Abstract
Despite effective antiretroviral therapy (ART) and undetectable HIV RNA in the plasma, latent replication-competent HIV persists indefinitely in long-lived cells. Cessation of ART results in rebound of HIV from these persistent reservoirs. While this was thought to be an insurmountable obstacle to viral eradication, recent cases suggest otherwise. To date one patient has been “cured” of HIV and several others have been able to interrupt ART without viral rebound for prolonged periods. These events have sparked renewed interest in developing strategies that will allow eradication of HIV in infected individuals. We review the current knowledge of HIV latency and the viral reservoir, describe the potential utility of emerging cancer therapeutics in HIV cure research with an emphasis on pathways implicated in reservoir persistence, and outline opportunities and challenges in the context of the current clinical trial and regulatory environment.
1 Introduction
Despite effective antiretroviral therapy (ART) and undetectable HIV RNA in the plasma, latent replication competent HIV persists indefinitely in long-lived cells in infected individuals [1–3]. Cessation of ART results in rebound of HIV from these persistent reservoirs. While this was thought to be an insurmountable obstacle to viral eradication, recent cases suggest otherwise. To date one patient has been “cured” of HIV and several others have been able to interrupt ART without viral rebound for prolonged periods of time. These events have sparked renewed interest in developing strategies that will allow individuals with HIV to stop ART without viral rebound: either with complete viral eradication, a “sterilizing cure,” or without clinically significant viral replication in the absence of ART, a sustained remission or a “functional cure.”
We review the current knowledge of HIV latency and the viral reservoir, describe the potential utility of emerging cancer therapeutics in HIV cure research with an emphasis on pathways implicated in reservoir persistence and potentially of use in achieving a sustained remission, and outline opportunities and challenges in the context of the current clinical trial and regulatory environment.
2 Background
2.1 The HIV Reservoir
The HIV reservoir is established early in HIV infection primarily as transcriptionally silent integrated proviral DNA in long-lived resting memory T cells in blood and lymphoid tissue [3–5]. The central memory T cell (TCM) population has been hypothesized to be the key niche for HIV persistence [6, 7].
Long-lived T cells are thought to be the major cellular reservoir. Specific T-cell subsets within this compartment have been described in which long-term HIV persistence is prominent, including an immature progenitor like memory stem cell T cells (TSCM), which resists apoptosis, self-renews, and can differentiate into effector memory T cells (TEM) and central memory cells (TCM) [6]. Once differentiated, TCM and TEM cells migrate to lymphoid organs and to peripheral tissues [7]. Although controversial, cells of other origins have also been implicated in reservoir maintenance; for example, some monocyte lineage cells harbor proviral DNA [8, 9]. Cells containing latent HIV can produce both infectious and non-infections virions when the host cell is reactivated in response to factors including its cognate antigen, activating cytokines, or persistent immune activation [10]. The presence of replication-competent HIV in these latent reservoirs and the absence of effective HIV specific immune responses are the critical barriers to eradicating HIV in infected patients. Neither ART nor HIV specific immune responses have been able to effectively target these cells.
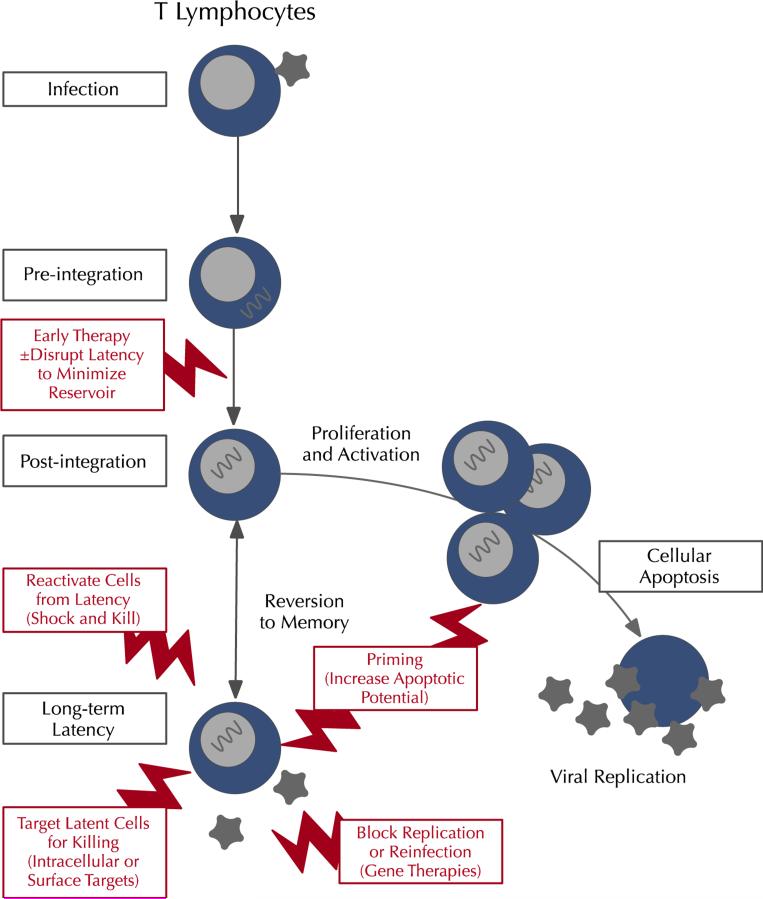
Latency is understood to be maintained by a combination of viral and cellular host processes (Fig. 1). Pre-integration mechanisms of latency may be important in reservoir establishment, and involve processes that are involved in packaging the pre-integration complex and the interaction of viral proteins with host restriction factors such as APOBEC3, SAMHD1, and MX2 [8, 9]. In resting CD4 T cells, repressive histone modifications and DNA methylation that regulate key host cellular transcription factors limit the initiation and elongation of viral transcription at the promoter site [11]. Cellular factors influencing latency include cellular transcription factors including PTEF B and nuclear factor (NF)-κB [7], intermittent viral production, and reinfection from reservoirs. These are driven by cytokines and mediated by low level immune activation and a dynamic interaction between resting memory, regulatory T cells, and activated T cells. Persistent low level immune activation may also change the immunologic environment and enable HIV persistence by promoting immune exhaustion and abrogating an effective T-cell response [12]. Recent studies demonstrating enrichment of HIV DNA integrated in or near to host growth promoter genes suggest an additional potential mechanism for provirus persistence and the survival and expansion of infected cells [13, 14].
Fig. 1.
Putative targets in HIV eradication. The HIV reservoir is established primarily in resting memory T cells and other T-cell subtypes (right), though other lineages including monocytes are also implicated (left). Cure strategies currently being explored target this reservoir at varying points, including early therapy to minimize reservoir size (as in the Mississippi baby); reactivation of HIV from its latent state; direct targeting of the reservoir cells; and interruption of maintenance of the reservoir by preventing cycles of reinfection. These approaches each have specific targets, and in many cases may be complementary. Other cell types including monocytes (not shown) have also been implicated in reservoir persistence
The major anatomic sites of the reservoir remain the subject of investigation. Low-level replication without inflammation at sites where ART penetration is decreased has been described in lymphoid tissue in the B-cell follicles [15] and in the gut [16]. HIV DNA has been demonstrated in the central nervous system, and expression of RNA has been described in the CNS of suppressed HIV-infected individuals [17, 18]. Phylogenetic studies have suggested evolutionary separation of plasma and CNS HIV; taken together this suggests that the CNS may be another important site of HIV persistence.
2.2 Emerging Approaches to Cure
Several potentially complementary strategies are being pursued to effect a sustained remission in the absence of ART. These include: early therapy to limit the size of the reservoir; reactivating latently infected cells and stimulating their clearance by the immune system; genetically modifying host CD4 cells to make them resistant to HIV infection; and use of endonucleases to target and digest critical portions of the integrated HIV DNA. It is also likely that early identification and treatment of HIV will reduce the size of the inducible replication competent reservoir (the “functional reservoir”) and aid cure efforts [19]. The “Mississippi Baby” was treated very early in infection and achieved a prolonged remission when ART was interrupted [20]. Similarly, 14 adults have been described who received ART during acute infection and were able to suppress HIV once ART was discontinued [21]. There are practical barriers to early ART intervention: patients with acute and early infection are difficult to identify and represent only a small portion of the HIV-infected population. For this reason chronically infected patients with long-term viral suppression are the likely population of interest and are likely to require different or additional interventions than those who are acutely infected.
Several of these strategies are illustrated in the case of the Berlin patient, the single person believed to be cured of HIV. He received bone marrow transplantation from a donor with the CCR5 delta 32 allele which prevented CCR5 proteins from being expressed on the surface of the transplanted cells, making the donor T cells resistant to the patient's CCR5 tropic HIV. Other factors including graft-versus-host disease and high-dose stem-cell toxic chemotherapy may also have contributed to his apparent cure [22]. Genetic engineering approaches are attempting to recapitulate this experience using autologous hematopoietic stem cell transplantation (HSCT) with some early promising results [23]. In contrast to the Berlin patient, allogeneic HSCT with HIV-susceptible cells (CCR5 wild type) and reduced-intensity conditioning failed to deliver HIV eradication in two adults who received clinically indicated HSCT. While on suppressive ART, HIV DNA was not detectable in CD4 cells and HIV RNA was not detectable in plasma. The loss of detectable HIV correlated temporally with full donor chimerism, development of graft-versus-host disease, and decrease in HIV specific antibody levels and avidity, which had also been reported in the Berlin patient. ART was interrupted and HIV replication became detectable several months after interruption in both patients, and was associated with acute antiretroviral syndrome [24, 25]. Ongoing work is attempting to assess whether specific immunologic factors in the graft-versus-host disease may have played a role in the temporary HIV remission.
Many current efforts are directed towards modifying or blocking the processes involved in the establishment and/or maintenance of HIV latency. It was initially thought that reactivating HIV in resting cells would cause their death by the host's residual HIV-specific immune responses; however, it is now known that a second step may be necessary to eliminate reactivated cells [26]. This strategy is referred to as “shock and kill.” There are several lines of research looking at mechanisms to reactivate latently infected cells as well as those that target the reactivated cells for elimination. Elimination might include priming cells to increase apoptotic potential prior to reactivation [27], involve immune mechanisms that target cellular markers involved in latency, boost the host HIV-specific immune response either to recall antigens or new targets, and the expression of HIV proteins on the surfaces of reactivated cells. Therapeutic vaccines, HIV-specific broadly neutralizing antibodies made toward HIV, processes involving regulatory T cells, and intracellular signaling processes involved in the maintenance of HIV persistence are also being assessed. In addition, non-neutralizing mAbs that recognize conserved epitopes on HIV envelope and have significant antibody-dependent cytotoxicity and potentially antibody-dependent cellular phagocytosis may help eliminate latently infected cells as they are reactivated [28, 29].
2.3 Cancer Therapeutics and Approaches to Cure
Established and emerging therapeutic strategies in oncology are likely to contribute agents with a potential to have an impact on HIV latency. Many of the pathways implicated in the maintenance of viral latency and persistence of the cellular reservoirs are highly conserved and have also been implicated either in oncogenesis or in the immune response to malignancies. Key examples include the histone deacytylase inhibitors (HDACi's), immune modulators including checkpoint inhibitors, and agents directed toward the apoptotic pathway. These agents are potentially attractive not only because they target cell types and pathways implicated in HIV persistence, but because their pharmacokinetic and toxicity profiles are already established, and they generally have a path to licensure through their primary oncologic indications. These factors potentially lower the barriers exploring their utility in HIV cure research and accelerate exploration of promising agents.
3 Reactivation from Latency
One of the most explored approaches to eradicating HIV has been the use of HDACi's to upregulate HIV expression in latently infected cells. These cells could then be lysed by HIV-specific T-lymphocytes or die from viral cytopathic effects (“shock and kill”) [30]. During HIV latency, host transcription factors recruit HDACs to the HIV 5′ LTR where the HIV promoter is packaged in chromatin [31]. HDACi's are able to reactivate latent HIV by allowing hyperacetylation of the LTR nuc-1 nucleosome or by HIV Tat activation of virus production [32, 33]. A recent study also suggests that HDACi's may eliminate HIV via autophagy and degradation of HIV in macrophages [34, 35]. However, the impact of reactivation on reservoir size remains to be demonstrated.
Clinical trials of HDACi's in HIV are ongoing or have been completed. One of the earliest proof-of-concept studies utilized valproic acid, which reported a decrease in latent infection in three out of four patients [36]. However, this was not reproduced in subsequent studies [37–39]. Vorinostat, a more potent HDACi, has also been extensively studied. A single dose of vorinostat was shown to increase the expression of HIV mRNA in resting CD4 T cells [40]; this was confirmed in a multidose study (with 14 daily doses) of vorinostat, but did not reduce HIV DNA [41]. No major clinical adverse events were reported [42]. A subsequent multidose study of vorinostat (given daily for 3 days per week for 8 weeks) confirmed that multidose vorinostat was well tolerated [43]. In this study however, three out of five study participants had an increase in resting CD4 T cell-associated HIV RNA, and in only one case was the magnitude of RNA induction comparable to the level seen after a single dose, suggesting that the kinetics of HDACi's need to be better delineated to improve response. The farnesyl transferase inhibitors may increase the amount of HIV expressed from latently infected cells when used with vorinostat [44], suggesting a potential synergy. Similar results were also reported from a small study with another potent HDACi, panobinostat [45]. Lastly, romidepsin has been the most potent HDACi identified for inducing HIV replication in vitro [46]. In a phase I/II trial in six participants, three doses of once-weekly romidepsin induced HIV expression resulting in plasma virema [47]. A dose-escalation study of romidepsin in HIV-infected individuals is currently underway (NCT1933594).
Bromodomain inhibitors, currently in early development, may have utility in reactivating latent HIV. Bromodomain proteins are involved in targeting chromatin modifying enzymes, and are important in regulating the transcription of growth-promoting genes and cell cycle regulators. They also are involved with the transcriptional control of proinflammatory cytokines via their interaction with acetylated NF-κB, a key transcription factor mediating inflammatory responses and important in reactivating HIV from latency [48]. A bromodomain inhibitor (JQ1) has been shown to reactivate HIV in a clonal cell model via the PTEF B mechanism [49]. Because this compound associates with a key transcription factor required for erythropoiesis, concerns have been raised about potential toxicities [50]. More selective bromodomain inhibitors may be useful in reversing latency [51].
4 Cell Surface Reservoir Targets
4.1 Immune Checkpoint Inhibitors and Negative Regulators of T-Cell Activation
The cytotoxic T-lymphocyte-associated protein 4 (CTLA4) and programmed death (PD) are T-cell regulatory pathways that regulate immune responses and maintain immune tolerance. Together they are referred to as checkpoint pathways. Both PD-1 and CTLA-4 inhibit immune responses through molecular mechanisms that involve protein kinase B (also known as Akt). PD-1 more effectively suppresses T-cell-specific transcription than CTLA-4 [7].
CTLA4 regulates the amplitude of early T-cell activation. The receptor is present on CD8 T cells but also has important effects on CD4 cells by down-regulating T-helper cells and enhancing the suppressive function of T-regulatory cells. Blockade of CTLA4 inhibits the ability to regulate both autoimmunity and antitumor immunity. In HIV infection increased expression of the CTLA4, the PD 1, and the LAG 3 receptor are markers of T-cell exhaustion, and may be responsible for or contribute to the ineffective HIV-specific immune responses. Blocking the effects of the checkpoint inhibitors may result in improved immune response to HIV, but in oncology studies have resulted in immune-related adverse events.
Ipilimumab and tremelimumab are anti-CTLA-4 monoclonal antibodies (mAbs). Ipilimumab is licensed for use by the US Food and Drug Administration (FDA) for metastatic melanoma, and both are in clinical trials for the treatment of other solid tumors. A trial is planned for ipilimumab in HIV-related cervical cancer that may give important mechanistic insights into the relationship of this agent to the HIV reservoir. The immune effects of ipilumumab were studied in a single HIV-infected individual receiving therapy for metastatic melanoma. Both total memory T cells and unspliced cell-associated RNA increased after therapy, suggesting reactivation and redistribution of latently infected T cells and perhaps their elimination [52]. Toxicities of this agent are not trivial, are generally immune mediated, and include a potentially fatal immune mediated enterocolitis [53] (Table 1).
Table 1.
Select cancer agents with potential utility in HIV cure approaches
| Agent(s)/class | Target(s) | Primary indication (established or in development for, if applicable) | Possible reservoir targets | Key toxicities | Regulatory status or phase of development | Current and completed cure trials |
|---|---|---|---|---|---|---|
| HDAC inhibitors | Inhibit histone deactylase to induce HIV transcription | Latency reversal | ||||
| Valproic acid | Anticonvulsant; mood stabilizer; migraine headache | Hepatotoxicity, pancreatitis, bleeding and other hematopoietic disorders, teratogenicity | FDA-approved as antiepileptic | Several clinical studies completed evaluating valproic acid in cure trials | ||
| Vorinostat | Cutaneous T-cell lymphoma (CTCL) | Myelosuppression, thrombosis, gastrointestinal toxicity, electrolyte abnormalities, hyperglycemia, teratogenicity | FDA-approved for CTCL | Several clinical studies completed evaluating vorinostat in cure trials. Phase I/II trial underway to evaluate the effect of a single dose compared with multiple dosing regimens of vorinostat | ||
| Romidepsin | Peripheral T-cell lymphoma (PTCL), CTCL | Myelosuppression, ECG changes, EBV/HBV reactivation, teratogenicity | FDA-approved for PTCL, CTCL | Two trials underway: A phase I/II trial currently underway to evaluate escalating single-dose romidepsin (ACTG 5315) and an open phase I/IIa study with romidepsin and vacc4x, an HIV vaccine | ||
| Panobinostat | Not yet FDA-approved | Fatigue, cutaneous toxicity, GI disturbances, sleeplessness | In clinical development for multiple myeloma treatment | Recent phase I/II trial completed to evaluate safety and effects on latency reactivation with multiple doses of panobinostat | ||
| Ipilumumab and tremelimumab | Immune checkpoint inhibitors by targeting CTLA4 | Malignant melanoma, potential applications for other solid tumors | Reverse T-cell exhaustion, improved HIV- specific immune response | Immune-mediated adverse reactions, which can be severe: enterocolitis, hepatitis, dermatitis (including toxic epidermal necrolysis), neuropathy, and endocrinopathy. Some delayed reactions occurred after therapy cessation | Ipilumumab FDA-approved for melanoma; tremelimumab in phase I/II trials | None current, but correlatives centered on cure objectives are planned in an AIDS Malignancy Consortium trial in solid tumors in people with HIV |
| Nivolumab, pembrolizumab, Lambrolizumab, BMS-936559 | Immune checkpoint inhibitors by targeting PD-1 or PDL1 | Malignant melanoma following ipilumumab; in development for multiple solid and hematologic tumors | Reverse T-cell exhaustion, improved HIV-specific immune response | Immune-mediated adverse reactions, which can be severe: colitis, pneumonitis, others | FDA-approved for nivolumab, pembrolizumab in metastatic melanoma | BMS 936559 planned for evaluation in ACTG 5326 |
| Sirolimus temsirolimus, everolimus and ridaforolimus | Mammalian target of rapamycin (mTor) | Immune suppression following transplantation | Arrest activated T cells in G phase | Increased risk of lymphomas, skin cancers, infection | All but ridaforolimus FDA approved. Indications: immunosuppressant, tuberous sclerosis complex, renal cell carcinoma | Sirolimus under evaluation against cure endpoints in ACTG 5337 |
| Immunomodulatory derivatives of thalidomide (IMIDs; thalidomide, lenalidomide, pomalidomide) | Multiple via cereblon, including T cell costimulation, cytokine modulation, cell microenvironment | Hematologic malignancies including myeloma and lymphoma, alone or in combination; Kaposi sarcoma | Immune stimulation to clear reservoir cells; stem cell disruption | Hematologic cytopenias Neurologic (neuropathy and sedation, less with newer agents) Gastrointestinal disturbance Teratogenicity |
FDA-approved for multiple myeloma in combination regimens | None cure-centered; trials in HIV associated malignancies are ongoing in Kaposi sarcoma for lenalidomide (AIDS Malignancy Consortium) and pomalidomide (National Cancer Institute) |
| Ibrutinib | Bruton's Tyrosine kinase and interleukin-2-inducible T-cell kinase (ITK) inhibitor | Mantle cell lymphoma, chronic lymphocytic leukemia, other hematological malignancies | Promotes apoptosis | Thrombocytopenia, renal impairment, cutaneous toxicity | FDA-approved for chronic lymphocytic leukemia | Trials in HIV associated malignancies underway (phase I safety study, AIDS Malignancy Consortium) |
| Imatinib | Multi-tyrosine kinase inhibitor including PDGFR, cKIT | Chronic myeloid leukemia, gastrointestinal stromal cell tumors | Promotes apoptosis | Cytopenias, gastroinstestinal toxicity | FDA approved for CML and GIST | None current |
| Gamma secretase inhibitors (PF-03084014, others) | Enzymatic components of the notch signaling pathway | Metastatic solid tumors; Kaposi sarcoma | Inhibits angiogenesis; may target stem cell cycling and renewal | Gastrointestinal toxicity dose limiting, but full safety profile not yet defined | Phase I/II studies under way in advanced solid tumors including breast and pancreatic, and planned in Kaposi sarcoma | Trial in HIV-associated Kaposi sarcoma is planned (AIDS Malignancy Consortium) |
CML chronic myelocytic leukemia; CTLA cytotoxic T-lymphocyte-associated protein; EBV Epstein-Barr virus; ECG electrocardiogram; GI gastrointestinal; GIST GI stromal tumor; HBV hepatitis B virus; PD programmed death; PDL PD ligand; PDGFR platelet-derived growth factor receptor
The PD-1 pathway dampens inflammatory responses of T cells in peripheral tissue. Its two ligands, PDL1 and PDL2, are expressed on T cells. PD-1 and PDL1 remain elevated in HIV-infected patients on suppressive ART, and expression of these molecules may be a marker for cells that are latently infected with HIV [54, 55]. Blockade of the PD-1/PDL1 pathway increases HIV-specific immunity in vitro and may improve HIV-specific immunity and clearance of HIV-expressing cells in patients on ART [56].
Pembrolizumab and nivolumumab are anti-PD-1 mAbs recently FDA-approved for the treatment of advanced melanoma, and other antibodies are in development. They are also being studied for the treatment of other solid tumors both alone and in combination with anti-CTLA-4 mAbs. No current trial includes HIV-infected subjects. Major toxicities include immune-mediated organ dysfunction which has resulted in death. An anti-PD-L1 antibody, BMS-936559, has also been studied for the treatment of various malignancies [57]; a dose-escalation trial evaluating its safety and efficacy in HIV-infected individuals suppressed on ART is underway but currently on clinical hold (NCT02028403). Finally, there is growing interest in indolamine 2, 3 deoxygenase (IDO) pathway which interacts with both the PD-1 and CTLA-4 pathways to suppress cytotoxic T cells [58]. There are two small-molecule, orally available inhibitors which are in early-phase cancer clinical trials (Indoximod, Newlink Genetics and INCB024360 Incyte Corporation) [59].
4.2 Engineered T Cells
Adoptively transferred T-cell-based immune strategies have been used successfully in oncology. In HIV, the rationale for using these strategies stems from the crucial role of HIV-specific CD8+ T-cell responses which have been associated with improved virologic control [60–62]. Adoptively transferred modified T cells have the potential to hone and lyse HIV-infected cells and develop into long-lived memory cells conferring lifetime protection. Notably, unmodified T-cell strategies have been used without meaningful results in HIV [63–65]. Genetic modifications include transduction with a chimeric antigen receptors (CARs), which combine antibody specificity with receptor signaling, and modification of specific T-cell receptors used for HIV binding [66]. The results of three trials evaluating the safety, durability, and functionality of a CAR expressing a CD4 molecule on its surface that was fused with the CD4zeta signaling domain (CD4zCAR) in viremic individuals were recently reported; this CAR mediated T-cell interaction with HIV-infected cell via gp120 [67]. These trials demonstrated safety and tolerability as well as long-term persistence of the CD4zCAR modified cells for up to 11 years with retained expression and function including trafficking to mucosal sites and lowering of HIV RNA in some patients [67–70]. The use of adoptively transferred T cells to reduce the reservoir is being explored in a phase I study, evaluating the safety and immunologic and virologic efficacy of ex vivo expanded HIV-1 multi-antigen-specific T cells in ART-suppressed HIV-infected patients (NCT02208167).
The observation that the Berlin patient achieved durable remission following bone marrow transplantation from a CCR5 delta homozygous donor resistant to HIV infection has led to interest in developing CCR5-resistant T cells for autologous transplantation. This offers the prospect of deriving a more specific HIV cure strategy with the use of a less toxic allogeneic transplantation. Autologous T-cell infusion following site-specific gene modification of the CCR5 gene using a zinc-finger nuclease (ZFN) has recently been described [23]. Infusion of autologous T cells, a minority of which expressed the desired genetic modification, resulted in a significant increase in CD4 T cell numbers. One patient in this study, who was heterozygous for CCR5 delta 32 prior to the T-cell modification, had an undetectable HIV RNA at week 12 off ART despite an earlier viral load rebound. This approach continues to be evaluated. Newer endonucleases may provide technical advantages and lead to the development of genetically modified hematopoetic stem cells resistant to HIV [71].
5 Intracellular Reservoir Targets
5.1 Rapamycin and the mTor Pathway
Rapamycin and its three derivatives, temsirolimus, everolimus, and ridaforolimus, are macrolides used as immunosuppressive agents in organ transplantation and as chemotherapeutic agents. They are inhibitors of the mammalian target of the rapamycin (mTor)-signaling pathway, which is involved in the control of cell growth and proliferation, induces cell cycle arrest at the G1 phase and cell death via apoptosis and autophagy [72]. Other effects include inhibition of interleukin (IL)-2 and other stimulatory cytokines and inhibition of T- and B-cell activation. Activated T cells are also arrested at the G1 phase by mTor inhibition, and this is responsible for many of the immune effects of these agents. The pathway is important in the expression of transcription factors that regulate CD8 T-cell activation and autophagy, and enhance the development of T-cell memory [73]. Together it is possible that these effects may enhance clearance of HIV in the setting of a “shock and kill” curative strategy. This strategy is planned for evaluation in an AIDS Clinical Trials Group interventional protocol (NCT02440789).
5.2 Immunomodulatory Derivatives of Thalidomide
Thalidomide and its derivatives (IMIDs), including lenalidomide (CC-5013) and pomalidomide (CC-4047), are small molecules with broad-based effects on immune activation, including T-cell activation and responsiveness, as well as anti-angiogenic properties [74–77]. Many of their activities are mediated through binding to and inactivation of cereblon, an E3 ubiquitin ligase with multiple targets that is highly expressed in lymphocytes as well as other tissues, though there may be additional mechanisms of action [75, 78]. Their downstream effects are diverse, and likely vary in different cells and tumor types [56]. However, their immunologic effects are well characterized, and include effects that may influence the HIV reservoir.
IMIDs have been shown in vitro to augment T-cell responsiveness and proliferation by several mechanisms, leading to increased production of IL-2 and interferon-γ (IFN-γ) and inhibition of pro-inflammatory cytokine and chemokine production [79–81]. They also enhance CD4- and CD8-positive T-cell co-stimulation. This reprogramming is mediated at least in part by induction of the transcription factor T-bet. In addition, T-regulatory cell expansion and FOXP3 expression on T-regulatory cells are inhibited without affecting survival or apoptosis, and T helper (Th)-1 cytokine production is enhanced [82–84]. These effects were confirmed in vivo in patients with multiple myeloma treated with IMIDs, who showed changes with activation of T cells and monocytes [64]. Similarly in people with HIV treated with IMIDs, increased T-cell activation both in vivo and in ex vivo stimulation assays, including enhanced IL-2 generation. Taken together, these raise the possibility that IMIDs may act to activate of T-cell subsets implicated in HIV reservoir maintenance and thus perhaps effect the maintenance of HIV latency. Given the established activity of IMIDs in hematologic and AIDS-associated malignancies, several clinical trials in people with HIV infection and cancer are now underway (NCT01495598, NCT01057121) and may offer an opportunity to explore the effect of these agents on the reservoir.
5.3 Pro-Apoptotic Agents, Including Ibrutinib
Identification and disruption of abnormally active pathways that promote cancer cell survival is a central focus of oncology drug development. While not yet explored in HIV-centered trials, many of the pathways disordered in malignancy may be of interest in HIV cure efforts. Abnormal B cell receptor (BCR) pathways have been studied in the context of lymphomas and leukaemias: propogation of the BCR signal leads to up-regulation of several nuclear transcription factors including NF-κB. In T cells this pathway is important in regulating both apoptosis and the genetic regulation of cell development, and can also activate HIV transcription through caspase effects on HIV toll-like receptors’ (TLR) transcriptional activity [85]. It is plausible that molecules that disrupt this pathway at different points may also have an effect on T-cell activation and HIV latency. Ibrutinib is an inhibitor of an IL-2-inducible T-cell kinase (ITK) in this pathway and has been shown to block T-cell differentiation to the Th2 phenotype [86]. This process has been implicated in tumor immune evasion [87] and may have utility in HIV cure efforts. Ibrutinib is approved for the treatment of certain B-cell leukemias, and it is being studied for other indications. Other tyrosine kinase inhibitors such as imatinib may also have effects on reversing HIV-infected T-cell persistence by promoting apoptosis and autophagic cell death [88].
Other apotoptic pathways are also being actively explored. In particular, the tumor-necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) induces cell death by binding to nuclear “death receptors” in a caspase-dependent pathway. In a murine cytomegalovirus (CMV) model, TRAIL-positive NK cells led to the elimination of activated CD4 cells [89], and a reduced TRAIL ligand with an altered TRAIL pathway has been demonstrated in the CD4 T-cells of HIV infected elite controllers [90]. There is intense interest in identifying agents that promote TRAIL-mediated apoptosis in cancer therapeutics [91, 92].
5.4 Pattern Recognition Pathways
TLR signaling, through their sentinel actions in recognizing pathogen-associated molecular patterns and initiating immune responses, could also lead to activation of T-cell subsets implicated in HIV reservoir maintenance [93]. This possibility warrants exploration as TLR agonists proceed into early-phase clinical trials for oncologic and other indications. Agonists of TLR 7 and 9 have already been explored in limited-cure-centered studies. GS-9620 is a selective orally administered TLR7 agonist that is in clinical development as a treatment of chronic hepatitis B. Ex vivo it has been shown to increase HIV RNA expression in peripheral blood mononuclear cells (PBMCs) from 11 of 12 ART-suppressed HIV-infected individuals [94]. In a multidose, dose-escalation study in chronically simian immunodeficiency virus (SIV)-infected ART-suppressed rhesus macaques, GS-9620 produced a consistent transient increase in plasma viremia after several doses, substantial reductions in viral DNA content in tissue samples, and a lower viral set point after cessation of antiretroviral therapy [95]. A phase I study is planned in suppressed HIV-infected individuals. CPG 7909 is an immunostimulatory TLR9 agonist that has been used in several cancers including B-cell leukemias [96, 97]. In a post hoc analysis of a phase I study, ART suppressed HIV-infected individuals who received a pneumococcal vaccine regimen plus CPG 7909 had a reduction in proviral DNA compared to the group that received the vaccine with placebo. Reductions in proviral DNA was correlated with increasing levels of HIV specific CD8 cells [98, 99].
5.5 Other Intracellular Pathways
Pathways implicated in stem cell cycling and renewal may also be fruitful avenues of exploration for interruption of HIV latency. Among the former, the notch, hedgehog, and wnt signaling pathways are emerging as key stem-cell regulators in adulthood in addition to their established roles in cell development, cell-to-cell communication, and cellular differentiation during angiogenesis [100, 101]. Overexpression of pathway constituents has also been implicated in a number of malignancies, and selective inhibitors are now in development. Any impact on stem-like T-memory cell persistence and cycling would have important implications for exploration for HIV latency.
6 Risk-Benefit and Regulatory Considerations
Clinical trials of HIV cure strategies offer little prospect of direct clinical benefit for individual study participants, while potentially placing them at risk of toxicities related to the investigational agent. Some of these toxicities may be more pronounced in individuals with HIV. Given the tolerability and effectiveness of current ART, HIV-infected individuals who can live healthy productive lives for decades on ART are now considered to be similar to healthy volunteers. Therefore, clinical trials evaluating one or more novel agents in support of the cure agenda need to be small, maintain a comprehensive risk mitigation strategy, have carefully described safety endpoints for individuals and for the study, and may require gating strategies to limit the number of participants initiating therapy at any one time until toxicity profiles are better established. Long-term follow-up for late effects, including possible oncogenic effects, is also crucial as the long-term complications of many of the potentially useful agents have not yet been established. The establishment of surrogate endpoints for reservoir disruption, short of structured interruptions to HIV treatment to observe for viral rebound, is currently a focus of discussion and would further aid efforts to speed and simplify trial design [74]. The FDA is engaged in a dialogue with investigators in the field regarding these issues [74, 102].
7 Leveraging Cancer Trials
Elevated rates of many malignancies in HIV-infected individuals are well described [103–105]. Effective and suppressive ART regimens with minimal toxicities are available for HIV-infected patients including those in need of cancer chemotherapy. These individuals, fully suppressed on ART, who are receiving cancer therapeutics that may have activity on the latent HIV reservoir activity, represent a unique opportunity to further our understanding about how to achieve a sustained remission of HIV. The risk-benefit profile for a trial subject with an established malignancy is considerably different to that of a healthy HIV-infected volunteer, potentially reducing the barrier to regulatory and institutional review and approval. Toxicity management in the setting of cancer therapeutics is well established, including approaches to interactions between ART and chemotherapeutic agents.
Thus correlative cure studies embedded in cancer trials which permit HIV-infected individuals to be enrolled provide an opportunity to explore effects on HIV latency and may provide the first signal for toxicity in people with HIV. Cure-centered correlative endpoints within trials for people with HIV and cancer would allow for the exploration of promising agents in this setting. The impact of tumor on T-cell activation and recruitment can be assessed, and its effect on HIV-treatment parameters and the HIV reservoir can also be determined. Most importantly, these trials can provide a pool of potential participants interested in making a contribution to HIV cure efforts while availing themselves of expanded options to treat their tumor and contributing to oncology knowledge. The number of agents that may be “screened” for utility in cure could therefore be expanded. Further development of promising agents might then be conducted through dedicated cure trials where more detailed assessment of their role may be made or drug combinations that are of specific interest to cure endpoints could be evaluated.
8 Conclusions
For the first time since the advent of effective ART, our emerging understanding of the role of the HIV reservoir and viral latency in persistence offers the realistic prospect of achieving a sustained remission or a sterilizing cure for HIV in infected individuals. The processes and pathways involved in latency are complex and overlapping, and our understanding of them is likely to evolve in parallel with efforts aimed at their disruption. At this early stage of exploration, the prospect of leveraging agents from cancer therapeutics and cancer clinical trials may accelerate evaluation of potentially useful agents and provide a foundation for dedicated cure studies.
Key Points.
Persistence of an HIV reservoir in long-lived immune cells has been a barrier to its eradication or cure.
Recent cases of cure or sustained remission suggest clearance or control of the HIV reservoir may possible.
Many therapies now being developed for cancer indications may have dual utility in targeting the HIV reservoir. These could be explored in trials in people with HIV and cancer prior to dedicated “cure” trials.
Acknowledgments
Funding This project has been funded in whole or in part with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN272200800014C and by the Intramural Research Program of the National Cancer Institute, National Institutes of Health, Department of Human Services
Footnotes
This paper was written by the authors in their capacity as NIH employees, but the views expressed in this paper do not necessarily represent those of the NIH.
Compliance with Ethical Standards
Conflicts of Interest M. N. Polizzotto, C. Godfrey, G. Chen and R. L. Tressler declare that they have no conflicts of interest.
References
- 1.Wong JK, Hezareh M, Gunthard HF, et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278(5341):1291–5. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- 2.Chomont N, El-Far M, Ancuta P, et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med. 2009;15(8):893–900. doi: 10.1038/nm.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hermankova M, Siliciano JD, Zhou Y, et al. Analysis of human immunodeficiency virus type 1 gene expression in latently infected resting CD4+ T lymphocytes in vivo. J Virol. 2003;77(13):7383–92. doi: 10.1128/JVI.77.13.7383-7392.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siliciano JD, Kajdas J, Finzi D, et al. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med. 2003;9(6):727–8. doi: 10.1038/nm880. [DOI] [PubMed] [Google Scholar]
- 5.Swiggard WJ, Baytop C, Yu JJ, et al. Human immunodeficiency virus type 1 can establish latent infection in resting CD4(+) T cells in the absence of activating stimuli. J Virol. 2005;79(22):14179–88. doi: 10.1128/JVI.79.22.14179-14188.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buzon MJ, Sun H, Li C, et al. HIV-1 persistence in CD4+ T cells with stem cell-like properties. Nat Med. 2014;20(2):139–42. doi: 10.1038/nm.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dahabieh MS, Battivelli E, Verdin E. Understanding HIV latency: the road to an HIV cure. Annu Rev Med. 2015;66:407–21. doi: 10.1146/annurev-med-092112-152941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar A, Abbas W, Herbein G. HIV-1 latency in monocytes/macrophages. Viruses. 2014;6(4):1837–60. doi: 10.3390/v6041837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell JH, Hearps AC, Martin GE, Williams KC, Crowe SM. The importance of monocytes and macrophages in HIV pathogenesis, treatment, and cure. Aids. 2014;28(15):2175–87. doi: 10.1097/QAD.0000000000000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ho YC, Shan L, Hosmane NN, et al. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell. 2013;155(3):540–51. doi: 10.1016/j.cell.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richman DD, Margolis DM, Delaney M, Greene WC, Hazuda D, Pomerantz RJ. The challenge of finding a cure for HIV infection. Science. 2009;323(5919):1304–7. doi: 10.1126/science.1165706. [DOI] [PubMed] [Google Scholar]
- 12.Hatano H, Jain V, Hunt PW, et al. Cell-based measures of viral persistence are associated with immune activation and programmed cell death protein 1 (PD-1)-expressing CD4+ T cells. J Infect Dis. 2013;208(1):50–6. doi: 10.1093/infdis/jis630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wagner TA, McLaughlin S, Garg K, et al. HIV latency. Proliferation of cells with HIV integrated into cancer genes contributes to persistent infection. Science. 2014;345(6196):570–3. doi: 10.1126/science.1256304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maldarelli F, Wu X, Su L, et al. HIV latency. Specific HIV integration sites are linked to clonal expansion and persistence of infected cells. Science. 2014;345(6193):179–83. doi: 10.1126/science.1254194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fukazawa Y, Lum R, Okoye AA, et al. B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nat Med. 2015;21(2):132–9. doi: 10.1038/nm.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fletcher CV, Staskus K, Wietgrefe SW, et al. Persistent HIV-1 replication is associated with lower antiretroviral drug concentrations in lymphatic tissues. Proc Natl Acad Sci. 2014;111(6):2307–12. doi: 10.1073/pnas.1318249111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruelas DS, Greene WC. An integrated overview of HIV-1 latency. Cell. 2013;155(3):519–29. doi: 10.1016/j.cell.2013.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Canestri A, Lescure FX, Jaureguiberry S, et al. Discordance between cerebral spinal fluid and plasma HIV replication in patients with neurological symptoms who are receiving suppressive antiretroviral therapy. Clin Infect Dis. 2010;50(5):773–8. doi: 10.1086/650538. [DOI] [PubMed] [Google Scholar]
- 19.Archin NM, Vaidya NK, Kuruc JD, et al. Immediate antiviral therapy appears to restrict resting CD4+ cell HIV-1 infection without accelerating the decay of latent infection. Proc Natl Acad Sci. 2012;109(24):9523–8. doi: 10.1073/pnas.1120248109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Persaud D, Gay H, Ziemniak C, et al. Absence of detectable HIV-1 viremia after treatment cessation in an infant. N Engl J Med. 2013;369(19):1828–35. doi: 10.1056/NEJMoa1302976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saez-Cirion A, Bacchus C, Hocqueloux L, et al. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog. 2013;9(3):e1003211. doi: 10.1371/journal.ppat.1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hutter G, Nowak D, Mossner M, et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med. 2009;360(7):692–8. doi: 10.1056/NEJMoa0802905. [DOI] [PubMed] [Google Scholar]
- 23.Tebas P, Stein D, Tang WW, et al. Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N Engl J Med. 2014;370(10):901–10. doi: 10.1056/NEJMoa1300662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henrich TJ, Hu Z, Li JZ, et al. Long-term reduction in peripheral blood HIV type 1 reservoirs following reduced-intensity conditioning allogeneic stem cell transplantation. J Infect Dis. 2013;207(11):1694–702. doi: 10.1093/infdis/jit086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henrich TJ, Hanhauser E, Marty FM, et al. Antiretroviral-free HIV-1 remission and viral rebound after allogeneic stem cell transplantation: report of 2 cases. Ann Intern Med. 2014;161(5):319–27. doi: 10.7326/M14-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shan L, Deng K, Shroff NS, et al. Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity. 2012;36(3):491–501. doi: 10.1016/j.immuni.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Badley AD, Sainski A, Wightman F, Lewin SR. Altering cell death pathways as an approach to cure HIV infection. Cell Death Dis. 2013;4(7):e718. doi: 10.1038/cddis.2013.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boesch AW, Alter G, Ackerman ME. Prospects for engineering HIV-specific antibodies for enhanced effector function and half-life. Curr Opin HIV AIDS. 2015;10(3):160–9. doi: 10.1097/COH.0000000000000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forthal D, Hope T, Alter G. New paradigms for functional HIV-specific non-neutralizing antibodies. Curr Opin HIV AIDS. 2013;8(5):393–401. doi: 10.1097/COH.0b013e328363d486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siliciano JD, Siliciano RF. Recent developments in the search for a cure for HIV-1 infection: targeting the latent reservoir for HIV-1. J Allergy Clin Immunol. 2014;134(1):12–9. doi: 10.1016/j.jaci.2014.05.026. [DOI] [PubMed] [Google Scholar]
- 31.Verdin E. DNase I-hypersensitive sites are associated with both long terminal repeats and with the intragenic enhancer of integrated human immunodeficiency virus type 1. J Virol. 1991;65(12):6790–9. doi: 10.1128/jvi.65.12.6790-6799.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manson McManamy ME, Hakre S, Verdin EM, Margolis DM. Therapy for latent HIV-1 infection: the role of histone deacetylase inhibitors. Antiviral Chem Chemother. 2014;23(4):145–9. doi: 10.3851/IMP2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shirakawa K, Chavez L, Hakre S, Calvanese V, Verdin E. Reactivation of latent HIV by histone deacetylase inhibitors. Trends Microbiol. 2013;21(6):277–85. doi: 10.1016/j.tim.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Campbell GR, Bruckman RS, Chu Y, Spector SA. Autophagy induction by histone deacetylase inhibitors inhibits HIV type 1. J Biol Chem. 2015;290(8):5028–40. doi: 10.1074/jbc.M114.605428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Lint C, Emiliani S, Ott M, Verdin E. Transcriptional activation and chromatin remodeling of the HIV-1 promoter in response to histone acetylation. EMBO J. 1996;15(5):1112–20. [PMC free article] [PubMed] [Google Scholar]
- 36.Lehrman G, Hogue IB, Palmer S, et al. Depletion of latent HIV-1 infection in vivo: a proof-of-concept study. Lancet. 2005;366(9485):549–55. doi: 10.1016/S0140-6736(05)67098-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Archin NM, Eron JJ, Palmer S, et al. Valproic acid without intensified antiviral therapy has limited impact on persistent HIV infection of resting CD4+ T cells. AIDS. 2008;22(10):1131–5. doi: 10.1097/QAD.0b013e3282fd6df4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Archin NM, Cheema M, Parker D, et al. Antiretroviral intensification and valproic acid lack sustained effect on residual HIV-1 viremia or resting CD4+ cell infection. PLoS One. 2010;5(2):e9390. doi: 10.1371/journal.pone.0009390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Routy JP, Tremblay CL, Angel JB, et al. Valproic acid in association with highly active antiretroviral therapy for reducing systemic HIV-1 reservoirs: results from a multicentre randomized clinical study. HIV Med. 2012;13(5):291–6. doi: 10.1111/j.1468-1293.2011.00975.x. [DOI] [PubMed] [Google Scholar]
- 40.Archin NM, Liberty AL, Kashuba AD, et al. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature. 2012;487(7408):482–5. doi: 10.1038/nature11286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elliott JH, Wightman F, Solomon A, et al. Activation of HIV transcription with short-course vorinostat in HIV-infected patients on suppressive antiretroviral therapy. PLoS Pathog. 2014;10(10):e1004473. doi: 10.1371/journal.ppat.1004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Glaser KB. HDAC inhibitors: clinical update and mechanism-based potential. Biochem Pharmacol. 2007;74(5):659–71. doi: 10.1016/j.bcp.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 43.Archin NM, Bateson R, Tripathy MK, et al. HIV-1 expression within resting CD4+ T cells after multiple doses of vorinostat. J Infect Dis. 2014;210(5):728–35. doi: 10.1093/infdis/jiu155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dolgin E. Underestimate of HIV reservoirs threatens purging approach. Nat Med. 2013;19(4):384–5. doi: 10.1038/nm0413-384. [DOI] [PubMed] [Google Scholar]
- 45.Rasmussen TATM, Brinkmann CR, Olesen R, Erikstrup C, Solomon A, Winckelmann A, Palmer S, Dinarello C, Buzon M, Lichterfeld M, Lewin SR, Ostergaard L, Sagaard O. Panobinostat, a histone deacetylase inhibitor, for latent virus reactivation in HIV-infected patients on suppressive antiretroviral therapy: a phase 1/2, single group, clinical trial. Lancet HIV. 2014;1(1):e13–21. doi: 10.1016/S2352-3018(14)70014-1. [DOI] [PubMed] [Google Scholar]
- 46.Wei DG, Chiang V, Fyne E, et al. Histone deacetylase inhibitor romidepsin induces HIV expression in CD4 T cells from patients on suppressive antiretroviral therapy at concentrations achieved by clinical dosing. PLoS Pathog. 2014;10(4):e1004071. doi: 10.1371/journal.ppat.1004071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sogaard OS, Graverson ME, Leth S, Brinkmann CR, Kjaer AS, Olesen R, Denton PW, Nissen S, Sommerfelt M, Rasmussen TA, Ostergaard L, Tolstrup M. The HDAC inhibitor romidepsin is safe and effectively reverses HIV-1 latency in vivo as measured by standard clinical assays.. 20th International AIDS conference, AIDS 2014; Melbourne, Australia. 2014. [Google Scholar]
- 48.Papavassiliou KA, Papavassiliou AG. Bromodomains: pockets with therapeutic potential. Trends Mol Med. 2014;20(9):477–8. doi: 10.1016/j.molmed.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 49.Budhiraja S, Rice AP. Reactivation of latent HIV: do all roads go through P-TEFb? Fut virol. 2013 doi: 10.2217/fvl.13.52. doi:10.2217/fvl.2213.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Belkina AC, Denis GV. BET domain co-regulators in obesity, inflammation and cancer. Nat Rev Cancer. 2012;12(7):465–77. doi: 10.1038/nrc3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Muller S, Filippakopoulos P, Knapp S. Bromodomains as therapeutic targets. Expert Rev Mol Med. 2011;13:e29. doi: 10.1017/S1462399411001992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wightman F, Solomon A, Kumar S, et al. Effect of ipilimumab on the HIV reservoir in an HIV-infected individual with meta-static melanoma. AIDS. 2015;29(4):504–6. doi: 10.1097/QAD.0000000000000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beck KE, Blansfield JA, Tran KQ, et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol. 2006;24(15):2283–9. doi: 10.1200/JCO.2005.04.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barber DL, Wherry EJ, Masopust D, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439(7077):682–7. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 55.Ferris RL, Lu B, Kane LP. Too much of a good thing? Tim-3 and TCR signaling in T cell exhaustion. J Immunol. 2014;193(4):1525–30. doi: 10.4049/jimmunol.1400557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Day CL, Kaufmann DE, Kiepiela P, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443(7109):350–4. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 57.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Favre D, Mold J, Hunt PW, et al. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of T(H)17 to regulatory T cells in HIV disease. Sci Transl Med. 2010;2(32):32ra36. doi: 10.1126/scitranslmed.3000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.De Lantigue J. [30 Apr 2015];Another immune checkpoint emerges as anti-cancer target 2013. 2015 [Google Scholar]
- 60.Walker BD, Chakrabarti S, Moss B, et al. HIV-specific cytotoxic T lymphocytes in seropositive individuals. Nature. 1987;328(6128):345–8. doi: 10.1038/328345a0. [DOI] [PubMed] [Google Scholar]
- 61.Saez-Cirion A, Lacabaratz C, Lambotte O, et al. HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation pheno-type. Proc Natl Acad Sci. 2007;104(16):6776–81. doi: 10.1073/pnas.0611244104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Koup RA, Safrit JT, Cao Y, et al. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68(7):4650–5. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Koenig S, Conley AJ, Brewah YA, et al. Transfer of HIV-1-specific cytotoxic T lymphocytes to an AIDS patient leads to selection for mutant HIV variants and subsequent disease progression. Nat Med. 1995;1(4):330–6. doi: 10.1038/nm0495-330. [DOI] [PubMed] [Google Scholar]
- 64.Riddell SR, Elliott M, Lewinsohn DA, et al. T-cell mediated rejection of gene-modified HIV-specific cytotoxic T lymphocytes in HIV-infected patients. Nat Med. 1996;2(2):216–23. doi: 10.1038/nm0296-216. [DOI] [PubMed] [Google Scholar]
- 65.Lieberman J, Skolnik PR, Parkerson GR, 3rd, et al. Safety of autologous, ex vivo-expanded human immunodeficiency virus (HIV)-specific cytotoxic T-lymphocyte infusion in HIV-infected patients. Blood. 1997;90(6):2196–206. [PubMed] [Google Scholar]
- 66.Lam S, Bollard C. T-cell therapies for HIV. Immunotherapy. 2013;5(4):407–14. doi: 10.2217/imt.13.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Scholler J, Brady TL, Binder-Scholl G, et al. Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. Science Transl Med. 2012;4(132):132ra153. doi: 10.1126/scitranslmed.3003761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mitsuyasu RT, Anton PA, Deeks SG, et al. Prolonged survival and tissue trafficking following adoptive transfer of CD4zeta gene-modified autologous CD4(+) and CD8(+) T cells in human immunodeficiency virus-infected subjects. Blood. 2000;96(3):785–93. [PubMed] [Google Scholar]
- 69.Deeks SG, Wagner B, Anton PA, et al. A phase II randomized study of HIV-specific T-cell gene therapy in subjects with undetectable plasma viremia on combination antiretroviral therapy. Mol Ther. 2002;5(6):788–97. doi: 10.1006/mthe.2002.0611. [DOI] [PubMed] [Google Scholar]
- 70.Mitsuyasu R. Curing HIV: lessons from cancer therapy. Curr Opin HIV AIDS. 2013;8(3):224–9. doi: 10.1097/COH.0b013e32835ef0a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Drake MJ, Bates P. Application of gene-editing technologies to HIV-1. Curr Opin HIV AIDS. 2015;10(2):123–7. doi: 10.1097/COH.0000000000000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Husseinzadeh N, Husseinzadeh H. mTOR inhibitors and their clinical application in cervical, endometrial and ovarian cancers: a critical review. Gynecol Oncol. 2014;133(2):375–81. doi: 10.1016/j.ygyno.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 73.Rao RR, Li Q, Odunsi K, Shrikant PA. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin. Immunity. 2010;32(1):67–78. doi: 10.1016/j.immuni.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jakubowiak AJ, Dytfeld D, Griffith KA, et al. A phase 1/2 study of carfilzomib in combination with lenalidomide and low-dose dexamethasone as a frontline treatment for multiple myeloma. Blood. 2012;120(9):1801–9. doi: 10.1182/blood-2012-04-422683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lu G, Middleton RE, Sun H, et al. The myeloma drug lenalidomide promotes the cereblon-dependent destruction of Ikaros proteins. Science. 2014;343(6168):305–9. doi: 10.1126/science.1244917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Richardson PG, Siegel D, Baz R, et al. Phase I study of pomalidomide MTD, safety and efficacy in patients with refractory multiple myeloma who have received lenalidomide and bortezomib. Blood. 2013;121(11):1961–7. doi: 10.1182/blood-2012-08-450742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lacy MQ, Allred JB, Gertz MA, et al. Pomalidomide plus low-dose dexamethasone in myeloma refractory to both bortezomib and lenalidomide: comparison of two dosing strategies in dual-refractory disease. Blood. 2011;118(11):2970–5. doi: 10.1182/blood-2011-04-348896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lopez-Girona A, Mendy D, Ito T, et al. Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia. 2012;26(11):2326–35. doi: 10.1038/leu.2012.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Escoubet-Lozach L, Lin I-L, Jensen-Pergakes K, et al. Pomalidomide and lenalidomide induce p21 WAF-1 expression in both lymphoma and multiple myeloma through a LSD1-mediated epigenetic mechanism. Cancer Res. 2009;69(18):7347–56. doi: 10.1158/0008-5472.CAN-08-4898. [DOI] [PubMed] [Google Scholar]
- 80.Görgün G, Calabrese E, Soydan E, et al. Immunomodulatory effects of lenalidomide and pomalidomide on interaction of tumor and bone marrow accessory cells in multiple myeloma. Blood. 2010;116(17):3227–37. doi: 10.1182/blood-2010-04-279893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kotla V, Goel S, Nischal S, et al. Mechanism of action of lenalidomide in hematological malignancies. J Hematol Oncol. 2009;2:36. doi: 10.1186/1756-8722-2-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kuwabara S, Misawa S, Kanai K, et al. Thalidomide reduces serum VEGF levels and improves peripheral neuropathy in POEMS syndrome. J Neurol Neurosurg Psychiatry. 2008;79(11):1255–7. doi: 10.1136/jnnp.2008.150177. [DOI] [PubMed] [Google Scholar]
- 83.Ito T, Ando H, Suzuki T, et al. Identification of a primary target of thalidomide teratogenicity. Science. 2010;327(5971):1345–50. doi: 10.1126/science.1177319. [DOI] [PubMed] [Google Scholar]
- 84.Xu W, Celeridad M, Sankar S, Webb DR, Bennett BL. CC-4047 promotes Th1 cell differentiation and reprograms polarized human Th2 cells by enhancing transcription factor T-bet. Clin Immunol. 2008;128(3):392–9. doi: 10.1016/j.clim.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 85.Bren GD, Whitman J, Cummins N, et al. Infected cell killing by HIV-1 protease promotes NF-kappaB dependent HIV-1 replication. PLoS One. 2008;3(5):e2112. doi: 10.1371/journal.pone.0002112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dubovsky JA, Beckwith KA, Natarajan G, et al. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood. 2013;122(15):2539–49. doi: 10.1182/blood-2013-06-507947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Horna P, Sotomayor EM. Cellular and molecular mechanisms of tumor-induced T-cell tolerance. Curr Cancer Drug Targets. 2007;7(1):41–53. doi: 10.2174/156800907780006940. [DOI] [PubMed] [Google Scholar]
- 88.Ertmer A, Huber V, Gilch S, et al. The anticancer drug imatinib induces cellular autophagy. Leukemia. 2007;21(5):936–42. doi: 10.1038/sj.leu.2404606. [DOI] [PubMed] [Google Scholar]
- 89.Schuster IS, Wikstrom ME, Brizard G, et al. TRAIL+ NK cells control CD4+ T cell responses during chronic viral infection to limit autoimmunity. Immunity. 2014;41(4):646–56. doi: 10.1016/j.immuni.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 90.Barblu L, Smith N, Durand S, et al. Reduction of death receptor 5 expression and apoptosis of CD4+ T cells from HIV controllers. Clin Immunol. 2014;155(1):17–26. doi: 10.1016/j.clim.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 91.Trivedi R, Mishra DP. Trailing TRAIL resistance: novel targets for TRAIL sensitization in cancer cells. Front Oncol. 2015;5:69. doi: 10.3389/fonc.2015.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Haselmann V, Kurz A, Bertsch U, et al. Nuclear death receptor TRAIL-R2 inhibits maturation of let-7 and promotes proliferation of pancreatic and other tumor cells. Gastroenterology. 2014;146(1):278–90. doi: 10.1053/j.gastro.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 93.Chen R, Alvero AB, Silasi DA, Steffensen KD, Mor G. Cancers take their Toll: the function and regulation of Toll-like receptors in cancer cells. Oncogene. 2008;27(2):225–33. doi: 10.1038/sj.onc.1210907. [DOI] [PubMed] [Google Scholar]
- 94.Sloan D IA, Tsai A, Kaur J, Murry J, Cihlar T, Lalezari JT. TLR7 agonist GS-9620 activates HIV-1 in PBMCs from HIV-infected patients on cART.. Conference on retroviruses and opportunistic infections; Seattle, Washington. February 23–26, 2015; 2015. Abstract 417. [Google Scholar]
- 95.Whitney J, et al. Treatment with a TLR7 agonist induced transient viremia in SIV-infected ART-suppressed monkeys.. Conference on retroviruses and opportunistic infections; Seattle, Washington. February 23–26, 2015; 2015. Abstract 417. [Google Scholar]
- 96.Zent CS, Smith BJ, Ballas ZK, et al. Phase I clinical trial of CpG oligonucleotide 7909 (PF-03512676) in patients with previously treated chronic lymphocytic leukemia. Leukemia Lymphoma. 2012;53(2):211–7. doi: 10.3109/10428194.2011.608451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lahoud MH, Ahmet F, Zhang JG, et al. DEC-205 is a cell surface receptor for CpG oligonucleotides. Proc Natl Acad Sci. 2012;109(40):16270–5. doi: 10.1073/pnas.1208796109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Offersen R, Melchjorsen J, Paludan SR, Ostergaard L, Tolstrup M, Sogaard OS. TLR9-adjuvanted pneumococcal conjugate vaccine induces antibody-independent memory responses in HIV-infected adults. Hum Vaccines Immunother. 2012;8(8):1042–7. doi: 10.4161/hv.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Winckelmann AA, Munk-Petersen LV, Rasmussen TA, et al. Administration of a Toll-like receptor 9 agonist decreases the proviral reservoir in virologically suppressed HIV-infected patients. PLoS One. 2013;8(4):e62074. doi: 10.1371/journal.pone.0062074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434(7035):843–50. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 101.Takebe N, Harris PJ, Warren RQ, Ivy SP. Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog pathways. Nat Rev Clin Oncol. 2011;8(2):97–106. doi: 10.1038/nrclinonc.2010.196. [DOI] [PubMed] [Google Scholar]
- 102.Administration FaD Guidance for Industry Codevelopment of Two or More New Investigational Drugs for Use in Combination. Research CfDEa. :ed2013. [Google Scholar]
- 103.Park LS, Tate JP, Rodriguez-Barradas MC, et al. Cancer incidence in HIV-infected versus uninfected veterans: comparison of cancer registry and ICD-9 Code diagnoses. J AIDS Clin Res. 2014;5(7):1000318. doi: 10.4172/2155-6113.1000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shiels MS, Pfeiffer RM, Gail MH, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst. 2011;103(9):753–62. doi: 10.1093/jnci/djr076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Robbins HA, Pfeiffer RM, Shiels MS, Li J, Hall HI, Engels EA. Excess cancers among HIV-infected people in the United States. J Natl Cancer Inst. 2015;107(4) doi: 10.1093/jnci/dju503. doi:10.1093/jnci/dju503. [DOI] [PMC free article] [PubMed] [Google Scholar]