Abstract
Background
Pulmonary exacerbations (PEx) are important CF clinical events.
Methods
We studied time to next PEx following intravenous (IV) antibiotic PEx treatment among Cleveland Ohio CF center patients occurring between January 2010 and September 2014. Patient demographics, clinical presentations, and treatments were modeled by Cox proportional hazards regression to identify covariates associated with time to next PEx.
Results
193 patients were treated for PEx; 155 had a subsequent IV-treated PEx. Six covariates were associated with future PEx hazard: number of PEx in the prior year (hazard ratio 25.1 for ≥3 and 4.4 for 1-2 prior-year PEx versus none; P<.0001), IV treatment duration in weeks (1.2; P=.0004), percent hospital treatment (1.1; P=.0018), and chronic inhaled aminoglycosides (2.5; P<.0001), leukotriene modifiers (1.8; P=.0031), and high dose ibuprofen (0.52; P=.0006).
Conclusions
Time to next PEx was profoundly associated with prior-year PEx, suggestive of high-risk PEx phenotypes that warrant recognition and further study.
BACKGROUND
Pulmonary exacerbations (PEx) are a hallmark of cystic fibrosis (CF) lung disease characterized by increased respiratory signs and symptoms, decreased pulmonary function, weight loss, and malaise (1,2). PEx have been associated with accelerated lung disease progression (3-7), increased resource utilization (8), decreased quality of life (9), and increased mortality risk (6,10,11). The clinical significance attached to PEx has justified use of reduced risk of future PEx and increased median time to next PEx as efficacy outcomes in the comparison of PEx treatments (12). In addition, randomized controlled trials of chronic CF respiratory therapies commonly include risk of future PEx and time to next PEx as efficacy outcomes (13). We conducted analyses of time to next PEx on a cohort of CF patients followed at the Cleveland Ohio CF Care Center (pediatric and adult programs) to identify patient phenotypic and treatment covariates associated with differences in hazard rates of experiencing a subsequent PEx.
METHODS
This analysis employs data from patients of record at the Cleveland CF Center obtained from the Cystic Fibrosis Foundation Patient Registry (CFFPR) through PortCF. Patients/parents contributing data to the CFFPR have provided informed consent for use of their data for observational research. To be included, patients had to have had a confirmed diagnosis of CF, to have been treated with IV antibiotics for PEx (a PEx care episode) on or after January 1, 2010, and to have had at least one subsequent clinic visit (encounter) after the end of IV treatment and by September, 2014.
For each patient’s first PEx care episode on or after January 1, 2010, IV antibiotic treatment duration in days, including days treated in hospital and days treated at home, were recorded. Sequential PEx care episodes separated by <7 days were combined and treated as single care episodes. The time to next PEx was calculated as the time in days from the end of the first PEx care episode to the beginning of a next PEx care episode. If no subsequent PEx care episode occurred, a censoring time was calculated from the end of the first PEx treatment to the last clinic visit recorded through September, 2014. Whether there was any home IV treatment during the PEx care episode was indicated by a categorical variable and the percentage of IV treatment received in hospital was calculated as a continuous variable.
A series of patient covariates were collected to be screened by univariate Cox proportional hazards regression modeling of time to next PEx. Patients were categorically identified as “positive’ for Staphylococcus aureus, methicillin-resistant S. aureus (MRSA), Haemophilus influenzae, Stenotrophomonas maltophilia, Achromobacter xylosoxidans, Mycobacterium abscessus, or Burkholderia cepacia complex if organisms were isolated from airway secretions at any clinic visit during the registry year in which the initial PEx care event occurred. Patients were identified as chronically infected with Pseudomonas aeruginosa if they had 2 or more recorded respiratory secretion cultures and if P. aeruginosa was isolated from greater than 50% of those cultures during the PEx care event year. Chronic pulmonary medications prescribed at any encounter during the registry year were recorded as categorical variables, as were identification of CF-related diabetes (CFRD), asthma, allergic bronchial pulmonary aspergillosis (ABPA), massive hemoptysis, pneumothorax, gastro-esophageal reflux disease (GERD), pancreatitis, liver disease, F508del homozygous and heterozygous genotype, race, and Hispanic heritage. For each patient, forced expiratory volume in 1 second (FEV1, calculated as percent predicted using the reference equations of Wang et al (14) for males through age 17 years and for females through age 15 years and of Hankinson et al (15) for patients over these ages) was calculated as the average of all values recorded at clinic encounters during the calendar year of the PEx care episode. Body mass index (BMI) in kg/m2 was calculated from weight and height measures collected at clinic encounters and averaged across the calendar year of the PEx care episode. Patients who were smokers or exposed to second-hand smoke were identified. The number of care episodes for IV treatment of PEx in the prior registry year was collected as a continuous variable. Selected continuous variables were also coded as categorical variables (e.g., FEV1 % predicted, prior-year PEx) to allow inclusion of patients with missing data or to simplify modeling.
Median time to next PEx for the entire population and among categorical subgroups was determined by Kaplan-Meier survival analyses. Categorical and continuous variables were screened for significance by univariate Cox proportional hazards regressions for time to next PEx. Only variables with P <.10 were included in a subsequent single model and analyzed by multivariate Cox proportional hazards regression using backwards selection in which covariates with P <.01 were retained. Analyses were performed using Excel 2013 (Microsoft Corp., Redmond WA USA) and MedCalc Statistical Software version 14.10.2 (MedCalc Software bvba, Ostend, Belgium).
RESULTS
IV-treated PEx care events were recorded for 193 patients. Median age at IV antibiotic treatment initiation was 23.0 years (range 0.1 to 76.0 years). Patients were treated with IV antibiotics for an average of 19.7 days (median 17 days, range 1 to 97), with an average of 12.2 days of treatment occurring in hospital (median 12 days, range 0 to 66). Patient demographics at initiation of PEx care events are provided in Table 1.
Table 1.
Demographics and Antibiotic Treatment Durations for Study Patients
| All (N=193) |
No prior-year PEx (N=105) |
One or two prior-year PEx (N=59) |
Three or more prior-year PEx (N=29) |
|
|---|---|---|---|---|
| Mean Age at PEx start, years (SD) | 24.8 (13.4) | 24.1 (14.1) | 26.6 (14) | 24.0 (9.1) |
| FEV1 category, N (%) | ||||
| <6 years of age | 9 (4.7) | 7 (6.7) | 2 (3.4) | 0 (0) |
| ≥100% predicted | 6 (3.1) | 5 (4.8) | 0 (0) | 1 (3.4) |
| 70 to <100% predicted | 56 (29) | 35 (33.3) | 15 (25.4) | 6 (20.7) |
| 40 to <70% predicted | 79 (40.9) | 38 (36.2) | 30 (50.8) | 11 (37.9) |
| <40% predicted | 32 (16.6) | 16 (15.2) | 9 (15.3) | 7 (24.1) |
| ≥6 years of age, no measure | 11 (5.7) | 4 (3.8) | 3 (5.1) | 4 (13.8) |
| Mean BMI, kg/m2 (SD) | 19.5 (4.6) | 19.4 (4.7) | 19.8 (4.7) | 19.3 (3.8) |
| IV Antibiotic Treatment for PEx | ||||
| Mean duration, days (SD) | 19.7 (12.0) | 17.6 (9.9) | 22.3 (14.8) | 22.0 (11.3) |
| Mean duration in hospital, days (SD) | 12.3 (10.8) | 10.0 (9.2) | 13.3 (10.6) | 18.3 (14.0) |
| Mean proportion in hospital, % (SD) | 68.9 (40.5) | 65.9 (41.2) | 69.1 (40.1) | 79.1 (38.1) |
| Home IV Treatment, N (%) | 75 (38.9) | 45 (42.9) | 23 (39.0) | 7 (24.1) |
| Median time to next PEx, days [95% CI] | 311 [242, 389] | 593 [514, 749] | 196 [154, 242] | 71 [47, 96] |
In all, 155 patients (80.3%) had at least one subsequent PEx care episode and 38 (19.7%) had subsequent clinic encounters but no PEx care episodes treated with IV antibiotics recorded as of September, 2014. Median time to next PEx was 311 days [95% CI 242, 389] among all 193 patients studied and 219 days [174, 286] among those 155 patients experiencing a subsequent PEx care event prior to September, 2014.
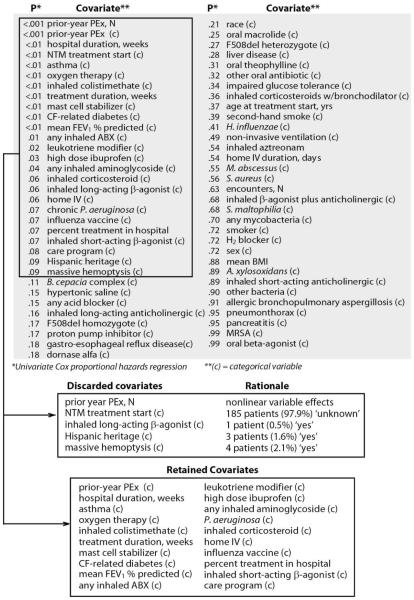
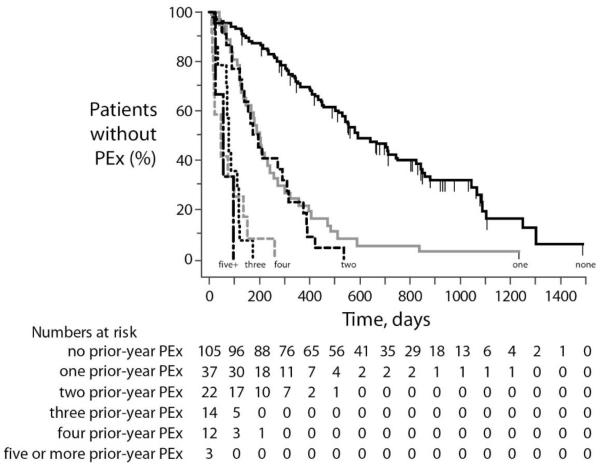
Of 65 studied screened covariates drawn from patient demographics, clinical presentations, and administered therapies, 25 had a univariate P value <.10 by Cox proportional hazards regression (Figure 1). Four covariates with univariate P <.10 were excluded from subsequent analyses because they affected <3% of patients (Figure 1). The covariate with the largest effect size was the number of PEx a patient had experienced in the prior calendar year (“Prior-year PEx”), whether studied as a continuous or categorical variable. Values of Prior-year PEx ranged from 0 (N=105) to 10 (N=1), but effects of increasing Prior-year PEx were not uniform with respect to time to next PEx by Kaplan-Meier survival analysis. Kaplan-Meier survival curves for the individual counts of prior-year PEx revealed a clustering of effects into three categories: “0”, “1 or 2”, and “3 or more” prior-year PEx (Figure 2). Unadjusted Cox proportional hazard ratios compared to patients with 0 prior-year PEx were 4.1 [95% CI 2.8, 6.0] for patients with 1 or 2 prior-year PEx and 20.9 [12.1, 36.1] for patients with 3 or more prior-year PEx.
Figure 1. Disposition of Covariates for Cox Proportional Hazards Regression.
A total of 65 covariates (gray shaded area) were analyzed by univariate Cox proportional hazards regression and sorted by P value. Among 25 covariates with P<.10 (upper box), 5 were discarded for reasons shown (middle box) and 20 were retained for modeling (bottom box).
Figure 2. Kaplan-Meier Survival Plots of Time to Next PEx by Prior-Year PEx.
Survival curves are shown for time from the end of PEx treatment to either censor (vertical dashes) or next PEx care episode stratified by the number of PEx care episodes experienced in the prior calendar year (in text at the end of each curve). Numbers of patients remaining at risk for PEx in each group are shown below the figure.
Prior-year PEx and the other 19 remaining covariates with univariate Cox regression P <.10 were modeled by multivariate Cox regression with backward selection and a threshold for retention in the model of P <.01 (Figure 1). Six covariates (prior-year PEx, IV treatment duration, percentage of treatment in hospital, chronic treatment with inhaled aminoglycosides, chronic treatment with leukotriene modifiers, and chronic treatment with high-dose ibuprofen) were retained in the final model (Figure 3).
Figure 3. Forest Plot of Final Cox Proportional Hazards Regression Model.
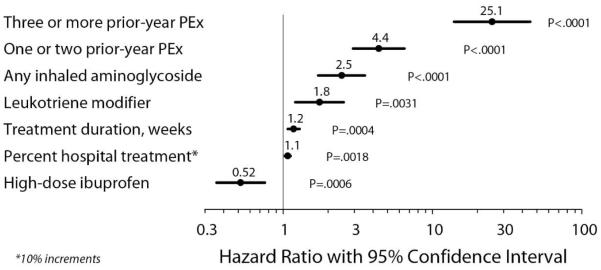
Four categorical variables and two continuous variables remaining from the original 20 retained covariates (Figure 1) after backwards selection with a requirement of covariate P <.01. Point estimates and associated P-values for covariates are shown.
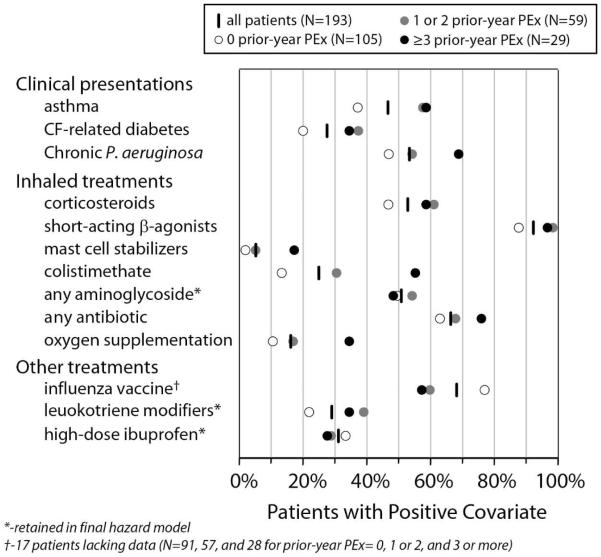
The effect of prior-year PEx on hazard rates was an order of magnitude greater than that of any of the remaining covariates, with three or more PEx in the prior year associated with a 25-fold higher hazard rate compared to none in the prior year, and one or two prior-year PEx associated with a more than 4-fold higher PEx hazard. Duration of IV treatment was associated with a modest increased hazard of future PEx (with each additional week of treatment associated with a ~20% hazard increase), as was an increased proportion of treatment in hospital (with each 10% increase in proportion of treatment in hospital associated with a 10% hazard increase). Patients receiving chronic inhaled aminoglycosides and leukotriene modifiers had 2.5-fold and 1.8-fold higher hazard rates for future PEx, while future PEx hazard was roughly halved among patients receiving high-dose ibuprofen (Figure 3). Covariate distributions differed among Prior-year PEx subgroups (Table 1, Figure 4).
Figure 4. Covariate distributions by Prior-year PEx subgroup.
Percentages of all patients (vertical dashes), and Prior-year PEx patient subgroups (circles) in which categorical covariates included in Cox proportional hazards regression (Figure 1) were positive.
DISCUSSION
Our results suggest that a patient’s history of PEx is a far more important factor associated with time to next PEx than any other of a number of readily available phenotypic and treatment-related covariates. By comparison, administration of selected chronic pulmonary therapies were much less strongly associated with time to next PEx. Impressively large hazard ratios associated with Prior-year PEx in univariate analyses increased after adjustment for other covariates.
The hazard ratios for the two covariates related to IV antibiotic treatment of PEx in the final model, total duration of IV treatment and proportion of IV treatment administered in the hospital, appear counterintuitive in that increasing treatment duration and proportion of treatment in hospital are associated with greater PEx hazard. This result may be due to indication bias resulting from more extended and aggressive treatment of patients considered by clinicians to be at greater risk for future PEx, although differences in mean treatment values between patients in different prior-year PEx categories are modest (Table 1).
It is unclear to what extent the presence of the remaining covariates in the model (chronic inhaled aminoglycosides, chronic leukotriene modifiers, and chronic high-dose ibuprofen) is a result of a direct effect on PEx hazard rate, of indication bias, or of both. Inhaled aminoglycosides have been shown to reduce the risk of PEx in prospective randomized controlled trials of chronically P. aeruginosa-infected persons with CF (16, 17) and we have previously shown that chronic P. aeruginosa infection is associated with an increased risk of IV treatment for PEx (18). These observations suggest that our observation of an increased PEx hazard associated with inhaled aminoglycosides, one of four covariates entered into modeling that were related to P. aeruginosa infection, was likely due to indication bias. Interestingly, inhaled antibiotic treatment choices differed across different Prior-year PEx subgroups at our center (Figure 4).
Similarly, increased PEx hazard associated with chronic administration of leukotriene modifiers may be an indirect result of a subgroup of patients with asthma being at higher risk for PEx, although diagnosis of asthma did not survive backwards selection as a covariate in our model. However, unlike the case with inhaled aminoglycosides, objective evidence from extended controlled trials that leukotriene modifiers do not increase risk of PEx is lacking. Although there are a number of different leukotriene modifiers that these patients may have been receiving, this level of granularity in treatment is not collected in the CFFPR.
The observation that patients receiving chronic high-dose ibuprofen had a lower hazard of future PEx may be reflective of the demographics of high-dose ibuprofen treatment at our center, in that high-dose ibuprofen has the greatest potential for slowing disease progression in younger patients with better associated lung function (19). However, it may also be that high-dose ibuprofen actually has an effect on future PEx hazard rate, or that it is a marker for patients with a tendency to be more adherent to all chronic therapies, and to have lower associated PEx hazard rates for that reason. There were no substantial differences in the proportion of patients receiving high-dose ibuprofen across Prior-year PEx subgroups (Figure 4).
There are important limitations to our study. Observational analyses can highlight associations but cannot evaluate causality. The fact that increasing duration of IV antibiotic treatment for PEx was associated with a modest increased hazard of subsequent PEx does not mean that a carefully-controlled prospective clinical study would reach the same conclusion. Further, our cohort is drawn from a limited geographic area, which affected both patient and management diversity. For instance, the average durations of IV antibiotic treatment for PEx among our patients <18 years and ≥18 years of age were 18.2 and 20.3 days, respectively; the corresponding medians across US CF Care Centers in 2012 were 13.0 days and 14.0 days (20).
Similarly, our average durations of hospital treatment of 11.8 days for children <18 years of age and 12.5 days for older patients are higher than the respective national medians across centers of 10.0 days and 9.2 days (20). Finally, these data are derived from one of only a handful of US CF Care Centers that ever prescribe high-dose ibuprofen (20), and there are likely other routine practices that are not shared broadly across CF care centers. Broader modeling including all eligible patients followed in the CFF Patient Registry would likely result in identification of additional and/or different significant covariates for hazard of future PEx, although we feel that it is almost certain that Prior-year PEx would remain highly predictive.
Incidence rates of IV antibiotic-treated PEx have been observed to increase with advancing lung disease stage and patient age (2, 18), and in a previous analysis of factors associated with time to next PEx in adults with CF that did not include prior-year PEx as a covariate, older age and lower FEV1 were associated with shorter time to next PEx. (21). Although we observed a trend where the frequency of prior-year PEx was somewhat higher among patients with more advanced lung disease (Table 1), neither patient age nor disease stage as captured by categorical FEV1% predicted were found to be significant covariates in regression modeling that included prior-year PEx as a covariate.
Epidemiologic data suggest there may be a variety of “causes” of PEx, including viral infection, inhaled pollutants, changes in bacterial infection, and other insults (1). Our results suggest that, independent of environmental factors, there appear to be fundamentally different phenotypes among persons with CF such that their hazard rates associated with future PEx can differ dramatically (by more than 25-fold). Interestingly, we saw no obvious trends among currently-collected demographics (other than history of PEx) that might be useful in identifying patients with higher PEx risk phenotypes. However, Sanders et al recently reported that high resolution computerized tomographic scores among school-age children are more strongly associated with IV-treated PEx rates over the following decade than are spirometric measures (22). Given that PEx are recognized as important clinical events associated with increased morbidity and mortality, and that there is substantial community interest in improving PEx management and outcomes, it will be important to account for these PEx phenotypic differences in future observational studies and clinical trial designs.
HIGHLIGHTS.
We studied intravenous (IV) antibiotic treatment for pulmonary exacerbations (PEx)
Covariates associated with time to next IV exacerbation treatment were studied
Number of PEx in the prior year had the greatest association with time to next PEx
ACKNOWLEDGEMENTS
The authors thank the patients, families, and CF caregivers associated with the Cleveland Ohio CF Center who contributed data to the Cystic Fibrosis Foundation Patient Registry. DRV and DJP did not receive an honorarium, grant, or other form of payment for their participation in this project. MWK was supported by NIH P30 DK027651 and the Cystic Fibrosis Foundation (CFFT KONSTA09Y0)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ferkol T, Rosenfeld M, Milla CE. Cystic fibrosis pulmonary exacerbations. J Pediatr. 2006 Feb;148(2):259–64. doi: 10.1016/j.jpeds.2005.10.019. Review. [DOI] [PubMed] [Google Scholar]
- 2.Goss CH, Burns JL. Exacerbations in cystic fibrosis. 1: Epidemiology and pathogenesis. Thorax. 2007 Apr;62(4):360–7. doi: 10.1136/thx.2006.060889. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Konstan M, Morgan W, Butler S, Pasta D, Craib M, Silva S, et al. Risk factors for rate of decline in forced expiratory volume in one second in children and adolescents with cystic fibrosis. J Pediatr. 2007;151:134–139. doi: 10.1016/j.jpeds.2007.03.006. 139. [DOI] [PubMed] [Google Scholar]
- 4.Amadori A, Antonelli A, Balteri I, Schreiber A, Bugiani M, De Rose V. Recurrent exacerbations affect FEV(1) decline in adult patients with cystic fibrosis. Respir Med. 2009 Mar;103(3):407–13. doi: 10.1016/j.rmed.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 5.VanDevanter DR, Wagener JS, Pasta DJ, Elkin E, Jacobs JR, Morgan WJ, Konstan MW. Pulmonary outcome prediction (POP) tools for cystic fibrosis patients. Pediatr Pulmonol. 2010;45:1156–66. doi: 10.1002/ppul.21311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Boer K, Vandemheen KL, Tullis E, Doucette S, Fergusson D, Freitag A, et al. Exacerbation frequency and clinical outcomes in adult patients with cystic fibrosis. Thorax. 2011 Aug;66(8):680–5. doi: 10.1136/thx.2011.161117. [DOI] [PubMed] [Google Scholar]
- 7.Waters V, Stanojevic S, Atenafu EG, Lu A, Yau Y, Tullis E, et al. Effect of pulmonary exacerbations on long-term lung function decline in cystic fibrosis. Eur Respir J. 2012 Jul;40(1):61–6. doi: 10.1183/09031936.00159111. [DOI] [PubMed] [Google Scholar]
- 8.Lieu T, Ray G, Farmer G, Shay G. The cost of medical care for patients with cystic fibrosis in a health maintenance organization. Pediatrics. 1999;103:e72. doi: 10.1542/peds.103.6.e72. [DOI] [PubMed] [Google Scholar]
- 9.Britto M, Kotagal U, Hornung R, Atherton H, Tsevat J, Wilmott R. Impact of recent pulmonary exacerbations on quality of life in patients with cystic fibrosis. Chest. 2002;121:64–72. doi: 10.1378/chest.121.1.64. [DOI] [PubMed] [Google Scholar]
- 10.Liou T, Adler F, Fitzsimmons S, Cahill B, Hibbs J, Marshall B. Predictive 5-year survivorship model of cystic fibrosis. Am J Epidemiol. 2001;153:345–352. doi: 10.1093/aje/153.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mayer-Hamblett N, Rosenfeld M, Emerson J, Goss C, Aitken M. Developing cystic fibrosis lung transplant referral criteria using predictors of 2-year mortality. Am J Respir Crit Care Med. 2002;166:1550–1555. doi: 10.1164/rccm.200202-087OC. [DOI] [PubMed] [Google Scholar]
- 12.Smith AL, Doershuk C, Goldmann D, Gore E, Hilman B, Marks M, et al. Comparison of a beta-lactam alone versus beta-lactam and an aminoglycoside for pulmonary exacerbation in cystic fibrosis. J Pediatr. 1999 Apr;134(4):413–21. doi: 10.1016/s0022-3476(99)70197-6. [DOI] [PubMed] [Google Scholar]
- 13.VanDevanter DR, Konstan MW. Outcome measures for clinical trials assessing treatment of cystic fibrosis lung disease. Clin Invest. 2012;2(2):163–175. doi: 10.4155/cli.11.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X, Dockery DW, Wypij D, Fay ME, Ferris BG. Pulmonary function between 6 and 18 years of age. Pediatr Pulmonol. 1993;15:75–88. doi: 10.1002/ppul.1950150204. [DOI] [PubMed] [Google Scholar]
- 15.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 16.Ramsey BW, Pepe MS, Quan JM, Otto KL, Montgomery AB, Williams-Warren J, et al. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. Cystic Fibrosis Inhaled Tobramycin Study Group. N Engl J Med. 1999 Jan 7;340(1):23–30. doi: 10.1056/NEJM199901073400104. [DOI] [PubMed] [Google Scholar]
- 17.Murphy TD, Anbar RD, Lester LA, Nasr SZ, Nickerson B, VanDevanter DR, et al. Treatment with tobramycin solution for inhalation reduces hospitalizations in young CF subjects with mild lung disease. Pediatr Pulmonol. 2004 Oct;38(4):314–20. doi: 10.1002/ppul.20097. [DOI] [PubMed] [Google Scholar]
- 18.VanDevanter DR, Yegin A, Morgan WJ, Millar SJ, Pasta DJ, Konstan MW. Design and powering of cystic fibrosis clinical trials using pulmonary exacerbation as an efficacy endpoint. J Cyst Fibros. 2011;10:453–9. doi: 10.1016/j.jcf.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Konstan MW, Byard PJ, Hoppel CL, Davis PB. Effect of high-dose ibuprofen in patients with cystic fibrosis. N Engl J Med. 1995 Mar 30;332(13):848–54. doi: 10.1056/NEJM199503303321303. [DOI] [PubMed] [Google Scholar]
- 20.Cystic Fibrosis Foundation Patient Registry . 2012 Annual data report to the Center Directors. Cystic Fibrosis Foundation; Bethesda Maryland: 2013. [Google Scholar]
- 21.Sequeiros IM, Jarad N. Factors associated with a shorter time until the next pulmonary exacerbation in adult patients with cystic fibrosis. Chron Respir Dis. 2012 Feb;9(1):9–16. doi: 10.1177/1479972311433575. [DOI] [PubMed] [Google Scholar]
- 22.Sanders DB, Li Z, Brody AS. Chest CT Predicts the Frequency of Pulmonary Exacerbations in Children with Cystic Fibrosis. Ann Am Thorac Soc. 2014 Dec 4; doi: 10.1513/AnnalsATS.201407-338OC. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]