Abstract
Objective
To provide a detailed summary of current scientific knowledge on uterine fibroids (leiomyomas) in vitro and in in vivo animal models, as well as to postulate the potential role of vitamin D3 as an effective, inexpensive, safe long-term treatment option for uterine fibroids.
Design
PubMed search articles were used to identify the most relevant studies on uterine fibroids as well as effects of vitamin D3 on uterine fibroid cells and fibroid tumor growth in in vivo animal models.
Setting
University research laboratory - affiliated infertility clinic.
Patient(s)
Not applicable.
Intervention(s)
None
Main Outcome Measure(s)
Not applicable.
Results
Despite numerous publications available on uterine fibroids, information about the role that vitamin D3 plays in the regulation of uterine fibroids are limited. Most of the recent vitamin D3-related studies on uterine fibroids were published from our group. Recent studies have demonstrated that vitamin D deficiency plays a significant role in the development of uterine fibroids. Our recent studies have demonstrated that vitamin D3 reduces leiomyoma cell proliferation in vitro, and leiomyoma tumor growth in in vivo animal models. These results postulate the potential role of vitamin D3 for an effective, safe non-surgical medical treatment option for uterine fibroids.
Conclusions
This article reviews human and animal studies and uncover new possibilities for understanding the vitamin D-based therapeutic option for an effective, safe long-term treatment of uterine fibroids. Based on these results, a clinical trial with vitamin D3 or a hypocalcemic analog, paricalcitol may be warranted for non-surgical medical treatment of uterine fibroids.
Keywords: Uterine leiomyomas, fibroids, vitamin D3, paricalcitol, VDR
Uterine Leiomyomas
Uterine leiomyomas (fibroids) are the most common gynecological tumors in females. They are benign clonal tumors that arise from the smooth muscle cells of the uterus and contain excessive extracellular matrix (ECM). Leiomyomas are well known estrogen-dependent tumors in the uterus of premenopausal women (1-4). Leiomyomas clinically affect more than 25% of reproductive age females and cause significant morbidity (5, 6). While the majority of females with uterine leiomyoma are asymptomatic, symptomatic leiomyomas can cause abnormal uterine bleeding, pelvic pressure and pain, and urinary incontinence or retention, and are associated with infertility and recurrent abortion (7-10). In addition, leiomyomas may grow rapidly during pregnancy and can cause obstructed labor necessitating cesarean section. Furthermore, symptomatic leiomyomas are among the most common indications for major surgery in pre-menopausal females, and the leading cause of hysterectomy in the United States (2, 3). They are responsible for about one-third of all hospital admissions respective to gynecological services. Classified as a major surgery, hysterectomies are not only associated with significantly increasing morbidity and mortality, but are all too often known for their huge economic impact on healthcare systems (4, 11). The etiology and pathophysiology of the leiomyoma formation is not completely understood; however, growing evidence has focused on investigating the molecular mechanism in the development of disease and the influence of ethnicity. Several studies have found mutations in exon 2 of the mediator complex subunit 12 (Med12) gene in up to 85% of cases of uterine fibroids from diverse populations from Finland, northern United States, and in South Africa (12-16). In a recent study, our team investigated the Med12 gene somatic mutations in females with symptomatic uterine fibroids from the southern United States. We identified four novel somatic mutations in the Med12 gene in uterine fibroids in this population (17). As expected, no mutations were identified in the Med12 gene in normal myometrium in these women (17). These finding highlight the molecular pathogenesis of uterine fibroids; understanding those molecular events may help to develop noninvasive therapeutic agents to effectively treat uterine fibroids.
Treatment of Leiomyomas
The primary treatment of uterine fibroids has been surgery, either myomectomy or hysterectomy. More than 600,000 hysterectomies are performed in the United States each year. Novel nonsurgical alternatives for the treatment of uterine fibroids have been investigated (18) and thus far, no definitive oral therapeutic agent has been developed. At present, medical treatments are only used for short-term therapy to avoid risks associated with long-term therapy. There is also a lack of evidence regarding the benefit and risks of long-term therapy with newer medical agents. Recent discoveries in the pathogenesis of leiomyoma with increasing knowledge of the mechanism of action of more recent candidate agents such as Vitamin D, green tea extract, and elagolix (oral GnRH antagonist), as well as older agents such as selective estrogen receptor modulators (SERMs) and gestrinone may lead to the development of an oral agent with the ability to decrease leiomyoma size with minimal side effects (19).
Leiomyomas Prevalence in African Americans
Several studies have shown that African American females are at increased risk for uterine fibroids identified by ultrasound or hysterectomy (20-23). While Hispanics and Asians in the United States have similar incidence as Caucasians (24), there is a disproportionate disease burden apparent in the number of hysterectomies performed on African American women, 75% of which were performed for uterine leiomyomas (25). Studies reveal a two to threefold higher incidence of uterine fibroids in African American females as compared to Caucasian females (21). Many studies have shown that African American females are disproportionately burdened by the clinical sequel associated with uterine leiomyomas. At the second NIH International Congress on the Advances in Uterine Leiomyoma Research in 2005, several reports reconfirmed the fact that African American is a risk factor in the development of uterine leiomyomas independent of other variables. However, the exact incidence of uterine leiomyoma remains to be determined. Baird et al.'s sub-analysis of leiomyoma phenotype as multiple or single revealed that 73% of African American females had multiple leiomyoma on ultrasound, whereas only 45% of Caucasian females demonstrated this phenotype (21). Myomectomy data suggest that leiomyoma in African American females are larger and more numerous than among a cohort of Caucasian females. Kjerulff et al. reported that African American females are at increased risk for hysterectomy second to symptomatic leiomyomas when compared with Caucasians (26, 27). Marshall et al. and others have concluded that African American women tend to have larger sized uterine leiomyomas (23, 24, 26, 28). The likelihood of being diagnosed with uterine fibroids is approximately three percent a year for reproductive-age African American females (22). Moreover, leiomyomas diagnosed at a younger age are more often multiple and larger in African American females with a cumulative incidence of 80% by age 50 (21, 29).
Vitamin D and African Americans
While several studies have demonstrated that African American females are at increased risk for vitamin D deficiency than Caucasian females, few studies have examined determinants of vitamin D deficiency in this population (30). African American females are 10 times more likely to have vitamin D deficiency than Caucasian females (30). Studies have also demonstrated a lower mean 25(OH)D3 concentration in African American females than in Caucasian females aged 20–40 years (31-33). Thomas et al examined determinants of low levels of 25(OH)D3 concentrations among older, predominantly white, hospitalized patients and found that vitamin D intake, winter season, and being housebound were independent predictors of vitamin D deficiency (34). Vitamin D may be obtained from dietary sources such as fatty fish, fish oils, fortified foods, and vitamin supplements; however, the main source of vitamin D is sunlight exposure (35). During exposure to sunlight, the solar UVB photons are absorbed by 7-dehydrocholesterol in the skin and converted to vitamin D (36). Factors that affect cutaneous absorption of vitamin D include the use of sunblock, levels of sunlight exposure (eg, season, latitude, and time of day), and skin pigmentation. Black pigmentation in African Americans decreases the absorption of ultraviolet rays from the sun. In addition, decreased milk consumption due to lactose intolerance reduces the levels of vitamin D in African Americans. In fact, African Americans may be at particularly higher risk for vitamin D deficiency because of their high melanin concentrations (36-38). Our group and others have recently demonstrated that vitamin D deficiency is an important risk factor for uterine fibroids (39-41). Our group also revealed that black women have lower levels of serum vitamin D3 as compared to white women (39). In addition, our group demonstrated that uterine fibroids express reduced levels of vitamin D receptor (VDR) when compared with adjacent myometrium (42). Therefore, it is possible that the loss of vitamin D functions, due to reduced levels of serum vitamin D3 and/or reduced expression of VDR could be an important risk factor for uterine fibroids, which could partially explain why African American women have increased incidence of uterine fibroids than other ethnic groups.
Vitamin D and Cancer
Both vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol) can be obtained from sunlight and dietary sources. Both are then converted to 25-hydroxyvitamin D3 [25(OH)D3] by the enzyme 25 α-hydroxylase in the liver (36, 37). 25(OH)D3 is the major circulating form of vitamin D and it is widely accepted as an index of vitamin D status in humans. However, 25(OH)D3 is biologically inert unless it is hydroxylated in the kidneys to form 1α,25-dihydroxyvitamin D [1α, 25(OH)2D3]. 1α, 25(OH)2D3 is a lipid-soluble hormone and the biologically active form of vitamin D3. This active form of vitamin D (1α, 25(OH)2D3) mediates its biological actions through a classic steroid hormone-like transcriptional mechanism thus, influencing the expression of many genes. We present a simple model of vitamin D metabolism and its action is figure 1. The VDR is a nuclear protein receptor activated by 1α, 25(OH)2D3 leading to alteration in transcription rate of target genes. The VDR undergoes conformational change and forms a heterodimer complex with the retinoid X receptor alpha (RXR-α). This heterodimer complex then binds to DNA elements in the promoter/enhancer regions of target genes and then mediate its genomic actions (43). In addition, 1α, 25(OH)2D3 appears to bind to one or more cell surface receptors through second messenger pathways mediating certain non-genomic effects (44-46). Thus, 1α, 25(OH)2D3 interacts with VDR to exert a variety of functions, including genomic and non-genomic actions.
Figure. 1.
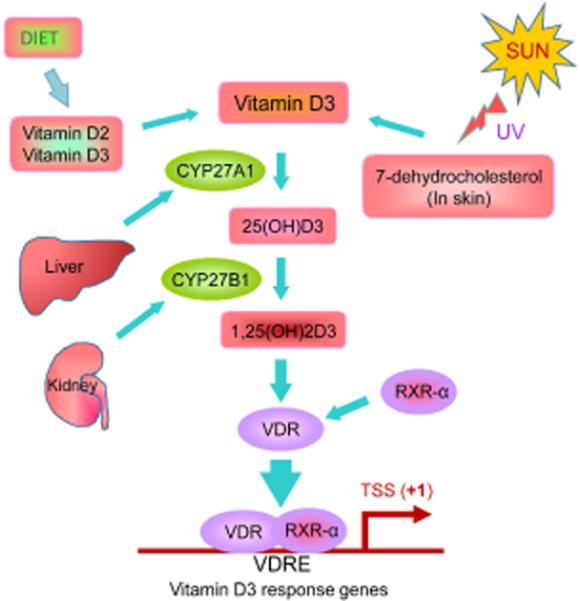
Schematic diagram of vitamin D metabolism and its physiological action. Sunlight and diet are the main sources of vitamin D. Vitamin D2 can only be obtained from plant sources. Vitamin D3 is derived from animal sources and is produced in the skin when 7-dehydrocholesterol reacts with ultraviolet (UV) B light from the sun at wavelengths between 270 and 290 nm. Vitamin D3 that is converted from 7-dehydrocholesterol transfer to the blood, which is hydroxylated by the liver hydroxylase, CYP27A1 to form circulating 25-hydroxyvitamin D3 [25(OH)D3]. The biologically active form of vitamin D3, 1,25-dihydroxivitamin D3 [1,25(OH)2D3] is synthesized by further hydroxylation of 25(OH)D3 by the kidney 1a, hydroxylase, CYP27B1. 1,25(OH)2D3 is mainly involved in the calcium homeostasis, and its formation is tightly regulated by the body. Once the bioactive vitamin D3 binds to its own nuclear vitamin D receptor (VDR), then VDR undergoes conformational change and forms a heterodimer with the retinoid X receptor-alpha (RXR-α), which in turn binds to vitamin D response element (VDRE) present not only in the proximal promoter regions, but also in the distal enhancers, intergenic regions, and introns of target genes, and then negatively or positively regulates the transcription of target genes.
The biologically active form of vitamin D hormone, 1α, 25(OH)2D3 is active in almost every tissue in the body. As mentioned, 1α, 25(OH)2D3 binds to the nuclear VDR, and then to a specific target DNA sequence known as the vitamin D response elements (VDREs) are present not only in the proximal promoter regions, but also in the distal enhancers, intergenic regions, and introns of target genes (43). 1α,25(OH)2D3 can modulate gene expression in a tissue-specific manner and can lead to the inhibition of cellular proliferation, induction of differentiation, and apoptosis. These processes can ultimately lead to the protection of cells from malignant transformation as well as inhibition of cancer cell growth. Recent studies have demonstrated that the systemic administration of 1α, 25(OH)2D3 can induce hypercalcemia and lead to kidney stone formation. To overcome this side effect of hypercalcemia, several analogs of 1α, 25(OH)2D3 have been tested in animal studies which exhibit potent growth inhibition with decreased hypercalcemic side effect (22). Studies with a 1α, 25(OH)2D3 analog, EB1089, have exhibited an antproliferative effect on colon cancer in an xenograft animal model (47). Another 1α, 25(OH)2D3 analog, OCT, exhibited an antitumor effect on a xenograft model using human breast cancer MCF-7 cell line (48). Additionally, another analog, LG190119, showed anti-proliferative activity when tested in a xenograft model using prostate cancer LNCaP cell line (49). Also, a 1α, 25(OH)2D3 analog, 22-oxa-1α,25(OH)2D3, can reduce growth of pancreatic cancer cell lines as well as inhibit the growth of a BxPC-3 tumor in xenograft nude mice model (50, 51). Therefore, 1α, 25(OH)2D3 analogs designed to avoid the hypercalcemic side effect may be more attractive anti-cancer agents for preclinical studies for the non-surgical and non-invasive treatment of cancers.
Vitamin D has been postulated to reduce cancer risk by regulating cellular proliferation and differentiation, inhibition of angiogenesis, and induction of apoptosis. Studies have shown that several cancer cell lines such as prostate, colon, breast, lung and melanoma undergo growth inhibition when exposed to 1α,25(OH)2D3 (37, 52-54). The anti-proliferative effects of 1α, 25(OH)2D3 are mainly due to the alterations in several key cell cycle regulators leading to the arrest of cells in the G0/G1 phase (55). In general, cell cycle progression is regulated by cyclins and their associated cyclin-dependent kinases (CDK) and cyclin-dependent kinase inhibitors (CDKIs). The CDKI genes such as p21 and/or p27 contain VDREs within their promoter regions and are the targets of the 1α, 25(OH)2D3/VDR complex in many cell types, which in turn induce G1 cell-cycle arrest and reduces cell growth (56-58). 1α, 25(OH)2D3 can also induce apoptosis in many cells by repressing the expression of the anti-apoptotic protein, Bcl-2, and the pro-survival protein, Bcl-xL. 1α, 25(OH)2D3 can also enhance the expression of pro-apoptotic proteins, Bax and Bad (59). In addition, 1α, 25(OH)2D3 can induce apoptosis by directly activating the caspase effector molecules (59). Inhibition of angiogenesis is also an important mechanism of anticancer action of 1α, 25(OH)2D3. Studies have reported that 1α, 25(OH)2D3 can reduce endothelial cell growth in vitro and can reduce angiogenesis in vivo (60-62). The anti-angiogenic effects of 1α, 25(OH)2D3 have been shown to reduce prostate and lung metastasis in rats animal models (63, 64).
Liver fibrosis is defined by excessive accumulation of extracellular matrix that ultimately lead to the loss of liver function. A recent study demonstrated the important role of VDR signaling in the suppression of liver fibrosis through the reduction of fibrotic gene expression in mice model (65). In that study, VDR expression was knocked down in mice treated with carbon tetrachloride (CCl4), a widely known hepatotoxic agent to develop liver fibrosis. However, treatment with low-calcemic analog of vitamin D3, calcipotrial attenuated liver fibrosis in those VDR knocked down mice, and they demonstrated that the VDR agonist possesses not only the ability to attenuate the liver fibrosis, but also has the potential to proactively prevent liver fibrosis in in vivo (65).
The Nuclear receptors (NRs) comprise a superfamily of transcription factors regulated by specific ligands and cellular signaling pathways. There are 48 NRs members of the NR superfamily in humans, and they directly regulate transcription of hormone-regulated genes. NRs play essential roles in human physiology and are major drug targets for treatment of reproductive abnormalities, cancer, diabetes, cardiovascular disease, and metabolic syndrome (66, 67). A recent study by Yin et al demonstrated the expression profiling of these NRs in uterine leiomyomas and matched myometrium (68). Their study demonstrated that several of these NRs are dysregulated in uterine leiomyomas, while NR4A subfamily members were dramatically underexpressed in leiomyomas as compared with normal myometrium (68). They further demonstrated that downregulation of NR4A plays functional role in leiomyoma cell proliferation and could be a potential regulator of uterine leiomyoma and other fibroproliferative diseases.
Vitamin D and Leiomyoma Growth
Vitamin D deficiency and Leiomyoma (clinical observations)
African American females are 2-3 times more likely to have uterine leiomyomas than White females. In addition, recent studies have found that vitamin D deficiency is associated with an increased risk of leiomyoma. Our group was the first to demonstrate an association between lower serum vitamin D levels and an increased risk of uterine leiomyoma in 2013 in a cohort of black and white females from North Africa (39). In addition, our results revealed a significant inverse relationship between vitamin D serum levels and the severity of fibroids in African American females, meaning the lower the vitamin D level, the more severe the leiomyoma burden. Our findings were confirmed by two other major studies by Baird et al and Paffoni et al (40, 41). Baird et al found that only 10% of African Americans and 50% of Caucasians had sufficient vitamin D levels. In addition, women with sufficient levels of vitamin D were less likely to have uterine fibroids with an adjusted odds ratio of 0.68. Similarly, Paffoni et al (40, 41) found that women with vitamin D deficiency were more likely to have leiomyomas with an adjusted odds ratio of 2.4. A detailed description of these studies have been presented in the table 1. The endocrine society recommends using the serum circulating 25-hydroxyvitamin D [25(OH)D] level, measured by a reliable assay, to evaluate vitamin D status in patients who are at risk for vitamin D deficiency. Vitamin D deficiency is defined as a 25(OH)D below 20 ng/ml (50 nmol/liter) and vitamin D insufficiency as a 25(OH) D of 21–29 ng/ml (52.5– 72.5) nmol/liter (69).
Table 1.
Recent investigation demonstrating the relationship between vitamin D and uterine fibroids.
| Sabry et al. (2013) | Baird et al. (2013) | Paffoni et al. (2013) | |
|---|---|---|---|
| Number of participants | N=154; 104 with fibroids & 50 controls; 87 black & 67 | 1036; 620 black & 416 white | 384; 128 with fibroids & 256 control |
| OR/CI | Not: reported | Adjusted protective OR: 0.68; 95% CI: 0.48–0.96 for 25OHD | 2.5 (95% CI: 1.2–4.9; p = 0.016) >20 ng/ml |
| Race/Ethnicity | Black and White | Black and White | Italian |
| Serum level 25(OH)D | UF: 19.7 ± 11.8 ng/ml Control: 22.3 ± 6.5 ng/ml |
Only 10% of blacks and 50% of whites had sufficient levels | UF: 18.0 ± 7.7 ng/ml Control: 20.8 ± 11.1 ng/ml |
| Assays | Radio-immunoassay | Radio-immunoassay | Chemiluminescence |
25(OH)D: 25-hydroxyvitamin D; OR: Odds ratio; UF: Uterine fibroid.
Several genes are involved in vitamin D3 metabolism and several single nucleotide polymorphism (SNPs) are associated with 25(OH)D3 concentrations. Wise et al recently investigated the incidence of uterine leiomyomas in relation to polymorphism in genes involved in vitamin D metabolism and skin pigmentation (70). They evaluated the risk of uterine leiomyomas in relation to 12 polymorphisms in eight genes of a large cohort of African American females. These polymorphisms were localized within or near genes involved in vitamin D transport (GC), cholesterol synthesis (DHCR7), and hydroxylation (CYP2R1 and CYP24A1, a major gene in vitamin D pathway). They examined uterine leiomyoma risk in relation to 12 polymorphisms in 8 genes: two in GC (71), two in VDR (72), two in CYP2R1 (71), two near DHCR7 (71, 73), and one each in SLC24A5 (74), OCA2 (75), ASIP (76), and CYP24A1 (72). They identified two single nucleotide polymorphisms, one near DHCR7 and other in ASIP, which are significantly associated with uterine leiomyoma. Genome-wide association studies (GWAS) suggest that polymorphisms in enzyme related to activation or degradation of vitamin D and its metabolites predict serum levels of 25-hydroxyvitamin D3 (77). This further demonstrates the association between nucleotide polymorphisms and risk of development of uterine fibroids in African American women.
Vitamin D and Leiomyomas (in vitro studies)
Although, estradiol and progesterone are the major stimulators of leiomyoma tumor growth, the precise pathophysiology of uterine leiomyomas remains unknown. Chromosomal abnormalities, hormonal deregulation, and growth and angiogenic factors are the most common concern for the etiology of these clonal smooth muscle cell proliferations (78-80). Catherino et al used global expression profiling to compare clonal tumors with normal myometrium (81). Contrary to the expected, their results revealed that genes involved in estrogen encoding were not differentially expressed between leiomyoma and normal myometrium. However, they found that genes encoding proteins from the ECM were overexpressed in leiomyomas. Moreover, analysis of the ECM in leiomyoma tissue revealed a disordered collagen fibril orientation and a decreased dermatopontin, which is a collagen-binding protein. The reduction in dermatopotin was associated with an increase in TGF-β3 mRNA levels. TGF-β has been found to be significantly involved in the accumulation of ECM proteins in leiomyoma (82). At present, TGF-β3 is the only growth factor found to be overexpressed in leiomyoma samples during the secretory phase (83). Recently, we examined COMT (Catechol-O-methyltransferase) and ER-α (estrogen receptor-alpha) polymorphism analyses in women from different ethnic groups (84, 85). Our investigation revealed that females with a high-activity genotype for COMT were 2.5 times more likely to develop leiomyomas than females with other genotypes. It is evident from these results and the results of the study on polymorphism for the ER-α in African American females, that submicroscopic genetic anomalies may be operational at different levels in African American females, likely leading to leiomyoma formation (85). Therefore, the estrogen hypothesis provides the most reasonable biological explanation for the increased risk of leiomyomas among African American females. Although, numerous studies have been conducted on uterine leiomyomas to understand the causes of their development, the role of vitamin D3 on regulation of leiomyoma had not been investigated until recently. To understand the biological role of vitamin D3 in the regulation of uterine leiomyomas growth, Blauer et al performed a study which demonstrated that the bioactive 1α, 25(OH)2D3 inhibits the growth of both leiomyoma and myometrial cells derived from human tissues of premenopausal females undergoing hysterectomy (86). Growth inhibition was concentration-dependent, and the level of inhibition was significant at a concentration of 100 nM-the physiological level (87, 88). We and others have recently demonstrated that women with uterine fibroids have lower levels of serum vitamin D3 than their healthy counterpart women (39-41). We also demonstrated that serum levels of vitamin D3 are inversely correlated with leiomyoma sizes (39), suggesting that vitamin D3 deficiency is a risk factor for the development of uterine leiomyoma. Based on published literature on uterine leiomyoma and factors that affect leiomyoma growth, we hypothesized that vitamin D3 could be a potential regulator for uterine leiomyoma growth, and thus we performed in vitro studies to elucidate vitamin D3 functions. We have demonstrated in our lab that 1, 25-dihydroxyvitamin D3 is an anti-fibrotic factor and inhibits the proliferation of the immortalized human uterine fibroid HuLM cells (42, 88). In one study, we evaluated the effect and mechanism of action of vitamin D on human uterine leiomyoma cell proliferation. Cells were treated with vitamin D3 followed by measurement of proliferation cell nuclear antigen (PCNA), BCL-2, BCL-w, CDK1, and COMT protein levels. Results revealed vitamin D3 inhibits growth and induces apoptosis in cultured human leiomyoma cells through the down-regulation of PCNA, CDK1, and BCL-2 and suppression of COMT expression and activity in human leiomyoma cells (87). In a subsequent study, we examined the effect of vitamin D3 on TGF-β3-induced fibrosis-related protein expression in human cells. We found that vitamin D3 suppressed the effect of TGF-β3 on the process of fibrosis in human leiomyoma cells (88).
Leiomyomas are characterized by excessive deposition of ECM as well as an increase in cell proliferation. The ECM undergoes degradation in a physiologic process designed to repair and remodel it. Disruption of this degradation process leads to pathology. The major enzymes involved in this degradation process are matrix metalloproteinases (MMPs), which are in turn regulated by tissue inhibitors of matrix metalloproteinases (TIMPs). In an investigation to evaluate the effect of vitamin D3 on the expression and activity of MMPs in human uterine fibroid cells, we found vitamin D3 significantly reduced the level of MMP-2 and MMP-9 activity (42). In addition, vitamin D3 increased levels of VDR and TIMP-2 in a concentration-dependent manner (42). In a subsequent study, we evaluated the risk associated with reduced levels of VDR protein in human uterine fibroid tumors and sought to determine the biological function of 1, 25(OH)D3 in regulation of ECM-associated proteins. We found that more than 60% of uterine fibroid tissue analyzed expressed low levels of VDR compared to adjacent normal myometrium (42). Treatment with bioactive 1, 25-dihydrozyvitamin D3 induced VDR in a concentration-dependent manner in human fibroid cells (42). Vitamin D3 also significantly reduced the protein expression of ECM-associated collagen type 1, fibronectin, and plasminogen activator-1 (PAI-1). Moreover, vitamin D decreased the abnormal expression of structural smooth muscle fibers in human uterine fibroid cells (42).
In a study to verify the ethnic differences in tumorigenic factors of uterine leiomyomas, Wei et al. identified selective genes by tissue microarray analyses and specific immunohistochemistry determinants involved in the development of leiomyomas and compared the results to matched myometrial tissue (89). The results indicated that progesterone receptor, PR-A was up-regulated in leiomyoma tissue of African American females as compared to other ethnic groups (89). Furthermore, the estrogen receptor ER-α was elevated in both the normal myometrial and leiomyoma tissues of African American females when compared with other ethnic groups (89). Recent observations from our group also confirmed elevated expressions of ER-α, PR-A, and PR-B in human uterine fibroids when compared with adjacent myometrium (90). We also observed that vitamin D3 reduced the expression of these sex steroid receptors in a concentration-dependent manner in an immortalized uterine fibroid cell line (90), indicating a possible therapeutic role of vitamin D3 in the treatment of uterine fibroids.
Vitamin D and fibroids (animal models)
In our recent animal study we evaluated the effect of vitamin D3 on leiomyoma growth in the Eker Rat model of uterine fibroids (91). We found that treatment with vitamin D3 significantly reduced leiomyoma size by suppressing cell growth and proliferation-related genes, anti-apoptotic genes and estrogen and progesterone receptors (91). Results revealed suppression of cell growth and proliferation-related genes (PCNA, cyclin D1 [Ccnd1], c-Myc, CDK1, CDK2, and CDK4). Anti-apoptotic genes (BCL2 and BCL-xl), estrogen receptor ER-α, and progesterone receptors PR-A and PR-B were all suppressed. Toxicity panels revealed similar levels of SGOT, SGPT, calcium and total bilirubin in vitamin D3 treated animals compared to untreated controls (91). In another study, we examined the effect of paricalcitol, an analog of 1, 25-dihydroxyvitqmin D3 with lower hypercalcemic and potential activator of VDR, on uterine fibroids as compared to both vitamin D3 and placebo (92). We found that both 1, 25-dihydroxyvitamin D3 and paricalcitol significantly reduced fibroid tumor size in female nude mice. A recent study demonstrated that administration of paricalcitol is associated with the reduction of cardiac fibrosis, and lower expression of profibrotic genes in the heart, and that ultimately can improve heart function in a murine model (93). Based on our in vitro and in vivo studies, we have proposed mechanism(s) by which vitamin D may function in regulating the proliferation of fibroid cells and fibroid tumor growth (Figure 2). These findings suggest that vitamin D3 or paricalcitol may be considered as a therapeutic option for the effective, safe non-surgical long-term medical treatment for uterine fibroids.
Figure 2.
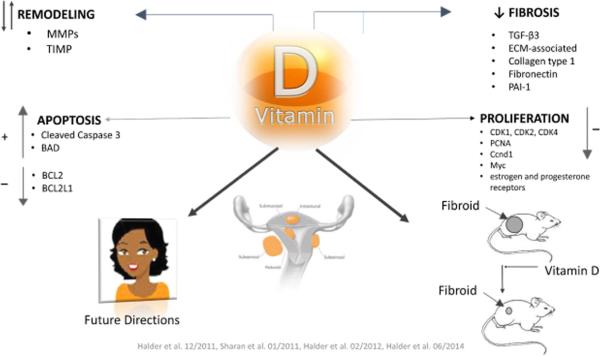
Mechanism of vitamin D action in uterine fibroid development.
Conclusions
Vitamin D is known as the main regulator of calcium hemostasis. Several studies have demonstrated that 1, 25-dihydroxyvitamin D is a potent anti-tumor agent that inhibits leiomyoma cell proliferation in vitro and decreases the size of uterine leiomyomas in vivo animal models. These observations and findings further support the potential role of 1α, 25(OH)2D3 or its potent analogues in the non-surgical treatment of uterine leiomyomas. Further research is necessary to fully understand the role of vitamin D3 on leiomyoma growth, but more importantly, in further investigating the utility and efficacy of vitamin D3 as a non-surgical and non-invasive treatment of leiomyomas. To date, no randomized controlled trial has been implemented to prospectively assess the efficacy of vitamin D in the management of uterine fibroids. Thus, randomized controlled trials are needed to investigate the therapeutic effects of vitamin D3 on uterine leiomyomas.
Future Direction
The physiological nontoxic circulating level of vitamin D3 (25-hydroxyvitamin D3) is 30 ng/ml and can usually be accomplished by an intake of 2,000 IU/day. The chronic or acute administration of higher doses of vitamin D3 can cause vitamin D toxicity, leading to hypercalcemia and functional hypoparathyroidism and resulting in frequent fractures and bone pain. A safe dose of 0.5 μg/kg/day equivalent to 1,400 IU for a 70 Kg adult was used in our studies (91, 92). The Endocrine Society practice guidelines on the treatment of vitamin D deficiency recommends that adults who are vitamin D deficient be treated with 50,000 IU of vitamin D2 or vitamin D3 once a week for 8 weeks or its equivalent of 6000 IU of vitamin D2 or vitamin D3 daily to achieve a blood level of 25(OH)D above 30 ng/ml, followed by maintenance therapy of 1500–2000 IU/d (69). Vitamin D toxicity could be prevented with short term vitamin D deficiency treatment then maintenance therapy. The yearly cost of maintenance therapy would be around 32 dollars. The cost of the 8 weeks treatment therapy would be less than 16 dollars. In our Eker model, we used 0.5 μg/kg per day dosage of vitamins D3, which is equivalent to 1400 IU for an adult having a body weight of 70 kg (91). Thus, 1400 IU is a possible nontoxic and effective treatment of uterine leiomyoma.
Moreover, analogs of vitamin D3 have been successfully synthesized and their anti-proliferative properties with reduced hypercalcemic effect have been previously demonstrated (94-96). Our investigation with paricalcitol confirm these findings and suggest that vitamin D3 analogs could be potential candidates for an effective, safe, and noninvasive medical treatment option for uterine fibroids (92). The next endeavor is to investigate these analogs through the conduct of a clinical trial for the evaluation of their effectiveness and safety in treating human uterine fibroids.
Precis.
Vitamin D3 or its hypocalcemic analog, paricalcitol may be a novel therapeutic approach as an effective, safe non-surgical treatment option for uterine fibroids.
Acknowledgements
The authors are grateful to Ms. Walidah Walker, Department of Obstetrics and Gynecology at Georgia Regents University for reviewing of the manuscript. This study was supported by a Georgia Regents University start-up package, by National Institutes of Health (NIH)/Research Centers in Minority Institutions Pilot Grant 2G12RR003032–26 (to S.K.H.), and by NIH/R01 Grant 2R01HD046228–11 (to A.A.-H.).
Abbreviations
1,25-dihydroxyvitamin D3; vitamin D3; uterine fibroids; leiomyomas; paricalcitol; VDR
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement: The authors in this paper have no conflict of interest or nothing to disclose.
References
- 1.Cook JD, Walker CL. Treatment strategies for uterine leiomyoma: the role of hormonal modulation. Seminars in reproductive medicine. 2004;22(2):105–11. doi: 10.1055/s-2004-828616. [DOI] [PubMed] [Google Scholar]
- 2.Eltoukhi HM, Modi MN, Weston M, Armstrong AY, Stewart EA. The health disparities of uterine fibroid tumors for African American women: a public health issue. American journal of obstetrics and gynecology. 2014;210(3):194–9. doi: 10.1016/j.ajog.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Metwally M, Farquhar CM, Li TC. Is another meta-analysis on the effects of intramural fibroids on reproductive outcomes needed? Reproductive biomedicine online. 2011;23(1):2–14. doi: 10.1016/j.rbmo.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Eldar-Geva T, Healy DL. Other medical management of uterine fibroids. Bailliere's clinical obstetrics and gynaecology. 1998;12(2):269–88. doi: 10.1016/s0950-3552(98)80064-3. [DOI] [PubMed] [Google Scholar]
- 5.Guo XC, Segars JH. The impact and management of fibroids for fertility: an evidence-based approach. Obstetrics and gynecology clinics of North America. 2012;39(4):521–33. doi: 10.1016/j.ogc.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan AT, Shehmar M, Gupta JK. Uterine fibroids: current perspectives. International journal of women's health. 2014;6:95–114. doi: 10.2147/IJWH.S51083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farhi J, Ashkenazi J, Feldberg D, Dicker D, Orvieto R, Ben Rafael Z. Effect of uterine leiomyomata on the results of in-vitro fertilization treatment. Human reproduction. 1995;10(10):2576–8. doi: 10.1093/oxfordjournals.humrep.a135748. [DOI] [PubMed] [Google Scholar]
- 8.Eldar-Geva T, Meagher S, Healy DL, MacLachlan V, Breheny S, Wood C. Effect of intramural, subserosal, and submucosal uterine fibroids on the outcome of assisted reproductive technology treatment. Fertility and sterility. 1998;70(4):687–91. doi: 10.1016/s0015-0282(98)00265-9. [DOI] [PubMed] [Google Scholar]
- 9.Hart R, Khalaf Y, Yeong CT, Seed P, Taylor A, Braude P. A prospective controlled study of the effect of intramural uterine fibroids on the outcome of assisted conception. Human reproduction. 2001;16(11):2411–7. doi: 10.1093/humrep/16.11.2411. [DOI] [PubMed] [Google Scholar]
- 10.Surrey ES, Lietz AK, Schoolcraft WB. Impact of intramural leiomyomata in patients with a normal endometrial cavity on in vitro fertilization-embryo transfer cycle outcome. Fertility and sterility. 2001;75(2):405–10. doi: 10.1016/s0015-0282(00)01714-3. [DOI] [PubMed] [Google Scholar]
- 11.Lepine LA, Hillis SD, Marchbanks PA, Koonin LM, Morrow B, Kieke BA, Wilcox LS. Hysterectomy surveillance--United States, 1980-1993. MMWR CDC surveillance summaries : Morbidity and mortality weekly report CDC surveillance summaries / Centers for Disease Control. 1997;46(4):1–15. [PubMed] [Google Scholar]
- 12.Makinen N, Mehine M, Tolvanen J, Kaasinen E, Li Y, Lehtonen HJ, Gentile M, Yan J, Enge M, Taipale M, et al. MED12, the mediator complex subunit 12 gene, is mutated at high frequency in uterine leiomyomas. Science. 2011;334(6053):252–5. doi: 10.1126/science.1208930. [DOI] [PubMed] [Google Scholar]
- 13.Makinen N, Heinonen HR, Moore S, Tomlinson IP, van der Spuy ZM, Aaltonen LA. MED12 exon 2 mutations are common in uterine leiomyomas from South African patients. Oncotarget. 2011;2(12):966–9. doi: 10.18632/oncotarget.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Makinen N, Vahteristo P, Butzow R, Sjoberg J, Aaltonen LA. Exomic landscape of MED12 mutation-negative and -positive uterine leiomyomas. International journal of cancer Journal international du cancer. 2014;134(4):1008–12. doi: 10.1002/ijc.28410. [DOI] [PubMed] [Google Scholar]
- 15.Markowski DN, Bartnitzke S, Loning T, Drieschner N, Helmke BM, Bullerdiek J. MED12 mutations in uterine fibroids--their relationship to cytogenetic subgroups. International journal of cancer Journal international du cancer. 2012;131(7):1528–36. doi: 10.1002/ijc.27424. [DOI] [PubMed] [Google Scholar]
- 16.McGuire MM, Yatsenko A, Hoffner L, Jones M, Surti U, Rajkovic A. Whole exome sequencing in a random sample of North American women with leiomyomas identifies MED12 mutations in majority of uterine leiomyomas. PLoS One. 2012;7(3):e33251. doi: 10.1371/journal.pone.0033251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halder SK, Laknaur A, Miller J, Layman LC, Diamond M, Al-Hendy A. Novel MED12 gene somatic mutations in women from the Southern United States with symptomatic uterine fibroids. Molecular genetics and genomics. Apr. 2015;290(2):505–11. doi: 10.1007/s00438-014-0938-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Hendy A, Badr M. Can vitamin D reduce the risk of uterine fibroids? Women's health. 2014;10(4):353–8. doi: 10.2217/whe.14.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sabry M, Al-Hendy A. Innovative oral treatments of uterine leiomyoma. Obstetrics and gynecology international. 2012;2012:943635. doi: 10.1155/2012/943635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen CR, Buck GM, Courey NG, Perez KM, Wactawski-Wende J. Risk factors for uterine fibroids among women undergoing tubal sterilization. American journal of epidemiology. 2001;153(1):20–6. doi: 10.1093/aje/153.1.20. [DOI] [PubMed] [Google Scholar]
- 21.Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. American journal of obstetrics and gynecology. 2003;188(1):100–7. doi: 10.1067/mob.2003.99. [DOI] [PubMed] [Google Scholar]
- 22.Wise LA, Palmer JR, Stewart EA, Rosenberg L. Age-specific incidence rates for self-reported uterine leiomyomata in the Black Women's Health Study. Obstetrics and gynecology. 2005;105(3):563–8. doi: 10.1097/01.AOG.0000154161.03418.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faerstein E, Szklo M, Rosenshein N. Risk factors for uterine leiomyoma: a practice-based case-control study. I. African-American heritage, reproductive history, body size, and smoking. American journal of epidemiology. 2001;153(1):1–10. doi: 10.1093/aje/153.1.1. [DOI] [PubMed] [Google Scholar]
- 24.Marshall LM, Spiegelman D, Barbieri RL, Goldman MB, Manson JE, Colditz GA, Willett WC, Hunter DJ. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstetrics and gynecology. 1997;90(6):967–73. doi: 10.1016/s0029-7844(97)00534-6. [DOI] [PubMed] [Google Scholar]
- 25.Farquhar CM, Steiner CA. Hysterectomy rates in the United States 1990-1997. Obstetrics and gynecology. 2002;99(2):229–34. doi: 10.1016/s0029-7844(01)01723-9. [DOI] [PubMed] [Google Scholar]
- 26.Kjerulff KH, Langenberg P, Seidman JD, Stolley PD, Guzinski GM. Uterine leiomyomas. Racial differences in severity, symptoms and age at diagnosis. The Journal of reproductive medicine. 1996;41(7):483–90. [PubMed] [Google Scholar]
- 27.Kjerulff KH, Guzinski GM, Langenberg PW, Stolley PD, Moye NE, Kazandjian VA. Hysterectomy and race. Obstetrics and gynecology. 1993;82(5):757–64. [PubMed] [Google Scholar]
- 28.Matchar DB, Myers ER, Barber MW, Couchman GM, Datta S, Gray RN, Gustilo-Ashby T, Kolimaga JT, McCrory DC. Management of uterine fibroids. Evidence report/technology assessment. 2001;34:1–6. [PMC free article] [PubMed] [Google Scholar]
- 29.Davis BJ, Haneke KE, Miner K, Kowalik A, Barrett JC, Peddada S, Baird DD. The fibroid growth study: determinants of therapeutic intervention. Journal of women's health. 2009;18(5):725–32. doi: 10.1089/jwh.2008.0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nesby-O'Dell S, Scanlon KS, Cogswell ME, Gillespie C, Hollis BW, Looker AC, Allen C, Doughertly C, Gunter EW, Bowman BA. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988-1994. The American journal of clinical nutrition. 2002;76(1):187–92. doi: 10.1093/ajcn/76.1.187. [DOI] [PubMed] [Google Scholar]
- 31.Bikle DD, Ettinger B, Sidney S, Tekawa IS, Tolan K. Differences in calcium metabolism between black and white men and women. Mineral and electrolyte metabolism. 1999;25(3):178–84. doi: 10.1159/000057442. [DOI] [PubMed] [Google Scholar]
- 32.Harris SS, Dawson-Hughes B. Seasonal changes in plasma 25-hydroxyvitamin D concentrations of young American black and white women. The American journal of clinical nutrition. 1998;67(6):1232–6. doi: 10.1093/ajcn/67.6.1232. [DOI] [PubMed] [Google Scholar]
- 33.Parisien M, Cosman F, Morgan D, Schnitzer M, Liang X, Nieves J, Forese L, Luckey M, Meier D, Shen V, et al. Histomorphometric assessment of bone mass, structure, and remodeling: a comparison between healthy black and white premenopausal women. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 1997;12(6):948–57. doi: 10.1359/jbmr.1997.12.6.948. [DOI] [PubMed] [Google Scholar]
- 34.Thomas MK, Lloyd-Jones DM, Thadhani RI, Shaw AC, Deraska DJ, Kitch BT, Vamvakas EC, Dick IM, Prince RL, Finkelstein JS. Hypovitaminosis D in medical inpatients. The New England journal of medicine. 1998;338(12):777–83. doi: 10.1056/NEJM199803193381201. [DOI] [PubMed] [Google Scholar]
- 35.Holick MF. Vitamin D: A millenium perspective. Journal of cellular biochemistry. 2003;88(2):296–307. doi: 10.1002/jcb.10338. [DOI] [PubMed] [Google Scholar]
- 36.Holick MF. Too little vitamin D in premenopausal women: why should we care? The American journal of clinical nutrition. 2002;76(1):3–4. doi: 10.1093/ajcn/76.1.3. [DOI] [PubMed] [Google Scholar]
- 37.Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. The American journal of clinical nutrition. 2004;80(6 Suppl):1678S–88S. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- 38.Clemens TL, Adams JS, Henderson SL, Holick MF. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet. 1982;1(8263):74–6. doi: 10.1016/s0140-6736(82)90214-8. [DOI] [PubMed] [Google Scholar]
- 39.Sabry M, Halder SK, Allah AS, Roshdy E, Rajaratnam V, Al-Hendy A. Serum vitamin D3 level inversely correlates with uterine fibroid volume in different ethnic groups: a cross-sectional observational study. International journal of women's health. 2013:593–100. doi: 10.2147/IJWH.S38800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baird DD, Hill MC, Schectman JM, Hollis BW. Vitamin d and the risk of uterine fibroids. Epidemiology. 2013;24(3):447–53. doi: 10.1097/EDE.0b013e31828acca0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paffoni A, Somigliana E, Vigano P, Benaglia L, Cardellicchio L, Pagliardini L, Papaleo E, Candiani M, Fedele L. Vitamin D status in women with uterine leiomyomas. The Journal of clinical endocrinology and metabolism. 2013;98(8):E1374–8. doi: 10.1210/jc.2013-1777. [DOI] [PubMed] [Google Scholar]
- 42.Halder SK, Osteen KG, Al-Hendy A. 1,25-dihydroxyvitamin d3 reduces extracellular matrix-associated protein expression in human uterine fibroid cells. Biology of reproduction. 2013;89(6):150. doi: 10.1095/biolreprod.113.107714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsai MJ, O'Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annual review of biochemistry. 1994;63:451–86. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- 44.Ross TK, Darwish HM, DeLuca HF. Molecular biology of vitamin D action. Vitamins and hormones. 1994;49:281–326. doi: 10.1016/s0083-6729(08)61149-8. [DOI] [PubMed] [Google Scholar]
- 45.Issa LL, Leong GM, Eisman JA. Molecular mechanism of vitamin D receptor action. Inflammation research : official journal of the European Histamine Research Society [et al] 1998;47(12):451–75. doi: 10.1007/s000110050360. [DOI] [PubMed] [Google Scholar]
- 46.Haussler MR, Whitfield GK, Haussler CA, Hsieh JC, Thompson PD, Selznick SH, Dominguez CE, Jurutka PW. The nuclear vitamin D receptor: biological and molecular regulatory properties revealed. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 1998;13(3):325–49. doi: 10.1359/jbmr.1998.13.3.325. [DOI] [PubMed] [Google Scholar]
- 47.Akhter J, Chen X, Bowrey P, Bolton EJ, Morris DL. Vitamin D3 analog, EB1089, inhibits growth of subcutaneous xenografts of the human colon cancer cell line, LoVo, in a nude mouse model. Diseases of the colon and rectum. 1997;40(3):317–21. doi: 10.1007/BF02050422. [DOI] [PubMed] [Google Scholar]
- 48.Abe-Hashimoto J, Kikuchi T, Matsumoto T, Nishii Y, Ogata E, Ikeda K. Antitumor effect of 22-oxa-calcitriol, a noncalcemic analogue of calcitriol, in athymic mice implanted with human breast carcinoma and its synergism with tamoxifen. Cancer research. 1993;53(11):2534–7. [PubMed] [Google Scholar]
- 49.Polek TC, Murthy S, Blutt SE, Boehm MF, Zou A, Weigel NL, Allegretto EA. Novel nonsecosteroidal vitamin D receptor modulator inhibits the growth of LNCaP xenograft tumors in athymic mice without increased serum calcium. The Prostate. 2001;49(3):224–33. doi: 10.1002/pros.1138. [DOI] [PubMed] [Google Scholar]
- 50.Kawa S, Nikaido T, Aoki Y, Zhai Y, Kumagai T, Furihata K, Fujii S, Kiyosawa K. Vitamin D analogues up-regulate p21 and p27 during growth inhibition of pancreatic cancer cell lines. British journal of cancer. 1997;76(7):884–9. doi: 10.1038/bjc.1997.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kawa S, Yoshizawa K, Tokoo M, Imai H, Oguchi H, Kiyosawa K, Homma T, Nikaido T, Furihata K. Inhibitory effect of 220-oxa-1,25-dihydroxyvitamin D3 on the proliferation of pancreatic cancer cell lines. Gastroenterology. 1996;110(5):1605–13. doi: 10.1053/gast.1996.v110.pm8613068. [DOI] [PubMed] [Google Scholar]
- 52.Colston K, Hirt M, Feldman D. Organ distribution of the cytoplasmic 1,25-dihydroxycholecalciferol receptor in various mouse tissues. Endocrinology. 1980;107(6):1916–22. doi: 10.1210/endo-107-6-1916. [DOI] [PubMed] [Google Scholar]
- 53.Chen TC, Holick MF. Vitamin D and prostate cancer prevention and treatment. Trends in endocrinology and metabolism: TEM. 2003;14(9):423–30. doi: 10.1016/j.tem.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 54.Skowronski RJ, Peehl DM, Feldman D. Vitamin D and prostate cancer: 1,25 dihydroxyvitamin D3 receptors and actions in human prostate cancer cell lines. Endocrinology. 1993;132(5):1952–60. doi: 10.1210/endo.132.5.7682937. [DOI] [PubMed] [Google Scholar]
- 55.Simboli-Campbell M, Narvaez CJ, van Weelden K, Tenniswood M, Welsh J. Comparative effects of 1,25(OH)2D3 and EB1089 on cell cycle kinetics and apoptosis in MCF-7 breast cancer cells. Breast cancer research and treatment. 1997;42(1):31–41. doi: 10.1023/a:1005772432465. [DOI] [PubMed] [Google Scholar]
- 56.Inoue T, Kamiyama J, Sakai T. Sp1 and NF-Y synergistically mediate the effect of vitamin D(3) in the p27(Kip1) gene promoter that lacks vitamin D response elements. The Journal of biological chemistry. 1999;274(45):32309–17. doi: 10.1074/jbc.274.45.32309. [DOI] [PubMed] [Google Scholar]
- 57.Hager G, Formanek M, Gedlicka C, Thurnher D, Knerer B, Kornfehl J. 1,25(OH)2 vitamin D3 induces elevated expression of the cell cycle-regulating genes P21 and P27 in squamous carcinoma cell lines of the head and neck. Acta oto-laryngologica. 2001;121(1):103–9. doi: 10.1080/000164801300006353. [DOI] [PubMed] [Google Scholar]
- 58.Wu G, Fan RS, Li W, Ko TC, Brattain MG. Modulation of cell cycle control by vitamin D3 and its analogue, EB1089, in human breast cancer cells. Oncogene. 1997;15(13):1555–63. doi: 10.1038/sj.onc.1201329. [DOI] [PubMed] [Google Scholar]
- 59.Ylikomi T, Laaksi I, Lou YR, Martikainen P, Miettinen S, Pennanen P, Purmonen S, Syvala H, Vienonen A, Tuohimaa P. Antiproliferative action of vitamin D. Vitamins and hormones. 2002;64:357–406. doi: 10.1016/s0083-6729(02)64010-5. [DOI] [PubMed] [Google Scholar]
- 60.Chung I, Wong MK, Flynn G, Yu WD, Johnson CS, Trump DL. Differential antiproliferative effects of calcitriol on tumor-derived and matrigel-derived endothelial cells. Cancer research. 2006;66(17):8565–73. doi: 10.1158/0008-5472.CAN-06-0905. [DOI] [PubMed] [Google Scholar]
- 61.Iseki K, Tatsuta M, Uehara H, Iishi H, Yano H, Sakai N, Ishiguro S. Inhibition of angiogenesis as a mechanism for inhibition by 1alpha-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 of colon carcinogenesis induced by azoxymethane in Wistar rats. International journal of cancer Journal international du cancer. 1999;81(5):730–3. doi: 10.1002/(sici)1097-0215(19990531)81:5<730::aid-ijc11>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 62.Mantell DJ, Owens PE, Bundred NJ, Mawer EB, Canfield AE. 1 alpha,25-dihydroxyvitamin D(3) inhibits angiogenesis in vitro and in vivo. Circulation research. 2000;87(3):214–20. doi: 10.1161/01.res.87.3.214. [DOI] [PubMed] [Google Scholar]
- 63.Getzenberg RH, Light BW, Lapco PE, Konety BR, Nangia AK, Acierno JS, Dhir R, Shurin Z, Day RS, Trump DL, et al. Vitamin D inhibition of prostate adenocarcinoma growth and metastasis in the Dunning rat prostate model system. Urology. 1997;50(6):999–1006. doi: 10.1016/S0090-4295(97)00408-1. [DOI] [PubMed] [Google Scholar]
- 64.Nakagawa K, Kawaura A, Kato S, Takeda E, Okano T. 1 alpha,25-Dihydroxyvitamin D(3) is a preventive factor in the metastasis of lung cancer. Carcinogenesis. 2005;26(2):429–40. doi: 10.1093/carcin/bgh332. [DOI] [PubMed] [Google Scholar]
- 65.Ding N, Yu RT, Subramaniam N, Sherman MH, Wilson C, Rao R, Leblanc M, Coulter S, He M, Scott C, et al. A vitamin D receptor/SMAD genomic circuit gates hepatic fibrotic response. Cell. 2013;153(3):601–13. doi: 10.1016/j.cell.2013.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Germain P, Staels B, Dacquet C, Spedding M, Laudet V. Overview of nomenclature of nuclear receptors. Pharmacological reviews. 2006;58(4):685–704. doi: 10.1124/pr.58.4.2. [DOI] [PubMed] [Google Scholar]
- 67.Jeong Y, Xie Y, Xiao G, Behrens C, Girard L, Wistuba, Minna JD, Mangelsdorf DJ. Nuclear receptor expression defines a set of prognostic biomarkers for lung cancer. PLoS medicine. 2010;7(12):e1000378. doi: 10.1371/journal.pmed.1000378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yin H, Lo JH, Kim JY, Marsh EE, Kim JJ, Ghosh AK, Bulun S, Chakravarti D. Expression profiling of nuclear receptors identifies key roles of NR4A subfamily in uterine fibroids. Molecular endocrinology. 2013;27(5):726–40. doi: 10.1210/me.2012-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM, Endocrine S. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. The Journal of clinical endocrinology and metabolism. 2011;96(7):1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 70.Wise LA, Ruiz-Narvaez EA, Haddad SA, Rosenberg L, Palmer JR. Polymorphisms in vitamin D-related genes and risk of uterine leiomyomata. Fertility and sterility. 2014;102(2):503–10. e1. doi: 10.1016/j.fertnstert.2014.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang TJ, Zhang F, Richards JB, Kestenbaum B, van Meurs JB, Berry D, Kiel DP, Streeten EA, Ohlsson C, Koller DL, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 2010;376(9736):180–8. doi: 10.1016/S0140-6736(10)60588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yao S, Zirpoli G, Bovbjerg DH, Jandorf L, Hong CC, Zhao H, Sucheston LE, Tang L, Roberts M, Ciupak G, et al. Variants in the vitamin D pathway, serum levels of vitamin D, and estrogen receptor negative breast cancer among African-American women: a case-control study. Breast cancer research : BCR. 2012;14(2):R58. doi: 10.1186/bcr3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kuan V, Martineau AR, Griffiths CJ, Hypponen E, Walton R. DHCR7 mutations linked to higher vitamin D status allowed early human migration to northern latitudes. BMC evolutionary biology. 2013;13:144. doi: 10.1186/1471-2148-13-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lamason RL, Mohideen MA, Mest JR, Wong AC, Norton HL, Aros MC, Jurynec MJ, Mao X, Humphreville VR, Humbert JE, et al. SLC24A5, a putative cation exchanger, affects pigmentation in zebrafish and humans. Science. 2005;310(5755):1782–6. doi: 10.1126/science.1116238. [DOI] [PubMed] [Google Scholar]
- 75.Shriver MD, Parra EJ, Dios S, Bonilla C, Norton H, Jovel C, Pfaff C, Jones C, Massac A, Cameron N, et al. Skin pigmentation, biogeographical ancestry and admixture mapping. Human genetics. 2003;112(4):387–99. doi: 10.1007/s00439-002-0896-y. [DOI] [PubMed] [Google Scholar]
- 76.Bonilla C, Boxill LA, Donald SA, Williams T, Sylvester N, Parra EJ, Dios S, Norton HL, Shriver MD, Kittles RA. The 8818G allele of the agouti signaling protein (ASIP) gene is ancestral and is associated with darker skin color in African Americans. Human genetics. 2005;116(5):402–6. doi: 10.1007/s00439-004-1251-2. [DOI] [PubMed] [Google Scholar]
- 77.Didriksen A, Grimnes G, Hutchinson MS, Kjaergaard M, Svartberg J, Joakimsen RM, Jorde R. The serum 25-hydroxyvitamin D response to vitamin D supplementation is related to genetic factors, BMI, and baseline levels. European journal of endocrinology / European Federation of Endocrine Societies. 2013;169(5):559–67. doi: 10.1530/EJE-13-0233. [DOI] [PubMed] [Google Scholar]
- 78.Flake GP, Andersen J, Dixon D. Etiology and pathogenesis of uterine leiomyomas: a review. Environmental health perspectives. 2003;111(8):1037–54. doi: 10.1289/ehp.5787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Arslan AA, Gold LI, Mittal K, Suen TC, Belitskaya-Levy I, Tang MS, Toniolo P. Gene expression studies provide clues to the pathogenesis of uterine leiomyoma: new evidence and a systematic review. Human reproduction. 2005;20(4):852–63. doi: 10.1093/humrep/deh698. [DOI] [PubMed] [Google Scholar]
- 80.Shushan A, Ben-Bassat H, Mishani E, Laufer N, Klein BY, Rojansky N. Inhibition of leiomyoma cell proliferation in vitro by genistein and the protein tyrosine kinase inhibitor TKS050. Fertility and sterility. 2007;87(1):127–35. doi: 10.1016/j.fertnstert.2006.05.056. [DOI] [PubMed] [Google Scholar]
- 81.Catherino WH, Leppert PC, Stenmark MH, Payson M, Potlog-Nahari C, Nieman LK, Segars JH. Reduced dermatopontin expression is a molecular link between uterine leiomyomas and keloids. Genes, chromosomes & cancer. 2004;40(3):204–17. doi: 10.1002/gcc.20035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ignotz RA, Massague J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. The Journal of biological chemistry. 1986;261(9):4337–45. [PubMed] [Google Scholar]
- 83.Arici A, Sozen I. Transforming growth factor-beta3 is expressed at high levels in leiomyoma where it stimulates fibronectin expression and cell proliferation. Fertility and sterility. 2000;73(5):1006–11. doi: 10.1016/s0015-0282(00)00418-0. [DOI] [PubMed] [Google Scholar]
- 84.Al-Hendy A, Salama SA. Catechol-O-methyltransferase polymorphism is associated with increased uterine leiomyoma risk in different ethnic groups. Journal of the Society for Gynecologic Investigation. 2006;13(2):136–44. doi: 10.1016/j.jsgi.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 85.Al-Hendy A, Salama SA. Ethnic distribution of estrogen receptor-alpha polymorphism is associated with a higher prevalence of uterine leiomyomas in black Americans. Fertility and sterility. 2006;86(3):686–93. doi: 10.1016/j.fertnstert.2006.01.052. [DOI] [PubMed] [Google Scholar]
- 86.Blauer M, Rovio PH, Ylikomi T, Heinonen PK. Vitamin D inhibits myometrial and leiomyoma cell proliferation in vitro. Fertility and sterility. 2009;91(5):1919–25. doi: 10.1016/j.fertnstert.2008.02.136. [DOI] [PubMed] [Google Scholar]
- 87.Sharan C, Halder SK, Thota C, Jaleel T, Nair S, Al-Hendy A. Vitamin D inhibits proliferation of human uterine leiomyoma cells via catechol-O-methyltransferase. Fertility and sterility. 2011;95(1):247–53. doi: 10.1016/j.fertnstert.2010.07.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Halder SK, Goodwin JS, Al-Hendy A. 1,25-Dihydroxyvitamin D3 reduces TGF-beta3-induced fibrosis-related gene expression in human uterine leiomyoma cells. The Journal of clinical endocrinology and metabolism. 2011;96(4):E754–62. doi: 10.1210/jc.2010-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wei JJ, Chiriboga L, Arslan AA, Melamed J, Yee H, Mittal K. Ethnic differences in expression of the dysregulated proteins in uterine leiomyomata. Human reproduction. 2006;21(1):57–67. doi: 10.1093/humrep/dei309. [DOI] [PubMed] [Google Scholar]
- 90.Al-Hendy A, Diamond MP, El-Sohemy A, Halder SK. 1,25-Dihydroxyvitamin D3 Regulates Expression of Sex Steroid Receptors in Human Uterine Fibroid Cells. The Journal of clinical endocrinology and metabolism. 2015;100(4):E572–E82. doi: 10.1210/jc.2014-4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Halder SK, Sharan C, Al-Hendy A. 1,25-dihydroxyvitamin D3 treatment shrinks uterine leiomyoma tumors in the Eker rat model. Biology of reproduction. 2012;86(4):116. doi: 10.1095/biolreprod.111.098145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Halder SK, Sharan C, Al-Hendy O, Al-Hendy A. Paricalcitol, a vitamin d receptor activator, inhibits tumor formation in a murine model of uterine fibroids. Reproductive sciences. 2014;21(9):1108–19. doi: 10.1177/1933719114537721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Meems LM, Cannon MV, Mahmud H, Voors AA, van Gilst WH, Sillje HH, Ruifrok WP, de Boer RA. The vitamin D receptor activator paricalcitol prevents fibrosis and diastolic dysfunction in a murine model of pressure overload. J Steroid Biochem Mol Biol. 2012;132(3-5):282–9. doi: 10.1016/j.jsbmb.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 94.Guyton KZ, Kensler TW, Posner GH. Cancer chemoprevention using natural vitamin D and synthetic analogs. Annual review of pharmacology and toxicology. 2001;41:421–42. doi: 10.1146/annurev.pharmtox.41.1.421. [DOI] [PubMed] [Google Scholar]
- 95.Bouillon R, Verstuyf A, Verlinden L, Allewaert K, Branisteanu D, Mathieu C, van Baelen H. Non-hypercalcemic pharmacological aspects of vitamin D analogs. Biochemical pharmacology. 1995;50(5):577–83. doi: 10.1016/0006-2952(95)00121-f. [DOI] [PubMed] [Google Scholar]
- 96.James SY, Mercer E, Brady M, Binderup L, Colston KW. EB1089, a synthetic analogue of vitamin D, induces apoptosis in breast cancer cells in vivo and in vitro. British journal of pharmacology. 1998;125(5):953–62. doi: 10.1038/sj.bjp.0702103. [DOI] [PMC free article] [PubMed] [Google Scholar]


