Abstract
Clinical trials show that opioid agonist therapy (OAT) with methadone or buprenorphine is more effective than behavioral treatments, but state policymakers remain ambivalent about covering OAT for long periods. We used Medicaid claims for 52,278 Massachusetts Medicaid beneficiaries with a diagnosis of opioid abuse or dependence between 2004 and 2010 to study associations between use of methadone, buprenorphine or other behavioral health treatment without OAT, and time to relapse and total healthcare expenditures. Cox Proportional Hazards ratios for patients treated with either methadone or buprenorphine showed approximately 50% lower risk of relapse than behavioral treatment without OAT. Expenditures per month were from $153 to $233 lower for OAT episodes compared to other behavioral treatment. Co-occurring alcohol abuse/dependence quadrupled the risk of relapse, other non-opioid abuse/dependence doubled the relapse risk and severe mental illness added 80% greater risk compared to those without each of those disorders. Longer current treatment episodes were associated with lower risk of relapse. Relapse risk increased as prior treatment exposure increased but prior treatment was associated with slightly lower total healthcare expenditures. These findings suggest that the effectiveness of OAT that has been demonstrated in clinical trials persists at the population level in a less controlled setting and that OAT is associated with lower total healthcare expenditures compared to other forms of behavioral treatment for patients with opioid addiction. Co-occurring other substance use and mental illness exert strong influences on cost and risk of relapse, suggesting that individuals with these conditions need more comprehensive treatment.
Introduction
Clinical trials have demonstrated the superiority of opioid agonist therapy (OAT) for individuals with opioid addiction and national policymakers urge its expanded use, but support for the leading forms of OAT, buprenorphine/naloxone and methadone maintenance, varies widely from one state to the next (Clark & Baxter, 2013; Clark & Baxter, 2013; Mattick, Kimber, Breen, & Davoli, 2014; Mattick et al., 2014; Rinaldo & Rinaldo, 2013; Volkow, Friedan, Hyde, & Cha, 2014). Public attitudes toward opioid substitution, concerns about over-utilization or diversion of buprenorphine, and perceptions that it is expensive contribute to policymakers' ambivalence about OAT. As OAT becomes more widely available, questions also arise about its effectiveness and cost for large populations in real world settings, where treatment access and implementation are monitored less carefully than in clinical trials (Cohen et al., 2008).
Additional concerns apply to Medicaid programs, which fund more than one-third of all substance abuse treatment in the United States and are the primary source of treatment in some states (Mark, Levit, Yee, & Chow, 2014). Individuals who qualify for Medicaid benefits have low incomes, high rates of co-morbidity and disability, and a range of other social risk factors that may limit the effectiveness of treatment (Kaiser Commission on Medicaid and the Uninsured, 2013). Specific co-occurring disorders, such as severe mental illness are more common among Medicaid beneficiaries and may require more intensive and specialized services to facilitate recovery than are typically available from specialty addiction providers. States have relatively little specific data on which to base decisions about who is at greatest risk of relapse and, thus, who may need additional assistance.
Opioid addiction is widely, but not universally, viewed as a chronic relapsing condition; but healthcare policies and public perceptions often treat it as a problem that can be addressed with short-term treatment (Heyman, 2009; McLellan, Lewis, O'Brien, & Kleber, 2000). Medicaid programs are particularly concerned about the longitudinal course of treatment, in which individuals may move into and out of treatment multiple times. Several studies suggest that patients who remain in treatment for longer periods have better treatment outcomes (Simpson & Joe, 2004; Hubbard, Craddock, & Anderson, 2003). But evidence for the benefits of previous treatment attempts for current treatment success is less clear. Some studies indicate that individuals with a history of prior treatment are often less successful than those enrolled in treatment for the first time (Cacciola, Dugosh, & Camilleri, 2009; Grella, Hser, Joshi, & Anglin, 1999; Hser, Grella, Hsieh, Anglin, & Brown, 1999). Lack of consensus about the chronic nature of substance abuse and confusion about the benefits of long-term treatment make it difficult to craft consistent policies for addiction treatment.
Each state designs its own set of Medicaid benefits within the limits of broad federal regulations. Massachusetts residents had comparatively better access to Medicaid and less restricted choice of addiction treatment modalities compared to residents of many other states during the past decade; thus, Massachusetts offers a particularly good opportunity to examine the potential population-level effects of opioid addiction treatment on Medicaid members and on healthcare expenditures as the Affordable Care Act expands coverage in other states (Rinaldo & Rinaldo, 2013).
In this study, we build on our previous work by following individuals with opioid addiction whose treatment was funded by MassHealth, Massachusetts' Medicaid program, between 2004 and 2010 (Clark, Samnaliev, Baxter, & Leung, 2011). We examine associations between treatment modality (buprenorphine/naloxone, methadone or behavioral health treatment without OAT), relapse rates during treatment and total healthcare expenditures during treatment, taking into account co-occurring behavioral health disorders, the duration of current treatment and previous treatment for opioid addiction.
Methods
We used MassHealth claims for services paid on a fee-for-service basis and encounter data for services provided by managed care organizations to identify members age 16 and older with a primary diagnosis of opioid abuse or dependence between 2004 and 2010, with additional claims from 2002 and 2003 used for measuring treatment and relapse history. Enrollment data provided information about when members were covered by MassHealth, their age, gender and some limited race and ethnicity information. In addition, we linked MassHealth claims with records from the Massachusetts Department of Public Health which funds some treatment services that MassHealth does not cover (e.g., residential care) and for individuals who do not qualify for Medicaid benefits at the time of use. This additional information improved our ability to identify treatment enrollment and to measure relapses.
Episodes of Treatment
Treatment episodes were the basic unit of our analysis. Using MassHealth claims, we constructed three types of treatment episodes: methadone maintenance, buprenorphine maintenance and behavioral health treatment without OAT. The latter category included a mix of outpatient and residential treatments that did not involve use of opioid agonists. To simplify further discussion, we refer to this category as non-OAT treatment. Methadone is typically dispensed in structured clinic settings with observed dosing. Buprenorphine was available in sublingual pill form during the period covered by this study. Following a brief induction period, patients were allowed to take their daily dose of buprenorphine at home without direct observation.
Some patients had multiple episodes of the same treatment or used different kinds of treatment during the seven year study period. Methadone episodes were identified with a Healthcare Common Procedure Code (H0020) in claims or encounter data. Episodes began on the date of the first service code and continued until the date of the last code. Buprenorphine episodes were identified through outpatient pharmacy claims for buprenorphine or buprenorphine/naloxone. A buprenorphine episode began on the day a prescription was filled and continued until the date that the final filled prescription supply ran out. Episodes of less than 30 days were excluded from our analysis for all treatment modalities because buprenorphine and methadone are sometimes used for detoxification without an intention for maintenance treatment. Non-OAT treatment episodes were identified with Current Procedure Terminology or Healthcare Common Procedure Codes for any form of behavioral health treatment. Eligibility for the non-OAT group was defined as having received behavioral health treatment with at least one claim having a primary diagnosis of opioid abuse or dependence. Episodes of non-OAT treatment began on the day that the first service was delivered and continued until the last date of service. When non-OAT behavioral treatment overlapped with a methadone or buprenorphine episode, the episode was coded as either methadone or buprenorphine. Treatment gaps of fewer than two months were treated as one episode. An exception to this rule occurred when there was a direct switch from one type of treatment to another (e.g., from buprenorphine to methadone.) These cases were treated a two separate episodes, with the first ending on the last day of treatment and the second beginning on the following day (i.e., the first day of the second treatment.)
Relapse Measure
As a proxy measure for clinically-defined relapses during treatment, we used claims for detoxification, inpatient admission with a primary diagnosis of substance use disorder (SUD), or an emergency department visit with a primary diagnosis of SUD to indicate that a relapse had occurred. This is a conservative measure which tends to identify more serious relapses and cannot account for clinical relapses not resulting in the use of these three services.
Expenditures
We used all Medicaid claims to identify total healthcare expenditures during treatment episodes for individuals who met the study criteria. Previous research shows that addiction and addiction treatment can significantly impact expenditures for care of other conditions as well treatment directly related to substance abuse (Clark, Samnaliev, & McGovern, 2009; Parthasarathy, Weisner, Hu, & Moore, 2001).
Total expenditures per patient were measured on a monthly basis and treated as a time-varying outcome measure.
Analytical Models
Relapse
To reduce bias that might be associated with patient selection into specific treatments, we included measures of demographic characteristics, overall disease burden, specific mental health and addiction co-morbidities, history of relapses prior to an episode, length of the current episode, and cumulative length of prior treatment episodes, measured in years. Examining the impact of these factors can also point to groups that may need additional assistance. Our conceptual model included a range of demographic and clinical factors that have been shown to affect treatment outcomes. These included the following: age at the beginning of an episode, gender, co-occurring mental health (severe mental illness, major depression and other psychiatric disorders) and addiction diagnoses (alcohol or other non-opioid drug disorders), a measure of overall disease burden (the Chronic Disease Payment System score (Kronick, Gilmer, Dreyfus, & Lee, 2000)), relapse rate during the six months preceding the current episode, the year in which the episode began to capture secular (time-related) changes in treatment or the population served, and the length of the current episode. To adjust for differences in overall exposure to MassHealth coverage after the beginning of an episode, which could affect detection of relapses, we included the number of days a person remained eligible for coverage after the beginning of the episode. Average MassHealth enrollment prior to the beginning of an episode was 2.8 years, with 12.8% of episodes having fewer than 6 months of prior enrollment. We also added separate measures of cumulative time spent receiving buprenorphine, methadone or behavioral health treatment before the current episode to explore the effect of prior treatment exposure on current treatment outcomes. Periods of treatment lasting less than 30 days, which were relatively infrequent, were not included in the cumulative measure. Treatment history was measured in years. We also tested prior treatment measures based on the number of previous episodes. Our key variables of interest were whether the current episode was for methadone, buprenorphine or non-OAT behavioral treatment, measured as binary variables for buprenorphine and for methadone, with non-OAT treatment as the omitted category. Coefficients for these variables represent the association between that type of treatment and the probability of relapse compared to behavioral health treatment without OAT.
Expenditures
Our multivariate model of associations between treatment modality and healthcare expenditures during treatment was similar to that used for relapses, with the exceptions that we substituted measures of prior expenditures for prior relapses and used a simple count of the number of previous treatment episodes for each modality. Episodes lasting fewer than 30 days were not counted in this measure. Based on preliminary analyses, we clustered the expenditure model by the year in which treatment began, to adjust for numerous policy, payment and treatment changes that may have occurred during the study period.
Statistical Analyses
We used Cox Proportional Hazards models to measure the association between treatment type and time to the first relapse following an episode start. Episodes were censored at the end of 2010 or if the member lost MassHealth coverage for any reason.
To estimate monthly total expenditures, we used hierarchical generalized estimating equations (GEE) to measure associations between treatment variables and covariates, and expenditures during each month of a treatment episode.
All analyses were performed using SAS statistical software (SAS Institute Inc., 1999). Cox models used the PHREG procedure. GEE models used the GENMOD procedure.
Results
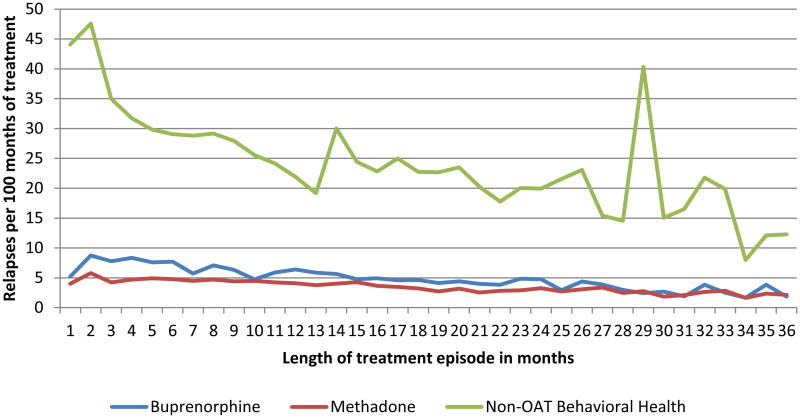
We identified 56,278 individuals who received a total 104,840 episodes of treatment between 2004 and 2010. Table 1 shows some variation in the characteristics of individuals who received the different types of treatment. Generally, MassHealth members not receiving OAT had higher rates of co-occurring mental illness and alcohol use disorders, as well as higher mean costs and more frequent (unadjusted) relapses. The median episode lasted 5 months for non-OAT behavioral health treatment, 8 months for buprenorphine and 13 months for methadone.
Table 1. MassHealth members who received treatment for opioid addiction between 2003 -2010.
| Characteristic | Total (N =56,278) | Type of Treatment Received1 | ||
|---|---|---|---|---|
|
| ||||
| Buprenorphine (N = 18,866) | Methadone (N = 24,309) | Non-OAT Behavioral Health (N =31,220) | ||
| Gender, n (%) | ||||
| Male | 32,636 (58.0) | 10,999 (58.3) | 14,089 (58.0) | 17,274 (55.3) |
| Female | 23,642 (42.0) | 7,867 (41.7) | 10,220 (42.0) | 13,946 (44.7) |
| Average age2, mean (SD) | 33.8 (10.4) | 32.1 (9.5) | 32.7 (9.8) | 34.5 (10.7) |
| CDPS2, mean (SD) | 3.2 (2.0) | 3.0 (1.7) | 2.8 (1.8) | 3.4 (2.2) |
| Behavioral health diagnosis2, n (%) | ||||
| SMI | 13,627 (24.2) | 3,878 (20.6) | 3,877 (16.0) | 10,311 (33.0) |
| Other | 13,647 (24.3) | 5,080 (26.9) | 5,397 (22.2) | 7,660 (24.5) |
| Major depression | 8,113 (14.5) | 2,564 (13.6) | 2,982 (12.3) | 5,397 (17.3) |
| Co-occurring substance use2, n (%) | ||||
| Alcohol | 12,861 (22.9) | 3,338 (17.7) | 3,030 (12.5) | 10,019 (32.1) |
| Other drug | 19,266 (34.2) | 7,783 (41.3) | 7,111 (29.3) | 11,157 (35.7) |
| Treatment episodes per person, mean (SD) | 1.9 (1.2) | 1.3 (0.7) | 1.3 (0.7) | 1.5 (0.8) |
| Medicaid expenditures during treatment3, mean (SD) | $1,429 (1,669) | $1,169 (1,168) | $1,079 (1,218) | $1,638 (2074) |
| Relapses per 100 months of treatment4, mean (SD) | 12.3 (28.4) | 5.6 (16.8) | 3.7 (12.5) | 22.1 (37.1) |
| Members with relapses during treatment5, n (%) | 19,578 (34.8) | 3,901 (20.7) | 4,786 (19.7) | 13,578 (43.7) |
Treatment groups are not mutually exclusive; members may have received more than one type of treatment during the study period
At first diagnosis of opioid addiction or abuse.
Average expenditures per person per month during treatment episode.
Relapses include detoxifications, emergency department visits and hospitalizations with a primary diagnosis of a drug or alcohol disorder
At least once during a treatment episode.
Relapses
Results of the Cox Proportional Hazards analysis show a number of statistically significant associations between covariates and relapse rates during treatment. Compared to non-OAT behavioral health treatment, episodes of buprenorphine or methadone maintenance treatment were less than one-half as likely to result in a relapse. Among co-morbidities, individuals with co-occurring alcohol abuse or dependence relapsed at four times the rate of those without alcohol disorders. Additional addiction to drugs other than opioids doubled the relapse risk. Having a diagnosis of schizophrenia, other psychoses or bipolar disorder increased the risk of relapse by 80%.
Each year of current treatment was associated with a decreased relapse rate of about 30%. However, substance abuse treatment prior to the current episode did not have a statistically significant effect on the risk of relapse. We also observed a secular time effect suggesting a general decline in the risk of relapse of about 9% per year during the study period.
Expenditures
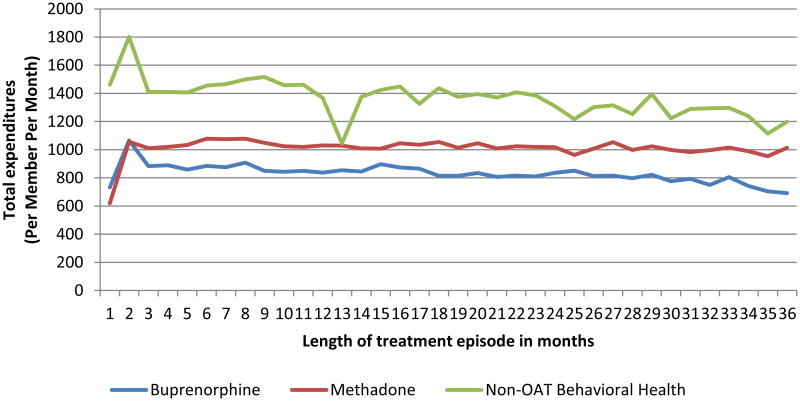
Adjusting for other factors, including treatment history and episode duration, methadone and buprenorphine treatment episodes were associated with $223 to $153 lower total healthcare expenditures per month than other non-OAT behavioral health treatment episodes, most likely due to their more effective reduction of relapse events. Co-occurring severe mental illness, alcohol disorders and other drug disorders collectively added $786 per month to the average per-person cost of treatment. Expenditure reductions associated with prior treatment episodes were modest but significantly higher for methadone than for non-OAT treatment.
Discussion
Our findings are consistent with other analyses showing that opioid agonist therapy is significantly more effective in reducing relapses and less costly than other forms of behavioral health treatment. This effect appears to be independent of other risk factors in our model that might cause patients or their providers to choose one treatment over another.
Co-occurring conditions clearly play a strong role in determining the cost and course of treatment. In particular, our findings suggest that, when patients have addictions to alcohol or other drugs, costs are significantly higher and OAT alone may not be adequate to prevent the frequent relapses associated with substances other than opioids. These associations strongly suggest the importance of addressing all addictions.
Like many other studies, we found that mental health disorders, particularly more severe illnesses such as schizophrenia or bipolar disorder, increase the risk of relapse and raise total healthcare expenditures. The need for effective integrated treatment of mental health and substance use disorders has long been recognized. Our findings suggest that it remains a significant problem to be addressed.
We did not find a dose-response association between previous treatment for opioid addiction and relapse rates. In fact, prior treatment was associated with a greater risk of relapse. This finding is consistent with research by John Cacciola and colleagues (Cacciola, Dugosh, Camillari, 2009), who found that substance abuse patients with two or more prior treatment episodes had more problems upon discharge from outpatient or residential treatment than those with a single prior treatment attempt or no prior treatment. These findings lend support to the interpretation by Michael Dennis and colleagues (Dennis, Scott, Funk & Foss, 2005) that longer treatment histories are indicators of chronicity. On a more positive note, patients who were able to remain engaged in their current episode of treatment for longer periods showed a significantly lower risk of relapse than those with shorter episodes. In a secondary analysis, not shown here, we found similar results when we included the number of previous treatment episodes of each type rather than the cumulative length of treatment in the various modalities.
Previous episodes of treatment were associated with slightly lower healthcare expenditures. Prior treatment's association with a greater risk of relapse and slightly lower costs is somewhat puzzling and requires further study. One possibility is that by bringing individuals into the healthcare system, even unsuccessful treatment for opioid addiction may reduce healthcare costs by preventing or treating other physical conditions such as infectious disease or chronic illnesses like asthma or cardiovascular disease. At the same time, individuals with multiple previous treatment attempts probably represent a subset of patients with particularly severe addiction or who face other barriers to successful treatment.
Methadone and buprenorphine performed similarly in our analysis but are often viewed quite differently by patients, providers and policymakers. Patients and providers often prefer one of these OAT forms over another, patient characteristics differ (Baxter, et. al., 2011), and access to methadone maintenance tends to be more limited than buprenorphine (Rinaldo & Rinaldo, 2013.) Treatment preferences are likely to be very important at the patient and provider level, thus the similarity of associations in our analysis should not be interpreted to mean that different forms of OAT are easily substitutable.
Strengths and Limitations
Using Medicaid claims and other service utilization records improved the comprehensiveness of some measures, such as service use across multiple providers, but may have led to systematic under-identification of relapses and prior treatment across all treatment types. Clinically defined relapses do not always result in detoxification, emergency department visits, or admission to hospitals; thus, it is likely that our relapse-related service use measure undercounts the number of clinical relapses. However, we see no indication that the effect varied across the three treatments studied.
Diagnoses, too, are likely measured with less precision with claims than in a randomized trial or an epidemiological study in which standardized instruments can be used to improve the accuracy and reliability of diagnoses. Given the large number of individuals and episodes used in our study, we believe that comparisons involving diagnoses represent true differences in the effect of co-morbidities, even though the actual rate of those co-morbidities may be higher or lower than that identified in epidemiologic studies. Other data-related limitations include the lack of other key outcome measures and covariates, such as days of abstinence or days of substance use, health-related quality of life, and severity of illness.
Measures of prior treatment in our study were limited by the available years of service data and the difficulty of accessing alternate sources of information on previous treatment. Treatment provided while members were incarcerated, living out of state, or covered by commercial insurance was not included in our analysis, thus treatment received in these circumstances was not included in our measures of prior treatment. For members who were MassHealth-enrolled for the entire study period, we captured an average of 2.8 years of Medicaid-funded treatment history. Additional data from Department of Public Health records extended the look-back period for most Medicaid members. However, for newly enrolled members the observation period could have been much shorter. Thus, our cumulative treatment measures cannot be considered lifetime treatment records.
The absence of random assignment to treatment in this study can be seen as both a weakness and a strength. Randomization of individuals to methadone, buprenorphine or other non-OAT behavioral health treatment would have improved the internal validity of the study, thereby increasing confidence that the effects reported for each type of treatment are a precise measure of its effectiveness and not due to an unobserved difference in the characteristics of patients who used one treatment versus another. Our findings are limited by the information available to us for this analysis and cannot be interpreted as causal. Given that the effectiveness of buprenorphine and methadone is well established in clinical trials, our purpose was to learn whether differences in effectiveness found in controlled studies were also evident in large populations, where patients often choose or are assigned to treatments based on assessments that they or their providers make about the likelihood that a particular treatment will be effective for them. Access to treatment also plays a large role in real world treatment assignment and implementation of various treatments, especially those included in the non-OAT behavioral health treatment category, varies widely. We believe these factors should be allowed to affect outcomes in a study of how treatments perform in real world settings.
Conclusions
Consistent with clinical trials and previous economic analyses, we find that treatment of opioid addiction with methadone or buprenorphine is more effective and less costly than most behavioral health treatment without OAT. These findings indicate that opioid agonist therapy is efficient and effective at the system level as well as in more controlled settings. Our analysis also underlines the importance of keeping patients engaged in treatment, particularly during early episodes.
As Medicaid programs expand to include larger populations of individuals at risk of addiction, we see no reasons to restrict access to these effective treatments from the perspective of patient outcomes or of public spending. Indeed, our findings strongly suggest that restrictive state policies will lead to less effective treatment for the growing number of individuals with opioid addiction and higher healthcare costs for taxpayers.
Figure 1. Relapses during treatment among MassHealth members who received treatment for opioid addiction between 2003 – 20101.
1N = 18,866 episodes of buprenorphine treatment, 24,309 episodes of methadone treatment and 31,220 episodes of non-OAT behavioral health treatment in month 1. 33% of buprenorphine episodes, 52% of methadone episodes, and 12% of non-OAT treatment episodes lasted 12 months or more. 13% of buprenorphine treatment episodes, 27% of methadone episodes, and 1% of non-OAT treatment episodes lasted 24 months or longer.
Figure 2. Per member per month (PMPM) total expenditures during treatment for MassHealth members who received treatment for opioid addiction between 2003 – 20101.
1N = 18,866 episodes of buprenorphine treatment, 24,309 episodes of methadone treatment and 31,220 episodes of non-OAT behavioral health treatment in month 1. 33% of buprenorphine episodes, 52% of methadone episodes, and 12% of non-OAT behavioral health treatment episodes lasted 12 months or more. 13% of buprenorphine treatment episodes, 27% of methadone episodes, and 1% of non-OAT treatment episodes lasted 24 months or longer.
Table 2. Cox proportional hazards analysis of factors related to relapse during treatment among MassHealth members who received treatment for opioid addiction between 2003 -2010.
| Factor | Hazard Ratio | (95% CI) |
|---|---|---|
| Age | 0.98 | (0.98, 0.98)*** |
| Gender (male = 1) | 1.34 | (1.31, 1.37)*** |
| CDPS | 1.05 | (1.04, 1.05)*** |
| Year treatment (episode) started | 0.91 | (0.90, 0.92)*** |
| Relapse rate in the 6 months prior to start of episode | 1.22 | (1.20, 1.25)*** |
| Co-occurring mental illness | ||
| Severe mental illness | 1.83 | (1.76, 1.90)*** |
| Other mental illness | 1.34 | (1.29, 1.40)*** |
| Major depression | 1.38 | (1.32, 1.45)*** |
| Co-occurring substance abuse disorder | ||
| Alcohol | 4.32 | (4.15, 4.49)*** |
| Other drug | 2.33 | (2.24, 2.43)*** |
| Type of episode (compared to non-OAT behavioral health treatment) | ||
| Buprenorphine | 0.42 | (0.40, 0.44)*** |
| Methadone | 0.43 | (0.41, 0.44)*** |
| Episode length (in years) | 0.71 | (0.69, 0.72)*** |
| Length of prior treatment (in years) | ||
| Buprenorphine | 1.00 | (0.93, 1.07) |
| Methadone | 0.97 | (0.93, 1.02) |
| Non-OAT Behavioral Health | 0.99 | (0.93, 1.04) |
| Total number of years of MH eligibility after start of treatment | 1.00 | (0.99, 1.00) |
p < .0001
Table 3. GEE analysis of factors related to total expenditures during treatment for MassHealth members who received treatment for opioid addiction between 2003 - 2010.
| Factor | Estimate | (95% CI) |
|---|---|---|
| Intercept | 384.20 | (320.06, 448.34)*** |
| Age | 3.89 | (2.65, 5.13)*** |
| Gender (male = 1) | 3.69 | (-19.31, 26.68) |
| CDPS | 179.10 | (162.96, 195.24)*** |
| Expenditures during the 6 months before the episode | 0.30 | (0.25, 0.36) *** |
| Co-occurring mental illness | ||
| Severe mental illness | 332.06 | (291.33, 372.79)*** |
| Other mental illness | -16.31 | (-43.02, 10.40) |
| Major depression | -9.75 | (-44.33, 24.84) |
| Co-occurring substance abuse disorder | ||
| Alcohol | 437.41 | (384.22, 490.60)*** |
| Other drug | 140.60 | (110.75, 170.44) *** |
| Type of current episode (compared to non-OAT behavioral health treatment) | ||
| Buprenorphine | -190.88 | (-222.71, -159.04)*** |
| Methadone | -183.64 | (-213.87, -153.41)*** |
| Number of prior buprenorphine treatment episodes | -58.82 | (-87.24, -30.39)*** |
| Number of prior methadone treatment episodes | -87.27 | (-116.24, -58.30)*** |
| Number of prior treatment episodes without OAT | -31.01 | (-55.63, -6.38)* |
p < .05
p < .0001
Highlights.
We identified 52,278 individuals with opioid abuse dependence between 2004-2010
There were 104,840 episodes of methadone, buprenorphine or behavioral health treatment without an opioid agonist.
Methadone & buprenorphine were associated with lower relapse rates and costs
Longer episodes were associated with lower relapse rates
Mental illness, alcohol & other drugs associated with more relapses & higher costs
Acknowledgments
This study was supported by National Institute of Drug Abuse grant number R01DA029741. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baxter JD, Clark RE, Samnaliev M, Leung GY, Hashemi L. Factors Associated with Medicaid Patients' Access to Buprenorphine Treatment. Journal of Substance Abuse Treatment. 2011;41(1):88–96. doi: 10.1016/j.jsat.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Cacciola JS, Dugosh KL, Camilleri AC. Treatment history: Relationship to treatment outcomes. Substance use & Misuse. 2009;44:305–321. doi: 10.1080/10826080802344732. [DOI] [PubMed] [Google Scholar]
- Clark RE, Baxter JD. Responses of state medicaid programs to buprenorphine diversion: Doing more harm than good? JAMA Internal Medicine. 2013;173(17):1571–1572. doi: 10.1001/jamainternmed.2013.9059. [DOI] [PubMed] [Google Scholar]
- Clark RE, Samnaliev M, Baxter JD, Leung GY. The evidence doesn't justify steps by state medicaid programs to restrict opioid addiction treatment with buprenorphine. Health Aff (Millwood) 2011;30(8):1425–1433. doi: 10.1377/hlthaff.2010.0532. [DOI] [PubMed] [Google Scholar]
- Clark RE, Samnaliev M, McGovern MP. Impact of substance disorders on medical expenditures for medicaid beneficiaries with behavioral health disorders. Psychiatric Services. 2009;60(1):35–42. doi: 10.1176/ps.2009.60.1.35. [DOI] [PubMed] [Google Scholar]
- Cohen DJ, Crabtree BF, Etz RS, Balasubramanian BA, Donahue KE, Leviton LC, et al. Green LW. Fidelity versus flexibility: Translating evidence-based research into practice. American Journal of Preventive Medicine. 2008;35(5S):S381–S389. doi: 10.1016/j.amepre.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Dennis ML, Scott CK, Funk R, Foss MA. The duration and correlates of addiction and treatment careers. Journal of Substance Abuse Treatment. 2005;28(2):S51–S62. doi: 10.1016/j.jsat.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Grella CE, Hser YI, Joshi V, Anglin MD. Patient histories, retention, and outcome models for younger and older adults in DATOS. Drug and Alcohol Dependence. 1999;57:151–166. doi: 10.1016/s0376-8716(99)00082-4. [DOI] [PubMed] [Google Scholar]
- Heyman GM. Addiction: A disorder of choice. Cambridge, Massachusetts: Harvard University Press; 2009. [Google Scholar]
- Hser YI, Grella CE, Hsieh SC, Anglin MD, Brown BS. Prior treatment experience related to process and outcomes in DATOS. Drug & Alcohol Dependence. 1999;57:137–150. doi: 10.1016/s0376-8716(99)00081-2. [DOI] [PubMed] [Google Scholar]
- Hubbard RL, Craddock SG, Anderson J. Overview of 5-year followup outcomes in the drug abuse treatment outcome studies (DATOS) Journal of Substance Abuse Treatment. 2003;25(3):125–134. doi: 10.1016/s0740-5472(03)00130-2. [DOI] [PubMed] [Google Scholar]
- Kaiser Commission on Medicaid and the Uninsured. Medicaid: A primer. Kaiser Family Foundation; 2013. Issue Brief. [Google Scholar]
- Kronick R, Gilmer T, Dreyfus T, Lee L. Improving health-based payment for Medicaid beneficiaries: CDPS(chronic illness and disability payment system) Health Care Financing Review. 2000;21(3):29. [PMC free article] [PubMed] [Google Scholar]
- Mark TL, Levit KR, Yee T, Chow CW. Spending on mental health and substance use disorders projected to grow more slowly than all health spending through 2020. Health Affairs. 2014;33:1407–1415. doi: 10.1377/hlthaff.2014.0163. [DOI] [PubMed] [Google Scholar]
- Mattick RP, Kimber J, Breen C, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database of Systematic Reviews pub4. 2014 doi: 10.1002/14651858.CD002207.pub3. Systematic Review No. CD002207. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Lewis DC, O'Brien CP, Kleber HD. Drug dependence, a chronic medical illness: Implications for treatment, insurance, and outcomes evaluation. Journal of the American Medical Association. 2000;284(13):1689–95. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- Parthasarathy S, Weisner C, Hu TW, Moore C. Association of outpatient alcohol and drug treatment with health care utilization and cost: Revisiting the offset hypothesis. Journal of Studies on Alcohol. 2001;62(1):89–97. doi: 10.15288/jsa.2001.62.89. [DOI] [PubMed] [Google Scholar]
- Rinaldo SG, Rinaldo DW. Advancing access to addiction medications. availability without accessibility? state medicaid coverage and authorization requirements for opioid dependence medications. (No. 1). online: American Society of Addiction Medicine; 2013. [Google Scholar]
- SAS Institute Inc. SAS version 8. Cary, NC: SAS Institute Inc; 1999. [Google Scholar]
- Simpson DD, Joe GW. A longitudinal evaluation of treatment engagement and recovery stages. Journal of Substance Abuse Treatment. 2004;27(2):89–97. doi: 10.1016/j.jsat.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Friedan TR, Hyde PS, Cha SS. Medication-assisted therapies--tackling the opioid overdose epidemic. New England Journal of Medicine. 2014;370:2063–2066. doi: 10.1056/NEJMp1402780. [DOI] [PubMed] [Google Scholar]