Abstract
Objective
To evaluate the relation of endometrial thickness (EnT) and endometrial pattern (EnP) to euploid embryo transfer (ET) outcomes.
Design
Retrospective cohort
Setting
Private academic clinic
Patients
Patients (n=277, 36.1±4.0 years) whose embryos (n=476) underwent aneuploidy screening with fresh (n=176) or frozen (n=180) ET from July 2010–March 2014.
Interventions
EnT and EnP were measured on trigger day and at ET. Patients were stratified by age and cycle type (fresh or frozen). Cycle data were combined at trigger day but separated at ET day.
Main Outcome Measures
Outcome measures were implantation (IR), pregnancy (PR) and clinical pregnancy (CPR) rates.
Analysis was conducted by chi-square and Fisher exact test with significance at p<0.05.
Results
234 gestational sacs, 251 pregnancies and 202 clinical pregnancies resulted from 356 cycles.
EnT (9.6±1.8mm; range 5–15mm) at trigger day (n=241 cycles) as a continuous or categorical (≤8 vs >8mm) variable was not associated with IR, PR or CPR.
EnT at day of fresh (9.7±2.2mm; range 4.4–17.9mm) (n=176 cycles) or frozen ET (9.1±2.1mm; range 4.2–17.7mm) (n=180 cycles) was not associated with IR, PR or CPR.
Type 3 EnP at trigger day was associated with increased serum progesterone at trigger and decreased IR compared with type 2 EnP.
EnP at fresh or frozen ET was not associated with IR, PR or CPR.
Conclusions
Within the study population, EnT was not significantly associated with clinical outcomes of euploid ETs. A type 3 EnP at trigger day suggests a prematurely closed window of implantation.
Keywords: Endometrial Thickness, Endometrial Pattern, Implantation Rate, Pregnancy Rate, Preimplantation Genetic Screening
Introduction
The identification of the optimal conditions for controlled ovarian hyperstimulation (COH) and embryo transfer (ET) is of substantial clinical interest. Improved clinical outcomes have been demonstrated with particular stimulation protocols (1,2), embryo handling and culture conditions (3), technical factors, such as the use of the transfer catheter and placement of the embryo during ET, (4–9) and embryo selection techniques (10,11). However, identification of clinical markers of endometrial receptivity for optimization during COH remains a challenge.
During a natural menstrual cycle and one under COH, the endometrium develops and matures within a complex hormonal environment, proliferating and thickening under the influence of estrogens and decidualizing under the influence of progestins (12–19). Despite recent advances in molecular assays (20–23), ultrasound (US) assessment is the only non-invasive tool in standard clinical use for assessing the endometrium. Endometrial thickness (EnT) directly reflects histological thickness, while endometrial pattern (EnP) changes in lockstep with the menstrual cycle, correlating closely with morphologic assessment of endometrial biopsies (24,25). While endometrial histology has long been recognized to inform the optimal window of implantation (26), the influence of EnT and EnP on endometrial receptivity and pregnancy rates (PR) has been intensively explored but not conclusively answered (27,28).
US measurements of endometrium at the day of ovulatory trigger (the earliest point of completed follicular development of oocytes) and at ET day (the first interaction between embryo(s) and the uterine environment) may provide a window into the developing egg and the implantation environment. Studies thus far focusing on the effect of EnT on embryo implantation and receptivity have yielded conflicting findings. Some have shown that increased EnT on human chorionic gonadotropin (hCG) trigger day correlated with improved pregnancy outcomes in in vitro fertilization (IVF) patients (29–34). EnT <6–7 millimeter (mm) (35–37) or >10–14mm (37,38) on hCG trigger day has been reported to adversely affect implantation rate (IR). Similar findings were noted in ovum donation (OD) cycles in recipients with EnT <8mm at day of ET (39).
Other studies have documented no association between implantation rates and EnT at trigger or ET day (40–48). Given reports of successful pregnancy with an EnT <4mm (49), a thick endometrium is certainly not a prerequisite for pregnancy. One study found an positive correlation between EnT and pregnancy rates in intrauterine insemination but not IVF cycles (50), although this has also been challenged (51). Recipient EnT at ET day in OD cycles was not predictive of pregnancy outcomes (52).
A number of interventions have been developed and employed clinically to increase EnT in the hope of improving endometrial receptivity, primarily by promoting estrogen-dependent endometrial proliferation (53,54). However, given the above conflicting studies, it remains unknown whether EnT is a parameter that should be considered for clinical optimization. A recent survey found that 30% of clinicians would defer embryo transfer if EnT were ≤6mm, with smaller percentages as EnT increased (55).
EnP reflects the anatomical changes associated with the menstrual cycle following progestin exposure and can be used to track the pre- and peri-implantation uterine environment (19,56). It is possible that an optimized EnP may lead to improved reproductive outcomes. However, an incomplete consensus on the predictive power of a patient’s EnP on reproductive outcomes persists. A triple-line EnP on US after ovarian stimulation before or on trigger day has been associated with improved PR versus a homogenous, hyperechogenic or intermediate EnP (57–60). Others have failed to observe this association (61) or only confirmed it in a subset of patients with EnT 7–14mm (36,62). Some have highlighted the importance of a homogeneous, hyperechogenic endometrium at the ET day in achieving implantation (63) while others have observed a triple-line pattern more frequently (64).
In all of the aforementioned studies, morphology prior to ET was used for embryo selection. However morphologic embryo selection alone carries potential limitations (65,66). The lack of preimplantation genetic assessment of embryos, a major source of variability in implantation across patients (67), limits the generalizability of findings from previous studies on the role of both EnT and EnP.
With the use of pre-implantation genetic screening (PGS) to detect aneuploid embryos (10,11), a more standardized and systematic analysis of the role of sonographic endometrial measurements on implantation can be performed. This study sought to evaluate the impact of EnT and EnP measured on trigger and ET day in IVF patients on cycle IR and PR after controlling for oocyte age and cycle type.
Materials and Methods
Patient population
A single-center retrospective cohort study was performed on patients whose embryos underwent TB and PGS by comprehensive 24-chromosome screening during IVF cycles between July 2010 and March 2014. Aneuploidy screening was offered during routine infertility care. Patient age at the initiation of the assisted reproductive technology (ART) cycle producing the euploid embryo was recorded as a categorical variable (A <35 years old (yo); B [35–38) yo; C [38–41) yo; D [41–43) yo; and E >43 yo).
Treatment protocol
IVF stimulation cycles and hormonal adjustments were performed according to standard clinical practice (68). All cycles were autologous. Patients were treated with one of three different protocols determined by clinician preference: Antagonist (ganirelix acetate, Antagon®, Organon USA Inc., Roseland, NJ or cetrorelix acetate, Cetrotide®, EMD Serono, Rockland, MA); Down-Regulation (leuprolide acetate, Lupron®, AbbVie Inc., North Chicago, IL); or Microflare (leuprolide acetate, Lupron®, AbbVie Inc., North Chicago, IL) (Table 1). In general, antagonist protocols were used in potential hyperresponders, microflare protocols in poor responders, and down-regulation or antagonist protocols in the remaining patients.
Final oocyte maturation (henceforth referred to as “trigger”) was induced with 6500 IU recombinant hCG alone (Ovidrel®, EMD Serono, Rockland, MA) or, in patients with strong ovarian response or at risk for ovarian hyperstimulation syndrome (OHSS) undergoing an antagonist protocol, with 40 IU of leuprolide acetate together with 1000 IU of hCG (Novarel®, Ferring Pharmaceuticals, Parsippany, NJ) following confirmation of ≥2 mature follicles ≥18 mm by US. Vaginal oocyte retrieval (VOR) was performed under transvaginal ultrasound guidance 36 hours later.
For frozen embryo transfers (FET), patients started oral estradiol (Estrace®, Teva Pharmaceuticals, Sellersville, PA) 2 mg twice daily for 1 week, then 2 mg three times daily, with EnT assessed weekly until a thickness of ≥8mm was observed. Immediately thereafter, 50 mg intramuscular progesterone (Watson Pharma Inc., Parsippany, NJ) was initiated daily. Thawing and transferring of the embryo(s) was performed after 5 days of progesterone supplementation.
Embryos reaching the blastocyst stage at day 5 post-fertilization underwent trophectoderm biopsy, overnight PGS interpretation and were transferred fresh on day 6 at 8am or frozen immediately after biopsy. All frozen embryos were thawed at 9am for transfer at 1pm into a day 5 endometrium (i.e. 5 days after starting progesterone) to avoid embryo-endometrium asynchrony. The decision to freeze day 5 embryos was made by patients following clinician consultation. All embryos reaching the blastocyst stage at day 6 were biopsied and frozen in the morning of day 6.
The EnT and EnP at trigger from both fresh and frozen cycles were considered together, while the EnT and EnP at ET from fresh and frozen cycles were considered separately.
EnT was measured by transvaginal US on trigger day and transabdominally at ET to the nearest 0.1 mm. While transabdominal measurements were imported automatically, transvaginal measurements were manually inputted into our database rounded to the nearest mm. EnP was recorded as being in one of 3 categories, as described by Grunfeld et al. (24): 1 (late proliferative: hyperechoic endometrium constituting less than 50% of the EnT with a hyperechoic basalis and a hypoechoic functionalis); 2 (early secretory: hyperechoic basalis and functionalis extending to more than 50% of the EnT, but not encompassing the entire endometrial cavity); and 3 (mid-late secretory: homogeneous hyperechoic functionalis extending from the basalis to the lumen). All assessments of EnT and EnP were performed by the clinician performing the US. The most commonly observed EnP (type 2 at trigger day and type 3 at ET day) was used as the reference factor in linear models.
A pregnancy was defined as the detection of β-hCG in serum 14 days after the VOR. A clinical pregnancy (CP) was defined as the detection of a gestational sac (GS) on an ultrasound examination 22–25 days after retrieval. Monozygotic twins were counted as a single GS. Implantation rates (IR), pregnancy rates (PR) and CP rates (CPR) were calculated from these statistics. IR was calculated as the ratio of the number of GS to the number of transferred euploid embryos. PR and CPR were calculated as the ratio of total pregnancies and CPs, respectively, to the number of ART cycles entailing ET.
Serum estradiol, FSH, progesterone and hCG levels were quantitatively assessed by solid-phase, competitive chemiluminescent immunometric assay (Immulite 2000, Siemens) with an analytical sensitivity of 15 pg/mL, 0.1 mIU/mL, 0.1 ng/mL and 0.4 mIU/mL, respectively. Progesterone was considered elevated at day of trigger if it exceeded 1.5 ng/mL (69–71).
Outcomes
The outcome measures were IR, PR and CPR. Outcomes were regressed against age group, cycle type, EnT (at trigger and ET) and EnP (at trigger and ET). EnT and EnP at ET were analyzed separately for fresh and frozen cycles. EnT was analyzed both as a categorical (≤8 mm or >8mm) variable and as a continuous variable.
Statistical methods
Statistical analysis was performed with the R programming language (72). Binomial regression was performed using a logistic link function. Statistical analysis of binomial regression model was calculated by chi-square for residual deviance with significance at p<0.05. Contribution of model terms was assessed by Akaike information criterion (AIC) using the “step” function in R (better models have smaller AICs). Differences between outcomes in two groups were assessed by Fisher’s exact test. 95% confidence intervals for implantation rates in binned samples were calculated by Clopper-Pearson method with the R package “binom” from CRAN. Linear correlation was calculated with a variable intercept and significance was tested by Pearson correlation coefficient. Power analysis calculations were performed using the R package “pwr” from CRAN. Levels of progesterone across patient samples as a function of EnP were compared by chi square test in a linear model.
Power analysis
The study was designed for 80% power with a 5% false positive rate to detect the difference between a 60% IR if EnT >8mm and a 40% IR if EnT ≤8mm. Power is achieved with 97 euploid embryos in each of two equally sized groups if each embryo is considered independent, but is also >79.9% if one group has 67 patients and the other group has 173 patients.
Review
This research was approved by the Western Institutional Review Board (WIRB). Because of its retrospective nature, an informed consent was not necessary.
Results
A total of 476 euploid embryos were transferred into 277 patients over 356 IVF cycles. One (n=247 cycles), 2 (n=101), 3 (n=5) or 4 (n=3) euploid embryos were replaced per cycle. Patients ranged from 23.4 to 44.4 years old (yo) (mean 36.1 ± 4.0 yo) at the day of the initiation of their IVF stimulation cycles (Table S1–S2). Aggregate IR was 49.5% (234/476), and aggregate PR and CPR were 70.5% (251/356) and 56.7% (202/356), respectively.
Age group
IR did not generally change as a function of maternal age group (p=0.08, 0.88 and 0.33 for age groups B, C, and E relative to group A, respectively), although there was an increased IR in patients in age group D (0.67 [0.46–0.83 95% CI] vs 0.45 [0.37–0.52] for group A, p=0.04) (Table S2). PR and CPR were no different across all age groups (p>0.05 for all comparisons). Addition of age group to a model of IR did not improve the AIC (611.1 with vs 610.8 without age group).
Fresh vs frozen
FETs (n=180 cycles in 152 patients) were compared with patients who underwent fresh ET (n=176 cycles in 166 patients) (Table S1–S2). Distinction of cycle type did not improve models of IR (AIC of 613.7 with vs 610.8 without cycle type), although it led to improved models of PR and CPR. IRs were similar in FETs vs fresh (0.52 [0.45–0.58 95% CI] vs 0.47 [0.40–0.53], p=0.27). Compared with patients who underwent fresh cycles, PR (0.76 [0.70–0.83 95% CI] vs 0.64 [0.57–0.71], p=0.01) and CPR (0.62 [0.55–0.69] vs 0.51 [0.44–0.59], p=0.03) were improved in FETs (Table S1).
EnT at Trigger
EnT ranged from 5 to 15mm (mean 9.6 ± 1.8mm) at trigger day. Presence of EnT ≤8mm at trigger day (n=71 cycles) was not associated with decreased or increased IR (p=0.90), PR (p=0.88) or CPR(p=0.78) compared with EnT >8mm (n=170 cycles) (Table S3).
EnT detected at trigger day and treated as a continuous variable was not associated with IR (p=0.77), PR (p=0.73) or CPR (p=0.98) (Table S3, Figure 1a).
Figure 1. EnT at trigger or ET day does not correlate with IR or PR.
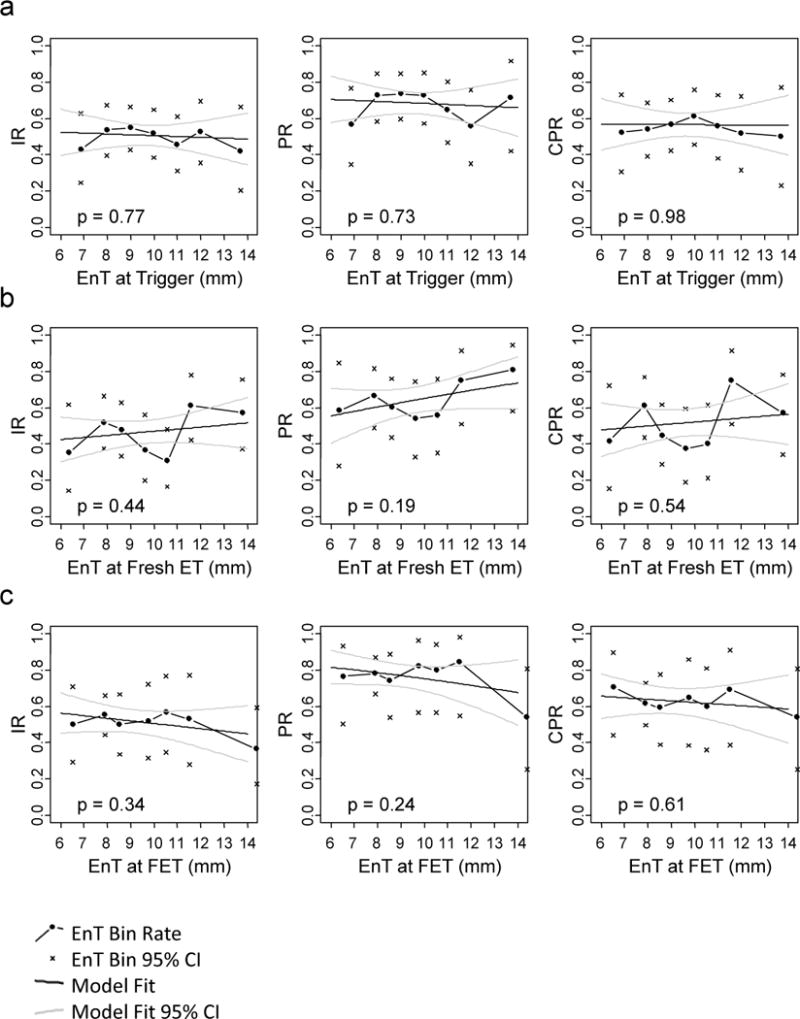
Clinical outcomes (connected points) with their respective 95% confidence intervals (x’s) are plotted at the mean EnT of each EnT bin. Model of IR, PR and CPR versus EnT treated as a continuous variable at A) trigger day B) fresh ET day or C) FET day is calculated and superimposed in black lines. Grey lines represent model 95% confidence intervals.
EnT at ET
EnT ranged from 4.4 to 17.9mm (mean 9.7 ± 2.2mm) at fresh ET day and from 4.2 to 17.7mm (mean 9.1 ± 2.1mm) at FET day. Presence of EnT ≤8mm at ET day in fresh cycles (n=48 cycles) was not associated with decreased or increased IR (p=0.8), PR (p=1.00) or CPR (p=0.50) compared with EnT >8mm (n=128 cycles) (Table 2). Presence of EnT ≤8mm at ET day in FET cycles (n=90 cycles) was not associated with decreased or increased IR (p=0.52), PR (p=0.86) or CPR (p=0.88) compared with EnT >8mm (n=90 cycles).
EnT at the time of fresh ET treated as a continuous variable was not associated with IR (p=0.44), PR (p=0.19) or CPR (p=0.54) (Figure 1b). EnT at the time of FETs treated as a continuous variable was not associated with IR (p=0.34), PR (p=0.24) or CPR (p=0.61) (Table S3, Figure 1c).
EnP at Trigger
The majority of patients had a type 2 EnP (n=79, 138 and 20 for type 1, 2 and 3 EnP, respectively) at trigger day (Table S1). Patients with a type 3 EnP at trigger day experienced decreased IR (0.31 [0.14–0.52 95% CI]) compared with a type 2 EnP (0.54 [0.47–0.62]) (p=0.03) (Figure 2a, left). IR for patients with a type 1 EnP (0.50 [0.40–0.60]) at trigger day did not differ from those with a type 2 or 3 EnP (p=0.49 and 0.08, respectively) (Figure 2a, left). No effect was observed from EnP at trigger day on PR (p=0.92, 0.22 and 0.18 for type 1 vs 2, 1 vs 3 and 2 vs 3, respectively) or CPR (p=0.33, 0.27 and 0.09 for type 1 vs 2, 1 vs 3 and 2 vs 3, respectively) (Figure 2a center and right, Table S3–S4).
Figure 2. EnP at trigger correlates with IR.
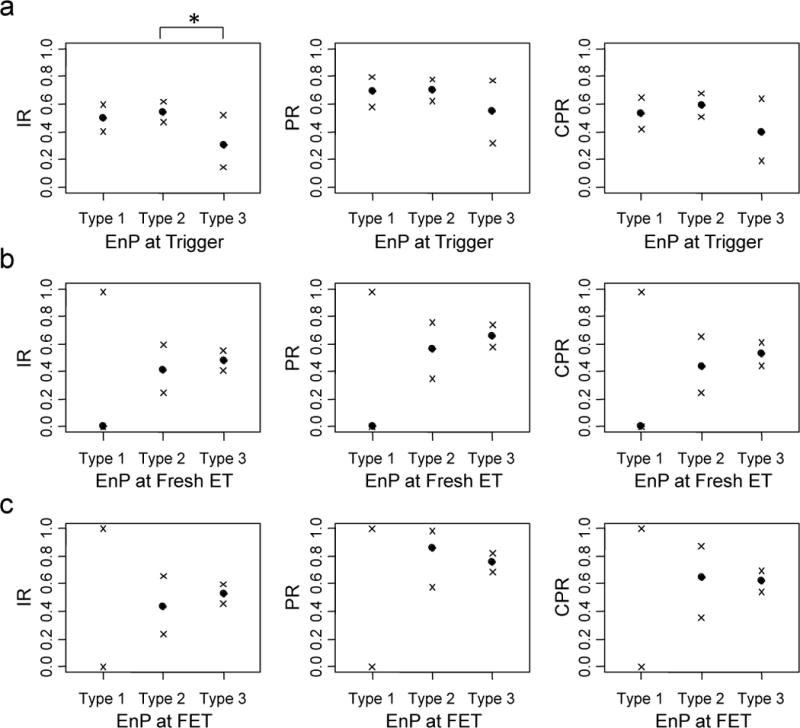
ETs were binned by EnP at A) trigger day B) fresh ET day or C) FET day. Clinical outcomes (points) reflecting IR (left), PR (center) and CPR (right) are plotted for each of the EnP categories with their respective 95% confidence intervals (x’s). * denotes p<0.05.
The serum progesterone levels at trigger day were compared across patients grouped by their same-day EnP. Patients with a type 3 pattern at trigger day had higher same-day serum progesterone levels (1.21 ± 0.54 ng/mL) compared with those with a type 2 (0.92 ± 0.39, p<0.006) or type 1 (0.94 ± 0.46, p<0.004) EnP (Figure 3). Progesterone levels in type 1 and type 2 patterns at trigger day were not significantly different (p=0.64).
Figure 3. Patients with Type 3 EnP at trigger day have elevated progesterone.
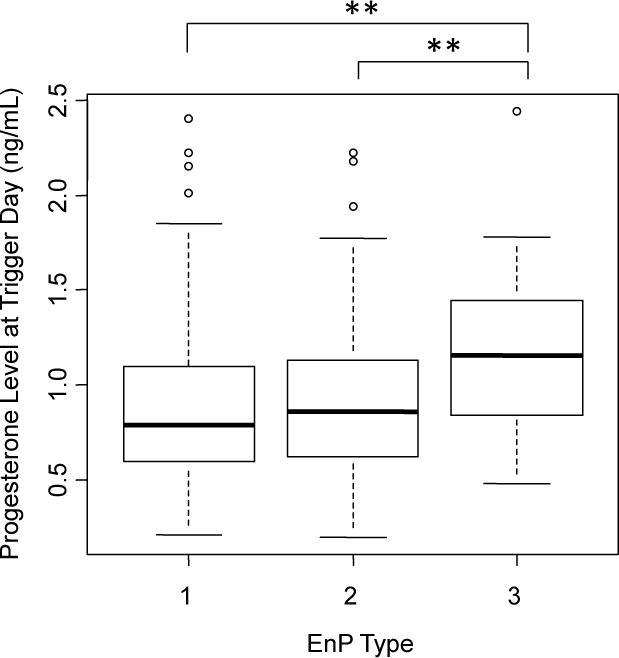
Boxplot for levels of progesterone at trigger day, grouped by EnP at trigger day. Boxes display quantiles, with whiskers extending to the most extreme data point no more than 1.5 times the interquartile range. ** denotes p<0.01.
Progesterone elevation (>1.5 ng/mL) at trigger was associated with a decreased IR (0.43 [0.35–0.50]) compared with non-elevation (0.53 [0.47–0.59], p=0.04) (Table S2). This effect remained strong in patients with type 2 EnP (p=0.02) but not in those with type 3 EnP (p=0.62) (Table S5). After controlling for progesterone elevation, the trend toward a decreased IR in type 3 EnP compared with type 2 EnP did not meet significance (p=0.07). In patients with non-elevated progesterone, the trend toward a decreased IR in type 3 (0.38 [0.09–0.76]) compared with type 2 EnP (0.61 [0.51–0.70], p=0.21) did not meet significance (Table S5).
EnP at ET
The majority of patients had a type 3 EnP at ET day (n=1, 25 and 150 for type 1, 2 and 3 EnP in fresh cycles, respectively and 0, 14, and 166 for frozen cycles, respectively) (Table S1). EnP at ET day had no detectable difference on IR, PR or CPR between types 2 and 3 in either fresh (p=0.47, 0.34 and 0.39, respectively) or frozen (p=0.41, 0.41 and 0.91, respectively) ETs (Figure 2bc, Table S3–S4).
Conclusions
While previous studies have suggested a significant effect of EnT and possibly EnP on implantation, they were limited by the unknown genetic composition of embryos prior to ET. After controlling for embryo quality by aneuploidy screening, we find that EnT either at trigger or ET day had no significant correlation with IR or clinical outcomes. However, type 3 EnP at trigger did correlate with poor IR.
The agreement of this work with the negative findings on the role of EnT in OD cycles (52) likely stems from the low aneuploidy rates of eggs from young donors (67). Given that the primary contributor to age-related fertility decline is aneuploidy (67), the lack of maternal age effect on IR of euploid embryos in this study is unsurprising. However, the existence of additional rare oocyte and genetic abnormalities that contribute to embryo failure (73,74) but are undetectable by current PGS technology cannot be ruled out. The strong ovarian response in this patient cohort (Table S1) is largely a consequence of the use of PGS in routine infertility care, including patients with normal ovarian reserve.
This study’s findings are consistent with others, placing importance on EnP at trigger day in IVF cycles (58). The decreased IR observed in patients with a type 3 EnP at trigger day compared with those with the typical type 2 EnP suggests that premature luteinization leading to uterine-embryo asynchrony is a significant contributor to implantation failure. The finding of elevated progesterone levels, a known trigger of premature ovulation (18,75), in patients with a type 3 EnP suggests that an early opening of the window of implantation leads to its premature closure, preventing successful embryo implantation. While the findings from this study may be limited due to clinician-to-clinician variability in grading EnP, the association between elevated serum progesterone and a hyperechogenic endometrium with associated decreased endometrial receptivity has been widely reported (76–78) in ETs performed without aneuploidy screening.
Although 7mm has been widely reported as a cutoff for a “thin” EnT (46,62), our clinic generally considered a “thin” EnT to be ≤8mm. While there was no protocol requiring “optimization” of EnT to this target in fresh cycles, most ETs were performed with a thicker EnT. EnT ≥8 mm was explicitly targeted in FETs (68), although achieving it was not always possible. Of the 180 frozen cycles (representing 236 FETs), 30 (39 FETs) needed to be performed with EnT <8mm at transfer. Consequently, there were too few patients with EnT ≤7mm in all groups to analyze this subset with statistical significance. A future study entailing randomization to different target EnTs, especially in FETs, would better address this limitation.
This study’s findings may not apply to euploid embryos derived from cycles that would have been cancelled in our clinical practice. Follicles from patients who were cancelled (prior to retrieval) in cycles with a thin or thick EnT or unusual EnP might have contained oocytes that were less competent for implantation.
The negative findings of this study are unlikely to generalize to patients whose EnT or EnP is altered due to endometrial pathology (e.g. Asherman’s syndrome, intrauterine tuberculosis or an autoimmune disorder), in whom an altered endometrium may be a marker of disease. A future study restricted to couples with male-factor infertility might better establish the role of EnT and EnP in implantation into an otherwise normal uterine environment.
While the study was appropriately powered to detect substantial differences between EnT types at trigger or ET day if each embryo was considered an independent trial, it lacked sufficient patients in age groups D (41–43 yo) and E (43+ yo). Confirmation of the observed negative effects of EnT on IR with statistical significance would mandate larger cohorts of up to ~20,000 patients.
Although an effect for EnP effect was fortuitously observed, this was not anticipated due to the small number of patients with type 3 EnP and consequently limited statistical power (<50%). This study lacked sufficient patients to rule out an independent association between EnP and adverse implantation outcomes after controlling for progesterone levels. Similarly, despite substantial evidence for the effects of stimulation protocols on the endometrium (75,79,80), the small number of patients undergoing downregulation protocols limited the ability to draw any conclusions.
A number of other factors besides EnT and EnP likely contribute to the variability in IR in euploid transfers. Hormonal and secreted factors produced by both the embryo and the endometrium (41,45,75,79–81), morphological differences (82–90) and genetic and epigenetic alterations not detected by PGS all potentially represent implantation and survival barriers. A more comprehensive understanding of these barriers is not likely be addressed by additional clinical studies of EnT or EnP alone.
While this study cannot currently make any definitive clinical recommendations, it found no evidence of improved IR or clinical outcomes with increasing EnT, nor did it find evidence of improved outcomes in euploid embryos derived from younger oocytes. The findings from this study suggest that aggressive “optimization” of EnT is unlikely to lead to substantial clinical benefits. However, attempts should be made to trigger ovulation prior to the transformation to a type 3 EnP.
Supplementary Material
Table S1: Study population characteristics. Patients were divided by age group and cycle type, as detailed in the methods section. Number of patients and their associated hormone levels, endometrial characteristics and embryological parameters were calculated for each group.
Table S2: Clinical outcomes by age group, cycle type, stimulation protocol and progesterone elevation. Patients were stratified by age group and further divided into fresh and frozen cycles; by cycle type; by stimulation protocol; and by presence of progesterone >1.5 ng/mL. For each category, GSs, pregnancies and CPs were tallied and IR, PR and CPR were calculated. 95% confidence intervals are calculated by Clopper-Pearson method.
Table S3: Clinical outcomes separately divided by EnT and EnP. EnT at either trigger or ET day, both fresh and frozen, was binned into thickness categories. EnP was binned as described in the methods section. Number of patients in each bin and number of total ET was noted. For each category, GSs, pregnancies and CPs were tallied and IR, PR and CPR were calculated. 95% confidence intervals are calculated by Clopper-Pearson method.
Table S4: Clinical outcomes divided by EnT and EnP in combination. EnT at either trigger or ET day, both fresh and frozen, was classified as thin (≤8mm) or thick (>8mm). EnP was binned as described in the methods section. Number of patients in each bin and number of total ET was noted. For each category, GSs, pregnancies and CPs were tallied and IR, PR and CPR were calculated. 95% confidence intervals are calculated by Clopper-Pearson method.
Table S5: Clinical outcomes divided by EnP and progesterone at trigger in combination. EnP at trigger was binned as described in the methods section. Progesterone at trigger was classified as non-elevated (≤1.5 ng/mL) or elevated (>1.5 ng/mL). Number of patients in each bin and number of total ET was noted. For each category, GSs, pregnancies and CPs were tallied and IR, PR and CPR were calculated. 95% confidence intervals are calculated by Clopper-Pearson method.
Footnotes
Disclosures: None
References
- 1.La Marca A, Sunkara SK. Individualization of controlled ovarian stimulation in IVF using ovarian reserve markers: from theory to practice. Hum Reprod Update. 2014;20(1):124–40. doi: 10.1093/humupd/dmt037. [DOI] [PubMed] [Google Scholar]
- 2.Polat M, Bozdag G, Yarali H. Best protocol for controlled ovarian hyperstimulation in assisted reproductive technologies: fact or opinion? Semin Reprod Med. 2014;32(4):262–71. doi: 10.1055/s-0034-1375178. [DOI] [PubMed] [Google Scholar]
- 3.Chronopoulou E, Harper JC. IVF culture media: past, present and future. Hum Reprod Update. 2014 doi: 10.1093/humupd/dmu040. [DOI] [PubMed] [Google Scholar]
- 4.Schoolcraft WB, Surrey ES, Gardner DK. Embryo transfer: techniques and variables affecting success. Fertil Steril. 2001;76(5):863–70. doi: 10.1016/s0015-0282(01)02731-5. [DOI] [PubMed] [Google Scholar]
- 5.Ghazzawi IM, Al-Hasani S, Karaki R, Souso S. Transfer technique and catheter choice influence the incidence of transcervical embryo expulsion and the outcome of IVF. Hum Reprod. 1999;14(3):677–82. doi: 10.1093/humrep/14.3.677. [DOI] [PubMed] [Google Scholar]
- 6.Friedman BE, Lathi RB, Henne MB, Fisher SL, Milki AA. The effect of air bubble position after blastocyst transfer on pregnancy rates in IVF cycles. Fertil Steril. 2011;95(3):944–7. doi: 10.1016/j.fertnstert.2010.07.1063. [DOI] [PubMed] [Google Scholar]
- 7.Coroleu B, Barri PN, Carreras O, Martínez F, Parriego M, Hereter L, et al. The influence of the depth of embryo replacement into the uterine cavity on implantation rates after IVF: a controlled, ultrasound-guided study. Hum Reprod. 2002;17(2):341–6. doi: 10.1093/humrep/17.2.341. [DOI] [PubMed] [Google Scholar]
- 8.Frankfurter D, Trimarchi JB, Silva CP, Keefe DL. Middle to lower uterine segment embryo transfer improves implantation and pregnancy rates compared with fundal embryo transfer. Fertil Steril. 2004;81(5):1273–7. doi: 10.1016/j.fertnstert.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 9.Rovei V, Dalmasso P, Gennarelli G, Lantieri T, Basso G, Benedetto C, et al. IVF outcome is optimized when embryos are replaced between 5 and 15 mm from the fundal endometrial surface: a prospective analysis on 1184 IVF cycles. Reprod Biol Endocrinol. 2013;11:114. doi: 10.1186/1477-7827-11-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wells D, Alfarawati S, Fragouli E. Use of comprehensive chromosomal screening for embryo assessment: microarrays and CGH. Mol Hum Reprod. 2008;14(12):703–10. doi: 10.1093/molehr/gan062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schoolcraft WB, Fragouli E, Stevens J, Munne S, Katz-Jaffe MG, Wells D. Clinical application of comprehensive chromosomal screening at the blastocyst stage. Fertil Steril. 2010;94(5):1700–6. doi: 10.1016/j.fertnstert.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 12.Okada H, Tsuzuki T, Shindoh H, Nishigaki A, Yasuda K, Kanzaki H. Regulation of decidualization and angiogenesis in the human endometrium: mini review. J Obstet Gynaecol Res. 2014;40(5):1180–7. doi: 10.1111/jog.12392. [DOI] [PubMed] [Google Scholar]
- 13.Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci U S A. 1993;90(23):11162–6. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katzenellenbogen BS. Biology and receptor interactions of estriol and estriol derivatives in vitro and in vivo. J Steroid Biochem. 1984;20(4B):1033–7. doi: 10.1016/0022-4731(84)90015-3. [DOI] [PubMed] [Google Scholar]
- 15.Harris HA. Estrogen receptor-beta: recent lessons from in vivo studies. Mol Endocrinol. 2007;21(1):1–13. doi: 10.1210/me.2005-0459. [DOI] [PubMed] [Google Scholar]
- 16.Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev. 1999;20(3):358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- 17.Hapangama DK, Kamal AM, Bulmer JN. Estrogen receptor β: the guardian of the endometrium. Hum Reprod. doi: 10.1093/humupd/dmu053. Update 2014;dmu053. [DOI] [PubMed] [Google Scholar]
- 18.Wetendorf M, DeMayo FJ. The progesterone receptor regulates implantation, decidualization, and glandular development via a complex paracrine signaling network. Mol Cell Endocrinol. 2012;357(1–2):108–18. doi: 10.1016/j.mce.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dehbashi S, Parsanezhad ME, Alborzi S, Zarei A. Effect of clomiphene citrate on endometrium thickness and echogenic patterns. Int J Gynecol Obstet. 2003;80(1):49–53. doi: 10.1016/s0020-7292(02)00341-7. [DOI] [PubMed] [Google Scholar]
- 20.Ruiz-Alonso M, Blesa D, Díaz-Gimeno P, Gómez E, Fernández-Sánchez M, Carranza F, et al. The endometrial receptivity array for diagnosis and personalized embryo transfer as a treatment for patients with repeated implantation failure. Fertil Steril. 2013;100(3):818–24. doi: 10.1016/j.fertnstert.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Garrido-Gómez T, Quiñonero A, Antúnez O, Díaz-Gimeno P, Bellver J, Simón C, et al. Deciphering the proteomic signature of human endometrial receptivity. Hum Reprod. 2014;29(9):1957–67. doi: 10.1093/humrep/deu171. [DOI] [PubMed] [Google Scholar]
- 22.Galliano D, Bellver J, Díaz-García C, Simón C, Pellicer A. ART and uterine pathology: how relevant is the maternal side for implantation? Hum Reprod Update. 2014 doi: 10.1093/humupd/dmu047. [DOI] [PubMed] [Google Scholar]
- 23.Revel A. Defective endometrial receptivity. Fertil Steril. 2012;97(5):1028–32. doi: 10.1016/j.fertnstert.2012.03.039. [DOI] [PubMed] [Google Scholar]
- 24.Grunfeld L, Walker B, Bergh PA, Sandler B, Hofmann G, Navot D. High-resolution endovaginal ultrasonography of the endometrium: a noninvasive test for endometrial adequacy. Obstet Gynecol. 1991;78(2):200–4. [PubMed] [Google Scholar]
- 25.Yoshimitsu K, Nakamura G, Nakano H. Dating sonographic endometrial images in the normal ovulatory cycle. Int J Gynaecol Obstet. 1989;28(1):33–9. doi: 10.1016/0020-7292(89)90541-9. [DOI] [PubMed] [Google Scholar]
- 26.Noyes RW. Dating the endometrial biopsy. Fertil Steril. 1950;1:23. doi: 10.1016/j.fertnstert.2019.08.079. [DOI] [PubMed] [Google Scholar]
- 27.Kwan I, Bhattacharya S, Kang A, Woolner A. Monitoring of stimulated cycles in assisted reproduction (IVF and ICSI) Cochrane database Syst Rev. 2014;8:CD005289. doi: 10.1002/14651858.CD005289.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teixeira DM, Dassunção LA, Vieira CVR, Barbosa MAP, Neto MAC, Nastri CO, et al. Ultrasound guidance during embryo transfer: a systematic review and meta-analysis of randomized controlled trials. Ultrasound Obstet Gynecol. 2014 doi: 10.1002/uog.14639. [DOI] [PubMed] [Google Scholar]
- 29.Al-Ghamdi A, Coskun S, Al-Hassan S, Al-Rejjal R, Awartani K. The correlation between endometrial thickness and outcome of in vitro fertilization and embryo transfer (IVF-ET) outcome. Reprod Biol Endocrinol. 2008;6:37. doi: 10.1186/1477-7827-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kovacs P, Matyas S, Boda K, Kaali SG. The effect of endometrial thickness on IVF/ICSI outcome. Hum Reprod. 2003;18(11):2337–41. doi: 10.1093/humrep/deg461. [DOI] [PubMed] [Google Scholar]
- 31.Noyes N, Liu HC, Sultan K, Schattman G, Rosenwaks Z. Endometrial thickness appears to be a significant factor in embryo implantation in in-vitro fertilization. Hum Reprod. 1995;10(4):919–22. doi: 10.1093/oxfordjournals.humrep.a136061. [DOI] [PubMed] [Google Scholar]
- 32.Zhang X, Chen C-H, Confino E, Barnes R, Milad M, Kazer RR. Increased endometrial thickness is associated with improved treatment outcome for selected patients undergoing in vitro fertilization-embryo transfer. Fertil Steril. 2005;83(2):336–40. doi: 10.1016/j.fertnstert.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 33.McWilliams GDE, Frattarelli JL. Changes in measured endometrial thickness predict in vitro fertilization success. Fertil Steril. 2007;88(1):74–81. doi: 10.1016/j.fertnstert.2006.11.089. [DOI] [PubMed] [Google Scholar]
- 34.Richter KS, Bugge KR, Bromer JG, Levy MJ. Relationship between endometrial thickness and embryo implantation, based on 1,294 cycles of in vitro fertilization with transfer of two blastocyst-stage embryos. Fertil Steril. 2007;87(1):53–9. doi: 10.1016/j.fertnstert.2006.05.064. [DOI] [PubMed] [Google Scholar]
- 35.Aydin T, Kara M, Nurettin T. Relationship between Endometrial Thickness and In Vitro Fertilization-Intracytoplasmic Sperm Injection Outcome. Int J Fertil Steril. 2013;7(1):29–34. [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao J, Zhang Q, Li Y. The effect of endometrial thickness and pattern measured by ultrasonography on pregnancy outcomes during IVF-ET cycles. Reprod Biol Endocrinol. 2012;10(1):100. doi: 10.1186/1477-7827-10-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dain L, Bider D, Levron J, Zinchenko V, Westler S, Dirnfeld M. Thin endometrium in donor oocyte recipients: enigma or obstacle for implantation? Fertil Steril. 2013;100(5):1289–95. doi: 10.1016/j.fertnstert.2013.07.1966. [DOI] [PubMed] [Google Scholar]
- 38.Weissman A, Gotlieb L, Casper RF. The detrimental effect of increased endometrial thickness on implantation and pregnancy rates and outcome in an in vitro fertilization program. Fertil Steril. 1999;71(1):147–9. doi: 10.1016/s0015-0282(98)00413-0. [DOI] [PubMed] [Google Scholar]
- 39.Noyes N, Hampton BS, Berkeley A, Licciardi F, Grifo J, Krey L. Factors useful in predicting the success of oocyte donation: a 3-year retrospective analysis. Fertil Steril. 2001;76(1):92–7. doi: 10.1016/s0015-0282(01)01823-4. [DOI] [PubMed] [Google Scholar]
- 40.Dietterich C, Check JH, Choe JK, Nazari A, Lurie D. Increased endometrial thickness on the day of human chorionic gonadotropin injection does not adversely affect pregnancy or implantation rates following in vitro fertilization-embryo transfer. Fertil Steril. 2002;77(4):781–6. doi: 10.1016/s0015-0282(01)03276-9. [DOI] [PubMed] [Google Scholar]
- 41.Yuval Y, Lipitz S, Dor J, Achiron R. The relationships between endometrial thickness, and blood flow and pregnancy rates in in-vitro fertilization. Hum Reprod. 1999;14(4):1067–71. doi: 10.1093/humrep/14.4.1067. [DOI] [PubMed] [Google Scholar]
- 42.Laasch C, Puscheck E. Cumulative embryo score, not endometrial thickness, is best for pregnancy prediction in IVF. J Assist Reprod Genet. 2004;21(2):47–50. doi: 10.1023/B:JARG.0000025937.43936.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garcia-Velasco JA, Isaza V, Caligara C, Pellicer A, Remohí J, Simón C. Factors that determine discordant outcome from shared oocytes. Fertil Steril. 2003;80(1):54–60. doi: 10.1016/s0015-0282(03)00545-4. [DOI] [PubMed] [Google Scholar]
- 44.Baruffi RLR, Contart P, Mauri AL, Petersen C, Felipe V, Garbellini E, et al. A uterine ultrasonographic scoring system as a method for the prognosis of embryo implantation. J Assist Reprod Genet. 2002;19(3):99–102. doi: 10.1023/A:1014795502401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Remohí J, Ardiles G, García-Velasco JA, Gaitán P, Simón C, Pellicer A. Endometrial thickness and serum oestradiol concentrations as predictors of outcome in oocyte donation. Hum Reprod. 1997;12(10):2271–6. doi: 10.1093/humrep/12.10.2271. [DOI] [PubMed] [Google Scholar]
- 46.Kasius A, Smit JG, Torrance HL, Eijkemans MJC, Mol BW, Opmeer BC, et al. Endometrial thickness and pregnancy rates after IVF: a systematic review and meta-analysis. Hum Reprod Update. 20(4):530–41. doi: 10.1093/humupd/dmu011. [DOI] [PubMed] [Google Scholar]
- 47.Rinaldi L, Lisi F, Floccari A, Lisi R, Pepe G, Fishel S. Endometrial thickness as a predictor of pregnancy after in-vitro fertilization but not after intracytoplasmic sperm injection. Hum Reprod. 1996;11(7):1538–41. doi: 10.1093/oxfordjournals.humrep.a019434. [DOI] [PubMed] [Google Scholar]
- 48.Bodri D, Colodron M, Vidal R, Galindo A, Durban M, Coll O. Prognostic factors in oocyte donation: an analysis through egg-sharing recipient pairs showing a discordant outcome. Fertil Steril. 2007;88(6):1548–53. doi: 10.1016/j.fertnstert.2007.01.054. [DOI] [PubMed] [Google Scholar]
- 49.Sundström P. Establishment of a successful pregnancy following in-vitro fertilization with an endometrial thickness of no more than 4 mm. Hum Reprod. 1998;13(6):1550–2. doi: 10.1093/humrep/13.6.1550. [DOI] [PubMed] [Google Scholar]
- 50.De Geyter C, Schmitter M, De Geyter M, Nieschlag E, Holzgreve W, Schneider HP. Prospective evaluation of the ultrasound appearance of the endometrium in a cohort of 1,186 infertile women. Fertil Steril. 2000;73(1):106–13. doi: 10.1016/s0015-0282(99)00484-7. [DOI] [PubMed] [Google Scholar]
- 51.Asante A, Coddington CC, Schenck L, Stewart EA. Thin endometrial stripe does not affect likelihood of achieving pregnancy in clomiphene citrate/intrauterine insemination cycles. Fertil Steril. 2013;100(6):1610–4. doi: 10.1016/j.fertnstert.2013.08.035. e1. [DOI] [PubMed] [Google Scholar]
- 52.Barker MA, Boehnlein LM, Kovacs P, Lindheim SR. Follicular and luteal phase endometrial thickness and echogenic pattern and pregnancy outcome in oocyte donation cycles. J Assist Reprod Genet. 2009;26(5):243–9. doi: 10.1007/s10815-009-9312-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lebovitz O, Orvieto R. Treating patients with “thin” endometrium – an ongoing challenge. Gynecol Endocrinol. 2014;30(6):409–14. doi: 10.3109/09513590.2014.906571. [DOI] [PubMed] [Google Scholar]
- 54.Glujovsky D, Pesce R, Fiszbajn G, Sueldo C, Hart RJ, Ciapponi A. Endometrial preparation for women undergoing embryo transfer with frozen embryos or embryos derived from donor oocytes. Cochrane database Syst Rev. 2010;(1):CD006359. doi: 10.1002/14651858.CD006359.pub2. [DOI] [PubMed] [Google Scholar]
- 55.Cohen MJ, Rosenzweig TS, Revel A. Uterine abnormalities and embryo implantation: clinical opinion altered by peer debate. Reprod Biomed Online. 2007;14(5):555–8. doi: 10.1016/s1472-6483(10)61045-0. [DOI] [PubMed] [Google Scholar]
- 56.Check JH, Dietterich C, Choe JK, Cohen R. Effect of triple line vs isoechogenic endometrial texture on pregnancy outcome following embryo transfer according to use of controlled ovarian stimulation (COH) or estrogen/progesterone replacement. Clin Exp Obstet Gynecol. 2013;40(1):37–9. [PubMed] [Google Scholar]
- 57.Järvelä IY, Sladkevicius P, Kelly S, Ojha K, Campbell S, Nargund G. Evaluation of endometrial receptivity during in-vitro fertilization using three-dimensional power Doppler ultrasound. Ultrasound Obstet Gynecol. 2005;26(7):765–9. doi: 10.1002/uog.2628. [DOI] [PubMed] [Google Scholar]
- 58.Zhao J, Zhang Q, Wang Y, Li Y. Endometrial pattern, thickness and growth in predicting pregnancy outcome following 3319 IVF cycle. Reprod Biomed Online. 2014;29(3):291–8. doi: 10.1016/j.rbmo.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 59.Hock DL, Bohrer MK, Ananth CV, Kemmann E. Sonographic assessment of endometrial pattern and thickness in patients treated with clomiphene citrate, human menopausal gonadotropins, and intrauterine insemination. Fertil Steril. 1997;68(2):242–5. doi: 10.1016/s0015-0282(97)81509-9. [DOI] [PubMed] [Google Scholar]
- 60.Bohrer MK, Hock DL, Rhoads GG, Kemmann E. Sonographic assessment of endometrial pattern and thickness in patients treated with human menopausal gonadotropins. Fertil Steril. 1996;66(2):244–7. doi: 10.1016/s0015-0282(16)58447-7. [DOI] [PubMed] [Google Scholar]
- 61.Rashidi BH, Sadeghi M, Jafarabadi M, Tehrani Nejad ES. Relationships between pregnancy rates following in vitro fertilization or intracytoplasmic sperm injection and endometrial thickness and pattern. Eur J Obstet Gynecol Reprod Biol. 2005;120(2):179–84. doi: 10.1016/j.ejogrb.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 62.Chen S-L, Wu F-R, Luo C, Chen X, Shi X-Y, Zheng H-Y, et al. Combined analysis of endometrial thickness and pattern in predicting outcome of in vitro fertilization and embryo transfer: a retrospective cohort study. Reprod Biol Endocrinol. 2010;8:30. doi: 10.1186/1477-7827-8-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Puerto B, Creus M, Carmona F, Cívico S, Vanrell JA, Balasch J. Ultrasonography as a predictor of embryo implantation after in vitro fertilization: a controlled study. Fertil Steril. 2003;79(4):1015–22. doi: 10.1016/s0015-0282(02)04854-9. [DOI] [PubMed] [Google Scholar]
- 64.Zácková T, Järvelä IY, Tapanainen JS, Feyereisl J. Assessment of endometrial and ovarian characteristics using three dimensional power Doppler ultrasound to predict response in frozen embryo transfer cycles. Reprod Biol Endocrinol. 2009;7:151. doi: 10.1186/1477-7827-7-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Scott L. The biological basis of non-invasive strategies for selection of human oocytes and embryos. Hum Reprod Update. 2003;9(3):237–49. doi: 10.1093/humupd/dmg023. [DOI] [PubMed] [Google Scholar]
- 66.Fragouli E, Lenzi M, Ross R, Katz-Jaffe M, Schoolcraft WB, Wells D. Comprehensive molecular cytogenetic analysis of the human blastocyst stage. Hum Reprod. 2008;23(11):2596–608. doi: 10.1093/humrep/den287. [DOI] [PubMed] [Google Scholar]
- 67.Lathi RB, Westphal LM, Milki AA. Aneuploidy in the miscarriages of infertile women and the potential benefit of preimplanation genetic diagnosis. Fertil Steril. 2008;89(2):353–7. doi: 10.1016/j.fertnstert.2007.02.040. [DOI] [PubMed] [Google Scholar]
- 68.Luna M, Grunfeld L, Mukherjee T, Sandler B, Copperman AB. Moderately elevated levels of basal follicle-stimulating hormone in young patients predict low ovarian response, but should not be used to disqualify patients from attempting in vitro fertilization. Fertil Steril. 2007;87:782–7. doi: 10.1016/j.fertnstert.2006.08.094. [DOI] [PubMed] [Google Scholar]
- 69.Keltz MD, Stein DE, Berin I, Skorupski J. Elevated progesterone-to-estradiol ratio versus serum progesterone alone for predicting poor cycle outcome with in vitro fertilization. J Reprod Med. 57:1–2. 9–12. doi: 10.1016/j.jrhm.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Papanikolaou EG, Pados G, Grimbizis G, Bili E, Kyriazi L, Polyzos NP, et al. GnRH-agonist versus GnRH-antagonist IVF cycles: is the reproductive outcome affected by the incidence of progesterone elevation on the day of HCG triggering? A randomized prospective study. Hum Reprod. 2012;27(6):1822–8. doi: 10.1093/humrep/des066. [DOI] [PubMed] [Google Scholar]
- 71.Kyrou D, Al-Azemi M, Papanikolaou EG, Donoso P, Tziomalos K, Devroey P, et al. The relationship of premature progesterone rise with serum estradiol levels and number of follicles in GnRH antagonist/recombinant FSH-stimulated cycles. Eur J Obstet Gynecol Reprod Biol. 2012;162(2):165–8. doi: 10.1016/j.ejogrb.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 72.R Development Core Team. R: A Language and Environment for Statistical Computing. R Found. Stat. Comput.; Vienna Austria: 2013. 0. [Google Scholar]
- 73.Fiorentino F, Bono S, Biricik A, Nuccitelli A, Cotroneo E, Cottone G, et al. Application of nextgeneration sequencing technology for comprehensive aneuploidy screening of blastocysts in clinical preimplantation genetic screening cycles. Hum Reprod. 2014 doi: 10.1093/humrep/deu277. [DOI] [PubMed] [Google Scholar]
- 74.Winand R, Hens K, Dondorp W, de Wert G, Moreau Y, Vermeesch JR, et al. In vitro screening of embryos by whole-genome sequencing: now, in the future or never? Hum Reprod. 2014;29(4):842–51. doi: 10.1093/humrep/deu005. [DOI] [PubMed] [Google Scholar]
- 75.Fanchin R, Righini C, Olivennes F, Ferreira AL, de Ziegler D, Frydman R. Consequences of premature progesterone elevation on the outcome of in vitro fertilization: insights into a controversy. Fertil Steril. 1997;68(5):799–805. doi: 10.1016/s0015-0282(97)00337-3. [DOI] [PubMed] [Google Scholar]
- 76.Sonigo C, Dray G, Roche C, Cédrin-Durnerin I, Hugues J-N. Impact of high serum progesterone during the late follicular phase on IVF outcome. Reprod Biomed Online. 2014;29(2):177–86. doi: 10.1016/j.rbmo.2014.03.027. [DOI] [PubMed] [Google Scholar]
- 77.Fanchin R, Righini C, Ayoubi JM, Olivennes F, de Ziegler D, Frydman R. New look at endometrial echogenicity: objective computer-assisted measurements predict endometrial receptivity in in vitro fertilization-embryo transfer. Fertil Steril. 2000;74(2):274–81. doi: 10.1016/s0015-0282(00)00643-9. [DOI] [PubMed] [Google Scholar]
- 78.Ochsenkühn R, Arzberger A, von Schönfeldt V, Gallwas J, Rogenhofer N, Crispin A, et al. Subtle progesterone rise on the day of human chorionic gonadotropin administration is associated with lower live birth rates in women undergoing assisted reproductive technology: a retrospective study with 2,555 fresh embryo transfers. Fertil Steril. 2012;98(2):347–54. doi: 10.1016/j.fertnstert.2012.04.041. [DOI] [PubMed] [Google Scholar]
- 79.Paulson RJ, Sauer MV, Lobo RA. Embryo implantation after human in vitro fertilization: importance of endometrial receptivity. Fertil Steril. 1990;53(5):870–4. doi: 10.1016/s0015-0282(16)53524-9. [DOI] [PubMed] [Google Scholar]
- 80.Tang B, Gurpide E. Direct effect of gonadotropins on decidualization of human endometrial stroma cells. J Steroid Biochem Mol Biol. 1993;47(1–6):115–21. doi: 10.1016/0960-0760(93)90064-4. [DOI] [PubMed] [Google Scholar]
- 81.Weimar CHE, Post Uiterweer ED, Teklenburg G, Heijnen CJ, Macklon NS. In-vitro model systems for the study of human embryo-endometrium interactions. Reprod Biomed Online. 2013;27(5):461–76. doi: 10.1016/j.rbmo.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 82.Racowsky C, Stern JE, Gibbons WE, Behr B, Pomeroy KO, Biggers JD. National collection of embryo morphology data into Society for Assisted Reproductive Technology Clinic Outcomes Reporting System: associations among day 3 cell number, fragmentation and blastomere asymmetry, and live birth rate. Fertil Steril. 2011;95(6):1985–9. doi: 10.1016/j.fertnstert.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 83.Cummins JM, Breen TM, Harrison KL, Shaw JM, Wilson LM, Hennessey JF. A formula for scoring human embryo growth rates in in vitro fertilization: its value in predicting pregnancy and in comparison with visual estimates of embryo quality. J In Vitro Fert Embryo Transf. 1986;3(5):284–95. doi: 10.1007/BF01133388. [DOI] [PubMed] [Google Scholar]
- 84.Puissant F, Van Rysselberge M, Barlow P, Deweze J, Leroy F. Embryo scoring as a prognostic tool in IVF treatment. Hum Reprod. 1987;2(8):705–8. doi: 10.1093/oxfordjournals.humrep.a136618. [DOI] [PubMed] [Google Scholar]
- 85.Dennis SJ, Thomas MA, Williams DB, Robins JC. Embryo morphology score on day 3 is predictive of implantation and live birth rates. J Assist Reprod Genet. 2006;23(4):171–5. doi: 10.1007/s10815-006-9027-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vernon M, Stern JE, Ball GD, Wininger D, Mayer J, Racowsky C. Utility of the national embryo morphology data collection by the Society for Assisted Reproductive Technologies (SART): correlation between day-3 morphology grade and live-birth outcome. Fertil Steril. 2011;95(8):2761–3. doi: 10.1016/j.fertnstert.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 87.Tesarik J, Greco E. The probability of abnormal preimplantation development can be predicted by a single static observation on pronuclear stage morphology. Hum Reprod. 1999;14(5):1318–23. doi: 10.1093/humrep/14.5.1318. [DOI] [PubMed] [Google Scholar]
- 88.Balaban B, Yakin K, Urman B, Isiklar A, Tesarik J. Pronuclear morphology predicts embryo development and chromosome constitution. Reprod Biomed Online. 2004;8(6):695–700. doi: 10.1016/s1472-6483(10)61651-3. [DOI] [PubMed] [Google Scholar]
- 89.Scott L, Alvero R, Leondires M, Miller B. The morphology of human pronuclear embryos is positively related to blastocyst development and implantation. Hum Reprod. 2000;15(11):2394–403. doi: 10.1093/humrep/15.11.2394. [DOI] [PubMed] [Google Scholar]
- 90.Berger DS, Zapantis A, Merhi Z, Younger J, Polotsky AJ, Jindal SK. Embryo quality but not pronuclear score is associated with clinical pregnancy following IVF. J Assist Reprod Genet. 2014;31(3):279–83. doi: 10.1007/s10815-013-0162-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Study population characteristics. Patients were divided by age group and cycle type, as detailed in the methods section. Number of patients and their associated hormone levels, endometrial characteristics and embryological parameters were calculated for each group.
Table S2: Clinical outcomes by age group, cycle type, stimulation protocol and progesterone elevation. Patients were stratified by age group and further divided into fresh and frozen cycles; by cycle type; by stimulation protocol; and by presence of progesterone >1.5 ng/mL. For each category, GSs, pregnancies and CPs were tallied and IR, PR and CPR were calculated. 95% confidence intervals are calculated by Clopper-Pearson method.
Table S3: Clinical outcomes separately divided by EnT and EnP. EnT at either trigger or ET day, both fresh and frozen, was binned into thickness categories. EnP was binned as described in the methods section. Number of patients in each bin and number of total ET was noted. For each category, GSs, pregnancies and CPs were tallied and IR, PR and CPR were calculated. 95% confidence intervals are calculated by Clopper-Pearson method.
Table S4: Clinical outcomes divided by EnT and EnP in combination. EnT at either trigger or ET day, both fresh and frozen, was classified as thin (≤8mm) or thick (>8mm). EnP was binned as described in the methods section. Number of patients in each bin and number of total ET was noted. For each category, GSs, pregnancies and CPs were tallied and IR, PR and CPR were calculated. 95% confidence intervals are calculated by Clopper-Pearson method.
Table S5: Clinical outcomes divided by EnP and progesterone at trigger in combination. EnP at trigger was binned as described in the methods section. Progesterone at trigger was classified as non-elevated (≤1.5 ng/mL) or elevated (>1.5 ng/mL). Number of patients in each bin and number of total ET was noted. For each category, GSs, pregnancies and CPs were tallied and IR, PR and CPR were calculated. 95% confidence intervals are calculated by Clopper-Pearson method.


