Summary
Background
Under severe stenotic conditions, von Willebrand Factor (VWF) multimerizes into large insoluble fibers at pathological shear rates.
Objective
Evaluate the mechanics and biology of VWF fibers without the confounding effects of endothelium or collagen.
Methods
Within a micropost-impingement microfluidic device, >100 µm long VWF fibers multimerized on the post within 10 min using EDTA-treated PFP perfused at wall shear rates >5000 s−1.
Results
VWF fiber thickness increased to >10 µm by increasing shear rate to 10,000 s−1. In a stress-strain test, fibrous VWF had an elastic modulus of ~50 MPa. The insoluble VWF fibers were non-amyloid since they rapidly dissolved in trypsin, plasmin, or 2% SDS, but were resistant to 50 nM ADAMTS13 or 100 nM tPA in plasma. Following fiber formation, perfusion of low corn trypsin inhibitor (CTI)-treated (4 µg/ml), recalcified citrated plasma at 1500 s−1 caused fibrin formation on the VWF fibers, a result not observed with purified type 1 collagen or a naked micropost. During VWF fiber formation, contact pathway factors accumulated on VWF since the use of EDTA/PPACK/apixaban/high CTI-treated PFP during VWF fiber formation prevented subsequent fibrin production from low CTI, recalcified citrated PFP. VWF fibers displayed FXIIa-immunostaining. When PPACK-inhibited whole blood was perfused over VWF fibers, platelets rolled and arrested on the surface of VWF, but only displayed P-selectin if prevailing shear rates were pathological. Platelet arrest on VWF fibers was blocked with αIIbβ3 antagonist GR144053.
Conclusions
We report VWF fiber-contact pathway crosstalk and mechanisms of thrombolytic resistance in hemodynamic settings of myocardial infarction.
Keywords: Blood Platelets, Coronary Stenosis, Thrombolytic Therapy, Tissue Plasminogen Activator, von Willebrand Factor
Introduction
Severe coronary stenosis can cause extremely high shear rates, boundary layer detachment, and flow recirculation zones downstream of the stenosis [1]. Only when the stenosis exceeds ~75% occlusion does the flow resistance cause symptoms of angina [2]. Stenotic coronary flows can even create rare instances of fluid mechanical turbulence, especially during the deceleration phase of diastole [3]. At physiological arterial shear rates, von Willebrand Factor (VWF) is essential for platelet capture via glycoprotein Ibα (GPIbα) binding followed by adhesion stabilization via αIIbβ3 binding to VWF [4]. However, in pathological flow conditions with shear rates exceeding 5000 s−1, VWF undergoes a complex coil-stretch transition to an extended conformation capable of association [5]. This complex aggregation process occurs both axially and laterally to form thick and long fiber bundles of greater than 100 µm in length [5]. The largest soluble VWF species above ~ 5 × 106 MW display remarkable platelet adhesivity [6,7] and may incorporate preferentially into VWF fibers. Additionally, extreme shearing flows in certain left ventricular assist devices cause an acquired von Willebrand’s Disease with associated bleeding risks [8].
Distinct from unprocessed VWF fibers secreted acutely from endothelium [9–11], intrathrombic VWF fibers deposited during arterial thrombosis are likely derived from ADAMTS13-processed plasma [12]. Microfluidic devices have enabled studies of plasma-derived VWF unfolding and fiber formation on collagen at extreme shear conditions using small volumes of blood or plasma. Colace et al. [13] utilized a stenosis shaped microfluidic device to show VWF fiber formation and embolic VWF-platelet aggregates on collagen mediated through GPIbα and αIIbβ3. Kragh et al. [14] also demonstrated self-assembly of VWF fibers on a collagen or VWF surface capable of rolling platelet aggregate formation under high shear rates. However, collagen in these VWF studies can have strong confounding effects on platelet function via activation of platelet GPVI. Other studies have coated the surfaces of glass slides [11] or microspheres [15] with plasma-derived VWF to explore how platelet thrombi formed on VWF-coated surfaces in response to shear. However, surface sorbed VWF from solution [7] lacks the structural and biomechanical attributes of VWF fibers formed from plasma at pathological shear.
A stenotic microfluidic channel was designed with a micropost positioned in the middle of flow field to capture soluble VWF multimers as they experienced extreme shear rates within the device. Using a microfluidic design capable of generating fibrous VWF from plasma in the absence of collagen or endothelium, the unique mechanical and biological attributes of VWF fibers were investigated. We report the first determination of the VWF fiber elastic modulus, the role of VWF fibers in promoting contact pathway function, and the thrombolytic resistance of VWF fibers to tissue plasminogen activator (tPA).
Materials and Methods
Blood Collection and Preparation
Whole blood was drawn from healthy volunteers who self-reported as free of any bleeding disorders or disease, as well as free of oral medication for at least 10 days. All blood was collected in accordance with the University of Pennsylvania’s Internal Review Board. Depending on the experiment, blood was anticoagulated with 5 mM EDTA (Sigma, St. Louis, MO) to chelate calcium, 100 µM D-Phe-Pro-Arg chloromethylketone (PPACK, Haematologic Industries, Essex, VT) to inhibit thrombin and FXIIa, 1 µM apixaban to inhibit Factor Xa, and/or corn trypsin inhibitor (CTI, 4 µg/mL or 40 µg/mL) to inhibit Factor βXIIa (see supplemental Fig. S1 on CTI use). GR144053 (2 µM) was used in some experiments to inhibit αIIbβ3. Platelet-free plasma (PFP) was generated by centrifugation of whole blood (300g, 10 min) to create platelet-rich plasma (PRP), followed by PRP centrifugation (10,000g, 5 min) to create platelet-poor plasma (PPP), followed by PPP centrifugation (10,000g, 5 min). Trypsin (Life Technologies, Grand Island, NY), ADAMTS13 (R&D Systems, Minneapolis, MN), plasmin (Haematologic Technologies, Inc., Essex Junction, VT), and tPA (Abcam), and fluorogenic substrates FRETS-VWF73 (Anaspec, Fremont, CA) and Boc-VPR-MCA (R&D Systems) were also used. Anti-CD41 and anti-CD62P (BD Biosciences, San Diego, CA) are both PE labeled. Anti-Factor alpha XIIa antibody (Abcam) was labeled using an Alexa Fluor® 647 Protein Labeling Kit (Life Technologies). FXII-deficient plasma (Haematologic Technologies, Inc.) was manufactured from normal citrated human plasma that was FXII immunodepleted to <1% activity. The antibody 14E11 was a kind gift from Dr. Andras Gruber (Oregon Health and Science University, Portland, OR).
Device Design and Fabrication
Microfluidic devices were fabricated out of polydimethylsiloxane (PDMS) (Ellsworth Adhesives) using previously described soft lithography techniques [16–18]. The device design consisted of a 500 µm channel that rapidly narrowed to a 60 µm wide stenosis region, which extended for 1000 µm before expanding again to a 500 µm wide channel. The channel height throughout the device was 60 µm. In the middle of the stenosis region, a 30-µm square micropost extended across the entire height of the channel, providing a physical location for VWF fiber capture and growth without the use of collagen.
Computational Fluid Dynamics (CFD)
Finite element simulations were conducted using COMSOL Multiphysics (Burlington, MA) to predict the plasma or blood flow profile (1.38 cP or 3.39 cP viscosity, respectively [19]) around the post, with and without a VWF deposit at steady flowrates of 1 to 20 µL/min. CFD simulation provided wall shear rates at regions of interest: (1) average centerline wall shear rate in the stenosis region upstream of the post, which is a metric of the shear force on VWF in solution to extend into its active form before reaching the post, and (2) wall shear rate on the micropost, which is a metric of the extensional force on fibers that multimerize with other VWF already tethered on the post.
Microfluidic Flow Experiments
Devices were sealed to Sigmacote® (Sigma-Aldrich)-treated glass slides. The flow channels were blocked with 5% bovine serum albumin (BSA, Sigma-Aldrich) for 30 min before sample perfusion. Whole blood or PFP (under various specified anticoagulation conditions) were perfused through the device using a syringe pump (Harvard Apparatus, Holliston, MA). The microfluidic device was mounted on an Olympus IX81 inverted microscope equipped with a charge-coupled camera (Hamamatsu, Bridgewater, NJ).
Results
Pathological shear rates generate fibrous VWF from plasma on a capturing micropost
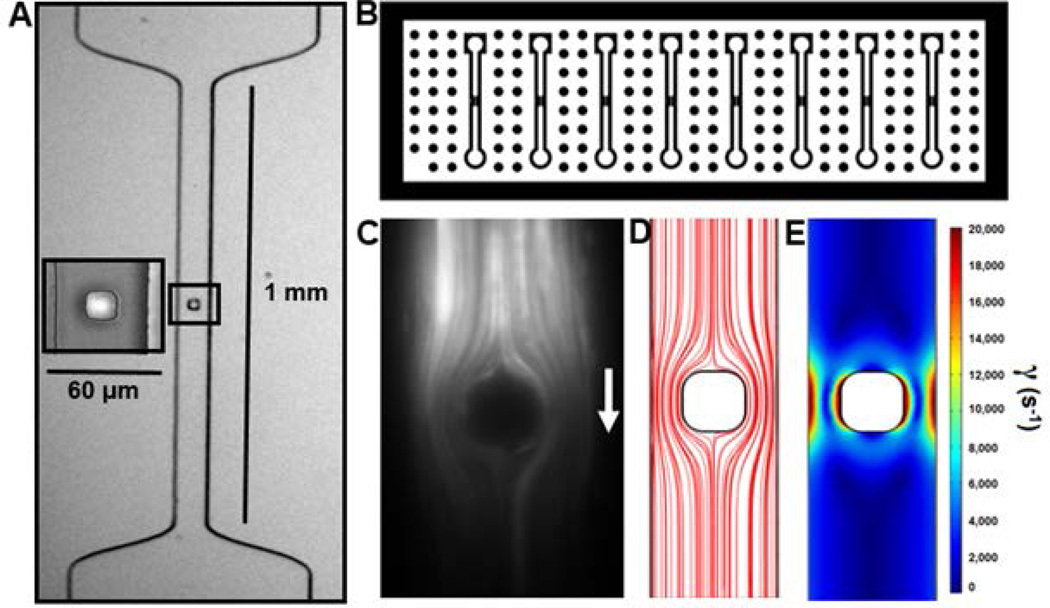
A PDMS microfluidic device with 8 individual lanes was vacuum-sealed to glass where each lane converged stenotically to a 60 µm wide×60 µm high flow path that then impinged on a 30-µm square micropost (Fig. 1A–B). Fluid streamlines were visualized with 10 µm fluorescent beads (5 µL/min) revealing the computationally-expected flow stagnation points on the front and back of the micropost (Fig. 1C–D). For a flow rate of 5 µL/min, wall shear rate increased from 0 at the front centerline stagnation point to >10,000 s−1 at the front corners of the micropost, resulting in a wall shear rate gradient of ~3.33 × 106 s−1/cm. The peak wall shear rates along the side walls of the micropost were calculated at 20,000 s−1 for a flow rate of 5 µL/min (Fig. 1E). As plasma impinges and flows around the post, a rapidly increasing extensional force is expected to help to orient fibers to maximize their contact with the post, increasing the probability of a VWF fiber sticking. Perfusion of EDTA-PFP resulted in VWF fiber formation at 5000, 8000, and 10000 s−1 upstream centerline wall shear rate (Fig. 2A). The thickness of the fibers at 5 min increased 5-fold (p<0.01) from 2.5 µm to 12.5 µm as the wall shear rate increased from 5000 to 10000 s−1 (Fig. 2B). Flow-aligned VWF fibers were stretched in the direction of flow as they formed since an acute reduction in flow triggered an observable retraction (not shown). To examine the elastic properties of VWF, a small post-attached single VWF fiber was formed at 10000 s−1 (13.7 µL/min) and then interrogated mechanically by alterations in the prevailing flow rate. Once the small fiber formed from plasma, the flow was switched to HBS and reduced to 4 µL/min and HBS was perfused over the tethered VWF fiber. The flow rate was increased stepwise from 4 to 13 µL/min, allowing the fiber to equilibrate at each flow rate before measuring its length (Fig. 2C). The wall shear stress and total force on the surface of the fiber as it was stretched was estimated with an axial annular flow approximation over a cylindrical fiber. Assuming the volume of the fiber was conserved over the experiment, the length of the fiber after each stretch was used to determine its new diameter. By integrating the drag force per length over the entire length of the fiber, the total stress on the fiber was calculated, assuming the initial 4 µL/min fiber as “relaxed” for the purpose of comparison to the “stretched” forms of the fiber under higher flow rates. Through this estimation, the calculated elastic modulus of fibrous plasma-derived human VWF was ~50 MPa (Fig. 2D), quite similar to the fibrin fiber elastic modulus in FXIIIa-crosslinked plasma clots [20].
Figure 1. Microfluidic device design and characterization.
(A) Stenosis-shaped channel with a small post located in the middle of flow. (B) Macroscopic device design allows for multiple channel VWF fiber generation. (C) Fluorescent beads were perfused past the post and imaged with a 25 ms exposure to visualize streamlines within the device. (D) COMSOL-simulated streamlines depicting diverging flow upstream of the post. (E) Wall shear rate heat map flow for a flow rate of 5 µL/min.
Figure 2. VWF fiber characteristics and mechanical properties.
(A) Representative immunofluorescent images of VWF fibers that were generated by flowing EDTA PFP at 5000, 8000, and 10000 s−1 upstream wall shear rate for 5 min from initial fiber capture. (B) Average fiber width was measured at 30 µm below the post at each shear rate. (C) A single VWF fiber was captured on the post by flowing EDTA PFP at 5000 s−1 for 30 s from initial fiber capture. The fluid was then switched to HBS to prevent additional VWF fiber growth and the flow was reduced to 4 µL/min. The flow rate was increased stepwise and the fiber was allowed to equilibrate between steps. (D) Stress-strain plot for the single VWF fiber in C. Stress was calculated by assuming axial annular flow around the fiber to calculate the drag force on the fiber. Strain was calculated assuming the 4 µL/min case was “at rest” and each subsequent flow rate as stretched forms at constant fiber volume.
Fibrous VWF is non-amyloid, tPA-resistant, but plasmin-sensitive
In order to further characterize the VWF fibers produced by micropost capture, various proteases/surfactants were assessed. Both 0.25 % trypsin and 2% SDS quickly dissolved fibrous VWF (Fig. 3A–B), demonstrating that insoluble VWF fibers were non-amyloid. In contrast, 30 mM N-acetylcysteine was not able to dissolve already formed VWF fibers (not shown). However, when ADAMTS13 (50 nM) was added to plasma and perfused over VWF fibers, no observable degradation took place at 10000 s−1 within 480 s (Fig. 3C). ADAMTS13 activity was confirmed with the fluorogenic substrate FRETS-VWF73 (Fig. S2). VWF fibers (formed from plasma treated with apixaban and PPACK to prevent any fibrin incorporation) were resistant to 100 nM tPA in apixaban/PPACK-treated plasma (Fig. 3D) demonstrating that VWF does not serve as a cofactor to enhance tPA activity toward plasminogen. However, 1 µM plasmin readily dissolved fibrous VWF fibers formed from EDTA-treated PFP in 40 s (Fig. 3E), a result consistent with that observed for trypsin. Both plasmin and trypsin are fairly non-specific peptidase family S1 (chymotrypsin family, clan PA) members [21]. Also, soluble VWF is a known substrate for plasmin [22].
Figure 3. Sensitivity of VWF fibers to various proteases and surfactants.
(A) VWF fibers were generated using EDTA inhibited PFP at 10000 s−1 for 5 min from initial fiber capture. VWF was then labeled using polyclonal fluorescent anti-VWF antibody in HBS, and then washed with HBS for 2 min to reduce background. When 0.25% trypsin solution was perfused over the VWF at 10000 s−1, the fibers were digested in 10 s. (B) When 2% SDS solution was perfused over the VWF at 10000 s−1, the fibers were dissolved in 80 s. (C) VWF fibers were generated using apixaban and PPACK inhibited PFP at 10000 s−1 for 5 min from initial fiber capture. VWF was then labeled using polyclonal fluorescent anti-VWF antibody in HBS, and then washed with HBS for 2 min to reduce background. The shear rate was reduced to 1500 s−1 and apixaban and PPACK inhibited PFP with 50 nM ADAMTS13 was perfused over the fibrous VWF for 480 s. (D) VWF fibers were generated in the same way as C. The shear rate was reduced to 1500 s−1 and apixaban and PPACK inhibited PFP with 100 nM tPA was perfused over the fibrous VWF for 300 s. (E) VWF fibers were generated in the same way as A. When 1 µM plasmin in HBS was perfused over the VWF fibers at 1500 s−1, the fibers were digested in 40 s.
Fibrous VWF accumulates procoagulant factors to drive fibrin formation
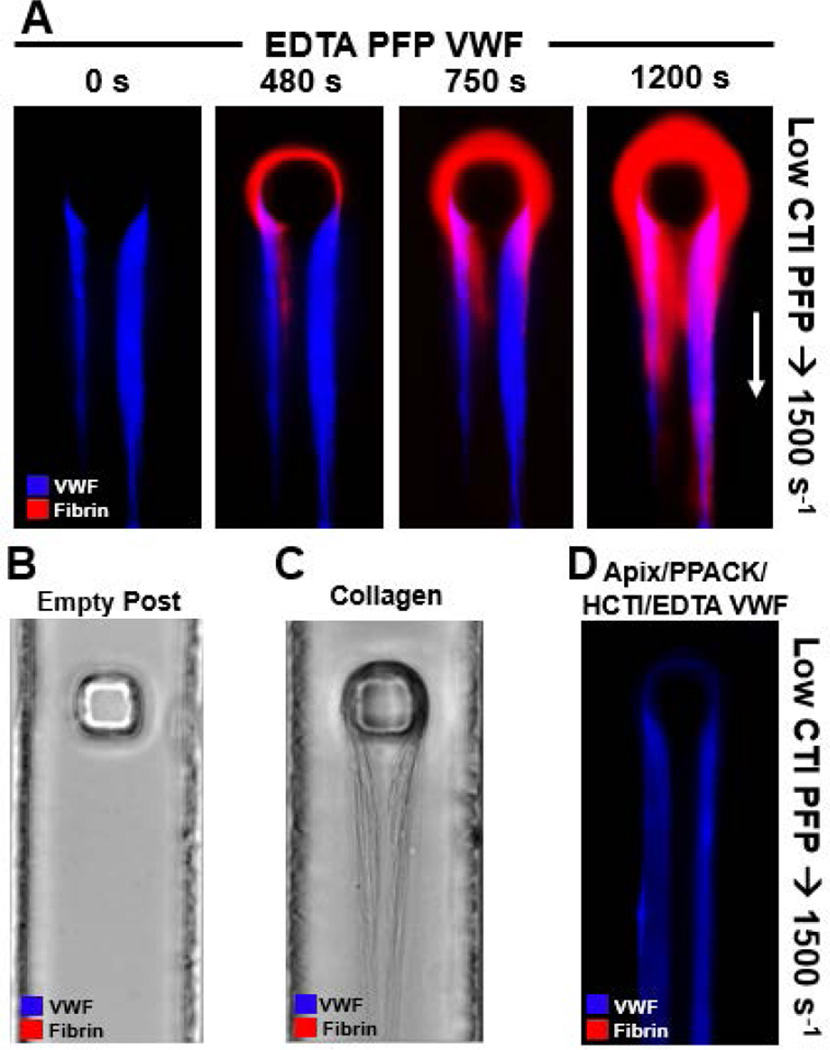
To analyze the role of VWF in the function of the contact pathway, the polymerization of fibrin was monitored on the surface of VWF fibers created under different conditions. To isolate the role of VWF from the effect of platelets, platelet-free plasma (PFP) was used for both fibrous VWF formation and the subsequent coagulating flow over the VWF. When Factor XIIa was only weakly inhibited with 4 µg/mL CTI in recalcified citrated PFP that was perfused over VWF fibers previously made in the presence of EDTA, fibrin began polymerizing on the surface of the VWF fibers within 7 min at an upstream wall shear rate of 1500 s−1 (Fig. 4A).
Figure 4. Fibrous VWF provides a surface for coagulation via contact pathway.
(A) VWF fibers were generated using EDTA PFP at 10000 s−1 for 5 min from initial fiber capture. VWF was then labeled using polyclonal fluorescent anti-VWF antibody in HBS, and then washed with HBS for 2 min to reduce background. The shear rate was reduced to 1500 s−1 and recalcified low CTI (4 µg/mL, LCTI) citrated PFP labeled with fluorescent anti-fibrin antibody was perfused over the fibrous VWF for 20 min. (B) No VWF fibers were generated on the post before perfusion of recalcified LCTI citrated PFP labeled with fluorescent anti-fibrin antibody at 1500 s−1 for 20 min. No fibrin formed on the post. (C) 10 µg/mL collagen type 1 solution in HBS was perfused over the post for at 10000 s−1 until the collagen fibers were approximately the same size as the previous VWF fibers. Recalcified LCTI citrated PFP labeled with fluorescent anti-fibrin antibody was then perfused over the collagen fibers at 1500 s−1 for 20 min. No fibrin formed on the collagen or post. (D) VWF fibers were generated using 40 µg/mL CTI, 1 µM apixaban, 100 µM PPACK, and 5 mM EDTA inhibited PFP at 10000 s−1 for 5 min from initial fiber capture. VWF was then labeled using polyclonal fluorescent anti-VWF antibody in HBS, and then washed with HBS for 2 min to reduce background. The shear rate was reduced to 1500 s−1 and recalcified LCTI citrated PFP labeled with fluorescent anti-fibrin antibody was perfused over the fibrous VWF for 20 min. No fibrin formed on the inhibited VWF fibers.
However, it was unclear whether: (case 1) the VWF fibers themselves were initiating the contact pathway as a surface activator of Factor XII, (case 2) the VWF fibers had accumulated contact pathway factors as they formed (EDTA does not inhibit FXIIa generation or thrombin activity), or (case 3) fibrin fibers were forming upstream in the device in low CTI plasma and were simply accumulating on the VWF. Surface-adsorbed VWF did not initiate significant contact activation in a well plate thrombin assay (Fig. S3). When the low CTI-plasma perfusion experiment was repeated with an empty post, no fibrin was formed or captured (Fig. 4B). Similarly, when repeating the experiment with micropost-captured fibrillar collagen in place of the VWF fibers, no fibrin formed on or was captured by the collagen fibers (equine fibrillary collagen type 1, Chronopar, Chronolog) (Fig. 4C). The shear rate of 1500 s−1 was too low for the plasma VWF to form massive VWF fibers on the collagen. These two results demonstrated that fibrin polymerization occurred on the VWF fibers in Fig. 4A conditions and was not simply forming upstream and capturing on the post or fibers (case 3). To distinguish case 1 (FXIIa generation) from case 2 (contact factor accumulation) the previous experiment was repeated with VWF fibers generated from fully inhibited plasma (apixaban/PPACK/high-CTI/EDTA). By inhibiting these active factors during VWF fiber multimerization, FXIIa activation and subsequent thrombin and fibrin generation would need to initiate on that VWF surface during perfusion of low CTI plasma. However, no fibrin formed on the fully inhibited fibers (Fig. 4D), eliminating case 1 and demonstrating case 2 where active coagulation factors in low CTI plasma were concentrated in the VWF fiber as it formed. No extrinsic pathway participation of tissue factor or FVIIa function on platelets can occur in these conditions [23].
To further investigate the possibility of upstream fibrin formation, we measured thrombin activity in calcium-containing plasma formed in the well prior to entrance into the microfluidic channel. Using the fluorogenic thrombin substrate boc-VPR-MCA, CTI-treated recalcified citrated plasma did not generate any detectable thrombin in 20 min (Fig. S4). With no thrombin present in the upstream reservoir under the conditions of the experiment, fibrin would not be able to polymerize upstream. We conclude that fibrin was generated on the VWF itself from local thrombin within the VWF, rather than deposition of fibrin formed upstream.
Additionally, VWF fibers were formed at pathological shear from EDTA-treated normal plasma or FXII-deficient plasma and then immunostained with fluorescent anti-FXII(a) in HBS. FXII(a) immunofluorescence was detected on VWF fibers from EDTA-plasma, but not on FXII-deficient plasma (Fig. S5). When FXIa generation was inhibited by 14E11 antibody (an antibody that binds FXI and prevents its activation by FXIIa) in the plasma used to generate VWF, no fibrin formed subsequently on the deposited VWF fibers when recalcified plasma was perfused (Fig. S6). This experiment demonstrated that FXIa (and its co-localized activator, FXIIa as seen in Fig. S5) were localized on the VWF during fiber formation under pathological flow.
A “bystander effect” was observed when fibrin was allowed to form on the VWF before perfusion of tPA. Though tPA was not able to convert plasminogen to plasmin on the surface of VWF fibers, plasmin generated by tPA on the surface of fibrin (co-localized with the VWF fiber) was able to cleave nearby VWF (Fig. S7).
Platelets roll and adhere on VWF fibers but pathological shear drives P-selectin display
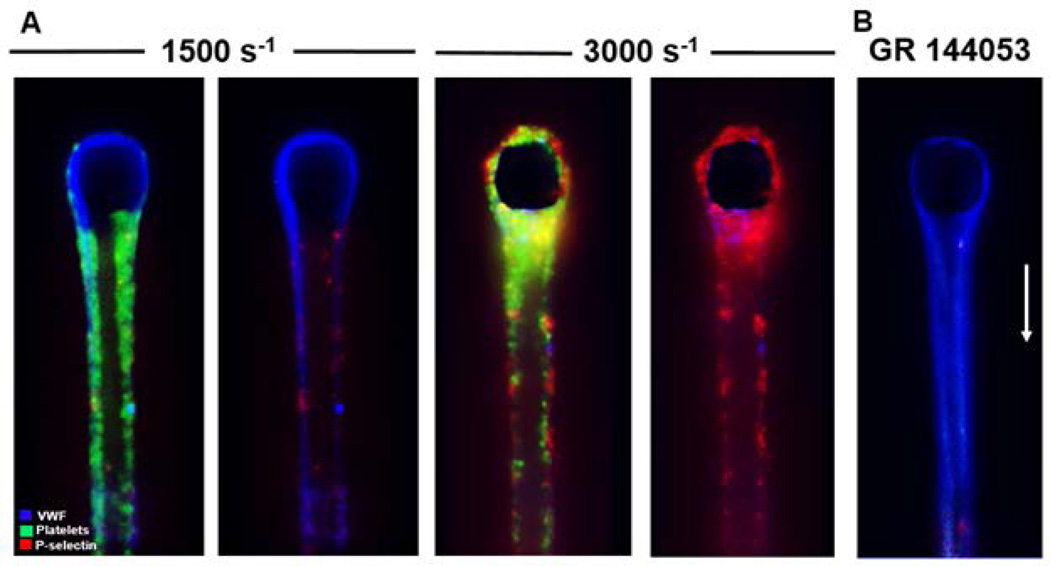
Whole blood anticoagulated with PPACK/apixaban and treated with fluorescent anti-CD41 and anti-CD62P was perfused over fibrous VWF (formed from PPACK/apixaban-treated plasma) at 1500 and 3000 s−1. Platelets attached, rolled, and firmly bound to the fibrous VWF at both shear rates (Fig. 5), demonstrating the previously observed behaviors seen on endothelial anchored VWF [24], immobilized plasma VWF [25,26] and fibrous VWF on collagen [13]. Additionally, platelets arrested on the fibrous VWF at either shear rate, but only displayed P-selectin at 3000 s−1 (Fig. 5A), consistent with a role for fibrous VWF supporting shear-induced platelet activation (SIPA). The wall shear rates seen on the surface of fibers (~5000 s−1) were obtained with an upstream approach wall shear rate of 1500 s−1 (Fig. S8). GR144053 at 2 µM to inhibit αIIbβ3 prevented platelet arrest on the VWF fibers (Fig. 5B). Therefore, fibrous VWF was a platelet activating surface under physiological arterial shear rates and a cofactor for SIPA at pathological shear rates to induce P-selectin display.
Figure 5. Platelets adhere to and activate on fibrous VWF.
(A) VWF fibers were generated using apixaban and PPACK inhibited PFP at 10000 s−1 for 5 min from initial fiber capture. VWF was then labeled using polyclonal fluorescent anti-VWF antibody in HBS, and then washed with HBS for 2 min to reduce background. The shear rate was reduced to either 1500 s−1 or 3000 s−1 and apixaban and PPACK inhibited whole blood labeled with fluorescent anti-CD41 (platelets) and fluorescent anti-CD62P (P-selectin) was perfused over the fibrous VWF for 10 min. The left and right images for each shear rate are included for clarity to highlight the activated platelets on the surface of VWF. (B) VWF fibers were generated and labeled using the same procedure as A. The addition of GR 144053 to the whole blood described in A resulted in total inhibition of firm platelet adhesion to the VWF fibers.
Discussion
By designing a microfluidic device capable of capturing multimerized VWF fibers in pathological shear flow, we were able to investigate mechanical, biochemical, and biological properties of fibrous VWF. The lack of collagen in the VWF capture method of this device allows the role of VWF to be examined without collagen present, and may be more representative of the VWF-rich regions of thrombotic clots seen in the work of Le Behot et al. [12], where VWF incorporated into the top region of nearly occlusive thrombi and was not directly bound to subsurface collagen [12]. The determination of the VWF fiber elastic modulus is potentially quite useful for whole clot mechanical modeling, particularly in studying thromboembolism [27,28]. Another advantage to this approach is that the VWF fibers are formed under single-pass shear flow, which is a more physiologically relevant condition than continuous exposure in a cone and plate viscometer or vortexing assays [29,30]. VWF aggregates produced in a cone and plate viscometer have previously been shown to partially dissociate after treatment by SDS [31], but we have shown that fibrous VWF made under elongational flow also dissociates in response to SDS. This single-pass flow approach demonstrates that the sensitivity of VWF fibers to trypsin, SDS, and plasmin does not require long incubation times to achieve substantial degradation of the fibers.
The focus of this study is on human plasma VWF, which unlike VWF secreted by endothelial cells, is made up of subunit fragments resulting from processing of endothelial VWF by ADAMTS13 [32,33]. For endothelial released ultra-large VWF, cleavage by ADAMTS13 regulates the release and size of VWF multimers by cleaving the A2 domain of VWF to release it into the blood stream [33]. As a result, the fibers observed in this study, and potentially those in the high shear exposed regions of large thrombi, would potentially have resistance to ADAMTS13 due to a lower fraction of structurally exposed A2 domains for cleavage of large VWF fibers. The ADAMTS13 sensitivity of VWF fibers in the absence of platelets has not been previously studied [34]. Although soluble VWF multimers have been shown to reduce in size in the presence of ADAMTS13 and shear [35], the ability of ADAMTS13 to cleave insoluble VWF fibers was observed in this assay to be undetectable. The effect of calcium, and therefore endogenous calcium-dependent ADAMTS13 activity, on the formation size of VWF fibers was found to be insignificant (Fig. S9), consistent with the fact that the plasma VWF multimers incorporated into the fiber at pathological shear rate were already processed by ADAMTS13. Distinct from observations of acutely released ULVWF from stimulated endothelium, the massive VWF fibers formed at high shear from processed plasma VWF were insensitive to ADAMTS13.
In typical thrombolytic therapy, tPA is used to dissolve fibrin rich thrombi by converting plasminogen into plasmin on the surface of fibrin, which then degrades fibrin [36]. In occlusive thrombi, degradation of clot-integrated VWF by plasmin could synergize with the cleavage of fibrin to contribute to overall thrombolysis. Both trypsin and plasmin can reduce the average multimer size of purified soluble VWF [22]. We show these activities are maintained on the insoluble fibrous form of VWF generated from plasma. However, fibrous VWF was not a cofactor for tPA. The resolution of VWF in an arterial occlusion during thrombolytic therapy with tPA would require the transfer of plasmin from degrading fibrin to the fibrous VWF without inhibition by soluble or fibrin-linked antiplasmin.
Coagulation on the surface of fibrous VWF could play an important role in propagating clot growth to occlusion in severe thrombosis. The role of the contact pathway in arterial thrombosis has received considerable attention recently [37–39]. Because collagen and apixaban/PPACK/high-CTI/EDTA inhibited VWF fibers do not lead to fibrin formation, we conclude that active FXIIa or FXIa accumulated on VWF fibers while they formed. Endothelial cell-anchored ultra-large VWF has been shown to cause localization of other blood proteins on VWF [40]. Coagulation factor accumulation could potentially lead to a second wave of coagulation at the top region of a thrombus, leading to vessel occlusion.
Though it is well established that platelets bind to and roll along endothelial bound multimeric VWF [24], it had not previously been observed on multimeric plasma VWF in the absence of collagen as a confounding platelet stimulus [13]. Rolling adhesion occurs through GPIbα, and firm adhesion occurs through αIIbβ3, but platelet activation that occurs when bound to VWF is thought to do so through GPIbα caused by GPIbα clustering on the surface of platelets [41]. The resulting proximity to the VWF fibers allows the slower but long-lived αIIbβ3 bond to take stably take hold of the platelet. Outside-in signaling via GPIb or αIIbβ3 could also contribute to platelet activation through a mechano-sensitive mechanism when bound to VWF [42]. Only if prevailing shear rates are pathological can the shear forces on the platelet drive P-selectin exposure (Fig. 5). In summary, we report that fibrous VWF formed under conditions of pathological stenotic flow are non-amyloid, plasmin sensitive but tPA-resistant, procoagulant through accumulation of contact pathway factors, and substrates for SIPA at pathological shear rates.
Supplementary Material
Acknowledgments
This work was supported by NIH predoctoral training grant 5T32HL007954-15 (B.A.H.) and NIH R01 HL103419 (S.L.D.).
Footnotes
Addendum
B. A. Herbig designed and performed the experiments, analyzed data, and contributed to the manuscript. S. L. Diamond designed the research, analyzed data, and contributed to the manuscript.
Disclosure of Conflicts of Interests
The authors have no conflicts of interest to declare.
References
- 1.Javadzadegan A, Yong ASC, Chang M, Ng ACC, Yiannikas J, Ng MKC, Behnia M, Kritharides L. Flow recirculation zone length and shear rate are differentially affected by stenosis severity in human coronary arteries. Am J Physiol Heart Circ Physiol. 2013;304:H559–H566. doi: 10.1152/ajpheart.00428.2012. [DOI] [PubMed] [Google Scholar]
- 2.Kim HJ, Vignon-Clementel IE, Coogan JS, Figueroa CA, Jansen KE, Taylor CA. Patient-specific modeling of blood flow and pressure in human coronary arteries. Ann Biomed Eng. 2010;38:3195–3209. doi: 10.1007/s10439-010-0083-6. [DOI] [PubMed] [Google Scholar]
- 3.Long Q, Xu X, Ramnarine K, Hoskins P. Numerical investigation of physiologically realistic pulsatile flow through arterial stenosis. J Biomech. 2001;34:1229–1242. doi: 10.1016/s0021-9290(01)00100-2. [DOI] [PubMed] [Google Scholar]
- 4.Savage B, Saldívar E, Ruggeri ZM. Initiation of Platelet Adhesion by Arrest onto Fibrinogen or Translocation on von Willebrand Factor. Cell. 1996;84:289–297. doi: 10.1016/s0092-8674(00)80983-6. [DOI] [PubMed] [Google Scholar]
- 5.Schneider SW, Nuschele S, Wixforth A, Gorzelanny C, Alexander-Katz A, Netz RR, Schneider MF. Shear-induced unfolding triggers adhesion of von Willebrand factor fibers. Proc Natl Acad Sci U S A. 2007;104:7899–7903. doi: 10.1073/pnas.0608422104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stockschlaeder M, Schneppenheim R, Budde U. Update on von Willebrand factor multimers: focus on high-molecular-weight multimers and their role in hemostasis. Blood Coagul Fibrinolysis. 2014;25:206–216. doi: 10.1097/MBC.0000000000000065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Federici AB, Bader R, Pagani S, Colibretti ML, Marco L, Mannucci PM. Binding of von Willebrand factor to glycoproteins Ib and IIb/IIIa complex: affinity is related to multimeric size. Br J Haematol. 1989;73:93–99. doi: 10.1111/j.1365-2141.1989.tb00226.x. [DOI] [PubMed] [Google Scholar]
- 8.Crow S, Chen D, Milano C, Thomas W, Joyce L, Piacentino V, Sharma R, Wu J, Arepally G, Bowles D, Rogers J, Villamizar-Ortiz N. Acquired von Willebrand syndrome in continuous-flow ventricular assist device recipients. Ann Thorac Surg. 2010;90:1263–1269. doi: 10.1016/j.athoracsur.2010.04.099. discussion 1269. [DOI] [PubMed] [Google Scholar]
- 9.Moake JL, Turner NA, Stathopoulos NA, Nolasco LH, Hellums JD. Involvement of large plasma von Willebrand factor (vWF) multimers and unusually large vWF forms derived from endothelial cells in shear stress-induced platelet aggregation. J Clin Invest. 1986;78:1456–1461. doi: 10.1172/JCI112736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong J, Moake JL, Nolasco L, Bernardo A, Arceneaux W, Shrimpton CN, Schade AJ, McIntire LV, Fujikawa K, López JA. ADAMTS-13 rapidly cleaves newly secreted ultralarge von Willebrand factor multimers on the endothelial surface under flowing conditions. Blood. 2002;100:4033–4039. doi: 10.1182/blood-2002-05-1401. [DOI] [PubMed] [Google Scholar]
- 11.Westein E, van der Meer AD, Kuijpers MJE, Frimat J-P, van den Berg A, Heemskerk JWM. Atherosclerotic geometries exacerbate pathological thrombus formation poststenosis in a von Willebrand factor-dependent manner. Proc Natl Acad Sci U S A. 2013;110:1357–1362. doi: 10.1073/pnas.1209905110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le Behot A, Gauberti M, De Lizarrondo SM, Montagne A, Lemarchand E, Repesse Y, Guillou S, Denis CV, Maubert E, Orset C, Vivien D. GpIbα-VWF blockade restores vessel patency by dissolving platelet aggregates formed under very high shear rate in mice. Blood. 2014;123:3354–3363. doi: 10.1182/blood-2013-12-543074. [DOI] [PubMed] [Google Scholar]
- 13.Colace TV, Diamond SL. Direct observation of von Willebrand factor elongation and fiber formation on collagen during acute whole blood exposure to pathological flow. Arterioscler Thromb Vasc Biol. 2013;33:105–113. doi: 10.1161/ATVBAHA.112.300522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kragh T, Napoleone M, Fallah MA, Gritsch H, Schneider MF, Reininger AJ. High shear dependent von Willebrand factor self-assembly fostered by platelet interaction and controlled by ADAMTS13. Thromb Res. 2014;133:1079–1087. doi: 10.1016/j.thromres.2014.03.024. [DOI] [PubMed] [Google Scholar]
- 15.Nesbitt WS, Westein E, Tovar-Lopez FJ, Tolouei E, Mitchell A, Fu J, Carberry J, Fouras A, Jackson SP. A shear gradient-dependent platelet aggregation mechanism drives thrombus formation. Nat Med. 2009;15:665–673. doi: 10.1038/nm.1955. [DOI] [PubMed] [Google Scholar]
- 16.Duffy DC, McDonald JC, Schueller OJ, Whitesides GM. Rapid Prototyping of Microfluidic Systems in Poly(dimethylsiloxane) Anal Chem. 1998;70:4974–4984. doi: 10.1021/ac980656z. [DOI] [PubMed] [Google Scholar]
- 17.Colace TV, Muthard RW, Diamond SL. Thrombus growth and embolism on tissue factor-bearing collagen surfaces under flow: role of thrombin with and without fibrin. Arterioscler Thromb Vasc Biol. 2012;32:1466–1476. doi: 10.1161/ATVBAHA.112.249789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neeves KB, Maloney SF, Fong KP, Schmaier aa, Kahn ML, Brass LF, Diamond SL. Microfluidic focal thrombosis model for measuring murine platelet deposition and stability: PAR4 signaling enhances shear-resistance of platelet aggregates. J Thromb Haemost. 2008;6:2193–2201. doi: 10.1111/j.1538-7836.2008.03188.x. [DOI] [PubMed] [Google Scholar]
- 19.Rosenson RS, McCormick A, Uretz EF. Distribution of Blood Viscosity Values and Biochemical Correlates in Healthy Adults. Clin Chem. 1996;42:1189–1195. [PubMed] [Google Scholar]
- 20.Collet J-P, Shuman H, Ledger RE, Lee S, Weisel JW. The elasticity of an individual fibrin fiber in a clot. Proc Natl Acad Sci U S A. 2005;102:9133–9137. doi: 10.1073/pnas.0504120102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rawlings ND, Waller M, Barrett AJ, Bateman A. MEROPS: the database of proteolytic enzymes, their substrates and inhibitors. Nucleic Acids Res. 2014:D503–D509. doi: 10.1093/nar/gkt953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamilton KK, Fretto LJ, Grierson DS, McKee PA. Effects of plasmin on von Willebrand factor multimers. Degradation in vitro and stimulation of release in vivo. J Clin Invest. 1985;76:261–270. doi: 10.1172/JCI111956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bouchard BA, Gissel MT, Whelihan MF, Mann KG, Butenas S. Platelets do not express the oxidized or reduced forms of tissue factor. Biochim Biophys Acta. 2014;1840:1188–1193. doi: 10.1016/j.bbagen.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bernardo A, Ball C, Nolasco L, Choi H, Moake JL, Dong JF. Platelets adhered to endothelial cell-bound ultra-large von Willebrand factor strings support leukocyte tethering and rolling under high shear stress. J Thromb Haemost. 2005;3:562–570. doi: 10.1111/j.1538-7836.2005.01122.x. [DOI] [PubMed] [Google Scholar]
- 25.Fredrickson BJ, Dong JF, McIntire LV, López JA. Shear-dependent rolling on von Willebrand factor of mammalian cells expressing the platelet glycoprotein Ib-IX-V complex. Blood. 1998;92:3684–3693. [PubMed] [Google Scholar]
- 26.Ruggeri ZM, Orje JN, Habermann R, Federici AB, Reininger AJ. Activation-independent platelet adhesion and aggregation under elevated shear stress. Blood. 2006;108:1903–1910. doi: 10.1182/blood-2006-04-011551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martinez MR, Cuker A, Mills AM, Crichlow A, Lightfoot RT, Chernysh IN, Nagaswami C, Weisel JW, Ischiropoulos H. Enhanced lysis and accelerated establishment of viscoelastic properties of fibrin clots are associated with pulmonary embolism. Am J Physiol Lung Cell Mol Physiol. 2014;306:L397–L404. doi: 10.1152/ajplung.00265.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skorczewski T, Erickson LC, Fogelson AL. Platelet motion near a vessel wall or thrombus surface in two-dimensional whole blood simulations. Biophys J. 2013;104:1764–1772. doi: 10.1016/j.bpj.2013.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Madabhushi SR, Shang C, Dayananda KM, Rittenhouse-Olson K, Murphy M, Ryan TE, Montgomery RR, Neelamegham S. von Willebrand factor (VWF) propeptide binding to VWF D’D3 domain attenuates platelet activation and adhesion. Blood. 2012;119:4769–4778. doi: 10.1182/blood-2011-10-387548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rauch A, Legendre P. Antibody-based prevention of von Willebrand factor degradation mediated by circulatory assist devices. Thromb Haemost. 2014:1014–1023. doi: 10.1160/TH14-02-0148. [DOI] [PubMed] [Google Scholar]
- 31.Shankaran H, Alexandridis P, Neelamegham S. Aspects of hydrodynamic shear regulating shear-induced platelet activation and self-association of von Willebrand factor in suspension. Blood. 2003;101:2637–2645. doi: 10.1182/blood-2002-05-1550. [DOI] [PubMed] [Google Scholar]
- 32.Zimmerman TS, Dent JA, Ruggeri ZM, Nannini LH. Subunit composition of plasma von Willebrand factor. Cleavage is present in normal individuals, increased in IIA and IIB von Willebrand disease, but minimal in variants with aberrant structure of individual oligomers (types IIC,IID, and IIE) J Clin Invest. 1986;77:947–951. doi: 10.1172/JCI112394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zanardelli S, Crawley JTB, Chion CKNCK, Lam JK, Preston RJS, Lane DA. ADAMTS13 substrate recognition of von Willebrand factor A2 domain. J Biol Chem. 2006;281:1555–1563. doi: 10.1074/jbc.M508316200. [DOI] [PubMed] [Google Scholar]
- 34.Donadelli R, Orje JN, Capoferri C, Remuzzi G, Ruggeri ZM. Size regulation of von Willebrand factor-mediated platelet thrombi by ADAMTS13 in flowing blood. Blood. 2006;107:1943–1950. doi: 10.1182/blood-2005-07-2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shim K, Anderson PJ, Tuley EA, Wiswall E, Sadler JE. Platelet-VWF complexes are preferred substrates of ADAMTS13 under fluid shear stress. Blood. 2008;111:651–657. doi: 10.1182/blood-2007-05-093021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Medved L, Nieuwenhuizen W. Molecular mechanisms of initiation of fibrinolysis by fibrin. Thromb Haemost. 2003:409–419. [PubMed] [Google Scholar]
- 37.Gailani D, Renné T. Intrinsic pathway of coagulation and arterial thrombosis. Arterioscler Thromb Vasc Biol. 2007;27:2507–2513. doi: 10.1161/ATVBAHA.107.155952. [DOI] [PubMed] [Google Scholar]
- 38.Woodruff RS, Sullenger B, Becker RC. The many faces of the contact pathway and their role in thrombosis. J Thromb Thrombolysis. 2011;32:9–20. doi: 10.1007/s11239-011-0578-5. [DOI] [PubMed] [Google Scholar]
- 39.Kuijpers MJE, van der Meijden PEJ, Feijge MAH, Mattheij NJA, May F, Govers-Riemslag J, Meijers JCM, Heemskerk JWM, Renné T, Cosemans JMEM. Factor XII regulates the pathological process of thrombus formation on ruptured plaques. Arterioscler Thromb Vasc Biol. 2014;34:1674–1680. doi: 10.1161/ATVBAHA.114.303315. [DOI] [PubMed] [Google Scholar]
- 40.Turner Na, Moake J. Assembly and Activation of Alternative Complement Components on Endothelial Cell-Anchored Ultra-Large Von Willebrand Factor Links Complement and Hemostasis-Thrombosis. PLoS One. 2013;8 doi: 10.1371/journal.pone.0059372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kroll MH, Harris TS, Moake JL, Handin RI, Schafer AI. von Willebrand factor binding to platelet GpIb initiates signals for platelet activation. J Clin Invest. 1991;88:1568–1573. doi: 10.1172/JCI115468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang W, Deng W, Zhou L, Xu Y, Yang W, Liang X, Wang Y, Kulman JD, Zhang XF, Li R. Identification of a juxtamembrane mechanosensitive domain in the platelet mechanosensor glycoprotein Ib-IX complex. Blood. 2015;125:562–569. doi: 10.1182/blood-2014-07-589507. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.