Abstract
The current study compared adherence rates as measured by two indirect measurement methods (pill count and daily medication diary) to two direct measurement methods (urine riboflavin and serum 6-OH-buspirone level measurement) among participants (n=109) in a medication treatment trial for cannabis dependence. Pill count and diary data showed high levels of percent agreement and strong kappa coefficients throughout the study. Riboflavin levels indicated lower level of percent in adherence during the study as compared to both pill count and self-report. In the subset of participants with 6-OH-buspirone levels (n=58), the kappa coefficient also showed low to moderate agreement between the pill count and medication diaries with 6-OH-buspirone levels. In contrast to pill count and medication diaries, adherence as measured by riboflavin and 6-OH-buspirone significantly decreased over time. The findings from this study support previous work demonstrating that pill count and patient self-report of medication taking likely overestimate rates of medication adherence, and may become less reliable as the duration of a clinical trial increases.
Keywords: Adherence, self-report, biological marker, medication diary, drug level monitoring
1. Introduction
Medication adherence is of critical importance in clinical trials, as poor medication adherence in research studies may obscure potential effects of a pharmacologic intervention (Farmer, 1999; Spilker, 1991; Williams et al., 2013). Adherence can be a particular challenge for researchers in the addiction field, as substance use can directly impair judgment, which may negatively impact treatment adherence. There can also be ambivalence surrounding the use of medications in this population, which could further reduce adherence (Sowers & Golden, 1999).
Both direct and indirect methods to measure medication adherence are commonly utilized in substance use clinical trials. Direct methods of adherence measurement include directly observed medication administration, detection of the drug or drug metabolite in a biologic fluid, and detection of a biological marker administered with the drug. Indirect methods of adherence measurement can be objective, such as pill counts and electronic medication event monitoring systems (MEMS), or subjective, such as participant or collateral self-report. Each method has advantages and limitations. Directly observing participant ingestion of a medication should ensure adherence, but places significant burden on both participants and research staff. Measurement of a drug, metabolite, or biological marker in a biological fluid provides objective confirmation that a dose of medication has been ingested by an individual; however, such “spot” levels may not be reflective of steady state drug confirmation. Advantages of pill counts include low burden and costs. However, pill counts may be inaccurate due to participants not returning all medication as directed. Further, although a total number of medication ingestions may be estimated from pill counts, it is not possible to confirm an individual took the medication as prescribed. Although MEMS are widely regarded as the gold standard measure of participant adherence, a potential shortcoming of this approach is that actual medication ingestion is not measured; rather, the system is limited to registering times when the medication container is opened and when it is closed. Cost is also a consideration with use of MEMS, as the product components and software required for data retrieval can be expensive. Participant self-report is the most common method used to assess medication adherence. Advantages of self-report include low participant and provider burden and essentially no cost. Disadvantages of self-report include recall bias and potential inaccuracy in reporting.
Given the critical importance of accurate adherence measurement in interpretation of medication trial results, data on validity of measurement methods are needed to inform clinical trial design. The current study expands on limited previous research in this area by comparing adherence rates as measured by two indirect measurement methods (pill count and daily medication diary) to two direct measurement methods (urine riboflavin and serum metabolite level measurement). Further, as clinical trials commonly exceed four to eight weeks in length, evaluations of reliability of measurement methods over an extended time period are needed. It was hypothesized that indirect methods of adherence would overestimate adherence as compared to direct methods, and that all adherence measurements would demonstrate a reduction in adherence over the course of the study.
2. Methods
2.1. Design and Procedures
Participants were primarily recruited through media and internet advertisements between November, 2009, and March, 2014, to participate in a 12-week, double-blind, placebo-controlled trial of a medication and behavioral intervention in cannabis-dependent individuals. All procedures were conducted in accordance with Good Clinical Practice Guidelines and the Declaration of Helsinki and received approval from the Medical University of South Carolina Institutional Review Board. All participants gave written, informed consent prior to study participation.
Following assessment to determine study eligibility, participants were randomized to receive either buspirone (flexible dose of up to 60 mg/day) or a matching placebo. Medication was dosed twice daily, with each dose containing 25 mg of riboflavin. Participants also completed three sessions of motivational enhancement therapy focused on cannabis use. Escalating contingency management compensation was used to reinforce study visit attendance and retention; compensation was not contingent on cannabis abstinence. Participants attended weekly clinic visits.
2.2. Adherence Measures
Medication adherence was assessed using (1) pill counts, (2) patient self-report, (3) quantitative urine riboflavin levels, and (4) serum measurement of 6-OH-buspirone, a major metabolite of buspirone. At each study visit, participants were provided with a supply of medication and a diary to record medication intake. Returned pills were then counted by study staff to determine the proportion of pills taken from what was prescribed at each previous visit, and diaries were also collected weekly. Participants received $10 weekly compensation for returned medication diaries, pill bottles, and unused pills. Urine samples were collected for riboflavin analysis at every other visit (visits 2, 4, 6, 8, 10, and 12). Analysis was conducted using a TECAN microplate reader. Samples were exposed to light at a wavelength of 444 nm, and emission fluorescence of riboflavin measured at 515 nm. A standard curve was established by measuring the intensity of emitted light of known amounts of riboflavin (concentrations ranging from 250 ng/ml to 8000 ng/ml); study samples were run against this standard curve. Serum samples were also obtained at every other visit (visits 2, 4, 6, 8, 10, and 12) for determination of 6-OH-buspirone concentrations. A liquid-liquid extraction procedure was developed following a previously described method (Dockens et al., 2006) and samples analyzed using a Waters 2695 HPLC (Waters Corp., Milford, MA) equipped with a photo diode array (PDA) detector capable of spectral analysis for peak purity and identity confirmation.
Adherence was defined as having reported ≥80% of doses taken (pill count/diary), riboflavin ≥ 900 ng/ml, and 6-OH-buspirone > 0 (in the active treatment group only). The riboflavin cut-off was based on recommendations by Herron and colleagues (2013). Since riboflavin was considered the gold standard of adherence measurement in this study, adherence was calculated at each visit where riboflavin data and at least one other measure was present. Participants taking a multivitamin containing riboflavin were excluded from the analysis.
2.3. Statistical Analysis
Baseline demographics and clinical characteristics were calculated as means and associated 95% confidence intervals for continuous variables and percentages (n) for categorical variables. A t-test was used to evaluate continuous baseline demographic and clinical measures while the normal Pearson Chi-Square test was used to assess the relationship for categorical and ordinal variables (Fisher’s exact test was used where appropriate) between the analysis cohort and the cohort of participants excluded from the analysis.
Various methods of measuring medication adherence were assessed across several visits during the treatment phase of the study (weeks 2, 4, 6, 8, 10, 12). Percentages in agreement and kappa coefficients were calculated between each measurement method. Due to very high prevalence of self-reported adherence in the study population, kappa coefficients are calculated as the prevalence adjusted bias adjusted kappa (PABAK) (Byrt et al., 1993). Using riboflavin determined adherence as the reference marker, a clustered logistic regression model using the methods of generalized estimating equations (Zeger & Liang, 1986) was applied to assess the differences in measurement methods on binary adherence outcomes over time. Working correlation structures were independently compared and the final model structure was chosen using the quasilikelihood under the independence model criterion statistic (Pan, 2001). The main effects of adherence measurement method and visit as well as the interaction between method and visit were examined for significance. Additionally, demographic and clinical characteristics were individually added to the primary analysis model to assess possible predictors of treatment adherence. All statistical analyses were conducted using SAS version 9.3 (SAS Institute Inc. Cary, NC, USA).
3. Results
Participant characteristics
The present study included all participants from the parent study (n=175) with at least one riboflavin adherence measure, either a concurrent pill count or medication diary measure and those not taking multivitamins that contain riboflavin (n=109). The cohort was primarily Caucasian (n=69, 63.3%) and male (n=82, 75.2 %). The average age was 23.1 years (SD=5.3) with an average age of cannabis dependence onset of 19.3 years (SD=3.5). Participants excluded from the analysis (n=66) were slightly older than those included (25.6±7.6 vs.23.1±5.3. p=0.017); however the excluded participants were otherwise clinically and demographically similar to those included in the analysis (Table 1). Demographic and clinical characteristics collected at the baseline visit were assessed for univariate associations with both direct and indirect measures of medication adherence. These characteristics were additionally added to full models to investigate possible confounding effects on the relationship between the measurement method and the level of adherence. Of the baseline characteristics, increased cannabis use prior to study entry was associated with decreased odds of riboflavin based adherence (p=0.025) but not adherence based on medication diaries (p=0.589). No other clinical or demographic characteristics were univariately associated with adherence measures during study treatment.
Table 1.
Demographic and clinical characteristics of the study cohort and those excluded from the study analysis.
| Characteristic | Study Cohort n=109 |
Excluded N=66 |
|---|---|---|
| Age (yrs) | 23.1 (22.0–24.1)* | 25.6 (23.7–27.5) |
| Male % (n) | 75.2 (82) | 78.8 (52) |
| Caucasian % (n) | 63.3 (69) | 65.2 (43) |
| Graduated HS % (n) | 90.8 (99) | 89.4 (59) |
| Buspirone Treatment % (n) | 53.2 (58) | 45.5 (30) |
| Age of Onset of Cannabis Dependence | 19.3 (18.7–20.0) | 20.6 (19.1–22.1) |
| Ounces used per week | 5.2 (4.8–5.7) | 5.3 (4.6–6.0) |
| Sessions Per Day | 3.1 (2.7–3.5) | 3.2 (2.8–3.7) |
Continuous characteristics are shown as their mean and associated 95% confidence interval while categorical characteristics are shown as percent (n). Continuous characteristics are compared using a t-test statistic while categorical characteristics are compared using a Pearson chi-square test statistic.
Age (p=0.017).
Comparison of riboflavin, pill counts and medication diaries
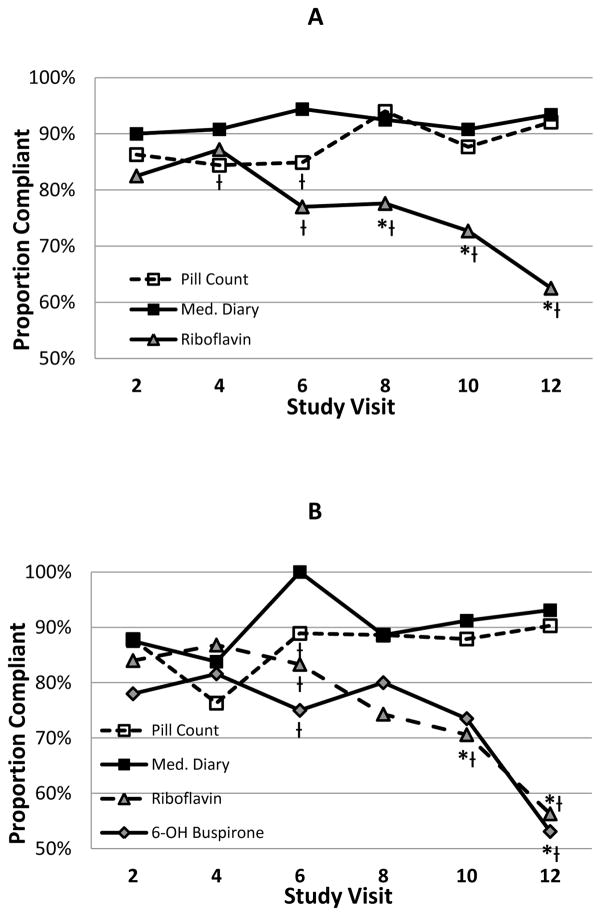
Pill count and medication diary data showed high weekly and overall proportion of doses taken and adherence percentages (Table 2). Participants reported taking greater than 94% of prescribed doses in medication diaries and pill count data showed greater than 93% of doses taken. Weekly adherence measures were consistent and high across weekly visits for both pill count and medication diaries (Weekly Adherence Percentages: Pill Count=84.4–94.0% and Medication Diary=90.0–94.4%). Pill count and diary data showed high levels of percent agreement (% in agreement: 90.0–100.0%) and strong kappa coefficients (PABAK=0.80–1.00) throughout the study. Riboflavin levels indicated lower level of percent in weekly adherence during the study as compared to both pill count and self-report (Adherence Percent: 62.5–87.2%). Consequently, the percent agreement between the indirect measures and the riboflavin measures of adherence are attenuated as compared to the agreement between pill count and diary data (% in agreement: Riboflavin/Pill Count=61.9–77.6%, p=0.005; Riboflavin/Diary=62.3–80.3%, p<0.001). Additionally, the kappa coefficients between weekly riboflavin and pill count/diary data were significantly lower than between pill count and diary reports. Further, there was a significant method x visit interaction (Figure 1a; X210=19.5, p=0.035) in the model, indicating that adherence profiles over the course of treatment were dissimilar between the different adherence measure methods. Medication adherence determined from pill counts and medication diary data are consistent over time while riboflavin determined adherence declines sharply as treatment continues. At study visits 2 and 4, riboflavin determined adherence was not statistically different from that determined by pill counts or medication diaries (p>0.10); however, from visits 6 to 12, riboflavin determined adherence was significantly lower than medication diaries (p<0.01) and from visits 8 to 12 it was lower than data from pill counts (p<0.025). Of note, at visits 4 and 6, adherence determined by pill counts was significantly lower than that determined by medication diaries (p=0.021 and 0.007, respectively). To determine if study attrition was associated with the decline in riboflavin determined adherence, an additional analysis was restricted to participants that attended at least 5 of the 6 treatment visits where metabolites were measured. Seventy of the 109 participants (64%) were considered to have complete data The analysis of those participants showed results consistent with the analysis of all available data (method x visit interaction; X210=19.0, p=0.040).
Table 2.
Overall and study visit adherence and agreement results for riboflavin, pill count and medication diary for the study cohort.
| Study Visit | Adherence Measurement | Agreement Statistics | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pill Counta | Diarya | Riboflavinb | Pill Count/Diary | Pill Count/Riboflavin | Diary/Riboflavin | ||||||||
|
|
|||||||||||||
| N | Mean (SD) | % Comp | Mean (SD) | % Comp | Mean (SD) | % Comp | % Agree | Kappa | % Agree | Kappa | % Agree | Kappa | |
| Visit 2 | 103 | 92.3 (14.9) | 86.3 % | 93.6 (12.6) | 90.0 % | 3829 (2581) | 82.5 % | 90.9 % | 0.82 | 74.5 % | 0.49 | 79.0 % | 0.59 |
| Visit 4 | 78 | 90.9 (14.0) | 84.4 % | 93.6 (12.2) | 90.8 % | 4211 (2579) | 87.2 % | 93.3 % | 0.87 | 74.0 % | 0.48 | 80.3 % | 0.61 |
| Visit 6 | 74 | 91.1 (18.8) | 84.9 % | 93.2 (18.7) | 94.4 % | 3216 (2515) | 77.0 % | 90.0 % | 0.80 | 72.6 % | 0.45 | 77.5 % | 0.55 |
| Visit 8 | 67 | 96.6 (10.1) | 94.0 % | 96.6 (10.6) | 92.5 % | 4212 (3024) | 77.6 % | 98.5 % | 0.97 | 77.6 % | 0.55 | 76.1 % | 0.52 |
| Visit 10 | 66 | 93.8 (15.1) | 87.7 % | 95.6 (10.2) | 90.8 % | 2864 (2463) | 72.7 % | 90.6 % | 0.81 | 69.2 % | 0.38 | 70.8 % | 0.42 |
| Visit 12 | 64 (11.5) | 95.6 % | 92.1 (9.0) | 96.5 % | 93.4 (2668) | 2784 % | 62.5 % | 100 | 1.00 % | 61.9 | 0.24 % | 62.3 | 0.25 |
| Overall | 93.2 (14.5) | 87.9 | 94.7 (12.7) | 91.8 | 3562 (2684) | 77.4 % | 93.6 % | 0.87 | 72. 0% | 0.44 | 75.0 % | 0.50 | |
Pill count and Diary data are shown as mean (SD) proportion of study doses taken as a function of the number of study doses prescribed. Percent compliant (% Comp) is shown as the proportion of participants with ≥ 80% of study doses taken at that visit.
Riboflavin data are shown as the mean (SD) ng/ml of riboflavin detected at each visit. Percent compliant is shown as the proportion of participants with ≥ 900 ng/ml riboflavin detected at that visit. The Prevalence and Bias Adjusted Kappa (PABAK) is shown.
Figure 1.
Medication adherence over study treatment visits by adherence measurement method for a) all study participants and b) study participants randomized to the buspirone treatment group. * p<0.05 as compared to pill count determined adherence; Ɨ p<0.05 as compared to medication diary determined adherence
Agreement with 6-OH-Buspirone
Fifty-eight of the 109 (53.2%) participants in the study cohort were randomized to receive active buspirone during the study treatment phase. In this sub-cohort, serum samples were analyzed for the presence of 6-OH-buspirone, a metabolite of the active treatment medication, and compared to the standard measures of adherence from same participants. Pill count, diary data, and riboflavin determined adherence were similar in the active treatment sub-cohort as compared to the full data cohort (Table 3). As with riboflavin, pill count and diary data were only in moderate agreement with the 6-OH-buspirone (% in agreement: Pill Count=45.2–77.6%; Medication Diary=44.8–77.1%); similarly, the kappa coefficient showed low to moderate agreement between the pill count and medication diaries with 6-OH-buspirone (PABAK: Pill Count=−0.10–0.55; Medication Diary=−0.10–0.54). Adherence data from riboflavin and 6-OH-buspirone showed high agreement during early visits (% in agreement: 80.6–94.3%) with reduced agreement at visit 10 and 12 (% in agreement: 73.5 and 65.6%).
Table 3.
Overall and study visit adherence and agreement results for 6-OH-buspirone, pill count and medication diary for the study cohort randomized to receive buspirone during treatment (n=58).
| Study Visit | Adherence Measurement | Agreement Statistics | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pill Counta | Diarya | Riboflavinb | 6-OH Buspironec | Pill Count/Buspirone | Diary/Buspirone | Riboflavin/Buspirone | |||||||||
|
|
|||||||||||||||
| N | Mean (SD) | % Comp | Mean (SD) | % Comp | Mean (SD) | % Comp | Mean (SD) | % Comp | % Agree | Kappa | % Agree | Kappa | % Agree | Kappa | |
| Visit 2 | 50 | 92.6 (12. 1) | 87.8 % | 93.8 (11. 8) | 87.5 % | 3755 (2603) | 84.0 % | 10.0 (9.4) | 78.0 % | 77.6 % | 0.55 | 77.1 % | 0.54 | 94.0 % | 0.88 |
| Visit 4 | 38 | 87.8 (16. 0) | 76.3 % | 89.9 (15. 5) | 83.8 % | 4257 (2551) | 86.8 % | 9.9 (10. 2) | 81.6 % | 63.2 % | 0.26 | 73.0 % | 0.46 | 89.5 % | 0.79 |
| Visit 6 | 36 | 94.6 (8.0) | 88.9 % | 96.0 (5.8) | 100 % | 3672 (2605) | 83.3 % | 10.5 (10. 1) | 75.0 % | 75.0 % | 0.50 | 73.5 % | 0.47 | 80.6 % | 0.61 |
| Visit 8 | 35 | 95.0 (13. 2) | 88.6 % | 94.8 (13. 6) | 88.6 % | 4105 (3035) | 74.3 % | 10.5 (13. 6) | 80.0 % | 74.3 % | 0.49 | 74.3 % | 0.49 | 94.3 % | 0.89 |
| Visit 10 | 34 | 92.4 (18. 6) | 87.9 % | 94.7 (12. 0) | 91.2 % | 2845 (2550) | 70.6 % | 8.1 (8.8) | 73.5 % | 63.6 % | 0.27 | 64.7 % | 0.29 | 73.5 % | 0.47 |
| Visit 12 | 32 | 96.5 (8.9) | 90.3 % | 96.2 (8.8) | 93.1 % | 2556 (2524) | 56.3 % | 4.4 (5.8) | 53.1 % | 45.2 % | − 0.10 | 44.8 % | − 0.10 | 65.6 % | 0.31 |
| Overall | 93.0 (14. 1) | 86.5 % | 94.1 (11. 9) | 90.3 % | 3573 (2686) | 76.9 % | 9.0 (10. 0) | 74.2 % | 67.6 % | 0.35 | 69.1 % | 0.38 | 84.0 % | 0.68 | |
Pill count and Diary data are shown as mean (SD) proportion of study doses taken as a function of the number of study doses prescribed. Percent compliant (% Comp) is shown as the proportion of participants with ≥ 80% of study doses taken at that visit.
Riboflavin data are shown as the mean (SD) ng/ml of riboflavin detected at each visit. Percent complaint is shown as the proportion of participants with ≥ 900 ng/ml riboflavin detected at that visit.
6-OH-buspirone data are shown as the mean (SD) ng/ml of the metabolite detected at each visit. Percent complaint is shown as the proportion of participants with > 0 ng/ml detected at that visit. The Prevalence and Bias Adjusted Kappa (PABAK) is shown.
Similar to the direct adherence measured in the whole study cohort, adherence determined from 6-OH-buspirone levels, significantly decreased as the study progresses compared to pill count and medication diary determined adherence (Figure 1b; X215=25.2, p=0.047). In this sub cohort, the indirect medication adherence measures (pill count and medication diary) showed similar and consistent patterns over time while the direct measures both indicated significant decreases in adherence over time.
Clinical Correlates of Medication Adherence
In addition to baseline predictors of medication adherence, study related treatment and outcomes were tested for associations with adherence during the treatment phase of the study. Randomized treatment assignment was not significantly associated with direct or indirect measures of medication adherence (all p>0.45). Clinical outcome data (number of reported weekly use days) was tested to determine if poor medication adherence was related to rates of cannabis use. It was hypothesized that cannabis use days during the week prior to compliance testing could be correlated with both direct and indirect measures of compliance. Lagged weekly use days were not significantly associated with either direct or indirect measures of compliance (all p>0.17). Similarly, it was of interest to assess whether adherence measures consequently affect treatment outcomes. Neither direct nor indirect weekly measures of adherence improved upon, or modified, treatment efficacy models (all p>0.08).
Medication side effects were evaluated weekly by a clinician by asking the participant open-ended questions such as “Have you had any problems or side effects since we saw you last (such as cold, flu, nausea, headache, or any other problem)?” The type of adverse event, severity of adverse event, relationship to study medication, action taken, and outcome were recorded. There was no difference in the rate of events noted between treatment assignments (χ21=1.34, p=0.25) nor was there a difference in the number of visits with reported events between treatment assignments (χ21=2.69, p=0.10). During weeks where newly reported adverse events were noted, the odds ratio of medication adherence as measured by both direct measures were significantly decreased [Riboflavin OR=0.91 (0.83–0.99); p=0.048 and 6-OH Buspirone OR=0.89 (0.81–0.99); p=0.046]. This relationship did not extend to compliance measured by indirect measures [Pill Count OR=0.97 (0.92–1.03); p=0.316 and Medication Diary OR=0.98 (0.93–1.03); p=0.414].
4. Discussion
In this evaluation, indirect measurements of medication adherence (pill count and self-report) were highly correlated, but, as hypothesized, overestimated adherence as compared to measurement of adherence by direct methods (quantitative riboflavin or 6-OH-buspirone measurement). This finding is consistent with a report by Mooney and colleagues (2004) in which self-report was found to estimate a much higher adherence rate than MEMS (87% and 28%, respectively). In contrast, Feinn and colleagues (2003) reported similar and concordant adherence rates with self-report and MEMS (91% and 87%, respectively). Of note, participants in the latter study completed medication diaries daily and were provided postage-paid, return envelopes to mail the diaries the next day; participants were compensated for each diary returned on time as indicated by postmark. In the present evaluation, participants were instructed to complete the self-report diary daily, but returned it at the next scheduled visit. As such, it is possible that participants delayed completing the diary, and did not accurately record medication ingestion.
Of interest, adherence profiles were dissimilar over the course of the study for the different adherence measures. Although riboflavin determined adherence did not differ from the indirect methods at early study visits, a statistically significant difference emerged as the study progressed, suggesting that self-report of medication taking and pill counts become less reliable as the duration of a clinical trial increases. Further, adherence as measured by riboflavin and 6-OH-buspirone significantly decreased over time. This phenomenon may be of particular significance in substance use disorder research, as abstinence outcomes in the final two weeks of treatment are often identified as primary outcome measures, and investigations have shown a relationship between medication adherence and positive outcomes in alcohol (Baros et al., 2007) and methamphetamine (Anderson et al., 2012) using populations. If medication adherence diminishes over time, adjustments to trial design may be needed to account for this effect.
Although the proportion of compliant participants was similar using riboflavin and 6-OH-buspirone measurement methods, agreement between these methods was reduced at later study visits, highlighting that even direct methods of adherence measurement have limitations. In the instances where participants were considered compliant based on riboflavin results but did not have measurable 6-OH-buspirone levels, it is possible that a “spillover” effect occurred and riboflavin was detected from previously taken doses (Babiker et al., 1989), or, alternatively, that participants may have consumed riboflavin containing vitamins or supplements that were not reported to study staff. Instances where participants were considered compliant based on 6-OH-buspirone levels but did not meet adherence criteria for riboflavin levels may be reflective of potential individual differences in drug absorption and metabolism. Given the half-life of 6-OH-buspirone (approximately six hours; Dockens et al., 2007), it is possible that an individual with slow metabolism had a detectable level of medication even after missing one or more medication doses.
Of the adherence measures used in this trial, the method with the least concordance with other methods was the medication diary. Although a medication diary may help a participant remember to take study medications, its accuracy decreased over time. This may be due to participants developing a relationship with the research staff over time, and not wanting to “disappoint” the staff by disclosing that they did not take study medication. There may also be some participant fatigue when participants have to complete assessments daily over a long period of time.
The findings of this investigation should be considered in light of some limitations. The population under study was composed of cannabis-dependent individuals; it is possible that individuals dependent on other substances may exhibit different patterns in adherence rates. As this was a medication treatment trial, it is possible that other factors, such as adverse effects as discussed above, may have affected adherence behavior. Finally, 6-OH-buspirone levels were only available for individuals randomized to the active treatment group, which reduced the size of the sample available for those analyses.
In spite of these limitations, the current study does significantly adds to the limited adherence literature, and supports previous work demonstrating that pill count and patient self-report of medication taking likely overestimate rates of medication adherence. Quantitative riboflavin and 6-OH-buspirone measurement likely provided a more realistic estimate of medication taking behavior. However, these methods may increase costs, and availability of laboratory assays may limit availability of some direct adherence measurements. Results from measures such as these also may not be immediately available to researchers, and as such opportunities for intervening to improve adherence are missed. Further research is needed to develop methods to not only accurately measure but also enhance medication adherence in clinical trials.
Highlights.
Indirect and direct methods of measuring medication adherence were compared.
Pill count and daily medication diary data had high agreement thought out the study.
Lower adherence was measured using riboflavin and 6-OH-buspirone levels.
Adherence as measured by riboflavin and 6-OH-buspirone also significantly decreased over time.
Acknowledgments
Role of the Funding Source
Funding for this study was provided by NIDA Grant R01DA026782 (McRae-Clark). NIDA had no further role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Conflict of Interest
The authors have no conflicts of interest related to this work to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson AL, Li S-H, Biswas K, et al. Modafinil for the treatment of methamphetamine dependence. Drug and Alcohol Dependence. 2012;120:135–41. doi: 10.1016/j.drugalcdep.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiker IE, Cooke PR, Gillett MG. How useful is riboflavin as a tracer of medication compliance? Journal of Behavioral Medicine. 1989;12:25–38. doi: 10.1007/BF00844747. [DOI] [PubMed] [Google Scholar]
- Baros AM, Latham PK, Moak DH, Voronin K, Anton RF. What role does measuring medication compliance play in evaluating the efficacy of naltrexone? Alcoholism Clinical and Experimental Research. 2007;31:596–603. doi: 10.1111/j.1530-0277.2007.00343.x. [DOI] [PubMed] [Google Scholar]
- Byrt T, Bishop J, Carlin JB. Bias, prevalence and kappa. Journal of Clinical Epidemiology. 1993;46:423–429. doi: 10.1016/0895-4356(93)90018-v. [DOI] [PubMed] [Google Scholar]
- Dockens RC, Salazar DE, Fulmor E, et al. Pharmacokinetics of a newly identified active metabolite of buspirone after administration of buspirone over its therapeutic dose range. Journal of Clinical Pharmacology. 2006;46:1308–1312. doi: 10.1177/0091270006292250. [DOI] [PubMed] [Google Scholar]
- Dockens RC, Tran AQ, Zeng J, Croop R. Pharmacokinetics of 6-hydroxybuspirone and its enantiomers administered individually or following buspirone administration in humans. Biopharmaceutics & Drug Disposition. 2007;28:393–402. doi: 10.1002/bdd.566. [DOI] [PubMed] [Google Scholar]
- Farmer KC. Methods for measuring and monitoring medication regimen adherence in clinical trials and clinical practice. Clinical Therapeutics. 1999;21:1074–1090. doi: 10.1016/S0149-2918(99)80026-5. [DOI] [PubMed] [Google Scholar]
- Feinn R, Tennen H, Cramer J, Kranzler HR. Measurement and prediction of medication compliance in problem drinkers. Alcoholism Clinical and Experimental Research. 2003;27:1286–1292. doi: 10.1097/01.ALC.0000080670.59386.6E. [DOI] [PubMed] [Google Scholar]
- Herron AJ, Mariani JJ, Pavlicova M, et al. Assessment of riboflavin as a tracer substance: Comparison of a qualitative to a quantitative method of riboflavin measurement. Drug and Alcohol Dependence. 2003;128:77–82. doi: 10.1016/j.drugalcdep.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney M, Sayre S, Green C, Rhoades H, Schmitz J. Comparing measures of medication taking in a pharmacotherapy trial for cocaine dependence. Addictive Disorders and Their Treatment. 2004;3:165–173. [Google Scholar]
- Pan W. Akaike’s information criterion in generalized estimating equations. Biometrics. 2001;57:120–125. doi: 10.1111/j.0006-341x.2001.00120.x. [DOI] [PubMed] [Google Scholar]
- Sowers W, Goden S. Psychotropic medication management in persons with co-occurring psychiatric and substance use disorders. Journal of Psychoactive Drugs. 1999;31:59–70. doi: 10.1080/02791072.1999.10471727. [DOI] [PubMed] [Google Scholar]
- Spilker B. Methods of assessing and improving patient compliance in clinical trials. In: Cramer JA, Spilker B, editors. Patient Compliance in Medical Practice and Clinical Trials. New York: Raven Press; 1991. pp. 37–56. [Google Scholar]
- Williams AB, Amico KR, Bova C, Womack JA. A proposal for quality standards for measuring adherence in research. AIDS Behavior. 2013;17:284–297. doi: 10.1007/s10461-012-0172-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]