Abstract
The concept of mammalian pheromones was established decades before the discovery of any bioactive ligands. Therefore, their molecular identity, native sources, and the meaning of their detection has been largely speculative. There has been recent success in identifying a variety of candidate mouse pheromones and other specialized odors. These discoveries reveal that mammalian pheromones come in a variety of ligand types and they are detected by sensory neurons that are pre-set to promote an array of social and survival behaviors. Importantly, recent findings show that they activate molecularly diverse sensory neurons that differ from canonical odorant detectors. These novel sensory neurons hold future promise to unlock the mystery of how their detection is hardwired to generate behavior.
Introduction
A simple sniff in a crowded environment causes an instant attraction towards the lucky emitter while others are hopelessly ignored. Such is the power of pheromones according to popular culture. The concept was established in the middle of the last century when studies with invertebrates such as ants, moths, and bees revealed the existence of powerful olfactory cues that are pre-programmed to direct and regulate cohesive social behavior [1–3]. These signals, named pheromones, are molecules that are produced by one individual and act as ‘ectohormones’ to hijack behavior when detected by another member of the same species [1]. In the intervening fifty years only a handful of mammalian pheromones have been discovered. This may be because they are extremely rare, act in complex blends, or initiate behaviors that are not observed in simplified laboratory environments. Their identification is further complicated because normal odors can appear to act as pheromones when coupled with experience, by similarly evoking a range of social behaviors with emotional valence such as fear and attraction [4]. Several new ligands have now been isolated that have specialized properties not evoked by normal odors.
Pheromones are specialized odors
Olfactory signals can be segregated into two classes: associative and specialized (Figure 1). In associative olfaction, one experiences an odor and interacts with the environment to determine its meaning such as whether it is pleasant or aversive. This association is not fixed; its significance can change with new experience and therefore varies between individuals. In contrast, specialized odors are thought to activate subsets of neurons that have a high probability of generating pre-set behavior. Therefore, their detection can elicit the same behavior in a subset of individuals irrespective of individual experience [3]. Specialized odors are proposed to include pheromones [1,3]. Based on these assumptions one could assume that there are at least two different types of olfactory sensory detectors; one set to generate flexible associative sensations and another hardwired to respond to ligands with pre-set function such as pheromones. To activate a response that is different from associative olfaction, the pheromone detection system may be composed of sensory detectors that have different physiological properties (perhaps in adaptation or temporal response) and/or they may project axons to special preset targets in the brain.
Figure 1. Two classes of olfactory sensory signals; associative and specialized.
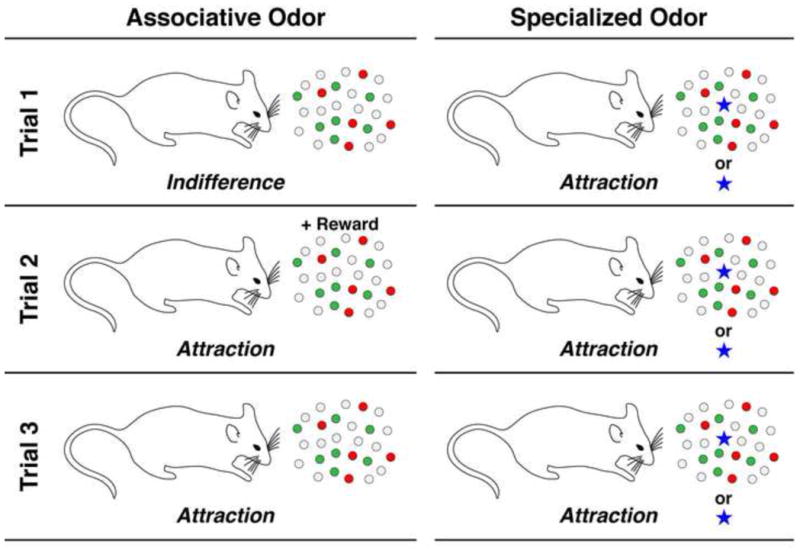
Left: Associative odors do not inherently encode behavioral meaning (trial 1). Upon either positive or negative experience (trial 2) further interaction with the odor will elicit a largely predictable behavior (trial 3). Right: Specialized odors activate neural pathways that are pre-set with meaning. Upon first detection of either odors detected in a complex environment or even purified ligands will elicit behavior (trial 1). The associated behavior is largely fixed, repeated exposure does not alter its pre-set meaning (trials 2–3).
The nasal cavity of almost all terrestrial vertebrates contains two anatomically separate chemosensory organs: the vomeronasal organ (VNO) and the main olfactory epithelium (MOE) [5]. Not only are they physically independent, but their sensory neurons largely express completely different repertoires of olfactory receptors and signal transduction components, they respond to different classes of ligands, their adaptation physiology differs, and they each project axons to different regions of the brain. Based on these differences it has been proposed that the MOE and the VNO may have two distinct functions [1]. One theory has been that the MOE generates traditional associative olfaction while the VNO serves to detect specialized ligands such as pheromones. While most candidate mouse pheromones have been found to be detected by VNO sensory neurons, it is now clear that the MOE functions to sense both specialized and associative odorants (Table 1).
Table 1.
Candidate Mouse Pheromones
| Sensory Organ | Pheromone | Source | Effect |
|---|---|---|---|
| VNO | 2-heptanone1 trans-5-hepten-2-one1 trans-4-hepten-2-one1 n-pentyl acetate1 cis-2-penten-1-yl-acetate1 2,5-dimethylpyrazine1 |
Female urine | Puberty delay |
| VNO | 2-sec-butyl-dihydrothiazole (SBT)1 2,3-dehydro-exo-brevicomin (DHB)1 |
Male urine | Estrus induction Intermale aggression Female attraction |
| VNO | α and β farnesenes1 | Male urine | Estrus induction Intermale aggression |
| VNO | 6-hydroxy-6-methyl-3-heptanone1 | Male urine | Puberty acceleration |
| VNO | MUPs (Major Urinary Proteins)2 | Male urine | Male countermarking |
| VNO | MUP32 | Male urine | Intermale aggression |
| VNO | MUP202–3 | Male urine | Intermale aggression Female attraction |
| VNO | ESP14 | Male tear | Female attraction |
| VNO | ESP225 | Juvenile tear | Inhibiting mating |
| VNO | Sulfated Estrogens6 | Female urine | Male attraction |
| VNO | MUPs7 | Cat saliva and Rat urine | Avoidance |
| VNO and MOE | MHC class I peptides8 | Urine | Individual recognition |
| MOE | 2-phenylethyamine9 | Carnivore urine | Avoidance |
| MOE | 2,5-dihydro-2,4,5-trimethylthiazoline (TMT)10 | Fox odor | Avoidance |
| MOE | (methylthio) methanethiol (MTMT)11 | Male urine | Female attraction |
| MOE | Trimethylamine4 | Male urine | Attraction |
References:
Dulac and Torello, 2003;
Kaur et al., 2014;
Roberts et al., 2010;
Haga et al., 2010;
Ferrero et al., 2013;
Haga-Yamanaka et al., 2014;
Papes et al., 2010;
Leinders-Zufall et al., 2004;
Dewan et al., 2013;
Kobayakawa et al., 2007;
Lin da et al., 2005; 12) Li et al., 2013
Mammals have two distinct olfactory sensory organs
Recent deep sequencing analysis has revealed dramatic molecular differences between sensory neurons located in the VNO and MOE [6,7]. The VNO of mice express 585 G-protein-coupled receptors (GPCRs) to detect sensory ligands which are largely absent from the MOE [6]. While the MOE sensory neurons each express a single sensory GPCR tuned to detect and respond to a set of chemical moieties [8,9], some VNO neurons express as many as three different receptors; two VNO-specific GPCRs and a non-classical major histocompatibility receptor of the H2-Mv family [10–12]. The functional significance of expressing of two VNO receptors remains unknown, however chromosome engineering to delete the entire family of H2-Mvs found that these membrane proteins are necessary for the VNO to be ultrasensitive to exceedingly low amounts of ligands in the environment [11]. The MOE can be similarly sensitive to very low concentration of ligands, therefore, whether the expression of H2-Mvs constitutes a functional difference between the VNO and MOE remains unknown. VNO-specific ligands and GPCRs have been found to promote social behaviors expected of pheromones such as mating, male aggression, attraction and male territory urine-marking [13–20]. Genetically ablating VNO signal transduction elements that are commonly expressed by many different VNO sensory neuron populations alters male and female parental behavior and male aggression [21–24]. The source of odor in these studies is from other members of the same species, which is a characteristic of pheromones. However, the tested animals don’t respond with detectable behavior until they are sexually mature. Since juvenile mice require adult mouse care throughout development, animals in these studies are continuously exposed to a complete repertoire of adult pheromones. This prior experience with their own, parental, or cage-mates’ emitted ligands, prior to first experimental testing, raises the formal possibility that the observed response is actually an associative one learned throughout development.
The Vomeronasal Organ detects specialized odors
To determine if chemosensory ligands and their receptor neurons are truly specialized (pre-programmed with meaning) one needs to test the innate response of animals upon their very first encounter with the ligand. The identification and purification of bioactive ligands that direct aggression, mating, and fear have recently made such experiments possible. Male-male territorial aggression is mediated by a protein excreted in male urine; MUP20 (darcin) [14,16] which is exclusively detected by VNO neurons [13,14]. Urine from BALB/c mice, a lab strain that fails to express MUP20, does not evoke aggression in other BALB/c males or males from the C57BL/6J strain which robustly expresses MUP20. BALB/c males therefore have no experience with MUP20 throughout their development yet they initiate the complete repertoire of aggressive behavior upon first exposure to MUP20 [14]. This indicates that MUP20 is a specialized olfactory ligand; able to activate sensory detectors hardwired to circuits that trigger aggressive behavior.
Specialized ligands are also pre-set to direct appropriate mating. Males secrete the small peptide ESP1 in their tears which is detected by a receptor in the VNO [18,25]. Receptive females investigate the face of males and upon detection of ESP1 initiate lordosis (a female mating posture) [18]. Analysis of females from a strain that does not produce ESP1 (C57BL/6J) showed an increase in lordosis with males from an ESP1-producing strain (BALB/c) compared to C57BL/6J males [17], confirming that experience with the ligand is not necessary for its specialized function. Further, juveniles have been found to secrete another small peptide (ESP22) whose detection inhibits mating advances from adult males [19]. Analysis of males from a strain that lacks ESP22 expression (C3H) finds that first exposure of adult males to this ligand appropriately inhibits sexual behavior [19].
Even specialized ligands emitted from other species activate sensory receptors that are evolved to promote pre-programmed significance upon detection by mice (kairomones) [3]. Some inbred strains of lab mice (such as C57BL/6J) have been housed for hundreds of generations in isolation from other species. Strikingly, first exposure to a cotton swab infused with the odor of cats, rats, or snakes is sufficient to promote a repertoire of fear-like responses including avoidance and cautious stretch-attend behavior as well as a rapid and robust increase in levels of the stress hormone ACTH [26]. Identification of the bioactive ligands produced by cats and rats has enabled their artificial synthesis. In turn, these were used to demonstrate that their activation of VNO sensory neurons is sufficient to generate fear-like behaviors upon an animals’ first encounter [26].
When considered together these experiments reveal that subsets of chemosensory ligands emitted by mice are specialized to activate neurons in the VNO that are preset with meaning to initiate behavior. It has recently been determined that in mice the majority of VNO sensory neurons are tuned to detect ligands emitted from other species, such as birds and snakes [26,27]. The isolation of the activating ligands and the associated behavior promoted by these cues largely remains to be determined so it is not known if they each have a specialized function [27]. It is reasonable to speculate that those ligands emitted from potential predators are preset to generate fear similar to the cat and rat purified cues [26].
Other subsets of VNO sensory neurons have been found to be tuned to specifically detect sulfated steroids, which include sulfated androgens, estrogens, and glucocorticoids [27–29]. Sulfated steroids strongly activate subsets of receptors in the VNO [27,29], however, their meaning to the receiving animal is largely unknown. It has been suggested that they act as pheromones to indicate the physiological status of the emitter (gender, ovulation stage, stress levels) in order to inform the receivers’ behavior [27,30,31]. Interestingly, a sulfated estrogen emitted by ovulating females has now been found to promote male courtship behavior, but only when detected in combination with a second, currently uncharacterized, female urine ligand (which also does not initiate behavior on its own) [20]. Each of these ligands may combine to form a multi-component pheromone, or they may be transmitting different kinds of information about the emitter. Many social behaviors require the receiver to simultaneously confirm several independent characteristics of the emitter (such as their species, gender, dominance, age, health) some of which are stable across an animal’s lifetime and others which rapidly change with environment and experience. Sulfated steroids may provide a mechanism to transmit dynamic information about the emitter’s internal state, which then may work in concert with other, more general, ligands that transmit stable information in order to appropriately guide the receiver’s response.
The MOE also detects specialized ligands
It has been clear from complementary experiments ablating either MOE or VNO function in mice that in addition to associative olfaction, the MOE also senses specialized odors [32–38]. However, with >1200 different types of sensory GPCRs expressed in the MOE, discovering the minor subset that is specialized has proven remarkably difficult [6]. The first candidates have now been identified through the assumption that the transcriptome of specialized sensory neurons may differ from those that perform associative olfaction (Figure 2). A subset of MOE neurons has been found to lack the expression of canonical odorant receptors (ORs) and instead express one of nine Trace Amine Associated Receptors (TAARs) [39,40]. These olfactory-specific receptors detect ultra-low concentrations of volatile amines as well as unknown ligands in predator urine [40–42]. Upon first exposure to this class of ligands individuals largely respond with innate aversion [41,43], with the exception of trimethylamine which promotes innate attraction [42]. In addition to TAAR-expressing neurons, canonical OR-expressing neurons may also function as detectors of amines in the MOE. ORs do not recognize entire ligands instead they detect molecular features (parts of the molecule) [9]. This means that many ORs recognize each ligand and each ligand is detected by several ORs; termed ‘combinatorial coding’ [9]. Following this principle, there likely exists ORs capable of detecting molecular features of TAAR activating ligands and the coincidental activity of ORs and TAARs may be expected to cooperatively generate the aversive behavior. Remarkably, genetic deletion of a single TAAR, TAAR4, entirely eliminates the aversive behavior evoked by a subset of amines and even converts the response towards predator odor from innately aversive to attractive [43]. This indicates that the TAAR4 expressing neurons are powerfully specialized and confirms that individual MOE neuron types can be preset with meaning to instruct an individual’s behavior.
Figure 2. Sensory neurons that have been implicated in pheromone detection.
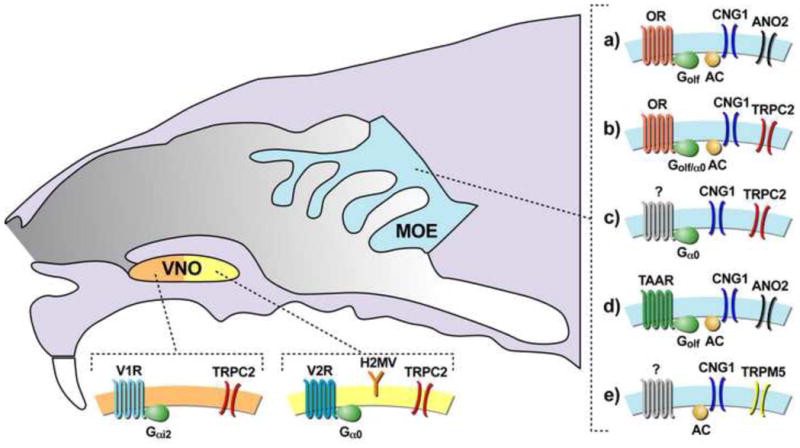
The mouse VNO is composed of two major sensory neuron subtypes those expressing V1R receptors (orange) and V2R expressing cells (yellow). Both have been implicated in pheromone detection. a–e) The mouse MOE (blue) is primarily populated with canonical sensory neurons (a) which express Ors, Golf, and CNG1. These neurons are thought to generate associative olfaction. b–e) Sensory neurons expressing signaling elements that differ from canonical odorant neurons have been implicated in pheromone detection.
In addition to identification of the TAARs, molecular profiling has discovered several other non-canonical MOE neuron types (Figure2). One type expresses the primary sensory transduction channel found in VNO neurons; TRPC2 [34,44,45]. TRPC2 neurons located in the MOE appear to be hybrids of canonical VNO and MOE neurons. Some TRPC2 neurons express the MOE-specific ORs and G-protein (Golf), yet in others the sensory receptors have not been found but they have been shown to express VNO-type G-proteins (Gαo) [45]. It remains unknown whether these unusual MOE neurons generate a specialized function. While most previous studies ensured the reported phenotypes were due to TRPC2 function in the VNO [34 (explicited tested in 13), 46], others attributed specialized function to the VNO based solely on analysis of TRPC2 mutants [33] which instead may have been due, partially or completely, to the loss of this new class of MOE sensory detection.
A third subset of MOE neurons, defined by the expression of another channel TRPM5, is also implicated to generate specialized function [47–49]. TRPM5 has been shown to be necessary for detection of 2-heptanone and 2-DMP at ultra-sensitive concentrations [47,50]. These ligands are secreted in the urine of adult males and have been reported to promote social behavior which is consistent with pheromone activity. However, 2-heptanone and 2-DMP also robustly activate VNO neurons [51] therefore whether TRPM5 expressing neurons located in the MOE contribute to innate behavioral responses to these odors remains to be determined.
Conclusions: Why utilize two olfactory systems to detect specialized ligands?
The recent success in identification of potential mammalian pheromone ligands as well as their sensory neurons and receptors has now confirmed that mice indeed produce and detect specialized chemosensory ligands that generate pre-programmed social and survival behavior. The biochemical properties of these ligands are diverse (from proteins, to peptides, to small organic volatiles) as is the molecular identities of their cognate sensory neurons. The discovery of these specialized sensory neurons provides the means to begin to determine how they differ from associative olfactory receptors to promote pre-programmed behavior.
The task of generating specialized olfactory responses now appears to be equally divided between the VNO and the MOE. If pre-programmed olfactory information can be processed by the MOE, then what is the purpose of an anatomically separated VNO? Genomic analysis indicates that the VNO-specific GPCRs have undergone rapid and substantial changes between species [52–54]. This has created a ‘semi-private’ repertoire of species-specific VNO receptors [54] which may be dedicated to pre-programmed tasks tailored to the need of each species: such as aversion towards relevant predators or mouse-specific behaviors such as lordosis, nest building, or scent marking. In contrast, many olfactory receptors have orthologs across evolution [55,56] and most of the specialized behaviors attributed to MOE sensation such as aversion to amines or attraction to the opposite sex are similarly performed by many mammal species using stereotyped motor outputs. Further comparative analysis of specialized olfactory ligands, sensory detectors, and the behavioral consequences of their sensation will be required to solve the puzzle of the logic of specialized olfactory coding.
Highlights.
A variety of mammalian pheromones have recently been isolated from the mouse
Mouse pheromones, cognate sensory neurons, and their elicited behaviors are diverse
Main olfactory epithelium and vomeronasal sensory neurons detect pheromones
The logic of why two olfactory organs function to detect pheromones remains unknown
Acknowledgments
We would like to thank J. Keller and DW Logan for helpful comments on the text. LS is supported by grants from the NIH-NIDCD, Ellison Medical Foundation, and Skaggs Trust.
Footnotes
Conflict of interest statement:
Nothing declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Lisa Stowers, Email: Stowers@scripps.edu.
Tsung-Han Kuo, Email: Kuoth@scripps.edu.
References Cited
- 1.Karlson P, Luscher M. Pheromones’: a new term for a class of biologically active substances. Nature. 1959;183:55–56. doi: 10.1038/183055a0. [DOI] [PubMed] [Google Scholar]
- 2.Wagner S, Gresser AL, Torello AT, Dulac C. A multireceptor genetic approach uncovers an ordered integration of VNO sensory inputs in the accessory olfactory bulb. Neuron. 2006;50:697–709. doi: 10.1016/j.neuron.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 3.Wyatt TD. Pheromones and signature mixtures: defining species-wide signals and variable cues for identity in both invertebrates and vertebrates. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2010;196:685–700. doi: 10.1007/s00359-010-0564-y. [DOI] [PubMed] [Google Scholar]
- 4.Choi GB, Stettler DD, Kallman BR, Bhaskar ST, Fleischmann A, Axel R. Driving opposing behaviors with ensembles of piriform neurons. Cell. 2011;146:1004–1015. doi: 10.1016/j.cell.2011.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liberles SD. Mammalian pheromones. Annu Rev Physiol. 2014;76:151–175. doi: 10.1146/annurev-physiol-021113-170334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ibarra-Soria X, Levitin MO, Saraiva LR, Logan DW. The olfactory transcriptomes of mice. PLOS Genetic. 2014 doi: 10.1371/journal.pgen.1004593. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pascarella G, Lazarevic D, Plessy C, Bertin N, Akalin A, Vlachouli C, Simone R, Faulkner GJ, Zucchelli S, Kawai J, et al. NanoCAGE analysis of the mouse olfactory epithelium identifies the expression of vomeronasal receptors and of proximal LINE elements. Front Cell Neurosci. 2014;8:41. doi: 10.3389/fncel.2014.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan M, Vaes E, Mombaerts P. Regulation of the probability of mouse odorant receptor gene choice. Cell. 2011;147:907–921. doi: 10.1016/j.cell.2011.09.049. [DOI] [PubMed] [Google Scholar]
- 9.Malnic B, Hirono J, Sato T, Buck LB. Combinatorial receptor codes for odors. Cell. 1999;96:713–723. doi: 10.1016/s0092-8674(00)80581-4. [DOI] [PubMed] [Google Scholar]
- 10.Ishii T, Mombaerts P. Coordinated coexpression of two vomeronasal receptor V2R genes per neuron in the mouse. Mol Cell Neurosci. 2011;46:397–408. doi: 10.1016/j.mcn.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Leinders-Zufall T, Ishii T, Chamero P, Hendrix P, Oboti L, Schmid A, Kircher S, Pyrski M, Akiyoshi S, Khan M, et al. A family of nonclassical class I MHC genes contributes to ultrasensitive chemodetection by mouse vomeronasal sensory neurons. J Neurosci. 2014;34:5121–5133. doi: 10.1523/JNEUROSCI.0186-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silvotti L, Cavalca E, Gatti R, Percudani R, Tirindelli R. A recent class of chemosensory neurons developed in mouse and rat. PLoS One. 2011;6:e24462. doi: 10.1371/journal.pone.0024462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chamero P, Marton TF, Logan DW, Flanagan K, Cruz JR, Saghatelian A, Cravatt BF, Stowers L. Identification of protein pheromones that promote aggressive behaviour. Nature. 2007;450:899–902. doi: 10.1038/nature05997. [DOI] [PubMed] [Google Scholar]
- 14•.Kaur AW, Ackels T, Kuo TH, Cichy A, Dey S, Hays C, Kateri M, Logan DW, Marton TF, Spehr M, et al. Murine pheromone proteins constitute a context-dependent combinatorial code governing multiple social behaviors. Cell. 2014;157:676–688. doi: 10.1016/j.cell.2014.02.025. A single protein ligand, MUP20, has been proposed to regulate both aggression and countermarking behaviors, but the neural mechanisms of regulating these two behaviors are still unknown. This study shows that the context of detection of MUP20 determines the extent to which it serves as a pheromone or generates a more associative response that is influenced by previous experience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roberts SA, Davidson AJ, McLean L, Beynon RJ, Hurst JL. Pheromonal induction of spatial learning in mice. Science. 2012;338:1462–1465. doi: 10.1126/science.1225638. [DOI] [PubMed] [Google Scholar]
- 16.Roberts SA, Simpson DM, Armstrong SD, Davidson AJ, Robertson DH, McLean L, Beynon RJ, Hurst JL. Darcin: a male pheromone that stimulates female memory and sexual attraction to an individual male’s odour. BMC Biol. 2010;8:75. doi: 10.1186/1741-7007-8-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haga S, Hattori T, Sato T, Sato K, Matsuda S, Kobayakawa R, Sakano H, Yoshihara Y, Kikusui T, Touhara K. The male mouse pheromone ESP1 enhances female sexual receptive behaviour through a specific vomeronasal receptor. Nature. 2010;466:118–122. doi: 10.1038/nature09142. [DOI] [PubMed] [Google Scholar]
- 18.Kimoto H, Haga S, Sato K, Touhara K. Sex-specific peptides from exocrine glands stimulate mouse vomeronasal sensory neurons. Nature. 2005;437:898–901. doi: 10.1038/nature04033. [DOI] [PubMed] [Google Scholar]
- 19•.Ferrero DM, Moeller LM, Osakada T, Horio N, Li Q, Roy DS, Cichy A, Spehr M, Touhara K, Liberles SD. A juvenile mouse pheromone inhibits sexual behaviour through the vomeronasal system. Nature. 2013;502:368–371. doi: 10.1038/nature12579. Adult mice recognize and respond to juveniles with behavior that differs from encounters with adults, but the sensory cues for recognition of juveniles are unknown. This study showed that ESP22 is released from the tears of juvenile animals and activates neurons in the vomeronasal organ. This pheromone inhibits male mating behavior to protect young animal from the sexual behavior of adult mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20••.Haga-Yamanaka S, Ma L, He J, Qiu Q, Lavis LD, Looger LL, Yu CR. Integrated action of pheromone signals in promoting courtship behavior in male mice. Elife. 2014;3:e03025. doi: 10.7554/eLife.03025. In mice, the majority of receptors for pheromones have not been identified. In particular, the receptors conveying the information about animal status remain elusive. By profiling the calcium response of individual VNO neurons, this study identified two groups of V1Rs responding to a female pheromone. The V1re clade members recognize gender-identifying cues in female urine, while multiple members of the V1rj clade are cognate receptors for sulfated estrogens. Intriguingly, neither signal is alone sufficient to induce male courtship, suggesting integrated information is required. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chamero P, Katsoulidou V, Hendrix P, Bufe B, Roberts R, Matsunami H, Abramowitz J, Birnbaumer L, Zufall F, Leinders-Zufall T. G protein G(alpha)o is essential for vomeronasal function and aggressive behavior in mice. Proc Natl Acad Sci U S A. 2011;108:12898–12903. doi: 10.1073/pnas.1107770108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montani G, Tonelli S, Sanghez V, Ferrari PF, Palanza P, Zimmer A, Tirindelli R. Aggressive behaviour and physiological responses to pheromones are strongly impaired in mice deficient for the olfactory G-protein-subunit G8. J Physiol. 2013;591:3949–3962. doi: 10.1113/jphysiol.2012.247528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oboti L, Perez-Gomez A, Keller M, Jacobi E, Birnbaumer L, Leinders-Zufall T, Zufall F, Chamero P. A wide range of pheromone-stimulated sexual and reproductive behaviors in female mice depend on G protein Galphao. BMC Biol. 2014;12:31. doi: 10.1186/1741-7007-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu Z, Autry AE, Bergan JF, Watabe-Uchida M, Dulac CG. Galanin neurons in the medial preoptic area govern parental behaviour. Nature. 2014;509:325–330. doi: 10.1038/nature13307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kimoto H, Sato K, Nodari F, Haga S, Holy TE, Touhara K. Sex- and strain-specific expression and vomeronasal activity of mouse ESP family peptides. Curr Biol. 2007;17:1879–1884. doi: 10.1016/j.cub.2007.09.042. [DOI] [PubMed] [Google Scholar]
- 26.Papes F, Logan DW, Stowers L. The vomeronasal organ mediates interspecies defensive behaviors through detection of protein pheromone homologs. Cell. 2010;141:692–703. doi: 10.1016/j.cell.2010.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Isogai Y, Si S, Pont-Lezica L, Tan T, Kapoor V, Murthy VN, Dulac C. Molecular organization of vomeronasal chemoreception. Nature. 2011;478:241–245. doi: 10.1038/nature10437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turaga D, Holy TE. Organization of vomeronasal sensory coding revealed by fast volumetric calcium imaging. J Neurosci. 2012;32:1612–1621. doi: 10.1523/JNEUROSCI.5339-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nodari F, Hsu FF, Fu X, Holekamp TF, Kao LF, Turk J, Holy TE. Sulfated steroids as natural ligands of mouse pheromone-sensing neurons. J Neurosci. 2008;28:6407–6418. doi: 10.1523/JNEUROSCI.1425-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arnson HA, Holy TE. Robust encoding of stimulus identity and concentration in the accessory olfactory system. J Neurosci. 2013;33:13388–13397. doi: 10.1523/JNEUROSCI.0967-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hammen GF, Turaga D, Holy TE, Meeks JP. Functional organization of glomerular maps in the mouse accessory olfactory bulb. Nat Neurosci. 2014;17:953–961. doi: 10.1038/nn.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim S, Ma L, Jensen KL, Kim MM, Bond CT, Adelman JP, Yu CR. Paradoxical contribution of SK3 and GIRK channels to the activation of mouse vomeronasal organ. Nat Neurosci. 2012;15:1236–1244. doi: 10.1038/nn.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leypold BG, Yu CR, Leinders-Zufall T, Kim MM, Zufall F, Axel R. Altered sexual and social behaviors in trp2 mutant mice. Proc Natl Acad Sci U S A. 2002;99:6376–6381. doi: 10.1073/pnas.082127599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stowers L, Holy TE, Meister M, Dulac C, Koentges G. Loss of Sex Discrimination and Male-Male Aggression in Mice Deficient for TRP2. Science. 2002;295:1493–1500. doi: 10.1126/science.1069259. [DOI] [PubMed] [Google Scholar]
- 35.Wang Z, Balet Sindreu C, Li V, Nudelman A, Chan GC, Storm DR. Pheromone detection in male mice depends on signaling through the type 3 adenylyl cyclase in the main olfactory epithelium. J Neurosci. 2006;26:7375–7379. doi: 10.1523/JNEUROSCI.1967-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Z, Storm DR. Maternal behavior is impaired in female mice lacking type 3 adenylyl cyclase. Neuropsychopharmacology. 2011;36:772–781. doi: 10.1038/npp.2010.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fraser EJ, Shah NM. Complex chemosensory control of female reproductive behaviors. PLoS One. 2014;9:e90368. doi: 10.1371/journal.pone.0090368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsuo T, Hattori T, Asaba A, Inoue N, Kanomata N, Kikusui T, Kobayakawa R, Kobayakawa K. Genetic dissection of pheromone processing reveals main olfactory system-mediated social behaviors in mice. Proc Natl Acad Sci U S A. 2015 doi: 10.1073/pnas.1416723112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liberles SD, Buck LB. A second class of chemosensory receptors in the olfactory epithelium. Nature. 2006;442:645–650. doi: 10.1038/nature05066. [DOI] [PubMed] [Google Scholar]
- 40.Pacifico R, Dewan A, Cawley D, Guo C, Bozza T. An olfactory subsystem that mediates high-sensitivity detection of volatile amines. Cell Rep. 2012;2:76–88. doi: 10.1016/j.celrep.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferrero DM, Lemon JK, Fluegge D, Pashkovski SL, Korzan WJ, Datta SR, Spehr M, Fendt M, Liberles SD. Detection and avoidance of a carnivore odor by prey. Proc Natl Acad Sci U S A. 2011;108:11235–11240. doi: 10.1073/pnas.1103317108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Q, Korzan WJ, Ferrero DM, Chang RB, Roy DS, Buchi M, Lemon JK, Kaur AW, Stowers L, Fendt M, et al. Synchronous evolution of an odor biosynthesis pathway and behavioral response. Curr Biol. 2013;23:11–20. doi: 10.1016/j.cub.2012.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43•••.Dewan A, Pacifico R, Zhan R, Rinberg D, Bozza T. Non-redundant coding of aversive odours in the main olfactory pathway. Nature. 2013 doi: 10.1038/nature12114. It is generally thought that odors in MOE are encoded by combinatorial coding, in which single receptor has little effect to the behavior. This study showed that deletion of TAAR4 in mice is sufficient to completely abolish aversion to volatile amines and to predator urine, suggesting that individual main olfactory receptor genes can contribute substantially to odor perception. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liman ER, Corey DP, Dulac C. TRP2. a candidate transduction channel for mammalian pheromone sensory signaling. Proc Natl Acad Sci U S A. 1999;96:5791–5796. doi: 10.1073/pnas.96.10.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45•.Omura M, Mombaerts P. Trpc2-expressing sensory neurons in the main olfactory epithelium of the mouse. Cell Rep. 2014;8:583–595. doi: 10.1016/j.celrep.2014.06.010. Although the function of TRPC2 in the VNO has been largely studied, the expression of TRPC2 in MOE, however, has never been addressed. Using in situ hybridization and immunohistology combined with TrpC2 knockout and TrpC2-lacZ knockin mice, this study provided the evidence that TRPC2 also expresses in the MOE, thus challenges the VSN-specific interpretation of the behavioral phenotypes of Trpc2-KO mice in previous studies. [DOI] [PubMed] [Google Scholar]
- 46.Kimchi T, Xu J, Dulac C. A functional circuit underlying male sexual behaviour in the female mouse brain. Nature. 2007;448:1009–1014. doi: 10.1038/nature06089. [DOI] [PubMed] [Google Scholar]
- 47.Lin W, Margolskee R, Donnert G, Hell SW, Restrepo D. Olfactory neurons expressing transient receptor potential channel M5 (TRPM5) are involved in sensing semiochemicals. Proc Natl Acad Sci U S A. 2007;104:2471–2476. doi: 10.1073/pnas.0610201104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thompson JA, Salcedo E, Restrepo D, Finger TE. Second-order input to the medial amygdala from olfactory sensory neurons expressing the transduction channel TRPM5. J Comp Neurol. 2012;520:1819–1830. doi: 10.1002/cne.23015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49•.Lopez F, Delgado R, Lopez R, Bacigalupo J, Restrepo D. Transduction for pheromones in the main olfactory epithelium is mediated by the Ca2+-activated channel TRPM5. J Neurosci. 2014;34:3268–3278. doi: 10.1523/JNEUROSCI.4903-13.2014. It is widely accepted that MOE plays a role in pheromone detection; however, the identity of the underlying neurons as defined by their signal transduction machinery is poorly understood. This study identified the expression of calcium activated cation channel TRPM5 in the neurons responding to putative pheromones in the MOE. Genetic and pharmacological blocking of TRPM5 abolished the neural responses to pheromone, indicating the requirement of TRPM5 for pheromone detection in MOE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rolen SH, Salcedo E, Restrepo D, Finger TE. Differential localization of NT-3 and TrpM5 in glomeruli of the olfactory bulb of mice. J Comp Neurol. 2014;522:1929–1940. doi: 10.1002/cne.23512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leinders-Zufall T, Lane AP, Puche AC, Ma W, Novotny MV, Shipley MT, Zufall F. Ultrasensitive pheromone detection by mammalian vomeronasal neurons. Nature. 2000;405:792–796. doi: 10.1038/35015572. [DOI] [PubMed] [Google Scholar]
- 52.Brykczynska U, Tzika AC, Rodriguez I, Milinkovitch MC. Contrasted evolution of the vomeronasal receptor repertoires in mammals and squamate reptiles. Genome Biol Evol. 2013;5:389–401. doi: 10.1093/gbe/evt013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shi P, Zhang J. Comparative genomic analysis identifies an evolutionary shift of vomeronasal receptor gene repertoires in the vertebrate transition from water to land. Genome Res. 2007;17:166–174. doi: 10.1101/gr.6040007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Young JM, Massa HF, Hsu L, Trask BJ. Extreme variability among mammalian V1R gene families. Genome Res. 2010;20:10–18. doi: 10.1101/gr.098913.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Adipietro KA, Mainland JD, Matsunami H. Functional evolution of mammalian odorant receptors. PLoS Genet. 2012;8:e1002821. doi: 10.1371/journal.pgen.1002821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Niimura Y, Matsui A, Touhara K. Extreme expansion of the olfactory receptor gene repertoire in African elephants and evolutionary dynamics of orthologous gene groups in 13 placental mammals. Genome Res. 2014;24:1485–1496. doi: 10.1101/gr.169532.113. [DOI] [PMC free article] [PubMed] [Google Scholar]


