Abstract
Despite recent advances in the identification of genomic alterations that lead to urothelial oncogenesis in vitro, advanced urothelial carcinomas continue to have poor clinical outcomes. In this review, we focus on targeted therapies that have yielded the most promising results alone or in combination with traditional chemotherapy, including the antiangiogenesis agent bevacizumab, the human epidermal growth factor receptor 2 antibody trastuzumab, and the tyrosine kinase inhibitor cabozantinib. We also describe ongoing and developing clinical trials that utilize innovative approaches, including dose-dense scheduling of singular chemotherapy combinations, prospective screening of tumor tissues for mutational targets and biomarkers to predict chemosensitivity prior to determination of therapeutic regimen, as well as novel agents that target proteins in the immune checkpoint regulation pathway (PD-1 and anti-PD-L1) which have shown significant potential in preclinical models and early clinical trials. New agents and targeted therapies, alone or in combination with traditional chemotherapy, will only be validated through accrual to developing clinical trials that aim to translate these therapies into individualized treatments and improved survival rates in urothelial carcinoma.
Keywords: bladder cancer, urothelial cancer, metastatic, targeted therapy, drug resistance, novel agents, immune checkpoints, clinical trials
Introduction
Urothelial carcinoma is the fifth most common cancer in the United States. Based on SEER data, it is estimated that approximately 75,000 new cases will have been reported in 2014. Due to relatively slow advances in the search for effective treatments, outcomes for patients with muscle-invasive and metastatic urothelial carcinomas are worse than for patients with other types of solid tumors. Fortunately, 70% of urothelial carcinomas are non-muscle invasive, for which local treatments can be effective. However, 15% to 20% of patients with non-muscle invasive disease will progress to muscle-invasive urothelial carcinoma. At diagnosis, 25% to 30% of patients will present with muscle-invasive disease, 25% of which already harbor lymph node metastases not visible on conventional imaging. Moreover, approximately 5% will present with distant metastatic urothelial carcinoma at diagnosis. In patients with locally advanced or metastatic disease, the 5-year survival rate is approximately 15%.1 Currently, the only approved treatments for locally advanced or metastatic disease are cisplatin-based chemotherapy combinations. Although almost 50% of patients respond to cisplatin combined with either gemcitabine (GC) or with methotrexate, vinblastine, and doxorubicin (MVAC), the duration of response is around 7 months.2 Patients who relapse after initial chemotherapy generally have a poor response to subsequent treatments and thus a poor prognosis.3 While there is clearly an urgent need for systemic treatments for metastatic urothelial carcinoma, only a few cytotoxic therapy combinations have been approved by the U.S. Food and Drug Administration (FDA) for first-line treatment, and none has been approved for second-line treatment. Indeed, no new agent has been approved for the treatment of metastatic urothelial carcinoma in the past 30 years. Among the more than 120 FDA-approved anticancer agents, only a small percentage has even been tested in urothelial carcinoma.
Barriers to Development of Effective Therapies for Urothelial Carcinoma
Multiple factors have impeded progress in developing effective treatments for urothelial carcinoma. First of all, many large randomized trials in urothelial carcinoma have closed prematurely due to poor patient accrual, the reasons for which appear to be complex.4–7 A significant number of urothelial cancer patients are cisplatin-ineligible based on a performance status of ≥ 2, reduced creatinine clearance, hearing loss, peripheral neuropathy, and New York Heart Association Class III heart failure.8 While these comorbidities present a challenge when assessing patients for clinical trial eligibility, renal insufficiency is especially significant due to its high prevalence in this patient population. A retrospective analysis found that 24%–50% (depending on the formula used to calculate creatinine clearance) of urothelial cancer patients had a glomerular filtration rate < 60 mL/min/1.73 m2 following cystectomy,9 which, for many trials, would compromise eligibility. On the other hand, although urothelial cancer is a disease of the elderly (median age 73),10 there is no evidence supporting an association between chronological age and greater toxicity with cisplatin-based chemotherapy.8 Furthermore, although lung cancer has a similar age distribution (median 70),11 this fact does not appear to compromise accrual into lung cancer trials and therefore should not be a factor in determining eligibility. Nevertheless, due to poor accrual, investigators usually design small, single-arm, phase II trials whose results are not likely to change the treatment paradigm, as demonstrated by a 2013 analysis of ongoing trials for metastatic urothelial carcinoma.12 Based on these observations, the Bladder Cancer Advocacy Network Clinical Trials Working Group released a report emphasizing the urgent need for communication and collaboration among investigators to overcome this major barrier to developing effective treatments for urothelial carcinoma.12
The Biology of Urothelial Carcinoma
Another obstacle to improved treatments for urothelial carcinoma is a lack of understanding of how this disease develops and progresses. Historically, the lack of effective therapies may also have contributed to poor accrual into clinical trials. In the past decade, investigators have made a tremendous effort to address this issue. The most detailed analysis, published earlier this year, was performed by the Cancer Genome Atlas Research Network.13 In this analysis, 131 samples of muscle-invasive bladder carcinoma were investigated for DNA copy number changes, somatic mutations, messenger RNA and microRNA expression, protein and phosphorylated protein expression, DNA methylation, transcript splice variation, gene fusion, viral integration, pathway perturbation, and clinical correlates in order to reveal the molecular landscape of urothelial carcinoma. The data collected identified several currently targetable genomic changes that are also supported by other research groups as important pathways in urothelial oncogenesis, i.e., the PI3K/AKT/mTOR pathway and RTK/RAS pathways, including HER2, ERBB3, and FGFR3 (Table 1).13–15 The analysis also found alterations in novel pathways like CDKN2A/CDK4/CCND1 and several epigenetic changes where many new targetable agents are being developed.13 Multiple clinical studies in the past decade have tested the efficacy of targeting several of these pathways in urothelial carcinoma, as summarized in comprehensive reviews.14–16 In this review, we highlight the most promising results from trials using targeted agents, report on ongoing clinical trials, and discuss novel trial designs for the treatment of muscle-invasive or metastatic urothelial carcinoma.
Table 1.
The Cancer Genome Atlas Project (TCGA) Most Commonly Mutated Genes in Urothelial Carcinoma Bladder Tumors and Potential Targeted Therapies in Muscle-Invasive or Metastatic Urothelial Carcinoma Currently under Investigation in Clinical Trials
| Gene | TCGA Reported Alteration (%) | Potential Agent | Target | Clinical Trial | Other notes |
|---|---|---|---|---|---|
| TP53 | 49 | ALT- 801 | p53 | NCT01326871 | Phase I/II with either GC or gemcitabine alone in muscle- invasive or metastatic urothelial cancer of bladder |
| KMT2D | 27 | ||||
| ARID1A | 25 | ||||
| KDM6A | 24 | ||||
| PIK3CA | 20 | everolimus | mTOR | NCT01182168 | Phase I first line with gemcitabine and split-dose cisplatin |
| everolimus | mTOR | NCT01215136 | Phase II first line +/− paclitaxel in cisplatin-ineligible patients | ||
| sirolimus | mTOR | NCT01938573 | Phase I/II with gemcitabine and cisplatin prior to cystectomy for muscle-invasive disease | ||
| sirolimus | mTOR | NCT01522820 | Phase I DEC-205-NY-ESO-1 fusion protein vaccine +/− sirolimus in solid tumors expressing NY-ESO-1 | ||
| AZD5363 | PI3K | NCT01226316 | Phase I refractory to standard treatment, PIK3CA or AKT1 mutation or dysregulation of PIK/AKT pathway | ||
| AZD8835 | PI3K | NCT02260661 | Phase I dose study for patients with advanced solid tumors | ||
| BGJ398/BYL719 | FGFR 1/2/3, PI3K | NCT01928459 | Phase Ib of oral BGJ398/BYL719 in solid tumors | ||
| buparlisib (BKM120) | PI3K | NCT01971489 | Phase I of buparlisib, gemcitabine, and cisplatin in patients with solid tumors | ||
| MLN1117 | PI3K | NCT01449370 | Phase I dose-escalation study of oral MLM1117 in patients with advanced cancer | ||
| MEK162 plus BYL719 | MEK1/2 and PI3K | NCT01449058 | Phase Ib of oral MEK162 plus BYL719 in patients with advanced solid tumors | ||
| nilotinib, everolimus, sorafenib, lapatinib, or pazopanib | multiple | NCT02029001 | Phase II adapting treatment to tumor molecular alterations for patients with advanced solid tumors: My Own Specific Treatment (MOST) | ||
| EP300 | 15 | mocetinostat | HDAC | NCT02236195 | Phase II refractory to standard treatment with EP300 or CREBBP mutation and advanced urothelial carcinoma |
| CDKN1A | 14 | ||||
| RB1 | 13 | ||||
| ERCC2 | 12 | ||||
| FGFR3 | 12 | dovitinib | FGFR3 | NCT01831726 | Phase II in patients with solid tumors with mutations or translocations of FGFR, PDGFR, VEGF, cKIT, FLT3, CSFR1, Trk, and RET |
| BGJ398 | FGFR 1/2/3 | NCT01004224 | Phase I advanced solid tumors with FGFR1 or FGFR2 amplification or FGFR3 mutation | ||
| BAY1163877 | FGFR | NCT01976741 | Phase I dose-escalation study in patients with solid tumors | ||
| BGJ398/BYL719 | FGFR 1/2/3, PI3K | NCT01928459 | Phase Ib of oral BGJ398/BYL719 in patients with solid tumors | ||
| Debio 1347 (CH5183284) | FGFR 1/2/3 | NCT01948297 | Phase I dose-escalation study of oral debio 1347 in patients with solid tumors | ||
| FPA144 | FGFR2 | NCT02318329 | Phase I 2-part safety/tolerability and PK study in patients with solid tumors | ||
| GSK3052230 | FGFR1 | NCT01868022 | Phase I GSK3052230 in combination with paclitaxel and carboplatin, or docetaxel or as single agent in patients with solid tumors and deregulated fibroblast growth factor pathway signaling | ||
| BIBF 1120 | VEGFR, FGFR and PDGFR | NCT01349296 | Phase I combined BIBF 1120 and RAD001 in patients with solid tumors | ||
| STAG2 | 11 | ||||
| ERBB3 | 11 | MM-141 | ERBB3, IGF-1R | NCT01733004 | Phase I in patients with solid tumors, designed to block PI3K/Akt/mTOR pathway |
| FBXW7 | 10 | ||||
| RXRA | 9 | ||||
| ELF3 | 8 | ||||
| NFE2L2 | 8 | ||||
| TSC1 | 8 | everolimus | mTOR | NCT02201212 | Phase II all advanced solid tumors with documented TSC1 or TSC2 mutation |
| everolimus | mTOR | NCT01182168 | Phase I first line with gemcitabine and split-dose cisplatin | ||
| everolimus | mTOR | NCT01215136 | Phase II first line +/− paclitaxel in cisplatin-ineligible patients | ||
| sirolimus | mTOR | NCT01938573 | Phase I/II with gemcitabine and cisplatin; prior to cystectomy for muscle-invasive disease | ||
| sirolimus | mTOR | NCT01522820 | Phase I DEC-205-NY-ESO-1 fusion protein vaccine +/− sirolimus in solid tumors expressing NY-ESO-1 | ||
| KLF5 | 8 | ||||
| TXNIP | 7 | ||||
| FOXQ1 | 5 | ||||
| CDKN2A | 5 | LEE011 | CDK4/6, cyclin D 1/3, p16 | NCT0218773 | Phase II oral LEE011 in patients with advanced solid tumors with CDK4 or CDK6 amplification or mutation, cyclin D1 or D3 amplification, CDKN2A mutation (SIGNATURE) |
| RHOB | 5 | ||||
| PAIP1 | 5 | ||||
| FOXA1 | 5 | ||||
| BTG2 | 5 | ||||
| HRAS | 5 | ||||
| ZFP36L1 | 5 | ||||
| RHOA | 4 | ||||
| CCND3 | 4 | LEE011 | CDK4/6, cyclin D 1/3, p16 | NCT0218773 | Phase II oral LEE011 in patients with advanced solid tumors with CDK4 or CDK6 amplification or mutation, cyclin D1 or D3 amplification, CDKN2A mutation (SIGNATURE) |
Abbreviations: AKT1, v-akt murine thymoma viral oncogene homolog 1; ARID1A, AT Rich Interactive Domain 1A; BCG, Bacille Calmette-Guerin vaccine; BTG2, B cell translocation gene family, member 2; CCND3, cyclin D3; CDKN1A, cyclin-dependent kinase inhibitor 1A; CDKN2A, cyclin-dependent kinase inhibitor 2A; CREBBP, CREB binding protein; ELF3, E74-like factor 3 (ets domain transcription factor, epithelial-specific; EP300, E1A binding protein p300; ERBB3, v-erb-b2 avian erythroblastic leukemia viral oncogene homolog 3; ERCC2, excision repair cross-complementation group 2; FBXW7, F-box and WD repeat domain containing 7, E3 ubiquitin protein ligase; FGFR3, fibroblast growth factor receptor 3; FOXA1, forkhead box A1; FOXQ1, forkhead box Q1; HRAS, Harvey rat sarcoma viral oncogene homolog; IGF-1R, insulin-like growth factor I receptor; KDM6A, lysine (K)-specific demethylase 6A; KLF5, Kruppel-like factor 5; KMT2D, lysine (K)-specific methyltransferase 2D; mTOR, mechanistic target of rapamycin (serine/threonine kinase); NFE2L2, nuclear factor, erythroid 2-like 2; PAIP1, poly(A) binding protein interacting protein; PI3K, phosphatidylinositide 3-kinase; PI3KCA, phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit alpha; PIK, phosphoinositide 3-kinase; RB1, retinoblastoma 1; RHOA, ras homolog family member A; RHOB, ras homolog family member B; RXRA, retinoid X receptor, alpha; STAG2, stromal antigen 2; TP53, tumor protein p53; TSC1, tuberous sclerosis 1; TSC2, tuberous sclerosis 2; TXNIP, thioredoxin interacting protein; ZFP36L1, ZFP36 ring finger protein-like 1.
Increasing the Efficacy of Cytotoxic Treatments for Urothelial Carcinoma
First-Line Therapy and Mechanisms of Resistance
Cisplatin, the backbone of combination chemotherapy for urothelial carcinoma, acts by forming inter- and intra-strand crosslinks in DNA, resulting in DNA damage and consequent cell death.17 Preclinical studies have identified the mechanisms of resistance to cisplatin as decreased influx or increased efflux of drug, glutathione or metallothionein conjugation, drug detoxification, and DNA repair.17 Although several key players underlying resistance to cisplatin have been identified,17–19 none of these discoveries has led to a therapeutic application. On the other hand, genes associated with chemoresistance may potentially become biomarkers for predicting treatment response. For example, somatic mutations of ERCC2, a gene involved in the nucleotide excision repair pathway, have been shown to correlate with cisplatin sensitivity,20 while activating missense mutations of ERBB2 are significantly more prevalent in tumor tissue from complete responders to neoadjuvant chemotherapy.21
While investigating clinical applications for the cellular mechanisms underlying cisplatin resistance, researchers have attempted to increase the efficacy of currently approved cisplatin-based combinations. The Gompertzian kinetics of tumor growth posit a negative correlation between tumor growth and tumor size,22 suggesting that administering cytotoxic chemotherapy in shorter intervals could maximize its effect by attacking the tumor while it is small and fast-growing. Dose-dense scheduling is supported with growth factors to increase the tolerability of this potentially toxic regimen. A randomized phase III trial compared a traditional MVAC regimen to dose-dense MVAC with growth factor support.23 Response rates with the dose-dense regimen improved (from 50% to 64%) along with tolerability; however, these positive outcomes did not culminate in a survival benefit. In another phase III trial, dose-dense GC was found to be equivalent to dose-dense MVAC in terms of survival, was associated with fewer instances of neutropenic fever, and was better tolerated.7 However, it should be noted that this trial randomized fewer patients than anticipated due to poor accrual and administrative issues.7
Neoadjuvant Therapy
To capitalize on their efficacy and tolerability, dose-dense regimens were also investigated in the neoadjuvant setting, where platinum-based treatment of resectable urothelial carcinoma has shown a survival benefit.24–26 It was recently reported that when 4 cycles of neoadjuvant dose-dense MVAC was given to patients, 43% of whom were lymph node-positive, 50% had down-staging to less than muscle-invasive disease at the time of surgery, and the treatment was well-tolerated.27 In another study, 3 cycles of the same neoadjuvant regimen resulted in down-staging in 52% of patients,28 demonstrating the lack of consensus for how many cycles of neoadjuvant therapy are adequate to achieve maximal therapeutic efficacy. Nevertheless, based on these promising findings with dose-dense MVAC, 2 phase II trials investigating dose-dense GC in the neoadjuvant setting (NCT01589094, NCT01611662) were initiated; however, one trial closed early due to high vascular toxicity with the dose-dense schedule.29
Two other trials have investigated the efficacy of novel platinum-based chemotherapy combinations prior to cystectomy. In a study by Siefker-Radtke et al. on the efficacy of sequential neoadjuvant ifosfamide-based chemotherapy (ifosfamide/doxorubicin/gemcitabine followed by cisplatin/gemcitabine/ifosfamide) in patients at high risk of noncurative cystectomy, 50% of patients obtained pathologic down-staging to ≤ pT1N0 at time of cystectomy.30 A phase II trial of neoadjuvant paclitaxel, carboplatin, and gemcitabine in patients with locally advanced bladder carcinoma demonstrated moderate efficacy but greater-than-anticipated toxicity.31 An ongoing phase II clinical trial (NCT01616875) is studying the efficacy of neoadjuvant cisplatin combined with cabazitaxel.
Second-Line Therapy
Chemotherapy has shown less promising results as second-line treatment for metastatic urothelial carcinoma. Numerous trials have investigated various cytotoxic treatments as monotherapies, with response rates ranging from 5% to 27% and no significant survival benefit.32 For example, a phase II study of nanoparticle albumin-bound (nab) paclitaxel reported a 27.7% overall response rate in previously treated patients,33 and an ongoing phase III trial (NCT02033993) is investigating nab paclitaxel vs. paclitaxel in patients with advanced urothelial carcinoma that has progressed on or after a platinum-based regimen. In Europe, vinflunine is approved as a second-line treatment following platinum-based chemotherapy, based on the results of a phase III study in which median overall survival (OS) showed only modest improvement compared to best supportive care (4.3 vs. 6.9 months, p = NS).34 Given this significant but suboptimal survival benefit, there is an urgent need for better agents in second-line treatment. On the other hand, we agree with previous commentators that second-line phase III trials should be delayed until therapies are discovered that significantly affect outcomes for untreated metastatic disease.3 Currently, only a few phase II trials are investigating the activity of novel cytotoxic agents (eribulin, amrubicin, cabazitaxel, and tesetaxel) in urothelial carcinoma that has relapsed after platinum-based therapy (NCT00365157, NCT01331824, NCT01437488, and NCT01215877, respectively). In the phase II clinical trials of cabazitaxel and tesetaxel, enrollment closed early based on lack of efficacy in the first stage of patients enrolled. The amrubicin study was closed due to loss of sponsorship.
Platinum-based chemotherapy has clearly advanced the treatment of urothelial carcinoma since its development in the 1980s. Several platinum-based combinations and novel cytotoxic agents have been investigated in both the first- and second-line settings. However, these attempts have not significantly improved outcomes for patients with metastatic disease, and therefore the focus of investigation has shifted from chemotherapy to targeted therapies given either in combination with cytotoxic agents or as single agents. The following is a review of clinical trials of targeted therapies that have yielded the most promising results.
The Most Promising Targeted Therapies for Urothelial Carcinoma
Antiangiogenic Agents
In numerous clinical trials in urothelial carcinoma, a few targeted therapies, given either with chemotherapy or as a single agent, have shown higher-than-expected activity and are currently undergoing further evaluation. The most promising result was seen in a phase II trial in which bevacizumab, a monoclonal antibody to vascular endothelial growth factor (VEGF), was combined with GC as first-line treatment in metastatic urothelial carcinoma. This study reported a 72% overall response rate, with a median OS of 19.1 months.35 In another phase II study in untreated cisplatin-ineligible patients with metastatic disease, whose expected survival was approximately 9 months, bevacizumab combined with gemcitabine and carboplatin led to a 63% response rate and OS of 13.9 months.36 Both of these studies showed better results than might be expected compared to historical controls. Bevacizumab has also been investigated in the neoadjuvant setting. In 2 phase II trials, it was combined with either GC or dose-dense MVAC, resulting in a 31% and 53% pathological response, respectively, of < T2.37,38 Based on these encouraging results, a phase III trial of GC with and without bevacizumab as first-line treatment in the metastatic setting and a phase II trial of bevacizumab with GC as neoadjuvant therapy followed by adjuvant paclitaxel have completed accrual (NCT00942331 and NCT00268450, respectively). Results of these studies are pending.
Although targeting angiogenesis via VEGF is a promising strategy in urothelial carcinoma, results with tyrosine kinase inhibitors that target VEGF receptors (VEGFR) have not been encouraging. Sunitinib (which targets VEGFR-1, -2, and -3 in addition to c-KIT, platelet-derived growth factor receptor (PDGFR)-alpha and -beta, Flt3, and RET) was given as a single-agent second-line therapy on 2 different dosing schedules. Partial response was seen in 5% of patients, with OS reported as 6.9 months.39 The treatment was not well tolerated; 74% of patients experienced grade 3/4 toxicities, with lymphopenia, thrombocytopenia, anemia, fatigue, and nausea being the most common adverse events.39 Moreover, when sunitinib was given as first-line treatment to patients who were cisplatin-ineligible due to renal impairment, an 8% response rate and 8.1-month OS were reported.40 While grade 3/4 toxicities were fewer compared to the second-line setting, 2 of 38 patients died (one from myocardial infarction and one from stroke), possibly due to sunitinib-related adverse events.40 Similarly, in trials in which sunitinib was combined with GC for either first-line metastatic or neoadjuvant treatment, intolerability was a major issue.41,42 Finally, sunitinib given as maintenance therapy in a phase II trial in patients who achieved stable disease or partial/complete response after 4 to 6 cycles of chemotherapy did not improve 6-month progression-free survival (PFS) compared to placebo.43 Due to these inauspicious results, there are no ongoing trials of sunitinib in metastatic urothelial carcinoma.
Other antiangiogenic agents have been investigated for efficacy in urothelial carcinoma. Sorafenib, which targets VEGFR-2 and -3 as well as B-Raf, c-Raf, and PDGFR-alpha and -beta, achieved no response either as first-line treatment for cisplatin-ineligible patients or as single-agent second-line treatment.44,45 These studies led researchers to conclude that sorafenib has little or no activity in urothelial carcinoma. A single-arm phase II trial investigating the combination of sorafenib with GC as neoadjuvant therapy in muscle-invasive bladder cancer has completed accrual and results are pending (NCT01222676). Similarly, in a trial of pazopanib, an antiangiogenic agent that targets VEGFR-1, -2, and -3, PDGFR-alpha and -beta, and c-kit, the drug effected no response when given as a single agent in second-line treatment46 and led to a 17% response rate in another trial, with a median OS of only 4.7 months.47 A trial of pazopanib in combination with gemcitabine as first-line treatment in cisplatin-ineligible patients (NCT01622660) closed due to hepatotoxicity. A trial of pazopanib in combination with vinflunine as second-line treatment was discontinued at the first dose level for safety reasons.48
While small molecule inhibitors of VEGFR have so far shown limited efficacy, a 3-arm randomized phase II study of recently developed monoclonal antibodies targeting VEGFR-1 (icrucumab) and -2 (ramucirumab) in combination with docetaxel in patients who progressed after first-line therapy (NCT01282463) has completed accrual. It will be interesting to compare the results of small molecule inhibitors to those of monoclonal antibodies, since the latter can lead to antibody-dependent cytotoxicity in addition to blocking VEGFR.
Results from all trials using antiangiogenic agents in either the first- or second-line setting are mixed. It appears that selectively targeting the VEGF pathway as sole therapy has limited activity, while combining this approach with chemotherapy is a feasible option, despite increased toxicity with selective agents. Data from the phase III trial of bevacizumab combined with chemotherapy (NCT00942331) will shed further light on this question. Disappointingly, small molecule inhibitors that target multiple pathways, including VEGF, have shown minimal clinical benefit in an unselected population. However, it is important to note that preclinical studies continue to identify novel pathways that underlie the oncogenesis of urothelial carcinomas. Thus, agents that target these pathways together with VEGF may show efficacy. For example, cabozantinib inhibits multiple tyrosine kinases, primarily targeting VEGFR-2 and c-met, which may be potential targets in urothelial carcinomas.49 Cabozantinib is currently being tested in a phase II trial as second-line treatment of metastatic urothelial carcinoma (NCT01688999).
Anti-EGFR Therapy
Epidermal growth factor receptor (EGFR) overexpression has been linked to progression of muscle-invasive urothelial carcinoma. In preclinical models, down-regulating EGFR with targeted antibody agents inhibited cell growth and angiogenesis.50–52 When 20 patients with muscle-invasive disease were treated with erlotinib prior to cystectomy, 60% were down-staged to ≤ pT1, suggesting single-agent activity with EGFR inhibitors.53 However, gefitinib administered as a single agent in second-line treatment produced only a 3% response rate and 3-month OS.54 Moreover, gefitinib in combination with GC, given either conventionally55 or at a fixed-dose rate,56 did not improve either response rate or OS in patients with untreated metastatic urothelial carcinoma compared to historical controls. Similarly, the combination of GC chemotherapy and cetuximab, a monoclonal antibody targeting EGFR, was intolerable, and both PFS and median OS showed trends toward a worse outcome compared to chemotherapy alone.57 A randomized phase II trial of another EGFR-specific monoclonal antibody, panitumumab, in combination with GC compared with chemotherapy alone as first-line treatment was terminated early due to insufficient recruitment (NCT01374789). Another randomized phase II trial in patients with previously treated metastatic disease reported a 25% response rate for cetuximab combined with paclitaxel, but no activity for cetuximab as a single agent.58 These data support preclinical data showing synergism between anti-EGFR monoclonal antibodies and taxanes.59 Overall, it appears that in unselected patients, targeting EGFR has limited efficacy. Therefore, it is imperative to identify biological markers, such as EGFR overexpression, that can identify patients most likely to be susceptible to anti-EGFR treatment.
The oral tyrosine kinase inhibitor vandetanib was studied in the second-line setting in combination with docetaxel in a phase II double-blind trial that reported no significantly improved overall response rate, PFS, or OS in patients with advanced urothelial carcinoma.60
The significance of testing targeted therapies in select populations was demonstrated in a single-arm phase II study of the safety and efficacy of the antihuman EGFR-2 (HER2) antibody trastuzumab combined with gemcitabine, carboplatin, and paclitaxel in patients who had HER2 overexpression by immunohistochemistry, gene amplification, and/or elevated serum HER2.61 Patient outcomes exceeded expectations, with an objective response rate of 70% and an OS of 14.1 months.61 Although the incidence of grade 1 to 3 cardiac toxicity (22.7%) was more frequent than anticipated, the results were sufficiently encouraging to prompt further testing. A randomized phase II trial compared trastuzumab (NCT01828736) with GC or gemcitabine and carboplatin vs. GC or gemcitabine and carboplatin alone in HER2-expressing bladder cancer.62 This study screened 563 patients with advanced urothelial carcinoma over 5 years and found only 75 patients (13.3%) with HER2-positive tumors (immunohistochemistry 2 or 3+ confirmed by fluorescence in situ hybridization). No difference was observed in overall response rate, PFS, or OS between the chemotherapy-alone arm and the chemotherapy-plus-trastuzumab arm. The authors did find that HER2 levels were predictive of PFS regardless of treatment. A study investigating trastuzumab in combination with standard GC chemotherapy in the first-line setting closed enrollment early (NCT02006667). Another study investigating trastuzumab as a single agent in the second-line setting (NCT02013765) closed early due to recruitment difficulties. Studies of ado-trastuzumab emtansine (TDM-1), a potentially more powerful drug, may be warranted in this disease.
Both EGFR and HER2 appear to play an important role in the pathogenesis of urothelial carcinoma, and therefore are still promising targets for treatment. Lapatinib, an agent that has selective activity against both EGFR and HER2, was hypothesized to have significant clinical activity. Although lapatinib had only a 1.7% response rate as a single agent in unselected, platinum-refractory patients with metastatic urothelial carcinoma, it led to a marked difference in OS in EGFR/HER2low patients compared to EGFR and/or HER2high patients.63 This subgroup analysis reinforces the concept of selecting appropriate patients for targeted therapies. The results of both a recently completed phase I trial that combined lapatinib with GC (NCT00623064) and a completed phase II/III trial (n = 223) comparing maintenance lapatinib to placebo following first-line chemotherapy (LAMB trial: NCT00949455) in patients expressing either EGFR or HER2 will shed more light on the role of dual-targeting these growth pathways in urothelial carcinoma. Results are expected in 2015.
Anti-PI3K/AKT/mTOR Therapy
An integrated analysis of urothelial carcinoma by the Cancer Genome Atlas Research Network demonstrated that 42% of tumors had mutations, copy number alterations, or RNA expression changes in the PI3K/AKT/mTOR pathway.13 Genomic changes included PI3K-alpha point mutations (17% of patients), mutation or deletion of tuberous sclerosis complex (TSC)-1 or TSC-2 (9%), and overexpression of AKT3 (10%). Other studies have reported inactivating mutations of PTEN, which result in activation of the PI3K pathway, in 30% of muscle-invasive urothelial carcinomas.64 Thus, targeting this pathway for the treatment of urothelial carcinoma is feasible. mTOR inhibition in urothelial carcinoma has thus far had disappointing results. The mTOR inhibitor everolimus, given as a single agent, produced only a 5% response rate in 2 trials in the second-line setting.65,66 Interestingly, in the second trial one patient with inactivating TSC-1 mutation had significant tumor shrinkage and a durable response, further highlighting the importance of patient selection in the use of targeted therapies.67
Currently, 3 clinical trials are testing mTOR inhibitors in unselected patient populations. A phase II trial that combined temsirolimus with GC in untreated metastatic urothelial carcinoma was recently completed (NCT01090466), and another trial of everolimus and gemcitabine with split-dose cisplatin in the same setting is ongoing (NCT01182168). In addition, everolimus plus paclitaxel is being tested as first-line therapy in cisplatin-ineligible patients (NCT01215136). Although these trials are in unselected patients, correlative studies should broaden our understanding of which subsets of patients are sensitive to mTOR inhibition. Finally, phase I trials of PI3K inhibitors, including BKM-120, an oral pan-PI3K inhibitor,68 and GSK1216458, an oral pan-PI3K, mTORC-1 and -2 inhibitor,69 will increase the clinical availability of PI3K inhibitors. BKM-120 is currently being tested as a second-line therapy in metastatic urothelial carcinoma (NCT01551030).
Checkpoint Inhibitors
Targeting immune checkpoints as a means of antitumor therapy has shown survival benefit in a variety of solid tumors.70–72 Ipilimumab is a first-in-class monoclonal antibody that targets cytotoxic T lymphocyte-associated protein 4 (CTLA-4), a potent immune checkpoint molecule that down-regulates T-cell activation after binding to antigen-presenting cells. Ipilimumab has demonstrated clinical activity and improved clinical outcomes in patients with metastatic melanoma.70 Programmed cell death protein 1 (PD-1) is another negative regulatory molecule expressed transiently following T-cell activation and on chronically stimulated T cells characterized by an “exhausted” phenotype. PD-1 and CTLA-4 are primarily expressed on T cells. PD ligand 1 (PD-L1) is expressed primarily on tumor cells, where it binds to PD-1 on T cells, effectively curtailing immune response by down-regulating T-cell recognition of tumor cells as non-self.
In a recent phase I trial of MPL3280A (a PD-L1 inhibitor), a 50% response rate was seen in patients with advanced/refractory bladder cancer whose tumor-infiltrating cells had high expression of PD-L1 by immunohistochemistry,73 suggesting that immune checkpoint inhibitors may have activity in advanced urothelial carcinoma. Responses were prolonged and grade 3 or 4 adverse events were uncommon. The drug received breakthrough designation status by the FDA in June 2014.74 This has led to a large phase II study (n = 400) in locally advanced/metastatic urothelial carcinoma patients who have previously failed platinum-based therapy or are chemotherapy-naive but unfit for cisplatin (NCT02108652). Also, a global randomized phase III trial of MPDL3280A vs. chemotherapy in patients who have previously failed chemotherapy has initiated enrollment (NCT02302807).
A recently reported study combined nivolumab, a PD-1 inhibitor, with ipilimumab, both of which target nonredundant immunoregulatory pathways, in patients with advanced melanoma.72 The response rate for concurrent administration was 40% at initial dose and 53% at maximum doses, while single-agent activity was approximately 20%. Following these results, a phase I/II study of nivolumab combined with ipilimumab has also initiated enrollment in patients with advanced or metastatic solid tumors, including bladder cancer (NCT01928394). A study of pembrolizumab, another antibody that blocks both PD-L1 and PD-L2, in urothelial cancer patients, 52% of whom had undergone ≥ 2 prior therapies, showed a 24.1% overall response rate.75 A phase II trial not yet open for recruitment will further study the efficacy of pembrolizumab in advanced/metastatic urothelial cancer patients who have not received any prior systemic therapy (unless it has been > 12 months since they completed neoadjuvant and adjuvant platinum-based chemotherapy) and are ineligible for cisplatin-based therapy (NCT02335424). Finally, a phase III trial is comparing pembrolizumab to paclitaxel, docetaxel, or vinflunine in patients who progressed following platinum-based therapy (NCT022456436).
In the phase II trial testing the novel VEGFR-2/c-met inhibitor cabozantinib in advanced bladder cancer, expression of PD-1 in regulatory T cells increased after 2 cycles of continuous treatment.76 A phase I trial is in development at the NCI CCR in which continuous cabozantinib will be administered with nivolumab or nivolumab combined with ipilimumab to patients with metastatic urothelial carcinoma and other genitourinary tumors who have failed at least one prior cytotoxic regimen (NCT02308943).
Additionally, a randomized phase II trial of maintenance PD-1 inhibition vs. placebo after first-line chemotherapy in patients with metastatic urothelial carcinoma is in development by the Alliance for Clinical Trials in Oncology. A seamless adaptive design77 has been proposed to select a patient population (full population vs. PD-1/PD-L1-positive subpopulation) after interim analysis, and also to provide a formal mechanism for performing statistical tests in both the full population and the biomarker-defined subpopulation. This novel adaptive design was developed to accommodate uncertainty as to whether PD-1/PD-L1 inhibitors are effective only in the PD-1/PD-L1-positive subpopulation or in the full patient population as well.
Novel Trial Designs in Urothelial Carcinoma
Results from the clinical trials in advanced urothelial carcinoma summarized above demonstrate that targeted molecular therapies may only be effective in appropriately selected patients. Therefore, in this era of individualized medicine, novel biomarker-based designs should be recommended for clinical trials in advanced urothelial carcinoma. It is equally important to develop the accompanying predictive biomarker for a targeted molecular therapy. Depending on the level of evidence, a predictive biomarker can be used for screening and treatment selection, or may be further validated or developed in a biomarker-based clinical trial design. Several clinical trials in metastatic urothelial carcinoma are being designed to exploit the potential of predictive biomarkers. Following is a summary of trials under development but not yet finalized for registration to the clinical trial database.
MATCH-UP (Molecular Allocation Trial to CHoose therapy for metastatic Urothelial carcinoma following Platinum-based chemotherapy) is a phase II trial designed to prospectively screen tumor tissues for molecular mutations through FoundationOne or local laboratories certified by the Clinical Laboratory Improvement Amendments (CLIA) and the College of American Pathologists to identify targetable molecular changes in patients with metastatic urothelial carcinoma previously treated with at least one platinum-containing regimen. MATCH-UP will be conducted by the Alliance for Clinical Trials in Oncology, and therefore will be a multicenter study. Based on data from the Cancer Genome Atlas Research Network13 and previous experience with molecular targeted agents (summarized above), this trial will initially focus on several of the most promising targets: FGFR3 fusion/mutation/amplification, Rb1 mutation, PIK3CA mutation, AKT1 mutation/amplification, mTOR mutation, TSC1 deletion/mutation, PTEN deletion/mutation, ERBB2 mutation/fusion/amplification, EGFR amplification, and histone acetyltransferase mutation (Figure 1). The trial arms will run parallel with a 2-stage design, so that if an arm fails, mutation selection criteria will be re-evaluated and an arm will potentially be re-opened or added. The co-primary endpoints are PFS and overall response rate. The study is based on historical data suggesting 40% of patients are progression-free and alive at 3 months with second-line chemotherapy, and a 15% overall response rate for platinum-resistant urothelial carcinoma treated with second-line chemotherapy. To be considered successful, an arm must demonstrate a 20% improvement in these characteristics (i.e., 60% progression-free and alive at 3 months, and/or 35% overall response rate). Using a phase II generalized enrichment design, 53 patients will be accrued to each arm, allowing for approximately 5% type 1 error and 84% power. Investigators have obtained approval from sponsors and the protocol is currently in development.
Figure 1.
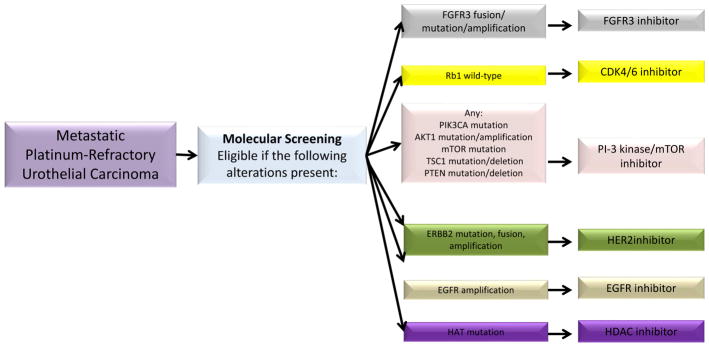
Schematic of chosen therapy for patients on MATCH-UP trial. Genetic testing of tumor sample is utilized to match patients to treatments that target tumor molecular abnormalities.
Abbreviations: PTEN, phosphatase and tensin homolog; HAT, histone acetyltransferase-like protein; CDK 4/6, cyclin-dependent kinase 4/6; HER2, human epidermal growth factor receptor 2; HDAC, histone deacetylase.
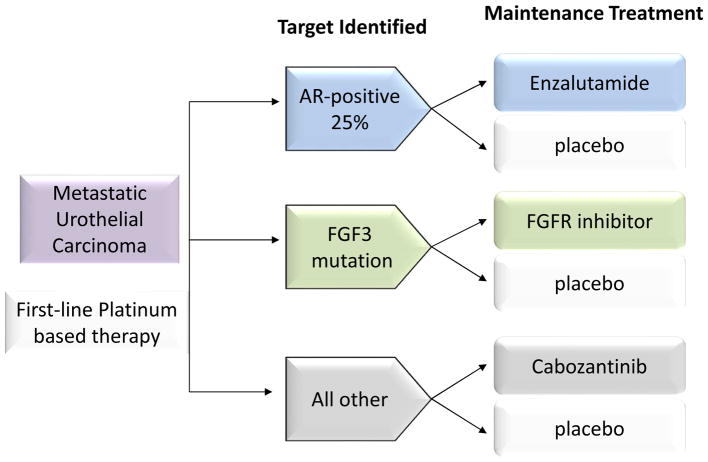
ATLANTIS, a multiarm randomized trial with an adaptive design, will start in 2015 in 40 sites in the United Kingdom. The aim is to test a number of different agents against placebo in the maintenance setting after completion of first-line chemotherapy. Tissue samples from urothelial tumors will be screened for expression of proteins (androgen receptor [AR]) and mutation (FGFR-3) during first-line chemotherapy (4 to 8 cycles of platinum-containing regimens). This trial will ultimately have at least 3 randomized arms in which the targeted therapy will be compared to placebo (Figure 2). The first arm will consist of patients whose tumors express AR, which is found in 25% to 30% of bladder tumors. Preclinical studies report that this pathway plays a role in the development and progression of urothelial carcinoma.78 In this arm, AR-positive patients will receive enzalutamide (an AR inhibitor) or placebo. The second arm will have patients whose tumors contain the FGFR3 mutation, which is present in 17% of urothelial carcinomas.13 These patients will receive an FGF3 inhibitor or placebo. The third arm will compare the VEGFR-2/c-met inhibitor cabozantinib to placebo. The statistical design will be based on results of the recently completed phase II/III lapatinib maintenance study (LAMB trial: NCT00949455), which will estimate PFS in control groups. Future arms are planned that will test immune checkpoint inhibitors and PI3K pathway inhibitors in specific subgroups.
Figure 2.
Randomization of patients to treatment arms in ATLANTIS trial. Tumor tissue is prospectively screened for clinically relevant biomarkers to identify patients who could potentially benefit from maintenance therapy following 4 to 8 cycles of first-line chemotherapy.
Whereas the specifics of MATCH-UP and ATLANTIS differ (single-arm 2-stage trial vs. randomized clinical trial with control), both clinical trials are exploiting clinical knowledge of genomic biomarkers for targeted therapies using the enrichment design.
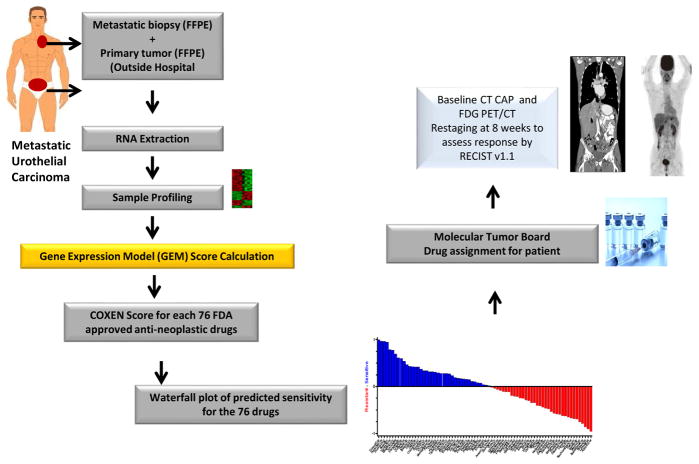
A joint study by the University of Colorado Comprehensive Cancer Center and the National Cancer Institute’s (NCI) Center for Cancer Research (CCR) in Bethesda, Maryland will investigate an algorithm for predicting chemosensitivity to a number of FDA-approved anticancer agents. The COXEN (CO-eXpression ExtrapolatioN) algorithm79–81 uses molecular profiles as a “Rosetta Stone” for translating the drug-sensitivity signatures of one set of cancers into those of another. COXEN’s ability to predict drug effectiveness in patients based solely on in vitro assays is unique and allows an a priori analysis of urothelial tumor responsiveness to anticancer agents. The study will prospectively test the hypothesis that the use of COXEN scores to select “best next therapy” for individual patients with platinum-refractory metastatic bladder cancer will provide superior response rates, PFS, and OS compared to historical controls (Figure 3). This is another example of employing a generalized enrichment design based on a predictive algorithm (COXEN score) to choose among multiple treatments rather than a single treatment. The primary endpoint of the pilot study is the feasibility of using the COXEN-assigned best next therapy to make real-time treatment decisions (within 3 weeks) for patients with advanced urothelial carcinoma. Secondary endpoints include PFS, response rate, and OS. The COXEN algorithm is also being investigated for its ability to predict response to chemotherapy in the neoadjuvant setting. A trial conducted by the Southwest Oncology Group (NCT02177695) is exploring the association between COXEN score and response to either GC or ddMVAC neoadjuvant chemotherapy. Thus, the COXEN algorithm may have a role in urothelial cancer in both the perioperative and advanced/metastatic settings.
Figure 3.
Study design of COXEN algorithm to investigate tumor tissue biomarkers as a means to predict chemosensitivity and select “best next therapy” for individual patients with platinum-refractory metastatic bladder cancer.
Conclusion
De novo metastatic bladder cancer and visceral metastatic disease after local treatment are incurable with currently available therapeutic modalities. At present there is no standard chemotherapy for patients with metastatic bladder cancer who have progressed on first-line treatment. A variety of cytotoxic agents used in this second-line, or salvage, setting have demonstrated response rates of approximately 5% to 20%, indicating that only a subset of patients with bladder cancer will benefit from these treatments. Despite the high incidence rate and lethality of urothelial carcinoma, only a small number of the more than 120 FDA-approved anticancer agents have been tested for efficacy in this disease, and an even smaller subset of those tested have shown some degree of clinical benefit. Furthermore, no molecular targeted agents have been approved for treatment of urothelial carcinoma, highlighting the urgent need for effective therapies, genomic predictors of chemosensitivity, and treatment modalities targeting immune checkpoints being tested in biomarker-based clinical trials. Despite the challenges, breakthroughs in detecting genomic alterations, the mechanisms of resistance to standard therapies, and the ability to exploit immune checkpoints should soon translate into individualized therapies and improved survival rates in urothelial carcinoma.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.von der Maase H, Sengelov L, Roberts JT, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23:4602–8. doi: 10.1200/JCO.2005.07.757. [DOI] [PubMed] [Google Scholar]
- 2.von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18:3068–77. doi: 10.1200/JCO.2000.18.17.3068. [DOI] [PubMed] [Google Scholar]
- 3.Dreicer R. Second-line chemotherapy for advanced urothelial cancer: because we should or because we can? J Clin Oncol. 2009;27:4444–5. doi: 10.1200/JCO.2009.23.8071. [DOI] [PubMed] [Google Scholar]
- 4.Stadler WM, Lerner SP, Groshen S, et al. Phase III study of molecularly targeted adjuvant therapy in locally advanced urothelial cancer of the bladder based on p53 status. J Clin Oncol. 2011;29:3443–9. doi: 10.1200/JCO.2010.34.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cognetti F, Ruggeri EM, Felici A, et al. Adjuvant chemotherapy with cisplatin and gemcitabine versus chemotherapy at relapse in patients with muscle-invasive bladder cancer submitted to radical cystectomy: an Italian, multicenter, randomized phase III trial. Ann Oncol. 2012;23:695–700. doi: 10.1093/annonc/mdr354. [DOI] [PubMed] [Google Scholar]
- 6.Sternberg CN, Skoneczna I, Kerst J. Final results of EORTC intergroup randomized phase III trial comparing immediate versus deferred chemotherapy after radical cystectomy in patients with pT3T4 and/or N+ M0 transitional cell carcinoma (TCC) of the bladder. J Clin Oncol. 2014;32(5S):abstr 4500. [Google Scholar]
- 7.Bamias A, Dafni U, Karadimou A, et al. Prospective, open-label, randomized, phase III study of two dose-dense regimens MVAC versus gemcitabine/cisplatin in patients with inoperable, metastatic or relapsed urothelial cancer: a Hellenic Cooperative Oncology Group study (HE 16/03) Ann Oncol. 2013;24:1011–7. doi: 10.1093/annonc/mds583. [DOI] [PubMed] [Google Scholar]
- 8.Galsky MD, Hahn NM, Rosenberg J, et al. Treatment of patients with metastatic urothelial cancer “unfit” for Cisplatin-based chemotherapy. J Clin Oncol. 2011;29:2432–8. doi: 10.1200/JCO.2011.34.8433. [DOI] [PubMed] [Google Scholar]
- 9.Dash A, Galsky MD, Vickers AJ, et al. Impact of renal impairment on eligibility for adjuvant cisplatin-based chemotherapy in patients with urothelial carcinoma of the bladder. Cancer. 2006;107:506–13. doi: 10.1002/cncr.22031. [DOI] [PubMed] [Google Scholar]
- 10.National Cancer Institute Surveillance, Epidemiology, and End Results Program. SEER Stat Fact Sheets: Bladder Cancer. 2015 Feb; Available from: http://seer.cancer.gov/statfacts/html/urinb.html.
- 11.National Cancer Institute Surveillance, Epidemiology, and End Results Program. SEER Stat Fact Sheets: Lung and Bronchus Cancer. 2015 Feb; Available from: http://seer.cancer.gov/statfacts/html/lungb.html.
- 12.Galsky MD, Hendricks R, Svatek R, et al. Critical analysis of contemporary clinical research in muscle-invasive and metastatic urothelial cancer: a report from the Bladder Cancer Advocacy Network Clinical Trials Working Group. Cancer. 2013;119:1994–8. doi: 10.1002/cncr.27973. [DOI] [PubMed] [Google Scholar]
- 13.Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507:315–22. doi: 10.1038/nature12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghosh M, Brancato SJ, Agarwal PK, et al. Targeted therapies in urothelial carcinoma. Curr Opin Oncol. 2014;26:305–20. doi: 10.1097/CCO.0000000000000064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bellmunt J, Teh BT, Tortora G, et al. Molecular targets on the horizon for kidney and urothelial cancer. Nat Rev Clin Oncol. 2013;10:557–70. doi: 10.1038/nrclinonc.2013.155. [DOI] [PubMed] [Google Scholar]
- 16.Bambury RM, Rosenberg JE. Actionable mutations in muscle-invasive bladder cancer. Curr Opin Urol. 2013;23:472–8. doi: 10.1097/MOU.0b013e328363a3cd. [DOI] [PubMed] [Google Scholar]
- 17.Rabik CA, Dolan ME. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat Rev. 2007;33:9–23. doi: 10.1016/j.ctrv.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265–79. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]
- 19.Galluzzi L, Senovilla L, Vitale I, et al. Molecular mechanisms of cisplatin resistance. Oncogene. 2012;31:1869–83. doi: 10.1038/onc.2011.384. [DOI] [PubMed] [Google Scholar]
- 20.Van Allen EM, Mouw KW, Kim P, et al. Somatic ERCC2 Mutations Correlate with Cisplatin Sensitivity in Muscle-Invasive Urothelial Carcinoma. Cancer Discov. 2014;4:1140–53. doi: 10.1158/2159-8290.CD-14-0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Groenendijk FH, de Jong J, Fransen van de Putte EE, et al. ERBB2 Mutations Characterize a Subgroup of Muscle-invasive Bladder Cancers with Excellent Response to Neoadjuvant Chemotherapy. Eur Urol. 2015 doi: 10.1016/j.eururo.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 22.Norton L, Simon R, Brereton HD, et al. Predicting the course of Gompertzian growth. Nature. 1976;264:542–5. doi: 10.1038/264542a0. [DOI] [PubMed] [Google Scholar]
- 23.Sternberg CN, de Mulder P, Schornagel JH, et al. Seven year update of an EORTC phase III trial of high-dose intensity M-VAC chemotherapy and G-CSF versus classic M-VAC in advanced urothelial tract tumours. Eur J Cancer. 2006;42:50–4. doi: 10.1016/j.ejca.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 24.Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–66. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- 25.Neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: a randomised controlled trial. International collaboration of trialists. Lancet. 1999;354:533–40. [PubMed] [Google Scholar]
- 26.Griffiths G, Hall R, Sylvester R, et al. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J Clin Oncol. 2011;29:2171–7. doi: 10.1200/JCO.2010.32.3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choueiri TK, Jacobus S, Bellmunt J, et al. Neoadjuvant dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin with pegfilgrastim support in muscle-invasive urothelial cancer: pathologic, radiologic, and biomarker correlates. J Clin Oncol. 2014;32:1889–94. doi: 10.1200/JCO.2013.52.4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Plimack ER, Hoffman-Censits JH, Viterbo R, et al. Accelerated methotrexate, vinblastine, doxorubicin, and cisplatin is safe, effective, and efficient neoadjuvant treatment for muscle-invasive bladder cancer: results of a multicenter phase II study with molecular correlates of response and toxicity. J Clin Oncol. 2014;32:1895–901. doi: 10.1200/JCO.2013.53.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Plimack ER, Hoffman-Censits J, Kutikov A, et al. Neoadjuvant dose-dense gemcitabine and cisplatin (DDGC) in patients (pts) with muscle-invasive bladder cancer (MIBC): Final results of a multicenter phase II study. J Clin Oncol. 2014;32(5S):abstr 4513. [Google Scholar]
- 30.Siefker-Radtke AO, Dinney CP, Shen Y, et al. A phase 2 clinical trial of sequential neoadjuvant chemotherapy with ifosfamide, doxorubicin, and gemcitabine followed by cisplatin, gemcitabine, and ifosfamide in locally advanced urothelial cancer: final results. Cancer. 2013;119:540–7. doi: 10.1002/cncr.27751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith DC, Mackler NJ, Dunn RL, et al. Phase II trial of paclitaxel, carboplatin and gemcitabine in patients with locally advanced carcinoma of the bladder. J Urol. 2008;180:2384–8. doi: 10.1016/j.juro.2008.08.075. discussion 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ortmann CA, Mazhar D. Second-line systemic therapy for metastatic urothelial carcinoma of the bladder. Future Oncol. 2013;9:1637–51. doi: 10.2217/fon.13.139. [DOI] [PubMed] [Google Scholar]
- 33.Ko YJ, Canil CM, Mukherjee SD, et al. Nanoparticle albumin-bound paclitaxel for second-line treatment of metastatic urothelial carcinoma: a single group, multicentre, phase 2 study. Lancet Oncol. 2013;14:769–76. doi: 10.1016/S1470-2045(13)70162-1. [DOI] [PubMed] [Google Scholar]
- 34.Bellmunt J, Theodore C, Demkov T, et al. Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J Clin Oncol. 2009;27:4454–61. doi: 10.1200/JCO.2008.20.5534. [DOI] [PubMed] [Google Scholar]
- 35.Hahn NM, Stadler WM, Zon RT, et al. Phase II trial of cisplatin, gemcitabine, and bevacizumab as first-line therapy for metastatic urothelial carcinoma: Hoosier Oncology Group GU 04–75. J Clin Oncol. 2011;29:1525–30. doi: 10.1200/JCO.2010.31.6067. [DOI] [PubMed] [Google Scholar]
- 36.Balar AV, Apolo AB, Ostrovnaya I, et al. Phase II study of gemcitabine, carboplatin, and bevacizumab in patients with advanced unresectable or metastatic urothelial cancer. J Clin Oncol. 2013;31:724–30. doi: 10.1200/JCO.2012.42.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chaudhary U, Golshayan A, Brisendine A, et al. Phase II trial of neoadjuvant cisplatin, gemcitabine, and bevacizumab followed by radical cystectomy (RC) in patients with muscle-invasive transitional cell carcinoma (TCC) of the bladder. J Clin Oncol. 2011;29(suppl 7):abstr 276. [Google Scholar]
- 38.Siefker-Radtke A, Kamat A, Corn P, et al. Neoadjuvant chemotherapy with DD-MVAC and be- vacizumab in high-risk urothelial cancer: Results from a phase II trial at the University of Texas M. D. Anderson Cancer Center. J Clin Oncol. 2012;30:abstr 4523. [Google Scholar]
- 39.Gallagher DJ, Milowsky MI, Gerst SR, et al. Phase II study of sunitinib in patients with metastatic urothelial cancer. J Clin Oncol. 2010;28:1373–9. doi: 10.1200/JCO.2009.25.3922. [DOI] [PubMed] [Google Scholar]
- 40.Bellmunt J, Gonzalez-Larriba JL, Prior C, et al. Phase II study of sunitinib as first-line treatment of urothelial cancer patients ineligible to receive cisplatin-based chemotherapy: baseline interleukin-8 and tumor contrast enhancement as potential predictive factors of activity. Ann Oncol. 2011;22:2646–53. doi: 10.1093/annonc/mdr023. [DOI] [PubMed] [Google Scholar]
- 41.Galsky MD, Hahn NM, Powles T, et al. Gemcitabine, cisplatin, and sunitinib for metastatic urothelial carcinoma and as preoperative therapy for muscle-invasive bladder cancer. Clin Genitourin Cancer. 2013;11:175–81. doi: 10.1016/j.clgc.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 42.Balar AV, Iyer G, Apolo AB, et al. Phase II trial of neoadjuvant gemcitabine (G) and cisplatin (C) with sunitinib in patients (pts) with muscle-invasive bladder cancer (MIBC) J Clin Oncol. 2012;30S:abstr 4581. [Google Scholar]
- 43.Grivas PD, Daignault S, Tagawa ST, et al. Double-blind, randomized, phase 2 trial of maintenance sunitinib versus placebo after response to chemotherapy in patients with advanced urothelial carcinoma. Cancer. 2014;120:692–701. doi: 10.1002/cncr.28477. [DOI] [PubMed] [Google Scholar]
- 44.Sridhar SS, Winquist E, Eisen A, et al. A phase II trial of sorafenib in first-line metastatic urothelial cancer: a study of the PMH Phase II Consortium. Invest New Drugs. 2011;29:1045–9. doi: 10.1007/s10637-010-9408-4. [DOI] [PubMed] [Google Scholar]
- 45.Dreicer R, Li H, Stein M, et al. Phase 2 trial of sorafenib in patients with advanced urothelial cancer: a trial of the Eastern Cooperative Oncology Group. Cancer. 2009;115:4090–5. doi: 10.1002/cncr.24467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pili R, Qin R, Flynn PJ, et al. A phase II safety and efficacy study of the vascular endothelial growth factor receptor tyrosine kinase inhibitor pazopanib in patients with metastatic urothelial cancer. Clin Genitourin Cancer. 2013;11:477–83. doi: 10.1016/j.clgc.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Necchi A, Mariani L, Zaffaroni N, et al. Pazopanib in advanced and platinum-resistant urothelial cancer: an open-label, single group, phase 2 trial. Lancet Oncol. 2012;13:810–6. doi: 10.1016/S1470-2045(12)70294-2. [DOI] [PubMed] [Google Scholar]
- 48.Gerullis H, Eimer C, Ecke TH, et al. Combined treatment with pazopanib and vinflunine in patients with advanced urothelial carcinoma refractory after first-line therapy. Anticancer Drugs. 2013;24:422–5. doi: 10.1097/CAD.0b013e32835efe78. [DOI] [PubMed] [Google Scholar]
- 49.Lee RJ, Smith MR. Cabozantinib and prostate cancer: inhibiting seed and disrupting soil? Clin Cancer Res. 2014;20:525–7. doi: 10.1158/1078-0432.CCR-13-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bellmunt J, Hussain M, Dinney CP. Novel approaches with targeted therapies in bladder cancer. Therapy of bladder cancer by blockade of the epidermal growth factor receptor family. Crit Rev Oncol Hematol. 2003;46 (Suppl):S85–104. doi: 10.1016/s1040-8428(03)00067-2. [DOI] [PubMed] [Google Scholar]
- 51.Nutt JE, Lazarowicz HP, Mellon JK, et al. Gefitinib (‘Iressa’, ZD1839) inhibits the growth response of bladder tumour cell lines to epidermal growth factor and induces TIMP2. Br J Cancer. 2004;90:1679–85. doi: 10.1038/sj.bjc.6601768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perrotte P, Matsumoto T, Inoue K, et al. Anti-epidermal growth factor receptor antibody C225 inhibits angiogenesis in human transitional cell carcinoma growing orthotopically in nude mice. Clin Cancer Res. 1999;5:257–65. [PubMed] [Google Scholar]
- 53.Pruthi RS, Nielsen M, Heathcote S, et al. A phase II trial of neoadjuvant erlotinib in patients with muscle-invasive bladder cancer undergoing radical cystectomy: clinical and pathological results. BJU Int. 2010;106:349–54. doi: 10.1111/j.1464-410X.2009.09101.x. [DOI] [PubMed] [Google Scholar]
- 54.Petrylak DP, Tangen CM, Van Veldhuizen PJ, Jr, et al. Results of the Southwest Oncology Group phase II evaluation (study S0031) of ZD1839 for advanced transitional cell carcinoma of the urothelium. BJU Int. 2010;105:317–21. doi: 10.1111/j.1464-410X.2009.08799.x. [DOI] [PubMed] [Google Scholar]
- 55.Philips GK, Halabi S, Sanford BL, et al. A phase II trial of cisplatin (C), gemcitabine (G) and gefitinib for advanced urothelial tract carcinoma: results of Cancer and Leukemia Group B (CALGB) 90102. Ann Oncol. 2009;20:1074–9. doi: 10.1093/annonc/mdn749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Philips GK, Halabi S, Sanford BL, et al. A phase II trial of cisplatin, fixed dose-rate gemcitabine and gefitinib for advanced urothelial tract carcinoma: results of the Cancer and Leukaemia Group B 90102. BJU Int. 2008;101:20–5. doi: 10.1111/j.1464-410X.2007.07226.x. [DOI] [PubMed] [Google Scholar]
- 57.Hussain M, Daignault S, Agarwal N, et al. A randomized phase 2 trial of gemcitabine/cisplatin with or without cetuximab in patients with advanced urothelial carcinoma. Cancer. 2014;120:2684–93. doi: 10.1002/cncr.28767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wong YN, Litwin S, Vaughn D, et al. Phase II trial of cetuximab with or without paclitaxel in patients with advanced urothelial tract carcinoma. J Clin Oncol. 2012;30:3545–51. doi: 10.1200/JCO.2012.41.9572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Inoue K, Slaton JW, Perrotte P, et al. Paclitaxel enhances the effects of the anti-epidermal growth factor receptor monoclonal antibody ImClone C225 in mice with metastatic human bladder transitional cell carcinoma. Clin Cancer Res. 2000;6:4874–84. [PubMed] [Google Scholar]
- 60.Choueiri TK, Ross RW, Jacobus S, et al. Double-blind, randomized trial of docetaxel plus vandetanib versus docetaxel plus placebo in platinum-pretreated metastatic urothelial cancer. J Clin Oncol. 2012;30:507–12. doi: 10.1200/JCO.2011.37.7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hussain MH, MacVicar GR, Petrylak DP, et al. Trastuzumab, paclitaxel, carboplatin, and gemcitabine in advanced human epidermal growth factor receptor-2/neu-positive urothelial carcinoma: results of a multicenter phase II National Cancer Institute trial. J Clin Oncol. 2007;25:2218–24. doi: 10.1200/JCO.2006.08.0994. [DOI] [PubMed] [Google Scholar]
- 62.Oudard S, Culine S, Viellefond A, et al. Multicenter randomized phase 2 trial of gemcitabine - platinum with or without trastuzumab (T) in advanced/metastatic urothelial carcinoma (a/mUC) with HER2 overexpression. ESMO Congress. 2012:abstr 786O. [Google Scholar]
- 63.Wulfing C, Machiels JP, Richel DJ, et al. A single-arm, multicenter, open-label phase 2 study of lapatinib as the second-line treatment of patients with locally advanced or metastatic transitional cell carcinoma. Cancer. 2009;115:2881–90. doi: 10.1002/cncr.24337. [DOI] [PubMed] [Google Scholar]
- 64.Kompier LC, Lurkin I, van der Aa MN, et al. FGFR3, HRAS, KRAS, NRAS and PIK3CA mutations in bladder cancer and their potential as biomarkers for surveillance and therapy. PLoS One. 2010;5:e13821. doi: 10.1371/journal.pone.0013821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Seront E, Rottey S, Sautois B, et al. Phase II study of everolimus in patients with locally advanced or metastatic transitional cell carcinoma of the urothelial tract: clinical activity, molecular response, and biomarkers. Ann Oncol. 2012;23:2663–70. doi: 10.1093/annonc/mds057. [DOI] [PubMed] [Google Scholar]
- 66.Milowsky MI, Iyer G, Regazzi AM, et al. Phase II study of everolimus in metastatic urothelial cancer. BJU Int. 2013;112:462–70. doi: 10.1111/j.1464-410X.2012.11720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Iyer G, Hanrahan AJ, Milowsky MI, et al. Genome sequencing identifies a basis for everolimus sensitivity. Science. 2012;338:221. doi: 10.1126/science.1226344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bendell JC, Rodon J, Burris HA, et al. Phase I, dose-escalation study of BKM120, an oral pan-Class I PI3K inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2012;30:282–90. doi: 10.1200/JCO.2011.36.1360. [DOI] [PubMed] [Google Scholar]
- 69.Munster P, Specht J, Werner T, et al. PI3K kinase inhibitor GSK2126458 (GSK458): Clinical activity in select patient (PT) populations defined by predictive markers (study P3K112826) Ann Oncol. 2013;23(suppl 10):abstr 442O. [Google Scholar]
- 70.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–33. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Powles T, Vogelzang NJ, Fine G. Inhibition of PD-L1 by MPDL3280A and clinical activity in pts with metastatic urothelial bladder cancer (UBC) J Clin Oncol. 2014;32(5S):abstr 5011. [Google Scholar]
- 74.Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558–62. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 75.Plimack ER, Gupta S, Bellmunt J, et al. A phase 1b study of pembrolizumab (Pembro; MK-3475) in patients (Pts) with advanced urothelial tract cancer. Ann Oncol. 2014;25:1–41. [Google Scholar]
- 76.Apolo AB, Tomita Y, Lee M, et al. Effect of cabozantinib on immunosuppressive subsets in metastatic urothelial carcinoma. J Clin Oncol. 2014;32s:abstr 4501. [Google Scholar]
- 77.Friede T, Parsons N, Stallard N. A conditional error function approach for subgroup selection in adaptive clinical trials. Stat Med. 2012;31:4309–20. doi: 10.1002/sim.5541. [DOI] [PubMed] [Google Scholar]
- 78.Miyamoto H, Zheng Y, Izumi K. Nuclear hormone receptor signals as new therapeutic targets for urothelial carcinoma. Curr Cancer Drug Targets. 2012;12:14–22. doi: 10.2174/156800912798888965. [DOI] [PubMed] [Google Scholar]
- 79.Lee JK, Havaleshko DM, Cho H, et al. A strategy for predicting the chemosensitivity of human cancers and its application to drug discovery. Proc Natl Acad Sci U S A. 2007;104:13086–91. doi: 10.1073/pnas.0610292104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Williams PD, Cheon S, Havaleshko DM, et al. Concordant gene expression signatures predict clinical outcomes of cancer patients undergoing systemic therapy. Cancer Res. 2009;69:8302–9. doi: 10.1158/0008-5472.CAN-09-0798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Smith SC, Baras AS, Lee JK, et al. The COXEN principle: translating signatures of in vitro chemosensitivity into tools for clinical outcome prediction and drug discovery in cancer. Cancer Res. 2010;70:1753–8. doi: 10.1158/0008-5472.CAN-09-3562. [DOI] [PMC free article] [PubMed] [Google Scholar]