Abstract
Pulmonary tuberculosis (TB) is characterized by oxidative stress and lung tissue destruction by matrix metalloproteinases (MMP). The interplay between these distinct pathological processes and the implications for TB diagnosis and disease staging are poorly understood. Heme oxygenase-1 (HO-1) levels have been shown to distinguish active from latent as well as successfully treated Mycobacterium tuberculosis (Mtb) infection. MMP-1 expression is also associated with active TB. Here, we measured plasma levels of these two important biomarkers in distinct TB cohorts from India and Brazil. Patients with active TB expressed either very high levels of HO-1 and low levels of MMP-1 or the converse. Moreover, TB patients with either high HO-1 or MMP-1 levels displayed distinct clinical presentations as well as plasma inflammatory marker profiles. In contrast, in an exploratory North American study, inversely correlated expression of HO-1 and MMP-1 was not observed in patients with other non-tuberculous lung diseases. To assess possible regulatory interactions in the biosynthesis of these two enzymes at the cellular level, we studied expression of HO-1 and MMP-1 in Mtb-infected human and murine macrophages. We found that infection of macrophages with live virulent Mtb is required for robust induction of high levels of HO-1, but not MMP-1. In addition, we observed that carbon monoxide, a product of Mtb induced HO-1 activity, inhibits MMP-1 expression by suppressing c-Jun/AP-1 activation. These findings reveal a mechanistic link between oxidative stress and tissue remodeling that may find applicability in the clinical staging of TB patients.
Introduction
Upon respiratory exposure to Mycobacterium tuberculosis (Mtb), individuals can develop a broad range of disease manifestations, varying from asymptomatic latent TB infection (LTBI) to aggressive pulmonary forms with extensive lung damage (1). Major challenges in TB diagnosis include the ability to distinguish active from latent infection (2, 3), and also the discrimination between TB and other lung diseases with similar clinical presentation, such as sarcoidosis and non-tuberculous mycobacterial (NTM) infection (4–6). Clinical and experimental animal studies have demonstrated that active TB is accompanied by systemic oxidative stress and augmented lipid peroxidation (7, 8). We have previously shown that plasma levels of heme oxygenase-1 (HO-1), a major antioxidant highly expressed in the lungs, can accurately distinguish active from latent TB cases or uninfected controls in both adult (9) and pediatric (10) populations in South India. These studies indicated that HO-1 can serve as an important biomarker of TB disease.
The pathology of pulmonary TB involves enzymatic degradation of lung tissue by matrix metalloproteinases (MMPs) (11–13). This process is reflected in the detection of increased MMP levels in sputum (14) and plasma (15) samples from TB patients which has been shown to correlate with clinical disease severity. Among the different MMPs, MMP-1 (interstitial collagenase) is thought to play a critical role in driving immunopathology in pulmonary TB (11) and appears to be selectively induced by Mtb infection (16). MMP-1 gene expression involves activation of the transcription factor activator protein 1 (AP-1) as well as c-Jun N-terminal kinases (JNKs), the extracellular signal-regulated kinases (ERKs) and the p38 kinases (17), factors which have been described to be induced in response to oxidative stress (18). Interestingly, biliverdin and carbon monoxide (CO), products of the reaction catalyzed by HO-1, have been shown to suppress expression of ERKs and p38 kinases in experimental models (19, 20). These observations led us to hypothesize that the clinical presentation of Mtb infection may be influenced by stress-induced HO-1 acting on MMP-driven lung damage/remodeling.
In the present study, we demonstrate that circulating levels of HO-1 and MMP-1 are elevated in active pulmonary TB patients compared to individuals with LTBI from two distinct South Indian and Brazilian cohorts and that expression of these two biomarkers is inversely correlated in active TB disease but not in North American subjects with pulmonary NTM or sarcoidosis. More importantly, our data reveal that the pattern of expression of HO-1 and MMP-1 in plasma identifies two subpopulations of active TB patients which exhibited different inflammatory profiles and clinical presentations. The inverse relationship between HO-1 and MMP-1 levels in TB patients was reflected in the dichotomous expression of the two enzymes in Mtb-infected macrophages which we were able to link with the suppression of MMP-1 production by HO-1 induced CO. Together, these findings reveal a pathway by which oxidative stress can negatively regulate tissue remodeling and demonstrate combined measurement of HO-1 and MMP-1 as a potential strategy for clinical staging of TB.
Materials and Methods
Ethics statement
All clinical investigations were conducted according to the principles expressed in the Declaration of Helsinki. Written informed consent was obtained from all participants or their legally responsible guardians before enrolling into the sub-studies. The South Indian study was approved by the Institutional Review Board (IRB) of the National Institute for Research in Tuberculosis (NIRT, protocol numbers: NCT01154959 and NCT00342017). The Brazilian study was approved by the Ethical Committee of the Centro de Pesquisas Gonçalo Moniz, Fundação Oswaldo Cruz (FIOCRUZ) (protocol number: 003.0.225.000–11). TB and NTM samples from the North American study were collected according to protocols approved by IRB of the National Institutes of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH) (protocol numbers: NCT00001355, NCT01212003 and NCT01611402). Sarcoidosis subjects selected for this study were screened for a pulmonary sarcoidosis treatment study, National Heart, Lung, and Blood Institute (NHLBI) protocol 06-H-0072, (NCT00279708). After written informed consent was obtained, sarcoidosis subjects were clinically screened under NHLBI protocol 82-H-0032, (NCT00001183), and research blood samples were collected under NHLBI protocol 96-H-0100, (NCT00001532).
Indian study
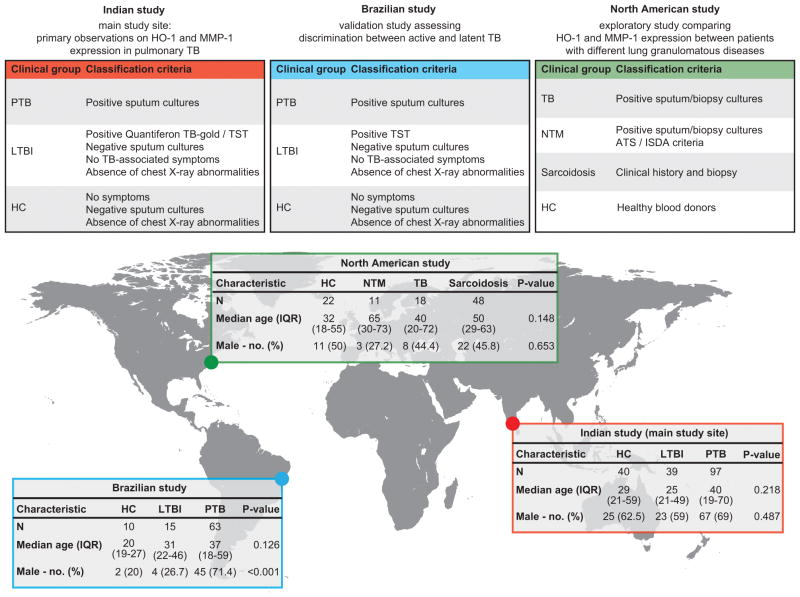
Cryopreserved heparinized plasma samples were collected from 97 subjects with active PTB, 39 individuals with LTBI and 40 uninfected healthy controls, recruited as part of a TB cohort study at the Government Stanley Medical Hospital and at TB clinics supported by the NIRT, Chennai, India as described previously (9) (Figure 1). TB diagnosis was based on culture positivity of sputum samples. Three sputum samples per subject were examined by fluorescence microscopy, processed by the modified Petroff’s method and cultured on Lowenstein-Jensen medium. Presence of acid-fast bacilli (AFB) in sputum smears was also documented. A posteroanterior chest x-ray was performed to determine the extent of lung disease (unilateral vs. bilateral lesions), which was scored by three independent expert physicians from National Institute for Research in Tuberculosis. LTBI diagnosis was based on Quantiferon TB-gold ELISA and tuberculin skin test (TST) positivity (≥10mm in diameter), absence of chest radiography abnormalities or pulmonary symptoms and negative sputum smears and cultures. Healthy controls individuals were health care professionals recruited in Government Stanley Medical Hospital and at TB clinics who agreed to participate in the study. To be designated as a healthy control, an individual needed to be asymptomatic with normal chest radiograph and negative sputum smears and cultures, Quantiferon results (<0.35) and TST induration (<5mm in diameter). All individuals were negative for type-2 diabetes on the basis of the American Diabetes Association criteria (HbA1c levels >6.5% and random plasma glucose > 200mg/dL). At time of enrollment, all individuals were HIV negative (all patients were actively screened), anti-TB treatment naïve, BCG-vaccinated and had no record of prior TB disease.
Figure 1. Classification criteria and characteristics of the study participants.
The present study uses data obtained from clinical investigation of patient cohorts from two geographically distinct, TB-endemic areas, India and Brazil, as well as from North America (see Material and Methods for details). Age was compared between the groups using the Kruskal-Wallis test. Frequency of male gender was compared using the chi-square test. All individuals enrolled in the different studies were screened negative for HIV infection (as indicated in the Material and Methods).
Brazilian study
Cryopreserved heparinized plasma samples were collected from a cohort of 63 subjects with active PTB, 15 individuals with LTBI and 10 healthy controls, recruited between May and November 2012 at the Hospital Especializado Octávio Mangabeira, Salvador, Brazil (Figure 1). PTB diagnosis included positive AFB in sputum smears and positive Mtb sputum cultures. Three sputum samples per subject were examined by fluorescence microscopy, processed by the modified Petroff’s method and cultured on Lowenstein-Jensen medium. LTBI diagnosis was performed in contacts of active TB cases who agreed to participate in the study and was based on TST positivity (≥10mm in diameter), absence of chest radiography abnormalities or pulmonary symptoms and negative sputum cultures. Healthy control individuals (health care professionals and medical students from the Hospital Especializado Octávio Mangabeira who agreed to participate) were asymptomatic with normal chest radiograph and negative sputum cultures and TST induration (<5mm in diameter). At time of enrollment, all individuals were HIV negative (all patients were actively screened), BCG-vaccinated and had no record of prior TB disease or anti-TB treatment.
North American study
We assessed cryopreserved EDTA plasma samples from 18 individuals with culture-confirmed TB and 11 individuals with NTM infection, recruited at the NIH Clinical Center under protocols from the NIAID, NIH, Bethesda, USA. Samples from 48 individuals with confirmed diagnosis of pulmonary sarcoidosis were recruited under a protocol from the NHLBI, NIH. Plasma samples from healthy controls were obtained from blood donors at the NIH Clinical Center. Recruited TB patients exhibited positive AFB as determined by smear, culture, or biopsy. Diagnostic criteria for pulmonary NTM was followed as established by the American Thoracic Society (ATS) guidelines (21), with microbiologic and radiographic evidence of active pulmonary NTM infection. All patients tested negative for antibodies to HIV, had normal numbers of CD4+ T cells and no HIV risk factors. Sarcoidosis patients selected for this study were recruited from a clinical protocol which accrues treatment-requiring patients with parenchymal lung disease and excludes those with manifest cardiac and neurological involvement and other serious disorders, such as HIV disease, TB and cancer. Forty-eight adult subjects who had a history compatible with sarcoidosis and a tissue biopsy demonstrating non-caseating granulomas were included in this analysis. Healthy controls were healthy blood donors from the NIH blood bank (Figure 1).
Immunoassays
Levels of HO-1 (Assay Designs, Ann Arbor, MI), Ferritin-heavy chain (Ferritin-H) (Abnova, Taipei City, Taiwan), IL-17, IFN-γ and TNF-α (R&D Systems, Minneapolis, MN) were measured using ELISA kits. Levels of C-reactive protein (CRP), serum amyloid protein-A (SAA), haptoglobin and α2macroglobulin were determined using a multiplex ELISA system (Bio-Rad, Hercules, CA). Levels of MMP-1, MMP-8, MMP-9, TIMP-1, TIMP-2, TIMP-3 and TIMP-4 were measured using a luminex kit from R&D Systems. Total heme concentrations were measured using a colorimetric assay from BioAssay Systems (Hayward, CA). Assessment of expression of 34 different human proteases in culture supernatants was performed using a standardized array (Proteome Profiler; R&D Systems) following the manufacturer’s instructions. Murine MMP-1a levels were determined using an ELISA kit (USCN Life Sciences, Houston, TX).
MMP1 gene expression assay
Total RNA was isolated from human macrophages using the RNAeasy Mini kit (Qiagen, Valencia, CA), and residual DNA was digested using RNAse-free DNAse (Qiagen). The RNA samples were reversely transcribed using SuperScript II reverse transcriptase (Invitrogen, Carlsbad, CA). Gene expression was measured using SYBR Green-based real-time quantitative PCR and 18S mRNA was used as the housekeeping gene. The following oligonucleotide primers were used: 18S, forward, 5′-CACGGCCGGTACAGTGAAAC-3′ and reverse, 5′-CCCGTCGGCATGTATTAGCT-3′; MMP1, forward, ′5′-GCTAACCTTTGATGCTATAACTACGA-3′ and reverse, 5′-TTTGTGCGCATGTAGAATCTG-3′. Fold induction of MMP1 gene expression was calculated using the ΔΔthreshold cycle method, normalizing mRNA levels for each sample to levels of 18S and comparing to mRNA levels in unstimulated cells.
In vitro assays
CD14+ column-purified human elutriated monocytes were obtained from peripheral blood of healthy donors from the NIH blood bank. Macrophages were generated by culturing monocytes in the presence of RPMI media containing 10% human AB serum and M-CSF 50 ng/mL (Prepotech, Rocky Hill, NJ) for seven days, with fresh media with growth factor being added every 48h as previously described (22). This method of macrophage differentiation was chosen based on a recently published guideline (23). Bone marrow cells from WT or Hmox1−/− mice in C57BL/6 genetic background (gift from Dr. Miguel Soares, Instituto Gulbenkian de Ciências, Portugal) were cultured for 7 days in 30% L929 supernatant media to differentiate bone marrow-derived macrophages. Cells were plated at the concentration of 106 cells/well in 24-well plates. Cells were exposed to H37Rv, ΔESAT6 (gift from Dr. Volker Briken, University of Maryland) or ΔRD1 (gift from Dr. Steven Derrick, U.S. Food and Drug Administration) Mtb strains at different multiplicities of infection (MOI) for 3h, washed to remove extracellular bacteria, and cultured in serum-free media for 24 hours in the presence or absence of the indicated concentrations of the HO-1 inducer cobalt (III) protoporphyrin IX dichloride (CoPPIX; Frontiers Scientific Inc., Logan UT), the inhibitor of HO-1 activity tin (IV) protoporphyrin IX dichloride (SnPPIX; Frontiers Scientific Inc.), the MAPK inhibitor SB 203580 (Tocris Bioscience, Bistrol, UK) used to inhibit MMP-1 production, FeSO4 (Sigma) as source of iron (Fe2+), the inhibitor of heme biosynthesis succinylacetone (SA; Sigma), the CO resealing molecule Ru2Cl4(CO)6 (CORM-II; Sigma, St. Louis, MO), a molecule with similar structure than CORM-II but with no CO releasing capability RuCl3 (Sigma), the CO scavenger oxyhemoglobin (HbO2, Sigma) and the inhibitor of AP-1 activity SR 11302 (Tocris, R&D Systems). In some experiments, recombinant ESAT6 was delivered into the cytosol of infected macrophages using a fusion protein system with the N-terminal fragment of the lethal factor of Bacillus anthracis. LFn-ESAT6-His6 consists of the N-terminal region of B. anthrax lethal factor fused at the C-terminus to ESAT6 which contains six histidine residues at its C-terminus. A DNA fragment encoding ESAT6 followed by a 6-residue linker and then a C-terminal hexa-histidine tag was synthesized by GeneArt (Life Technologies). The fragment was subcloned into the expression plasmid FP59AGGpYS (24) using MluI and XmaI restriction sites in frame with the first 255 N-terminal amino acids of anthrax lethal factor. A B. anthracis strain deficient in extracellular proteases (25) was transformed with the resulting plasmid, and LFn-ESAT6-His6 fusion protein was purified from culture supernatants by nickel-affinity chromatography. The molecular mass of the protein was confirmed by electrospray ionization mass spectrometry. Anthrax protective antigen (PA) was prepared as described previously (26). Indicated doses of the proteins were used in cell cultures 3h after Mtb infection and left for 24 h. Culture supernatants were collected, sterile filtered and stored at −80°C until use. Cell extracts were prepared following instructions of the HO-1 ELISA kit. Nuclear extracts were obtained using a kit from Active Motif (Carlsbad, CA). Activation of the transcription factors NFE2L2 and c-JUN/AP-1 was determined in nuclear extracts using the TransAM® DNA-binding ELISA kits (Active Motif) following the manufacturer’s instructions. Cell viability was estimated using the XTT assay kit (Cayman Chemical Ann Arbor, MI) following the manufacturer’s instructions.
Data analysis
The median values with interquartile ranges (IQR) were used as measures of central tendency. For the in vitro experiments, bars represent mean and standard deviations. The Mann-Whitney test (for two groups) or Kruskal-Wallis with Dunn’s multiple comparisons or linear trend post-hoc tests (for more than two groups) were used to compare continuous variables. Fisher’s or chi-square tests were used to compare variables displayed as percentage. Spearman rank tests were used to assess correlations. Receiver Operator Characteristics (ROC) curves was employed to test the power of individual or combined markers to distinguish active from latent TB. Three models of principal component analysis (PCA) indicated in the text were designed to assess how different combinations of plasma mediators contributed to the differentiation between patients with distinct expression profiles of HO-1 and MMP-1. Unsupervised two-way hierarchical cluster analyses (Ward’s method) with bootstrap, and where dendogram branches spaces is proportional to distance, were utilized to test whether PTB patients with different expression profiles of HO-1 and MMP-1 could be grouped separately. Multinomial logistic regression analyses adjusted for age and gender were performed to assess the odds ratios (OR) of the associations between hematological and clinical parameters and the different expression profiles of HO-1 and MMP-1. A p-value <0.05 was considered statistically significant. The statistical analyses were performed using GraphPad Prism 6.0 (GraphPad Software Inc., USA), STATA 9.0 (StataCorp, TX, USA), JMP 10.0 (SAS, Cary, NC, USA) and R 3.1.0 (R Development Core Team, NZ) programs.
Results
Patients with active TB display higher plasma levels of both HO-1 and MMP-1 than those with latent TB infection
We have previously shown that plasma HO-1 levels are increased in active pulmonary TB (PTB) patients compared to non-infected individuals and to those with LTBI in a cohort from South India (9). To validate these results, we measured HO-1 levels in a geographically distinct cohort from the Northeast region of Brazil. HO-1 concentrations were again found to be higher in patients with active PTB disease than in non-infected individuals (P<0.001) or those with LTBI (P<0.001; Figure 2). Although median HO-1 levels were found to be higher in active PTB than in LTBI cases, we observed that some patients from these different clinical groups exhibit similar values (9) (Figure 2).
Figure 2. Plasma levels of HO-1 and MMP-1 in a Brazilian TB patient cohort.
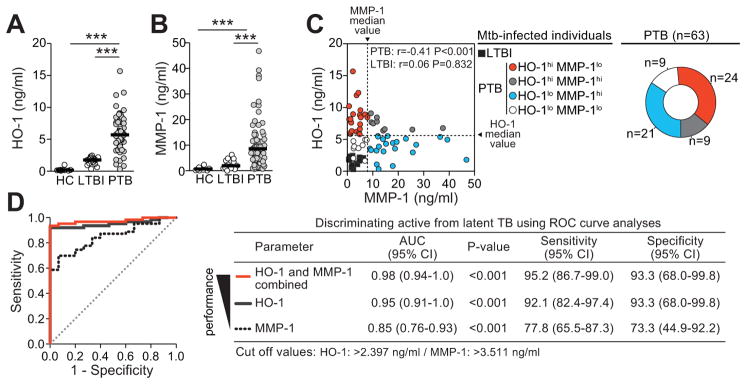
Plasma levels of HO-1 (A) and MMP-1 (B) were measured in a cohort of individuals with PTB (n=63), LTBI (n=15) and HC (n=10) from Northeast Brazil and compared using Kruskal-Wallis test with Dunn’s multiple comparisons post hoc test. Bars represent median values. (C) Correlation graph of plasma MMP-1 and HO-1 in individuals with PTB. Data were analyzed using Spearman rank correlation test. Dotted lines on the X-axis represent the median value of MMP-1 within the group of patients with pulmonary disease, while dotted lines on each Y-axis indicate median values for HO-1. Using this approach, it was possible to stratify the PTB patients according to the patterns of expression of MMP-1 and HO-1 in plasma (right panel). (D) Receiver Operator Characteristics (ROC) curves were employed to assess the performance of HO-1, MMP-1 or both in discriminating active from latent TB cases. CI, confidence interval; hi, values higher than median in PTB patients; lo, values lower than median in PTB patients; NPV, negative predictive value; PPV, positive predictive value. ***, P<0.001.
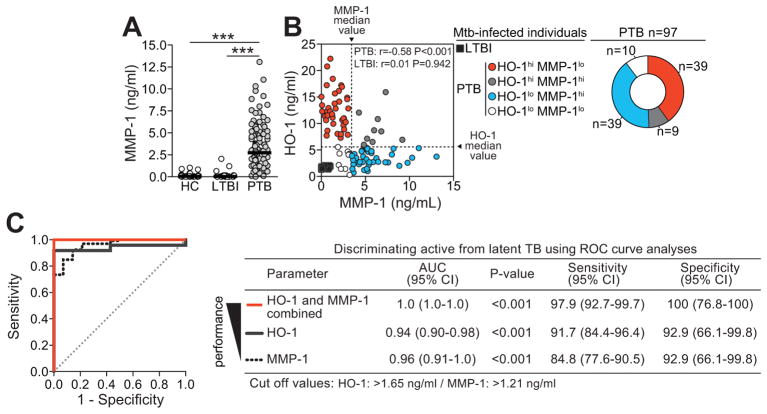
Using the larger cohort of patients from India, we next tested whether expression of MMP-1 in plasma also identifies individuals with active TB. In parallel to our findings with HO-1, we observed significantly higher levels of MMP-1 in active PTB patients than in healthy controls (P<0.001) or LTBI cases (P<0.001) (Figure 3A). Again, similar findings were obtained in the Brazilian validation cohort (Figure 2B). Interesting, as observed for HO-1 values, some patients with active TB exhibited MMP-1 levels which were non-distinguishable from those observed in LTBI cases. These results indicate that although HO-1 or MMP-1 alone can distinguish active from latent TB, some disease cases with overlapping values with LTBI can still be misclassified.
Figure 3. Combined assessment of HO-1 and MMP-1 plasma levels distinguishes active from latent TB infection.
(A) Plasma levels of MMP-1 were compared in 97 individuals with active pulmonary TB (PTB), 39 with latent TB infection (LTBI) and 40 healthy controls (HC) age-matched for age and gender using Kruskal-Wallis test with Dunn’s multiple comparisons post-hoc test (see Figure 7 for details of the study groups). (B) Correlation between MMP-1 and HO-1 plasma concentrations in individuals with LTBI or PTB was assessed using Spearman rank test. Dotted lines on the X-axis represent the median value of MMP-1 within the group of patients with pulmonary disease, while dotted lines on each Y-axis indicate median values for HO-1. Using this approach, it was possible to stratify the PTB patients according to the patterns of expression of MMP-1 and HO-1 in plasma. (C) Receiver Operator Characteristics (ROC) curve analyses were employed to assess the performance of HO-1, MMP-1 or both in discriminating active from latent TB cases. CI, confidence interval; hi, values higher than median in PTB patients; lo, values lower than median in PTB patients; ***, P<0.001.
Expression of HO-1 and MMP-1 in plasma delineates two subpopulations of patients with active TB
Having demonstrated that plasma levels of both HO-1 and MMP-1 are elevated in active pulmonary TB compared to LTBI, we then addressed whether combined measurement of HO-1 and MMP-1 would increase the power to discriminate these two patient groups. We found that although each marker individually displayed a high degree of accuracy, the combined assessment resulted in close to maximum (100%) performance in distinguishing active TB from LTBI (Figure 2D and Figure 3C). In Brazil, because the isolated markers had already been shown to be accurate in distinguishing active TB from LTBI, the gain in accuracy with the combined approach was less pronounced than in the Indian cohort (Figure 2D and Figure 3C). Based on this observation we hypothesized that the levels of these two biomarkers might be positively correlated. Surprisingly, we observed a striking negative correlation in HO-1 and MMP-1 expression in patients with active PTB, but not in individuals with LTBI (Figure 2C and Figure 3B). Thus patients with PTB in both the Indian and Brazilian cohorts expressed either very high levels of HO-1 (HO-1hiMMP-1lo) or of MMP-1 (HO-1loMMP-1hi), revealing a dichotomy within this clinical group (Figure 2C and Figure 3B). Of note, the median values of HO-1 and MMP-1 used to define the expression profiles were found to be different between the two patient cohorts. These assays were performed separately in the different countries and not simultaneously in a single facility. The distinct median values likely represent inter-assay variability and/or genetic end environmental differences. Importantly, despite the distinction in median values, the expression of the biomarkers relative to each other was very similar in the two geographically distinct patient groups.
Patients with active TB, atypical mycobacterial infection, or sarcoidosis show distinct HO-1 and MMP-1 expression profiles
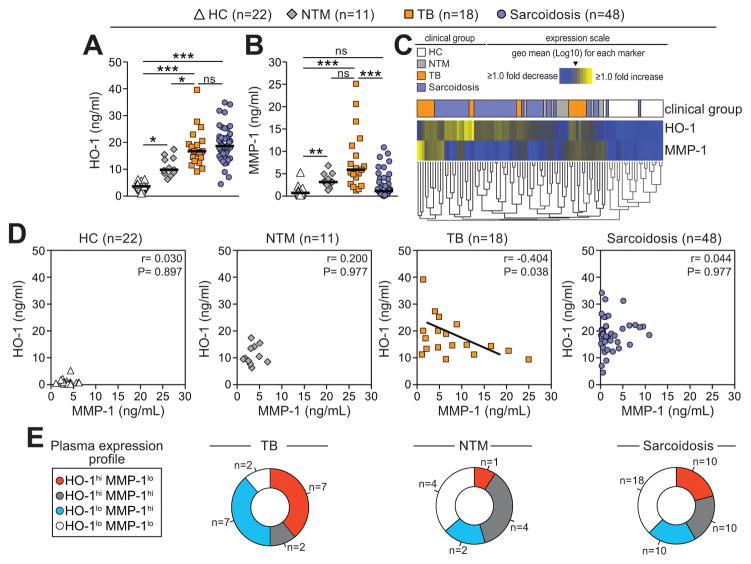
In an exploratory study of a limited number of patients from North America, we next examined whether the expression profile of HO-1 and MMP-1 in plasma differs between TB and other lung granulomatous diseases. HO-1 levels were increased in patients with active TB, NTM or sarcoidosis compared to healthy controls (Figure 4A). While active TB patients exhibited markedly higher HO-1 expression than NTM patients (P=0.008), they were not significantly different from sarcoid patients (Figure 4A). In contrast, systemic concentrations of MMP-1 were dramatically elevated in TB patients compared to healthy controls (P<0.001) and sarcoid patients (P<0.001) but were not significantly different from NTM patients (P=0.051; Figure 4B). On average, plasma MMP-1 values in sarcoid patients were indistinguishable from healthy controls (Figure 4B). A hierarchical clustering analysis of the plasma concentrations of these two enzymes revealed the existence of two major subgroups of TB patients with very distinct HO-1 and MMP-1 expression profiles whereas NTM and sarcoid patients exhibited more heterogeneous profiles (Figure 4C). Importantly, an inverse correlation between HO-1 and MMP-1 levels was observed in TB but not in the other lung diseases examined (Figure 4D). As described in the India and Brazil cohorts, patients with active TB preferentially displayed HO-1hiMMP-1lo or HO-1loMMP-1hi expression profiles whereas individuals with the other clinical groups exhibited a more heterogeneous profile (chi-square P<0.0001; Figure 4E). Thus, these preliminary findings indicated that the pattern of the relationship between plasma levels of HO-1 and MMP-1 is different between TB and other granulomatous lung diseases.
Figure 4. HO-1 and MMP-1 expression profiles in plasma from patients with active TB and individuals with other lung granulomatous lung diseases.
Plasma levels of HO-1 (A) and MMP-1 (B) were quantified in patients with confirmed active TB, non-TB mycobacteria (NTM) infection, sarcoidosis, and age- and gender- matched healthy controls (HC) from North America. Bars represent median values. Data were analyzed using Kruskal-Wallis tests with Dunn’s multiple comparisons post hoc tests. (C) An unsupervised cluster analysis (Ward’s method) was employed to identify overall differences in the expression profiles of HO-1 and MMP-1 in this study population. In the heat map, individual patients are listed in columns and each biomarker was placed in a different rows. The squares represent values below or above the geometric mean levels (Log10) of a given biomarker in the study population. (D) Correlation between MMP-1 and HO-1 plasma concentrations was assessed using Spearman rank test in the different study groups. (E) Distribution of the patients from the different lung disease groups with regard to expression profile of HO-1 and MMP-1 in plasma is shown. Data was compared using the chi-square test (P<0.0001). ns, non significant; hi, values higher than median in the indicated clinical group; lo, values lower than median in the indicated clinical group. *, P<0.05; **, P<0.01; ***, P<0.001.
Active TB patients with distinct HO-1 vs. MMP-1 expression patterns display markedly different inflammatory profiles
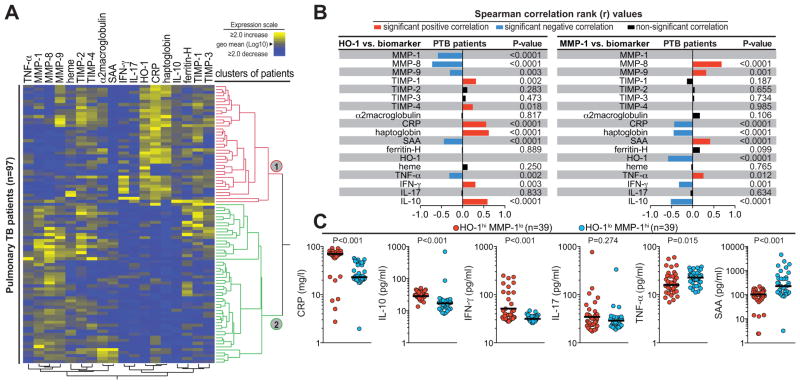
The data shown above demonstrated an inverse correlation between HO-1 and MMP-1 expression amongst patients with active TB in Indian, Brazilian and North American cohorts but not in healthy controls, individuals with latent TB or those with other lung diseases. We next examined the associations between HO-1 and MMP-1 and other biomarkers of inflammation or tissue damage/remodeling in PTB patients from our major study site in South India (n=97). Interestingly, we observed that the TB patients could be separated into two major clusters based on plasma protein expression of these markers (Figure 5A). Within these populations, HO-1 levels displayed significant negative correlations with other MMPs such as MMP-8 and MMP-9 as well as TNF-α and serum amyloid protein-A (SSA). On the other hand, HO-1 concentrations were positively correlated with TIMP-1, TIMP-4, CRP, haptoglobin, IFN-γ and IL-10 (Figure 5B). In addition, the correlations involving HO-1 resulted in identification of several unique expression profiles in a number of these markers (Supplemental Figure 1A–B). Visualization of the data by means of density plots clearly showed that the subpopulation of high HO-1 expressing individuals displayed relatively decreased levels of MMP-1, MMP-8, and MMP-9 compared to the group of individuals exhibiting low plasma values of HO-1, but they also accounted for all of the patients with greatly elevated CRP and haptoglobin levels (Supplemental Figure 1B). Strikingly, the correlations involving plasma MMP-1 concentrations exhibited a strongly inverted profile compared to those found for HO-1 levels (Figure 5B–C).
Figure 5. Pulmonary tuberculosis patients with different HO-1 and MMP-1 ratios display distinct plasma inflammatory biomarker profiles.
(A) Protein levels of several plasma biomarkers of inflammation and tissue damage/remodeling were determined by ELISA in active TB patients from India (n=97). An unsupervised hierarchical cluster with bootstrap analysis was employed to identify overall differences in the expression profiles of the markers in this population. To visualize the clusters, the dendogram branch spacing was set to be proportional to the hierarchical distance. The two larger clusters are highlighted with colors. In the heat map, individual patients are listed in rows and each biomarker was placed in a different column. The squares represent values below or above the geometric mean levels (Log10) of a given biomarker in the study population. (B) Plasma levels of HO-1 (left panel) and MMP-1 (right panel) were tested for correlations with each one of the biomarkers measured. Bars represent the strength of associations (Spearman rank values). (C) Circulating levels of indicated plasma mediators were compared between the groups of patients exhibiting opposite patterns of expression of HO-1 and MMP-1 in plasma using the Mann-Whitney test (See Supplemental Table 1 for details).
To better assess which of the two processes, inflammation or tissue remodeling, is more relevant in describing differences between patients with HO-1hiMMP-1lo and HO-1loMMPhi expression profiles, we employed three Principal Component Analyses (PCA). In the first model, we inputted data on all the biomarkers (Supplemental Table 1 and Supplemental Figure 2A). Using this approach, we found that the groups of patients with either HO-1hiMMP-1lo or HO-1loMMPhi clustered separately, although there was a notable dispersion of the data points within each group (Supplemental Figure 2B). In the second model, which incorporated only the biomarkers of inflammation, we observed that the groups remained separately clustered with considerably less dispersion (Supplemental Figure 2B). The third model utilized data from markers of tissue remodeling alone. In this analysis, both the intersection between the groups and the dispersion of the data points within each group were significantly greater than that observed with the other two models (Supplemental Figure 2B). The above PCA analyses indicated that patients with HO-1hiMMP-1lo or HO-1loMMPhi can be better distinguished on the basis of their inflammatory marker profiles than by the differential expression of tissue remodeling markers in plasma. This conclusion was supported by hierarchical clustering as well as ROC curve analyses of the different combinations of biomarkers used in the PCA models (Supplemental Figure 2C–D).
HO-1hiMMP-1lo and HO-1loMMP-1hi active TB patients display distinct disease presentation profiles
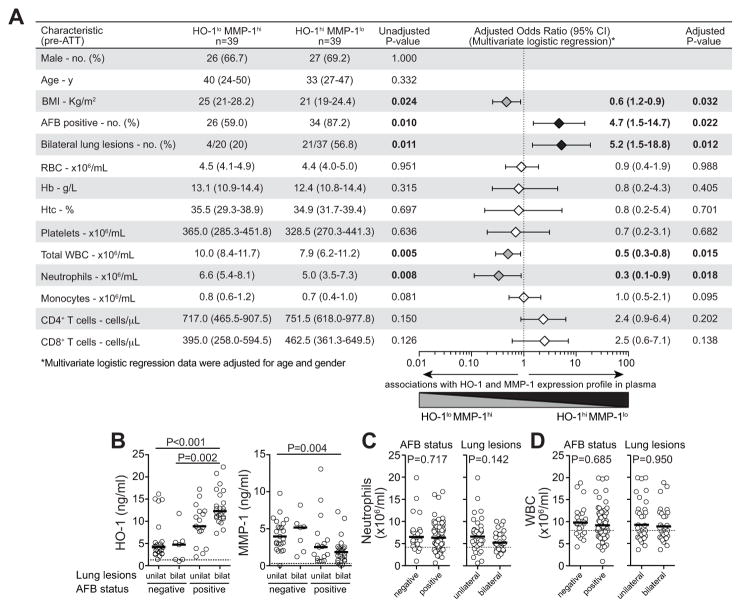
We next compared the HO-1hiMMP-1lo and HO-1loMMPhi patient subpopulations with regard to clinical, microbiological, hematological and radiological parameters to test if these populations diverge in terms of TB disease extension/severity. HO-1hiMMP-1lo and HO-1loMMPhi patients did not differ significantly with regard to age, gender and most hematological parameters (Figure 6A). HO-1hiMMP-1lo patients, when compared to HO-1loMMPhi patients, displayed significantly lower body mass index (BMI) and more frequently exhibited positive acid-fast bacilli (AFB) sputum smears and bilateral lung lesions (Figure 6A). Among the entire population of active PTB patients, individuals presenting with positive sputum smears and bilateral lung lesions simultaneously exhibited the highest levels of plasma HO-1 and the lowest MMP-1 values (Figure 6B). Total leukocyte and absolute neutrophil counts in the blood were higher in the subpopulation of TB patients with HO-1loMMPhi than in those with HO-1hiMMP-1lo expression profiles (Figure 6A). Nevertheless, there were no significant differences in either neutrophil or total leukocyte counts between the subgroups of patients with positive or negative sputum smears (Figure 6C–D) and also between individuals with unilateral or bilateral lung lesions (Figure 6C–D). Together, these analyses revealed that the patient subpopulations identified by combined measurement of plasma HO-1 and MMP-1 exhibit distinct disease presentations.
Figure 6. HO-1hiMMP-1lo and HO-1loMMP-1hi active TB patients display distinct disease presentation profiles.
(A) Patients with active pulmonary TB exhibiting different patterns of expression of HO-1 and MMP-1 in plasma were compared with regard to demographical, clinical and laboratory characteristics, using the Mann-Whitney test (for continuous variables) or the Fisher’s exact test (for categorical variables). The associations between the variables and the pattern of HO-1/MMP-1 expression were also assessed using multivariate logistic regression adjusted for age and gender. Odds ratios (ORs) are per SD increase after log transformation. ORs for percent of AFB positive and bilateral lung lesions are for comparisons between AFB positive vs. negative and for unilateral vs. bilateral lung lesions, respectively. (B) PTB patients were stratified according to sputum positivity and lung disease extension, and plasma levels of HO-1 (left panel) and MMP-1 (right panel) were compared using Kruskal-Wallis test with Dunn’s multiple comparisons post hoc test. (C) Absolute neutrophil counts in the blood were assessed in TB patients according to the AFB positivity in sputum (left panel) and also to the location of lung lesions (right panel). (D) Absolute white blood cell (WBC) counts in the blood were assessed in PTB patients according to the AFB positivity in sputum (left panel) and also to the location of lung lesions (right panel). In B–D, bars represent median values and dotted lines denote median values observed in the group of healthy controls. In C–D, data were analyzed using Mann-Whitney tests. AFB, acid fast bacilli; CI, confidence interval; Hb, hemoglobin; Htc, hematocrit; RBC, red blood cells; unilat, unilateral lung disease; bilat, bilateral lung disease; hi, higher than median values; lo, lower than median values; y, years.
Regulation of HO-1 and MMP-1 expression in Mycobacterium tuberculosis-infected macrophages
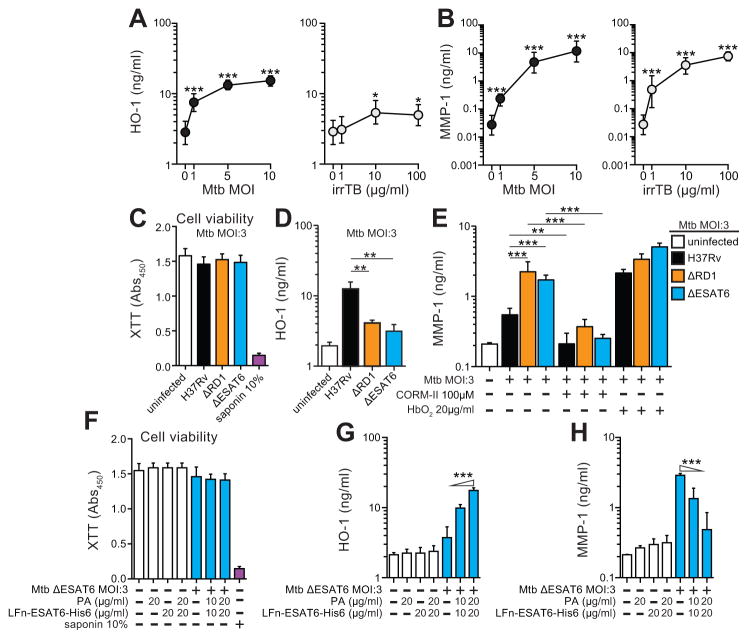
The above clinical observations on HO-1 and MMP-1 levels led us to hypothesize that the expression of these two biomarkers might be cross-regulated. We directly tested this hypothesis in in vitro studies employing human and murine macrophages. Macrophages have been described as an important source of HO-1 in several disease models including murine mycobacterial infection (27), and MMPs and other tissue proteases are known to be induced by Mtb infection in the same myeloid cell type (11). In the present study, we observed that MMP-1 is induced in a more selective manner than other MMPs or proteases in human macrophages infected with Mtb (Supplemental Figure 3). For this reason, we assessed the expression of intracellular protein HO-1 in cell lysates and the secreted protein MMP-1 in supernatants from macrophages infected with increasing multiplicities of virulent Mtb. We observed a significant dose-dependent induction of both proteins in the cell cultures (Figure 7A–B). In the case of HO-1, the response obtained was dependent on replicating bacilli as irradiated Mtb triggered only minor levels of the enzyme (Figure 7A). In contrast, Mtb irradiation failed to diminish the MMP-1 response in the same cultures (Figure 7B) suggesting that the two biomarkers have distinct bacterial triggers.
Figure 7. Infection of human macrophages with live virulent M. tuberculosis is required for robust induction of high levels of HO-1, but not MMP-1.
(A) Levels of HO-1 were determined in cell lysates of human monocyte-differentiated macrophages after 24h of infection with M. tuberculosis H37Rv strain (Mtb) at different multiplicities of infection (MOI) (left panel) or stimulation with irradiated Mtb at distinct concentrations (right panel) using ELISA. (B) MMP-1 levels were determined in culture supernatants of the same conditions listed in (A). Macrophages were infected with H37Rv, ΔRD1 or ΔESAT6 Mtb strains for 24 h in the presence or absence of the CO-releasing molecule CORM-II or the CO scavenger HbO2, and cell viability (XTT assay) (C), levels of HO-1 in cell lysates (D) and MMP-1 in culture supernatants (E) were assessed. Dashed lines represent values observed in uninfected cells. Cells infected with the ΔESAT6 Mtb strain were treated with different doses of the fusion Lfn-ESA6 with the anthrax protective antigen PA cytosolic delivery system and cell viability (XTT assay) (F), levels of HO-1 (G) and MMP-1 (H) were measured after 24h. In cell viability assays, saponin 10% was used as positive control to induce cell lysis and death. Data are from at least 3 experiments using cells from a total of up to 6 healthy donors. Data were compared using the Kruskal-Wallis test with Dunn’s multiple comparisons and/or linear trend post hoc analyses (triangles indicate the direction of the trend variation). *, P<0.05; **, P<0.01; ***, P<0.001.
A recent study has indicated that the Mtb virulence-associated secreted protein ESAT6 plays a role in induction of HO-1 in a murine macrophage cell line (28). In the present study, we observed that infection of human macrophages with mutant Mtb strains lacking the region of deletion 1 (RD1) or the ESAT6 protein (29) induced significantly lower HO-1 production (Figure 7C–D) but higher MMP-1 secretion (Figure 7E) than the wild type H37Rv Mtb strain. Delivery of ESAT6 recombinant protein into the cytosol of macrophages infected with ESAT6 deficient Mtb using a fusion protein with the N-terminal fragment of the lethal factor of Bacillus anthracis restored HO-1 induction and led to a decrease in MMP-1 secretion to levels similar to those induced by infection with Mtb H37Rv wild type strain (Figure 7F–H).
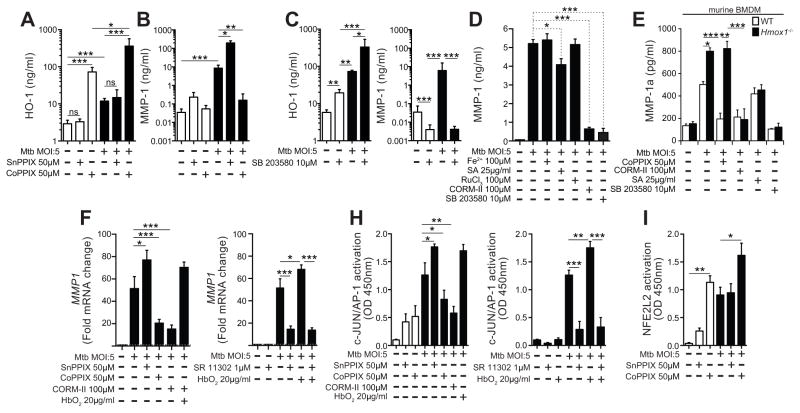
Given that HO-1 is described as a potent antioxidant and immunomodulator and that induction of MMP-1 by Mtb in macrophages involves activation of inflammatory transcription factors (17), we hypothesized that in conditions characterized by high HO-1 expression, MMP-1 production would be down-regulated. Indeed, drug-induced overexpression of HO-1 triggered by cobalt protoporphyrin (CoPPIX) caused a major (>2log) reduction in MMP-1 concentrations in supernatants of Mtb-infected macrophages (Figure 8A–B). Conversely, treatment of macrophages with tin protoporphyrin (SnPPIX), a potent inhibitor of HO-1 activity, resulted in a pronounced increase in MMP-1 production upon Mtb infection (Figure 8B). Interestingly, treatment of macrophages with a MAP kinase inhibitor (SB 203580) caused a significant increase in HO-1 expression while strongly inhibiting MMP-1 production by infected macrophages (Figure 8C). This data demonstrated that HO-1 and MMP-1 are differentially regulated in Mtb exposed macrophages and suggested that HO-1 induction is strongly associated with inhibition of MMP-1 expression in these cells.
Figure 8. HO-1 and MMP-1 expression are differentially regulated in Mycobacterium tuberculosis-infected human macrophages.
Human monocyte-differentiated macrophages were infected with H37Rv Mtb for 24h in the absence or presence of an inhibitor of HO-1 activity (SnPPIX) or a potent HO-1 inducer (CoPPIX) and levels of HO-1 in cell lysates (A) and of MMP-1 in supernatants (B) were quantified. (C) Macrophages were also treated with a MAPK inhibitor (SB 203580) in the presence of Mtb for 24h, and levels of HO-1 and MMP-1 were determined in cell lysates and supernatants, respectively. MMP-1 protein in supernatants (D) was quantified in cells treated with free iron (Fe2+; FeSO4), an inhibitor of heme biosynthesis (succinylacetone, SA), a CO-releasing agent (CORM-II), a molecule similar to CORM-II but with no CO-releasing capability (RuCl3), SB 203580, SnPPIX, CoPPIX, and the CO scavenger (oxyhemoglobin, HbO2) for 24h post Mtb infection. (E) These in vitro experiments were repeated using BMDM from C56BL/6 WT mice or Hmox1−/− animals, and the MMP-1a (an ortholog of the human MMP-1) was measured in culture supernatants by ELISA. (F) MMP1 mRNA levels were assessed in cultures of Mtb-infected human macrophages upon indicated treatments with for 24h. For the mRNA analysis, fold induction over mRNA levels in untreated cells is shown. Activation of the transcription factors c-JUN/AP-1 (H) and NFE2L2 (I) were determined after 12 hours post infection and/or stimulation using a colorimetric DNA-binding ELISA kit. Bars represent means and standard deviation and data were compared using the Kuskall-Wallis test with Dunn’s multiple comparisons post-test. Data are from at least 3 experiments using cells from a total of up to 6 healthy donors. In (E), 4 experiments were performed with samples run in triplicates. Data from different biological groups were analyzed using Kruskal-Wallis test with Dunn’s multiple comparisons while matched analyses were performed using Wilcoxon matched pairs test. *, P<0.05; **, P<0.01; ***, P<0.001; ns, non-significant.
Carbon monoxide derived from HO-1 activity down regulates MMP-1 expression by decreasing activation of c-JUN/AP-1
We next asked whether downstream products of HO-1 activity, such as free iron (Fe+2) and CO, or heme catabolism by HO-1 might explain the down modulation of MMP-1 expression induced by the former enzyme. To do so, we cultured Mtb-infected macrophages with FeSO4 to mimic increased availability of Fe+2 but this treatment failed to alter MMP-1 production (Figure 8D). In contrast, Mtb-infected cells treated with CORM-II, a CO-releasing molecule, led to a dramatic reduction in MMP-1 production, not reproduced by the control drug RuCl3 which lacks CO-releasing activity (P<0.001; Figure 8D). The CORM-II concentration used was not cytotoxic (cell viability in treated cultures: 92.5% ± 3.8 vs. 95.6% ± 5.2 in untreated cells, P=0.857). We also treated the macrophage cultures with succinylacetone, an inhibitor of heme biosynthesis, to mimic the reduction of heme availability triggered by HO-1 overexpression. We observed a substantial decrease in MMP-1 production by Mtb-infected macrophages in the succinylacetone treated cultures (P=0.022; Figure 8D) but with still much higher levels than those seen in cultures treated with CO-releasing molecule (P<0.001; Figure 8D). We next infected bone marrow-derived macrophages from WT or Hmox1−/− mice and assessed secretion of the MMP-1a, the murine ortholog of human MMP-1. Mtb-infected macrophages from Hmox1−/− mice secreted significantly higher amounts of MMP-1a than WT cells (Figure 8E). The secretion of MMP-1a by Hmox1−/− macrophages was dramatically reduced by treatment with CO-releasing molecule but only partially so following addition of the inhibitor of heme biosynthesis succinylacetone (Figure 8E), thus reinforcing the role of CO in the modulation of MMP-1 secretion.
In further experiments, we verified that CO affects MMP-1 expression at the transcriptional level, as mRNA levels of MMP1 were dramatically reduced in Mtb-infected macrophages treated with CORM-II and restored by addition of oxyhemoglobin (HbO2), a CO scavenger (Figure 8F). Given the central importance of the transcription factor AP-1 in MMP-1 expression in the context of Mtb infection (30), we hypothesized that CO derived from HO-1 activity would impair MMP-1 expression by interfering with AP-1 activation. We confirmed in our in vitro system that human macrophages infected with Mtb display increased activation of c-JUN/AP-1 in nuclear extracts 12 hours post infection (Figure 8H). Interestingly, we detected a significant reduction in c-JUN/AP-1 activation in infected macrophages treated with the HO-1 inducer, accompanied by increases in expression of the Hmox1 transcription factor NFE2L2 (Figure 8I). The inhibitory effects on c-JUN/AP-1 activation observed with treatment with the HO-1 inducer were reproduced when cells were treated with the CO-releasing molecule (Figure 8H). Importantly, inhibition of HO-1 activity or removal of CO by HbO2 restored the activation of c-JUN/AP-1 in infected macrophages (Figure 8H). These findings argue that HO-1 activity in Mtb-infected macrophages down-modulates MMP-1 expression via CO-mediated suppression of c-JUN/AP-1 activation.
Discussion
The development of reliable biomarkers for active TB is important both for identifying patients in need of antibiotic therapy and for a better understanding of the pathological mechanisms involved in the progression of Mtb infection to active disease that could serve as targets for immunotherapies. Here we demonstrate that combined measurement of HO-1 and MMP-1 in plasma reveals a dichotomy in PTB patients that reflects their different disease presentation profiles. This dichotomy was absent in both LTBI individuals as well as in the patients with other lung diseases, highlighting potential differences in immunopathology between these clinical conditions.
HO-1 is a potent antioxidant enzyme associated with cytoprotection in a number of different disease settings. MMP-1, on the other hand, is a major collagenase involved in tissue remodeling. Since both enzymes have been used as biomarkers for TB (9, 12, 14, 15) it was of interest to determine whether their expression is linked. We found in human macrophages infected with Mtb that while both HO-1 and MMP-1 are induced, MMP-1 expression is regulated by HO-1. Previous data has shown that HO-1 can regulate MMP-1 production in a human chondrocyte cell line stimulated with IL-1β by CO-driven inhibition of the NOX4 pathway (31). This finding suggested that HO-1 mediated inhibition of MMP-1 expression in Mtb-infected macrophages might involve a similar CO-dependent mechanism. In support of this hypothesis, we showed that HO-1 driven CO down regulates MMP-1 expression at the transcriptional level by inhibiting activation of c-JUN/AP-1. In addition, the observed suppression of MMP-1 by CO in macrophages was associated with increased activation of the HO-1 transcriptional regulator NFE2L2, suggesting an upstream interaction between the pathways controlling these two transcription factors. Additional experiments are necessary to confirm this relationship between HO-1 and MMP-1 at the single cell level and to further delineate its molecular basis.
The finding that CO is a major regulator of MMP-1 expression in Mtb infection has important implications for pathogenesis. In vitro exposure of several bacterial species such as Pseudomonas aeruginosa and Escherichia coli to increasing doses of CO results in microbial death by inhibition of critical enzymes involved in respiratory electron transport chains (32, 33). The importance of CO in controlling mycobacterial growth in vitro has been previously investigated (34). HO-1 derived CO has been shown to alter Mtb gene transcription and activate the mycobacterial dormancy regulon in experimental studies with mouse macrophages (27, 35). The recent identification of a gene mutation in Mtb strains that confers resistance to CO and leads to increased pathogenicity capacity in a murine TB model (36) suggests that CO resistance may be critical for Mtb survival and persistence in vivo. Our results demonstrating that CO strongly inhibits expression of MMP-1 argue that in addition to its antimicrobial effects, this metabolic product could have regulatory effects on collagen degradation in the lungs of TB patients. Inhibition of MMP-1 has been proposed as an adjunct treatment strategy for TB and other lung diseases (37). The findings presented here suggest that pharmacological induction of HO-1 expression leading to CO production could be employed as a strategy for MMP-1 inhibition in TB and perhaps other fibrotic diseases.
We observed that the majority of patients with active TB express either high HO-1 and low MMP-1 or vice-versa. However, up to 19.5% of the TB patient population in India and 28.6% in Brazil did not fall into the HO-1hiMMP-1lo or HO-1loMMP-1hi expression profiles. In addition, in our in vitro experiments, significant induction of HO-1 was seen only when macrophages were exposed to live virulent ESAT6 expressing Mtb whereas MMP-1 production was potently induced by both live and irradiated mycobacteria as well as by Mtb lacking ESAT6 expression. Thus, dichotomous expression of HO-1 and MMP-1 may reflect some aspect of the cellular response triggered by actively replicating virulent Mtb (and with capacity to secrete proteins), such as the ability to escape from the macrophage phagosome. In addition, we observed that pharmacologic inhibition of MMP-1 with SB 203580 results in increased HO-1 expression in Mtb-infected macrophages, suggesting that MMP-1 related pathways could also inhibit HO-1 production and that the expression of these two critical enzymes is indeed cross-regulated. Such a mechanism triggered by Mtb infection might explain why a distinct HO-1 and MMP-1 expression profile with a strong inverse correlation in plasma values occurs in the majority of active TB cases and is not clearly observed in patients with other lung granulomatous diseases such as sarcoidosis and NTM infection.
The two subpopulations of PTB patients identified by dual measurement of HO-1 and MMP-1 are of special interest as they could reflect either distinct stages of TB disease progression (early vs. advanced disease) or differences in disease severity or anatomical location. In the present study, patients were recruited at the time of disease presentation (none were exposed to anti-tuberculous treatment prior or at study enrollment), and for this reason we did not have reliable data on the history of TB disease prior to plasma collection. However, we were able to compare HO-1hiMMP-1lo and HO-1loMMPhi patients with regard to clinical, microbiological, hematological and radiological parameters to test if these populations diverge in terms of TB disease severity. Multivariate regression analysis confirmed that lower BMI, AFB positivity in sputum smears and presentation with bilateral lung lesions in chest X-rays were strongly associated with the HO-1hiMMP-1lo expression profile whereas total leukocyte and neutrophil counts were linked to HO-1loMMPhi profile. This observation is consistent with a scenario in which HO-1hiMMP-1lo patients are those with increased TB disease severity. A longitudinal study would be needed to determine whether these individuals derive from HO-1loMMPhi patients at an earlier stage of disease progression. Interestingly, single nucleotide polymorphisms that impact both HO-1 and MMP-1 expression have been previously described in a variety of conditions (38–45) and it is thus possible that the two patient populations described here reflect this genetic heterogeneity. Finally, as alluded to above, the distinct HO-1hiMMP-1lo and HO-1loMMPhi patient sets may reflect differences in the virulence of the Mtb strains infecting individuals in each subgroup within the cohort. Genetic analyses of both the infected patients and their corresponding Mtb isolates could be used to address the latter two hypotheses.
Together, our results reveal two distinct subgroups of active TB, each marked by increased HO-1 or MMP-1 expression and deriving from a potential cross-talk between these molecules. Both the nature of these clinical subpopulations and the possible role of HO-1 and MMP-1 in their pathogenesis await further definition.
Supplementary Material
Acknowledgments
Funding: This work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health. J.M., V.C.M., J.R.F. were supported by the NHLBI Intramural Research Program. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The Brazilian study was funded by Fundação de Amparo à Pesquisa do Estado da Bahia and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil [grant number: 028/2010]. T.B. is a senior investigator from CNPq. E.L.C. received a research fellowship and B.B.A. received a research award from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil.
The authors would like to thank Dr. Miguel Soares (Instituto Gulbenkian de Ciência, Portugal) for supplying bone marrow from Hmox1−/− mice and WT controls. In addition, we are grateful to Mr. Michael Rocha and Drs. Jamocyr Marinho (Hospital Especializado Octávio Mangabeira, Brazil), Eleanor Wilson (NIAID, NIH), Sonia Qasba (TB Control Program, Montgomery County Department of Health and Human Services, USA), Douglas B. Kuhns (Clinical Services Program, SAIC–Frederick, National Cancer Institute–Frederick, USA) as well as Delmyra Turpin (TB Control Program, Montgomery County DHS, USA) and Sandra MacDonald (NHLBI, Cardiovascular and Pulmonary Branch, NIH) for critical help in recruitment of patients and logistical issues. We also thank the staff of Department of Clinical Research and the Department of Social Work, NIRT, especially Ms. Kalaiselvi and Government Stanley Hospital, Chennai, for valuable assistance in recruiting the patients for the South India study, R. Anuradha, V. Gopinath and Jovvian George (NIH-ICER, India) for technical assistance and Dr. Dragana Jankovic (NIAID, NIH) for critical review of the manuscript.
New or special abbreviations
- AFB
acid-fast bacilli
- ATT
anti-tuberculous treatment
- CRP
C-reactive protein
- CoPPIX
cobalt (III) protoporphyrin IX dichloride
- HC
healthy control
- HO
heme oxygenase
- LTBI
latent tuberculosis infection
- MMP
metalloproteinase
- Mtb
Mycobacterium tuberculosis
- NTM
non-tuberculous mycobacteria
- PCA
principal component analysis
- PTB
pulmonary tuberculosis
- SAA
serum amyloid protein
- SnPPIX
tin (IV) protoporphyrin IX dichloride
- ROC
receiver operator characteristics
- TB
tuberculosis
- TIMP
tissue inhibitor of metalloproteinase
References
- 1.Zumla A, Raviglione M, Hafner R, von Reyn CF. Tuberculosis. N Engl J Med. 2013;368:745–755. doi: 10.1056/NEJMra1200894. [DOI] [PubMed] [Google Scholar]
- 2.Davies PD, Pai M. The diagnosis and misdiagnosis of tuberculosis. Int J Tuberc Lung Dis. 2008;12:1226–1234. [PubMed] [Google Scholar]
- 3.Frahm M, Goswami ND, Owzar K, Hecker E, Mosher A, Cadogan E, Nahid P, Ferrari G, Stout JE. Discriminating between latent and active tuberculosis with multiple biomarker responses. Tuberculosis (Edinb) 2011;91:250–256. doi: 10.1016/j.tube.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gopinath K, Singh S. Non-tuberculous mycobacteria in TB-endemic countries: are we neglecting the danger? PLoS Negl Trop Dis. 2010;4:e615. doi: 10.1371/journal.pntd.0000615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta D, Agarwal R, Aggarwal AN, Jindal SK. Sarcoidosis and tuberculosis: the same disease with different manifestations or similar manifestations of different disorders. Curr Opin Pulm Med. 2012;18:506–516. doi: 10.1097/MCP.0b013e3283560809. [DOI] [PubMed] [Google Scholar]
- 6.Wallis RS, Kim P, Cole S, Hanna D, Andrade BB, Maeurer M, Schito M, Zumla A. Tuberculosis biomarkers discovery: developments, needs, and challenges. Lancet Infect Dis. 2013;13:362–372. doi: 10.1016/S1473-3099(13)70034-3. [DOI] [PubMed] [Google Scholar]
- 7.Madebo T, Lindtjorn B, Aukrust P, Berge RK. Circulating antioxidants and lipid peroxidation products in untreated tuberculosis patients in Ethiopia. Am J Clin Nutr. 2003;78:117–122. doi: 10.1093/ajcn/78.1.117. [DOI] [PubMed] [Google Scholar]
- 8.Palanisamy GS, Kirk NM, Ackart DF, Shanley CA, Orme IM, Basaraba RJ. Evidence for oxidative stress and defective antioxidant response in guinea pigs with tuberculosis. PLoS One. 2011;6:e26254. doi: 10.1371/journal.pone.0026254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andrade BB, Pavan Kumar N, Mayer-Barber KD, Barber DL, Sridhar R, Rekha VV, Jawahar MS, Nutman TB, Sher A, Babu S. Plasma heme oxygenase-1 levels distinguish latent or successfully treated human tuberculosis from active disease. PLoS One. 2013;8:e62618. doi: 10.1371/journal.pone.0062618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pavan Kumar N, Anuradha R, Andrade BB, Suresh N, Ganesh R, Shankar J, Kumaraswami V, Nutman TB, Babu S. Circulating biomarkers of pulmonary and extrapulmonary tuberculosis in children. Clin Vaccine Immunol. 2013;20:704–711. doi: 10.1128/CVI.00038-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elkington P, Shiomi T, Breen R, Nuttall RK, Ugarte-Gil CA, Walker NF, Saraiva L, Pedersen B, Mauri F, Lipman M, Edwards DR, Robertson BD, D’Armiento J, Friedland JS. MMP-1 drives immunopathology in human tuberculosis and transgenic mice. J Clin Invest. 2011;121:1827–1833. doi: 10.1172/JCI45666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elkington PT, Ugarte-Gil CA, Friedland JS. Matrix metalloproteinases in tuberculosis. Eur Respir J. 2011;38:456–464. doi: 10.1183/09031936.00015411. [DOI] [PubMed] [Google Scholar]
- 13.Sundararajan S, Babu S, Das SD. Comparison of localized versus systemic levels of Matrix metalloproteinases (MMPs), its tissue inhibitors (TIMPs) and cytokines in tuberculous and non-tuberculous pleuritis patients. Hum Immunol. 2012;73:985–991. doi: 10.1016/j.humimm.2012.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ugarte-Gil CA, Elkington P, Gilman RH, Coronel J, Tezera LB, Bernabe-Ortiz A, Gotuzzo E, Friedland JS, Moore DA. Induced sputum MMP-1, -3 & -8 concentrations during treatment of tuberculosis. PLoS One. 2013;8:e61333. doi: 10.1371/journal.pone.0061333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sathyamoorthy T, Sandhu G, Tezera LB, Thomas R, Singhania A, Woelk CH, Dimitrov BD, Agranoff D, Evans CA, Friedland JS, Elkington PT. Gender-dependent differences in plasma matrix metalloproteinase-8 elevated in pulmonary tuberculosis. PLoS One. 2015;10:e0117605. doi: 10.1371/journal.pone.0117605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elkington PT, Nuttall RK, Boyle JJ, O’Kane CM, Horncastle DE, Edwards DR, Friedland JS. Mycobacterium tuberculosis, but not vaccine BCG, specifically upregulates matrix metalloproteinase-1. Am J Respir Crit Care Med. 2005;172:1596–1604. doi: 10.1164/rccm.200505-753OC. [DOI] [PubMed] [Google Scholar]
- 17.Vincenti MP, Brinckerhoff CE. Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in arthritis: integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res. 2002;4:157–164. doi: 10.1186/ar401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chuang SM, Liou GY, Yang JL. Activation of JNK, p38 and ERK mitogen-activated protein kinases by chromium(VI) is mediated through oxidative stress but does not affect cytotoxicity. Carcinogenesis. 2000;21:1491–1500. [PubMed] [Google Scholar]
- 19.Tongers J, Fiedler B, Konig D, Kempf T, Klein G, Heineke J, Kraft T, Gambaryan S, Lohmann SM, Drexler H, Wollert KC. Heme oxygenase-1 inhibition of MAP kinases, calcineurin/NFAT signaling, and hypertrophy in cardiac myocytes. Cardiovasc Res. 2004;63:545–552. doi: 10.1016/j.cardiores.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 20.Zhang M, Zhang BH, Chen L, An W. Overexpression of heme oxygenase-1 protects smooth muscle cells against oxidative injury and inhibits cell proliferation. Cell Res. 2002;12:123–132. doi: 10.1038/sj.cr.7290118. [DOI] [PubMed] [Google Scholar]
- 21.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M, Olivier K, Ruoss S, von Reyn CF, Wallace RJ, Jr, Winthrop K A. T. S. M. D. Subcommittee, S. American Thoracic, and A. Infectious Disease Society of. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 22.Mayer-Barber KD, Andrade BB, Barber DL, Hieny S, Feng CG, Caspar P, Oland S, Gordon S, Sher A. Innate and adaptive interferons suppress IL-1alpha and IL-1beta production by distinct pulmonary myeloid subsets during Mycobacterium tuberculosis infection. Immunity. 2011;35:1023–1034. doi: 10.1016/j.immuni.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege JL, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, Suttles J, Udalova I, van Ginderachter JA, Vogel SN, Wynn TA. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bachran C, Gupta PK, Bachran S, Leysath CE, Hoover B, Fattah RJ, Leppla SH. Reductive methylation and mutation of an anthrax toxin fusion protein modulates its stability and cytotoxicity. Sci Rep. 2014;4:4754. doi: 10.1038/srep04754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pomerantsev AP, Pomerantseva OM, Moayeri M, Fattah R, Tallant C, Leppla SH. A Bacillus anthracis strain deleted for six proteases serves as an effective host for production of recombinant proteins. Protein Expr Purif. 2011;80:80–90. doi: 10.1016/j.pep.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu S, Leung HJ, Leppla SH. Characterization of the interaction between anthrax toxin and its cellular receptors. Cell Microbiol. 2007;9:977–987. doi: 10.1111/j.1462-5822.2006.00845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shiloh MU, Manzanillo P, Cox JS. Mycobacterium tuberculosis senses host-derived carbon monoxide during macrophage infection. Cell Host Microbe. 2008;3:323–330. doi: 10.1016/j.chom.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar R, Halder P, Sahu SK, Kumar M, Kumari M, Jana K, Ghosh Z, Sharma P, Kundu M, Basu J. Identification of a novel role of ESAT-6-dependent miR-155 induction during infection of macrophages with Mycobacterium tuberculosis. Cell Microbiol. 2012;14:1620–1631. doi: 10.1111/j.1462-5822.2012.01827.x. [DOI] [PubMed] [Google Scholar]
- 29.Abdalla H, Srinivasan L, Shah S, Mayer-Barber KD, Sher A, Sutterwala FS, Briken V. Mycobacterium tuberculosis infection of dendritic cells leads to partially caspase-1/11-independent IL-1beta and IL-18 secretion but not to pyroptosis. PLoS One. 2012;7:e40722. doi: 10.1371/journal.pone.0040722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Green JA, Elkington PT, Pennington CJ, Roncaroli F, Dholakia S, Moores RC, Bullen A, Porter JC, Agranoff D, Edwards DR, Friedland JS. Mycobacterium tuberculosis upregulates microglial matrix metalloproteinase-1 and -3 expression and secretion via NF-kappaB- and Activator Protein-1-dependent monocyte networks. J Immunol. 2010;184:6492–6503. doi: 10.4049/jimmunol.0903811. [DOI] [PubMed] [Google Scholar]
- 31.Rousset F, Nguyen MV, Grange L, Morel F, Lardy B. Heme oxygenase-1 regulates matrix metalloproteinase MMP-1 secretion and chondrocyte cell death via Nox4 NADPH oxidase activity in chondrocytes. PLoS One. 2013;8:e66478. doi: 10.1371/journal.pone.0066478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Desmard M, Davidge KS, Bouvet O, Morin D, Roux D, Foresti R, Ricard JD, Denamur E, Poole RK, Montravers P, Motterlini R, Boczkowski J. A carbon monoxide-releasing molecule (CORM-3) exerts bactericidal activity against Pseudomonas aeruginosa and improves survival in an animal model of bacteraemia. FASEB J. 2009;23:1023–1031. doi: 10.1096/fj.08-122804. [DOI] [PubMed] [Google Scholar]
- 33.Nobre LS, Al-Shahrour F, Dopazo J, Saraiva LM. Exploring the antimicrobial action of a carbon monoxide-releasing compound through whole-genome transcription profiling of Escherichia coli. Microbiology. 2009;155:813–824. doi: 10.1099/mic.0.023911-0. [DOI] [PubMed] [Google Scholar]
- 34.Zacharia VM, Shiloh MU. Effect of carbon monoxide on Mycobacterium tuberculosis pathogenesis. Med Gas Res. 2012;2:30. doi: 10.1186/2045-9912-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar A, Deshane JS, Crossman DK, Bolisetty S, Yan BS, Kramnik I, Agarwal A, Steyn AJ. Heme oxygenase-1-derived carbon monoxide induces the Mycobacterium tuberculosis dormancy regulon. J Biol Chem. 2008;283:18032–18039. doi: 10.1074/jbc.M802274200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zacharia VM, Manzanillo PS, Nair VR, Marciano DK, Kinch LN, Grishin NV, Cox JS, Shiloh MU. cor, a novel carbon monoxide resistance gene, is essential for Mycobacterium tuberculosis pathogenesis. MBio. 2013;4:e00721–00713. doi: 10.1128/mBio.00721-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ong CW, Elkington PT, Friedland JS. Tuberculosis, pulmonary cavitation, and matrix metalloproteinases. Am J Respir Crit Care Med. 2014;190:9–18. doi: 10.1164/rccm.201311-2106PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Exner M, Minar E, Wagner O, Schillinger M. The role of heme oxygenase-1 promoter polymorphisms in human disease. Free Radic Biol Med. 2004;37:1097–1104. doi: 10.1016/j.freeradbiomed.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 39.Ganachari M, Ruiz-Morales JA, Gomez de la Torre Pretell JC, Dinh J, Granados J, Flores-Villanueva PO. Joint effect of MCP-1 genotype GG and MMP-1 genotype 2G/2G increases the likelihood of developing pulmonary tuberculosis in BCG-vaccinated individuals. PLoS One. 2010;5:e8881. doi: 10.1371/journal.pone.0008881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hsieh MH, Chou PC, Chou CL, Ho SC, Joa WC, Chen LF, Sheng TF, Lin HC, Wang TY, Chang PJ, Wang CH, Kuo HP. Matrix metalloproteinase-1 polymorphism (-1607G) and disease severity in non-cystic fibrosis bronchiectasis in Taiwan. PLoS One. 2013;8:e66265. doi: 10.1371/journal.pone.0066265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mendonca VR, Luz NF, Santos NJ, Borges VM, Goncalves MS, Andrade BB, Barral-Netto M. Association between the haptoglobin and heme oxygenase 1 genetic profiles and soluble CD163 in susceptibility to and severity of human malaria. Infect Immun. 2012;80:1445–1454. doi: 10.1128/IAI.05933-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ninomiya S, Niimi T, Shimizu S, Sato S, Achiwa H, Ito H, Akita K, Maeda H, Ueda R. Matrix metalloproteinase-1 polymorphism of promoter region in sarcoidosis and tuberculosis patients. Sarcoidosis Vasc Diffuse Lung Dis. 2004;21:19–24. doi: 10.1007/s11083-004-3344-x. [DOI] [PubMed] [Google Scholar]
- 43.Saukkonen K, Lakkisto P, Kaunisto MA, Varpula M, Voipio-Pulkki LM, Varpula T, Pettila V, Pulkki K. Heme oxygenase 1 polymorphisms and plasma concentrations in critically ill patients. Shock. 2010;34:558–564. doi: 10.1097/SHK.0b013e3181e14de9. [DOI] [PubMed] [Google Scholar]
- 44.Sheu CC, Zhai R, Wang Z, Gong MN, Tejera P, Chen F, Su L, Thompson BT, Christiani DC. Heme oxygenase-1 microsatellite polymorphism and haplotypes are associated with the development of acute respiratory distress syndrome. Intensive Care Med. 2009;35:1343–1351. doi: 10.1007/s00134-009-1504-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sri Manjari K, Nallari P, Balakrishna N, Vidyasagar A, Prabhakar B, Jyothy A, Venkateshwari A. Influence of matrix metalloproteinase-1 gene -1607 (1G/2G) (rs1799750) promoter polymorphism on circulating levels of MMP-1 in chronic pancreatitis. Biochem Genet. 2013;51:644–654. doi: 10.1007/s10528-013-9594-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.