Abstract
Background
Obstructive sleep apnea (OSA) has been associated with clinical deterioration in postoperative patients and patients hospitalized with pneumonia. Paradoxically, OSA has also been associated with decreased risk of inpatient mortality in these same populations.
Objectives
To investigate the association between OSA and in-hospital mortality in a large cohort of surgical and nonsurgical ward patients.
Design
Observational cohort study.
Setting
A 500-bed academic tertiary care hospital in the United States.
Patients
93,676 ward admissions from 53,150 unique adult patients between November 1, 2008 and October 1, 2013.
Intervention
None.
Measurements
OSA diagnoses and comorbidities were identified by ICD-9-CM codes. Logistic regression was used to control for patient characteristics, location prior to ward admission, and admission severity of illness. The primary outcome was in-hospital death. Secondary outcomes included RRT activation, ICU transfer, intubation, and cardiac arrest on the wards.
Main results
OSA was identified in 5,625 (10.6%) patients. Patients with OSA were more likely to be older, male, obese, and had higher rates of comorbidities. OSA patients had more frequent RRT activations (1.5% vs 1.1%) and ICU transfers (8% vs 7%) than controls (p < 0.001 for both comparisons), but a lower inpatient mortality rate (1.1% vs 1.4%, p < 0.05). OSA was associated with decreased adjusted odds for ICU transfer (OR 0.91 [0.84-0.99]), cardiac arrest (OR 0.72 [0.55-0.95]), and in-hospital mortality (OR 0.70 [0.58-0.85]).
Conclusions
After adjustment for important confounders, OSA was not associated with clinical deterioration on the wards and was associated with significantly decreased in-hospital mortality.
Keywords: obstructive sleep apnea, hospital mortality, hospital rapid response team, in-hospital cardiac arrest
INTRODUCTION
Obstructive sleep apnea (OSA) is an increasingly prevalent condition characterized by intermittent airway obstruction during sleep, which leads to hypoxemia, hypercapnia, and fragmented sleep. The current prevalence estimates of moderate to severe OSA (apnea-hypopnea index ≥ 15, measured as events/hour) in middle-aged adults are approximately 13% in men and 6% in women.1 OSA is a well-described independent risk factor for long-term neurocognitive, cardiovascular and cerebrovascular morbidity and mortality.2–6
Recent studies have also identified OSA as an independent risk factor for adverse perioperative outcomes, including endotracheal intubation, ICU transfer, and increased length of stay.7–11 Paradoxically, despite an increase in the risk of complications, several of these studies did not find an association between in-hospital death and OSA even after controlling for potential confounders.9–11 Further, a recent study of patients hospitalized for pneumonia reported increased rates of clinical deterioration and mechanical ventilation, but also lower odds of inpatient mortality in patients with OSA.12
These studies may have been limited by absence of physiologic data, which prevented controlling for severity of illness. It is also unclear whether these previously described associations between OSA and adverse clinical outcomes hold true for general hospital inpatients. OSA may be worsened by medications frequently used in hospitals, such as narcotics and benzodiazepines. Opiate use contributes to both central and obstructive sleep apneas,13,14 and benzodiazepines are known to produce airway smooth muscle relaxation and can cause respiratory depression.15 In fact, the use of benzodiazepines has been implicated in the unmasking of OSA in patients with previously undiagnosed sleep-disordered breathing.16 These findings suggest mechanisms by which OSA could contribute to an increased risk in hospital ward patients for rapid response team (RRT) activation, intensive care unit (ICU) transfer, cardiac arrest (CA), and in-hospital death.
The aim of this study was to determine the independent association between OSA and in-hospital mortality in ward patients. We also aimed to investigate the association of OSA with clinical deterioration on the wards, while controlling for patient characteristics, initial physiology, and severity of illness.
MATERIALS AND METHODS
Setting and Study Population
This observational cohort study was performed at an academic tertiary care medical center with approximately 500 beds. Data were obtained from all adult patients hospitalized on the wards between November 1, 2008, and October 1, 2013. Our hospital has utilized a rapid response team (RRT), led by a critical care nurse and respiratory therapist with hospitalist and pharmacist consultation available upon request, since 2008. This team is separate from the team that responds to a cardiac arrest. Criteria for RRT activation include “tachypnea,” “tachycardia,” “hypotension,” and “staff worry,” but specific vital sign thresholds are not specified.
The study analyzed de-identified data from the hospital’s Clinical Research Data Warehouse (CRDW), which is maintained by the Center for Research Informatics (CRI) at The University of Chicago. The study protocol was approved by the University of Chicago Institutional Review Board (IRB #16995A).
Data Collection
Patient age, sex, race, body mass index (BMI), and location prior to ward admission (i.e. whether they were admitted from the emergency department, transferred from the intensive care unit (ICU), or directly admitted from clinic or home) were collected. Patients who underwent surgery during their admission were identified using the hospital’s admission-transfer-discharge database. In addition, routinely collected vital signs (e.g. respiratory rate, blood pressure, heart rate) were obtained from the electronic health record (EPIC, Verona, WI). To determine severity of illness, the first set of vital signs measured on hospital presentation were utilized to calculate the cardiac arrest risk triage (CART) score, a vital-sign-based early warning score we previously developed and validated for predicting adverse events in our population.17 The CART score ranges from 0 to 57, with points assigned for abnormalities in respiratory rate, heart rate, diastolic blood pressure, and age. If any vital sign was missing, the next available measurement was pulled into the set. If any vital sign remained missing after this change, the median value for that particular location (i.e. wards, ICU, or emergency department) was imputed as previously described.18,19
Patients with OSA were identified by the following ICD-9 codes using inpatient and outpatient medical records: 278.03, 327.20, 327.23, 327.29, 780.51, 780.53, and 780.57 (Table 1). Data on other patient comorbidities, including coronary artery disease (CAD), congestive heart failure (CHF), arrhythmias, uncomplicated and complicated diabetes mellitus, hypertension, and cerebrovascular disease were collected using specific ICD-9 codes from both inpatient and outpatient records. Information on insurance payer was also collected from the hospital’s billing database. Insurance payers were grouped into the following categories: private payer, Medicare/Medicaid, and no insurance. Patients with both public and private payers were counted as being privately insured.
Table 1.
Diagnosis codes and prevalence of obstructive sleep apnea.
| Diagnosis Code |
Description | % of sleep apnea diagnoses* |
|---|---|---|
| 327.23 | Obstructive sleep apnea | 65.6 |
| 780.57 | Unspecified sleep apnea | 19.4 |
| 780.53 | Hypersomnia with sleep apnea, unspecified | 11.7 |
| 780.51 | Insomnia with sleep apnea, unspecified | 1.5 |
| 327.2 | Organic sleep apnea, unspecified | 0.2 |
| 278.03 | Obesity hypoventilation syndrome | 1.7 |
Percentages add to > 100% as a small number of patients carried more than one sleep apnea diagnosis
Outcomes
The primary outcome of the study was in-hospital mortality. Secondary outcomes included length of stay (LOS), RRT activation, transfer to the ICU, endotracheal intubation, cardiac arrest (defined as a loss of pulse with attempted resuscitation) on the wards, and a composite outcome of RRT activation, ICU transfer, and death. Because cardiac arrests on the wards result either in death or ICU transfer following successful resuscitation, this variable was omitted from the composite outcome. Cardiac arrests were identified using a prospectively validated quality improvement database that has been described previously.20 ICU transfer was identified using the hospital’s admission-transfer-discharge database. Only the index cardiac arrest, intubation, RRT, or ICU transfer for each admission was used in the study, but more than one type of outcome could occur for each patient (e.g. a patient who died following an unsuccessful resuscitation attempt would count as both a cardiac arrest and a death).
Statistical Analysis
Patient characteristics were compared using Student’s t tests, Wilcoxon rank-sum tests, and chi-squared statistics, as appropriate. Unadjusted logistic regression models were fit to estimate the change in odds of each adverse event and a composite outcome of any event for patient admissions with OSA compared to those without OSA. Adjusted logistic regression models were then fit for each outcome to control for patient characteristics (age, sex, body-mass index (BMI), insurance status, and individual co-morbidities), location immediately prior to ward admission, and admission severity of illness (as measured by CART score). In the adjusted model, CART score, age, and number of comorbidities were entered linearly, with the addition of squared terms for age and CART score, as these variables showed nonlinear associations with the outcomes of interest. Race, surgical status, insurance payer, location prior to ward, and BMI (underweight, < 18.5 kg/m2; normal weight, 18.5–24.9 kg/m2; overweight, 25.0–29.9 kg/m2; obese, 30–39.9 kg/m2; and severely obese, (≥ 40 mg/m2) were modeled as categorical variables.
Given that an individual patient could experience multiple hospitalizations during the study period, we performed a sensitivity analysis of all adjusted and unadjusted models using a single randomly selected hospitalization for each unique patient. In addition, we performed a sensitivity analysis of all patients who were not admitted to the ICU prior to their ward stay. Finally, we performed subgroup analyses of all unadjusted and adjusted models for each BMI category and surgical status.
All tests of significance used a two-sided p-value less than 0.05. Statistical analyses were completed using Stata version 12.0 (StataCorp, College Station, TX).
RESULTS
Patient Characteristics
During the study period, 93,676 patient admissions from 53,150 unique patients resulted in the occurrence of 1,069 RRT activations, 6,305 ICU transfers, and 1,239 in-hospital deaths. Within our sample, 40,034 patients had at least one inpatient record and at least one outpatient record. OSA diagnosis was present in 5,625 patients (10.6% of the total sample), with 4,748 patients having an OSA diagnosis code entered during a hospitalization, 2,143 with an OSA diagnosis code entered during an outpatient encounter, and 877 with both inpatient and outpatient diagnosis codes. These patients identified as having OSA contributed 12,745 (13.6%) hospital admissions.
Patients with an OSA diagnosis were more likely to be older (median age 59 [IQR 49-68] vs. 55 [38-68]), male (49% vs 42%), overweight or obese (88% vs 62%), and more likely to carry diagnoses of diabetes (53.8% vs 25.5%), hypertension (45.3% vs 18.2%), arrhythmias (44.4% vs 26.7%), coronary artery disease (46.8% vs 23.5%), heart failure (35.8% vs 13.5%), and cerebrovascular disease (13.5% vs 8.1%) than patients without an OSA diagnosis (all comparisons significant, p < 0.001) (Table 2).
Table 2.
Patient characteristics for admissions with and without OSA diagnosis.
| Characteristic | Patient admissions with OSA diagnoses (n = 12,745) |
Patient admissions without OSA diagnoses (n = 80,931) |
p value |
|---|---|---|---|
| Age, years, median (IQR) | 59 (49–68) | 55 (38–68) | <0.001 |
| Female, n (%) | 6,514 (51%) | 47,202 (58%) | <0.001 |
| Race, n (%) | <0.001 | ||
| White | 4,205 (33%) | 30,119 (37%) | |
| Black / African-American | 7,024 (55%) | 38,561 (48%) | |
| Asian | 561 (4.4%) | 3,419 (4.2%) | |
| American Indian or Native Alaskan | 20 (0.2%) | 113 (0.1%) | |
| More than one race | 127 (1%) | 843 (1%) | |
| Race unknown | 808 (6%) | 7,876 (10%) | |
| Insurance status, n (%) | <0.001 | ||
| Private | 4,484 (35%) | 32,467 (40%) | |
| Medicare/Medicaid | 8,201 (64%) | 42,208 (58%) | |
| Uninsured | 53 (0.4%) | 1,190 (1%) | |
| Unknown | 4 (<0.1%) | 16 (<0.1%) | |
| Location prior to wards, n (%) | <0.001 | ||
| ICU | 1,400 (11%) | 8,065 (10%) | |
| Emergency Department | 4,633 (36%) | 25,170 (31%) | |
| Ambulatory Admission | 6,712 (53%) | 47,696 (59%) | |
| Body Mass Index, kg/m2, n (%) | <0.001 | ||
| Normal (18.5 - 25) | 1,431 (11%) | 26,560 (33%) | |
| Underweight (< 18.5) | 122 (1%) | 4,256 (5%) | |
| Overweight (25 - 30) | 2,484 (20%) | 23,761 (29%) | |
| Obese (30–40) | 4,959 (39%) | 19,132 (24%) | |
| Severely obese (> 40) | 3,745 (29%) | 7,171 (9%) | |
| Initial Cardiac Arrest Risk Triage (CART) Score, median (IQR) | 4 (0–9) | 4 (0–9) | <0.001 |
| Underwent surgery, n (%) | 4,482 (35%) | 28,843 (36%) | 0.3 |
| Comborbidities | |||
| Number of Comorbidities, median (IQR) | 2 (1–4) | 1 (0–2) | <0.001 |
| Arrhythmia | 5,659 (44%) | 21,581 (27%) | <0.001 |
| Diabetes Mellitus | 6,855 (54%) | 20,641 (26%) | <0.001 |
| Hypertension | 5,777 (45%) | 14,728 (18%) | <0.001 |
| Coronary Artery Disease | 5,958 (47%) | 18,979 (23%) | <0.001 |
| Cerebrovascular Accident | 1,725 (14%) | 6,556 (8%) | <0.001 |
| Congestive Heart Failure | 4,559 (36%) | 10,919 (13%) | <0.001 |
Abbreviations: OSA = obstructive sleep apnea; IQR = interquartile range; ICU = intensive care unit
Complications and Adverse Outcomes
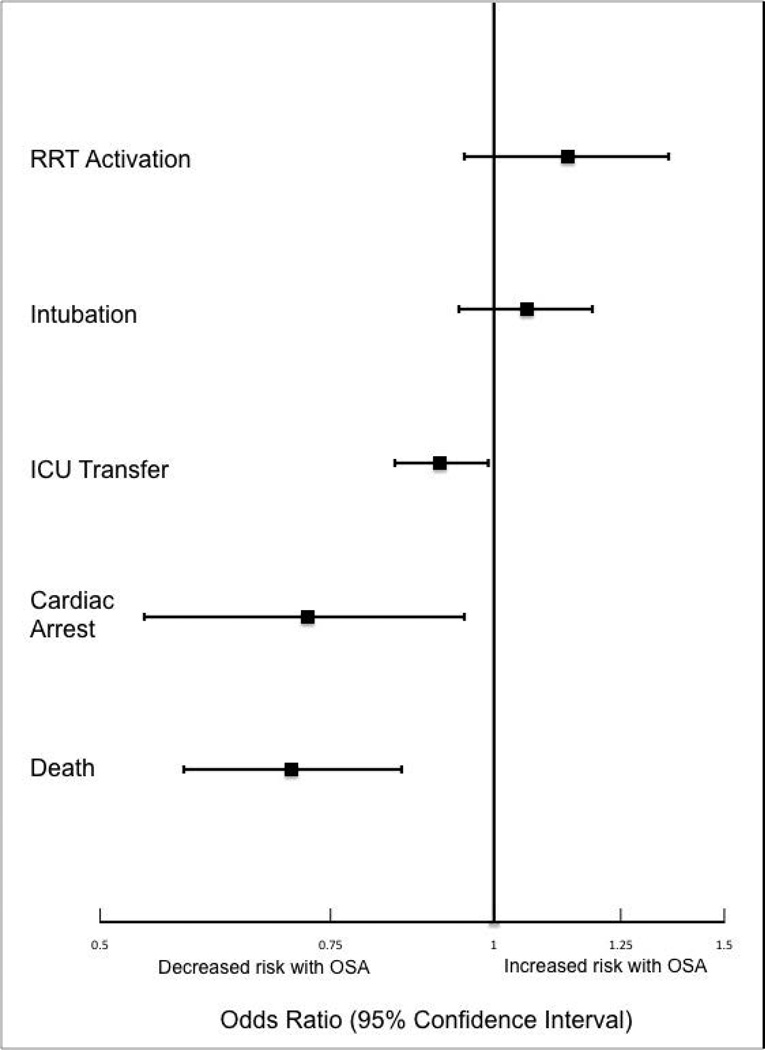
In the unadjusted analyses, the overall incidence of adverse outcomes was higher among patient admissions with a diagnosis of OSA compared to those without OSA (Table 3). Those with OSA were more likely to experience RRT activation (1.5% vs 1.1%), ICU transfer (8% vs 7%), and endotracheal intubation (3.9% vs 2.9%) than those without OSA diagnoses (p < 0.001 for all comparisons). There was no significant difference in the incidence of cardiac arrest between the two groups, nor was there a significant difference in length of stay. Unadjusted inpatient mortality for OSA patient admissions was lower than that for non-OSA hospitalizations (1.1% vs 1.4%, p < 0.05). A diagnosis of OSA was associated with increased unadjusted odds for RRT activation (OR 1.36 [1.16-1.59]) and ICU transfer (OR 1.28 [1.20-1.38]). However, after controlling for confounders, OSA was not associated with increased odds for RRT activation (OR 1.14 [0.95-1.36]) or intubation (OR 1.06 [0.94-1.19]), and was associated with slightly decreased odds for ICU transfer (OR 0.91 [0.84-0.99]) (Figure 1). Those with OSA had decreased adjusted odds of cardiac arrest (OR 0.72 [0.55-0.95]) compared to those without OSA. OSA was also associated with decreased odds of in-hospital mortality before (OR 0.83 [0.70-0.99]) and after (OR 0.70 [0.58-0.85]) controlling for confounders.
Table 3.
Unadjusted outcomes for patient admissions with and without OSA diagnosis.
| Characteristic | Patient admissions with OSA diagnoses (n = 12,745) |
Patient admissions without OSA diagnoses (n = 80,931) |
p value |
|---|---|---|---|
| Outcomes, n (%) | |||
| Composite Outcome* | 1,137 (9%) | 5,792 (7%) | <0.001 |
| In-hospital Death | 144 (1.1%) | 1,095 (1.4%) | 0.04 |
| Rapid Response Team Call | 188 (1.5%) | 881 (1.1%) | <0.001 |
| ICU transfer | 1,045 (8%) | 5,260 (7%) | <0.001 |
| Cardiac Arrest | 413 (0.5%) | 73 (0.6%) | 0.36 |
denotes experiencing RRT call, ICU transfer, or in-hospital death
Abbreviations: OSA = obstructive sleep apnea; ICU = intensive care unit; RRT = rapid response team
Figure 1. Adjusted models for the association of OSA with clinical deterioration outcomes.
Odds of RRT activation, intubation, ICU transfer, cardiac arrest, and in-hospital death for patient admissions with OSA diagnosis as compared to patient admissions without OSA diagnosis.
Sensitivity Analyses
The sensitivity analysis involving one randomly selected hospitalization per patient included a total of 53,150 patients. The results were similar to the main analysis, with adjusted odds of 1.01 (0.77-1.32) for RRT activation, 0.86 (0.76-0.96) for ICU transfer, and 0.69 (0.53-0.89) for inpatient mortality. An additional sensitivity analysis included only patients who were not admitted to the ICU prior to their ward stay. This analysis included 84,211 hospitalizations and demonstrated similar findings, with adjusted odds of 0.70 for in-hospital mortality (0.57-0.87). Adjusted odds for RRT activation (1.12 [0.92-1.37]) and ICU transfer (0.88 [0.81-0.96] were also similar to the results of our main analysis.
Subgroup Analyses
Surgical and Nonsurgical Patients
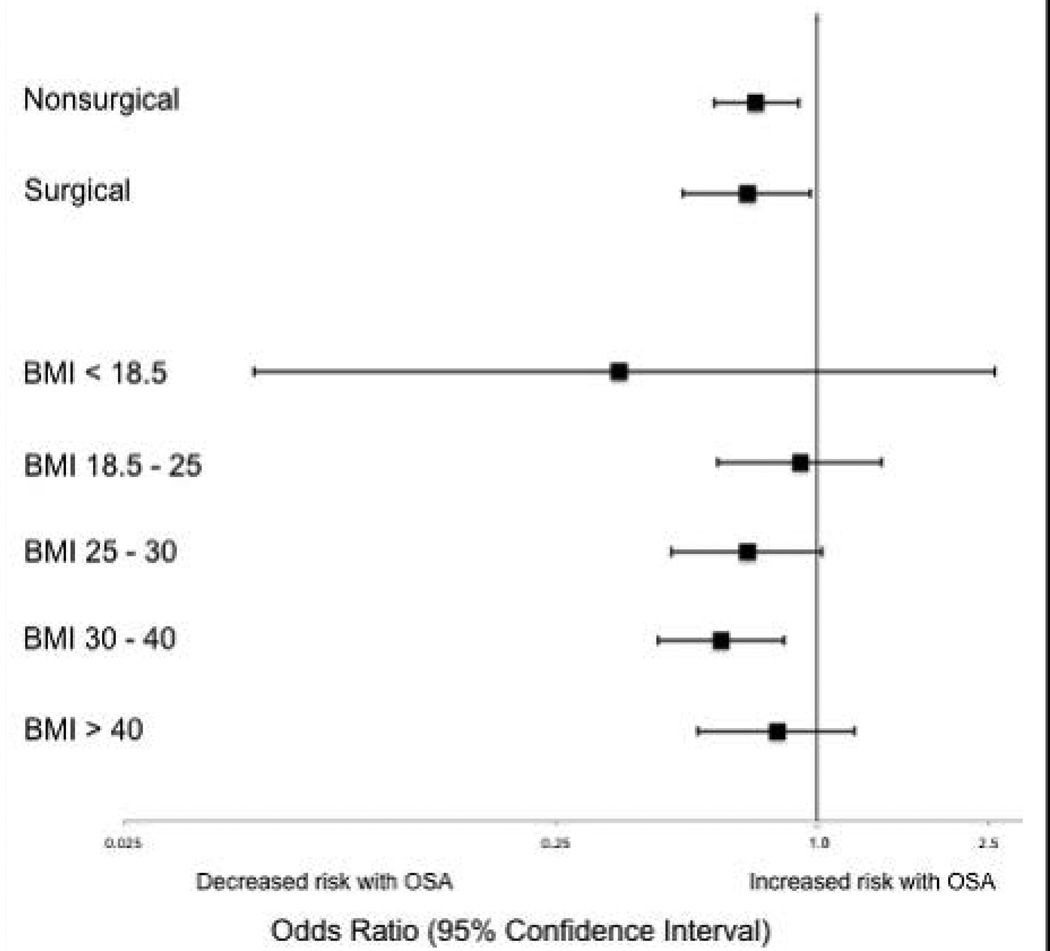
Subgroup analyses of surgical versus nonsurgical patients (Figure 2) revealed similarly decreased adjusted odds of in-hospital death for OSA patients in both groups (surgical OR 0.69 [0.49-0.97]; nonsurgical OR 0.72 [0.58-0.91]). Surgical patients with OSA diagnoses had decreased adjusted odds for ICU transfer (surgical OR 0.82 [0.73-0.92], but this finding was not seen in nonsurgical patients (OR 1.03 [0.92-1.15]).
Figure 2. Adjusted models for the association of OSA with death, by surgical status and body-mass index.
Odds of in-hospital death for patient admissions with OSA diagnosis as compared to patient admissions without OSA diagnosis, stratified by surgical status and body-mass index.
Patients Stratified by BMI
Examination across BMI categories (Figure 2) showed a significant decrease in adjusted odds of death for OSA patients with BMI 30–40 kg/m2 (OR 0.60 [0.43-0.84]). A nonsignificant decrease in adjusted odds of death was seen for OSA patients in all other groups. Adjusted odds ratios for the risk of RRT activation and ICU transfer in OSA patients within the different BMI categories were not statistically significant.
DISCUSSION
In this large observational single-center cohort study, we found that OSA was associated with increased odds of adverse events, such as ICU transfers and RRT calls, but this risk was no longer present after adjusting for demographics, comorbidities, and presenting vital signs. Interestingly, we also found that patients with OSA had decreased adjusted odds for cardiac arrest and mortality. This mortality finding was robust to multiple sensitivity analyses and subgroup analyses. These results have significant implications for our understanding of the short-term risks of sleep-disordered breathing in hospitalized patients, and suggest the possibility that OSA is associated with a protective effect with regard to inpatient mortality.
Our findings are in line with other recent work in this area. In two large observational cohorts of surgical populations drawn from the nationally representative Nationwide Inpatient Sample administrative database, our group reported decreased odds of in-hospital postoperative mortality in OSA patients.10,11 Using the same Nationwide Inpatient Sample, Lindenauer et al. showed that among inpatients hospitalized with pneumonia, OSA diagnosis was associated with increased rates of clinical deterioration but lower rates of inpatient mortality.12 While these three studies have identified decreased inpatient mortality among certain surgical populations and patients hospitalized with pneumonia, they are limited by using administrative databases that do not provide specific data on vital signs, presenting physiology, BMI, or race. Another important limitation of the Nationwide Inpatient Sample is the lack of any information on RRT activations and ICU transfers. Moreover, the database does not include information on outpatient diagnoses, which may have led to significantly lower prevalence of OSA than expected in these studies. Despite the important methodological differences, our study corroborates this finding among a diverse cohort of hospitalized patients. Unlike these previous studies of postoperative patients or those hospitalized with pneumonia, we did not find an increased risk of adverse events associated with OSA after controlling for potential confounders.
The decreased mortality seen in OSA patients could be explained by these patients receiving more vigilant care, showing earlier signs of deterioration, or displaying more easily treatable forms of distress than patients without OSA. For example, earlier identification of deterioration could lead to earlier interventions, which could decrease inpatient mortality. Indeed, in two studies of postsurgical patients,10,11 those with OSA diagnosis who developed respiratory failure were intubated earlier and received mechanical ventilation for a shorter period of time, suggesting that the cause of respiratory failure was rapidly reversible (e.g. upper airway complications due to over-sedation or excessive analgesia). However, we did not find increased adjusted odds of RRT activation or ICU transfer for OSA patients in our study, and so it is less likely that earlier recognition of decompensation occurred in our sample. In addition, our hospital did not have standardized practices for monitoring or managing OSA patients during the study period, which makes systematic early recognition of clinical deterioration among the OSA population in our study less likely.
Alternatively, there may be a true physiologic phenomenon providing a short-term mortality benefit in those with OSA. It has been observed that patients with obesity (but without severe obesity) often have better outcomes after acute illness, whether by earlier or more frequent contact with medical care or heightened levels of metabolic reserve.21,22 However, our findings of decreased mortality for OSA patients remained even after controlling for BMI. An additional important possibility to consider is ischemic preconditioning, a well-described phenomenon in which episodes of sublethal ischemia confer protection on tissues from subsequent ischemia/reperfusion damage.23 Ischemic preconditioning has been demonstrated in models of cardiac and neural tissue24–26 and has been shown to enhance stem cell survival by providing resistance to necrosis and lending functional benefits to heart, brain, and kidney models after transplantation.25–31The fundamentals of this concept may have applications in transplant and cardiac surgery,32,33 in the management of acute coronary syndromes and stroke32,34,35 and in athletic training and performance.35,36 Although OSA has been associated with long-term cardiovascular morbidity and mortality,2–6 the intermittent hypoxemia OSA patients experience could actually improve their ability to survive clinical deterioration in the short-term (i.e. during a hospitalization).
Limitations of our study include its conduction at a single center, which may prevent generalization to populations different than ours. Further, during the study period our hospital did not have formal guidelines or standardized management or monitoring practices for patients with OSA. Additionally, practices for managing OSA may vary across institutions. Therefore, our results may not be generalizable to hospitals with such protocols in place. However, as mentioned above, similar findings have been noted in studies using large, nationally representative administrative databases. In addition, we identified OSA via ICD-9-CM codes, which is likely insensitive for estimating the true prevalence of OSA in our sample. Despite this, our reported OSA prevalence of over 10% falls within the prevalence range reported in large epidemiological studies.37–39 Finally, we did not have data on polysomnograms or treatment received for patients with OSA, so we do not know the severity of OSA or adequacy of treatment for these patients.
Notwithstanding our limitations, our study has several strengths. First, we included a large number of hospitalized patients across a diverse range of medical and surgical ward admissions, which increases the generalizability of our results. We also addressed potential confounders by including a large number of comorbidities and controlling for severity of presenting physiology with the CART score. The CART score, which contains physiologic variables such as respiratory rate, heart rate, and diastolic blood pressure, is an accurate predictor of cardiac arrest, ICU transfer, and in-hospital mortality in our population.40 Finally, we were able to obtain information about these diagnoses from outpatient as well as inpatient data.
In conclusion, we found that after adjustment for important confounders OSA was associated with a decrease in hospital mortality and cardiac arrest but not with other adverse events on the wards. These results may suggest a protective benefit from OSA with regard to mortality, an advantage that could be explained by ischemic preconditioning or a higher level of care or vigilance not reflected by the number of RRT activations or ICU transfers experienced by these patients. Further research is needed to confirm these findings across other populations, to investigate the physiologic pathways by which OSA may produce these effects, and to examine the mechanisms by which treatment of OSA could influence these outcomes.
ACKNOWLEDGMENTS
We would like to thank Nicole Babuskow for administrative support, as well as Brian Furner and Timothy Holper for assistance with data acquisition.
Conflicts of Interest and Source of Funding: Drs. Churpek and Edelson have a patent pending (ARCD. P0535US.P2) for risk stratification algorithms for hospitalized patients. Dr. Churpek and Dr. Edelson are both supported by career development awards from the National Heart, Lung, and Blood Institute (K08 HL121080 and K23 HL097157, respectively). Dr. Churpek has received honoraria from Chest for invited speaking engagements. In addition, Dr. Edelson has received research support and honoraria from Philips Healthcare (Andover, MA), research support from the American Heart Association (Dallas, TX) and Laerdal Medical (Stavanger, Norway), and an honorarium from Early Sense (Tel Aviv, Israel). She has ownership interest in Quant HC (Chicago, IL), which is developing products for risk stratification of hospitalized patients. Dr. Mokhlesi is supported by National Institutes of Health grant R01HL119161. Dr. Mokhlesi has served as a consultant to Philips/Respironics and has received research support from Philips/Respironics.
Abbreviations
- OSA
obstructive sleep apnea
- ICU
intensive care unit
- RRT
rapid response team
- ICD-9-CM
International Classification of Diseases, 9th Revision, Clinical Modification
- OR
odds ratio
- CA
cardiac arrest
- CART Ccore
Cardiac Arrest Risk Triage Score
- CAD
coronary artery disease
- CHF
congestive heart failure
- BMI
body mass index
Footnotes
Author contributions: Study concept and design: P.L., D.P.E, B.M., M.C.; acquisition of data: P.L.; analysis and interpretation of data: all authors; first drafting of the manuscript: P.L.; critical revision of the manuscript for important intellectual content: all authors; statistical analysis: P.L., F.Z., M.C.; obtained funding: D.P.E., M.C.; administrative, technical, and material support: F.Z., D.P.E.; study supervision: D.P.E, B.M., M.C. Data access and responsibility: P.L. and M.C. had full access to all the data in the study, and take responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- 1.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marin JM, Carrizo SJ, Vicente E, Agusti AGN. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: An observational study. Lancet. 2005;365(9464):1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 3.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 4.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353(19):2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 5.Kendzerska T, Gershon AS, Hawker G, Leung RS, Tomlinson G. Obstructive Sleep Apnea and Risk of Cardiovascular Events and All-Cause Mortality: A Decade-Long Historical Cohort Study. PLoS Med. 2014;11(2):e1001599. doi: 10.1371/journal.pmed.1001599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marshall NS, Wong KK, Liu PY, Cullen SRJ, Knuiman MW, Grunstein RR. Sleep apnea as an independent risk factor for all-cause mortality: the Busselton Health Study. Sleep. 2008;31(8):1079–1085. [PMC free article] [PubMed] [Google Scholar]
- 7.Kaw R, Pasupuleti V, Walker E, Ramaswamy A, Foldvary-Schafer N. Postoperative complications in patients with obstructive sleep apnea. Chest. 2012;141(2):436–441. doi: 10.1378/chest.11-0283. [DOI] [PubMed] [Google Scholar]
- 8.Kaw R, Chung F, Pasupuleti V, Mehta J, Gay PC, Hernandez AV. Meta-analysis of the association between obstructive sleep apnoea and postoperative outcome. Br J Anaesth. 2012;109(6):897–906. doi: 10.1093/bja/aes308. [DOI] [PubMed] [Google Scholar]
- 9.Memtsoudis SG, Stundner O, Rasul R, et al. The impact of sleep apnea on postoperative utilization of resources and adverse outcomes. Anesth Analg. 2014;118(2):407–418. doi: 10.1213/ANE.0000000000000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mokhlesi B, Hovda MD, Vekhter B, Arora VM, Chung F, Meltzer DO. Sleep-disordered breathing and postoperative outcomes after bariatric surgery: Analysis of the nationwide inpatient sample. Obes Surg. 2013;23(11):1842–1851. doi: 10.1007/s11695-013-0991-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mokhlesi B, Hovda MD, Vekhter B, Arora VM, Chung F, Meltzer DO. Sleep-disordered breathing and postoperative outcomes after elective surgery: Analysis of the nationwide inpatient sample. Chest. 2013;144(3):903–914. doi: 10.1378/chest.12-2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindenauer PK, Stefan MS, Johnson KG, Priya A, Pekow PS, Rothberg MB. Prevalence, Treatment and Outcomes Associated with Obstructive Sleep Apnea Among Patients Hospitalized with Pneumonia. Chest. 2014;145(5):1032–1038. doi: 10.1378/chest.13-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doufas AG, Tian L, Padrez KA, et al. Experimental Pain and Opioid Analgesia in Volunteers at High Risk for Obstructive Sleep Apnea. PLoS One. 2013;8(1):e54807. doi: 10.1371/journal.pone.0054807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gislason T, Almqvist M, Boman G, Lindholm CE, Terenius L. Increased CSF opioid activity in sleep apnea syndrome. Regression after successful treatment. Chest. 1989;96(2):250–254. doi: 10.1378/chest.96.2.250. [DOI] [PubMed] [Google Scholar]
- 15.Koga Y, Sato S, Sodeyama N, et al. Comparison of the relaxant effects of diazepam, flunitrazepam and midazolam on airway smooth muscle. Br J Anaesth. 1992;69(1):65–69. doi: 10.1093/bja/69.1.65. [DOI] [PubMed] [Google Scholar]
- 16.Dolly FR, Block AJ. Effect of flurazepam on sleep-disordered breathing and nocturnal oxygen desaturation in asymptomatic subjects. Am J Med. 1982;73(2):239–243. doi: 10.1016/0002-9343(82)90185-1. [DOI] [PubMed] [Google Scholar]
- 17.Churpek MM, Yuen TC, Park SY, Meltzer DO, Hall JB, Edelson DP. Derivation of a cardiac arrest prediction model using ward vital signs*. Crit Care Med. 2012;40(7):2102–2108. doi: 10.1097/CCM.0b013e318250aa5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Churpek MM, Yuen TC, Park SY, Gibbons R, Edelson DP. Using electronic health record data to develop and validate a prediction model for adverse outcomes in the wards*. Crit Care Med. 2014;42(4):841–848. doi: 10.1097/CCM.0000000000000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knaus WA, Wagner DP, Draper EA, et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100(6):1619–1636. doi: 10.1378/chest.100.6.1619. [DOI] [PubMed] [Google Scholar]
- 20.Churpek MM, Yuen TC, Huber MT, Park SY, Hall JB, Edelson DP. Predicting cardiac arrest on the wards: A nested case-control study. Chest. 2012;141(5):1170–1176. doi: 10.1378/chest.11-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Memtsoudis SG, Bombardieri AM, Ma Y, Walz JM, Chiu YL, Mazumdar M. Mortality of patients with respiratory insufficiency and adult respiratory distress syndrome after surgery: the obesity paradox. J Intensive Care Med. 2012;27(4):306–311. doi: 10.1177/0885066611411410. [DOI] [PubMed] [Google Scholar]
- 22.Bucholz EM, Rathore SS, Reid KJ, et al. Body mass index and mortality in acute myocardial infarction patients. Am J Med. 2012;125(8):796–803. doi: 10.1016/j.amjmed.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74(5):1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 24.Murry CE, Richard VJ, Reimer KA, Jennings RB. Ischemic preconditioning slows energy metabolism and delays ultrastructural damage during a sustained ischemic episode. Circ Res. 1990;66(4):913–931. doi: 10.1161/01.res.66.4.913. [DOI] [PubMed] [Google Scholar]
- 25.Hu X, Yu SP, Fraser JL, et al. Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J Thorac Cardiovasc Surg. 2008;135(4):799–808. doi: 10.1016/j.jtcvs.2007.07.071. [DOI] [PubMed] [Google Scholar]
- 26.Yu X, Lu C, Liu H, et al. Hypoxic preconditioning with cobalt of bone marrow mesenchymal stem cells improves cell migration and enhances therapy for treatment of ischemic acute kidney injury. PLoS One. 2013;8(5):e62703. doi: 10.1371/journal.pone.0062703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Francis KR, Wei L. Human embryonic stem cell neural differentiation and enhanced cell survival promoted by hypoxic preconditioning. Cell Death Dis. 2010;1:e22. doi: 10.1038/cddis.2009.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamota T, Li TS, Morikage N, et al. Ischemic Pre-Conditioning Enhances the Mobilization and Recruitment of Bone Marrow Stem Cells to Protect Against Ischemia/Reperfusion Injury in the Late Phase. J Am Coll Cardiol. 2009;53(19):1814–1822. doi: 10.1016/j.jacc.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 29.Hu X, Wei L, Taylor TM, et al. Hypoxic preconditioning enhances bone marrow mesenchymal stem cell migration via Kv2.1 channel and FAK activation. Am J Physiol Cell Physiol. 2011;301(2):C362–C372. doi: 10.1152/ajpcell.00013.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Theus MH, Wei L, Cui L, et al. In vitro hypoxic preconditioning of embryonic stem cells as a strategy of promoting cell survival and functional benefits after transplantation into the ischemic rat brain. Exp Neurol. 2008;210(2):656–670. doi: 10.1016/j.expneurol.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 31.Wei L, Fraser JL, Lu ZY, Hu X, Yu SP. Transplantation of hypoxia preconditioned bone marrow mesenchymal stem cells enhances angiogenesis and neurogenesis after cerebral ischemia in rats. Neurobiol Dis. 2012;46(3):635–645. doi: 10.1016/j.nbd.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kharbanda RK, Nielsen TT, Redington AN. Translation of remote ischaemic preconditioning into clinical practice. Lancet. 2009;374(9700):1557–1565. doi: 10.1016/S0140-6736(09)61421-5. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt MR, Pryds K, Bøtker HE. Novel adjunctive treatments of myocardial infarction. World J Cardiol. 2014;6(6):434–443. doi: 10.4330/wjc.v6.i6.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ara J, De montpellier S. Hypoxic-preconditioning enhances the regenerative capacity of neural stem/progenitors in subventricular zone of newborn piglet brain. Stem Cell Res. 2013;11(2):669–686. doi: 10.1016/j.scr.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 35.Foster GP, Giri PC, Rogers DM, Larson SR, Anholm JD. Ischemic preconditioning improves oxygen saturation and attenuates hypoxic pulmonary vasoconstriction at high altitude. High Alt Med Biol. 2014;15(2):155–161. doi: 10.1089/ham.2013.1137. [DOI] [PubMed] [Google Scholar]
- 36.Jean-St-Michel E, Manlhiot C, Li J, et al. Remote preconditioning improves maximal performance in highly trained athletes. Med Sci Sports Exerc. 2011;43(7):1280–1286. doi: 10.1249/MSS.0b013e318206845d. [DOI] [PubMed] [Google Scholar]
- 37.Durán J, Esnaola S, Rubio R, Iztueta Á. Obstructive sleep apnea-hypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med. 2001;163(3 pt 1):685–689. doi: 10.1164/ajrccm.163.3.2005065. [DOI] [PubMed] [Google Scholar]
- 38.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 39.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 40.Churpek MM, Yuen TC, Edelson DP. Risk stratification of hospitalized patients on the wards. Chest. 2013;143(6):1758–1765. doi: 10.1378/chest.12-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]