Abstract
Cisplatin is a cytotoxic chemotherapeutic drug frequently used to treat many solid tumors, including head and neck squamous cell carcinoma (HNSCC). EGFR inhibitors have also shown efficacy as alternatives to cisplatin in some situations. However, large clinical trials have shown no added survival benefit from the use of these two drugs in combination. Possible explanations for this include overlapping downstream signaling cascades. Using in vitro studies, we tested the hypothesis that cisplatin and EGFR inhibitors rely on the activation of the tumor suppressor STAT1, characterized by its phosphorylation at serine (S727) or tyrosine (Y701) residues. Cisplatin consistently increased the levels of p-S727-STAT1, and STAT1 siRNA knockdown attenuated cisplatin-induced cell death. EGFR stimulation also activated p-S727-STAT1 and p-Y701-STAT1 in a subset of cell lines, whereas EGFR inhibitors alone decreased levels of p-S727-STAT1 and p-Y701-STAT1 in these cells. Contrary to our hypothesis, EGFR inhibitors added to cisplatin treatment caused variable effects among cell lines, with attenuation of p-S727-STAT1 and enhancement of cisplatin-induced cell death in some cells and minimal effect in other cells. Using HNSCC tumor specimens from a clinical trial of adjuvant cisplatin plus the anti-EGFR antibody panitumumab, higher intratumoral p-S727-STAT1 appeared to correlate with worse survival. Together, these results suggest that cisplatin-induced cell death is associated with STAT1 phosphorylation, and the addition of anti-EGFR therapy to cisplatin has variable effects on STAT1 and cell death in HNSCC.
Keywords: head and neck cancer, STAT1, cetuximab, cisplatin, EGFR
INTRODUCTION
Head and neck squamous cell carcinoma (HNSCC) is a prevalent disease associated with carcinogens such as tobacco and alcohol as well as the human papillomavirus (HPV). Cisplatin is the most widely used cytotoxic drug for HNSCC. Drugs targeting the epidermal growth factor receptor (EGFR) have also been employed based on high EGFR expression levels in most HNSCC tumors (1). Anti-EGFR monoclonal antibodies have been shown to improve survival compared with radiation alone for HNSCC, and in addition to cytotoxic drugs in the setting of recurrent or metastatic disease (2, 3). However, recent large clinical trials of anti-EGFR monoclonal antibodies or tyrosine kinase inhibitors in addition to definitive platinum-based chemoradiation regimens have been disappointing (4, 5). While the addition of EGFR inhibitors to standard-of-care chemoradiation no longer seems to be a promising treatment strategy, it offers an intriguing opportunity to better understand the signaling events involved in tumor cell death following exposure to these two drug classes. Potential explanations for the lack of additive clinical benefit with cisplatin and EGFR inhibitors include: 1) overlapping cell death signaling cascades that are maximally activated by each drug, and 2) opposing effects of the two drugs on cell death signaling cascades.
Signal transducer and activator of transcription (STAT) proteins are transcription factors downstream of cytokines and growth factors. The tyrosine phosphorylation site of STAT proteins is usually activated by Janus kinase (JAK) proteins in response to cytokine or growth factor receptor activation, resulting in STAT dimerization and translocation to the nucleus for activation of gene transcription. The serine phosphorylation residue can be activated by a myriad of kinases, and its functions are not well understood, though it is thought to be necessary for maximal transcription of STAT-dependent genes (6). While overexpression of oncogenic STAT3 in HNSCC has been well described (7), the impact of STAT1, considered to be a tumor suppressor, has been less well studied. Previous work from our laboratory suggests that STAT1 activation is important for antigen presentation and activation of cytotoxic T lymphocytes in HNSCC and is suppressed at baseline (8, 9). Additionally our laboratory showed that the clinically effective anti-EGFR monoclonal antibody cetuximab may enhance adaptive anti-tumor immunity in a STAT1-dependent manner (10). Cisplatin has been shown to activate STAT1 in other (non-tumor) cell types (11-14). STAT1-null, non-tumor cells are relatively resistant to cisplatin-induced cell death (11, 14), and antitumor efficacy of cisplatin may depend on adaptive immunity in HNSCC (15). Two small clinical trials suggest that high STAT1 staining in tumor specimens may be a good prognostic indicator in HNSCC patients treated with platinum-based chemotherapy (16, 17).
Based on these findings, we hypothesized that signaling cascades activated by cisplatin and EGFR inhibitors might converge upon STAT1, and that this could potentially explain the lack of additive benefit seen in large clinical trials using these two drugs in combination. In this study, we compared the STAT1 effects on HNSCC cells treated with these drugs alone or in combination as well as the impact on STAT1-induced apoptosis. Tumor specimens were obtained from a clinical trial of HNSCC patients treated with radiation, cisplatin, and the anti-EGFR antibody panitumumab in the adjuvant setting, and staining for phosphorylated STAT1 was compared with survival. These findings have novel implications for combining tumor-targeted antibodies and chemoradiation.
MATERIALS AND METHODS
Cell lines
The HNSCC cell line JHU029 (HPV-negative) was obtained as a kind gift from Dr. James Rocco (Harvard Medical School, Boston, MA) in 2007. PCI13 (HPV-negative) and SCC90 (HPV-positive) cell lines were isolated and cultured from patients at the University of Pittsburgh. All cell lines were authenticated and validated by short tandem repeat profiling and HLA genotyping every six months (18, 19). The cell lines were not personally validated by the authors, but were validated routinely by Drs. Jennifer Grandis and Susanne Gollin at the University of Pittsburgh Cancer Institute. Cell lines were maintained in IMDM Glutamax supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin at 37°C and 5% CO2.
Reagents
Cisplatin was manufactured by Fresenius Kabi (Lake Zurich, IL) and obtained from the pharmacy at the University of Pittsburgh Cancer Institute. Cetuximab was from Brystol-Myers Squibb (New York, NY), and panitumumab was from Amgen (Thousand Oaks, CA). Water-soluble gefitinib and SB203580 were obtained from Tocris Bioscience. Anti-human antibodies used for flow cytometry staining included Alexa 488-p-S727-STAT1er727, PE-pSTAT1Tyr701, Alexa 647-STAT1 N terminus, Pacific Blue-p-p38 and corresponding isotype controls, all from BD Biosciences. Anisomycin, antibodies to p-S727-STAT1 and p-Y701-STAT1 for immunohistochemistry of tumor specimens, and antibodies for Western blots (total STAT1 primary and anti-rabbit secondary) were obtained from Cell Signaling Technology. Annexin V stain and Annexin buffer were obtained from BD Biosciences. Zombie Aqua viability dye was obtained from BioLegend and used at a dilution of 1/500. Lipopolysaccharide and β-actin antibody were obtained from Sigma. The APO-BrdU TUNEL assay kit for flow cytometry was from Life Technologies.
Drug treatments
Cells were plated into 6- or 12-well plates at 100,000 cells/ml of culture medium. Cells were serum starved for 24 hours prior to each experimental treatment to synchronize the cell growth cycle. All drugs were dissolved in saline or water to avoid deactivation of cisplatin by DMSO (20). Complete medium with 10% FBS and 1% penicillin/streptomycin was used during the drug treatments.
Flow cytometry
Following each experimental treatment, cells were harvested with trypsin/EDTA, rinsed in PBS, incubated in Zombie Aqua viability dye for 20 minutes, fixed in 2% paraformaldehyde for 20 minutes, and then permeabilized in 100% methanol overnight at −20° C. Cells were then rinsed in FACS buffer and stained at room temperature for 90 minutes with fluorescent-conjugated antibodies or isotype controls, using dilutions obtained from preliminary titration experiments. After further washing, flow cytometry was then performed immediately after staining, using a BD LSR Fortessa flow cytometer and BD FACSDiva software. Values obtained for isotype controls were subtracted for each condition.
Cell death assays
Following drug treatments, cells were harvested with trypsin/EDTA, rinsed in PBS, and stained with Zombie Aqua viability dye for 20 minutes. Cells were then stained with FITC-conjugated Annexin V staining kit according to the manufacturer’s instructions. Flow cytometry was then performed immediately. Untreated cells were used to delineate the gates for Zombie Aqua and Annexin dyes, since even viable cells demonstrate low-level Zombie Aqua staining. Double-negative cells were determined to be viable, Annexin-positive cells as early apoptotic, and double-positive cells as late apoptotic. For the TUNEL assay, drug-treated cells were harvested with trypsin/EDTA, rinsed in PBS, fixed in 1% paraformaldehyde, permeabilized in 70% ethanol, stained with the APO-BrdU TUNEL assay kit according to the manufacturer’s instructions, and analyzed by flow cytometry. Gating was performed on untreated cells to determine TUNEL positive vs. negative cells.
STAT1 Small Interfering RNA (siRNA)
Cells were transfected at 30-40% confluence with 10 nM STAT1 or control siRNA with Lipofectamine RNAiMAX and Opti-MEM (Life Technologies) according to the manufacturer’s instructions. Cells were incubated in antibiotic- and serum-free IMDM Glutamax with siRNA for 48 hours, then rinsed with PBS prior to drug treatments. STAT1 siRNA from Ambion (siRNA ID #s277) was used with the following sequences:
5’ – CCUACGAACAUGACCCUAUtt – 3’ (sense)
5’ – AUAGGGUCAUGUUCGUAGGtg – 3’ (antisense)
For verification, a second STAT1 siRNA from Ambion (siRNA ID #s279) was used with the following sequences:
5’ – GGUUCACUAUAGUUGCGGAtt – 3’ (sense)
5’ – UCCGCAACUAUAGUGAACCag – 3’ (antisense)
Western blots
Following knockdown experiments, cells were rinsed with PBS. Whole cell extracts were obtained by incubating on ice for 30 minutes in radioimmunoprecipitation assay (RIPA) buffer including protease inhibitors (Roche), then centrifuged at 15,000 × g for 20 minutes at 4 degrees C and supernatants collected. Protein concentration was standardized using a BCA Protein Assay kit (Pierce). Samples were resolved by SDS-PAGE (Lonza), then transferred to a PVDF membrane (Millipore). Membranes were then immunoblotted with STAT1 and β–actin antibodies.
Immunohistochemistry of tumor specimens
Tumor specimens were obtained from a clinical trial (UPCI 06-120, NCT00798655) of patients with high-risk, advanced (stage III or IV) resectable HNSCC treated with surgery followed by adjuvant radiation and weekly cisplatin (30 mg/m2) and panitumumab (2.5 mg/Kg). Most tumors were from oral cavity or laryngeal sites, and all but one were HPV-negative. Tumor specimens were obtained at the time of surgical resection, prior to adjuvant therapy. Preparation of paraffin sections, H&E staining and immunohistochemistry for phosphoSTAT1 serine and tyrosine residues were performed as previously described (8). A staining score was obtained by a pathologist blinded to patient characteristics and was based on the intensity of staining and percentage of stained cells. The mean staining score for each phosphorylation residue was used to divide specimens into high and low pSTAT1 staining groups. Survival data were obtained from clinical records. This clinical trial was approved by the Institutional Review Board at the University of Pittsburgh, and informed consent was obtained from all patients.
Statistical Analysis
Data were analyzed by student’s t test, one- or two-way ANOVA where appropriate. GraphPad Prism software was used for statistical tests and creation of Kaplan-Meier survival curves. A p value of <0.05 was used to determine statistical significance.
RESULTS
Cisplatin and cetuximab induce cell death in multiple HNSCC cell lines
To investigate the degree of cell death of HNSCC cells treated with cisplatin or EGFR inhibitors, two cell lines (JHU029 and PCI13, HPV-negative) were treated with a range of doses of cisplatin and cetuximab, and cell death was investigated using Annexin V and TUNEL assays (Table 1). Based on these results, further experiments were done using cisplatin (6 μg/ml), an anti-EGFR monoclonal antibody (cetuximab or panitumumab, 10 μg/ml) or the EGFR tyrosine kinase inhibitor gefitinib (1 μM), alone or in combination for 24 hours. The chosen dose of cisplatin is consistent with the peak serum concentration following standard-of-care doses of cisplatin for head and neck squamous cell carcinoma (100 mg/m2) (21). The dose of cetuximab and panitumumab has been found in prior studies to effectively block EGFR signaling but is well below the peak serum concentration following most standard clinical regimens (22-24). The gefitinib dose was chosen to provide EGFR blockade (based on preliminary experiments) while limiting off-target effects. Cisplatin and cetuximab alone induced significant apoptosis in both cell lines, which was more pronounced when the drugs were used together (Table 1; Figures 1A and 2A, top panels).
Table 1.
Cell death and STAT1 phosphorylation following a range of cisplatin and cetuximab doses in two cell lines. Cells were treated with the indicated drug doses for 24 hours to assess cell death or 8 hours to assess STAT1 phosphorylation. Mean cell death according to these assays was 2% or less for untreated cells. Levels of phosphorylated STAT1 are reported as fold change in median fluorescence intensity by flow cytometry, compared with untreated cells. Data are mean ± SEM.
| Cell Line |
Drug | Dose (μg/ml) |
% Apoptotic Cells (Annexin V positive) |
% Apoptotic Cells (TUNEL positive) |
p-S727-STAT1 (Fold Change vs. Untreated) |
p-Y701-STAT1 (Fold Change vs. Untreated) |
|---|---|---|---|---|---|---|
| JHU029 | Cisplatin | 2 | 20 ± 1.66 | 9.07 ± 1.88 | 0.82 ± 0.16 | 1.22 ± 0.27 |
| 6 | 22.07 ± 0.45 | 22.6 ± 4.92 | 1.42 ± 0.27 | 1.05 ± 0.13 | ||
| 12 | 45.7 ± 1.56 | 43.5 ± 6.47 | 1.94 ± 0.58 | 0.96 ± 0.04 | ||
| Cetuximab | 1 | 53.37 ± 4.86 | 26.3 ± 2.77 | 0.81 ± 0.07 | 1.41 ± 0.04 | |
| 10 | 60.57 ± 1.27 | 28.4 ± 1.07 | 0.37 ± 0.16 | 0.80 ± 0.24 | ||
| 20 | 62.8 ± 3.59 | 34.7 ± 1.31 | 0.63 ± 0.05 | 0.98 ± 0.11 | ||
| PCI13 | Cisplatin | 2 | 30.36 ± 9.16 | 27.0 ± 0.44 | 1.52 ± 0.04 | 1.00 ± 0.02 |
| 6 | 44.8 ± 2.28 | 67.5 ± 4.50 | 1.75 ± 0.07 | 0.89 ± 0.03 | ||
| 12 | 46.4 ± 2.32 | 73.4 ± 7.14 | 1.35 ± 0.08 | 0.80 ± 0.02 | ||
| Cetuximab | 1 | 18.67 ± 1.16 | 9.9 ± 2.33 | 1.25 ± 0.41 | 1.04 ± 0.05 | |
| 10 | 29.07 ± 1.19 | 14.5 ± 5.55 | 1.15 ± 0.09 | 1.16 ± 0.05 | ||
| 20 | 27.2 ± 2.21 | 17.5 ± 0.44 | 1.63 ± 0.35 | 1.03 ± 0.02 |
Figure 1.
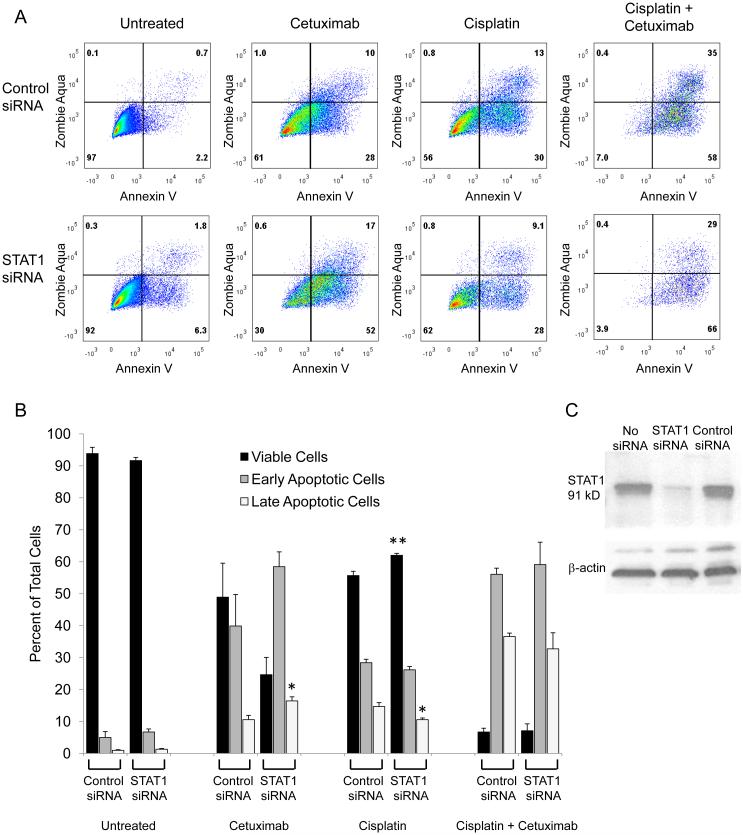
STAT1 siRNA knockdown modestly attenuates cisplatin-induced cell death and exacerbates cetuximab-induced cell death in JHU029 cells. JHU029 cells were treated with cisplatin, cetuximab, or the drugs in combination following treatment with control or STAT1 siRNA for 48 hours, then stained with Annexin V to detect apoptosis and Zombie Aqua to detect viability. A) Flow cytometry plots are shown, representative of at least 4 samples from 2 independent experiments. B) Cells were categorized as viable (double negative staining), early apoptotic (Annexin V only), or late apoptotic (double positive staining). Very few cells were dead (Zombie Aqua only). Data are mean + SEM, p <0.05, ** p <0.01 compared with untreated. C) After 48 hours of treatment with no siRNA, STAT1 siRNA or control siRNA, levels of total STAT1 in JHU029 cells were assessed by Western blot.
Figure 2.
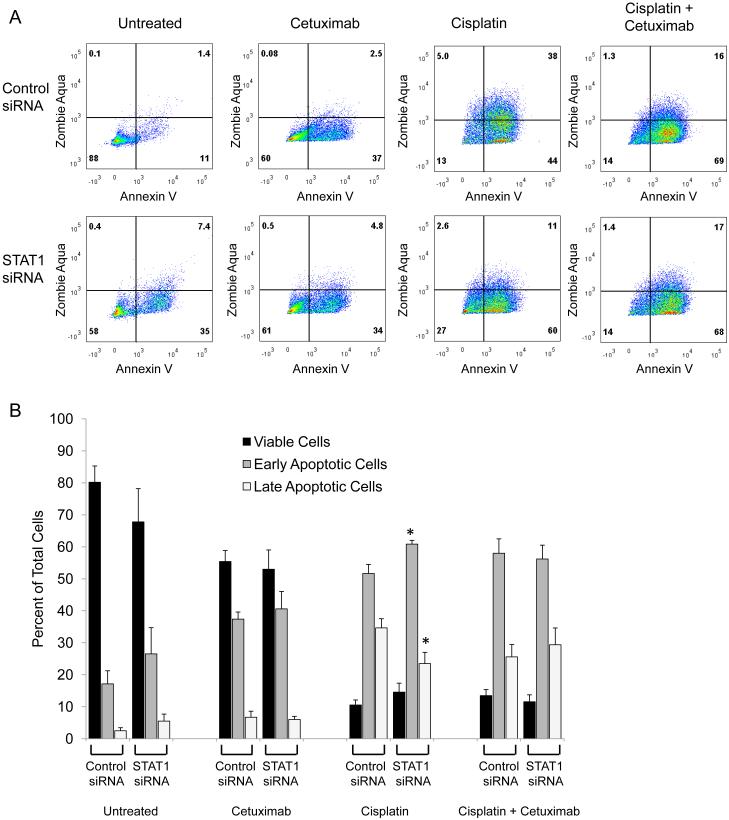
STAT1 siRNA knockdown modestly attenuates cisplatin-induced cell death but does not affect cetuximab-induced cell death in PCI13 cells. PCI13 cells were treated with cisplatin, cetuximab, or the drugs in combination following treatment with control or STAT1 siRNA for 48 hours, then stained with Annexin V to detect apoptosis and Zombie Aqua to detect viability. A) Flow cytometry plots are shown, representative of at least 4 samples from 2 independent experiments. B) Cells were categorized as viable (double negative staining), early apoptotic (Annexin V only), or late apoptotic (double positive staining). Very few cells were dead (Zombie Aqua only). Data are mean + SEM, *p <0.05 compared with untreated.
STAT1 siRNA knockdown modestly attenuates cisplatin-induced cell death but may enhance cetuximab-induced cell death in some HNSCC cell lines
STAT1 activation often induces cell death, and cisplatin-induced p-S727-STAT1 has been associated with cell death in other non-tumor cell types (11, 12, 14). We hypothesized that siRNA knockdown of STAT1 would attenuate cisplatin-induced cell death in HNSCC cells. Cisplatin-induced cell death was modestly attenuated by STAT1 siRNA knockdown in both cell lines (Figures 1 and 2). Cetuximab-induced cell death was unaffected by STAT1 siRNA knockdown in PCI13 cells and exacerbated in JHU029 cells. When cetuximab was added to cisplatin, cell death was increased in JHU029 cells but unaffected in PCI13 cells, with no effect of STAT1 siRNA knockdown in either cell line. STAT1 siRNA knockdown assessed by flow cytometry was found to be 69 ± 4% (mean ± SEM) for JHU029 cells and 80 ± 1% for PCI13 cells. Effective STAT1 siRNA knockdown was also verified by Western blot in the JHU029 cells (Figure 1C). These results suggest that STAT1 levels have a modest impact on tumor cell death following treatment with cisplatin and a variable impact on cetuximab-induced cell death.
Cisplatin induces phosphorylation of STAT1, which was either attenuated or unaffected by EGFR inhibition in different HNSCC cell lines
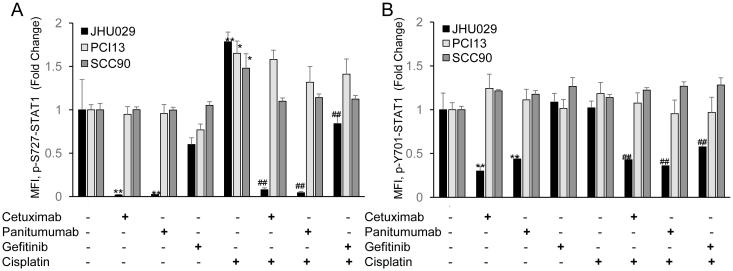
Based on the different patterns of STAT1-induced apoptosis seen in JHU029 and PCI13 cells, we hypothesized that STAT1 activation might differ in these two cell lines following treatment with cisplatin and/or cetuximab. In preliminary experiments, we treated these two cell lines with a range of cisplatin and cetuximab doses and measured levels of phosphorylated STAT1 by flow cytometry (Table 1). Based on these results, we then treated these two cell lines and a third, HPV-positive cell line (SCC90) with cisplatin (6 μg/ml), an anti-EGFR monoclonal antibody (cetuximab or panitumumab, 10 μg/ml) or the EGFR tyrosine kinase inhibitor gefitinib (1 μM), alone or in combination. In all three cell lines, cisplatin alone increased levels of STAT1 phosphorylation at the serine 727 residue, but not the tyrosine 701 residue, as previously found in other non-tumor cell types (11, 12, 14). Cisplatin-induced p-S727-STAT1 was noted as early as 4 hours and peaked at 8 hours (Figure 3). While EGFR inhibitors had no effect on STAT1 phosphorylation in PCI13 or SCC90 cells, all three EGFR inhibitors decreased both baseline and cisplatin-induced STAT1 phosphorylation at both residues in JHU029 cells (Figure 3). The degree of decreased p-S727-STAT1 noted with EGFR inhibition in JHU029 cells suggests that EGFR may account for some or all of the STAT1 serine phosphorylation in these cells, but not in PCI13 or SCC90 cells.
Figure 3.
STAT1 is activated by cisplatin and variably affected by EGFR inhibitors. Cell lines were treated with cisplatin with or without the EGFR inhibitors cetuximab, panitumumab and gefitinib for 8 hours. Intracellular p-S727-STAT1 (A) and p-Y701-STAT1 (B) staining were assessed by flow cytometry, comparing median fluorescence intensity (MFI) for each treatment to MFI for untreated cells. Data are mean + SEM. * p <0.05, ** p <0.01 compared with untreated; ## p <0.01 compared with cisplatin alone.
EGF activates STAT1 in Some HNSCC cell lines
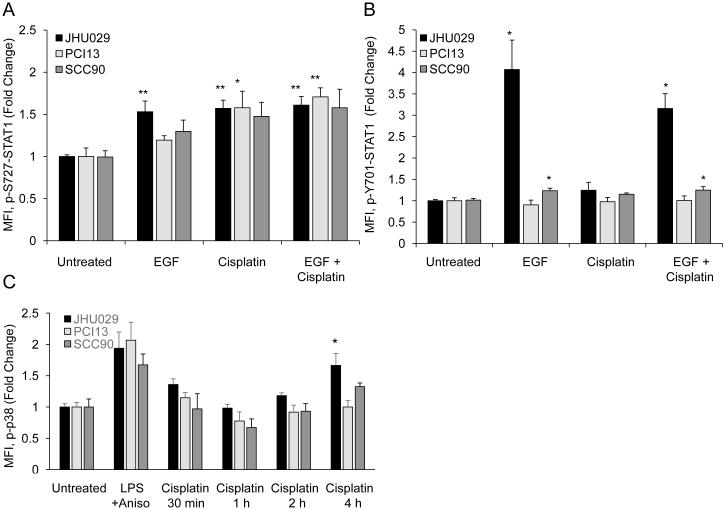
While it is well established that EGFR can activate STAT3, STAT1 activation by EGFR has been reported rarely in the literature and primarily in cells that overexpress EGFR (25). Noting that STAT1 phosphorylation was decreased by EGFR inhibitors in JHU029 cells, we hypothesized that EGF could activate STAT1 in these cells. All cell lines were treated with EGF (10 ng/ml) or cisplatin (6 μg/ml) alone or in combination. EGF alone caused an increase of p-S727-STAT1 only in JHU029 cells, with no additive effect when cisplatin was added (Figure 4A). EGF caused p-Y701-STAT1 to increase dramatically in JHU029 cells and modestly in SCC90 cells (Figure 4B).
Figure 4.
EGF, but not p38, may be upstream of STAT1 in some cell lines. A, B) Cell lines were treated with EGF or cisplatin alone or in combination. Intracellular p-S727-STAT1 and p-Y701-STAT1 staining were assessed by flow cytometry, comparing median fluorescence intensity (MFI) for each treatment to MFI for untreated cells. Note different scales for the two graphs. C) p38 MAPK phosphorylation increased modestly only in JHU029 cells following cisplatin treatment. Cell lines were treated with a combination of LPS and anisomycin for 30 minutes (positive control) or with cisplatin from 30 minutes to 4 hours. Intracellular phospho-p38 was assessed by flow cytometry, comparing median fluorescence intensity (MFI) for each treatment to MFI for untreated cells. Data are mean + SEM. * p <0.05, ** p <0.01 compared with untreated.
p38 MAP kinase is not responsible for cisplatin-induced STAT1 serine phosphorylation
STAT1 serine phosphorylation in the absence of tyrosine phosphorylation has been reported following various forms of cell stress such as UV irradiation (26). Upstream kinases implicated in STAT1 serine phosphorylation include EGFR, ERK, JNK, and PKC delta, but perhaps the best studied upstream kinase is p38 MAP kinase (26, 27). Since EGFR inhibition did not seem to affect cisplatin-induced p-S727-STAT1 in PCI13 and SCC90 cells, we investigated whether p38 activation might occur following treatment with cisplatin. Since p-S727-STAT1 was first noted at 4 hours, we treated all cell lines with cisplatin for 30 minutes, 1 hour, 2 hours, or 4 hours and checked intracellular levels of phospho-p38 (Figure 4C). A combination of anisomycin (25 μg/ml) and lipopolysaccharide (LPS, 1 μg/ml) was used to stimulate p38 phosphorylation as a positive control. Cisplatin caused a modest increase in phospho-p38 levels by 4 hours only in JHU029 cells; consistent with this, 12-hour pretreatment with the p38 inhibitor SB203580 decreased cisplatin-induced p-S727-STAT1 by 15% at 8 hours in this cell line. There was no increase in phospho-p38 noted in SCC90 or PCI13 cells following cisplatin treatment, suggesting that p38 is not the primary kinase responsible for STAT1 serine phosphorylation following cisplatin treatment in these cell lines.
STAT1 phosphorylation in HNSCC tumors does not appear to be a favorable prognostic marker in patients treated with adjuvant cisplatin and panitumumab
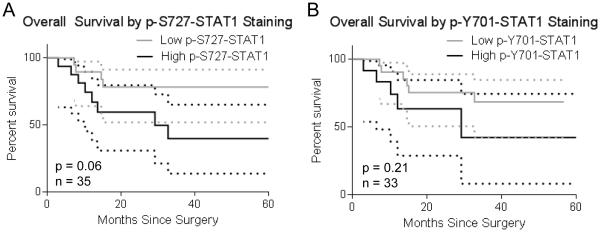
As described above, large clinical trials have shown no added benefit from EGFR inhibitors when used with standard platinum-based chemoradiation regimens for HNSCC. It is unclear, however, whether specific tumor biomarkers might help identify patients more likely to benefit from the combination of cisplatin, radiation and EGFR inhibitors. Two prior small clinical studies suggest that high levels of intratumoral STAT1 might be a favorable prognostic marker for HNSCC patients treated with chemotherapy (16, 17). To explore whether STAT1 might be a biomarker of response to cisplatin and EGFR inhibition, we obtained tumor specimens from patients with advanced, high-risk HNSCC treated with surgery followed by adjuvant radiation, cisplatin and panitumumab. Specimens from untreated patients were stained for p-S727-STAT1 and p-Y701-STAT1, and survival data were collected following surgery and adjuvant chemoradiation plus panitumumab. Interestingly, a trend toward worse overall survival was observed in patients whose tumors displayed higher than the mean level of p-S727-STAT1 (p = 0.06; Figure 5). A similar trend was seen for progression-free survival, again without reaching statistical significance (data now shown).
Figure 5.
pSTAT1 is not a favorable prognostic indicator in HNSCC patients treated with adjuvant cisplatin and panitumumab. Tumor specimens from patients with advanced, high risk HNSCC were collected prior to adjuvant radiation, cisplatin, and panitumumab, then stained for p-S727-STAT1 (A) and p-Y701-STAT1 (B). Patients were divided into high or low phospho-STAT1 groups (above or below mean), and survival was assessed.
DISCUSSION
STAT1 is a tumor suppressor that may contribute to cell death following several types of cell stress (6). Based on the current study and prior studies, STAT1 appears to contribute to cisplatin-induced cell death in tumor cells and in cisplatin-sensitive normal cells (11-14). To our knowledge, this is the first study of cisplatin-induced STAT1 activation in tumor cells. Activation was specifically seen at the serine phosphorylation residue, and STAT1 siRNA knockdown reduced cisplatin-induced cell death in multiple cell lines. While attenuation of cisplatin-induced cell death with STAT1 siRNA knockdown in the current study was modest, it was consistent and statistically significant, despite the fact that STAT1 siRNA knockdown itself induced some degree of death in otherwise untreated cells. Absence of STAT1 without the off-target effects of siRNA transfection might result in greater attenuation of cisplatin-induced cell death.
EGFR inhibitors had more variable effects on STAT1 activation, dramatically decreasing baseline and cisplatin-induced pSTAT1 levels in JHU029 cells but not in SCC90 or PCI13 cells. Similar results were seen in JHU029 cells with two different anti-EGFR monoclonal antibodies and with an anti-EGFR tyrosine kinase inhibitor, indicating that this effect is EGFR-specific. The results were not as pronounced with gefitinib, which may be due to submaximal EGFR inhibition at a dose of 1 μM. Our laboratory has seen improved EGFR inhibition using a gefitinib dose of 6 μM, however a dose of 1μM was chosen in order to limit potential off-target effects. Consistent with reduced levels of phosphorylated STAT1 seen with EGFR inhibitors in JHU029 cells, EGF markedly increased p-S727-STAT1 and p-Y701-STAT1 levels in this cell line with no additive effects seen with cisplatin. Taken together, these data suggest that EGFR may be the primary upstream kinase responsible for cisplatin-induced STAT1 serine phosphorylation in some cell lines. Interestingly, though a similar degree of cisplatin-induced STAT1 serine phosphorylation was seen in PCI13 and SCC90 cells, our data suggest that neither EGFR nor p38 is the primary kinase responsible for increased p-S727-STAT1 in these cell lines.
While tyrosine phosphorylation of STAT1 has been well studied, the events upstream and downstream of STAT1 serine phosphorylation are not as well understood. It has been suggested that serine phosphorylation contributes to STAT1-induced apoptosis and maximization of STAT1 transcriptional activity following tyrosine phosphorylation (6, 26, 27). Though p38 is perhaps the best studied upstream kinase to date, other kinases described in the literature as directly or indirectly upstream of p-S727-STAT1 include protein kinase C delta, JNK, MEK, MSK1, PI3K/Akt, and ERK (26-29). In addition to the current study, we were able to find one other report of STAT1 serine phosphorylation following treatment of cells with EGF (26). Our study suggests that in different cell lines varying upstream pathways are activated by cisplatin, converging on p-S727-STAT1 activation and contributing to cell death in HNSCC cells.
Though cisplatin-induced STAT1 serine phosphorylation and cell death appear to be consistent across cell lines and cell types, the addition of EGFR inhibitors may have dramatically different effects depending on the cell line or type. In the current study, the addition of EGFR inhibitors to cisplatin resulted in decreased pSTAT1, yet enhanced cell death in JHU029 cells; in contrast, EGFR inhibitors had no significant effect on levels of phosphorylated STAT1 or cell death in PCI13 cells. A study of diverse cell lines that overexpress EGFR (similar to HNSCC cells) showed cisplatin-induced EGFR activation, and EGFR inhibition protected the cells from cisplatin-induced cell death (30). Similarly, in a study of HNSCC cell lines cisplatin-induced EGFR phosphorylation and subsequent degradation were found to correlate with cisplatin-induced cell death, which was attenuated by EGFR inhibition (31). Taken together, data from the current study and the literature suggest that adding an EGFR inhibitor to cisplatin can have highly variable effects on STAT1 phosphorylation and cell death, even among different cell lines from the same cancer type. It would thus be of great benefit to find a biomarker allowing identification of patients whose tumor cells are likely to be further damaged, rather than protected, from the use of anti-EGFR therapy in addition to standard platinum-based regimens.
Based on our in vitro findings, we hypothesized that phosphorylated STAT1 might be a biomarker of response to combination cisplatin and anti-EGFR therapy. We obtained specimens from patients later treated with adjuvant cisplatin and radiation combined with panitumumab. Panitumumab is a fully humanized, non-immunogenic anti-EGFR antibody, and thus a good choice for approximation of our in vitro experiments which did not include any immune cells. We noted a trend toward worse survival with high levels of intratumoral phosphorylated STAT1, particularly for p-S727-STAT1. This did not quite reach statistical significance (p = 0.06), perhaps due to the small number of patients. The finding that increased levels of phosphorylated STAT1 might be a poor prognostic indicator is in contrast to our in vitro results showing that p-S727-STAT1 contributes to cell death following cisplatin; however, persistent/constitutive activation of STAT1 has been shown to enhance survival despite treatment with cytotoxic drugs and other forms of cell stress (32). These data indicate that high baseline STAT1 activation may reflect an escape phenotype different from inducible, ligand-dependent STAT1 activation, which is reversible by oncologic EGFR inhibition or cisplatin.
Two other small clinical studies have explored STAT1 as a biomarker in HNSCC. Laimer and colleagues (16) found that high levels of STAT1 in HNSCC tumor specimens correlated with improved survival in patients treated with adjuvant platinum-based chemotherapy, which is opposite the trend seen in our study. This study was similar to ours in terms of the number of patients, tumor characteristics such as anatomic subsite and stage, and method for dividing patients between high and low STAT1 staining groups. The authors stained for total STAT1 and assessed nuclear staining, so presumably the STAT1 that they assessed was phosphorylated. However, the majority of the included patients were treated by surgery followed by adjuvant chemotherapy alone, without radiation, which is not a common regimen recommended by National Comprehensive Cancer Network (NCCN) guidelines. In contrast, all of our patients were treated with adjuvant chemoradiation consistent with NCCN guidelines. Another study by Pappa and colleagues (17) found that high p-Y701-STAT1 staining correlated with improved survival in oral cavity carcinoma patients treated with adjuvant chemotherapy. This study assessed STAT1 staining in a similar manner to our study; however, there was a high proportion of lower-stage tumors, and the specific drugs or regimens of chemotherapy were not described. Despite the different methodologies in these two studies, it is possible that the opposite survival trend noted in our study may be in part attributable to panitumumab. However, based on the limited number of patients included in all three studies, no definitive conclusions can be made on the role of STAT1 as a biomarker for response to cisplatin and/or anti-EGFR therapy in HNSCC patients.
In conclusion, cisplatin appears to consistently activate STAT1, contributing to cell death, but the addition of EGFR inhibitors can have unpredictable effects on STAT1 activation and cell death in HNSCC. Larger studies are needed with cetuximab alone in a separate treatment arm, to determine whether STAT1 may be a useful biomarker for response to these drugs alone or in combination.
ACKNOWLEDGEMENTS
The authors thank Dr. Lin Wang for assistance with IHC of tumor specimens.
Financial Support: This work was supported by NIH R01 DE019727 (R. L. Ferris), NCI P50 CA097190 University of Pittsburgh Cancer Institute Head & Neck Cancer Specialized Program of Research Excellence (SPORE) grant (R. L. Ferris), and a joint grant from the American Head and Neck Society and American Academy of Otolaryngology – Head and Neck Surgery (N. C. Schmitt). The University of Pittsburgh received financial support from Bristol-Myers Squibb and Amgen to fund the trials described in this manuscript.
Footnotes
Disclosure of potential conflicts of interest: R. L. Ferris has served as a consultant for Bristol-Myers Squibb.
REFERENCES
- 1.Kalyankrishna S, Grandis JR. Epidermal growth factor receptor biology in head and neck cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24(17):2666–72. doi: 10.1200/JCO.2005.04.8306. [DOI] [PubMed] [Google Scholar]
- 2.Vermorken JB, Stohlmacher-Williams J, Davidenko I, Licitra L, Winquist E, Villanueva C, et al. Cisplatin and fluorouracil with or without panitumumab in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck (SPECTRUM): an open-label phase 3 randomised trial. The Lancet Oncology. 2013;14(8):697–710. doi: 10.1016/S1470-2045(13)70181-5. [DOI] [PubMed] [Google Scholar]
- 3.Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. The New England journal of medicine. 2006;354(6):567–78. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 4.Martins RG, Parvathaneni U, Bauman JE, Sharma AK, Raez LE, Papagikos MA, et al. Cisplatin and radiotherapy with or without erlotinib in locally advanced squamous cell carcinoma of the head and neck: a randomized phase II trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31(11):1415–21. doi: 10.1200/JCO.2012.46.3299. [DOI] [PubMed] [Google Scholar]
- 5.Ang KK, Zhang Q, Rosenthal DI, Nguyen-Tan PF, Sherman EJ, Weber RS, et al. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32(27):2940–50. doi: 10.1200/JCO.2013.53.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frank DA. STAT signaling in the pathogenesis and treatment of cancer. Molecular medicine. 1999;5(7):432–56. [PMC free article] [PubMed] [Google Scholar]
- 7.Leeman RJ, Lui VW, Grandis JR. STAT3 as a therapeutic target in head and neck cancer. Expert opinion on biological therapy. 2006;6(3):231–41. doi: 10.1517/14712598.6.3.231. [DOI] [PubMed] [Google Scholar]
- 8.Leibowitz MS, Srivastava RM, Andrade Filho PA, Egloff AM, Wang L, Seethala RR, et al. SHP2 is overexpressed and inhibits pSTAT1-mediated APM component expression, T-cell attracting chemokine secretion, and CTL recognition in head and neck cancer cells. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19(4):798–808. doi: 10.1158/1078-0432.CCR-12-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leibowitz MS, Andrade Filho PA, Ferrone S, Ferris RL. Deficiency of activated STAT1 in head and neck cancer cells mediates TAP1-dependent escape from cytotoxic T lymphocytes. Cancer immunology, immunotherapy : CII. 2011;60(4):525–35. doi: 10.1007/s00262-010-0961-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Srivastava RM, Trivedi S, Concha-Benavente F, Jie HB, Wang L, Seethala RR, et al. STAT1 Induced HLA class I Upregulation Enhances Immunogenicity and Clinical Response to anti-EGFR mAb Cetuximab Therapy in HNC Patients. Cancer Immunol Res. 2015 May 13; doi: 10.1158/2326-6066.CIR-15-0053. pii: canimm.0053.2015. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmitt NC, Rubel EW, Nathanson NM. Cisplatin-induced hair cell death requires STAT1 and is attenuated by epigallocatechin gallate. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29(12):3843–51. doi: 10.1523/JNEUROSCI.5842-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaur T, Mukherjea D, Sheehan K, Jajoo S, Rybak LP, Ramkumar V. Short interfering RNA against STAT1 attenuates cisplatin-induced ototoxicity in the rat by suppressing inflammation. Cell death & disease. 2011;2:e180. doi: 10.1038/cddis.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Youlyouz-Marfak I, Gachard N, Le Clorennec C, Najjar I, Baran-Marszak F, Reminieras L, et al. Identification of a novel p53-dependent activation pathway of STAT1 by antitumour genotoxic agents. Cell death and differentiation. 2008;15(2):376–85. doi: 10.1038/sj.cdd.4402270. [DOI] [PubMed] [Google Scholar]
- 14.Townsend PA, Scarabelli TM, Davidson SM, Knight RA, Latchman DS, Stephanou A. STAT-1 interacts with p53 to enhance DNA damage-induced apoptosis. The Journal of biological chemistry. 2004;279(7):5811–20. doi: 10.1074/jbc.M302637200. [DOI] [PubMed] [Google Scholar]
- 15.Spanos WC, Nowicki P, Lee DW, Hoover A, Hostager B, Gupta A, et al. Immune response during therapy with cisplatin or radiation for human papillomavirus-related head and neck cancer. Archives of otolaryngology--head & neck surgery. 2009;135(11):1137–46. doi: 10.1001/archoto.2009.159. [DOI] [PubMed] [Google Scholar]
- 16.Laimer K, Spizzo G, Obrist P, Gastl G, Brunhuber T, Schafer G, et al. STAT1 activation in squamous cell cancer of the oral cavity: a potential predictive marker of response to adjuvant chemotherapy. Cancer. 2007;110(2):326–33. doi: 10.1002/cncr.22813. [DOI] [PubMed] [Google Scholar]
- 17.Pappa E, Nikitakis N, Vlachodimitropoulos D, Avgoustidis D, Oktseloglou V, Papadogeorgakis N. Phosphorylated signal transducer and activator of transcription-1 immunohistochemical expression is associated with improved survival in patients with oral squamous cell carcinoma. Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons. 2014;72(1):211–21. doi: 10.1016/j.joms.2013.06.198. [DOI] [PubMed] [Google Scholar]
- 18.Lin CJ, Grandis JR, Carey TE, Gollin SM, Whiteside TL, Koch WM, et al. Head and neck squamous cell carcinoma cell lines: established models and rationale for selection. Head & neck. 2007;29(2):163–88. doi: 10.1002/hed.20478. [DOI] [PubMed] [Google Scholar]
- 19.Zhao M, Sano D, Pickering CR, Jasser SA, Henderson YC, Clayman GL, et al. Assembly and initial characterization of a panel of 85 genomically validated cell lines from diverse head and neck tumor sites. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17(23):7248–64. doi: 10.1158/1078-0432.CCR-11-0690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hall MD, Telma KA, Chang KE, Lee TD, Madigan JP, Lloyd JR, et al. Say no to DMSO: dimethylsulfoxide inactivates cisplatin, carboplatin, and other platinum complexes. Cancer research. 2014;74(14):3913–22. doi: 10.1158/0008-5472.CAN-14-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Go RS, Adjei AA. Review of the comparative pharmacology and clinical activity of cisplatin and carboplatin. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1999;17(1):409–22. doi: 10.1200/JCO.1999.17.1.409. [DOI] [PubMed] [Google Scholar]
- 22.Ceze N, Ternant D, Piller F, Degenne D, Azzopardi N, Dorval E, et al. An enzyme-linked immunosorbent assay for therapeutic drug monitoring of cetuximab. Ther Drug Monit. 2009;31(5):597–601. doi: 10.1097/FTD.0b013e3181b33da3. [DOI] [PubMed] [Google Scholar]
- 23.Galizia G, Lieto E, De Vita F, Orditura M, Castellano P, Troiani T, et al. Cetuximab, a chimeric human mouse anti-epidermal growth factor receptor monoclonal antibody, in the treatment of human colorectal cancer. Oncogene. 2007;26(25):3654–60. doi: 10.1038/sj.onc.1210381. [DOI] [PubMed] [Google Scholar]
- 24.Lopez-Albaitero A, Ferris RL. Immune activation by epidermal growth factor receptor specific monoclonal antibody therapy for head and neck cancer. Archives of otolaryngology--head & neck surgery. 2007;133(12):1277–81. doi: 10.1001/archotol.133.12.1277. [DOI] [PubMed] [Google Scholar]
- 25.Andersen P, Pedersen MW, Woetmann A, Villingshoj M, Stockhausen MT, Odum N, et al. EGFR induces expression of IRF-1 via STAT1 and STAT3 activation leading to growth arrest of human cancer cells. International journal of cancer Journal international du cancer. 2008;122(2):342–9. doi: 10.1002/ijc.23109. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Cho YY, Petersen BL, Zhu F, Dong Z. Evidence of STAT1 phosphorylation modulated by MAPKs, MEK1 and MSK1. Carcinogenesis. 2004;25(7):1165–75. doi: 10.1093/carcin/bgh115. [DOI] [PubMed] [Google Scholar]
- 27.DeVries TA, Kalkofen RL, Matassa AA, Reyland ME. Protein kinase Cdelta regulates apoptosis via activation of STAT1. The Journal of biological chemistry. 2004;279(44):45603–12. doi: 10.1074/jbc.M407448200. [DOI] [PubMed] [Google Scholar]
- 28.Harvey EJ, Li N, Ramji DP. Critical role for casein kinase 2 and phosphoinositide-3-kinase in the interferon-gamma-induced expression of monocyte chemoattractant protein-1 and other key genes implicated in atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 2007;27(4):806–12. doi: 10.1161/01.ATV.0000258867.79411.96. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen H, Ramana CV, Bayes J, Stark GR. Roles of phosphatidylinositol 3-kinase in interferon-gamma-dependent phosphorylation of STAT1 on serine 727 and activation of gene expression. The Journal of biological chemistry. 2001;276(36):33361–8. doi: 10.1074/jbc.M105070200. [DOI] [PubMed] [Google Scholar]
- 30.Benhar M, Engelberg D, Levitzki A. Cisplatin-induced activation of the EGF receptor. Oncogene. 2002;21(57):8723–31. doi: 10.1038/sj.onc.1205980. [DOI] [PubMed] [Google Scholar]
- 31.Ahsan A, Hiniker SM, Ramanand SG, Nyati S, Hegde A, Helman A, et al. Role of epidermal growth factor receptor degradation in cisplatin-induced cytotoxicity in head and neck cancer. Cancer research. 2010;70(7):2862–9. doi: 10.1158/0008-5472.CAN-09-4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khodarev NN, Roizman B, Weichselbaum RR. Molecular pathways: interferon/stat1 pathway: role in the tumor resistance to genotoxic stress and aggressive growth. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18(11):3015–21. doi: 10.1158/1078-0432.CCR-11-3225. [DOI] [PubMed] [Google Scholar]