Abstract
Background
Abdominal aortic aneurysm (AAA) rupture is an adverse arterial remodeling event with high mortality risk. Since females have increased rupture risk with smaller AAAs (<5.5 cm), many recommend elective repair prior to 5.5 cm. Elective repair improves survival for large AAAs, but long-term benefits of endovascular aneurysm repair (EVAR) for small AAAs in females remains less understood. The objective of this study is to identify if differences in late mortality exist between females undergoing elective EVAR at our institution for small/slow-growing AAAs compared to those who meet standard criteria.
Methods
We retrospectively analyzed all patients that underwent EVAR for infrarenal AAA from 6/2009–6/2013. We excluded patients that were male, treated emergently or for iliac artery aneurysm, and that received renal/mesenteric artery stenting. Patients did not meet anatomic criteria if preoperative AAA diameter was <5.5 cm or enlarged <0.5 cm over 6 months. Late mortality was assessed from the Social Security Death Index.
Results
36/162 (22.2%) elective EVAR patients were female (mean follow-up 37.2 months). 20 (55.6%) patients met AAA size/growth criteria while 16 (44.4%) did not meet criteria. Despite comparable demographics, comorbidities, and complications, patients that did not meet criteria had higher late mortality (37.5% vs. 5%; P= .03) with a trend towards increased reoperation rate (25% vs. 5%; P= .48). Meeting size/growth criteria decreased odds of late death (OR .09; 95% CI 0.01–0.83).
Conclusion
There is increased late mortality in females receiving elective EVAR at our institution for small/slow-growing AAAs. This late mortality may limit the benefits of EVAR for this population.
Keywords: Endovascular Aneurysm Repair (EVAR), Abdominal Aortic Aneurysm (AAA), Female, Mortality, Aneurysm Diameter, Size Threshold
1. Introduction
Abdominal aortic aneurysm (AAA) rupture is associated with significant mortality and is lethal in 90% of patients.[1] Despite declining global incidence over the past two decades, AAA burden in the United States remains high, with aortic rupture accounting for >13,000 deaths annually.[2,3] Over this same time period, women have experienced a smaller decrease in AAA rupture rates compared to males, and continue to represent a disproportionate amount of AAA-related mortality.[4,5] Although AAA prevalence in women is approximately six times lower than men, females account for over 40% of AAA-attributable deaths.[6,7]
High AAA mortality rates in females have been partially attributed to gender variations in AAA rupture risk. Compared to AAAs in males, aneurysms in females rupture at smaller average diameters and are up to four times as likely to rupture at the same aneurysm size.[8–11] Additionally, females are more likely to present emergently and at an older age, while less likely to receive surgical intervention or be eligible for endovascular aneurysm repair (EVAR). These factors have contributed to the comparatively poor outcomes for females following elective and emergent AAA repair, including longer lengths of stay, lower rates of discharge to home, and increased early mortality. [5,12–15]
Increased rupture risk, worse outcomes after emergent repair, and potential for losing EVAR eligibility have led many surgeons to suggest smaller AAA size/growth thresholds for elective EVAR in females.[4,5,16–18] The survival benefit for elective open repair of large (≥5.5 cm) and/or fast growing (≥0.5 cm in 6 months) AAAs is well established, but the size indications and long-term outcomes for elective EVAR in females are less well understood. Elective EVAR has demonstrated improved early outcomes compared to traditional open repair, but these benefits appear to decrease over time and are less pronounced in females.[14,19–23] Additionally, Society for Vascular Surgery practice guidelines acknowledge increased rupture risk and potential benefit of repairing small aneurysms in women, but give weak recommendation for elective EVAR of AAAs <5.5 cm in females.[24,25] These recommendations are based on results from the Comparison of Surveillance Versus Aortic Endografting for Small Aneurysm Repair (CAESAR)[26] and Positive Impact of Endovascular Options for Treating Aneurysms Early (PIVOTAL)[27] trials. Both studies failed to demonstrate survival benefit for elective EVAR of small AAAs, but were underpowered to allow subgroup analysis in female patients.[26,27]
Data is lacking regarding late outcomes after EVAR in females, especially those with small AAAs. In order to better assess the benefit of EVAR in female patients at our institution, the objective of this study is to identify if there are differences in late mortality between female patients undergoing elective EVAR for small, slow-growing AAAs compared to those that meet standard criteria.
2. Methods
Medical records from patients undergoing endovascular intervention from June 2009 to June 2013 were used to identify all patients that had received EVAR at our institution. Under an approved Institutional Review Board protocol and in accordance with the Helsinki Declaration of 1975 ethical standards on human experimentation, we performed retrospective evaluation of identified electronic medical records (EMRs) for study inclusion.
We collected patient demographics, insurance status, comorbidities, medication use, imaging studies, perioperative data, and clinical follow-up reports for all patients that underwent elective EVAR for infrarenal AAA during the study period. Charlson Comorbidity Index (CCI) was calculated based upon patient preoperative comorbidities.[28,29] All procedures were performed at a single institution by fellowship-trained vascular surgeons. Patients were excluded from analysis if they were male, had symptomatic or ruptured AAA, underwent concomitant treatment for iliac artery aneurysm, or received renal or mesenteric artery stenting at time of EVAR. Arterial vessel diameters, lengths, and angles were collected from preoperative 3-dimensional (3D) imaging, intraoperative angiograms, and operative reports. Iliac artery and aortic neck dimensions were compared to graft manufacturers’ Instructions for Use (IFU) guidelines to determine if patients met endoprosthesis-specific IFU criteria. Preoperative AAA size was determined from last documented 3D imaging prior to intervention and was measured in axial views at level of maximum external aortic diameter. Preoperative AAA growth rate was determined from both 3D imaging and ultrasound surveillance reports and was compared with corresponding notes from our vascular clinic and outside records. These imaging variables were used to group female EVAR patients by whether they did or did not meet preoperative AAA size and/or growth criteria for elective intervention. Patients were considered to have met criteria if maximum AAA diameter was ≥5.5 cm or AAA diameter was <5.5 cm, but had grown ≥0.5 cm in ≤6 months. Conversely, patients were classified as not meeting aneurysm criteria if AAA diameter was <5.5 cm and without rapid growth.
The primary objective of this study was to compare late mortality rates after EVAR between females that did and did not meet preoperative AAA size or growth criteria. Secondary outcomes measures included comparison of 30-day morbidity/mortality and graft-related reoperation rates between cohorts.
Late mortality was defined as all-cause death >30 days after EVAR and was determined from the EMR and the Social Security Death Index. Criteria for 30-day morbidity were determined prior to data collection. Major complications were prospectively defined prior to data collection as new dysrhythmia requiring cardioversion or not resolved by discharge, acute decline in renal function (rise in postoperative Creatinine ≥0.5 mg/dL or new-onset dialysis), myocardial infarction (confirmed with EKG and troponin elevation), respiratory compromise (prolonged/repeat intubation or ventilator-associated pneumonia), clinically significant bowel ischemia or pulmonary embolism, and iliac artery rupture. Minor complications were defined as clinically significant events that did not meet major complication criteria.
Continuous variables were reported as means with standard deviations and categorical variables were reported as frequencies with percentages. Independent samples t-test, Mann-Whitney U test, χ2 test, and Fisher’s Exact Test were used to compare patient variables where appropriate. Binary logistic regression was used to determine odds ratios (ORs) and confidence intervals (CIs) for late mortality. Kaplan-Meier plots were generated for survival analysis, with log-rank tests used to compare survival distributions between cohorts. All tests were performed in SPSS Version 22.0 (IBM Corp, Armonk, NY) using P< .05 as threshold for statistical significance.
3. Results
3.1 Baseline Characteristics
From June 2009 to June 2013, female patients accounted for 36 (22.2%) of the 162 elective EVARs performed at our institution. Of these 36 female patients, 20 (55.6%) had preoperative AAA diameter ≥5.5 cm or with rapid growth while 16 (44.4%) patients did not meet AAA size or growth criteria. Indications for intervention in patients that did not meet size or growth criteria included patient inability to tolerate rupture risk (13 [81.3%]), surgeon preference (two [12.5%]), and family history of AAA rupture (one [6.3%]). Decision to pursue surgical intervention in these patients were multifactorial, but were based upon surgeons’ concern for AAA rupture without elective intervention. All patients had fusiform AAA morphology on 3D imaging and were admitted electively to the hospital with AAA as the primary diagnosis. There were no differences in preoperative demographics, insurance status, or individual comorbidities between groups, although patients that did not meet criteria had smaller mean AAA diameter (5.0 cm vs. 5.8 cm; P= .004) and were less likely to be taking an angiotensin-converting-enzyme inhibitor (ACE-I) or angiotensin receptor blocker (ARB) (31.3% vs. 65%; P= .04). Patients that met criteria had higher mean CCI score compared to patients that did not meet criteria (2.25 vs. 1.38; P= .08), but this trend was not significant. (Table 1)
Table I.
Preoperative characteristics of female elective EVAR patients.
| Met AAA size and/or growth criteria? | |||
|---|---|---|---|
| Yes (n = 20) | No (n = 16) | P value | |
| Demographics: | |||
| Age (y) | 75.7 ± 7.2 | 73.4 ± 8.9 | 0.39 |
| Private Insurance | 10 (50) | 11 (69) | 0.26 |
| AAA diameter (cm) | 5.8 ± 1.1 | 5.0 ± 0.2 | 0.004** |
| Comorbidities: | |||
| CCI | 2.25 ± 1.59 | 1.38 ± 1.26 | 0.08 |
| Smoker | 18 (90) | 15 (94) | 1.00 |
| COPD | 12 (60) | 9 (56) | 0.82 |
| Hypertension | 18 (95) | 15 (94) | 1.00 |
| Diabetes mellitus | 6 (30) | 2 (13) | 0.26 |
| Hyperlipidemia | 15 (75) | 12 (75) | 1.00 |
| Myocardial infarction | 5 (25) | 2 (13) | 0.43 |
| PCI | 5 (25) | 5 (31) | 0.72 |
| CABG | 4 (20) | 3 (19) | 1.00 |
| Heart failure | 0 (0) | 1 (6) | 0.44 |
| Stroke | 3 (15) | 1 (6) | 0.61 |
| Renal failure | 3 (15) | 0 (0) | 0.24 |
| Medication Use: | |||
| ACE-I or ARB | 13 (65) | 5 (31) | 0.04* |
| Statin | 17 (85) | 10 (63) | 0.15 |
| Aspirin | 16 (80) | 12 (75) | 0.72 |
| Coumadin or Plavix | 7 (35) | 7 (44) | 0.59 |
| Beta blocker | 9 (45) | 10 (63) | 0.30 |
EVAR = endovascular aneurysm repair; AAA = abdominal aortic aneurysm; CCI = Charlson Comorbidity Index; COPD = chronic obstructive pulmonary disease; PCI = percutaneous coronary intervention; CABG = coronary artery bypass graft; ACE-I = angiotensin-converting-enzyme inhibitor; ARB = angiotensin receptor blocker.
AAA size/growth criteria for elective EVAR defined by Society for Vascular Surgery Practice Guidelines, 2009; CCI calculated using methods of Charlson et al., 1986.
Data presented as mean ± standard deviation where indicated or raw number (percentage).
p< .05
p< .01
Bifurcated endoprosthesis graft use varied by surgeon and included GORER (17 [47.2%]), Medtronic® (seven [19.4%]), Endologix® (six [16.7%]), Cook® (four [11.1%]), and Trivascular® (two [5.6%]) devices. 11 (68.8%) patients with AAA <5.5 cm and without rapid growth met device-specific IFU criteria compared to 16 (80.8%) patients with AAA ≥5.5 cm or with rapid growth, but this difference did not meet significance (P= .47). Further comparisons of device usage and IFU criteria between cohorts are listed in Table 2.
Table II.
Endoprosthesis use and IFU failure criteria for female elective EVAR patients.
| Met AAA size and/or growth criteria? | |||
|---|---|---|---|
| Yes (n = 20) | No (n = 16) | P value | |
| Endoprosthesis Device: | |||
| GORE® EXCLUDER® | 6 (30) | 11 (69) | 0.04* |
| COOK® Zenith® | 3 (15) | 1 (6) | 0.61 |
| Endologix AFX™ | 5 (25) | 1 (6) | 0.20 |
| Medtronic Talent® | 1 (5) | 3 (19) | 0.30 |
| Medtronic Endurant® | 3 (15) | 0 (0) | 0.24 |
| TriVascular Ovation™ | 2 (10) | 0 (0) | 0.49 |
| Failed IFU Criteria | 4 (20) | 5 (31) | 0.47 |
| Insufficient neck length | 1 (5) | 5 (31) | 0.05 |
| Neck angle >60° | 3 (15) | 2 (13) | 0.52 |
EVAR = endovascular aneurysm repair; AAA = abdominal aortic aneurysm; IFU = instructions for use.
AAA size/growth criteria for elective EVAR defined by Society for Vascular Surgery Practice Guidelines, 2009.
Data presented as raw number (percentage).
p< .05
3.2 Thirty-day Mortality & Morbidity
There were no deaths within 30-days of EVAR in either cohort. Patients that did and did not meet AAA criteria had similar major perioperative complication rates during this period (25% vs. 18.8%; P= .74). Major complications in the cohort that met criteria (N=5) included three cases of acute renal failure, one case of iliac artery rupture requiring graft extension, and one instance of new-onset arrhythmia not resolved at time of discharge. Major complications in the cohort that did not meet criteria (N=3) included two iliac artery ruptures requiring graft extension or bypass and one pre-discharge myocardial infarction (full complications provided in Table 3). Frequency of vessel damage (requiring patch angioplasty, graft extension, or bypass) during access manipulation or graft deployment was also higher in patients that did not meet criteria (six [37.5%] vs. three [15.0%]; P= .39), but this did not reach significance. Additionally, patients that did and did not meet criteria had comparable ≤30 day minor complication rates (25% vs. 25%; P= .86) and ≤30 day readmission rates (10% vs. 6.3%; P= .86), with similar lengths of hospital stay and rates of discharge to home. (Table 4)
Table III.
Complication frequencies for female elective EVAR patients.
| Met AAA size and/or growth criteria? | ||
|---|---|---|
| Yes (n = 20) | No (n = 16) | |
| Major complications: | ||
| Iliac artery rupturea | 1 (5) | 2 (13) |
| Renal failureb | 3 (15) | 0 (0) |
| Myocardial infarction | 0 (0) | 1 (6) |
| Arrhythmiac | 1 (5) | 0 (0) |
| Minor complications: | ||
| Access vessel dissectiond | 2 (10) | 4 (25) |
| Transfusione | 1 (5) | 1 (6) |
| Altered mental status | 0 (0) | 2 (13) |
| Pneumoniaf | 1 (5) | 0 (0) |
| Heart failure exacerbationf | 0 (0) | 1 (6) |
| Wound dehiscence | 1 (5) | 0 (0) |
| Seromag | 1 (5) | 0 (0) |
EVAR = endovascular aneurysm repair; AAA = abdominal aortic aneurysm.
Rupture defined as extravasation of contrast on intraoperative angiography that required bypass or unplanned graft extension;
Renal failure classified as new-onset Cr rise >0.5 mg/dL and/or need for emergent dialysis;
Arrhythmias were new-onset and unresolved by time of discharge;
Dissection required femoral endarterectomy and/or patch angioplasty for vessel closure;
Transfusions performed in patients without history of chronic anemia and pre to post-operative hemoglobin change >3 g/dL;
Exacerbations required hospital readmission;
Seromas present at surgical access site and required drainage.
AAA size/growth criteria for elective EVAR defined by Society for Vascular Surgery Practice Guidelines, 2009.
Data presented as raw number (percentage).
Table IV.
Perioperative and late events for female elective EVAR patients.
| Met AAA size and/or growth criteria? | |||
|---|---|---|---|
| Yes (n = 20) | No (n = 16) | P value | |
| Perioperative Events | |||
| Postoperative endoleaks | 6 (30) | 6 (37.5) | 0.64 |
| Postoperative LOS (days) | 2.9 ± 2.1 | 2.6 ± 2.3 | 0.46 |
| Discharged home | 18 (90) | 15 (94) | 1.00 |
| ≤30 Day Adverse Events | |||
| Major complications | 5 (25) | 3 (19) | 0.74 |
| Minor complications | 7 (35) | 8 (50) | 0.42 |
| Vessel damage | 3 (15) | 6 (38) | 0.39 |
| Hospital readmissions | 2 (10) | 1 (6) | 0.86 |
| Late Events | |||
| All-cause mortality | 1 (5) | 6 (38) | 0.03* |
| Reoperations | 1 (5) | 4 (25) | 0.48 |
| Follow-up (months) | 37.2 ± 11.6 | 37.2 ± 19.8 | 0.99 |
EVAR = endovascular aneurysm repair; AAA = abdominal aortic aneurysm; LOS = length of stay.
Perioperative events occurred during original hospital admission for elective EVAR; Late events occurred >30 days after date of initial procedure; AAA size/growth criteria for elective EVAR defined by Society for Vascular Surgery Practice Guidelines, 2009.
Data presented as mean ± standard deviation where indicated or raw number (percentage).
p< .05
3.3 Late Mortality and Reoperation
Average patient follow-up was 37.2 ± 15.5 months from date of EVAR. There were a total of 7 patient deaths during this follow-up period (mean time to death 21.6 ± 15.8 months) with an all-cause mortality rate of 19.4%. Patients that did not meet AAA size or growth criteria had significantly higher late mortality compared to patients that did meet criteria (six [37.5%] vs. one [5%]; P= .03). Mean time to death in patients that did not meet criteria was 18.9 ± 15.5 months and the one death in the cohort that did meet criteria occurred at 37.7 months. There were a total of 5 graft-related reoperations during the follow-up period (mean time to reoperation 21.1 ± 16.5 months) with an overall reoperation rate of 13.8%. Indications for reoperation included critical limb ischemia, new-onset lower extremity claudication, external iliac artery dissection, and type 1 endoleak on surveillance imaging. Although reoperations were also more frequent in patients that did not meet AAA size or growth criteria (four [25%] vs. one [5%]; P= .48) and patients that did not meet IFU (two [22%] vs. three [11%]; P= .97), these trends did not achieve significance. Of note, there were no mortalities in patients that underwent graft-related reoperations during the follow-up period.
Using univariate logistic regression for the dependent variable death, patients that met preoperative AAA size and/or growth criteria had significantly decreased odds of late mortality (OR .09; 95% CI 0.01–0.83). Age, aneurysm size, meeting IFU criteria, and major complication were not found to have significant influence on odds of late mortality. (Table 5) Kaplan-Meier analysis found significant difference in survival distributions between patients that did and did not meet criteria (x2= 4.55, P= .03), with cumulative proportion surviving at 4-years of 90% and 60.2% respectively. (Figure 1)
Table V.
Univariate regression for late mortality risk in female elective EVAR patients.
| Late Mortality? | OR | 95% CI | P value | ||
|---|---|---|---|---|---|
| Yes (n = 6) | No (n = 30) | ||||
| Age (y) | |||||
| Mean | 76.6 | 74.2 | 1.04 | 0.93–1.16 | 0.48 |
| SD | (± 10.5) | (± 7.4) | |||
| Major complication (rate) | |||||
| Mean | 0.29 | 0.21 | 1.28 | 0.31–5.36 | 0.73 |
| SD | (± 0.76) | (± 0.49) | |||
| AAA diameter <5.5 cm? | |||||
| Yes | 6 | 19 | 0.32 | 0.03–3.00 | 0.32 |
| No | 1 | 10 | |||
| Met AAA size/growth criteria? | |||||
| Yes | 6 | 10 | 0.09 | 0.01–0.83 | 0.03* |
| No | 1 | 19 | |||
| Met IFU criteria? | |||||
| Yes | 5 | 22 | 0.80 | 0.13–5.05 | 0.81 |
| No | 2 | 7 | |||
EVAR = endovascular aneurysm repair; AAA = abdominal aortic aneurysm; OR = odds ratio; CI = confidence interval; IFU = instructions for use.
AAA size/growth criteria for elective EVAR defined by Society for Vascular Surgery Practice Guidelines, 2009.
p< .05
Figure 1.
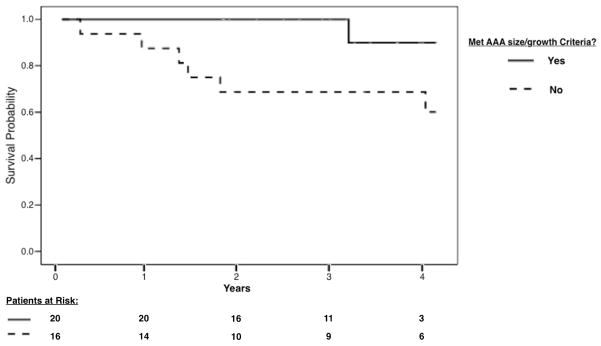
Kaplan-Meier curves of postoperative survival in female elective EVAR patients that did (solid line) and did not meet (dotted line) preoperative abdominal aortic aneurysm size and/or growth criteria. Log-rank test found significant difference in survival distributions between cohorts during the follow-up period (x2= 4.55, P= .03).
4. Discussion
Surgical guidelines and IFU guide evidence-based care and promote patient safety, yet in this real-world experience we found high rates of elective EVAR in females that did not meet traditional surgical criteria (44.4%) or device IFU (25%). Clinical evaluation for elective AAA treatment must balance a patient’s individual rupture risk with the short and long-term morbidity/mortality associated with intervention. Females have increased rupture risk and rupture at smaller diameters compared to males.[9–11] Higher rupture risk combined with more recent findings of improved perioperative outcomes of EVAR compared to open AAA repair[30–32] have led many to suggest lower size thresholds for elective EVAR in women.[4,5,16–18]
Lawrence et al. have previously described differences in treatment rates for men and women with similar AAA diagnosis. Analysis of over 110,000 patients with AAA from the National Hospital Discharge Survey database between 1984 and 1994 found that despite a higher diagnosis of ruptured AAA in females compared to males (9.4% vs. 7.4%), women were less likely to undergo elective AAA repair compared to men (15.4% vs. 37.3%; P< .001) over the same time period.[4] Dillavou et al. observed similar trends in their analysis of Medicare patients with diagnosis of AAA between 1994 and 2003. Women had significantly less decline in rates of ruptured AAA compared to men (12.2% decrease vs. 29.3% decrease; P< .001) and were less likely to receive elective or emergent AAA repair, were older at time of repair, and had more prolonged hospital courses with decreased survival. Additionally, data from 2003 showed lower EVAR utilization rates in women compared to men (28% vs 44.3%; P< .001).[5] Both papers suggest that inappropriately high AAA size thresholds in females contribute to elevated rupture rates in women and limit the benefit of newer, less-morbid elective AAA interventions for this population.[4,5] Our study included only patients that received intervention and was not designed to control for patients that did not receive EVAR, ultimately limiting our determination of prevented ruptures due to early elective intervention in females that did not meet criteria.
Randomized studies comparing surveillance to open AAA repair (ADAM and UK Small Aneurysm trials) found overall rupture risk to be low in patients with small aneurysms (~1% per year) and failed to show benefit of immediate open repair compared to surveillance for AAAs <5.5 cm,[11,33–36] but neither study were powered for subgroup analysis of females. Meta-analysis of both studies found no survival benefit of immediate open repair vs. surveillance for women (HR .96; 95% CI, 0.52–1.77), but cautioned against therapeutic certainty of these findings given the small number of females in the combined cohort (N=179).[37] More recent randomized studies comparing immediate EVAR vs. surveillance of small aneurysms (CAESAR and PIVOTAL trials) also found no mortality benefit for prophylactic treatment of AAAs <5.5 cm, but again these findings could not be fit to the female population given the small percentage of female enrollment (4.2% and 13.3% respectively).[26,27]
Our results support withholding treatment for female patients who do not meet current AAA size or growth guidelines for intervention. In this series of females that underwent elective EVAR at our institution, those with small/slow-growing AAAs tended to have higher intraoperative vessel damage and reoperation rates with significantly increased risk of late mortality compared to those with large or fast-growing AAAs. In addition, meeting size or growth criteria significantly decreased odds of late death. These outcomes were observed despite a trend in higher mean CCI in females that did meet criteria. Although the practice of “watchful waiting” raises the potential of losing EVAR eligibility and operating on an older patient population, the perioperative and long-term morbidity/mortality risks associated with elective intervention in females should not be devalued in the treatment decision-making process.
Finally, there are several limitations that must be addressed in our study. The number of females that met inclusion criteria for analysis was relatively small. Our total population size prevented development of a multivariate logistic regression model to control for variable interaction and to perform subpopulation analysis. Small sample size also increases our risk of type II error, or failing to detect true effects that are potentially present. Continued development of a prospective EVAR database at our institution will benefit further analysis of AAA diameter thresholds and EVAR outcome variables of interest. Additionally, while utilization of the Social Security Death Index to determine all-cause mortality in our cohort increases the validity of our late mortality data, we are unable to report specific cause of death for analysis of EVAR-specific mortality. Finally, as a single institution case series from a large tertiary referral center in the southeast United States, our findings may differ from other vascular practice groups and hospital systems. Larger collaborative studies are critical to the definitive validation of AAA size thresholds for EVAR in women.
5. Conclusion
Late outcomes for women following EVAR are understudied in the United States. While inherently limited by the smaller proportion of AAAs in the female vs. male population, the disproportionate percentage of AAA ruptures and deaths in women make this a topic of grave importance in need of further evaluation. There continues to be uncertainty regarding the optimum size threshold for elective EVAR in females. As displayed in this study, there is the potential for increased morbidity, reoperation, and late mortality with prophylactic intervention in females with small AAAs. Withholding intervention in women that do not meet elective guidelines, improving small aneurysm surveillance strategies, and incorporating this data into the treatment discussion with our patients may have long-term benefits on female EVAR outcomes.
Acknowledgments
Research support provided by NHLBI KO8HL119592 & Society for Vascular Surgery/American College of Surgeons Scientific Development Grant (LB), American Heart Award Innovative Research Grant IRG14740001 (LB), and Emory Department of Surgery Startup Funds (LB). In addition, Mr. Preiss’s time was supported by Emory School of Medicine’s Discovery research program.
Footnotes
Author Contributions:
Conceiving and designing study: JP, LB
Collecting data: JP, LB
Analyzing/interpreting data: All authors
Writing or critical revision: All authors
Approving final version: All authors
Disclosure:
The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bengtsson H, Bergqvist D. Ruptured abdominal aortic aneurysm: A population-based study. J Vasc Surg. 1993 Jul;18(1):74–80. doi: 10.1067/mva.1993.42107. [DOI] [PubMed] [Google Scholar]
- 2.Sampson UK, Norman PE, Fowkes FG, Aboyans V, Song Y, Harrell FE, Jr, Forouzanfar MH, Naghavi M, Denenberg JO, McDermott MM, Criqui MH, Mensah GA, Ezzati M, Murray C. Estimation of Global and Regional Incidence and Prevalence of Abdominal Aortic Aneurysms 1990 to 2010. Glob Heart. 2014 Mar;9(1):159–170. doi: 10.1016/j.gheart.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 3.Heron M, Hoyert DL, Murphy SL, Xu J, Kochanek KD, Tejada-Vera B. Natl Vital Stat Rep. 2009 Apr 17;57(14):1–134. [PubMed] [Google Scholar]
- 4.Lawrence PF, Gazak C, Bhirangi L, Jones B, Bhirangi K, Oderich G, Treiman G. The epidemiology of surgically repaired aneurysms in the United States. J Vasc Surg. 1999;30:632–640. doi: 10.1016/S0741-5214(99)70102-3. [DOI] [PubMed] [Google Scholar]
- 5.Dillavou ED, Muluk SC, Makaroun MS. A decade of change in abdominal aortic aneurysm repair in the United States: Have we improved outcomes equally between men and women? J Vasc Surg. 2006;43:230–238. doi: 10.1016/j.jvs.2005.09.043. [DOI] [PubMed] [Google Scholar]
- 6.Scott RA, Bridgewater SG, Ashton HA. Randomized clinical trial of screening for abdominal aortic aneurysm in women. Br J Surg. 2002 Mar;89(3):283–5. doi: 10.1046/j.0007-1323.2001.02014.x. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention National Center for Health Statistics. [Accessed at on 12/1/2014];Underlying Cause of Death 1999–2012 on CDC WONDER Online Database, released 2014. Data are from the Multiple Cause of Death Files, 1999–2012, as compiled from data provided by the 57 vital statistics jurisdictions through the Vital Statistics Cooperative Program. http://wonder.cdc.gov/ucd-icd10.html.
- 8.Forbes T, Lawlor DK, DeRose G, Harris KA. Gender differences in relative dilatation of abdominal aortic aneurysms. Ann Vasc Surg. 2006 Sep;20(5):564–8. doi: 10.1007/~10016-006-9079. [DOI] [PubMed] [Google Scholar]
- 9.Brown LC, Powell JT. Risk Factors for Aneurysm Rupture in Patients Kept Under Ultrasound Surveillance. Ann Surg. 1999 Sep;230(3):289–96. doi: 10.1097/00000658-199909000-00002. discussion 296–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sweeting MJ, Thompson SG, Brown LC, Powell JT RESCAN collaborators. Meta-analysis of individual patient data to examine factors affecting growth and rupture of small abdominal aortic aneurysms. Br J Surg. 2012 May;99(5):655–65. doi: 10.1002/bjs.8707. [DOI] [PubMed] [Google Scholar]
- 11.United Kingdom Small Aneurysm Trial Participants. Long-term outcomes of immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002 May 9;346(19):1445–52. doi: 10.1056/NEJMoa013527. [DOI] [PubMed] [Google Scholar]
- 12.Abedi NN, Davenport DL, Xenos E, Sorial E, Minion DJ, Endean ED. Gender and 30-day outcome in patients undergoing endovascular aneurysm repair (EVAR): an analysis using the ACS NSQIP dataset. J Vasc Surg. 2009 Sep;50(3):486–91. 491.e1–4. doi: 10.1016/j.jvs.2009.04.047. [DOI] [PubMed] [Google Scholar]
- 13.Grootenboer N, Hunink MG, Hendriks JM, van Sambeek MR, Buth J EUROSTAR collaborators. Sex differences in 30-day and 5-year outcomes after endovascular repair of abdominal aortic aneurysms in the EUROSTAR study. J Vasc Surg. 2013 Jul;58(1):42–9.e1. doi: 10.1016/j.jvs.2013.01.028. [DOI] [PubMed] [Google Scholar]
- 14.Grootenboer N, van Sambeek MRHM, Arends LR, Hendriks JM, Hunink MGM, Bosch JL. Systematic review and meta-analysis of sex differences in outcome after intervention for abdominal aortic aneurysm. Br J Surg. 2010 Aug;97(8):1169–79. doi: 10.1002/bjs.7134. [DOI] [PubMed] [Google Scholar]
- 15.Sweet MP, Fillinger MF, Morrison TM, Abel D. The influence of gender and aortic aneurysm size on eligibility for endovascular abdominal aortic aneurysm repair. J Vasc Surg. 2011 Oct;54(4):931–7. doi: 10.1016/j.jvs.2011.02.054. [DOI] [PubMed] [Google Scholar]
- 16.Brown PM, Zelt DT, Sobolev B. The risk of rupture in untreated aneurysms: The impact of size, gender, and expansion rate. J Vasc Surg. 2003 Feb;37(2):280–4. doi: 10.1067/mva.2003.119. [DOI] [PubMed] [Google Scholar]
- 17.Brewster DC, Cronenwett JL, Hallett JW, Jr, Johnston KW, Krupski WC, Matsumura JS Joint Council of the American Association for Vascular Surgery and Society for Vascular Surgery. Guidelines for the treatment of abdominal aortic aneurysms. Report of a subcommittee of the Joint Council of the American Association for Vascular Surgery and Society for Vascular Surgery. J Vasc Surg. 2003 May;37(5):1106–17. doi: 10.1067/mva.2003.363. [DOI] [PubMed] [Google Scholar]
- 18.Paraskevas KI, Mikhailidis DP, Andrikopoulos V, Bessias N, Bell SP. Should the size threshold for elective abdominal aortic aneurysm repair be lowered in the endovascular era? Yes. Angiology. 2010 Oct;61(7):617–9. doi: 10.1177/0003319710375084. [DOI] [PubMed] [Google Scholar]
- 19.EVAR trial participants. Endovascular aneurysm repair versus open repair in patients with abdominal aortic aneurysm (EVAR trial 1): randomised controlled trial. Lancet. 2005 Jun-Jul;365(9478):2179–86. doi: 10.1016/S0140-6736(05)66627-5. [DOI] [PubMed] [Google Scholar]
- 20.Stather PW, Sidloff D, Dattani N, Choke E, Bown MJ, Sayers RD. Systematic review and meta-analysis of the early and late outcomes of open and endovascular repair of abdominal aortic aneurysm. J Surg. 2013 Jun;100(7):863–72. doi: 10.1002/bjs.9101. [DOI] [PubMed] [Google Scholar]
- 21.De Bruin JL, Baas AF, Buth J, Prinssen M, Verhoeven EL, Cuypers PW, van Sambeek MR, Balm R, Grobbee DE, Blankensteijn JD DREAM Study Group. Long-term outcome of open or endovascular repair of abdominal aortic aneurysm. N Engl J Med. 2010 May 20;362(20):1881–9. doi: 10.1056/NEJMoa0909499. [DOI] [PubMed] [Google Scholar]
- 22.Egorova NN, Vouyouka AG, McKinsey JF, Faries PL, Kent KC, Moskowitz AJ, Gelijns A. Effect of gender on long-term survival after abdominal aortic aneurysm repair based on results from the Medicare national database. J Vasc Surg. 2011 Jul;54(1):1–12.e6. doi: 10.1016/j.jvs.2010.12.049. discussion 11–2. [DOI] [PubMed] [Google Scholar]
- 23.Mehta M, Byrne WJ, Robinson H, Roddy SP, Paty PS, Kreienberg PB, Feustel P, Darling RC. Women derive less benefit from elective endovascular aneurysm repair than men. J Vasc Surg. 2012 Apr;55(4):906–13. doi: 10.1016/j.jvs.2011.11.047. [DOI] [PubMed] [Google Scholar]
- 24.Chaikof EL, Brewster DC, Dalman RL, Makaroun MS, Illig KA, Sicard GA, Timaran CH, Upchurch GR, Jr, Veith FJ. The care of patients with an abdominal aortic aneurysm: the Society for Vascular Surgery practice guidelines. J Vasc Surg. 2009 Oct;50(4 Suppl):S2–49. doi: 10.1016/j.jvs.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Chaikof EL, Brewster DC, Dalman RL, Makaroun MS, Illig KA, Sicard GA, TimaranCH, Upchurch GR, Jr, Veith FJ. SVS practice guidelines for the care of patients with an abdominal aortic aneurysm: Executive summary. J Vasc Surg. 2009;50(4):880–896. doi: 10.1016/j.jvs.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Cao P, De Rango P, Verzini F, Parlani G, Romano L, Cieri E CAESAR Trial Group. Comparison of surveillance versus aortic endografting for small aneurysm repair (CAESAR): results from a randomised trial. Eur J Vasc Endovasc Surg. 2011 Jan;41(1):13–25. doi: 10.1016/j.ejvs.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 27.Ouriel K, Clair DG, Kent KC, Zarins CK Positive Impact of Endovascular Options for treating Aneurysms Early (PIVOTAL) Investigators. Endovascular repair compared with surveillance for patients with small abdominal aortic aneurysms. J Vasc Surg. 2010 May;51(5):1081–7. doi: 10.1016/j.jvs.2009.10.113. [DOI] [PubMed] [Google Scholar]
- 28.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of Chronic Diseases. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 29.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. Journal of Clinical Epidemiology. 1994;47(11):1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 30.Brown LC, Powell JT, Thompson SG, Epstein DM, Sculpher MJ, Greenhalgh RM. The UK EndoVascular Aneurysm Repair (EVAR) trials: randomised trials of EVAR versus standard therapy. Health Technol Assess. 2012;16(9):1–218. doi: 10.3310/hta16090. [DOI] [PubMed] [Google Scholar]
- 31.United Kingdom EVAR Trial Investigators. Greenhalgh RM, Brown LC, Powell JT, Thompson SG, Epstein D, Sculpher MJ. Endovascular versus open repair of abdominal aortic aneurysm. N Engl J Med. 2010 May 20;362(20):1863–71. doi: 10.1056/NEJMoa0909305. [DOI] [PubMed] [Google Scholar]
- 32.Lederle FA, Freischlag JA, Kyriakides TC, Padberg FT, Jr, Matsumura JS, Kohler TR, Lin PH, Jean-Claude JM, Cikrit DF, Swanson KM, Peduzzi PN Open Versus Endovascular Repair (OVER) Veterans Affairs Cooperative Study Group. Outcomes Following Endovascular vs Open Repair of Abdominal Aortic Aneurysm. JAMA. 2009 Oct 14;302(14):1535–42. doi: 10.1001/jama.2009.1426. [DOI] [PubMed] [Google Scholar]
- 33.Lederle FA, Wilson SE, Johnson GR, Reinke DB, Littooy FN, Acher CW, Ballard DJ, Messina LM, Gordon IL, Chute EP, Krupski WC, Busuttil SJ, Barone GW, Sparks S, Graham LM, Rapp JH, Makaroun MS, Moneta GL, Cambria RA, Makhoul RG, Eton D, Ansel HJ, Freischlag JA, Bandyk D Aneurysm Detection and Management Veterans Affairs Cooperative Study Group. Immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002 May 9;346(19):1437–44. doi: 10.1056/NEJMoa012573. [DOI] [PubMed] [Google Scholar]
- 34.Powell JT, Brown LC, Forbes JF, Fowkes FG, Greenhalgh RM, Ruckley CV, Thompson SG. Final 12-year follow-up of surgery versus surveillance in the UK Small Aneurysm Trial. Br J Surg. 2007 Jun;94(6):702–8. doi: 10.1002/bjs.5778. [DOI] [PubMed] [Google Scholar]
- 35.The UK Small Aneurysm Trial Participants. Mortality results for randomised controlled trial of early elective surgery or ultrasonographic surveillance for small abdominal aortic aneurysms. Lancet. 1998 Nov 21;352(9141):1649–55. [PubMed] [Google Scholar]
- 36.Brady AR, Fowkes FG, Greenhalgh RM, Powell JT, Ruckley CV, Thompson SG. Risk factors for postoperative death following elective surgical repair of abdominal aortic aneurysm: results from the UK Small Aneurysm Trial. Br J Surg. 2000 Jun;87(6):742–749. doi: 10.1046/j.1365-2168.2000.01410.x. [DOI] [PubMed] [Google Scholar]
- 37.Filardo G, Lederle FA, Ballard DJ, Hamilton C, da Graca B, Herrin J, Harbor J, Vanbuskirk JB, Johnson GR, Powell JT. Immediate open repair vs surveillance in patients with small abdominal aortic aneurysms: survival differences by aneurysm size. Mayo Clin Proc. 2013 Sep;88(9):910–9. doi: 10.1016/j.mayocp.2013.05.014. [DOI] [PubMed] [Google Scholar]


