Abstract
BACKGROUND
Thyroglobulin antibodies (TgAb) are produced by 10-25% of thyroid cancer patients and interfere with thyroglobulin (Tg) measurement, a marker of residual or recurrent cancer after surgery. Our purpose was to describe the TgAb resolution time-course and the significance of persistent antibody elevation after thyroidectomy.
METHODS
A database of 247 consecutive patients with preoperative TgAb measured who underwent thyroidectomy for differentiated thyroid cancer (DTC) between January 2007 and May 2013 was reviewed. Patients were stratified by TgAb status (positive or negative) and recurrence (defined as biopsy proven disease or unplanned second surgery). Survival and regression analysis was used to determine TgAb resolution time-course. Log-Rank was used to determine an association between persistent antibody elevation and recurrence.
RESULTS
Of 247 patients (77% women, 23% men; mean 45.7±1.0 years) with TgAb measured preoperatively, 34 (14%) were TgAb-positive (greater than or equal to 20 IU/ml) (mean 298.1±99.2 IU/ml). Median time to TgAb resolution was 11.0±2.3 months, and the majority resolved by 32.4 months. Regression analysis of patients with antibody resolution yielded an average decline of -11% IU/ml per month±2.2%. Disease free survival was equivalent between TgAb-positive and TgAb-negative groups (p=0.8). In 9 of 34 patients, antibodies had not resolved at last follow up but imaging could not identify recurrent disease.
CONCLUSIONS
TgAb are common in patients with thyroid cancer but resolve after treatment at approximately −11% IU/ml per month from preoperative levels with median resolution at 11.0 months. Persistently elevated levels after thyroidectomy were not associated with disease recurrence in our series.
Keywords: thyroglobulin antibody, thyroglobulin, thyroid cancer, recurrence, total thyroidectomy
Introduction
The thyroid produces thyroglobulin in its follicles as a precursor to the active T3 and T4 forms of thyroid hormone. Thyroglobulin is produced by thyroid tissue in both benign and malignant states. In differentiated thyroid cancer, the thyroid is usually removed but residual tissue can continue to synthesize and secrete thyroglobulin in the presence of high TSH concentrations. Thyroglobulin (Tg) levels can be used post-operatively as a tumor marker to identify residual or recurrent thyroid cancer after surgical treatment. Antibodies to thyroglobulin (TgAb), however, are produced in 10-25% of patients and can interfere with thyroglobulin measurement [1]. TgAb interfere with both immunometric (IMA) and radioimmunometric (RIA) assays. Although RIA is less susceptible to interference by TgAb and IMA usually underestimates Tg, patient characteristics can also affect Tg measurement both positively and negatively. Therefore, reliable detection of TgAb is necessary before evaluating the validity of a given Tg measurement [2].
If Tg cannot be measured as a tumor marker because of TgAb interference, then it may be possible to use TgAb as a surrogate marker of thyroglobulin levels, but that link is not well established. It is thought that TgAb are produced by lymphocytes within the thyroid, therefore if all thyroid is removed one would anticipate resolution of TgAb as well, but TgAb may persist even after total thyroidectomy. This could indicate that cervical lymph nodes possibly promulgate the response or Tg persist in antigen producing cells [2]. Other studies show that the disappearance of TgAb is related to the disappearance of auto-antigen or thyroid tissue; however, the literature is equivocal about the link between TgAb and recurrent cancer. Some cross sectional studies and longitudinal studies report a higher frequency of cancer recurrence in TgAb positive patients and inversely a lower frequency of cancer recurrence in TgAb negative patients [1,3], but other researchers did not find the same results [4-7].
To further investigate we sought first to understand how TgAb resolve and then to determine the relationship between TgAb positive patients and recurrent cancer. While the current standard of care is to measure serum Tg and TgAb postoperatively [8], preoperative data have also been collected at our institution which provide a useful baseline measurement that could be used to identify TgAb positive patients and to establish a time course for postoperative TgAb resolution [1]. The three aims of this study were to describe the time course of TgAb resolution, determine if recurrent cancer is more common in patients with positive preoperative TgAb, and determine if recurrent cancer is more common in patients with persistently elevated antibody levels.
Methods
A retrospective review of a prospectively collected thyroid database was conducted. Patients undergoing surgical treatment for differentiated thyroid cancer (DTC) between January 2007 and May 2013 were included. Only patients treated with a total thyroidectomy or completion thyroidectomy were included. Patients without preoperative TgAb measurement were excluded. In the end, 247 consecutive patients with preoperative TgAb measured who underwent surgical treatment were reviewed.
TgAb was measured using Beckman Coulter Access Dxl method and Siemens Immulite 2000. Since 2011, testing has been performed at ARUP a national reference laboratory (Salt Lake City, Utah). The currently used TgAb assay, which has only been used since 2012, uses a cutoff of < 4 IU/mL to define negative antibodies, but the version used prior to 2012 used a cutoff of <20 IU/mL. For consistency, positive TgAb status was defined as a preoperative measurement equal to or above 20 IU/mL. TgAb resolution in those patients was defined as postoperative levels less than 20 IU/mL. If TgAb resolved temporarily, recurred, then resolved again, the later date of resolution was used. Recurrent disease was defined by biopsy proven disease or unplanned second surgery. Patients who underwent completion thyroidectomies were not considered to have unplanned second surgery.
Survival and regression analysis was used to determine the time course of TgAb resolution. Log Rank (Mantel-Cox) was used to determine an association between positive preoperative TgAb levels and cancer recurrence. Fisher's Exact Test was used to determine an association between persistently elevated TgAb levels and cancer recurrence. SPSS v. 21 statistical software was used for analysis (IBM, Armonk, NY).
Results
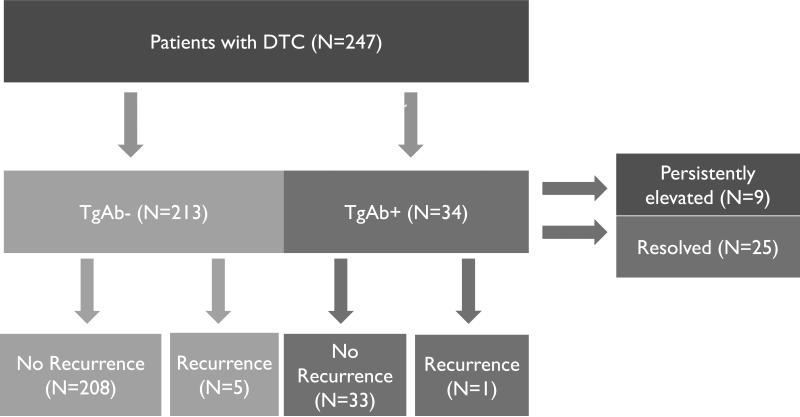
Our cohort consisted of 247 patients with TgAb measured preoperatively who underwent a total (n=188) or completion (n= 59) thyroidectomy for DTC. The mean age of the patients was 45.7 ± 1.0 years and 77% were women. Preoperative TgAb were positive (mean 298.1 ± 99.2 IU/ml) in 34 (14%) patients. The average tumor size was 1.8 ± 0.2 cm and lymph node involvement was seen in 8.9% of patients. Extra thyroidal extension was present in 5.3%. There was a significant difference in the proportion of autoimmune thyroid disease between the TgAb+ and TgAb- groups (35.3% vs 15.5% p = 0.01). Subgroup analysis also showed a significant difference in Hashimoto's thyroiditis prevalence between the two groups (29.4% vs 14.6% p = 0.05) (Table 1). The cohort was first stratified into TgAb negative (n=213) and TgAb positive (n=34) groups and then further stratified into those with recurrent cancer and those without (FIGURE 1). Recurrence rates were similar in patients with positive or negative TgAb (2.9% vs. 2.3%, p= 0.6). The patients with positive TgAb were also analyzed for TgAb resolution.
Table 1.
Cohort demographics
| Metric | Total | TgAb− | TgAb+ | p-values |
|---|---|---|---|---|
| N=247 | N=213 | N=34 | ||
| Sex | 77.3% women, 22.7% men | 75.6% women 24.4% men |
85.3%women, 14.7% men | 0.28 |
| Age (years) | 45.7 ± 1.0 years | 46.2 ± 1.1 years | 43.0 ± 2.5 years | 0.28 |
| Tumor Size (cm) | 1.8 ± 0.2 | 1.8 ± 0.2 | 1.8 ± 0.3 | 0.99 |
| Histopathological Subtypes | 96.0% PTC | 95.3% PTC | 100% PTC | 0.37 |
| 4.0% FTC | 4.7% FTC | |||
| Autoimmune Thyroid Disease | 16.6% HT | 14.6% HT | 29.4% HT | 0.05 |
| 1.6% GD | 0.9% GD | 5.9% GD | 0.09 | |
| 18.2% Total | 15.5% Total | 35.3% Total | 0.01 | |
| Lymph Node Involvement | 8.9% | 8.5% | 11.8% | 0.75 |
| Extrathyroidal extension | 5.3% | 5.1% | 5.9% | 0.70 |
| Surgery Type | 76.2% Total | 75.1% Total | 85.3% Total | 0.28 |
| 23.8% Completion | 24.9% Completion | 14.7% Completion | ||
Figure 1.
All patients were stratified to TgAb+/- groups and then further divided into those with recurrent cancer and those without. Additionally, TgAb+ positive patients were also stratified into those with persistent TgAb elevation and those without.
TgAb Resolution
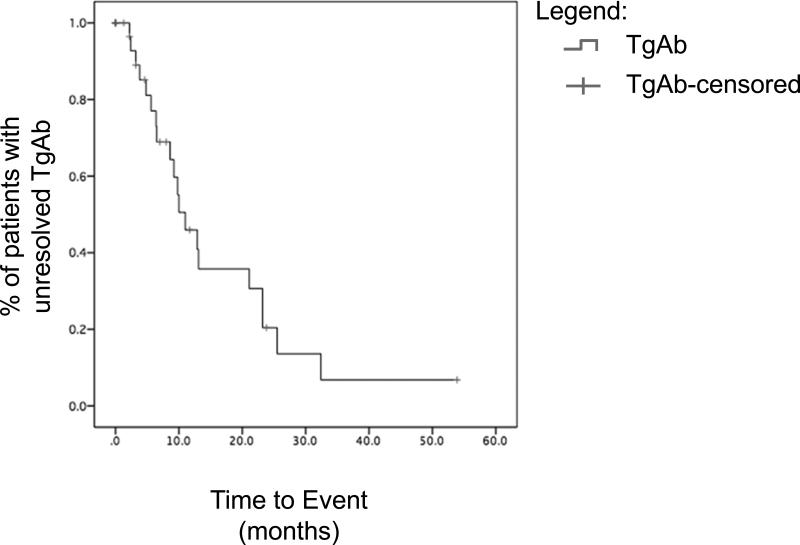
Of the 34 patients with positive TgAb, 25 (73.5%) had complete TgAb resolution in follow-up. The median time to TgAb resolution was 11.0 ± 2.3 months, and the majority resolved by 32.4 months. Regression analysis of the patients with completely resolved antibodies yielded an average decline of -11% IU/ml per month ± 2.2% (Figure 2). 9 patients had persistently elevated TgAb levels at last follow-up with a mean follow up 14.2 ± 4.7 months.
Figure 2.
TgAb Resolution. Log-Rank analysis showed that patients resolved at a median 11 months.
Recurrence
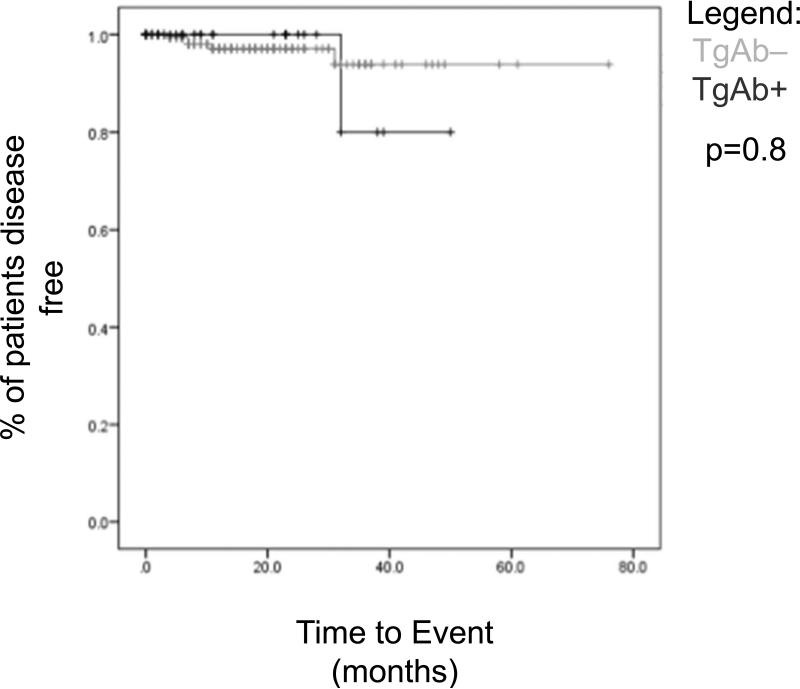
Recurrence occurred in 2.4% (n=6) of patients. Disease free survival was equivalent between TgAb positive and TgAb negative groups (p = 0.8). For the TgAb positive patients, the only recurrence occurred after 32 months (Figure 3).
Figure 3.
Disease Free Survival based on TgAb status. Log-Rank analysis could not determine a relationship between TgAb status and recurrent cancer
The TgAb positive group was further stratified into those with persistently elevated antibodies and those with resolution. Of 34 antibody positive patients, 9 had not resolved at most recent follow up (Table 1). Mean follow up time of those 9 patients was 14.2 ± 4.7 months. Follow up imaging for these patients did not reveal any evidence of recurrent disease.
Discussion
We studied the time course of TgAb resolution and the relationship between the presence of TgAb and for the risk of recurrence in 247 patients with thyroid cancer who received total thyroidectomy. Our study shows that TgAb occur in 14% of patients with thyroid cancer but they resolve after successful treatment at approximately −11% IU/ml per month from their preoperative levels with median resolution at 11.0 months. TgAb, when present preoperatively or persistently present post-treatment were not associated with disease recurrence in our series.
In our study we found that about 14% of patients had preoperative TgAb, which is consistent with published values of 10-25% range [5, 6, 9, 10]. The median resolution time for TgAb is uncertain and our study found a median resolution time of 11.0 months which is lower than the 3 years found by other investigators [7, 11, 12]. This could be due to more complete removal of thyroid tissue at the time of surgery [13] as well as more routine treatment with radioactive iodine, as almost all patients in this series received radioactive iodine postoperatively [13]. Since RAI was given to almost all patients, we could not analyze if there was an impact of RAI treatment on Ab resolution. We also selected patients based on preoperative TgAb positivity, not post-operative values. Therefore we may have included some low level Ab Positive patients that resolved early, which shortened the median time to resolution.
Whether or not the presence of TgAb impacts cancer recurrence is controversial. While some investigators have found a relationship between TgAbs and cancer recurrence, this has not been consistent. Rubello et al [9] looked at 43 patients with DTC and TgAb and found they were more likely to recur. However 19 patients had either lymph node involvement or lung metastasis, which means that total thyroidectomy alone was unlikely to completely remove their thyroid disease. These patients were likely more predisposed to recurrence because of their advanced disease stage – not necessarily because of the existence of TgAb. Kim et al also found an association between TgAb concentration and cancer recurrence [1]. However, they did not measure pre-thyroidectomy TgAb and used a TgAb measurement at time of remnant ablation, which can be prone to instability. On the other hand, Quevedo et al [4] looked at 26 patients retrospectively with thyroid cancer and found that concentration of TgAb was not associated with cancer recurrence. Gorges et al [7] studied 112 patients with thyroid cancer in a cross sectional study and found that neither initial TgAb levels nor persistent levels were correlated with cancer recurrence. A notable finding in his series was that 3 of these patients experienced de novo development of TgAb although none of them had disease recurrence and the mechanism remains unclear. Our study adds to the evidence that TgAb levels are not associated with cancer recurrence.
Since the presence of TgAb makes Tg unreliable as a tumor marker, clinicians need to rely on less sensitive methods to diagnose recurrence. Although scans like whole body scan and ultrasonography are critical tools for postoperative evaluation of thyroid cancer they are less sensitive than serum Tg levels and will not detect disease until it is macroscopic in size. We did find that our only recurrence in a TgAb positive patient occurred after 32 months and this occurred in a patient that had resolved their antibodies prior to developing the recurrence. While we did not have any recurrences in our 9 patients with persistently elevated thyroglobulin levels after surgery, the mean follow up time in the cohort was just over a year, which means that many of these patients may have resolved their TgAb with further follow up. This follow up period may also be too short of a time to detect an anatomic recurrence on imaging and with longer follow-up this cohort may develop evidence of imageable recurrent disease. Hopefully with more patients and longer follow up, the significance of a persistently elevated post-operative TgAb level can be better addressed.
There were several limitations in this study. First, there was a small sample size and only 34 of our 247 patients had positive TgAb. While the numbers are not large, because we had both pre and postoperative Thyroglobulin levels on this cohort as well as detailed clinical follow-up data we were able to answer some important questions regarding the significance of TgAbs. Second, there is inherent variability in lab assays. We chose a cut off of < 20 IU/mL but assays varied in sensitivity and it is conceivable that the time to event could change if we had used a different cut-off value. Third, we could not examine the impact of radioactive iodine treatment on TgAb resolution because the majority of our cohort received it. Last, new high sensitivity assays using mass spectroscopy may account for antibody interference in their measurements and possibly one day obviate the need for antibody measurement [14]. As of now, however, they are not universally available and expected time course to antibody resolution still remains clinically relevant.
In conclusion, our study shows that TgAb occur in 14% of patients with thyroid cancer but they resolve after successful treatment at approximately −11% IU/ml per month from their preoperative levels with median resolution at 11.0 months. TgAb, either present preoperatively or persistently elevated post-treatment were not associated with disease recurrence in our series. Given time, most patients will have resolution of their TgAb, but this can take several years to occur. In the interim a persistently elevated TgAb should not be considered indicative of residual disease.
Acknowledgement
This research was supported by NIH T35 DK062709 Grant and the University of Wisconsin Shapiro Summer Research Program. Many thanks to Glen Leverson and Chee Paul Lin for their invaluable statistics guidance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions Conception and design: Jimmy Xu and Rebecca Sippel Analysis and Interpretation: Jimmy Xu, Ryan Bergren, Rebecca Sippel Data collection: Jimmy Xu, David Schneider, Herbert Chen, Rebecca Sippel Critical revision: Jimmy Xu, Ryan Bergren, David Schneider, Herbert Chen, Rebecca Sippel
Disclosure
The authors reported no conflicts of interest.
References
- 1.WG, Yoon JH, Kim WB, et al. Change of serum antithyroglobulin antibody levels is useful for prediction of clinical recurrence in thyroglobulin-negative patients with differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2008;93:4683–9. doi: 10.1210/jc.2008-0962. [DOI] [PubMed] [Google Scholar]
- 2.Spencer CA. Clinical review: Clinical utility of thyroglobulin antibody (TgAb) measurements for patients with differentiated thyroid cancers (DTC). J Clin Endocrinol Metab. 2011;96:3615–27. doi: 10.1210/jc.2011-1740. [DOI] [PubMed] [Google Scholar]
- 3.Chung JK, Park YJ, Kim TY, et al. Clinical significance of elevated level of serum antithyroglobulin antibody in patients with differentiated thyroid cancer after thyroid ablation. Clin Endocrinol (Oxf) 2002;57:215–21. doi: 10.1046/j.1365-2265.2002.01592.x. [DOI] [PubMed] [Google Scholar]
- 4.Quevedo I, Campino C, Rodríguez Portales JA, et al. Anti thyroglobulin antibodies in the follow up of patients with differentiated thyroid cancer: residual or relapsing disease markers? Rev Med Chil. 2002;130:167–72. [PubMed] [Google Scholar]
- 5.Kumar A, Shah DH, Shrihari U, Dandekar SR, Vijayan U, Sharma SM. Significance of antithyroglobulin autoantibodies in differentiated thyroid carcinoma. Thyroid. 1994;4:199–202. doi: 10.1089/thy.1994.4.199. [DOI] [PubMed] [Google Scholar]
- 6.Pacini F, Mariotti S, Formica N, et al. Thyroid autoantibodies in thyroid cancer: incidence and relationship with tumour outcome. Acta Endocrinol (Copenh) 1988;119:373–80. doi: 10.1530/acta.0.1190373. [DOI] [PubMed] [Google Scholar]
- 7.Görges R, Maniecki M, Jentzen W, et al. Development and clinical impact of thyroglobulin antibodies in patients with differentiated thyroid carcinoma during the first 3 years after thyroidectomy. Eur J Endocrinol. 2005;153:49–55. doi: 10.1530/eje.1.01940. [DOI] [PubMed] [Google Scholar]
- 8.Heemstra KA, Liu YY, Stokkel M, et al. Serum thyroglobulin concentrations predict disease-free remission and death in differentiated thyroid carcinoma. Clin Endocrinol (Oxf) 2007;66:58–64. doi: 10.1111/j.1365-2265.2006.02685.x. [DOI] [PubMed] [Google Scholar]
- 9.Rubello D, Girelli ME, Casara D, Piccolo M, Perin A, Busnardo B. Usefulness of the combined antithyroglobulin antibodies and thyroglobulin assay in the follow-up of patients with differentiated thyroid cancer. J Endocrinol Invest. 1990;13:737–42. doi: 10.1007/BF03349612. [DOI] [PubMed] [Google Scholar]
- 10.Spencer CA, Takeuchi M, Kazarosyan M, et al. Serum thyroglobulin autoantibodies: prevalence, influence on serum thyroglobulin measurement, and prognostic significance in patients with differentiated thyroid carcinoma. J Clin Endocrinol Metab. 1998;83:1121–7. doi: 10.1210/jcem.83.4.4683. [DOI] [PubMed] [Google Scholar]
- 11.Chiovato L, Latrofa F, Braverman LE, et al. Disappearance of humoral thyroid autoimmunity after complete removal of thyroid antigens. Ann Intern Med. 2003;139:346–51. doi: 10.7326/0003-4819-139-5_part_1-200309020-00010. [DOI] [PubMed] [Google Scholar]
- 12.Thomas D, Liakos V, Vassiliou E, Hatzimarkou F, Tsatsoulis A, Kaldrimides P. Possible reasons for different pattern disappearance of thyroglobulin and thyroid peroxidase autoantibodies in patients with differentiated thyroid carcinoma following total thyroidectomy and iodine-131 ablation. J Endocrinol Invest. 2007;30:173–80. doi: 10.1007/BF03347421. [DOI] [PubMed] [Google Scholar]
- 13.Schneider DF, Ojomo KA, Chen H, Sippel RS. Remnant uptake as a postoperative oncologic quality indicator. Thyroid. 2013;23:1269–76. doi: 10.1089/thy.2012.0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giovanella L, Feldt-Rasmussen U, Verburg FA, Grebe SK, Plebani M, Clark PM. Thyroglobulin measurement by highly sensitive assays: focus on laboratory challenges. Clin Chem Lab Med. 2014 doi: 10.1515/cclm-2014-0813. [DOI] [PubMed] [Google Scholar]