Abstract
Ipilimumab (IPI) 10 mg/kg with sargramostim (GM-CSF; GM) improved overall survival (OS) and safety of patients with advanced melanoma over IPI in a randomized phase II trial. The FDA-approved dose of IPI 3 mg/kg has not been assessed with GM (IPI-GM). Consecutive patients treated with IPI-GM at a single institution were reviewed. Treatment included IPI every 3 weeks x 4 and GM 250 mcg subcutaneous injection days 1–14 of each IPI cycle. Efficacy, clinical characteristics, toxicities and blinded radiology review of tumor burden were evaluated. 32 patients were identified with 25 (78%) having immune-related response criteria (irRC) measurable disease and 41% with CNS metastases. 88.6% of GM doses were administered. Response rate by irRC and disease control rate at 12 weeks were 20% and 44%, respectively (median follow-up 37 weeks). Immune-related adverse events (irAE) were observed in 10 (31.3%) patients, with 3 (9.4%) Grade 3 events. Patients with Grade 3 irAEs had prior autoimmunity, advanced age and poor performance status. The median OS from first dose of ipilimumab was 41 weeks. Ipi-GM treatment is feasible and in this poor-risk advanced melanoma population, efficacy appeared similar but safety appeared improved relative to historical IPI alone.
Keywords: ipilimumab, CTLA-4, sargramostim, GM-CSF, immunotherapy
Introduction
Malignant melanoma is an aggressive disease with an annual incidence of greater than 70,000 cases in the United States (1). Ipilimumab is a fully human IgG1 monoclonal antibody that inhibits cytotoxic T lymphocyte antigen-4 (CTLA-4). Ipilimumab was shown to induce an overall survival (OS) advantage in patients with melanoma in two randomized phase III studies (2, 3).
Sargramostim (granulocyte-macrophage colony-stimulating factor or GM-CSF) is a cytokine that increases antigen presentation by dendritic cells and increases antitumor activity of T- and B-lymphocyte populations (4–6). Administration of GM-CSF has been evaluated in multiple tumor types including melanoma and other cancers (7, 8). The clinical properties of GM-CSF are somewhat controversial as several studies have suggested a potential immunosuppressive role in certain contexts (9). GM-CSF also plays a role in pulmonary and mucosal homeostasis (10, 11) and may modulate some forms of autoimmunity, especially involving the gastrointestinal tract (12).
A randomized multi-center phase II study of ipilimumab 10 mg/kg with sargramostim demonstrated improvements in OS and safety profile over ipilimumab alone (Eastern Cooperative Oncology Group (ECOG) study 1608) (13). Specifically, the incidence of high-grade immune-related adverse events (irAE), including colitis and pneumonitis, were significantly reduced. To date, no experience of ipilimumab at 3 mg/kg (the FDA approved dose) with sargramostim has been reported.
To assess the feasibility as well as preliminary safety and efficacy of ipilimumab 3 mg/kg with sargramostim, we conducted a single center, retrospective analysis of 32 patients with metastatic cutaneous melanoma treated with ipilimumab and sargramostim in standard clinical practice. Herein, we report the clinical activity and toxicity observed.
Methods
Patients and Clinical Characteristics
Consecutive patients who were not eligible for or declined participation in clinical trials underwent informed consent for treatment with ipilimumab 3 mg/kg and sargramostim. Clinical data were collected under institutional review board approval. Relevant clinical parameters were collected including age, gender, ECOG performance status, site(s) of metastatic disease, lines of prior therapy and number of sargramostim doses administered. Laboratory parameters were collected such as lactate dehydrogenase (LDH) and absolute lymphocyte count (ALC) were collected at baseline and at 7 weeks. Treatment response and safety data were also determined. All data were aggregated following patient de-identification.
Treatment
Ipilimumab was given as per standard practice 3 mg/kg every 3 weeks for 4 doses. Sargramostim was given as a subcutaneous injection of 250 mcg flat dose by the patient or family member at home on days 1–14 of each ipilimumab cycle.
Efficacy and Toxicity Assessment
Efficacy and toxicity were evaluated in all patients who received 1 dose of ipilimumab and sargramostim. Beneficial effects of ipilimumab were categorized as complete response (CR), partial response (PR) or stable disease (SD). Disease control rate was calculated as the percentage of patients without progression at 12 weeks after starting ipilimumab treatment.
Response Evaluation Criteria In Solid Tumors (RECIST) version 1.1 and immune-related response criteria (irRC) were applied to determine response in those patients with baseline measurable disease (14–17). Overall survival was calculated by Kaplan-Meier methodology from first dose of ipilimumab to date of death by any cause. Toxicity was assessed through chart review and graded using Common Terminology Criteria for Adverse Events (version 4.0) with attention on irAEs including dermatitis, colitis, hepatitis, pneumonitis, thyroiditis and hypophysitis.
Univariate comparisons of OS for baseline LDH, ECOG performance status, tumor mutational status, central nervous system (CNS) metastases and ALC were conducted using Kaplan-Meier estimates; differences were assessed using the log-rank test. LDH was divided as above or below the institutional upper limit of normal; ALC was divided into low (<1000 cells/μL) or normal (≥1000 cells/μL). ECOG performance status was classified as fully active versus any restriction (0 versus 1–2). Covariates of survival were calculated by univariate comparisons for patients who received ≥2 doses of ipilimumab as well as with measurable disease at baseline. Conditional landmark analyses were conducted to compare OS according to 7-week ALC levels (low versus normal). To minimize the potential for guarantee-time bias, patients who died before 7 weeks or who did not have 7-week ALC data were removed from the analysis. The remaining patients were followed forward in time. Statistical significance was defined as p<0.05.
Results
Patients, Clinical Characteristics and Drug Administration
The clinical characteristics of the 32 patients included in the analysis are shown in Table 1. Patients were predominately male with a median age of 63 years and median ECOG status of 1. The number of prior therapies was (median) zero, LDH 169 (23% elevated) while 56% of patients with 3 or more sites of metastatic disease and 41% of patients with CNS metastases. Median number of ipilimumab doses administered was 4. Three patients who were consented for sargramostim were unable to obtain the drug through insurance either due to denial or high patient out-of-pocket cost. These patients were not included in any analysis. 1302 of 1470 (88.6%) planned doses of sargramostim were administered.
Table 1.
Patient Characteristics
| Total study subjects | 32 | ||
| Age, median (Range), years | 63 | 26–95 | |
| Sex | Male | 17 | 53% |
| Female | 15 | 47% | |
| ECOG PS pretreatment (median ECOG 1, range 0–2) | 0 | 14 | 44% |
| 1 | 15 | 47% | |
| 2 | 3 | 9% | |
| Mutational status | BRAF | 6 | 19% |
| NRAS | 11 | 34% | |
| Non-BRAF or NRAS | 15 | 47% | |
| Pretreatment median LDH (range) | 169 | 112–2090 | |
| Patients with elevated LDH (%) | 11 | 23% | |
| Pretreatment median ALC | 1.13 | ||
| Prior lines of therapy, n (%) | 0 | 21 | 66% |
| 1 | 5 | 16% | |
| 2 | 3 | 9% | |
| ≥3 | 3 | 9% | |
| Median prior lines of therapy | 0 | ||
| Prior radiation | 18 | 56% | |
| Number of metastatic sites | 1 | 6 | 19% |
| M1b | 5 | 16% | |
| M1c | 1 | 3% | |
| 2 | 8 | 25% | |
| ≥3 | 18 | 56% | |
| Brain | 13 | 41% | |
| Median doses ipilimumab, n (range) | 4 | (1–4) | |
| Total doses of ipilimumab | 105 | ||
| Doses of GM-CSF* | 1302/1470 | 88.6% |
LDH = lactate dehydrogenase in units/L
ALC = absolute lymphocyte count (Kcells/μL)
14 doses possible per dose of ipilimumab or 56 for treatment course
Response Analysis
Measurable lesions were present on baseline scans in 24 patients according to RECIST and in 25 patients according to irRC. One patient had a cervical lymph node measuring 1.2x1.0 cm alone as the baseline tumor burden; the lesion was non-measurable according to RECIST which requires at least 1.5 cm in short axis for nodes to be measurable, however, was measurable according to irRC because it was ≥ 0.5x0.5 cm. Patients without measurable lesions at baseline had additional tumor burden from non-measurable lesions that was deemed significant enough to initiate treatment. These patients were assessed qualitatively and followed until a progression event, and were not included in the best overall response analysis.
Best overall response included 5 patients with PR (5/24, 21%) and 7 patients with SD at 12 weeks (7/24, 29%) by RECIST, and 5 patients with PR (5/25, 20%) and 6 patients with SD (6/25, 24%) by irRC among those with measurable disease at baseline. Overall disease control rate of ≥12 weeks was 12 of 24 patients (50%) by RECIST and 11 of 25 patients (44%) by irRC (Table 2A). Median follow-up was 37 weeks. The median time to progression (TTP) was 13.7 weeks and was similar between patients with evaluable (14.0 weeks) and non-evaluable (13.2 weeks) disease. Similarly TTP did not vary significantly by mutational status (BRAF:NRAS:non-BRAF/NRAS) or presence or absence of CNS metastases. Given that treatment was administered in standard practice, the timing of restaging imaging was somewhat variable. In patients with measurable disease by irRC, the first three scans took place at medians of 13.0, 18.0, and 25.7 weeks.
Table 2.
Clinical outcomes by RECIST and irRC (A) and toxicities (B).
| A | ||||
|---|---|---|---|---|
| RECIST | irRC | |||
| Patients | % | Patients | % | |
| PD | 7/24 | 50% | 9/25 | 56% |
| SD | 7/24 | 29% | 6/25 | 24% |
| PR | 5/24 | 21% | 5/25 | 20% |
| CR | 0/24 | 0% | 0/25 | 0% |
| SD + PR | 12/24 | 50% | 11/25 | 44% |
| B. Toxicities | ||||
|---|---|---|---|---|
| irAE | Any G | % | G3–4 | % |
| Dermatitis* | 5 | 18.5% | 1 | 3.7% |
| Colitis | 2 | 7.4% | 2 | 7.4% |
| Thyroiditis | 0 | 0.0% | 0 | 0.0% |
| Uveitis | 0 | 0.0% | 0 | 0.0% |
| Pancreatitis | 0 | 0.0% | 0 | 0.0% |
| Hepatitis | 0 | 0.0% | 0 | 0.0% |
| Hypophysitis | 2 | 7.4% | 0 | 0.0% |
| Pneumonitis | 1 | 3.7% | 0 | 0.0% |
| Total | 10 | 31.3% | 3 | 9.4% |
One case of dermatitis was dermatomyositis
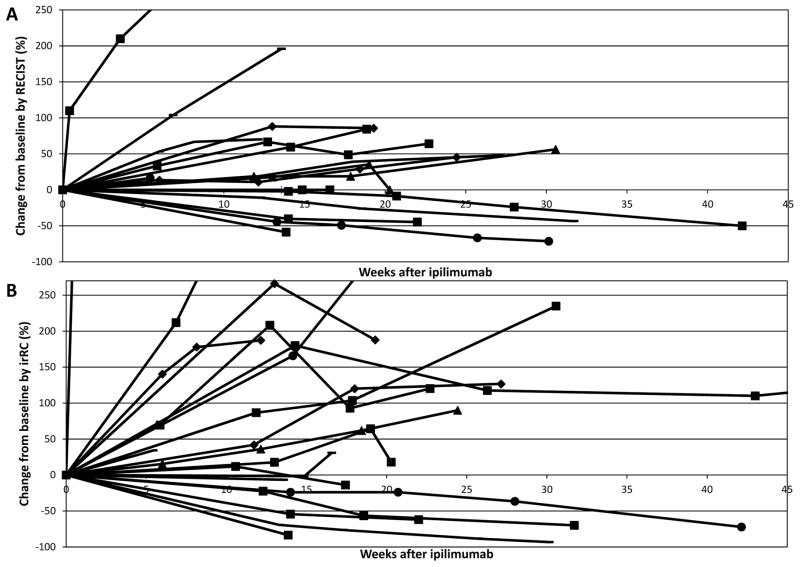
The changes in disease burden from baseline according to RECIST and irRC are shown in Figures 1A and 1B. No patients had initial progression followed by a formal tumor response; however, several patients had initial progressive disease with subsequent tumor shrinkage and clinical stability. Patients receiving clinical benefit ranged from 26–95 years of age, and were of heterogeneous molecular status and included patients with CNS metastases.
Figure 1.
Figure 1A and 1B. Change in disease burden for each patient over time by RECIST and irRC.
The biochemical parameters of patients experiencing clinical benefit included LDH level that was within normal limits in all but two patients. All patients experiencing disease control had a rise in ALC from baseline to week 7 (median increase of 430 cells/μL), except one patient who had a decrease of 500 cells/μL and another without a follow-up ALC value at 7 weeks.
Overall Survival Analysis
The median OS was 41 weeks (95% CI: 30 to ∞ - Figure 2). Subsequent treatment within the entire cohort included six patients who received anti-Programmed Death-1 (PD-1) antibodies and four patients who received BRAF plus MEK inhibitors. In univariate comparisons of survival according to covariates, only LDH level (normal/elevated) was statistically significantly different between survivors and patients who died (log-rank p=0.0001). Median survival among patients with normal LDH levels was not reached; in patients with elevated levels, the median survival was 16 weeks (95% CI: 1 to 36). Other factors that did not correlate with survival in the total population included ALC, mutational status and CNS metastases.
Figure 2. Overall survival for entire cohort.
The median overall survival for the total cohort was 41 weeks (95% CI 30 to ∞).
The conditional landmark analysis based on ALC at 7 weeks reduced the sample size from 32 patients to 24 patients. There was no difference in OS noted between patients with baseline ALC ≥1000 cells/μL (n=19 patients) versus ALC < 1000 cells/μL (n=5) (log-rank p=0.71). Cox proportional hazards model of OS stratified by LDH (normal/elevated) showed that that hazard of death for patients with low 7-week ALC was 1.49 times the hazard of patients with normal 7-week ALC. However, the comparison did not reach statistical significance (HR: 1.49, 95% CI: 0.27 to 8.3, p=0.65).
In the restricted cohort of 25 patients with evaluable disease at baseline, median OS was 37 weeks (95% CI 19 to ∞). As with the total cohort, only LDH level (normal vs. elevated) was statistically significantly associated with OS (log-rank p=0.0001). Conditional landmark analysis of survival based on 7- week ALC again did not show a significant relationship (log-rank p=0.43).
Toxicity Analysis
The overall incidence of irAE in all patients was 31.3% with 9.4% Grade-3 (G3) events and no treatment-related deaths (Table 2B). Dermatitis was the most common irAE, affecting five patients. Three G3 irAE were described including two events of colitis and one event of dermatitis. The three patients who experienced G3 irAEs were complex. One patient had previously discontinued ipilimumab as a single agent due to G3 rash one year earlier and upon re-induction with ipilimumab plus sargramostim developed G3 rash again with eventual evolution into dermatomyositis. Two patients developed G3 colitis, including one with a history of collagenous colitis who was on chronic therapy with oral aminosalicylates, and another who was of advanced age (90 years old). These toxicities were treated using standard management algorithms with intravenous corticosteroids followed by slow tapers of oral steroids.
Discussion
This retrospective study evaluating the feasibility and clinical characteristics of ipilimumab 3 mg/kg with sargramostim is the first report of this combination being administered with the currently approved dose of ipilimumab. From a practical stand point, subcutaneous injection of sargramostim was feasible with 88% of expected doses being administered.
From a clinical perspective, the toxicity and preliminary patient outcomes observed were generally similar with those reported in the randomized phase II study of ipilimumab 10 mg/kg with sargramostim compared with ipilimumab alone (ECOG 1608). The best overall response rate (RR) by RECIST and irRC were found to be 21% and 20%, respectively. This is in previous single agent studies or the approximately 15% reported in ECOG 1608. However given the small sample size of the population and lack of randomization within this study, the RR reported here does not appear to be a clinically meaningful difference. In the ECOG 1608 study the RR of ipilimumab with sargramostim was not different compared with ipilimumab alone or from historical ipilimumab controls. Median OS in the current study was 41 weeks. The length of follow up, as well as improved treatment options after ipilimumab plus sargramostim, potentially confound the ability to compare long-term outcomes of patients followed in this study. Notably however, this was a poor-risk patient population as 41% had active or treated brain metastases, 56% had ≥3 sites of metastatic disease and 5 patients who passed away shortly after the first dose of ipilimumab due to disease progression. Subsequent therapy included 12.5% receiving BRAF inhibitor combination therapy, and 19% receiving anti-PD-1 antibodies.
As in the ECOG 1608 study, a more favorable toxicity profile was seen when ipilimumab was administrated with sargramostim compared to historical data. Three (9.4%) high-grade events were observed in this study which is lower than the rate of high-grade toxicity seen with administration of ipilimumab 3 mg/kg alone. Further, in this study, those patients with high-grade events had high-risk features including autoimmunity, poor performance status and advanced age (90 years old). Similar patients were generally excluded from the clinical trials evaluating ipilimumab.
Some investigators disputed the benefit in survival seen in ECOG 1608 as no concomitant improvement in progression-free survival was observed. In the current series, a clinically similar RR was seen relative to historical ipilimumab alone and the median time to progression was 13 weeks (the median time of first restaging). The authors of ECOG 1608 pointed out that improvement in OS without progression-free survival is not unprecedented and that the GM-CSF-containing treatment approach sipuleucel-T similarly showed this pattern (18). Progression-free survival estimates were not provided from the current study given the heterogeneity of the patient population (some without baseline measurable disease); however, a median of 23 weeks of treatment was observed which would compare favorably with historical data of ipilimumab alone.
The ECOG 1608 trial reported a reduction in high-grade toxicity with ipilimumab 10 mg/kg plus sargramostim relative to single-agent ipilimumab. This ipilimumab 3 mg/kg plus sargramostim experience is consistent with the observation of lower toxicity with the caveats regarding patient selection as described above. As with ECOG 1608, a decrease in the incidence of high-grade colitis and pneumonitis events was observed in this data set. One proposed explanation for the improvement in OS observed in ECOG 1608 was that a greater number of ipilimumab doses may have been possible secondary to the reduction in toxicity facilitated by the addition of sargramostim. The data from this single institutional analysis would support this conclusion as only 3 (9.4%) patients were unable to complete the standard 4 doses of ipilimumab due to toxicity.
Our investigation is limited by several factors. Chart review was used to capture toxicity and thus may have led to a bias toward under-reporting of lower-grade events. The study sample size of 32 patients is relatively small and statistical comparisons based on the data would be of reduced power. Finally, the patient cohort was heterogeneous including many with high-risk features (e.g., CNS metastases) which could bias the outcome relative to clinical trial populations. This would seem to further boost the utility of this approach, however, given that such a bias would skew the data toward worse clinical outcomes.
In conclusion, this report represents the first description of ipilimumab 3 mg/kg administered with sargramostim. In clinical practice, patients are able to tolerate treatment. This retrospective analysis suggests similar efficacy but importantly decreased toxicity relative to historical reports of ipilimumab alone. A randomized clinical trial combining ipilimumab, nivolumab with and without sargramostim is planned (ClinicalTrials.gov Identifier: NCT02339571). Due to the retrospective and non-randomized nature of this study, definitive statements regarding the role of GM-CSF in combination with immune-checkpoint blocking antibodies in the clinical management of patients with melanoma is not possible. However, further exploration of GM-CSF in combination with immune-checkpoint blocking antibodies is warranted.
Acknowledgments
Funding: Dr. Luke acknowledges funding from the Paul Calabresi Career Development in Clinical Oncology Award (5K12CA139160). Dr. Nishino was supported by 1K23CA157631 (NCI).
Footnotes
Disclosure: Drs. Ott and Nishino served as consultant to Bristol-Myers Squibb. Dr. Hodi has served as a non-paid consultant to Bristol-Myers Squibb and Sanofi as well as research support to institution from Bristol-Myers Squibb.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–26. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 3.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fischer HG, Frosch S, Reske K, Reske-Kunz AB. Granulocyte-macrophage colony-stimulating factor activates macrophages derived from bone marrow cultures to synthesis of MHC class II molecules and to augmented antigen presentation function. J Immunol. 1988;141:3882–8. [PubMed] [Google Scholar]
- 6.Weisbart RH, Golde DW, Clark SC, Wong GG, Gasson JC. Human granulocyte-macrophage colony-stimulating factor is a neutrophil activator. Nature. 1985;314:361–3. doi: 10.1038/314361a0. [DOI] [PubMed] [Google Scholar]
- 7.Small EJ, Reese DM, Um B, Whisenant S, Dixon SC, Figg WD. Therapy of advanced prostate cancer with granulocyte macrophage colony-stimulating factor. Clin Cancer Res. 1999;5:1738–44. [PubMed] [Google Scholar]
- 8.Everly JJ, Lonial S. Immunomodulatory effects of human recombinant granulocyte-macrophage colony-stimulating factor (rhuGM-CSF): evidence of antitumour activity. Expert Opin Biol Ther. 2005;5:293–311. doi: 10.1517/14712598.5.3.293. [DOI] [PubMed] [Google Scholar]
- 9.Slingluff CL, Jr, Petroni GR, Olson WC, Smolkin ME, Ross MI, Haas NB, et al. Effect of granulocyte/macrophage colony-stimulating factor on circulating CD8+ and CD4+ T-cell responses to a multipeptide melanoma vaccine: outcome of a multicenter randomized trial. Clin Cancer Res. 2009;15:7036–44. doi: 10.1158/1078-0432.CCR-09-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dranoff G, Crawford AD, Sadelain M, Ream B, Rashid A, Bronson RT, et al. Involvement of granulocyte-macrophage colony-stimulating factor in pulmonary homeostasis. Science. 1994;264:713–6. doi: 10.1126/science.8171324. [DOI] [PubMed] [Google Scholar]
- 11.Bernasconi E, Favre L, Maillard MH, Bachmann D, Pythoud C, Bouzourene H, et al. Granulocyte-macrophage colony-stimulating factor elicits bone marrow-derived cells that promote efficient colonic mucosal healing. Inflamm Bowel Dis. 2010;16:428–41. doi: 10.1002/ibd.21072. [DOI] [PubMed] [Google Scholar]
- 12.Egea L, Hirata Y, Kagnoff MF. GM-CSF: a role in immune and inflammatory reactions in the intestine. Expert Rev Gastroenterol Hepatol. 2010;4:723–31. doi: 10.1586/egh.10.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hodi FS, Lee S, McDermott DF, Rao UN, Butterfield LH, Tarhini AA, et al. Ipilimumab plus sargramostim vs ipilimumab alone for treatment of metastatic melanoma: a randomized clinical trial. JAMA. 2014;312:1744–53. doi: 10.1001/jama.2014.13943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbé C, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–20. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 15.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 16.Nishino M, Jagannathan JP, Ramaiya NH, Van den Abbeele AD. Revised RECIST guideline version 1.1: What oncologists want to know and what radiologists need to know. AJR Am J Roentgenol. 2010;195:281–9. doi: 10.2214/AJR.09.4110. [DOI] [PubMed] [Google Scholar]
- 17.Nishino M, Jagannathan JP, Krajewski KM, O’Regan K, Hatabu H, Shapiro G, et al. Personalized tumor response assessment in the era of molecular medicine: cancer-specific and therapy-specific response criteria to complement pitfalls of RECIST. AJR Am J Roentgenol. 2012;198:737–45. doi: 10.2214/AJR.11.7483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–22. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]