Summary
Codon usage bias is a universal feature of eukaryotic and prokaryotic genomes and has been proposed to regulate translation efficiency, accuracy and protein folding based on the assumption that codon usage affects translation dynamics. The roles of codon usage in translation, however, are not clear and have been challenged by recent ribosome profiling studies. Here we used a Neurospora cell-free translation system to directly monitor the velocity of mRNA translation. We demonstrated that the preferred codons enhance rate of translation elongation, whereas non-optimal codons slow elognatioon. Codon usage also controls ribosome traffic on mRNA. These conclusions were further supported by ribosome profiling results in vitro and in vivo with template mRNAs designed to increase signal to noise. Finally, we demonstrate that codon usage regulates protein function by affecting co-translational protein folding. These results resolve a long-standing fundamental question and suggest the existence of a codon usage code for protein folding.
Keywords: Codon usage bias, translation elongation, Neurospora, protein folding
Introduction
The translation of mRNAs into polypeptides by the ribosome is one of the most fundamental biological processes. Most amino acids are encoded by two to six synonymous codons. Synonymous codons are not used with equal frequencies and codon usage bias is an essential feature of most genomes (Comeron, 2004; Ikemura, 1985; Plotkin and Kudla, 2011; Sharp et al., 1986). Selection for efficient translation is thought to be the major cause of codon usage bias , and highly expressed genes are encoded predominantly by codons that correspond to highly abundant tRNAs, which is thought to enable rapid, high-fidelity translation (Akashi, 1994; Drummond and Wilke, 2008; Hershberg and Petrov, 2008). However, codon usage bias is uneven across open-reading frames, and many genes exhibit little or no codon usage bias.
It has been suggested that protein folding and functions can be affected by synonymous codon substitutions for heterologously expressed genes (Komar et al., 1999; Siller et al., 2010; Spencer et al., 2012; Zhang et al., 2009). We previously showed that codon usage in a circadian clock gene, frequency (frq), is critical for the in vivo function of FRQ in Neurospora (Zhou et al., 2013). In addition, a single synonymous SNP in the human multidrug resistance 1 (MDR1) gene results in an altered protein function (Kimchi-Sarfaty et al., 2007). Bioinformatic analyses have identified correlations between codon usage and propensities to form certain protein structural motifs (Pechmann et al., 2014; Pechmann and Frydman, 2013; Zhou et al., 2009). The fitness of viruses is also proposed to be affected by codon modifications of viral genomes (Bull et al., 2012). We recently found that regions predicted to be unstructured are preferentially encoded by non-optimal codons, whereas regions likely to be well-structured are preferentially encoded by preferred codons (Zhou et al., 2015). These studies led to the hypothesis that codon usage and protein structures co-evolved to allow proper folding of proteins.
Underlying all hypotheses regarding the role of codon usage is the assumption that codon identity affects the rate of translation elongation. Clear evidence supporting this assumption is lacking, however. Studies concerning this issue relied on indirect measurements and protein overexpression and have resulted in conflicting conclusions (Bonekamp et al., 1989; Charneski and Hurst, 2013; Chevance et al., 2014; Sorensen et al., 1989). Ribosome profiling has recently emerged as a powerful tool to study protein translation dynamics with codon-level resolution of ribosome locations (Ingolia et al., 2009; Ingolia et al., 2011). In principle, ribosome densities at a particular codon should inversely correlate with translation elongation velocity. Surprisingly, however, a series of studies based on ribosome profiling data found no correlations between codon usage and levels of ribosome protected fragments (RPFs) (Charneski and Hurst, 2013; Ingolia et al., 2011; Li et al., 2012; Qian et al., 2012). Moreover, bioinformatic analyses of ribosome profiling results on potential pausing sites often resulted in conflicting conclusions (Artieri and Fraser, 2014; Charneski and Hurst, 2013; Pop et al., 2014; Tuller et al., 2010a). Recently, it was shown that ribosomal profiling results can be influenced by growth conditions, depth of coverage, cloning/sequencing biases, methods of bioinformatic analysis, and experimental noise (Artieri and Fraser, 2014; Gardin et al., 2014; Lareau et al., 2014; Nakahigashi et al., 2014). Thus, the conventional ribosome profiling method may not be sensitive enough to detect a clear effect of codon usage on elongation rate.
The filamentous fungus Neurospora crassa exhibits a strong codon usage bias. We previously demonstrated that the use of non-optimal codons in the frq gene is important for the expression, function and structure of the FRQ protein (Zhou et al., 2013). More recently, we discovered genome-wide correlations between codon usage and predicted protein structures (Zhou et al., 2015). Our data suggest an important role for codon usage in regulating co-translational protein folding. In this study, we sought to determine the role of codon usage on translation elongation speed and co-translational protein folding.
RESULTS
Codon optimization enhances translation rates of both heterologous and endogenous genes
We directly visualized the rate of mRNA translation using a Neurospora cell-free translation system that was previously shown to accurately reflect protein translation in vivo (Hood et al., 2009; Wang et al., 1999; Wang and Sachs, 1997; Wei et al., 2013; Wu et al., 2007). Cellular mRNAs are depleted from this system by micrococcal nuclease digestion. Firefly luciferase (Luc) mRNAs with different extents of codon manipulation were used as templates in the cell-free system. Because the luminescence signal generated by luciferase can be easily detected in real time, we used the time of first appearance (TFA) of luminescence signal (i.e. the first time point that the luminescence signal is significantly above background level) as a measure of translation velocity (Wang et al., 1998). The time needed for translation initiation should be the same for the Luc mRNAs because they share the same initiation context. It should be noted that other factors, such as protein folding, translation termination and ribosome recycling processes, may also contribute to TFA values (Dever and Green, 2012). As luciferase is known to be rapidly folded (within a few seconds) after translation (Kolb et al., 1994), the difference in TFA values should largely reflect the difference in translation elongation rates.
We first created three Luc constructs with identical 5' untranslated region (UTR), AUG start codon context and poly-A length: WT (wild-type Luc mRNA), OPT (the entire Luc open reading frame [ORF] codons optimized according to the N. crassa codon usage table), and de-OPT (the least preferred codon used for every amino acid). Their calculated average codon adaptation indices (CAI) (Sharp and Li, 1987) are 0.65, 1.00, and 0.36, respectively. As shown in Figure 1A, the TFA values of the Luc mRNAs correlated nicely with the CAI values: the TFA of WT was 1.5 min later than the TFA of the OPT mRNA but was ~6 min sooner than that of the de-OPT mRNA. Because the translation of de-OPT mRNA is very slow, which may causes unwanted effects in the translation extracts, the following experiments were focused on WT and OPT constructs. To rule out the possibility that the differences in TFAs were due to variations in translation initiation or in RNA structures that formed near the start codon, we replaced the first ten codons of the OPT mRNA with the WT sequence and found that the ~1.5 min differences in TFAs between WT and OPT mRNAs were maintained (Figure S1, related Figure 1).
Figure 1. Codon usage affects translation elongation rate.
(A) Real-time measurement of firefly luciferase (Luc) activity using Neurospora cell-free translation system at 26 °C. Recorded relative light units (RLU) were plotted versus translation reaction time in 30 seconds intervals. Time of first appearances (TFAs) are indicated by arrows. Data are represented as mean +/− standard deviations. (B) SDS-PAGE analysis showing the [35S]methionine-labeled translational products from WT and OPT Luc mRNAs obtained from micrococcal nuclease-treated Neurospora cell-free lysates. NC is background translation (without added mRNA). The arrow indicates the position of full-length LUC. (C and D) The Scatter plots show the effect of codon usage frequency on elongation speed by comparison the TFA of the indicated Luc mRNAs. In (C), N-OP-WT and M-OP-WT have codons corresponding to amino acids 2–223 and 224–423 optimized, respectively. In (D), N-WT-OPT and M-WT-OPT have codons corresponding to amino acids 2–223 and 224–423 as WT sequences, respectively. *P < 0.05, **P < 0.01. (E) Plot shows the real-time LUC activity of FRQ-WT Luc fusion proteins. The TFA of each construct is indicated by an arrow. Means indicated by horizontal bars for all measurements were derived from four to ten independent experiments. Data are represented as mean +/− standard deviations. See also Figure S1.
To further confirm these results, we performed in vitro translation in the presence of [35S] methionine (Fang et al., 2004). In agreement with the TFA results, the [35S]-labeled full-length LUC protein of the OPT mRNA appeared ~ 2 min earlier than the TFA of LUC protein encoded by the WT mRNA (Figure 1B), confirming that differences in TFA correlate with differences in the rates of translation.
To evaluate potential effects of RNA structure within the Luc mRNA and accumulative effects of codon usage along the ORF, we created additional constructs. In the N-OPT-WT and M-OPT-WT constructs, the N-terminal region (codons 2–223) and middle region (codons 223–423) of the WT Luc were optimized, respectively. In the N-WT-OPT and M-WT-OPT constructs, the N-terminal region (codons 2–223) and middle region (codons 223–423), respectively, of OPT Luc are changed to WT Luc sequence. The TFA values of N-OPT-WT and M-OPT-WT were each 0.5 min sooner than that of WT (Figure 1C). Conversely, the TFA values of N-WT-OPT and M-WT-OPT were each 0.5 min later than that of OPT (Figure 1D). These results suggest that the effect of codon usage on elongation rate is due to cumulative effects of codon usage in the coding sequence rather than changes in specific RNA structure.
To determine whether codon usage influences translation speed of endogenous Neurospora coding regions, we created two constructs (WT-FRQ-Luc and m-FRQ-Luc), in which the N-terminal region (codons 1–169) of the clock gene frq was fused with the WT Luc sequence. In the m-FRQ-Luc construct, the least preferred synonymous codons of frq were changed to the preferred codons. We previously showed that the codon optimization of this region of frq altered both activity and structure of FRQ and abolished circadian rhythms (Zhou et al., 2013). As expected, the TFA of m-FRQ-Luc mRNA was ~0.7 min sooner than that of the WT-FRQ-Luc in the cell-free translation system (Figure 1E). This result indicates that codon usage impacts elongation rate of endogenous Neurospora coding regions and that the effect of codon usage on FRQ protein structure is due to effects on elongation rate. Together, these results suggest that optimal codon usage increases translation elongation rate, whereas non-optimal codon usage slows down elongation in Neurospora.
The effect of codon usage is maintained over a range of temperatures
Temperature is an important factor regulating translation dynamics. To further confirm the effect of codon usage, we performed in vitro translation under different temperatures. As expected, elevation of temperature from 22°C to 26°C and to 30°C led to faster TFAs for both WT and OPT mRNAs (Figure 2A), indicating that increasing temperature increases elongation rates. However, the relative differences in TFAs between WT and OPT mRNAs were maintained at each temperature examined. Comparison of TFAs showed that the effect of altering codon usage is greater than that of altering temperature by 4°C.
Figure 2. Elongation rate is affected by codon usage frequency rather than by codon-tRNA balance or mRNA secondary structures.
(A) Scatter plot shows the TFA of WT and OPT Luc using Neurospora cell-free lysates at different temperatures. (B) Scatter plot shows the TFA of WT and OPT Luc in micrococcal nuclease-treated and untreated Neurospora cell-free lysates. (C) Scatter plot shows the TFAs of programmed translation reactions using micrococcal-nuclease-treated Neurospora lysates with indicated concentrations of WT and OPT Luc mRNAs. (D) Plot shows the real-time Luc activity of WT and OPT mRNAs using nuclease-treated yeast cell-free extracts. The TFAs are indicated by arrows. Means are indicated by horizontal bars and were derived from three to six independent experiments. Data are represented as mean +/− standard deviations.
The effect of codon usage is not consistent with the balanced codon usage model
To explain the previously observed lack of correlation between the codon selection time inferred from ribosome profiling data and codon usage bias in yeast, a balanced codon usage model was proposed (Qian et al., 2012). This model proposes that the decoding rate for each synonymous codon is determined by the balance between tRNA concentrations (supply) and number of synonymous codon used in mRNA (demand). Under normal physiological conditions, codon usage bias does not affect decoding speed due to the balance of supply and demand. Codon usage bias only impacts rate of decoding if this balance is broken. In our cell-free translation system, such a model can be directly tested by maintaining the amount of translation extracts (supply) and changing the Luc mRNA levels or cellular mRNAs (demand).
We first compared the TFAs of WT and OPT Luc mRNAs in the nuclease-treated and untreated cell-free translation lysates. The presence of cellular mRNAs in the untreated lysates is expected to dramatically increase the mRNA demand in the reaction. By using RNA-seq and qRT-PCR results to determine the relative levels of Luc mRNA levels (60 ng/reaction) in the untreated cell-free translation lysates (Yunkun Dang, unpublished), we estimated that Luc mRNA represents only ~2% of mRNA pool in the lysate. As shown in Figure 2B, the TFAs of WT and OPT Luc mRNAs were similar in the RNase-treated and untreated lysates despite their dramatic difference in mRNA demand. More importantly, the differences between the TFAs of WT and OPT Luc were almost identical in these two lysates. In addition, a 10-fold reduction in Luc mRNA concentration also had no significant effect on the TFA of either WT or OPT Luc mRNA or on the difference in TFAs (Figure 2C). Together, these results further suggest that preferred codons are decoded more rapidly than non-preferred codons, presumably due to higher concentrations of the tRNAs that recognize the preferred codons. Consistent with this notion, we previously showed that the preferred codon is always the codon with fastest predicted elongation rate based on tRNA gene copy numbers and the nature of anticodon-codon interactions (Zhou et al., 2013).
Codon effect on translation corresponds to the codon bias of the organism
Changing codon usage may cause RNA structural changes. Highly structured mRNA regions are known to impede ribosome progression (Somogyi et al., 1993; Wen et al., 2008). Because Neurospora prefers C/G at the wobble position for almost every codon family, codon optimization is therefore predicted to reduce translation velocity due to increase in stability of RNA structures. This is opposite from what we observed. Thus, it is unlikely that the effect of codon optimization/de-optimization on translation rate is due to changes in mRNA structure. Note that the analyses of yeast ribosome profiling data suggested that RNA structures do not play significant roles in regulating decoding rate (Charneski and Hurst, 2013).
To further examine this issue, we performed in vitro translation using Saccharomyces cerevisiae cell-free extracts. The structures of our WT and OPT Luc mRNAs should be similar as those in experiments with Neurospora extracts. Unlike the Neurospora codon preference for C/G, T/A is preferred in S. cerevisiae (CAIs of the WT and OPT Luc are 0.709 and 0.531, respectively). In contrast to the Neurospora extracts, the TFA of the OPT Luc was about 2 min slower than that of WT Luc (Figure 2D). This result indicates that the effect of codon usage on translation rate correlates with the codon usage bias of the organism. Moreover, changes in RNA structures cannot explain the effect of codon choices on elongation rate.
Codon usage controls local ribosome traffic on mRNA
Comparison of the [35S]-methionine labeled protein products of the WT and OPT mRNAs also revealed dramatic differences in labeled LUC protein profiles in the Neurospora cell-free system (Figure 1B and 3A). Translation of the WT Luc mRNA resulted in many non-full-length LUC bands. For the OPT mRNA, however, the levels of these species decreased dramatically and some disappeared. After in vitro translation, we isolated ribosome-associated nascent chains (RNCs) and showed that these intermediates are nascent peptides associated with ribosomes (Figure 3A).
Figure 3. Codon usage affects local ribosome traffic on mRNA.
(A) SDS-PAGE analysis of [35S]methionine-labeled total translation products and isolated RNCs of WT and OPT Luc after 12 min of translation reaction. The reaction was terminated by cycloheximide (0.5 mg/ml, final concentration). (B) SDS-PAGE analysis of [35S]methionine-labeled translation products of WT and OPT Luc after 12 min at indicated temperatures. The full-length Luc protein is indicated by an arrow. (C) SDS-PAGE analysis of [35S]methionine-labeled translation products of the indicated Luc mRNAs. The colored bar on the left of each lane demonstrates the codon usage patterns of the Luc gene: red indicates optimized codons; blue indicates wild-type codons.
Because temperature influences elongation rate, we performed the translation reaction at different temperatures. As expected, low temperature increased the relative levels of the intermediate LUC peptides, and high temperature decreased their relative amounts (Figure 3B). Thus, these intermediates are caused by decreases in the rate of ribosome progression during the elongation process.
Remarkably, when different regions (codon 2–223, 224–423 and 424–550) of the WT Luc were individually optimized (Figure 3C, indicated by red bars), we saw specific reduction or elimination of the intermediates within the predicted size-ranges (Figure 3C, N-OPT-WT, M-OPT-WT and C-OPT-WT). In contrast, when these regions of the OPT Luc were individually changed to WT sequences (indicated by the blue bars), the intermediates reappeared at the expected molecular weights (Figure 3C, N-WT-OPT, M-WT-OPT and C-WT-OPT). Together, these results indicate that preferred codons locally enhance the rate of ribosome elongation on the mRNA.
The effects of codon usage on ribosome occupancy in vitro and in vivo revealed by ribosome profiling
Since tRNA selection is a major rate-limiting step of ribosome progression (Gromadski and Rodnina, 2004; Lee et al., 2007), it is not clear why previous ribosome profiling studies failed to reveal the relationship between codon usage and elongation rates. A recent study suggested that the sensitivity of ribosome profiling is limited due to coverage depth, biases in cloning/sequencing, and experimental noise (Artieri and Fraser, 2014). We, therefore, sought to reexamine this question by performing ribosome profiling experiments in Neurospora, which has a stronger codon usage bias than that of yeast (Zhou et al., 2015). In addition, we performed the analyses with reporter mRNAs that have specifically engineered alterations in codon usage to increase signal to noise ratio.
We first performed ribosome profiling in the cell-free system using a Luc mRNA in which a region of the ORF was codon optimized. Thus, the relative RPF levels in the wild-type and optimized regions of a single mRNA could be compared. As shown in Figure 4A, although there were measureable peaks on each mRNA and in each region, the relative RPF levels in different Luc regions showed a remarkable negatively correlation with CAI of the corresponding mRNA region– that is, RPF increased as CAI decreased. Specifically, for the N-OPT-WT, M-OPT-WT and C-OPT-WT Luc mRNAs, with N-terminal, middle, and C-terminal regions of the Luc ORF optimized, respectively, the regions that were codon optimized all showed dramatically lower levels of RPF than the wild-type part of the mRNA, suggesting that codon optimization increased the rate of ribosome movement on mRNA.
Figure 4. Ribosome profiling analyses reveals that ribosome occupancy on mRNA negatively correlates with codon usage frequency in vitro and in vivo.
(A) RPF profile on Luc mRNAs translated in vitro. The sequenced RPFs of indicated Luc constructs from Neurospora in vitro translation are mapped to the corresponding mRNAs. The average CAI values of each gene fragment are indicated. (B) RPF profile on Luc mRNAs translated in vivo. The RPFs of indicated ccg-1-driven Luc constructs, which are transformed into his-3 locus of Neurospora genome, were mapped to the corresponding Luc gene. See also Figure S2.
To confirm these results in vivo, we placed OPT or C-WT-OPT Luc expression constructs into Neurospora at the his-3 locus, which resulted in comparable levels of Luc mRNA (Figure S2, related to Figure 4). In the C-WT-OPT construct, the C-terminal region of the OPT Luc has the wild-type sequence. Ribosome profiling of these two strains was performed. For the OPT mRNA, the RPF level was similar in the C-terminal and N-terminal regions of the gene (Figure 4B). In contrast, the RPF levels within the wild-type region of the C-WT-OPT mRNA were much higher than those in the optimized region. The increased RPF in the wild-type region is not likely to be due to mRNA structure changes since the wild-type mRNA sequence is predicted to have less stable RNA structures due to it lower GC content. Together, these results demonstrate the role of codon usage in regulating elongation rate in vivo and in vitro and indicate that RPF can reflect ribosome traffic on mRNA if the signal-to-noise ratio is high enough.
We next examined the genome-wide correlation between codon usage frequencies and RPF-derived decoding rates based on our in vivo ribosomal profiling results (Charneski and Hurst, 2013; Ingolia et al., 2011; Li et al., 2012; Qian et al., 2012). To compare the relative decoding rates of 61 codons, the RPF read of each codon was normalized to that of the most occupied codon (CCA, proline) to generate the relative codon decoding rate (RCDR) value. The relative codon usage frequency (RCUF) for each codon was determined by calculating the relative frequency of a given codon among all annotated N. crassa coding sequences. A modestly strong negative (ρ value of −0.48) correlation was observed between RCDR and RCUF (Figure 5). This result suggests that frequently used codons are generally decoded faster than non-preferred codons in Neurospora.
Figure 5. Genome-wide negative correlations between RPFs and synonymous codon usage in vivo.
A scatter plot shows the negative correlation between the relative codon decoding rate (RCDR) and relative codon usage frequency (RCUF) for all coding genes. Spearman's rank correlation coefficient (ρ) and the associated P value are indicated.
Codon usage regulates protein activity by affecting co-translational protein folding
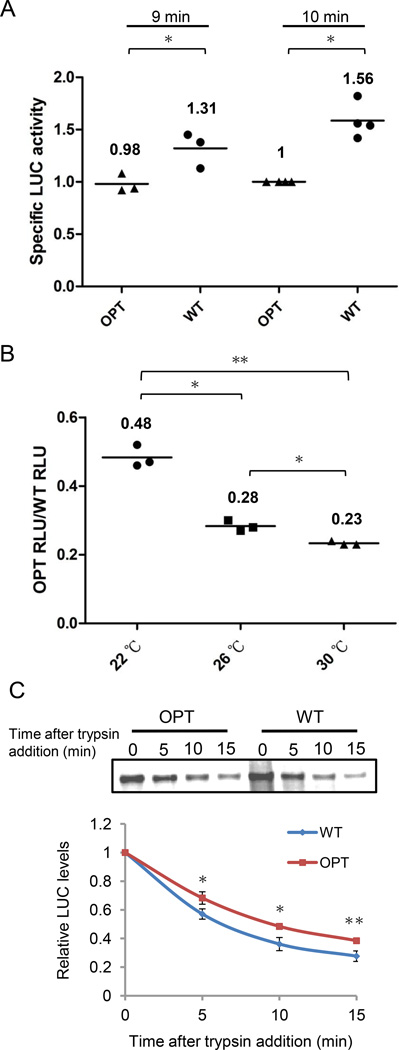
Because protein folding occurs co-translationally, the elongation rate and codon usage have been proposed to regulate co-translational folding. Our ability to use the in vitro system to monitor elongation rate and protein activity at the same time allowed us to directly test this hypothesis. In addition to the difference in TFA, the time-dependent profiles of bioluminescent signal were also different between the WT and OPT Luc mRNAs (Figure 1A). Although the OPT Luc was translated much more rapidly than the WT mRNA, the level of luciferase activity produced by translation of WT mRNA became greater than that from the OPT mRNA after 9.5 min. At 9 and 10 min, even though the levels of the full-length LUC protein were lower for the WT than for the OPT Luc mRNA as assessed by [35S]Met incorporation, the specific activity of LUC (bioluminescent level/protein level) were significantly higher for the WT than the OPT Luc mRNA (Figure 6A).
Figure 6. Codon usage regulates protein activity by affecting co-translational protein folding.
(A) Scatter plot shows the comparison of WT and OPT LUC specific activities in Neurospora cell-free lysate. (B) Scatter plot shows the ratio of relative LUC activity between OPT to the WT mRNA after 12 min of in vitro translation at indicated temperatures. (C) Top panel: SDS-PAGE analysis shows the levels of WT and OPT LUC at the indicated time points after the addition of trypsin (20 µg/ml). Lower panel: Densitometric analyses of LUC levels from four independent experiments. Means are indicated by horizontal bars and were derived from three to four independent experiments. *P < 0.05, **P < 0.01.
We compared the luciferase activities produced from the WT and OPT mRNAs at different temperatures because the effect of temperature on elongation speed (Figure 2A). The ratios of luciferase activity produced from OPT and WT Luc mRNAs inversely correlated with reaction temperatures: Low temperature reduced the difference between OPT and WT mRNAs (Figure 6B). This result suggests that the difference in luciferase activity is due to the difference in elongation rate, which increases as temperature increases. Furthermore, we found that the OPT-encoded luciferase was significantly more resistant to partial trypsin digestion than the WT luciferase (Figure 6C), indicating that the synthesized polypeptides differ structurally despite having identical amino acid sequences. Collectively, these results strongly suggest that codon usage regulates protein function by affecting the co-translational folding process.
A N-terminal domain of luciferase is sensitive to codon usage
To determine the LUC region that is most sensitive to codon usage changes, we compared the specific luciferase activities of proteins encoded by the N-OPT-WT, M-OPT-WT, and C-OPT-WT mRNAs. As shown in Figure 7A, codon optimization of the N-terminal region (amino acids 2–223) of Luc resulted in largest reduction in specific luciferase activity. To verify this result, the same three regions of the OPT mRNA were individually changed to the WT sequence (N-WT-OPT, M-WT-OPT and C-WT-OPT). As expected, the WT codon usage in the N-terminal region of Luc (N-WT-OPT mRNA) led to largest increase in specific luciferase activity (Figure 7B). These results indicate that the folding of the N-terminal region is more sensitive to codon usage than the other regions of the protein. Interestingly, these data are consistent with the previous finding that the co-translational folding of N-terminal domain of LUC is critical for luciferase folding (Frydman et al., 1999).
Figure 7. Codon usage in a defined structural region affects LUC activity in vitro and in vivo.
(A) Top panel: Diagrams shows the codon-optimized region (red) of each WT-based Luc construct. Bottom panel: The relative specific LUC activities of in vitro translated indicated Luc mRNAs are shown by scatter plot. (B) Top panel: Diagrams indicate the regions in which codons are WT sequence (blue) in otherwise OPT Luc constructs. Bottom panel: Scatter plot shows the comparison of the specific Luc activities of in vitro translated Luc mRNAs. (C) Top panel: Diagrams show the regions in which codons are optimized (Red) in the WT Luc. Bottom panel: The relative specific Luc activities of indicated Luc constructs. Means are indicated by horizontal bars and were derived from three to five independent experiments. (D) Scatter plot shows the comparison of LUC specific activity in vivo. The frq promoter-driven OPT Luc and (172–223)WT-Luc were transformed into Neurospora at the his-3 locus. The 24-hr germinating conidia were harvested followed by LUC activity (RLU) measurement and LUC protein quantification by western blot. The RLU and LUC protein levels were normalized to the value of one of the OPT samples. The specific Luc activities were calculated by dividing the normalized RLU by normalized Luc protein level. *P < 0.05, **P < 0.01. See also Figure S3.
Comparison of specific luciferase activity of three different constructs with a small region optimized showed that codon optimization of aa 172–223 resulted in a significant decrease in specific luciferase activity, whereas optimization of other regions had little effect (Figure 7C). Since these Luc mRNAs have the same first 37 translated codons, the observed effects are not due to a potential ribosomal ramping issue near the initiation site (Pechmann and Frydman, 2013; Tuller et al., 2010a). The aa 172–223 is highly conserved among luciferase homologs and contains an unstructured loop in the solved luciferase crystal structure (Fig. S3, related to Figure 7) (Conti et al., 1996). Interestingly, this result is consistent with our previous finding that non-optimal codon usage are preferentially used in regions that are predicted to be unstructured or coiled (Zhou et al., 2015), suggesting that the adaptation between codon usage and protein structure is important co-translation protein folding.
To determine whether the codons for residues 172–223 are also important for LUC folding in vivo, we changed the codons of this region in an OPT construct to wild-type residues. We predicted that the wild-type codons in this region would slow down elongation and facilitate proper folding, resulting in an increase of specific activity of luciferase. Both OPT and the resulting (172–223)WT-OPT constructs were separately introduced into Neurospora. Homokaryotic strains were obtained from the resulting transformants. As expected, the specific activity of LUC in the (172–223)WT-OPT strain was significantly higher than that in the OPT strains (Figure 7D), indicating that codon usage within this domain is important for the proper folding of luciferase in vivo.
DISCUSSION
Codon usage bias is observed in all organisms examined and has been proposed to regulate protein translation efficiency and accuracy and protein folding (Akashi, 1994; Drummond and Wilke, 2008; Gingold and Pilpel, 2011; Hershberg and Petrov, 2008; Plotkin and Kudla, 2011; Spencer et al., 2012; Xu et al., 2013). Despite previous studies, whether codon usage regulates translation dynamics is not clear. Recent studies based on ribosome profiling challenged the long-held assumption that preferred codons increase translation elongation rate (Charneski and Hurst, 2013; Ingolia et al., 2011; Li et al., 2012; Qian et al., 2012). In this study, by developing a cell-free system that allowed us to directly monitor the velocity of protein translation, our results indicate that codon usage regulates ribosome elongation rate: Optimal codons increased and non-optimal codons decreased elongation rate. This conclusion is supported by directly measured translation velocities and by nascent peptide profiles (Figures 1 & 3). In addition, we showed that the effect of codon usage on translation rate corresponds to the codon usage of the organism and is not due to changes in RNA structures. Collectively, our results provide a clear answer to the long-debated question of the role of codon bias.
Our use of TFA provided a simple method to compare translation elongation time of different Luc mRNAs. Our conclusions based on TFA are further supported by the [35S] methionine labeling result and several additional lines of evidences (Fig. 1B, Fig. 3A–C, and Fig. 4), indicating that it is a good metrics to study how codon usage regulates elongation speed. It is should be noted that luciferase is rapidly folded after translation (within seconds) (Kolb et al., 1994) and 30 sec time window was used to measure TFA. In addition, under our experimental condition, the concentration of mRNA did not significantly affect TFA values, indicating that luciferase translation did not result in depletion of translation components in the extracts.
Previous studies based on ribosome profiling were unable to draw a clear conclusion on the role of codon usage on elongation rate. This may be due to technical limitations of method and intrinsic noises/biases of the deep sequencing-based approach (Artieri and Fraser, 2014; Gardin et al., 2014). The use of a cell-free translation system allowed us to uncouple the transcription and translation processes and to directly monitor the rate of mRNA translation. Moreover, our conclusion was further confirmed by ribosomal profiling analyses in vivo and in vitro. The use of codon manipulated mRNA targets allowed us to convincingly show the impact of codon usage on elongation rate by ribosomal profiling (Figure 4). These results indicate that ribosomal profiling can be used to measure differences in elongation rates if the signal-to-noise ratio is high. In addition, we observed a significant genome-wide negative correlation between ribosome occupancy and codon usage frequency for vast majority of codon families in Neurospora (Figures 4 & 5).
The balanced codon usage model was previously used to explain the lack of correlation between codon usage and RPF levels on individual codons from ribosome profiling results (Charneski and Hurst, 2013; Qian et al., 2012). This model reasoned that the lack of correlation is due to the balance of effective tRNA concentrations and codon usage frequency on mRNAs. It should be noted that the experimental evidence that led to this model were based on protein activity levels, which reflect a combined effect of mRNA levels and translation efficiency. Changing the level of mRNA used for translation, which effectively broke the balance between the supply and demand, had no significant effect on the rate of elongation (Figure 2B–C). Thus, the balance between supply (tRNA levels) and demand (codon usage frequency on mRNA) does not appear to influence elongation rates. Even though the tRNA concentrations and codon usage frequencies may be balanced in cells, tRNAs that recognize preferred codons are still more abundant than those that recognize non-preferred codons. As a result, the preferred codons will still be decoded more rapidly by ribosomes than the non-preferred codons.
Codon usage biases have been proposed to affect co-translational protein folding (Komar et al., 1999; Siller et al., 2010; Spencer et al., 2012; Zhang et al., 2009). This hypothesis is supported by our previous demonstration in vivo that codon usage affects FRQ protein structure and function (Zhou et al., 2013). In addition, bioinformatic analyses revealed correlations between codon usage biases and protein structural motifs (Pechmann et al., 2014; Pechmann and Frydman, 2013; Zhou et al., 2015; Zhou et al., 2009). Here we provide strong biochemical evidence to support the hypothesis that codon usage affects protein folding and identified a specific unstructured loop-containing domain of the luciferase that is sensitive to codon usage (Figures 5 & 6). Interestingly, this result is consistent with our recent finding that there are genome-wide preferences for use of non-optimal codons in unstructured protein regions in eukaryotes (Zhou et al., 2015). In addition, elongation speed may also influence co-translational folding by affecting the binding of chaperones to nascent chains. Together, these results suggest that the co-evolution of codon usage and protein structures result in rhythms of ribosome decoding rates on mRNAs that match the co-translational folding process (O'Brien et al., 2012). We propose that this non-uniform decoding rate across an mRNA is a “code” within genetic codons that promote proper protein folding.
It was recently shown that codon usage plays a role in determining yeast mRNA halflives, likely due to its role in affecting elongation speed (Presnyak et al., 2015). It should be noted that there was no significant difference in the in vivo Luc mRNA levels between the OPT and C-WT-OPT strains (Fig. S2). This may be due to that only a relatively small region of Luc mRNA has non-optimal codons or that the effect of codons on mRNA stability is not strong in Neurospora.
As highly expressed genes have codons that are recognized by highly abundant tRNAs, it has been hypothesized that codon usage bias increases translation efficiency (output of proteins) by accelerating elongation (Hershberg and Petrov, 2008). However, recent studies are not consistent with such a hypothesis and suggested that overall translation efficiency is mainly determined by translation initiation efficiency, a process that is mostly determined by RNA structure near the translational start site (Kudla et al., 2009; Pop et al., 2014; Tuller et al., 2010b). The results we report here indicate that codon usage affects translation elongation rate and in doing so, it impacts protein function by regulating co-translational folding.
EXPERIMENTAL PROCEDURES
Additional materials and methods can be found in the Supplemental Experimental Procedures.
Codon manipulation and indices calculation
The codons of Firefly luciferase (Luc) was optimized or de-optimized based on the Neurospora codon usage frequency calculated from the annotated coding sequences published in Broad Institute N. crassa database (http://www.broadinstitute.org/annotation/genome/neurospora/MultiHome.html). The S. cerevisiae codon usage frequency table was obtained from http://www.kazusa.or.jp/codon/cgi-bin/showcodon.cgi?species=4932. The CAI indexes of Luc mutants was calculated through CAIcal server (Puigbo et al., 2008).
Preparation of N. crassa and S. cerevisiae cell free lysates
The N. crassa and S. cerevisiae cell free lysates were prepared as previously described (Wu et al., 2007).
In vitro transcription and In vitro translation using N. crassa and S. cerevisiae cell free lysate
Details of in vitro transcription and in vitro translation can be found in the Supplemental Experimental Procedures. To monitor firefly luciferase expression, the luminescence signals were recorded continuously in 30-second intervals with indicated time. Alternatively, the protein products were labeled by [35S]Met followed by resolving the samples on SDS-PAGE.
RNA isolation and qRT-PCR
RNA extraction and quantitative reverse transcriptase PCR (qRT–PCR) were performed as previously described (Zhou et al., 2013). For qRT–PCR, the primer sequences used for Luc were 5'-AAGTACAAGGGCTATCAGGT-3' (forward) and 5'-GCGTCGAAGATGTTGGGGTG-3' (reverse). The Neurospora gene coding for β-tubulin was used as an internal control. The primer sequences specific for β -tubulin were 5'-ATAACTTCGTCTTCGGCCAG-3'(forward) and 5'-ACATCGAGAACCTGGTCAAC-3' (reverse).
Isolation of ribosome-associated nascent chains (RNCs)
The RNCs were isolated as previously described (McCallum et al., 2000) with minor modification. See the Supplemental Experimental Procedures for details.
In vitro and in vivo ribosome profiling
For in vivo ribosome profiling, conidia were grown in minimal medium (1 × Vogel’s, 2% glucose) for 12 hr at room temperature, harvested by vacuum filtration through filter paper, followed by flash freezing in liquid nitrogen. The extraction and library constructions for both in vitro and in vivo ribosome-protected mRNA fragments were based on the ARTseq yeast ribosome profiling kit (Illumina) according to the accompanying protocol. Sequencing was performed on an Illumina HiSeq 2000 system (BGI). The detail protocols were described in the Supplemental Experimental Procedures.
Data analyses of ribosome profiling results
The sequenced reads (accession number GSE71032) were mapped to the built references by Bowtie (ver 1.0.0), and the results were visualized using on IGV browser (Robinson et al., 2011). The CDR (codon decoding rate) is calculated based on the sum of RPF reads of each 61 codons extracted from translated genes normalized to the corresponding mRNA quantities. To calculate RCDR, the CDR of each codon was normalized to the highest occupied codon (CCA, proline). The detailed procedure can be found in the Supplemental Experimental Procedures.
Measurement of specific Luc reporter activity in vivo
The specific Luc activity in vivo is derived by dividing the measured luminescence signal with corresponding protein quantity (determined by Western Blot). The results were normalized to one of the triplicate of the OPT construct. Details can be found in the Supplemental Experimental Procedures.
Supplementary Material
Acknowledgements
We thank the members of our laboratories for technical assistance and Dr. Andrey Karamyshey for suggesting the RNCs isolation experiment. This work is supported by grants from the National Institutes of Health and the Welch Foundation (I-1560) to Yi Liu.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions: Y.L., C.Y., and M.S.S designed the study. C.Y., Z. Z., C.W., and F.Z. performed the experiments. Y.D. performed bioinformatic analyses. C.Y. and Y.D. performed statistical analyses. All authors interpreted the results. Y.L. and C.Y. wrote the paper.
All authors declare that there is no financial conflict of interest that might be construed to influence the results or interpretation of our manuscript.
Accession Number
The GEO Accession number for sequencing data is GSE71032.
References
- Akashi H. Synonymous codon usage in Drosophila melanogaster: natural selection and translational accuracy. Genetics. 1994;136:927–935. doi: 10.1093/genetics/136.3.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artieri CG, Fraser HB. Accounting for biases in riboprofiling data indicates a major role for proline in stalling translation. Genome Res. 2014;24:2011–2021. doi: 10.1101/gr.175893.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonekamp F, Dalboge H, Christensen T, Jensen KF. Translation rates of individual codons are not correlated with tRNA abundances or with frequencies of utilization in Escherichia coli. J Bacteriol. 1989;171:5812–5816. doi: 10.1128/jb.171.11.5812-5816.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull JJ, Molineux IJ, Wilke CO. Slow fitness recovery in a codon-modified viral genome. Mol Biol Evol. 2012;29:2997–3004. doi: 10.1093/molbev/mss119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charneski CA, Hurst LD. Positively charged residues are the major determinants of ribosomal velocity. PLoS biology. 2013;11:e1001508. doi: 10.1371/journal.pbio.1001508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevance FF, Le Guyon S, Hughes KT. The effects of codon context on in vivo translation speed. PLoS Genet. 2014;10:e1004392. doi: 10.1371/journal.pgen.1004392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comeron JM. Selective and mutational patterns associated with gene expression in humans: influences on synonymous composition and intron presence. Genetics. 2004;167:1293–1304. doi: 10.1534/genetics.104.026351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti E, Franks NP, Brick P. Crystal structure of firefly luciferase throws light on a superfamily of adenylate-forming enzymes. Structure. 1996;4:287–298. doi: 10.1016/s0969-2126(96)00033-0. [DOI] [PubMed] [Google Scholar]
- Dever TE, Green R. The elongation, termination, and recycling phases of translation in eukaryotes. Cold Spring Harbor perspectives in biology. 2012;4:a013706. doi: 10.1101/cshperspect.a013706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Nilsson L, Kurland CG. Co-variation of tRNA abundance and codon usage in Escherichia coli at different growth rates. J Mol Biol. 1996;260:649–663. doi: 10.1006/jmbi.1996.0428. [DOI] [PubMed] [Google Scholar]
- Drummond DA, Wilke CO. Mistranslation-induced protein misfolding as a dominant constraint on coding-sequence evolution. Cell. 2008;134:341–352. doi: 10.1016/j.cell.2008.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang P, Spevak CC, Wu C, Sachs MS. A nascent polypeptide domain that can regulate translation elongation. Proc Natl Acad Sci U S A. 2004;101:4059–4064. doi: 10.1073/pnas.0400554101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitag M, Hickey PC, Raju NB, Selker EU, Read ND. GFP as a tool to analyze the organization, dynamics and function of nuclei and microtubules in Neurospora crassa. Fungal Genet Biol. 2004;41:897–910. doi: 10.1016/j.fgb.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Frydman J, Erdjument-Bromage H, Tempst P, Hartl FU. Co-translational domain folding as the structural basis for the rapid de novo folding of firefly luciferase. Nat Struct Biol. 1999;6:697–705. doi: 10.1038/10754. [DOI] [PubMed] [Google Scholar]
- Gardin J, Yeasmin R, Yurovsky A, Cai Y, Skiena S, Futcher B. Measurement of average decoding rates of the 61 sense codons in vivo. Elife. 2014;3 doi: 10.7554/eLife.03735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerashchenko MV, Lobanov AV, Gladyshev VN. Genome-wide ribosome profiling reveals complex translational regulation in response to oxidative stress. Proc Natl Acad Sci U S A. 2012;109:17394–17399. doi: 10.1073/pnas.1120799109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingold H, Pilpel Y. Determinants of translation efficiency and accuracy. Mol Syst Biol. 2011;7:481. doi: 10.1038/msb.2011.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooch VD, Mehra A, Larrondo LF, Fox J, Touroutoutoudis M, Loros JJ, Dunlap JC. Fully codon-optimized luciferase uncovers novel temperature characteristics of the Neurospora clock. Eukaryotic cell. 2008;7:28–37. doi: 10.1128/EC.00257-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromadski KB, Rodnina MV. Kinetic determinants of high-fidelity tRNA discrimination on the ribosome. Molecular cell. 2004;13:191–200. doi: 10.1016/s1097-2765(04)00005-x. [DOI] [PubMed] [Google Scholar]
- Hershberg R, Petrov DA. Selection on codon bias. Annu Rev Genet. 2008;42:287–299. doi: 10.1146/annurev.genet.42.110807.091442. [DOI] [PubMed] [Google Scholar]
- Hood HM, Neafsey DE, Galagan J, Sachs MS. Evolutionary roles of upstream open reading frames in mediating gene regulation in fungi. Annu Rev Microbiol. 2009;63:385–409. doi: 10.1146/annurev.micro.62.081307.162835. [DOI] [PubMed] [Google Scholar]
- Ikemura T. Codon usage and tRNA content in unicellular and multicellular organisms. Mol Biol Evol. 1985;2:13–34. doi: 10.1093/oxfordjournals.molbev.a040335. [DOI] [PubMed] [Google Scholar]
- Ingolia NT, Brar GA, Rouskin S, McGeachy AM, Weissman JS. The ribosome profiling strategy for monitoring translation in vivo by deep sequencing of ribosome-protected mRNA fragments. Nat Protoc. 2012;7:1534–1550. doi: 10.1038/nprot.2012.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingolia NT, Ghaemmaghami S, Newman JR, Weissman JS. Genome-wide analysis in vivo of translation with nucleotide resolution using ribosome profiling. Science. 2009;324:218–223. doi: 10.1126/science.1168978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingolia NT, Lareau LF, Weissman JS. Ribosome profiling of mouse embryonic stem cells reveals the complexity and dynamics of mammalian proteomes. Cell. 2011;147:789–802. doi: 10.1016/j.cell.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimchi-Sarfaty C, Oh JM, Kim IW, Sauna ZE, Calcagno AM, Ambudkar SV, Gottesman MM. A "silent" polymorphism in the MDR1 gene changes substrate specificity. Science. 2007;315:525–528. doi: 10.1126/science.1135308. [DOI] [PubMed] [Google Scholar]
- Kolb VA, Makeyev EV, Spirin AS. Folding of firefly luciferase during translation in a cell-free system. The EMBO journal. 1994;13:3631–3637. doi: 10.1002/j.1460-2075.1994.tb06670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komar AA, Lesnik T, Reiss C. Synonymous codon substitutions affect ribosome traffic and protein folding during in vitro translation. FEBS Lett. 1999;462:387–391. doi: 10.1016/s0014-5793(99)01566-5. [DOI] [PubMed] [Google Scholar]
- Kudla G, Murray AW, Tollervey D, Plotkin JB. Coding-sequence determinants of gene expression in Escherichia coli. Science. 2009;324:255–258. doi: 10.1126/science.1170160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lareau LF, Hite DH, Hogan GJ, Brown PO. Distinct stages of the translation elongation cycle revealed by sequencing ribosome-protected mRNA fragments. Elife. 2014;3:e01257. doi: 10.7554/eLife.01257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TH, Blanchard SC, Kim HD, Puglisi JD, Chu S. The role of fluctuations in tRNA selection by the ribosome. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:13661–13665. doi: 10.1073/pnas.0705988104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GW, Oh E, Weissman JS. The anti-Shine-Dalgarno sequence drives translational pausing and codon choice in bacteria. Nature. 2012;484:538–541. doi: 10.1038/nature10965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, Genome Project Data Processing S. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum CD, Do H, Johnson AE, Frydman J. The interaction of the chaperonin tailless complex polypeptide 1 (TCP1) ring complex (TRiC) with ribosome-bound nascent chains examined using photo-cross-linking. J Cell Biol. 2000;149:591–602. doi: 10.1083/jcb.149.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahigashi K, Takai Y, Shiwa Y, Wada M, Honma M, Yoshikawa H, Tomita M, Kanai A, Mori H. Effect of codon adaptation on codon-level and gene-level translation efficiency in vivo. BMC Genomics. 2014;15:1115. doi: 10.1186/1471-2164-15-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien EP, Vendruscolo M, Dobson CM. Prediction of variable translation rate effects on cotranslational protein folding. Nat Commun. 2012;3:868. doi: 10.1038/ncomms1850. [DOI] [PubMed] [Google Scholar]
- Pechmann S, Chartron JW, Frydman J. Local slowdown of translation by nonoptimal codons promotes nascent-chain recognition by SRP in vivo. Nat Struct Mol Biol. 2014;21:1100–1105. doi: 10.1038/nsmb.2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechmann S, Frydman J. Evolutionary conservation of codon optimality reveals hidden signatures of cotranslational folding. Nat Struct Mol Biol. 2013;20:237–243. doi: 10.1038/nsmb.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin JB, Kudla G. Synonymous but not the same: the causes and consequences of codon bias. Nat Rev Genet. 2011;12:32–42. doi: 10.1038/nrg2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pop C, Rouskin S, Ingolia NT, Han L, Phizicky EM, Weissman JS, Koller D. Causal signals between codon bias, mRNA structure, and the efficiency of translation and elongation. Mol Syst Biol. 2014;10:770. doi: 10.15252/msb.20145524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presnyak V, Alhusaini N, Chen YH, Martin S, Morris N, Kline N, Olson S, Weinberg D, Baker KE, Graveley BR, et al. Codon optimality is a major determinant of mRNA stability. Cell. 2015;160:1111–1124. doi: 10.1016/j.cell.2015.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puigbo P, Bravo IG, Garcia-Vallve S. CAIcal: a combined set of tools to assess codon usage adaptation. Biol Direct. 2008;3:38. doi: 10.1186/1745-6150-3-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian W, Yang JR, Pearson NM, Maclean C, Zhang J. Balanced codon usage optimizes eukaryotic translational efficiency. PLoS Genet. 2012;8:e1002603. doi: 10.1371/journal.pgen.1002603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan AR, Hall IM. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics. 2010;26:841–842. doi: 10.1093/bioinformatics/btq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radford A, Parish JH. The genome and genes of Neurospora crassa. Fungal Genet Biol. 1997;21:258–266. doi: 10.1006/fgbi.1997.0979. [DOI] [PubMed] [Google Scholar]
- Robinson JT, Thorvaldsdottir H, Winckler W, Guttman M, Lander ES, Getz G, Mesirov JP. Integrative genomics viewer. Nat Biotechnol. 2011;29:24–26. doi: 10.1038/nbt.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp PM, Li WH. The codon Adaptation Index--a measure of directional synonymous codon usage bias, and its potential applications. Nucleic acids research. 1987;15:1281–1295. doi: 10.1093/nar/15.3.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp PM, Tuohy TM, Mosurski KR. Codon usage in yeast: cluster analysis clearly differentiates highly and lowly expressed genes. Nucleic Acids Res. 1986;14:5125–5143. doi: 10.1093/nar/14.13.5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siller E, DeZwaan DC, Anderson JF, Freeman BC, Barral JM. Slowing bacterial translation speed enhances eukaryotic protein folding efficiency. J Mol Biol. 2010;396:1310–1318. doi: 10.1016/j.jmb.2009.12.042. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Jenner AJ, Brierley I, Inglis SC. Ribosomal pausing during translation of an RNA pseudoknot. Molecular and cellular biology. 1993;13:6931–6940. doi: 10.1128/mcb.13.11.6931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen MA, Kurland CG, Pedersen S. Codon usage determines translation rate in Escherichia coli. J Mol Biol. 1989;207:365–377. doi: 10.1016/0022-2836(89)90260-x. [DOI] [PubMed] [Google Scholar]
- Spencer PS, Barral JM. Genetic code redundancy and its influence on the encoded polypeptides. Computational and structural biotechnology journal. 2012;1:e201204006. doi: 10.5936/csbj.201204006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer PS, Siller E, Anderson JF, Barral JM. Silent substitutions predictably alter translation elongation rates and protein folding efficiencies. J Mol Biol. 2012;422:328–335. doi: 10.1016/j.jmb.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuller T, Carmi A, Vestsigian K, Navon S, Dorfan Y, Zaborske J, Pan T, Dahan O, Furman I, Pilpel Y. An evolutionarily conserved mechanism for controlling the efficiency of protein translation. Cell. 2010a;141:344–354. doi: 10.1016/j.cell.2010.03.031. [DOI] [PubMed] [Google Scholar]
- Tuller T, Waldman YY, Kupiec M, Ruppin E. Translation efficiency is determined by both codon bias and folding energy. Proceedings of the National Academy of Sciences of the United States of America. 2010b;107:3645–3650. doi: 10.1073/pnas.0909910107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Fang P, Sachs MS. The evolutionarily conserved eukaryotic arginine attenuator peptide regulates the movement of ribosomes that have translated it. Mol Cell Biol. 1998;18:7528–7536. doi: 10.1128/mcb.18.12.7528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Gaba A, Sachs MS. A highly conserved mechanism of regulated ribosome stalling mediated by fungal arginine attenuator peptides that appears independent of the charging status of arginyl-tRNAs. J Biol Chem. 1999;274:37565–37574. doi: 10.1074/jbc.274.53.37565. [DOI] [PubMed] [Google Scholar]
- Wang Z, Sachs MS. Arginine-specific regulation mediated by the Neurospora crassa arg-2 upstream open reading frame in a homologous, cell-free in vitro translation system. The Journal of biological chemistry. 1997;272:255–261. doi: 10.1074/jbc.272.1.255. [DOI] [PubMed] [Google Scholar]
- Wei J, Zhang Y, Ivanov IP, Sachs MS. The stringency of start codon selection in the filamentous fungus Neurospora crassa. J Biol Chem. 2013;288:9549–9562. doi: 10.1074/jbc.M112.447177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen JD, Lancaster L, Hodges C, Zeri AC, Yoshimura SH, Noller HF, Bustamante C, Tinoco I. Following translation by single ribosomes one codon at a time. Nature. 2008;452:598–603. doi: 10.1038/nature06716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Amrani N, Jacobson A, Sachs MS. The use of fungal in vitro systems for studying translational regulation. Methods in enzymology. 2007;429:203–225. doi: 10.1016/S0076-6879(07)29010-X. [DOI] [PubMed] [Google Scholar]
- Xu Y, Ma P, Shah P, Rokas A, Liu Y, Johnson CH. Non-optimal codon usage is a mechanism to achieve circadian clock conditionality. Nature. 2013;495:116–120. doi: 10.1038/nature11942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Hubalewska M, Ignatova Z. Transient ribosomal attenuation coordinates protein synthesis and co-translational folding. Nat Struct Mol Biol. 2009;16:274–280. doi: 10.1038/nsmb.1554. [DOI] [PubMed] [Google Scholar]
- Zhou M, Guo J, Cha J, Chae M, Chen S, Barral JM, Sachs MS, Liu Y. Non-optimal codon usage affects expression, structure and function of clock protein FRQ. Nature. 2013;495:111–115. doi: 10.1038/nature11833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Wang T, Fu J, Xiao G, Liu Y. Non-optimal codon usage influences protein structure in intrinsically disordered regions. Molecular Microbiology. 2015 doi: 10.1111/mmi.13079. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T, Weems M, Wilke CO. Translationally optimal codons associate with structurally sensitive sites in proteins. Mol Biol Evol. 2009;26:1571–1580. doi: 10.1093/molbev/msp070. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.