Abstract
Background
Patients with cancer frequently transition between different types of specialists and across care settings. We explored how frequently the medical and surgical oncologic care of stage III colon cancer patients occurs across more than one hospital and whether this is associated with mortality and costs.
Methods
This is a retrospective SEER-Medicare cohort study of 9,075 stage III colon cancer patients diagnosed between 2000 and 2009 receiving both surgical and medical oncologic care within one year of diagnosis. Patients were assigned to the hospital where they had their cancer surgery and to their oncologist's primary hospital, and then characterized according to whether these hospitals were same or different. Outcomes included all-cause mortality, subhazards for colon cancer specific mortality, and cost of care at 12 months.
Results
37% of patients received their surgical and medical oncologic care from different hospitals. Rural patients were less likely than urban patients to receive medical oncologic care from the same hospital (OR 0.62, 95%CI 0.43-0.90). Care from the same hospital was not associated with reduced all-cause or colon cancer specific mortality but resulted in lower costs at 12 months (dollars saved $5493, 95%CI $1799, $9525), 8% of median cost.
Conclusions
Delivery of surgical and medical oncology care at the same hospital was associated with lower costs; however, reforms which seek to improve outcomes and cost through integrating complex care will need to address the significant proportion of patients receiving care across more than one hospital.
Keywords: delivery of health care, integrated health care systems, colon cancer, patient care management, health care costs, mortality
Introduction
Fragmentation of care is a central cause of poor quality and high costs in the U.S.1,2 Because of its resource intensity, complexity for patients, and inequities in quality, cancer care has been identified by the Institute of Medicine as a priority area in which to address care fragmentation.3 One strategy for reducing fragmentation involves improving continuity during transitions in care--that is, junctures at which a patient's care switches between providers, settings, or institutions.3,4 Many current health care reforms, including accountable care organizations, seek to create continuity during transitions by developing integrated networks of providers and institutions to deliver complex care.5
At the same time, more cancer patients are receiving care from high volume surgeons located at a few high volume regional surgical centers.6,7 Patients who travel to a hospital for surgical care while receiving oncologic care from a different local hospital may experience increased fragmentation. In some settings, receiving treatment for an illness from more than one hospital is associated with poorer outcomes and delays in care.8,9
For most cancer care, transitioning between specialists (i.e., surgeon and oncologist) and settings (i.e., inpatient and outpatient) is inevitable. However, it is plausible that patient outcomes and costs may improve when cancer patients receive “one-hospital” care—that is, surgical and oncologic care delivered at the same hospital. One-hospital care may ease coordinating follow-up care, decrease barriers to physician communication, and reduce redundancy in care. The effect of this type of care fragmentation (one-hospital versus two-hospital care) on cancer mortality and costs of care is unknown.
Stage III colon cancer provides an important model for examining fragmentation due to two-hospital care. Colorectal cancer is the second most expensive cancer and the third leading cause of cancer mortality in the U.S.10 Further, guidelines for stage III colon cancer recommend timely surgery and adjuvant chemotherapy to improve survival.11 As this requires coordination between two separate providers, across different settings (inpatient surgical care and outpatient medical oncologic care) and possibly at different institutions, stage III colon cancer is particularly vulnerable to care fragmentation. Indeed, a significant proportion of patients do not receive guideline-concordant care and disparities exist.11
To evaluate the association between care fragmentation and outcomes in stage III colon cancer, we examined the associations between one-hospital versus two-hospital care on overall survival, colon cancer-specific survival, and twelve-month costs of care.
Methods
Study Population
We used SEER-Medicare files for patients with colon cancer diagnosed between 2000 and 2009. SEER-Medicare is a population-based cancer registry encompassing approximately 28% of the US population and is linked to claims for approximately 93% of the patients with Medicare.12
Patients with continuous Part A and B Medicare coverage during the 12 months before and after diagnosis date were eligible for inclusion. Patients were excluded if younger than 66, enrolled in an HMO during the two-year interval, diagnosed at autopsy or death, diagnosed with a second cancer within twelve months of the colon cancer diagnosis, had missing information on covariates, were not stage III, or could not be assigned to their surgeon, oncologist, and respective surgical/oncologic hospitals. We only included patients receiving care at hospitals capable of providing both surgical and oncologic care (i.e., hospitals which had both medical oncology and surgical claims associated with it in a given year). The final analytic cohort consisted of 9,075 patients (Figure 1).
Figure 1. Flow chart of inclusions & exclusions for analytic cohort.
Measures
Outcomes
The primary outcome was all-cause mortality; survival time was calculated from date of colon cancer diagnosis to Medicare date of death, or a censor date of December 31, 2011. Colon cancer specific mortality was a secondary outcome; the censor date was December 31, 2009, as cause of death is not available after this date. We also examined total cost of care at 12 months after diagnosis, calculated using inpatient claims (Medicare Provider Analysis and Review file), physician fees (Carrier Claims file), and outpatient claims (Outpatient Statistical Analysis file).
Provider and Hospital Assignment
For surgical care, the operative surgeon was identified as the patient's surgeon, and the location of the procedure was the patient's surgical hospital. For the 713 (7.9%) patients who had more than one colon cancer surgery, assignment of surgical care was based on the first operation. For oncologic care, we assigned patients to a medical oncologist who billed for the plurality of their visits in the year following diagnosis (using BETOS codes M1-M6 to identify appropriate claims and specialty codes 83 and 90), and then designated the hospital where these oncologists were most likely to practice.13 Following Bynum and colleagues’ approach, oncologists were assigned to the hospital in which they billed for the most inpatient care.14 Oncologists who did not bill for any inpatient claims were assigned to the hospital where most of their patients were admitted. Patients were classified as experiencing one-hospital care if they underwent their operation at the same hospital where their oncologist was assigned, and were classified as receiving two-hospital care if the hospitals were different.
Patient Level Covariates
Covariates included age, gender, self-reported Medicare race (Black, White, other), census tract median household income (in quartiles), year of diagnosis, Charlson comorbidity score in the 12 months prior to diagnosis, urban/rural residence, and SEER site. Cancer characteristics included tumor grade, adequate lymph node resection during surgery (≥12 lymph nodes), and, for patients diagnosed 2004 onward, cancer substage.15
Physician Level Covariates
Yearly surgical volume was tabulated using the total number of all colon cancer patients on whom surgeons operated in a given year16, and modeled in quartiles (<2, 2, 3, >3 cases/year). Similarly, the yearly panel size of all colon cancer patients attributed to each medical oncologist was calculated and modeled in quartiles (<2, 2-3, 4-5, >5 cases/year).
Hospital Level Covariates
Hospital characteristics from SEER included National Cancer Institute (NCI)-recognized status, academic hospital status, and for-profit status. We determined the volume of patients who undergo colon cancer surgery at each hospital by summing the total number of all colon cancer patients between 2000 and 2009. Hospital volume was analyzed in quartiles with the following cutoffs: surgical hospital volume (0-111, 112-198, 199-312, >312 cases), oncologic hospital volume (<130, 130-210, 211-320, >320).16,17
Analysis
Multivariable logistic regression controlling for patient, provider, and hospital characteristics was performed to identify characteristics independently associated with one-hospital versus two-hospital care. Survival analysis for all-cause mortality was conducted using Cox proportional hazard models. Fine and Gray's method for competing risk regression was used to model mortality due to colon cancer where death from other causes was a competing risk.18 To assess the proportional hazards assumption, we used a test of non-zero slope.19
Our cost analyses estimated the difference in costs between those receiving one-hospital versus two-hospital care with generalized linear models. Using modified Park tests to determine the distribution and link function20, we modeled non-extreme costs (bottom 95% of patient costs) with a gamma variance distribution and extreme costs (top 5%) using an inverse Gaussian variance distribution. All dollar values were inflated to 2009 using the annual Gross Domestic Product price index.21
For both survival and cost modeling, we used propensity-matched doubly robust regression models. Propensity scores were modeled as a function of all patient, physician, and hospital characteristics using psmatch2 version 3.0 in STATA.22 Balance was optimized with nearest neighbor 1:1 matching with caliper of 0.01 with no replacement. To retain equivalence between patients with one-hospital and two-hospital care, hospital characteristics were coded twice, once as the surgical and once as the oncologic hospital. We corrected for clustering using generalized estimating equations (GEE) and robust standard errors. The clustering unit was the medical-surgical hospital pair in which patients received care.
We performed sensitivity analyses to ensure robustness of findings. First, we modeled all analyses controlling for substage in the subcohort of patients diagnosed 2004 onward. Second, we modeled total cost of care at six months, and costs at both six and twelve months only amongst those surviving to each time respectively. Third, because physician and hospital characteristics may be unknown to patients prior to receiving their care, we re-ran our propensity models including only patient characteristics. Fourth, with known disparities, we tested for interactions between hospital care with race/ethnicity, median census tract income, and urban/rural residence. Fifth, we ran analyses excluding those patients with more than one surgery to avoid misclassification bias.
Data were analyzed used STATA IC 12.1. Our study received approval from the Johns Hopkins University School of Medicine Institutional Review Board.
Results
In our cohort of 9,075 patients, 37% of patients received two-hospital care—that is, they received their medical oncology care from an oncologist not assigned to the patient's surgical hospital (Table 1). Patients in rural areas (population <250,000) were less likely to receive one-hospital surgical and medical oncologic care compared to those in large cities, Odds Ratio (OR) 0.62, 95% Confidence Interval (CI) 0.43-0.90. Patients who received care from higher volume surgeons or from higher volume surgical hospitals were significantly more likely to receive one-hospital care compared to those who received their surgical care from lower counterparts.
Table 1. Characteristics of stage III colon cancer patients, by receipt of one- versus-two hospital medical oncologic and surgical care.
| One-Hospital Care n=5758 (63.45%) | Two-Hospital Care n=3317 (36.55%) | p-value | Odds Ratio (95%CI) for Receiving One Hospital Care* | |
|---|---|---|---|---|
|
| ||||
| PATIENT-LEVEL PREDICTORS | ||||
|
| ||||
| Age | ||||
| >65-70 | 941 (16.3) | 524 (15.8) | 0.066 | Ref |
| 71-75 | 1303 (22.6) | 807 (24.3) | 0.92 (0.79-1.07) | |
| 76-80 | 1473 (25.6) | 896 (27.0) | 0.92 (0.80-1.07) | |
| 81-85 | 1256 (21.8) | 677 (20.4) | 1.02 (0.88-1.18) | |
| ≥86 | 785 (13.6) | 413 (12.5) | 1.04 (0.87-1.24) | |
|
| ||||
| Female | ||||
| 3282 (57.0) | 1886 (56.9) | 0.73 | 0.99 (0.90-1.09) | |
|
| ||||
| Race | ||||
| White | 5003 (86.9) | 2778 (84.1) | <0.001 | Ref |
| Black | 427 (7.4) | 263 (7.9) | 1.07 (0.86-1.33) | |
| Other | 328 (5.7) | 266 (8.0) | 0.82 (0.64-1.05) | |
|
| ||||
| Census Tract | ||||
| Median Income | ||||
| Lowest Quartile | 1369 (23.8) | 1052 (31.7) | <0.001 | Ref |
| 2nd Quartile | 1345 (23.4) | 829 (25.0) | 1.04 (0.88-1.22) | |
| 3rd Quartile | 1390 (24.1) | 721 (21.7) | 1.09 (0.90-1.31) | |
| Highest Quartile | 1654 (28.7) | 715 (21.6) | 1.20 (0.97-1.47) | |
|
| ||||
| Urban/Rural Residence | ||||
| ≥1 million population | 3409 (59.2) | 1689 (50.9) | <0.001 | Ref |
| ≥250000 to < 1 mil | 1679 (29.2) | 880 (26.5) | 0.91 (0.66-1.25) | |
| <250,000 | 670 (11.6) | 748 (22.6) | 0.62 (0.43-0.90) | |
|
| ||||
| Charlson Comorbidity Score | ||||
| 0 | 3226 (56.0) | 1854 (55.9) | 0.89 | Ref |
| 1 | 1435 (24.9) | 840 (25.3) | 1.02 (0.92-1.14) | |
| ≥2 | 1097 (19.1) | 623 (18.8) | 1.07 (0.95-1.21) | |
|
| ||||
| Tumor Grade | ||||
| Well Differentiated | 301 (5.2) | 162 (4.9) | 0.099 | Ref |
| Moderately | 3601 (62.5) | 2131 (64.2) | 0.87 (0.68-1.13) | |
| Poorly | 1716 (29.8) | 966 (29.1) | 0.85 (0.65-1.12) | |
| Undifferentiated | 140 (2.4) | 58 (1.8) | 1.20 (0.78-1.84) | |
|
| ||||
| Adequate lymph node resection | ||||
| <12 | 2000 (34.7) | 1237 (37.3) | Ref | |
| ≥12 | 3758 (65.3) | 2080 (62.7) | 0.014 | 0.97 (0.86-1.09) |
|
| ||||
| Substage† | ||||
| IIIA | 368 (10.5) | 205 (10.3) | 0.96 | Ref |
| IIIB | 1993 (56.8) | 1134 (57.2) | 0.99 (0.81-1.20) | |
| IIIC | 1146 (32.7) | 623 (32.4) | 0.99 (0.81-1.22) | |
|
| ||||
| PROVIDER-LEVEL CHARACTERISTICS | ||||
|
| ||||
| Yearly surgical volume | ||||
| Lowest Quartile | 1161 (20.2) | 901 (27.2) | <0.001 | Ref |
| 2nd Quartile | 1499 (26.0) | 890 (26.8) | 1.03 (0.88-1.10) | |
| 3rd Quartile | 1570 (27.3) | 779 (23.5) | 1.29 (1.14-1.40) | |
| Highest Quartile | 1528 (26.5) | 747 (22.5) | 1.38 (1.02-1.77) | |
|
| ||||
| Yearly oncologist panel size | ||||
| Lowest Quartile | 1432 (24.9) | 885 (26.7) | <0.001 | Ref |
| 2nd Quartile | 1499 (26.0) | 767 (23.1) | 0.88 (0.69-1.21) | |
| 3rd Quartile | 1401 (24.3) | 750 (22.6) | 0.93 (0.77-1.15) | |
| Highest Quartile | 1426 (24.8) | 915 (27.6) | 0.90 (0.72-1.13) | |
|
| ||||
| HOSPITAL-LEVEL CHARACTERISTICS | ||||
|
| ||||
| Volume of Patients | ||||
| Surgical Hospital | ||||
| Lowest Quartile | 862 (15.0) | 1305 (39.3) | <0.001 | Ref |
| 2nd Quartile | 1507 (26.2) | 714 (21.5) | 2.94 (2.20-3.94) | |
| 3rd Quartile | 1719 (29.9) | 656 (19.8) | 3.68 (2.59-5.23) | |
| Highest Quartile | 1670 (29.0) | 642 (19.4) | 3.99 (2.51-6.33) | |
| Med Onc Hospital | ||||
| Lowest Quartile | 1095 (19.0) | 1002 (30.2) | <0.001 | Ref |
| 2nd Quartile | 1529 (26.6) | 822 (24.8) | 0.98 (0.74-1.30) | |
| 3rd Quartile | 1531 (26.6) | 724 (21.8) | 0.91 (0.66-1.24) | |
| Highest Quartile | 1603 (27.8) | 769 (23.2) | 0.86 (0.59-1.25) | |
|
| ||||
| NCI Status | ||||
| Surgical Hospital | 134 (2.3) | 126 (3.8) | <0.001 | 0.52 (0.28-0.94) |
| Med Onc Hospital | 134 (2.3) | 103 (3.1) | 0.025 | 1.07 (0.59-1.94) |
|
| ||||
| Academic center | ||||
| Surgical Hospital | 3202 (55.6) | 1578 (47.6) | <0.001 | 1.06 (0.79-1.42) |
| Med Onc Hospital | 3202 (55.6) | 1833 (55.3) | 0.75 | 0.83 (0.65-1.06) |
|
| ||||
| For Profit Status | ||||
| Surgical Hospital | 437 (7.6) | 522 (15.7) | <0.001 | 0.66 (0.48-0.91) |
| Med Onc Hospital | 437 (7.6) | 391 (11.8) | <0.001 | 0.98 (0.70-1.37) |
|
| ||||
| OUTCOME MEASURES | ||||
|
| ||||
| Number of Deaths | 3157 (54.8) | 1892 (57.0) | 0.041 | N/A |
|
| ||||
| Median Total costs for first year of care (IQR) | $69406 ($45223-$115287) | $73908 ($46615-$125622 ) | 0.001‡ | N/A |
Odds Ratios are fully adjusted for all other variables listed here as well as diagnosis year and SEER site which are not shown
Sample restricted to those diagnosed between 2004 onward, for whom this data is available
Wilcox-Mann-Whitney test
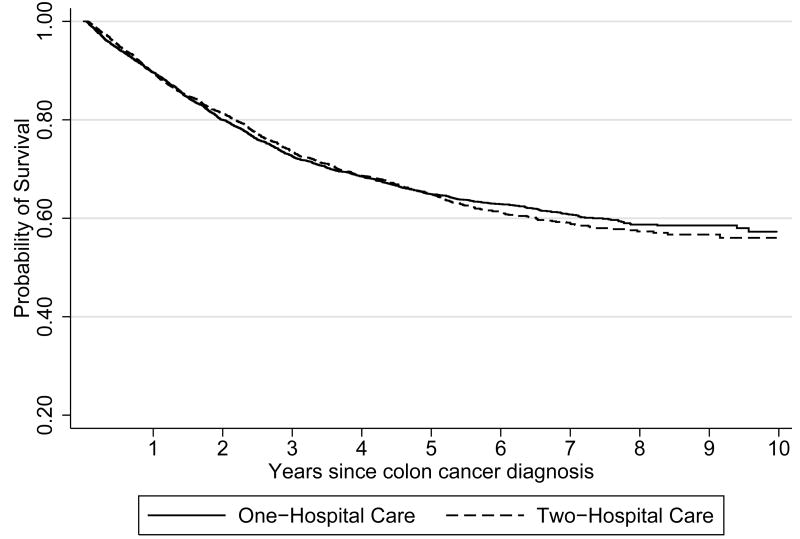
As shown in figures 2 and 3, there were no significant differences in all-cause mortality or colon cancer specific mortality between patients receiving one- versus two-hospital care. Follow-up times for all-cause and colon cancer specific mortality averaged 4.3 years (total 38836 years) and 3.3 years (total of 29807 years), respectively. Median unadjusted cost of care at 12 months was approximately $4500 lower for patients receiving one-hospital care ($73,908 versus $69,406, p=0.001).
Figure 2. Kaplan Meier survival curve for all-cause mortality by one- versus two-hospital care.
Log rank survival function: chi square=1.66, p=0.197
Figure 3. Kaplan Meier survival curve for colon-cancer specific mortality by one- versus two-hospital care.
Log rank survival function: chi square=0.15, p=0.70
Table 2 shows the final propensity score-matched doubly robust Cox proportional hazards regression for all-cause mortality, competing risks subhazard regression for colon cancer specific mortality, and total cost of care at 12 months. Appropriate balance was obtained on covariates (Supplemental Materials, Table 1). Receiving one-hospital medical oncologic and surgical care compared to two-hospital care was not associated with all-cause (Hazard Ratio 0.97, 95%CI 0.92-1.05) or colon cancer specific mortality (Subhazard Ratio 0.98, 95%CI 0.89-1.07). However, patients with non-extreme costs who received one-hospital care had significantly lower total costs than those who received two-hospital care (dollars saved $5493, 95%CI $1799, $9525). In particular, we found a statistically significant savings in inpatient claims (dollar saved: $3076, 95%CI: $520, $4893) among those receiving one- compared to two-hospital care; this includes the inpatient costs of the primary surgery. There was no statistically significant savings in physician fees ($523, 95%CI: -$786, $1702) or outpatient claims ($1041, 95%CI: -$628, $2277). When examining only those patients diagnosed 2004 onward to account for substage (Supplemental Materials, Table 2), total cost difference was even greater for one-hospital care (dollars saved $7531, 95% CI $1783 - $13043). Notably, there was no cost savings amongst those patients with the most extreme costs (Table 2).
Table 2. All-cause & colon cancer specific mortality and cost at 12 months associated with one- versus two-hospital care from propensity score-matched doubly robust models (Estimate, 95%CI).
| Hazard Ratio for All-Cause Mortality (n=5482)* | Subhazard Ratio for Colon Cancer Specific Mortality (n=5482)* | Dollars Saved from Generalized Linear Model Estimates For Patients with Non-Extreme Costs (n=5186) *,†,‡ | Dollars Saved from Generalized Linear Model Estimates For Patients with Extreme Costs (n=296) *,†,‡ | |
|---|---|---|---|---|
|
| ||||
| One-Hospital Care | Ref | Ref | Ref | Ref |
| Two-Hospital Care | 0.97 (0.92-1.05) | 0.98 (0.89-1.07) | $5493 ($1799-$9525) | $23244 (-$100606, $132636) |
Estimates are fully adjusted for all patient, provider, and hospital characteristics listed in Table 1, excluding substage (which is available only for those diagnosed 2004 onward).
All dollar values were inflated to 2009 using the annual Gross Domestic Product price index.
Patients with extreme cost are those patients with the top 5% of costs
Results did not significantly change in any of the sensitivity analyses (Supplemental Materials, Table 2), and all pre-specified testing of statistical interactions terms were non-significant.
Discussion
Over one-third of patients with stage III colon cancer received their medical oncologic and surgical care from different hospitals. While we did not observe significant differences in either all-cause or colon cancer specific mortality related to this type of care fragmentation, we found significant differences in the twelve-month cost of care. Patients receiving medical oncologic and surgical care at the same hospital had, on average, 8% lower median cost than patients receiving two-hospital care. These results raise important concerns regarding attempts to reduce cancer care fragmentation in the setting of current health care reform.
A substantial minority of patients received two-hospital surgical and medical oncologic care. Notably, rural residence was associated with receiving two-hospital medical oncologic and surgical care. This finding is consistent with known barriers to cancer care in rural communities: namely, limited access to physicians who provide colorectal cancer screening and treatment23-25 and geographic distance to health care facilities.24,25
Because there is some evidence that more colon cancer patients are receiving surgical care at high volume centers (which has been linked to improved outcomes), we had anticipated that patients would travel to receive high volume surgical care but receive their medical oncologic care from a different and potentially local hospital.6,7,16,17,26 Instead, we found that patients who received surgery from high volume surgeons or centers were more likely to receive one-hospital care. With evidence suggesting that high volume surgeons are more likely to collaborate in decisions about adjuvant chemotherapy with oncologists within their institution compared to lower volume surgeons,27 patients may prefer to remain at a high volume cancer center for their medical oncologic care. A deeper understanding of why patients select certain providers and institutions is still needed to improve continuity during transitions in specialist care.
Contrary to our expectations, we did not observe differences in mortality between patients who received one-hospital or two-hospital care in our study. Outcomes other than mortality such as process quality measures, delays in care, and satisfaction with care have been shown to vary with care fragmentation and warrant further study in the setting of one- versus two-hospital cancer care.8,9 Further, informal connections between different hospitals, such as collaboration between providers or the emergence of patient navigators and care managers, may mitigate the mechanisms through which two-hospital care potentially impacts patient outcomes.28,29
We did observe lower costs among patients who received one-hospital cancer care, suggesting that perhaps less fragmented delivery of complex cancer care helps reduce healthcare costs. Inpatient hospital costs in particular were significantly lower among those receiving one-hospital care. These savings nearly doubled between six months and at one year from diagnosis, possibly reflecting that cost differences may be related to both early treatment and ongoing care, or complications of early care. Single-hospital care delivery did not, however, decrease the expenditure of those patients with the most extreme costs. Further study is required to investigate the extent to which cost differences are driven by length of hospital stay, number of hospitalizations, preventable complications, duplication of services, or other factors. Electronic medical records and electronic referral systems, shared by physicians affiliated with the same hospital, have been associated with small but significant reduction in costs and improvements in timely care delivery and may have a role in explaining these findings, but, to our knowledge, have not ben tested with regard to cancer care.30,31
There are limitations to our study. First, a large portion of medical oncologic care occurs in outpatient settings and oncologists may be affiliated with more than one hospital, both of which creates challenges when trying to assign patients to hospitals. To test the face validity of our assignment method, we examined to which hospitals patients got admitted in the year following their diagnosis. 86% of patients had the plurality of their inpatient admissions at the assigned oncologic hospital and 93% had their admissions at either the assigned oncologic or surgical hospital. Second, in line with previous studies,32,33 a substantial proportion of patients could not be assigned to a medical oncologist; we relied on specialty codes to identify physicians who were medical oncologists, which would lead to misclassification if providers were missing a specialty code or identified under an alternate specialty code. Third, due to concerns regarding completeness of chemotherapy claims after changes in billing codes during the study period, we did not use administration of chemotherapy to classify doctors as medical oncologists nor could we confidently determine how one-hospital care affects timely receipt of chemotherapy. Fourth, some patients receiving care from two different hospitals may be part of a health system that coordinates care between hospitals. As of 2012, only 26% of registered U.S. hospitals were part of such an integrated network.34 Failing to account for these connections between hospitals would bias our findings towards the null. Fifth, we are unable to assess the reasons why patients and their referring providers select particular surgeons and medical oncologists. Some patients receiving two-hospital care may purposefully seek out specific providers based on reputation, compatibility, or other personal preferences, without regard to where the providers practice; these patients are likely to be more activated and capable of bridging deficits in coordination across health care systems. To the extent that these patients also have better health outcomes, this may bias our mortality findings towards the null. While we used propensity score methods to produce patient groups resembling each other on observed covariates, there may still be differences due to unmeasured characteristics. Sixth, our volume measures are based solely on Medicare data; however, volume measures constructed using Medicare data are highly correlated with those constructed from all-payer data.35 Finally, this study focuses on patients in fee-for-service Medicare and may not be generalizable to younger patients or those in preferred provider organizations, health maintenance organizations or with other types of insurance.
Notwithstanding these limitations, the current work suggests the potential that integrated delivery systems may have in reducing cancer costs while underscoring the challenges of doing so. While many current health care delivery reforms including accountable care organizations, patient-centered medical homes, and bundled payments seek to reduce fragmentation, they do not lock patients into receiving care through a single hospital or health system. Efforts to create continuity in complex care may be hampered by the large proportion of patients who receive treatment delivered by providers from more than one hospital or system. At the same time, our findings suggest costs are lower among patients who receive care from a single hospital, reinforcing the potential financial benefits of delivery models that reduce fragmentation in complex cancer care.
Supplementary Material
Acknowledgments
Funding: The Maryland Cigarette Restitution Fund and career development award from the NCI and OBSSR (K07CA151910) to Pollack; and the NCI's Center to Reduce Cancer Health Disparities’ Community Networks Program (CNP # U54CA153710) and NHLBI Training Grant (5T32HL007180-38) to Hussain.
Footnotes
Disclosures: Authors do not have any conflicts of interest to disclose.
References
- 1.Institute of Medicine (U.S.) Crossing the quality chasm : a new health system for the 21st century. Washington, D.C: National Academy Press; 2001. Committee on Quality of Health Care in America. [PubMed] [Google Scholar]
- 2.Crosson FJ. 21st-century health care--the case for integrated delivery systems. The New England journal of medicine. 2009;361(14):1324–1325. doi: 10.1056/NEJMp0906917. [DOI] [PubMed] [Google Scholar]
- 3.Levit LA, Balogh E, Nass SJ, Ganz P, Institute of Medicine (U.S.) Delivering high-quality cancer care : charting a new course for a system in crisis. Washington, D.C: National Academies Press; 2013. Committee on Improving the Quality of Cancer Care: Addressing the Challenges of an Aging Population. [PubMed] [Google Scholar]
- 4.Snow V, Beck D, Budnitz T, et al. Transitions of Care Consensus Policy Statement American College of Physicians-Society of General Internal Medicine-Society of Hospital Medicine-American Geriatrics Society-American College of Emergency Physicians-Society of Academic Emergency Medicine. Journal of general internal medicine. 2009;24(8):971–976. doi: 10.1007/s11606-009-0969-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisher ES, McClellan MB, Safran DG. Building the path to accountable care. The New England journal of medicine. 2011;365(26):2445–2447. doi: 10.1056/NEJMp1112442. [DOI] [PubMed] [Google Scholar]
- 6.Stitzenberg KB, Meropol NJ. Trends in centralization of cancer surgery. Annals of surgical oncology. 2010;17(11):2824–2831. doi: 10.1245/s10434-010-1159-0. [DOI] [PubMed] [Google Scholar]
- 7.Kronebusch K. Assessing changes in high-volume hospital use: hospitals, payers, and aggregate volume trends. Medical care research and review : MCRR. 2009;66(2):197–218. doi: 10.1177/1077558708326528. [DOI] [PubMed] [Google Scholar]
- 8.Hempstead K, Delia D, Cantor JC, Nguyen T, Brenner J. The fragmentation of hospital use among a cohort of high utilizers: implications for emerging care coordination strategies for patients with multiple chronic conditions. Medical care. 2014;52(Suppl 3):S67–74. doi: 10.1097/MLR.0000000000000049. [DOI] [PubMed] [Google Scholar]
- 9.Unni N, Peddinghaus M, Tormey CA, Stack G. Record fragmentation due to transfusion at multiple health care facilities: a risk factor for delayed hemolytic transfusion reactions. Transfusion. 2014;54(1):98–103. doi: 10.1111/trf.12251. [DOI] [PubMed] [Google Scholar]
- 10.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010-2020. Journal of the National Cancer Institute. 2011;103(2):117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desch CE, McNiff KK, Schneider EC, et al. American Society of Clinical Oncology/National Comprehensive Cancer Network Quality Measures. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26(21):3631–3637. doi: 10.1200/JCO.2008.16.5068. [DOI] [PubMed] [Google Scholar]
- 12. [Accessed October 18, 2013];About the SEER-Medicare Database. http://appliedresearch.cancer.gov/seermedicare/overview/linked.html.
- 13.Veenstra C, Epstein A, Liao K, Morris A, Pollack C, Armstrong K. The effect of care setting in delivery of high value colon cancer care. Cancer. 2014 doi: 10.1002/cncr.28874. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bynum JP, Bernal-Delgado E, Gottlieb D, Fisher E. Assigning ambulatory patients and their physicians to hospitals: a method for obtaining population-based provider performance measurements. Health services research. 2007;42(1 Pt 1):45–62. doi: 10.1111/j.1475-6773.2006.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quah HM, Chou JF, Gonen M, et al. Identification of patients with high-risk stage II colon cancer for adjuvant therapy. Diseases of the colon and rectum. 2008;51(5):503–507. doi: 10.1007/s10350-008-9246-z. [DOI] [PubMed] [Google Scholar]
- 16.Schrag D, Panageas KS, Riedel E, et al. Surgeon volume compared to hospital volume as a predictor of outcome following primary colon cancer resection. Journal of surgical oncology. 2003;83(2):68–78. doi: 10.1002/jso.10244. discussion 78-69. [DOI] [PubMed] [Google Scholar]
- 17.Begg CB, Cramer LD, Hoskins WJ, Brennan MF. Impact of hospital volume on operative mortality for major cancer surgery. JAMA : the journal of the American Medical Association. 1998;280(20):1747–1751. doi: 10.1001/jama.280.20.1747. [DOI] [PubMed] [Google Scholar]
- 18.Fine J, Gray R. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 19.Grambsch P, Therneau T. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- 20.Manning WG, Mullahy J. Estimating log models: to transform or not to transform? Journal of health economics. 2001;20(4):461–494. doi: 10.1016/s0167-6296(01)00086-8. [DOI] [PubMed] [Google Scholar]
- 21.Agency for Healthcare Research and Quality. Using appropriate price indices for analyses of health care expenditures or income across multiple years. [Accessed December 1, 2014];2014 http://meps.ahrq.gov/about_meps/Price_Index.shtml-c1.
- 22.Leuven E, Sianesi B. PSMATCH2: Stata module to perform full Mahalanobis and propensity score matching, common support graphing, and covariate imbalance testing. [Accessed January 12, 2014];2003 http://ideas.repec.org/c/boc/bocode/s43.
- 23.Aboagye JK, Kaiser HE, Hayanga AJ. Rural-Urban Differences in Access to Specialist Providers of Colorectal Cancer Care in the United States: A Physician Workforce Issue. JAMA surgery. 2014 doi: 10.1001/jamasurg.2013.5062. [DOI] [PubMed] [Google Scholar]
- 24.Onega T, Duell EJ, Shi X, Wang D, Demidenko E, Goodman D. Geographic access to cancer care in the U.S. Cancer. 2008;112(4):909–918. doi: 10.1002/cncr.23229. [DOI] [PubMed] [Google Scholar]
- 25.Casey MM, Thiede Call K, Klingner JM. Are rural residents less likely to obtain recommended preventive healthcare services? American journal of preventive medicine. 2001;21(3):182–188. doi: 10.1016/s0749-3797(01)00349-x. [DOI] [PubMed] [Google Scholar]
- 26.Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. The New England journal of medicine. 2002;346(15):1128–1137. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 27.Rogers SO, Jr, Ayanian JZ, Ko CY, et al. Surgeons' volume of colorectal cancer procedures and collaborative decision-making about adjuvant therapies. Annals of surgery. 2009;250(6):895–900. doi: 10.1097/SLA.0b013e3181afe0c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elliott TE, Elliott BA, Regal RR, et al. Improving rural cancer patients' outcomes: a group-randomized trial. The Journal of rural health : official journal of the American Rural Health Association and the National Rural Health Care Association. 2004;20(1):26–35. doi: 10.1111/j.1748-0361.2004.tb00004.x. [DOI] [PubMed] [Google Scholar]
- 29.Hedlund N, Risendal BC, Pauls H, et al. Dissemination of patient navigation programs across the United States. Journal of public health management and practice : JPHMP. 2014;20(4):E15–24. doi: 10.1097/PHH.0b013e3182a505ec. [DOI] [PubMed] [Google Scholar]
- 30.Amarasingham R, Plantinga L, Diener-West M, Gaskin DJ, Powe NR. Clinical information technologies and inpatient outcomes: a multiple hospital study. Archives of internal medicine. 2009;169(2):108–114. doi: 10.1001/archinternmed.2008.520. [DOI] [PubMed] [Google Scholar]
- 31.Chen AH, Murphy EJ, Yee HF., Jr eReferral--a new model for integrated care. The New England journal of medicine. 2013;368(26):2450–2453. doi: 10.1056/NEJMp1215594. [DOI] [PubMed] [Google Scholar]
- 32.Phelip JM, Molinie F, Delafosse P, et al. A population-based study of adjuvant chemotherapy for stage-II and -III colon cancers. Gastroenterologie clinique et biologique. 2010;34(2):144–149. doi: 10.1016/j.gcb.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 33.Schrag D, Cramer LD, Bach PB, Begg CB. Age and adjuvant chemotherapy use after surgery for stage III colon cancer. Journal of the National Cancer Institute. 2001;93(11):850–857. doi: 10.1093/jnci/93.11.850. [DOI] [PubMed] [Google Scholar]
- 34.Fast facts on U.S. Hospitals. [Accessed May 17, 2014]; http://www.aha.org/research/rc/stat-studies/fast-facts.shtml.
- 35.Begg CB, Riedel ER, Bach PB, et al. Variations in morbidity after radical prostatectomy. The New England journal of medicine. 2002;346(15):1138–1144. doi: 10.1056/NEJMsa011788. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.