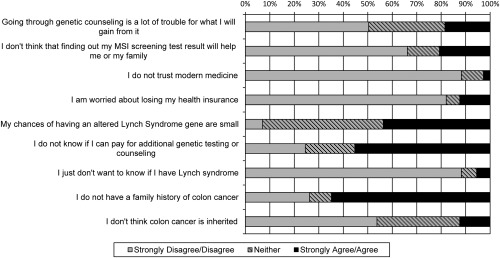
Participants' endorsement of potential barriers to screening for Lynch syndrome. MSI indicates microsatellite instability.
Intention to Share MSI Screening Test Results With Health Care Providers
The majority of participants (133 participants; 91.7%) indicated they intended to share their results with a health care provider (Fig. 5). Women were more likely to indicate they would share their results with their surgeon compared with men (OR, 3.23; 95% CI, 1.09‐9.61). Only 21 women (36.8%) indicated they would share their results with their obstetrician/gynecologist (OBGYN). Because women may be less likely to see an OBGYN as they age, the electronic medical records of the female participants were searched for OBGYN appointments within the past 3 years. Of those who had seen an OBGYN (28.1%), 56.3% indicated they would share their result with their OBGYN.
Figure 5.
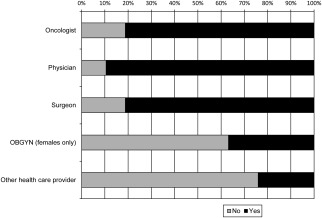
Participants' intention to share microsatellite instability screening results with a health care provider. OBGYN indicates obstetrician/gynecologist.
Distress Associated With the MSI Screening Test
Overall, participants were found to have low levels of distress associated with MSI screening as measured by the IES‐R for intrusion (mean, 1.04; SD, 0.16), avoidance (mean, 1.12; SD, 0.40), hyperarousal (mean, 1.06; SD, 0.34), and total distress (mean, 22.91; SD, 2.64). The majority of participants (112 participants; 77.2%) had an IES‐R score of 0, indicating a lack of distress. A high distress score (≥22) was noted for only 3 participants (2.1%). The level of distress was not found to be associated with age, CRC stage, expected MSI results, perceived risk of Lynch syndrome, or endorsement of benefits or barriers (data not shown).
DISCUSSION
Although universal CRC tumor screening for Lynch syndrome has been recommended,22, 23 the success of such a screening program lies in the attitudes of and uptake of further evaluation by patients with CRC identified through tumor screening as having a high risk of developing Lynch syndrome. Addressing barriers and facilitating benefits to tumor screening during implementation of a screening program will maximize its impact and success. Thus, patient perspectives toward tumor screening for Lynch syndrome need to be explored. The results of the current study are significant because they assess these perspectives among unselected patients newly diagnosed with CRC, which is the target population of universal tumor screening.
Overall, the majority of participants indicated that they did not expect to be at high risk of developing Lynch syndrome. However, participants had a positive attitude toward tumor screening, endorsing many potential benefits of screening to themselves and their family. Patients' opinions regarding potential barriers to screening were more diverse. Surprisingly, only 12% of participants were concerned about the potential loss of health insurance. This rate is substantially lower than reported in previous studies among patients undergoing screening for Lynch syndrome after identification via family history, in which >30% of patients with CRC reported losing insurance as a concern.36, 37 The lower level of concern regarding health insurance discrimination in the population in the current study could be due to an increasing awareness of the Genetic Information Nondiscrimination Act, passed in 2008, or the recent passage of the Patient Protection and Affordable Care Act, which prevents health insurance companies from discriminating against patients with genetic disorders due to the presence of a preexisting condition. In addition, it is likely that many participants in the current study population have KPNW insurance through their employer, which may decrease their concerns about losing their health insurance.
A family history of CRC (defined as the number of relatives with a prior CRC diagnosis) was found to be associated with the expectation of receiving a high MSI score, a higher suspicion of being a gene carrier, and the endorsement of fewer barriers to screening. Lack of a family history of CRC was the most common reason given to not undergo screening for Lynch syndrome. The results of the current study highlight the need to educate patients and their health care providers that Lynch syndrome is not always associated with a positive family history.11, 12, 13, 14, 15
Ongoing concerns about universal tumor screening for Lynch syndrome include potentially unnecessary burdens to patients, including those who are older or have advanced cancer.25 However, the results of the current study demonstrated that age and CRC stage were not significantly associated with patient endorsement of the benefits of and barriers to screening, indicating that older patients and those with advanced cancer are no less likely to see the value in Lynch syndrome screening. In addition, there was no significant distress associated with screening, and the distress that was experienced was not found to be associated with age or CRC stage. Previous studies have reported higher levels of distress associated with screening for Lynch syndrome among patients with CRC identified via family history.36, 37, 38, 39, 40 The lower levels of distress in the current study population could be due to KPNW being an integrated health care system, which might relieve stress due to a lack of referral requirements, for example. In addition, universal screening of all patients with CRC, as opposed to those patients identified as being at high risk only, might lead to less overall distress because the majority of patients will likely believe that they are at low risk, as reflected in the results presented herein.
Given the association between Lynch syndrome and endometrial cancer, it is concerning that women in the current study did not recognize the importance of sharing their screening results with their OBGYN. Women who are screened due to a diagnosis of CRC should be educated regarding this association as well as the recommended surveillance for endometrial and ovarian cancer.23, 24 In addition, health care facilities could implement a system to facilitate colonic and extracolonic surveillance recommendations for all patients identified as having Lynch syndrome.
It is important to address the limitations of the current study. First, perspectives on screening for Lynch syndrome among patients who consented to participate were assessed and therefore the survey responses may be biased toward those individuals interested in screening. Second, the use of MSI testing and IHC to screen tumors for MMR deficiency can vary across screening programs.41 Therefore, the results of the current study using MSI testing may not be directly applicable to those that use IHC. In addition, all participants were insured members of KPNW, an integrated health care system with comprehensive medical services including access to physicians, laboratories, pharmacies, and genetic counseling. Thus, these participants may not share the same concerns as other patients with CRC without KPNW coverage, such as worry over the loss of health insurance or costs. Finally, the population in the current study was not racially or ethnically diverse. Given these limitations, the data presented herein may not represent the attitudes and beliefs of patients with CRC in general.
Additional issues surrounding the implementation of universal tumor screening for Lynch syndrome not addressed in the current study remain unresolved. Future analyses of this study population will focus on interest in tumor screening and genetic testing with respect to patient characteristics and the presence of at‐risk relatives. In addition, participants will be evaluated for uptake of genetic counseling and testing, their understanding of their tumor screening and genetic test results, and the communication of these results to family members and health care providers.
The results of the current study indicate that patients with CRC have a positive attitude toward screening for Lynch syndrome, endorse the benefits of screening to themselves and their families, and do not report significant distress associated with the screening process. In addition, these findings identify specific barriers to screening that could be addressed to maximize the success of tumor screening programs and guide the implementation of universal CRC tumor screening. Specifically, patients could be educated during screening regarding the potential costs associated with additional genetic counseling or testing. Overall, these results can inform the development of patient education tools to assist in decision‐making regarding screening for Lynch syndrome (Table 2). However, further study is needed to explore additional factors associated with tumor screening and genetic testing to confirm Lynch syndrome.
Table 2.
Summary of Patient Perspectives and Further Points to Consider Regarding the Implementation of Universal Tumor Screening for Lynch syndrome
|
• Most patients with CRC endorse the benefits of universal tumor screening for Lynch syndrome. • Most patients reported minimal distress associated with tumor screening, and distress was not associated with age or stage of disease. • Most patients were not concerned about the potential loss of health insurance. • Most patients recognized the importance of sharing their tumor screening results with their health care providers. • Health care providers and patients should be educated that a lack of family history of CRC does not rule out Lynch syndrome. • Health care providers should educate female patients regarding the association between Lynch syndrome and endometrial cancer and facilitate appropriate clinical care. • Health care providers and/or health plans should be prepared to provide information to patients regarding the potential costs of additional genetic counseling and testing associated with a positive tumor screen. |
Abbreviation: CRC, colorectal cancer.
FUNDING SUPPORT
Supported through a grant from the National Cancer Institute of the National Institutes of Health (grant 5R01CA140377 to Dr. Goddard).
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
We thank Elizabeth Hess for her editorial support as well as all the participants who made this work possible.
REFERENCES
- 1. Lynch HT, Lynch PM, Lanspa SJ, Snyder CL, Lynch JF, Boland CR. Review of the Lynch syndrome: history, molecular genetics, screening, differential diagnosis, and medicolegal ramifications. Clin Genet. 2009;76:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Moreira L, Balaguer F, Lindor N, et al. Identification of Lynch syndrome among patients with colorectal cancer. JAMA. 2012;308:1555–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hampel H, Frankel W, Panescu J, et al. Screening for Lynch syndrome (hereditary nonpolyposis colorectal cancer) among endometrial cancer patients. Cancer Res. 2006;66:7810–7817. [DOI] [PubMed] [Google Scholar]
- 4. Hampel H, Panescu J, Lockman J, et al. Comment on: Screening for Lynch Syndrome (Hereditary Nonpolyposis Colorectal Cancer) among Endometrial Cancer Patients. Cancer Res. 2007;67:9603. [DOI] [PubMed] [Google Scholar]
- 5. Kempers MJ, Kuiper RP, Ockeloen CW, et al. Risk of colorectal and endometrial cancers in EPCAM deletion‐positive Lynch syndrome: a cohort study. Lancet Oncol. 2011;12:49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rumilla K, Schowalter KV, Lindor NM, et al. Frequency of deletions of EPCAM (TACSTD1) in MSH2‐associated Lynch syndrome cases. J Mol Diagn. 2011;13:93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vasen HF, Mecklin JP, Khan PM, Lynch HT. The International Collaborative Group on Hereditary Non‐Polyposis Colorectal Cancer (ICG‐HNPCC). Dis Colon Rectum. 1991;34:424–425. [DOI] [PubMed] [Google Scholar]
- 8. Vasen HF, Watson P, Mecklin JP, Lynch HT. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative Group on HNPCC. Gastroenterology. 1999;116:1453–1456. [DOI] [PubMed] [Google Scholar]
- 9. Rodriguez‐Bigas MA, Boland CR, Hamilton SR, et al. A National Cancer Institute Workshop on Hereditary Nonpolyposis Colorectal Cancer Syndrome: meeting highlights and Bethesda guidelines. J Natl Cancer Inst. 1997;89:1758–1762. [DOI] [PubMed] [Google Scholar]
- 10. Umar A, Boland CR, Terdiman JP, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96:261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Van Lier MG, De Wilt JH, Wagemakers JJ, et al. Underutilization of microsatellite instability analysis in colorectal cancer patients at high risk for Lynch syndrome. Scand J Gastroenterol. 2009;44:600–604. [DOI] [PubMed] [Google Scholar]
- 12. Wong C, Gibbs P, Johns J, et al. Value of database linkage: are patients at risk of familial colorectal cancer being referred for genetic counselling and testing? Intern Med J. 2008;38:328–333. [DOI] [PubMed] [Google Scholar]
- 13. Perez‐Carbonell L, Ruiz‐Ponte C, Guarinos C, et al. Comparison between universal molecular screening for Lynch syndrome and revised Bethesda guidelines in a large population‐based cohort of patients with colorectal cancer. Gut. 2012;61:865–872. [DOI] [PubMed] [Google Scholar]
- 14. Cross DS, Rahm AK, Kauffman TL, et al; CERGEN Study Team . Underutilization of Lynch syndrome screening in a multisite study of patients with colorectal cancer. Genet Med. 2013;15:933–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Canard G, Lefevre JH, Colas C, et al. Screening for Lynch syndrome in colorectal cancer: are we doing enough? Ann Surg Oncol. 2012;19:809–816. [DOI] [PubMed] [Google Scholar]
- 16. Hampel H, Frankel WL, Martin E, et al. Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer). N Engl J Med. 2005;352:1851–1860. [DOI] [PubMed] [Google Scholar]
- 17. Hampel H, Frankel WL, Martin E, et al. Feasibility of screening for Lynch syndrome among patients with colorectal cancer. J Clin Oncol. 2008;26:5783–5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Julie C, Tresallet C, Brouquet A, et al. Identification in daily practice of patients with Lynch syndrome (hereditary nonpolyposis colorectal cancer): revised Bethesda guidelines‐based approach versus molecular screening. Am J Gastroenterol. 2008;103:2825–2835. [DOI] [PubMed] [Google Scholar]
- 19. Morrison J, Bronner M, Leach BH, Downs‐Kelly E, Goldblum JR, Liu X. Lynch syndrome screening in newly diagnosed colorectal cancer in general pathology practice: from the revised Bethesda guidelines to a universal approach. Scand J Gastroenterol. 2011;46:1340–1348. [DOI] [PubMed] [Google Scholar]
- 20. Bonis PA, Trikalinos TA, Chung M, et al. Hereditary nonpolyposis colorectal cancer: diagnostic strategies and their implications. Evid Rep Technol Assess (Full Rep). 2007;(150):1–180. [PMC free article] [PubMed] [Google Scholar]
- 21. Palomaki GE, McClain MR, Melillo S, Hampel HL, Thibodeau SN. EGAPP supplementary evidence review: DNA testing strategies aimed at reducing morbidity and mortality from Lynch syndrome. Genet Med. 2009;11:42–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group . Recommendations from the EGAPP Working Group: genetic testing strategies in newly diagnosed individuals with colorectal cancer aimed at reducing morbidity and mortality from Lynch syndrome in relatives. Genet Med. 2009;11:35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Provenzale D, Jasperson K, Ahnen DJ, et al. Genetic/familial high‐risk assessment: colorectal. National Comprehensive Cancer Network . Version 1.2014. Available at: www.nccn.org/professionals/physician_gls/pdf/genetics_colon.pdf. Accessed June 16, 2014.
- 24. Giardiello FM, Allen JI, Axilbund JE, et al. Guidelines on genetic evaluation and management of Lynch syndrome: a consensus statement by the US Multi‐society Task Force on colorectal cancer. Am J Gastroenterol. 2014;109:1159–1179. [DOI] [PubMed] [Google Scholar]
- 25. Hall MJ. Counterpoint: implementing population genetic screening for Lynch Syndrome among newly diagnosed colorectal cancer patients–will the ends justify the means? J Natl Compr Canc Netw. 2010;8:606–611. [DOI] [PubMed] [Google Scholar]
- 26. Cragun D, DeBate RD, Vadaparampil ST, Baldwin J, Hampel H, Pal T. Comparing universal Lynch syndrome tumor‐screening programs to evaluate associations between implementation strategies and patient follow‐through. Genet Med. 2014;16:773–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ladabaum U, Wang G, Terdiman J, et al. Strategies to identify the Lynch syndrome among patients with colorectal cancer: a cost‐effectiveness analysis. Ann Intern Med. 2011;155:69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mvundura M, Grosse SD, Hampel H, Palomaki GE. The cost‐effectiveness of genetic testing strategies for Lynch syndrome among newly diagnosed patients with colorectal cancer. Genet Med. 2010;12:93–104. [DOI] [PubMed] [Google Scholar]
- 29. Cragun D, Malo TL, Pal T, Shibata D, Vadaparampil ST. Colorectal cancer survivors' interest in genetic testing for hereditary cancer: implications for universal tumor screening. Genet Test Mol Biomarkers. 2012;16:493–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kinney AY, Choi YA, DeVellis B, Millikan R, Kobetz E, Sandler RS. Attitudes toward genetic testing in patients with colorectal cancer. Cancer Pract. 2000;8:178–186. [DOI] [PubMed] [Google Scholar]
- 31. Kinney AY, DeVellis BM, Skrzynia C, Millikan R. Genetic testing for colorectal carcinoma susceptibility: focus group responses of individuals with colorectal carcinoma and first‐degree relatives. Cancer. 2001;91:57–65. [DOI] [PubMed] [Google Scholar]
- 32. Ramsey SD, Wilson S, Spencer A, Geidzinska A, Newcomb P. Attitudes towards genetic screening for predisposition to colon cancer among cancer patients, their relatives and members of the community. Results of focus group interviews. Community Genet. 2003;6:29–36. [DOI] [PubMed] [Google Scholar]
- 33. Champion VL, Skinner CS. The Health Belief Model In: Glanz K, Rimer BK, Viswanath K, eds. Health Behavior and Health Education: Theory, Research, and Practice. San Francisco, CA: Jossey‐Bass; 2008:45–65. [Google Scholar]
- 34. Weiss DS, Marmar CR. The Impact of Event Scale‐revised In: Wilson JP, Tang CS, eds. Assessing Psychological Trauma and PTSD: A Practitioner's Handbook. 2nd ed New York: Guilford Press; 2004:168–189. [Google Scholar]
- 35. Acheson LS, Zyzanski SJ, Stange KC, Deptowicz A, Wiesner GL. Validation of a self‐administered, computerized tool for collecting and displaying the family history of cancer. J Clin Oncol. 2006;24:5395–5402. [DOI] [PubMed] [Google Scholar]
- 36. Esplen MJ, Madlensky L, Butler K, et al. Motivations and psychosocial impact of genetic testing for HNPCC. Am J Med Genet. 2001;103:9–15. [PubMed] [Google Scholar]
- 37. Keller M, Jost R, Kadmon M, et al. Acceptance of and attitude toward genetic testing for hereditary nonpolyposis colorectal cancer: a comparison of participants and nonparticipants in genetic counseling. Dis Colon Rectum. 2004;47:153–162. [DOI] [PubMed] [Google Scholar]
- 38. Esplen MJ, Madlensky L, Aronson M, et al. Colorectal cancer survivors undergoing genetic testing for hereditary non‐polyposis colorectal cancer: motivational factors and psychosocial functioning. Clin Genet. 2007;72:394–401. [DOI] [PubMed] [Google Scholar]
- 39. Hasenbring MI, Kreddig N, Deges G, et al. Psychological impact of genetic counseling for hereditary nonpolyposis colorectal cancer: the role of cancer history, gender, age, and psychological distress. Genet Test Mol Biomarkers. 2011;15:219–225. [DOI] [PubMed] [Google Scholar]
- 40. Keller M, Jost R, Haunstetter CM, et al. Comprehensive genetic counseling for families at risk for HNPCC: impact on distress and perceptions. Genet Test. 2002;6:291–302. [DOI] [PubMed] [Google Scholar]
- 41. Cohen SA. Current Lynch syndrome tumor screening practices: a survey of genetic counselors. J Genet Couns. 2014;23:38–47. [DOI] [PubMed] [Google Scholar]


