Abstract
Introduction
It remains unknown whether dipeptidyl peptidase-4 (DPP-4) inhibitors improve early-phase insulin secretion in Japanese patients with type 2 diabetes (T2D), a disease characterized by impaired insulin secretion. We investigated the changes in insulin secretion before and after treatment with the DPP-4 inhibitor teneligliptin in patients with T2D with a low insulinogenic index (IGI) determined by the oral glucose tolerance test (OGTT).
Methods
An open-label, prospective clinical study was conducted. Thirteen drug-naïve patients (mean age 55.5 ± 3.9 years) with T2D underwent OGTT before and after teneligliptin 20 mg/day monotherapy. Plasma levels of glucose (PG), insulin, and C-peptide were measured at 0, 30, 60, 90, and 120 min after glucose loading in the OGTT. Homeostasis model assessment (HOMA)-β, IGI, and the total or incremental area under the curve (AUC) for PG and insulin were measured. AUC120min for the secretory units of islets in transplantation (SUIT) index was also measured.
Results
HbA1c significantly decreased from 8.3 ± 0.4 % at baseline to 6.3 ± 0.2 % after 12 weeks of teneligliptin treatment (p < 0.05). Incremental AUC120min PG also significantly decreased, and β-cell function assessed by IGI30min, AUC120min insulin, and the AUC120min SUIT index significantly increased (0.16 ± 0.05 vs. 0.28 ± 0.06, 2692 ± 333 µU·2h/mL vs. 3537 ± 361 µU·2h/mL, and 4261 ± 442 vs. 8290 ± 1147, respectively; all p < 0.05). HOMA-β was unchanged. The reduction in incremental AUC120min PG was significantly associated with the augmentation of IGI30min and the AUC120min SUIT index. No severe adverse events were observed.
Conclusions
Twelve weeks of teneligliptin treatment improved IGI30min, AUC120min, and the SUIT index in drug-naïve Japanese patients with T2D.
Key Points
| It remains unknown whether Dipeptidyl Peptidase (DPP)-4 inhibitors improve early-phase insulin secretion in Japanese patients with Type 2 diabetes (T2D) characterized by impaired insulin secretion. |
| We investigated changes of insulin secretion before and after 12 weeks treatment with a DPP-4 inhibitor, teneligliptin, in drug naïve patients with T2D with low insulinogenic index (IGI) determined by an oral glucose tolerance test. IGI [30-min insulin − 0-min insulin)/(30-min glucose − 0-min glucose) = IGI30min] and AUC120min for secretory units of islets in transplantation index {C-peptide (ng/mL) × 1500/[PG (mg/dL) − 61.7] = SUIT-index) were measured. |
| HbA1c significantly decreased from 8.3 ± 0.4 % to 6.3 ± 0.2 % (p < 0.05). IGI30min, AUC120min Insulin, and AUC120min SUIT-index significantly increased (0.16 ± 0.05 vs. 0.28 ± 0.06, 2692 ± 333 µU·2h/mL vs. 3537 ± 361 µU·2h/mL, and 4261 ± 442 vs. 8290 ± 1147, respectively; all p < 0.05). |
| Twelve weeks of teneligliptin treatment markedly improved β-cell function in drug naïve Japanese patients with T2D. |
| No severe hypoglycemia or major side effects occurred. |
Introduction
The prevalence of type 2 diabetes (T2D) has been rapidly increasing worldwide [1], particularly in Asian countries [1, 2]. Dipeptidyl peptidase-4 (DPP-4) inhibitors have recently emerged as a new class of oral hypoglycemic drugs (OADs). Although several clinical studies have shown that DPP-4 inhibitors improve glycemic control and β-cell function, the influence of DPP-4 inhibitors on insulin secretion is controversial in patients with T2D. Several reports on DPP-4 inhibitors in patients with T2D have shown no significant increases of plasma insulin during meal tolerance tests [3–6], while others have shown significant increases in insulin during oral glucose tolerance tests (OGTT) [7, 8]. The apparent discrepancy in insulin levels between these methods may be partly explained by the stronger glucose stimulation provided by the OGTT versus the meal tolerance test. Teneligliptin is a novel DPP-4 inhibitor that is now commercially available in Japan. The agent potently inhibits DPP-4, with a 50 % inhibitory concentration (IC50) of 1 nmol, but its effects on β-cell function and early-phase insulin secretion during OGTT have yet to be studied.
The present investigation sought to evaluate the effects of teneligliptin 20 mg/day monotherapy on insulin secretion and postprandial glucose excursions during OGTT in drug-naïve Japanese patients with T2D.
Materials and Methods
Subjects
Japanese patients aged 20–76 years with T2D were eligible to participate if they had inadequate glycemic control with diet and exercise and had not taken OADs for 48 weeks prior to enrollment. We excluded patients with type 1 diabetes, gestational diabetes, overt nephropathy (urine albumin-to-creatinine ratio >300 mg/g creatinine or estimated glomerular filtration rate <60 ml/min/1.73 m2), liver disease, previous treatment with DPP-4 inhibitors, insulin treatment within 1 year of enrollment (except if used during hospitalization), infections, or malignant tumors. Patients who were taking drugs with diabetogenic effects, such as corticosteroids, were also excluded.
Study Methods
Subjects were administered 20 mg of teneligliptin once daily after breakfast for 12 weeks. Plasma glucose, insulin, and C-peptide immunoreactivity (CPR) were measured after an overnight 12-h fast, and at 30, 60, 90, and 120 min during the 75-g OGTT, before and after a 12-week period of teneligliptin treatment. In the OGTT after the 12-week treatment period, patients were administered teneligliptin before the test commenced. There were no changes in teneligliptin dose during the study period, no diabetic drugs added, and no changes in the doses of antihypertensive drugs or lipid-improving drugs that the subjects were already taking. Diabetic retinopathy was graded as simple, pre-proliferative, or proliferative retinopathy by ophthalmologists. The study was approved by the Ethical Committee of Showa University (approval no. 1519), and all patients provided informed consent.
Measurement of Pancreatic β-Cell Function and Insulin Sensitivity
Pancreatic β-cell function was evaluated based on the CPR index, homeostasis model assessment of β-cell function (HOMA-β), the insulinogenic index (IGI; an estimate of early insulin secretion), and the secretory units of islets in transplantation (SUIT) index during a 75 g OGTT. IGI was calculated by dividing the increment in insulin during the first 30 min by the increment in glucose over the same period [(30 min insulin − 0 min insulin)/(30 min glucose − 0 min glucose) = IGI30min].
The SUIT index was calculated at 0, 30, 60, 90, and 120 min during the OGTT (SUIT index0, SUIT index30, SUIT index60, SUIT index90, and SUIT index120, respectively) using the following formula: CPR (ng/mL) × 1500/[PG (mg/dL) − 61.7] [9]. The CPR index and HOMA-β were calculated as follows: CPR index, fasting CPR (ng/mL) × 100/fasting (mg/dL) [10]; HOMA-β, fasting insulin (μU/mL) × 360/[fasting PG (mg/dl) − 63] [11]. Insulin sensitivity was estimated using a homeostasis model assessment of insulin resistance (HOMA-IR), calculated using the following formula: fasting PG (mg/dL) × fasting insulin (μU/mL)/405 [11].
The total or incremental areas under the curve (AUC) during the OGTT for PG, insulin, and SUIT (incremental AUC120min PG, AUC120min insulin, and AUC120min SUIT index, respectively) were calculated using a trapezoidal method.
Biochemical Measurements
Plasma glucose levels were measured using the glucose-oxidase method, and plasma insulin and CPR concentrations were measured by immunoenzymometric assay (ST E test Tosoh II C-peptide; Tosoh Corporation, Tokyo, Japan). The 1,5-anhydro-d-glucitol (1,5-AG) enzyme assay was measured using standard methods, and glucagon was measured using a Glucagon RIA Kit (EMD Millipore, Billerica, MA, USA). The value for HbA1c (%) was estimated as a National Glycohemoglobin Standardization Program (NGSP) equivalent value (%) calculated using the following formula: HbA1c (%) = HbA1c [Japan Diabetes Society (JDS)] (%) + 0.4 % [12].
Results
Patient characteristics are listed in Table 1. The mean baseline values for HbA1c, fasting PG, body mass index, and duration of diabetes were 8.3 ± 0.4 %, 142.5 ± 6.3 mg/dL, 24.4 ± 1.0 kg/m2, and 3.6 ± 1.5 years, respectively. None of the patients had diabetic retinopathy, but ten patients (77 %) had normoalbuminuria and three patients (23 %) had microalbuminuria.
Table 1.
Baseline characteristics of patients (n = 13)
| Sex [male/female] (n) | 10/3 |
| Age (years) | 55.5 ± 3.9 |
| BMI (kg/m2) | 24.4 ± 1.0 |
| Diabetic retinopathy [NDR/SDR/PDR/PPDR] (n) | 13/0/0/0 |
| Diabetic nephropathy [none/microalbuminuria] (n) | 10/3 |
| Duration of diabetes (years) | 3.6 ± 1.5 |
| Baseline HbA1c (%) | 8.3 ± 0.4 |
| Fasting plasma glucose (mg/dL) | 142.5 ± 6.3 |
Data are expressed as mean ± standard error
BMI body mass index, NDR non-diabetic retinopathy, SDR simple diabetic retinopathy, PPDR pre-proliferative diabetic retinopathy, PDR proliferative diabetic retinopathy
Eight of the 13 patients were treated with teneligliptin alone, and the remaining five patients received teneligliptin with angiotensin receptor blockers (n = 3), hydrochlorothiazide (n = 1), or statins (n = 3). Table 2 shows the changes of glycemic parameters, pancreatic β-cell function, and insulin sensitivity between baseline and week 12. HbA1c levels significantly decreased from 8.3 ± 0.4 % at baseline to 6.3 ± 0.2 % after 12 weeks of teneligliptin treatment (p < 0.05). The 1.5-AG levels significantly improved from 6.5 ± 1.3 to 12.5 ± 1.6 µg/mL. Fasting plasma glucose and incremental AUC120min PG at week 12 were significantly lower than the baseline values.
Table 2.
Changes in glycemic parameters, pancreatic β-cell function, and insulin sensitivity between baseline and week 12
| Pre | Post | p value | |
|---|---|---|---|
| HbA1c (%) | 8.3 ± 0.4 | 6.3 ± 0.2 | <0.01 |
| 1,5-AG (μg/mL) [n = 11] | 6.48 ± 1.3 | 12.53 ± 1.6 | <0.01 |
| Fasting plasma glucose (mg/dL) | 142.5 ± 6.3 | 113.3 ± 4.8 | <0.01 |
| AUC120 PG (mg·2h/dL) | 14,097 ± 941 | 9193 ± 1060 | <0.01 |
| AUC120 insulin (µU·2h/mL) | 2692 ± 333 | 3537 ± 361 | <0.01 |
| AUC120 SUIT index | 4261 ± 442 | 8290 ± 1147 | <0.01 |
| CPR index | 1.55 ± 0.2 | 1.55 ± 0.1 | 0.99 |
| Insulinogenic index | 0.16 ± 0.05 | 0.28 ± 0.06 | <0.05 |
| HOMA-β (%) | 32.9 ± 4.4 | 44.9 ± 6.95 | 0.09 |
| HOMA-R | 2.52 ± 0.40 | 1.71 ± 0.26 | <0.05 |
Data are expressed as mean ± standard error
1,5-AG 1,5-anhydro-d-glucitol, PG plasma glucose, AUC area under the curve, SUIT secretory units of islets in transplantation, HOMA-β homeostasis model assessment of β-cell function, HOMA-R homeostasis model assessment of insulin resistance, CPR C-peptide immunoreactivity
The AUC120min insulin, AUC120min SUIT index, and IGI30min at week 12 were significantly higher than the baseline values [2692 ± 333 µU·2h/mL vs. 3537 ± 361 µU·2h/mL, 4261 ± 442 vs. 8290 ± 1147 (both p < 0.01), and 0.16 ± 0.05 vs. 0.28 ± 0.06 (p < 0.05), respectively]. There were no significant differences in HOMA-β or the CPR index at week 12 compared with baseline values. HOMA-IR at week 12 was significantly improved compared with baseline. In addition, we analyzed the changes of glycemic parameters, pancreatic β-cell function, and insulin sensitivity between baseline and week 12 after teneligliptin monotherapy (n = 8) without the patients treated with teneligliptin combined with angiotensin receptor blockers (n = 3), hydrochlorothiazide (n = 1), or statins (n = 3), which are well known to affect the glycemic parameters. After the 12-week treatment period, the eight patients treated with teneligliptin alone showed significant decreases in HbA1c, fasting plasma glucose, and AUC120 PG, significant increases in AUC120 insulin and AUC120 SUIT index, and a tendency towards increased IGI30min (0.13 ± 0.1 vs. 0.30 ± 0.19; p = 0.06) [data not shown].
Table 3 shows the correlations of the change of incremental AUC120min PG with the changes of pancreatic β-cell function and insulin sensitivity. The changes of incremental AUC120min PG were not correlated with the change of AUC120min insulin or HOMA-IR but were positively correlated with the changes of IGI30min and the AUC120min SUIT index (r = 0.68 and 0.63, respectively; p < 0.05).
Table 3.
Simple correlation between the changes of iAUC120min PG and those of pancreatic β-cell function and insulin sensitivity
| R | p value | |
|---|---|---|
| AUC120 insulin | −0.03 | 0.92 |
| Insulinogenic index | 0.68 | 0.01 |
| HOMA-R | −0.46 | 0.11 |
| AUC120 SUIT | 0.63 | 0.02 |
PG plasma glucose, R correlation coefficient, AUC area under the curve, iAUC incremental area under the curve, HOMA-R homeostasis model assessment of insulin resistance, SUIT secretory units of islets in transplantation
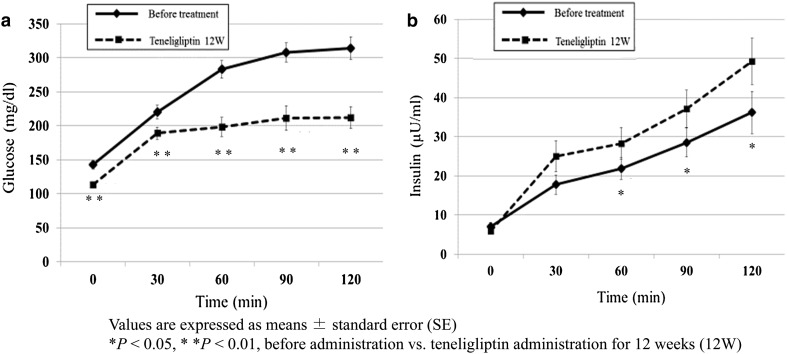
Figure 1a shows the changes in PG during the OGTT between baseline and week 12. After 12 weeks of teneligliptin treatment, PG levels significantly decreased at all time points during the OGTT compared with baseline values. Figure 1b shows the changes in insulin levels during the OGTT between baseline and week 12. Insulin levels after 12 weeks of treatment were higher than baseline levels at all time points, except at fasting, and the differences between baseline and post-treatment levels were significant at 60, 90, and 120 min.
Fig. 1.
Changes in a plasma glucose and b serum insulin levels in response to the oral glucose tolerance test before and after 12 weeks of teneligliptin administration. Data are expressed as mean ± standard error (SE). *p < 0.05, **p < 0.01, before vs. after 12 weeks of teneligliptin administration. 12W 12 weeks
Figure 2 shows the changes in the SUIT index during the OGTT between baseline and 12 weeks. The SUIT index after the 12-week treatment period was significantly higher at all time points during the OGTT compared with baseline values.
Fig. 2.
Changes in secretory units of islets in transplantation in response during oral glucose tolerance test before and after 12 weeks of teneligliptin administration
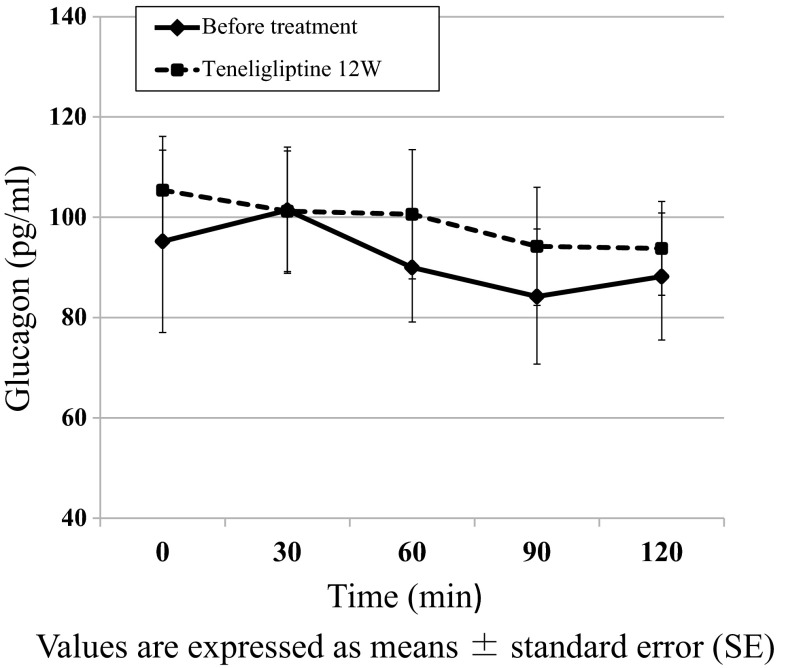
Figure 3 shows the changes in glucagon during the OGTT between baseline and week 12. No significant effect was observed for glucagon after the 12-week treatment period (n = 5).
Fig. 3.
Changes in glucagon in response during oral glucose tolerance test before and after 12 weeks of teneligliptin administration (n = 5)
No hypoglycemia or other side effects were observed during the study period.
Discussion
We demonstrated that 12-week treatment with teneligliptin 20 mg/day provided a substantial reduction in HbA1c and incremental AUC120min PG in drug-naïve Japanese patients with inadequately controlled T2D. Teneligliptin also significantly increased insulin secretion and improved insulin sensitivity without inducing clinically significant hypoglycemia or weight alteration. Most notably of all, teneligliptin significantly improved IGI30min and the AUC120min SUIT index. While it has already been confirmed that teneligliptin improves inadequate insulin secretion in fasting blood samples from T2D patients [13], the present study is the first to indicate the favorable effects of teneligliptin on glucose-mediated insulin secretion in the OGTT.
IGI30min reflects early-phase insulin secretion, a process impaired in most Japanese patients with T2D [14]. Recent investigations have shown that treatment with DPP-4 inhibitors improves IGI30min in patients with T2D [8, 16]. DPP-4 inhibitors induce a two- to threefold increase in glucagon-like peptide-1 (GLP-1) concentrations. The effects of GLP-1 on glucose-mediated insulin secretion are mainly regulated by cyclic adenosine monophosphate (cAMP)/protein kinase A and cAMP-Epac2 signaling pathways in a glucose-dependent manner. Shibasaki et al. reported that the cAMP-Epac2 signaling pathway amplifies early-phase insulin secretion [15]; therefore, we can speculate that the teneligliptin-induced increase in GLP-1 production brought about a pronounced improvement of IGI30min in the present study.
The SUIT index, an index of β-cell function, is generally calculated in fasting samples. In the present study we investigated the augmentation of CPR in response to the increase in glucose during the OGTT (i.e. AUC120min SUIT index). After the 12-week treatment we found that teneligliptin markedly increased not only SUIT index0 but also the AUC120min SUIT index compared with baseline values (Fig. 2). The AUC120min SUIT index before teneligliptin treatment was greatly impaired, suggesting that T2D had lost the incretin effect. Losses of incretin effect in T2D are mainly associated with reduced secretion of GLP-1 [16] and a lack of insulinotropic activity of glucose-dependent insulinotropic polypeptide [17].
The mechanisms contributing to the effect of teneligliptin in improving the AUC120min SUIT index were poorly understood in the present study. Recent finding has shown that DPP-4 inhibitors increase GLP-1-mediated insulin secretion by acting directly on islet DPP-4 [18]. In addition to their action on early-phase insulin secretion, this finding suggests that a local islet mechanism of DPP-4 inhibitors may also contribute to the improvement of the AUC120min SUIT index.
Although it is estimated that DPP-4 inhibitors may augment postload insulin, the significant increases in insulin or CPR levels during the meal tolerance test have not been observed in some clinical studies [3–6]. The insulin enhancement we observed during the OGTT after teneligliptin treatment may have important clinical implications regarding the effects of DPP-4 inhibitors on insulin secretion per se in patients with T2D. In comparison with the meal tolerance test, the OGTT appears to be a more direct method for determining glucose-mediated insulin secretion in patients with T2D taking DPP-4 inhibitors.
In the present study, teneligliptin was not found to increase HOMA-β or CPR index in spite of its effects in increasing IGI30min, AUC120min insulin, and the AUC120min SUIT index. HOMA-β and the CPR index have often been used to estimate β-cell function [10, 11] but have limitations because they only measure β-cell function under the fasting state. According to Meier et al., the CPR-to-glucose ratio after OGTT appears to better predict the β-cell area in individual patients with diabetes than fasting measures, such as the HOMA-β [19]. This suggests that fasting measures may not well reflect β-cell function.
Glucagon plays an essential role in glucose metabolism. Eto et al. reported that teneligliptin provided significant improvements in inappropriate postprandial glucagon during the meal tolerance test [4]. While no significant change in AUC120min glucagon was observed in the present study, the differential effects on glucagon suppression might be attributable to the small number of patients or inaccurate methods for glucagon measurement. According to a report published after we completed our experiments for the present study, the Glucagon RIA kit from EMD Millipore, the method we used for glucagon measurement, appears to be inadequate under certain conditions, such as when glucagon secretion is suppressed [20]. Other studies have found no significant changes in postprandial glucagon levels following the meal tolerance test in DPP-4 inhibitor groups compared with placebo groups [21, 22].
We would like to point out three potentially significant limitations of the present study: (1) the study was conducted under an open-label design with no control arm; (2) the 12-week study duration may not have been long enough to assess long-term results; and (3) the number of patients was relatively small.
Conclusions
Once-daily teneligliptin improved glycemic control in Japanese patients with T2D. Twelve weeks of teneligliptin treatment clearly improved IGI30min and the AUC120min SUIT index in drug naïve Japanese patients with T2D. The OGTT may be a useful method for estimating insulin secretion per se in patients with T2D receiving DPP-4 inhibitors.
Conflicts of interest
Dr Ito received Speakers Bureau from Mitsubishi Tanabe Pharma Corporation. Dr Fukui received Speakers Bureau from Daiichi Sankyo Co., Ltd., Mitsubishi Tanabe Pharma Corporation., Nippon BoehringerIngelheim Co., Ltd., Novo Nordisk Pharma Ltd., Takeda Pharmaceutical Co., Ltd., AstraZeneca K.K., MSD K.K., Sanofi K.K., Astellas Pharma Inc., Eli Lilly Japan K.K., and Novartis Pharma K.K. Dr Hayashi received Speakers Bureau from Daiichi Sankyo Co., Ltd., Mitsubishi Tanabe Pharma Corporation., Novo Nordisk Pharma Ltd., Takeda Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., Sanofi K.K., Astellas Pharma Inc., Eli Lilly Japan K.K., Novartis Pharma K.K., Shionogi & Co., Ltd., and Kissei Pharmaceutical Co., Ltd. Dr Osamura received Speakers Bureau from Mitsubishi Tanabe Pharma Corporation., Eli Lilly Japan K.K., and Sanwa Kagaku Kenkyusho Co., Ltd. Dr Ohara received Speakers Bureau from Daiichi Sankyo Co., Ltd., Mitsubishi Tanabe Pharma Corporation., Nippon BoehringerIngelheim Co., Ltd., Novo Nordisk Pharma Ltd., Takeda Pharmaceutical Co., Ltd., Eli Lilly Japan K.K., Kowa Pharmaceutical Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Kissei Pharmaceutical Co., Ltd., and Astellas Pharma Inc. Dr Hara received Speakers Bureau from Astellas Pharma Inc. and Mitsubishi Tanabe Pharma Corporation. Dr Yamamoto received Speakers Bureau from Sanofi K.K., Novo Nordisk Pharma Ltd., Novartis Pharma K.K., Eli Lilly Japan K.K., Daiichi Sankyo Co., Ltd., Mitsubishi Tanabe Pharma Corporation., Nippon BoehringerIngelheim Co., Ltd., Takeda Pharmaceutical Co., Ltd., MSD K.K., Ono Pharmaceutical Co., Ltd., and Kissei Pharmaceutical Co., Ltd. Dr Hirano received Research Grant from Mitsubishi Tanabe Pharma Corporation., Novo Nordisk Pharma Ltd., Takeda Pharmaceutical Co., Ltd., AstraZeneca K.K., MSD K.K., Ono Pharmaceutical Co., Ltd., Eli Lilly Japan K.K., Novartis Pharma K.K., and Sanwa Kagaku Kenkyusho Co., Ltd.; Speakers Bureau from Mitsubishi Tanabe Pharma Corporation., Novo Nordisk Pharma Ltd., Takeda Pharmaceutical Co., Ltd., AstraZeneca K.K., MSD K.K., Ono Pharmaceutical Co., Ltd., Eli Lilly Japan K.K., Kowa Pharmaceutical Co., Ltd., Novartis Pharma K.K., Sanwa Kagaku Kenkyusho Co., Ltd., and Shionogi & Co., Ltd.
Contributor Information
Rika Ito, Email: rikarikaakaoka@yahoo.co.jp.
Tomoyasu Fukui, Phone: +81-3-3784-8947, Email: showauft@med.showa-u.ac.jp.
Toshiyuki Hayashi, Email: t-hayashi@med.showa-u.ac.jp.
Anna Osamura, Email: annatoku@gmail.com.
Makoto Ohara, Email: s6018@nms.ac.jp.
Noriko Hara, Email: nori-nnori@hotmail.co.jp.
Akiko Higuchi, Email: tottytotty1981@gmail.com.
Takeshi Yamamoto, Email: tak3105@aol.com.
Tsutomu Hirano, Email: hirano@med.showa-u.ac.jp.
References
- 1.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–787. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 2.Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009;301:2129–2140. doi: 10.1001/jama.2009.726. [DOI] [PubMed] [Google Scholar]
- 3.Ahrén B, Landin-Olsson M, Jansson PA, et al. Inhibition of dipeptidyl peptidase-4 reduces glycemia, sustains insulin levels, and reduces glucagon levels in type 2 diabetes. J Clin Endocrinol Metab. 2004;89:2078–2084. doi: 10.1210/jc.2003-031907. [DOI] [PubMed] [Google Scholar]
- 4.Eto T, Inoue S, Kadowaki T. Effects of once-daily teneligliptin on 24-h blood glucose control and safety in Japanese patients with type 2 diabetes mellitus: a 4-week, randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2012;14:1040–1046. doi: 10.1111/j.1463-1326.2012.01662.x. [DOI] [PubMed] [Google Scholar]
- 5.Rauch T, Graefe-Mody U, Deacon CF, et al. Linagliptin increases incretin levels, lowers glucagon, and improves glycemic control in type 2 diabetes mellitus. Diabetes Ther. 2012;3:10. doi: 10.1007/s13300-012-0010-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kadowaki T, Kondo K. Efficacy and safety of teneligliptin in combination with pioglitazone in Japanese patients with type 2 diabetes mellitus. J Diabetes Investig. 2013;27:576–584. doi: 10.1111/jdi.12092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He YL, Wang Y, Bullock JM, et al. Pharmacodynamics of vildagliptin in patients with type 2 diabetes during OGTT. J Clin Pharmacol. 2007;47:633–641. doi: 10.1177/0091270006299137. [DOI] [PubMed] [Google Scholar]
- 8.Lim S, An JH, Shin H, et al. Factors predicting therapeutic efficacy of combination treatment with sitagliptin and metformin in type 2 diabetic patients: the COSMETIC study. Clin Endocrinol. 2012;77:215–223. doi: 10.1111/j.1365-2265.2011.04240.x. [DOI] [PubMed] [Google Scholar]
- 9.Yamada Y, Fukuda K, Fujimoto S, et al. SUIT, secretory units of islets in transplantation: an index for therapeutic management of islet transplanted patients and its application to type 2 diabetes. Diabetes Res Clin Pract. 2006;74:222–226. doi: 10.1016/j.diabres.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 10.Funakoshi S, Fujimoto S, Hamasaki A, et al. Analysis of factors influencing pancreatic beta-cell function in Japanese patients with type 2 diabetes: association with body mass index and duration of diabetic exposure. Diabetes Res Clin Pract. 2008;82:353–358. doi: 10.1016/j.diabres.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 11.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 12.Committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes Mellitus. Seino Y, Nanjo K, et al. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Invest. 2010;1:212–228. doi: 10.1111/j.2040-1124.2010.00074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kutoh E, Hirate M, Ikeno Y. Teneligliptin as an initial therapy for newly diagnosed, drug naive subjects with type 2 diabetes. J Clin Med Res. 2014;6:287–294. doi: 10.14740/jocmr1841e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshinaga H, Kosaka K. Heterogeneous relationship of early insulin response and fasting insulin level with development of non-insulin-dependent diabetes mellitus in non-diabetic Japanese subjects with or without obesity. Diabetes Res Clin Pract. 1999;44:129–136. doi: 10.1016/S0168-8227(99)00019-4. [DOI] [PubMed] [Google Scholar]
- 15.Shibasaki T, Takahashi H, Miki T, et al. Essential role of Epac2/Rap1 signaling in regulation of insulin granule dynamics by cAMP. Proc Natl Acad Sci USA. 2007;104:19333–19338. doi: 10.1073/pnas.0707054104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toft-Nielsen MB, Damholt MB, Madsbad S, et al. Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J Clin Endocrinol Metab. 2001;86:3717–3723. doi: 10.1210/jcem.86.8.7750. [DOI] [PubMed] [Google Scholar]
- 17.Nauck MA, Heimesaat MM, Orskov C, et al. Preserved incretin activity of glucagon-like peptide 1 [7–36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest. 1993;91:301–307. doi: 10.1172/JCI116186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Omar B, Ahrén B. Pleiotropic mechanisms for the glucose-lowering action of DPP-4 inhibitors. Diabetes. 2014;63:2196–2202. doi: 10.2337/db14-0052. [DOI] [PubMed] [Google Scholar]
- 19.Meier JJ, Menge BA, Breuer TG, et al. Functional assessment of pancreatic beta-cell area in humans. Diabetes. 2009;58:1595–1603. doi: 10.2337/db08-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bak MJ, Albrechtsen NW, Pedersen J, et al. Specificity and sensitivity of commercially available assays for glucagon and oxyntomodulin measurement in humans. Eur J Endocrinol. 2014;170:529–538. doi: 10.1530/EJE-13-0941. [DOI] [PubMed] [Google Scholar]
- 21.Kadowaki T, Kondo K. Efficacy, safety and dose–response relationship of teneligliptin, a dipeptidyl peptidase-4 inhibitor, in Japanese patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2013;15:810–818. doi: 10.1111/dom.12092. [DOI] [PubMed] [Google Scholar]
- 22.Utzschneider KM, Tong J, Montgomery B, et al. The dipeptidyl peptidase-4 inhibitor vildagliptin improves beta-cell function and insulin sensitivity in subjects with impaired fasting glucose. Diabetes Care. 2008;31:108–113. doi: 10.2337/dc07-1441. [DOI] [PubMed] [Google Scholar]