Abstract
Endometriosis is a chronic, inflammatory disease characterized by the growth of endometrial tissue in aberrant locations outside the uterus. Neo-angiogenesis or establishment of new blood supply is one of the fundamental requirements of endometriotic lesion survival in the peritoneal cavity. IL-17A is emerging as a potent angiogenic and pro-inflammatory cytokine involved in the pathophysiology of several chronic inflammatory diseases such as rheumatoid arthritis and psoriasis. However, sparse information is available in the context of endometriosis. In this study, we demonstrate the potential importance of IL-17A in the pathogenesis and pathophysiology of endometriosis. The data show a differential expression of IL-17A in human ectopic endometriotic lesions and matched eutopic endometrium from women with endometriosis. Importantly, surgical removal of lesions resulted in significantly reduced plasma IL-17A concentrations. Immunohistochemistry revealed localization of IL-17A primarily in the stroma of matched ectopic and eutopic tissue samples. In vitro stimulation of endometrial epithelial carcinoma cells, Ishikawa cells and human umbilical vein endothelial cells with IL-17A revealed significant increase in angiogenic (VEGF, IL-8), pro-inflammatory (IL-6, IL-1β) and chemotactic cytokines (G-CSF, CXCL12, CXCL1, CX3CL1). Furthermore, IL-17A promoted tubulogenesis of HUVECs plated on matrigel in a dose-dependent manner. Thus we provide the first evidence that endometriotic lesions produce IL-17A and that the removal of the lesion via laparoscopic surgery leads to the significant reduction in the systemic levels of IL-17A. Taken together, our data shows a likely important role of IL-17A in promoting angiogenesis and pro-inflammatory environment in the peritoneal cavity for the establishment and maintenance of endometriosis lesions.
Keywords: Angiogenesis, inflammation, plasma, endothelial cells, endometriosis, Interleukin-17A
Introduction
Endometriosis, one of the most prevalent causes of hysterectomy, infertility and chronic pelvic pain (1), is characterized by the growth of endometrial tissue at ectopic sites, including the peritoneal cavity. Among multiple factors involved in the complex pathophysiology of this disease (2), both a pro-inflammatory peritoneal environment and active neo-angiogenesis at the site of lesion development are thought to be integral to endometriotic lesion establishment and persistence. This notion is supported with the effectiveness of anti-inflammatory and anti-angiogenic drugs in minimizing pain and lesion size in mice and humans (3–5). Theorized to originate from menstrual material normally shed into the peritoneal cavity through the patent fallopian tube (6), the endometrial fragments, only in some women, initiate inflammatory response associated with tissue damage (7). This leads to the recruitment and activation of neutrophils and macrophages (8), which secrete and increase the concentrations of chemotactic and angiogenic cytokines such as CCL11 (9) and VEGF (10) in the peritoneal fluid. Indeed, the immune system of women who develop endometriosis is postulated to be abnormal, and the atypical activation of macrophages and subsequent recruitment of neutrophils (8), dendritic cells (11), T helper (12) and natural killer (NK) cells are suggested to play a crucial role in the pathogenesis of endometriosis (13, 14).
Interleukin (IL)-17A, a cytokine that was once believed to be primarily produced by T helper 17 (Th17) cells, is the most studied cytokine among the family of IL-17 cytokines. Since its discovery as a pro-inflammatory mediator (15), IL-17A has been recognized in its critical role in the promotion of disease progression and pathogenesis of autoimmune diseases (16). Furthermore, expression of IL-17A is associated with the pathogenesis of a variety of tumors, where it has been documented to exhibit both tumorigenic and anti-tumor effects depending on the tumor microenvironment (17). IL-17A mediates its action by binding to a heterodimeric complex of receptor composed of IL-17RA and IL-17RC for downstream cell signaling (18). Early in its discovery, majority of its production was thought to originate from Th17 cells; however further investigations have documented the expression of IL-17A from innate immune cells such as γδT (19–21), NK (22), neutrophils (16, 23) as well as mast cells (24), adding complexity to our understanding of the pleiotropic functions of this cytokine.
The pro-inflammatory nature of IL-17A was recognized in a seminal experiment conducted by Fossiez et al. (15) where rheumatoid synovial fibroblasts produced IL-6, IL-8, G-CSF and PGE2 in response to human recombinant IL-17A treatment. This effect was promptly abolished with the addition of anti-IL-17A monoclonal antibody. Since then, IL-17A has been extensively linked to the pathogenesis of chronic inflammatory diseases including rheumatoid arthritis and psoriasis (25). In addition, IL-17A promotes vascular remodeling of the airways in the mouse model of asthma via the recruitment of endothelial progenitor cells to the allergen exposed airway (26). Furthermore, it confers tumor resistance to anti-VEGF therapy via induction and secretion of angiogenic and inflammatory factors such as Bv8 and IL-6 from tumor stromal cells (27). Recently, IL-17A was demonstrated to be involved in the promotion of ovarian cancer growth in mice via up-regulation of pro-angiogenic and inflammatory mediators from small peritoneal macrophages (21). Despite much advancement made in understanding the role of IL-17A in other disease pathogenesis, little is known regarding its role in endometriosis. So far, IL-17A has been documented to induce the production of IL-8 and COX-2 from endometriotic stromal cells, in addition to promoting proliferation of the cells (28). Furthermore, IL-17A concentration in the peritoneal fluid of women with endometriosis correlated with endometriosis disease severity and infertility of the patients (29). These reports clearly point towards the potential involvement of IL-17A in the pathogenesis of endometriosis that warrants further investigation.
Endometriosis is a chronic disease characterized by the elevation of pro-inflammatory cytokines in the peritoneal fluid. Concentrations of cytokines such as TNF-α, IL-6 and IL-8 are increased in the peritoneal fluid and are augmented in production from activated peritoneal macrophages, which primarily contribute to the inflammatory milieu associated with pathogenesis of the disease. Furthermore, secretory factors from immune cells mediate vascularization of endometriotic lesions through both neo-angiogenesis stimulated by VEGF (30) and de novo vasculogenesis at the site of implantation through recruitment of bone-marrow derived endothelial progenitor cells (EPCs) (31, 32). Emerging evidences from cancer and autoimmune literature suggest that IL-17A contributes to the neo-angiogenesis and perpetuates inflammatory responses (25, 33), but there are few reports investigating the role of IL-17A in the pathogenesis of endometriosis. Here we show that endometriotic lesions are capable of producing variable amounts of IL-17A, depending on the disease severity, and that systemic concentrations of IL-17A drop significantly in endometriosis patients after surgical removal of endometriotic lesions. In addition, we document the effects of recombinant IL-17A on the induction of pro-angiogenic, chemotactic, and growth promoting cytokines from EECCs, Ishikawa cells and HUVECs.
Materials and Methods
Ethics approval for human samples
Human eutopic endometrial, ectopic endometriotic tissue samples and plasma samples from endometriosis patients and normal eutopic endometrium and plasma samples from women without known pathologies were collected and stored after informed consent using approved protocols by the Institutional Review Committees at Greenville Health System, Greenville, SC, USA, University of North Carolina, Chapel Hill, NC, USA, and Ottawa General Hospital, Ottawa, ON, Canada. Normal endometrial tissue and ectopic endometriotic tissue sections embedded in paraffin were obtained from the Kingston General Hospital, Kingston, ON, Canada with informed consent from the patients. Ethics approval for this study was provided by the Health Sciences Research Ethics Board, Queen’s University, Kingston, ON, Canada.
Human endometrium, endometriosis and plasma samples
Human eutopic endometrium, ectopic lesion tissue samples and plasma samples were obtained from Greenville Health Systems, Greenville, SC, USA after informed consent from patients between ages of 21 and 39 with confirmed endometriosis at the time of laparoscopic surgery. Patients received no hormonal therapy for minimal of 3 months prior to laparoscopic surgery, and for the duration of participation in the study. Patient characteristics including age, BMI, parity, and stage of endometriosis are provided in Table 1. Eutopic endometrium samples from patients were obtained by Pipelle sampling and ectopic lesions were obtained during laparoscopic surgery by excision. Patients were consented for additional blood draws at the 2 week post-op visit and at 3 months. Upon collection, samples were snap-frozen in liquid nitrogen and stored in −80°C. All plasma samples obtained from the patients and healthy women were separated from peripheral blood and stored in −80°C.
Table 1.
Endometriosis study patients and healthy control subject characteristics
| Parameters | Endometriosis | Control | |
|---|---|---|---|
| Number of patients participated in study | n = 24a | n = 10 | |
| Age (years) | 26.7 ± 7.6 | 27.3 ± 5.5 | |
| BMI (kg/m2) | 25.3 ± 6.1 | 22.5 ± 2.4 | |
| Parity | 25%b | 20%c | |
| Stage of endometriosis | |||
| Stages I–II | n = 16 | ||
| Stages III–IV | n = 8 | ||
patients on oral contraceptive therapy, progestins, gonadotrophin releasing hormone agonist/antagonist, aromatase inhibitors, or any other medications used towards the management of endometriosis during the study were excluded.
6 out of 24 endometriosis patients were parity 1 ; all other patients were nulliparous.
2 out of 10 control subjects were parity 1; all other control subjects were nulliparous.
± Standard deviation
Multiplex cytokine assay
To assess the concentration of IL-17A in human samples, a Bioplex Pro human cytokine 27-plex assay (M50-0KCAF0Y, Bio-Rad Laboratories, Mississauga, Canada) was conducted on eutopic endometrial and ectopic endometriotic tissue plasma and peritoneal fluid samples from women with endometriosis as per kit instructions. Approximately 20 mg of endometriotic tissue samples or normal endometrial samples were placed in 1.5 mL microcentrifuge tubes containing protease inhibitor (1ul/0.01g of tissue, Sigma-Aldrich, St. Louis, MO, USA) with Tissue Extraction Reagent I (100ul/0.01g of tissue, Invitrogen Corp., CA, USA). The tissue was homogenized using a rotor-stator homogenizer on ice for 1 minute and then centrifuged at 18,000 rpm for 15 min at 4°C. The supernatant was collected, and protein concentration measured using Pierce BCA Protein Assay Kit as per kit protocol (Pierce Biotechnology, IL, USA). The samples were normalized and stored at −80°C until later assessment. Plasma samples from healthy subjects were also included as control for the plasma samples from endometriosis patients. Briefly, the 96-well cell culture plate was coated with assay buffer and magnetic beads, followed by two wash steps using the wash buffer. Samples, blanks, and diluted standards were transferred onto the 96-well plate. The plate was then incubated in the dark at room temperature on shaker for 30 minutes. After three washes using the wash buffer, detection antibody was added to the plate for 30 minutes followed by a wash step. The plate was incubated with Streptavidin-Phycoerythrine for additional 10 minutes. Finally, the plate was washed before analysis using Bioplex 2000 Suspension Array System (Bio-Rad Laboratories, Mississauga, ON, Canada).
IL-17A Immunohistochemistry on matched eutopic endometrium and ectopic lesions
Immunohistochemistry was performed on paraffin embedded sections of matched eutopic endometrium and ectopic lesion samples obtained from patients. The slides with tissue sections cut at 5µm were deparaffinized in Cytrisolv (Fisher Scientific, Ottawa, ON, Canada) and subsequent re-hydration in decreasing gradients of ethanol. Heat-induced antigen retrieval was conducted using sodium citrate buffer (0.01M, pH 6.0) heated to 95°C in a water bath. Endogenous peroxide activity was blocked using 3% H2O2, followed by incubation with 1% BSA to block non-specific binding. Sections were incubated with anti-human IL-17A rabbit polyclonal antibody (1:250 in 1% BSA/PBS, ab79056, Abcam, Inc, Cambridge, MA, USA) or isotype antibody as a negative control. After overnight incubation in a humidified chamber at 4°C, sections were incubated with biotinylated secondary polyclonal goat anti-rabbit IgG antibody (1:500, Dako, Glostrup, Denmark), stained with DAB chromogen (Dako, Glostrup, Denmark) then counter stained with Harris hematoxylin (Fisher Scientific, Ottawa, ON, Canada). The sections were dehydrated in increasing gradients of ethanol and Citrisolv, coversliped with Permount mounting media (Fisher Scientific, Ottawa, ON, Canada), then viewed under the microscope.
Cell culture
Endometrial epithelial carcinoma cells (EECCs, CRL-1671, ATCC, VA, USA) and HUVECs (200-05f, Cell applications Inc., San Diego, CA, USA), and Ishikawa Cells (99040201-1VL, Sigma-Aldrich, St. Louis, MO, USA) were incubated in a standard cell incubator at 37°C with 5% CO2. EECCs and Ishikawa cells were maintained in DMEM supplemented with 10% FBS and 1% penicillin and streptomycin (Sigma-Aldrich, St. Louis, MO, USA). HUVECs were maintained in All-in-one ready to use Endothelial Cell Growth Medium (211–500, Cell Applications, Inc., San Diego, CA, USA). All cell lines were grown in T75 cell culture flasks (Corning Inc., NY, USA) up to 70–80% confluence prior to experimental use.
WST-1 proliferation assay
EECCs and HUVECs were harvested with Trypsin-EDTA and seeded onto a 96-well tissue culture plate (Sarstedt, Inc. Newton, NC, USA) at 1.25×104 cells/well, followed by recombinant IL-17A stimulation at different concentrations (1, 5, 25,50, 100 ng/ml, R&D Systems, MN, USA) in triplicates. PBS was used as a control. Post 24 hour incubation, WST-1 cell proliferation reagent (Roche Diagnostics, Laval, QC, Canada) was added to each well for additional 2 hours at 37°C. Using spectrophotometer, absorbance at 450nm and 690 nm were measured. Optical density was calculated by subtracting absorbance at 690nm from 450nm.
Propidium Iodide Cell Cycle analysis
EECCs were plated onto a 6-well plate (Sarstedt, Inc. Newton, NC, USA) at 5×105 cells/well and were incubated with different concentrations of IL-17A in triplicates (25, 50, 100ng/ml, R&D systems, MN, USA) and PBS control for 24 hours at 37°C with 5% CO2. Post treatment, cells harvested from the plate, pelleted and were fixed in 70% ethanol at 4°C overnight. Post fixation and centrifugation to pellet the cells, 1ml of propidium iodide (0.04mg/ml, BioShop Canada Inc. Burlington, ON, Canada) and RNase A (0.625mg/ml, Sigma-Aldrich, MO, USA) were added to each condition and incubated for 3 hours at 4°C in the dark. Beckman Coulter Cytomics FC500 (Beckman Coulter Inc., Mississauga, ON, Canada) was used to conduct single-color analysis.
Flow cytometric analysis for IL-17RA
After removal of the growth media from the flask, EECCs and HUVECs were harvested using Trypsin-EDTA, pelleted and washed with cell staining buffer (1% BSA, 0.1% sodium azide in PBS). Approximately 1×105 cells were incubated with either mouse anti-human IL-17RA antibody conjugated with PE (1:50, FAB177P, R&D systems, MN, USA) or Mouse IgG1 isotype control conjugated with PE (1:100, IC002P, R&D systems, MN, USA) for 30 minutes in room temperature. Cells were fixed with ice cold 2% Paraformaldehyde in PBS for 15 minutes and were kept at 4°C prior to conducting flow cytometric analysis (Beckman Coulter Cytomics FC500, Beckman Coulter Inc., Mississauga, ON, Canada).
Cell culture supernatant cytokine analysis from EECCs, Ishikawa cells, and HUVECs treated with IL-17A
EECCs and Ishikawa cells were seeded at 5×105 cells/well onto 6 well plate in triplicate (Sarstedt, Inc. Newton, NC, USA) and stimulated with different concentrations of IL-17A (10, 25, 50, and 100ng/ml, R&D Systems, MN, USA) and PBS as control. HUVECs were seeded at 1×105 cells/well onto 6 well plate in triplicate (Sarstedt, Inc. Newton, NC, USA) and were incubated with different concentrations of IL-17A (5 and 50 ng/ml, R&D Systems, MN, USA) and PBS as control. The cells were incubated for 24 hours in a standard cell culture incubator at 37°C with 5% CO2. The conditioned media was collected and were analyzed using Human multiplex cytokine analysis (Eve Technologies Corporation, Calgary, AL, Canada).
Endothelial cell tubulogenesis assay
Tubulogenesis assay was conducted as per kit protocol (CBA-200, Cell applications Inc., San Diego, CA, USA). HUVECs grown to 70–80% confluence in T75 cell culture flask (Corning Inc., Corning, NY, USA) were trypsinized, pelleted and seeded onto matrigel coated ibidi µ-angiogenesis slide (Ibidi USA Inc., WI, USA) at a density of 5×103 cells/well with a complete medium containing different concentrations of IL-17A (1, 5, 50 ng/ml, R&D Systems, MN, USA) in triplicate. VEGF (50ng/ml, R&D Systems, MN, USA) and PBS was used as a positive and negative control respectively.
Statistical analysis
All statistical analysis was performed using GraphPad Prism 6.0. Ordinary one-way ANOVA with Tukey post-hoc test was used to analyze the results of WST-1 proliferation assay, supernatant cytokine analysis and tubulogenesis assay. Unpaired t-test was used to analyze the results of human tissue and plasma data and flow cytometric data. Data are represented as mean ± SD, unless otherwise stated in the figure. Values of p≤0.05 were considered statistically significant.
Results
IL-17A concentration in matched tissue sample and plasma sample from women with endometriosis is variable compared to healthy controls
In the present study, we evaluated the concentration of IL-17A in tissues and plasma samples from patients with or without endometriosis (Fig. 1A–C). Patients were further stratified into Stage I, II, III or IV of endometriosis as per the guidelines from American Society for Reproductive Medicine. We further grouped Stage I and II patient into Early and Stage III and IV into Advanced disease categories. Although statistically non-significant, we observed a trend of increasing IL-17A concentration in the ectopic lesions compared to the matched eutopic endometrium obtained from same patients (Fig. 1A). In the plasma samples, IL-17A concentration was significantly increased in women with endometriosis compared to healthy controls (Fig. 1B). When the matched ectopic lesions and eutopic endometrium were stratified by disease severity, we observed persistent increase in IL-17A in ectopic lesion samples compared to eutopic endometrium across severity (Fig. 1C). Note that the concentration of IL-17A from normal endometrial tissues of disease-free women was less than 10pg/mL (n=4, 3.13±1.92 pg/mL, data not shown) and the concentration of IL-17A in the peritoneal fluid was negligible (n=24, data not shown).
Figure 1.
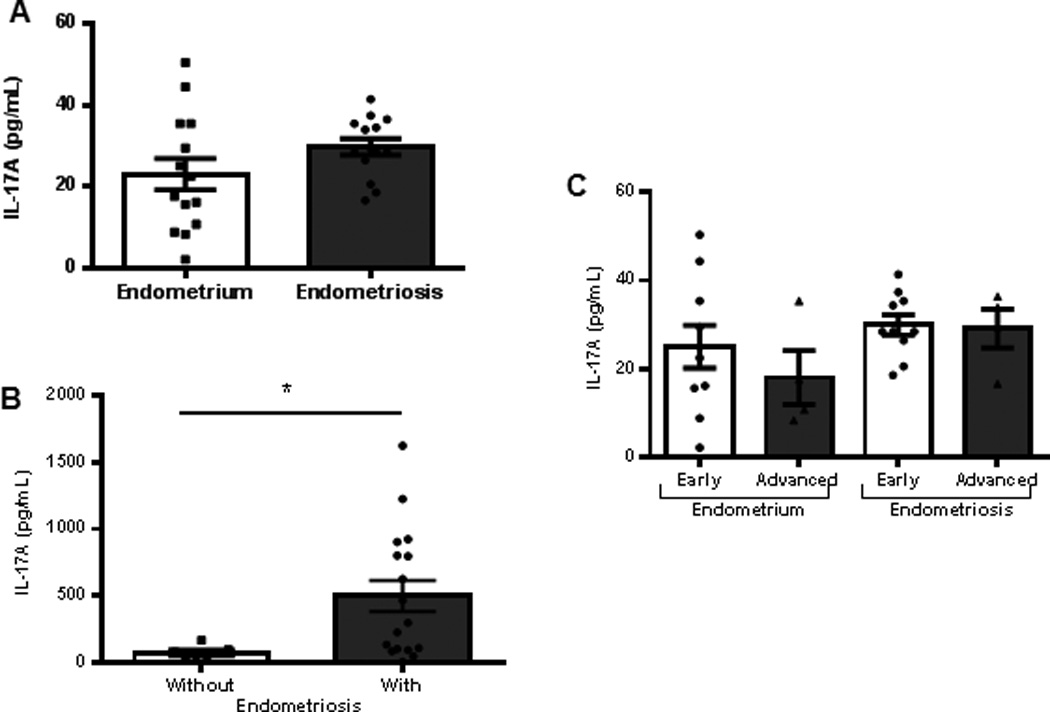
IL-17A expression in tissue and plasma samples. (A) Matched eutopic endometrium (n=14, 23.0±3.84pg/ml) and ectopic lesions (n=14, 29.77±1.97pg/ml) (B) Plasma samples from women without (n=7, 72.90±18.94pg/ml) and with (n=17, 499.4±116.4pg/ml) endometriosis. IL-17A levels were significantly higher in women with endometriosis compared to healthy controls (*p<0.05). (C) IL-17A concentration in matched eutopic endometrium and ectopic lesions distributed by disease severity following ASRM staging criteria (early denotes Stages I-II, and advanced denotes Stages III-IV). All data are represented as mean ± SEM.
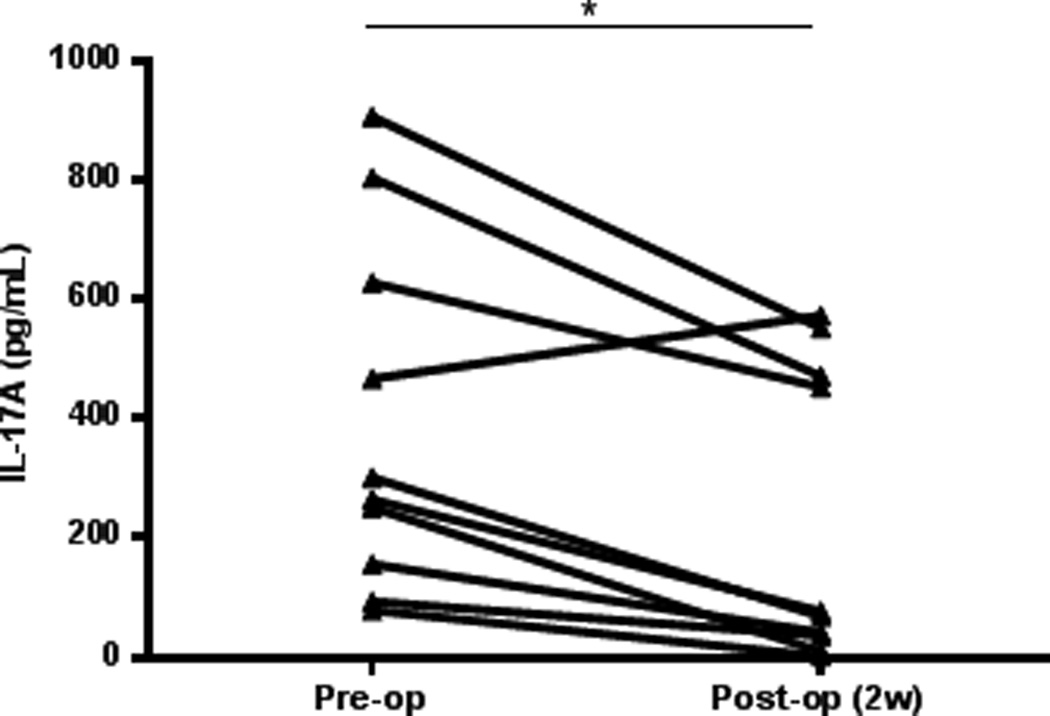
The plasma concentration of IL-17A diminishes after laparoscopic lesion removal
The systemic concentration of IL-17A in women with endometriosis significantly declined after surgical removal of lesions, with mean IL-17A plasma concentrations of 395.1±93.20 pg/ml pre-surgery and 228.6±78.33 pg/ml, 2 weeks after surgery (Fig. 2).
Figure 2.
IL-17A concentration in plasma of women (n=10) undergoing laparoscopy surgery for the removal of endometriosis. IL-17A concentration was measured from the patient prior to undergoing surgery and 2 weeks post-surgical visit to the clinic. The concentration of IL-17A diminishes significantly in the peripheral blood of women with endometriosis after lesion is removed (*p=0.0016).
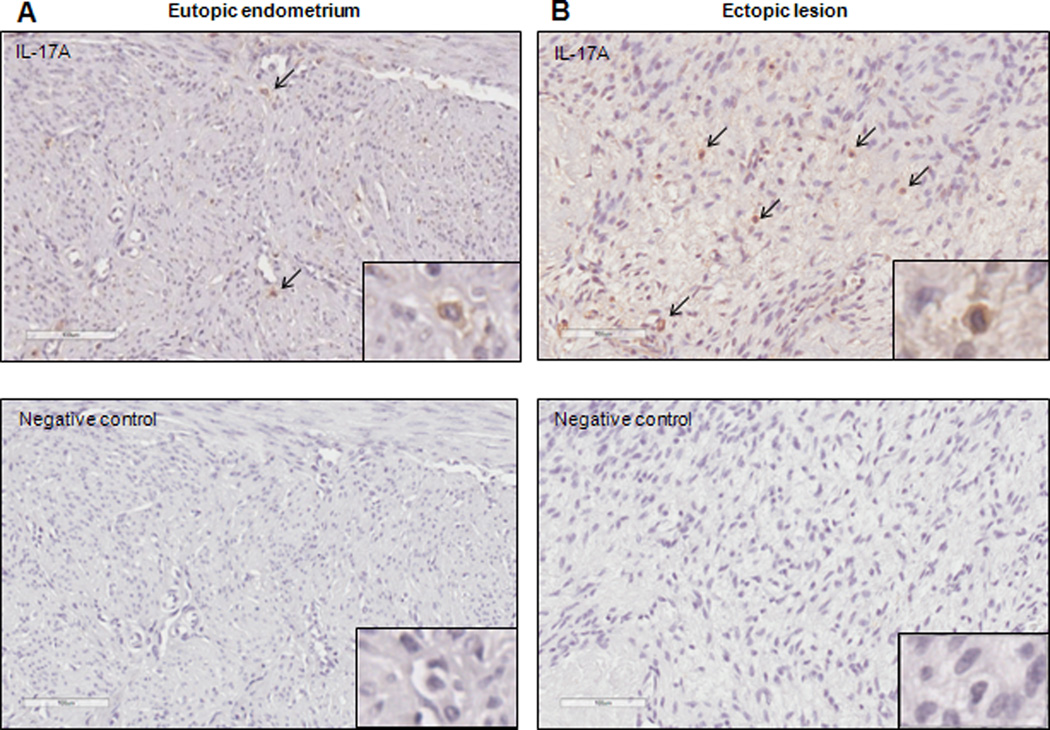
Immunolocalization of IL-17A in human eutopic endometrium and ectopic endometriotic lesions
In the extant literature, there are no reports of IL-17A immunolocalization in the eutopic endometrium or peritoneal endometriosis lesions. A single study showed localization of IL-17A positive cells in the stroma of an ovarian endometrioma lesion; however, expression in peritoneal lesions or matched eutopic endometrium was not examined (28). Here, we show the localization of IL-17A positive cells within the stroma and surrounding the vasculature in matched eutopic endometrium and ectopic lesion samples from women with endometriosis (Fig. 3A and B). Because of the heterogeneous nature of the ectopic lesions, we were unable to concretely conclude whether IL-17A staining was increased in ectopic lesions as compared to matched eutopic endometrium from same patients.
Figure 3.
IL-17A positive cells are detected in the matched eutopic (A) and ectopic lesion (B) samples from women with endometriosis. Immunohistochemistry images are representative of 5 matched tissue samples immunostained with anti-human IL-17A. 200× magnification with digitally magnified inlet; scale bar represents 100µm.
Interleukin-17 Receptor A is expressed in EECCs and HUVECs
IL-17RA, the primary receptor for IL-17A is reported to be ubiquitously expressed in different cell types in mouse (34) and in human (35). Before conducting functional assays with IL-17A, we first wanted to establish whether IL-17RA is present on EECCs and HUVECs to rationalize their responsiveness to IL-17A. Both EECCs and HUVECs were incubated with anti-IL-17RA antibody conjugated with PE at 4°C overnight. Flow cytometric analysis revealed that EECCs (Fig. 4A) and HUVECs (Fig. 4B) indeed express IL-17RA on the cell surface, which suggests their capacity to specifically respond to IL-17A stimulation.
Figure 4.
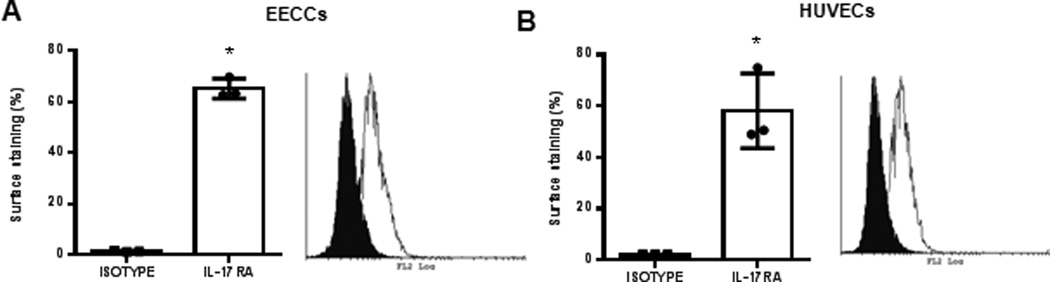
IL-17RA is expressed on EECCs and HUVECs. EECCs (A) and HUVECs (B) were stained with either PE conjugated mouse anti-human IL-17RA or PE conjugated Mouse IgG isotype control in room temperature. On average 65.2±3.9% of EECCs stained for IL-17RA whereas 58.1±14.6% cell surface staining was seen for HUVECs. Representative of three separate experiments. *p<0.05 compared to isotype.
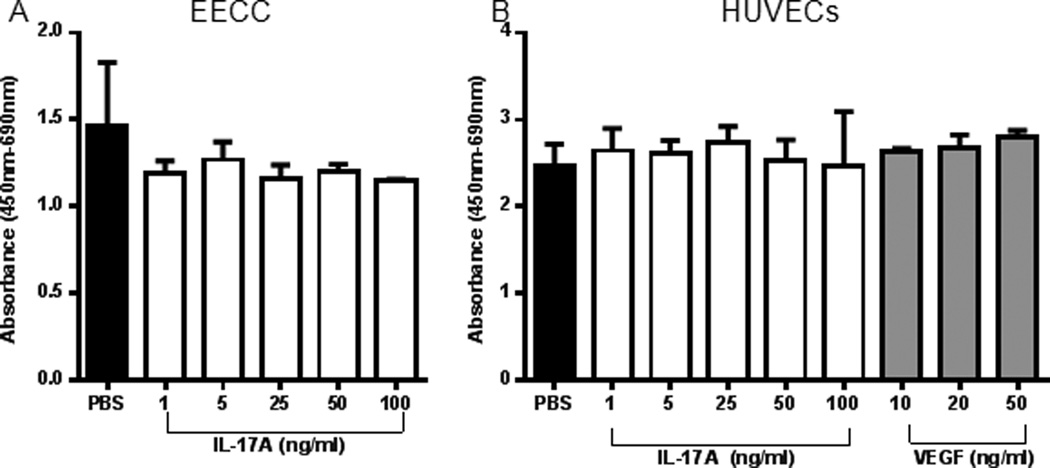
IL-17A does not induce proliferation in EECCs or HUVECs
To investigate whether IL-17A exhibit mitotic effect on epithelial and endothelial cells, we incubated EECCs (Fig. 5A) and HUVECs (Fig. 5B) with different concentrations of recombinant IL-17A and PBS control. After 24 hours of incubation at 37°C, we did not observe any significant difference in proliferative capacity compared to the PBS treated wells on either cell line. Therefore, IL-17A does not directly induce mitosis on the EECCs or HUVECs in vitro. To determine whether recombinant IL-17A would induce apoptosis in EECCs, we performed propidium iodide (PI) flow cytometric assay to determine DNA abundance in EECCs treated with IL-17A (25, 50, 100ng/ml) and PBS control. We did not observe any differences between IL-17A treated and PBS control groups (data not shown). This further strengthens the notion that IL-17A may not directly have proliferative or apoptotic effects of epithelial and endothelial cells.
Figure 5.
WST-1 proliferation assay indicate that IL-17A does not induce proliferation of EECCs and HUVECs in vitro. (A) EECCs were treated with different concentrations of IL-17A (1, 5, 25, 50 and 100 ng/ml) or PBS control to assess the effect of IL-17A on proliferation. (B) HUVECs were treated with different concentrations of IL-17A (1, 5, 25, 50 and 100 ng/ml) or PBS control to assess the effect of IL-17A on proliferation. VEGF (10, 20, 50 ng/ml) was used as a positive control. Three separate WST-1 proliferation assay were conducted on both (A) and (B) as per standard protocol.
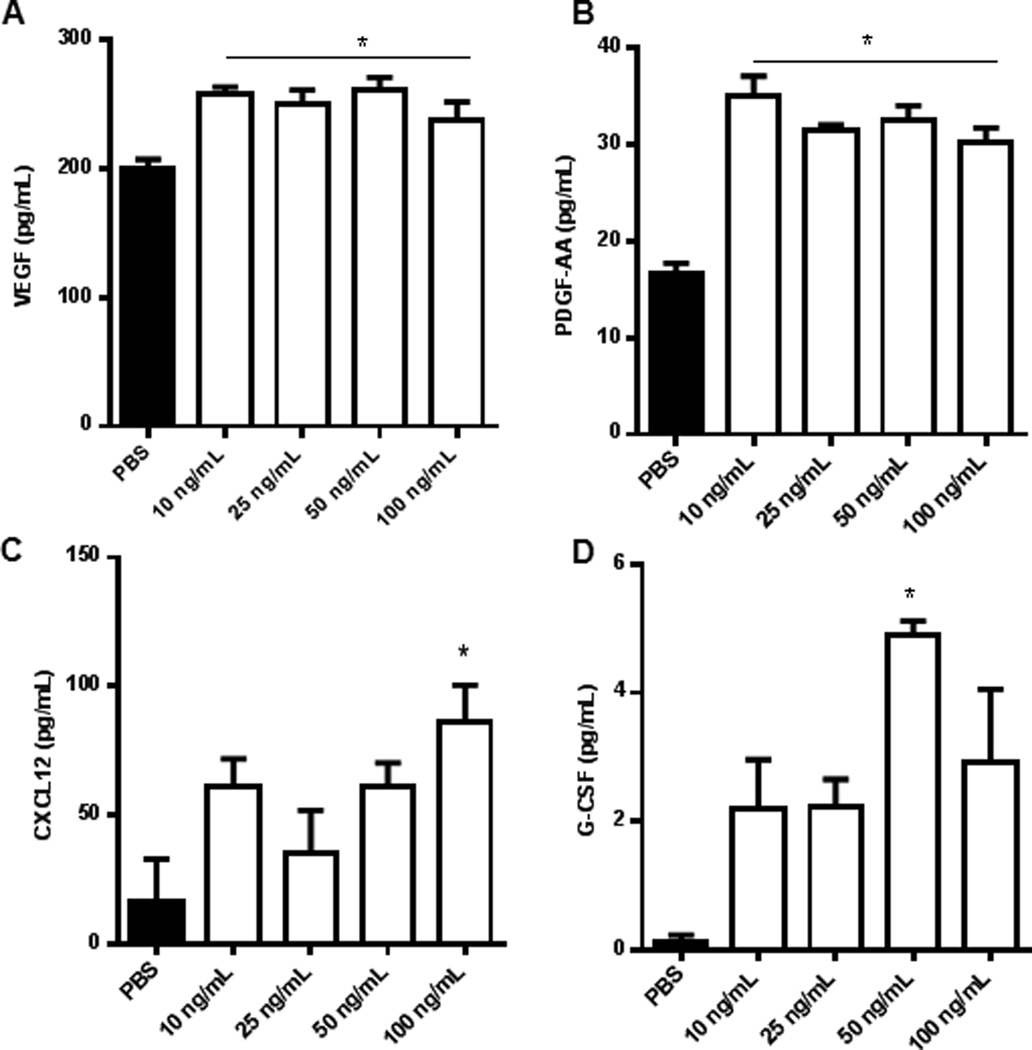
IL-17A induces the production of chemokine and angiogenic cytokines from EECCs
IL-17A is known to induce a variety of cytokines from different tissue types with pleiotropic downstream effects. We wanted to investigate the cytokine profile of EECCs when induced with varying concentration of IL-17A. Stimulation of EECCs with IL-17A (10, 25, 50, 100ng/ml) led to the significant increase in the production of angiogenic and chemotactic cytokines, namely VEGF, PDGF-AA, CXCL12 and G-CSF (Fig. 6A–D respectively). The cytokine profile suggests a potential involvement of IL-17A in mediating neo-angiogenesis and recruitment of lymphocytes and bone-marrow derived cells to the site of lesion development. In earlier studies, we (31) and others (32) showed that CXCL12 contributes to the recruitment of endothelial progenitor cells at the endometriotic lesions and aid neo-angiogenesis.
Figure 6.
IL-17A induces production of chemokine and angiogenic cytokines from EECCs. Endometrial epithelial carcinoma cells (EECCs) were plated onto a 96-well cell culture plate in triplicate at a density of 5×105cells/well and incubated with different concentrations of IL-17A (10, 25, 50, 100ng/mL) for 24 hours at 37°C with 5% CO2. The conditioned supernatants of EECCs were collected and screened for cytokine expression from which PDGF-AA, VEGF, CXCL12 and G-CSF showed statistical significance. *p < 0.05 compared with PBS.
IL-17A induces the production of pro-inflammatory cytokines and chemokines from HUVECs
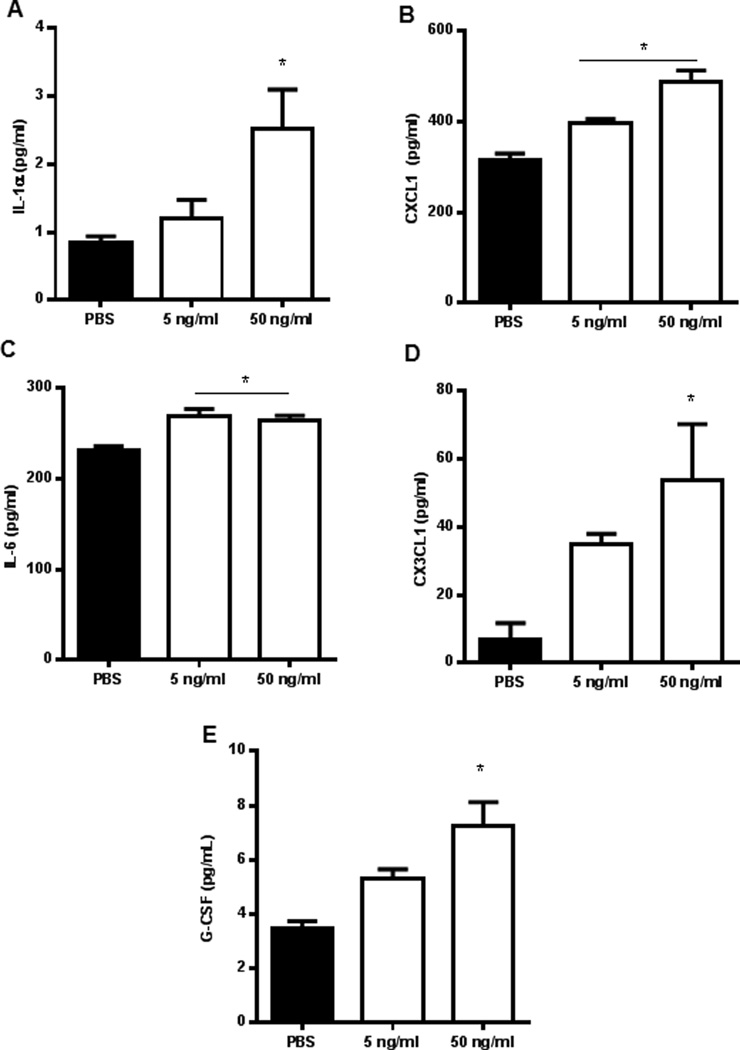
Similar to EECCs, different concentrations of IL-17A (5 and 50ng/ml) induced the production of pro-inflammatory cytokines and chemokines from HUVECs, namely IL-1α, CXCL1, IL-6 and CX3CL1 (Fig. 7A–D, respectively) in a dose dependent fashion. These cytokines are well known for their potent pro-inflammatory actions. This data suggests the ability of IL-17A to regulate the expression of pro-inflammatory, angiogenic cytokines and chemokines in the peritoneal environment.
Figure 7.
IL-17A induces the production of pro-inflammatory cytokines and chemokines from HUVECs in a dose dependent manner. HUVECs were plated onto a 6-well plate in triplicate at a density of 1×105 cells/plate and were incubated with different concentrations of IL-17A (5 and 50 ng/ml) for 24 hours in a standard cell culture incubator at 37°C with 5% CO2. Conditioned supernatants were collected and screened for cytokine expression from which IL-1α, CXCL1, IL-6, CX3CL1, and G-CSF (A-E, respectively) showed statistical significance. *p<0.05 compared with PBS.
IL-17A induces the production of pro-inflammatory cytokines from Ishikawa cells
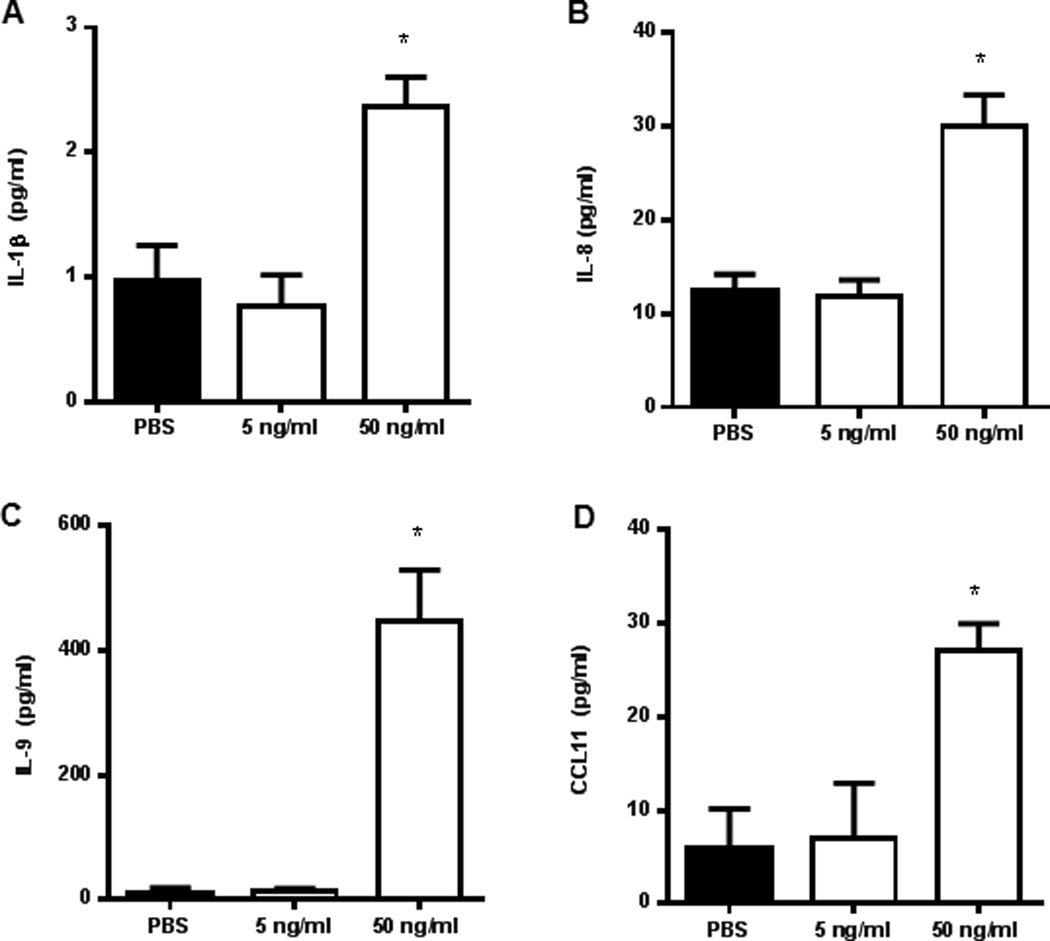
In the endometriosis literature, EECCs and Ishikawa cells have been widely used to understand molecular mechanisms involved in the pathogenesis of endometriosis. We wanted to establish how Ishikawa cells would respond to IL-17A stimulation. Here we report that stimulation of Ishikawa cells with different concentrations of IL-17A lead to increased expression of pro-inflammatory and chemotactic cytokines in a dose dependent manner, namely IL-1β, IL-8, IL-9, and CCL11. (Fig. 8A–D, respectively). It is well described in literature that IL-1β, IL-8, and CCL11 are increased in concentration in the peritoneal fluid of women with endometriosis and are thought to contribute to the pathogenesis of the disease.
Figure 8.
IL-17A induces production of chemokine, angiogenic and pro-inflammatory cytokines from Ishikawa cells. Ishikawa cells were plated onto a 96-well cell culture plate in triplicate at a density of 5×105cells/well and incubated with different concentrations of IL-17A (5 and 50 ng/mL) or PBS control for 24 hours at 37°C with 5% CO2. The conditioned supernatants of each treatment were collected and screened for cytokine expression from which IL-1β, IL-8, IL-9 and CCL11 showed statistical significance. *p < 0.05 compared with PBS.
IL-17A promotes tubulogenesis from HUVECs in a dose dependent fashion
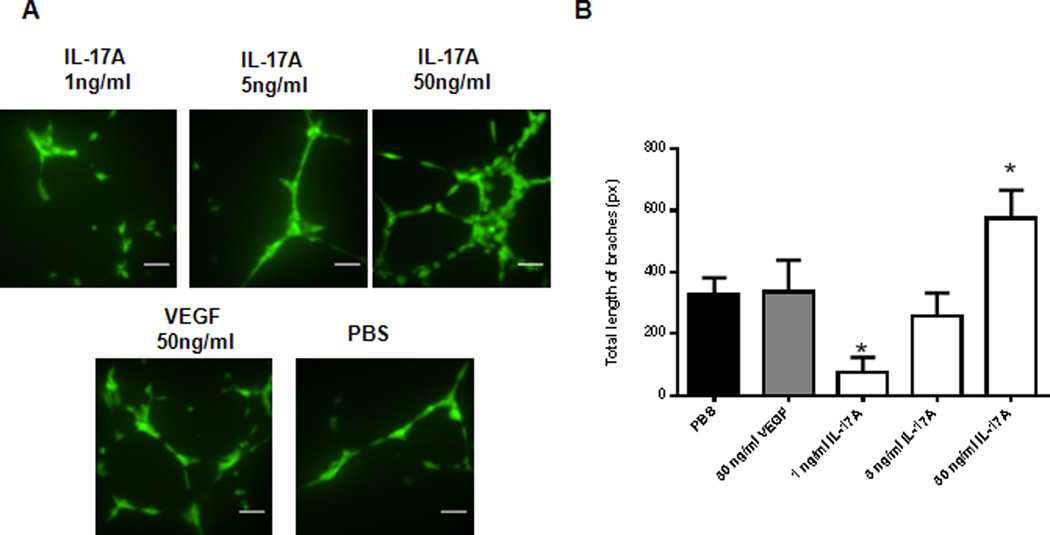
The endothelial tube formation assay is a fast, quantifiable method for measuring in vitro angiogenesis and is a standard method used to investigate the effects of an angiogenic stimulant or inhibitor on endothelial cells. To investigate whether IL-17A can induce direct tubulogenesis, HUVECs seeded on a coat of matrigel were treated with different concentrations of IL-17A in complete endothelial cell growth medium for 16 hours at 37°C prior to the analysis of tubulogenesis, unlike other methods where endothelial cells are serum starved prior to tubulogenesis assay (26). Our data showed that IL-17A induces tubulogenesis of HUVECs on matrigel in a dose dependent manner (Fig. 9A and B), suggesting that IL-17A is capable of inducing direct tubulogenesis of endothelial cells in vitro.
Figure 9.
IL-17A promotes in vitro tubulogenesis in HUVECs. (A) HUVECs were plated on Matrigel at 5×103 cells/well using Ibidi µ-slide angiogenesis plate (Cat. #81506) in triplicate per treatment. VEGF (50ng/ml) and PBS were used as a positive and negative control, respectively. Scale bar represents 10µm. 150× magnification using a confocal microscope (Quorum Wave Effects Spinning Disc Confocal, Queen’s University Cancer Research Institute Imaging Facility) (B) Total length of branches in the field of image was measured using ImageJ Angiogenesis Analyzer Macro with HUVECs phase contrast setting. *p<0.05 compared with PBS.
Discussion
Interleukin (IL)-17A has been implicated in several chronic, inflammatory and autoimmune disorders; however its association with the pathogenesis of endometriosis has not previously been well described. We provide the first evidence that endometriotic lesions produce IL-17A protein in variable amounts depending on the stage of the disease. Traditionally, IL-17A was thought to be produced only by Th17 cells; however, recent reports suggest that variety of cell types produce IL-17A including stromal cells, fibroblasts, and endothelial cells (34). Immunohistochemical analysis provides further evidence that IL-17A is expressed by the endometriotic lesions, but the complexity and heterogeneous nature of the endometriotic lesions precludes identification of the specific cell types. Strikingly, we demonstrated significant decline in plasma concentration of IL-17A after surgical removal of endometriotic lesions. These findings strongly suggest that IL-17A may be a contributory factor to the inflammatory peritoneal milieu associated with endometriosis. The association between the removal of the lesion and decrease in IL-17A further suggest the role of the ectopic lesion as a potential reservoir of IL-17A, or the removal of the lesion simply leads to the diminishment in the pro-inflammatory environment, where IL-17A is a component that mediates the pro-inflammatory status. It is likely that, with the removal of the lesion, the tissue resident Th17 cells, and other potential producers of IL-17A are also removed, thereby contributing to the significantly lowered concentration of IL-17A detected in the peripheral blood.
The source of IL-17A can be speculated by analyzing the data available on the peritoneal fluid of women with endometriosis. If the main source of IL-17A is from peritoneal fluid resident immune cells, the concentration of IL-17A in the peritoneal fluid will be elevated. Zhang et al. (29) documented elevated concentration of IL-17A in the peritoneal fluid in minimal or mild endometriosis as compared to severe disease, ranging between 5–6 pg/ml. This study did not find a significant difference in the peritoneal fluid concentration of IL-17A between women with endometriosis and without disease. On the contrary, we could not detect measurable levels of IL-17A in the peritoneal fluid from women with endometriosis (n=24, data not shown). Since we show that the removal of the lesion led to the significant decrease of IL-17A in the plasma, the effect of removal likely involves changes within the systemic immune system. It is possible that, unlike other proinflammatory cytokines, IL-17A is primarily produced by tissue resident immune cells, and as such may not be detectable in the peritoneal fluid. To clarify these findings, we need to establish whether IL-17A positive, tissue-resident immune cells in women with endometriosis are indeed the source of IL-17A in this disease.
Endometriosis is a disease mediated by inflammatory and angiogenic peritoneal environment. Using cell lines well established in the endometriosis literature, we document the unique cytokine signatures induced by IL-17A in EECCs, Ishikawa cells and HUVECs. In particular, IL-17A induced production of chemotactic and angiogenic factors including G-CSF, VEGF, CXCL12, and IL-8 from EECCs and Ishikawa cells. In addition, IL-17A induced the production of chemotactic and pro-inflammatory cytokines such as CXCL1, CX3CL1 and IL-6 from HUVECs. Taken together, our data suggests that IL-17A may be involved in the orchestration of a paracrine network of cytokines between the adjacent cells that leads to the promotion of angiogenesis and inflammation in the peritoneal cavity. Specifically, our data suggests that IL-17A has the potential to enhance vascularization of the lesion through VEGF and IL-8 mediated pathways. Evidence for de novo vasculogenesis at endometriosis lesions has been previously suggested by a report of the recruitment of EPCs to the lesion site via a CXCL12 mediated pathway (36).
IL-17A may also play a crucial role in the promotion of inflammation via the recruitment of immune cells by inducing the production of chemokines such as G-CSF, CCL11, CXCL1 and CX3CL1 from the endometriotic lesion. For instance, IL-17A-induced CX3CL1 may play a critical role in the mobilization of pro-inflammatory monocytes and other immune cells into the lesion. CX3CL1 is both an adhesion molecule and chemotactic cytokine for T cells and monocytes that acts by adhering and immobilizing the cells to the endothelial cell surface (37, 38), and whose production by HUVECs can be induced by IFN-γ (39). Thus, IL-17A may not only initiate the process of inflammation in endometriosis, but also sustain it through the indirect mobilization of pro-inflammatory immune cells by inducing the production CX3CL1 from the endothelial cells of the lesion. Taken together, the data suggests a potential therapeutic effect of IL-17A blockade in endometriosis. Decoding the paracrine network established between different cell types in the lesion will enhance our understanding of the mechanisms employed by IL-17A in establishing the pro-inflammatory and pro-angiogenic environment in endometriosis.
Current research suggests inherent molecular differences in eutopic endometrium of women with endometriosis that allows the menstrual exudate to escape immunesurveillance and develop into ectopic foci in the pelvic cavity. In addition, the immune system of women with endometriosis behaves curiously in the presence of endometrial fragments. As such, the pathogenesis of endometriosis may be two-fold: women with endometriosis have endometrial cell dysfunction that stimulate aberrant innate and adaptive immune responses towards the endometrial fragments found in ectopic locations, allowing for the fragments to survive and implant to grow into endometriosis. These immune cells are not only producing increased amount of pro-inflammatory and growth promoting cytokines, but also exhibit diminished cytotoxicity/adaptive responses towards the fragments. Such immune cell activity is reflected in the inflammatory milieu known to be associated with the peritoneal environment of endometriosis. We hypothesize that the immune cells would be the major producer of IL-17A, which trigger other cells in the vicinity that express its receptor to make cytokine that are typically found in endometriosis environment.
In literature, IL-17A is shown to promote direct endothelial cell tubulogenesis in vitro (26, 40). Here, we also show direct effect of IL-17A in promoting tubulogenesis of HUVECs. Typically other studies use serum starved media on matrigel to study the angiogenic effect of IL-17A. In this study, we opted to use complete growth media supplemented with growth factors and FBS. The rational for using the complete endothelial cell growth media for our experiment is to achieve the similar angiogenic environment of peritoneal fluid that bathes endometriotic lesions in the peritoneal cavity. This allows us to elucidate if the presence of IL-17A, in addition to other growth factors, would elicit an additive effect in driving tubulogenesis. This method also allows our data to be translatable to in vivo situation as endometriotic lesions are exposed to growth promoting cytokines in the peritoneal fluid.
Overall, the limitation of the current study is inherent in endometriosis research in general. To conduct proper interpretation and comparison of data, the tissue samples between patients and controls must be matched in menstrual stage, disease stage, and age of the individual. In addition, the medical history of individuals and therapeutic regimens needs to be taken into account to consider the effect of estrogen and progesterone on the disease state. The analysis is further complicated by the complexity of endometriosis which comes in multiple stages and phenotypes. Furthermore, only old-world primate species, including humans, develop spontaneous endometriosis, increasing the complexity of using animal models. Finally, both the diagnosis and staging of endometriosis depends upon surgery, making it difficult to study and establish true control subjects. For all these reasons, understanding of the disease remains rudimentary and simple classifications lack biological uniformity. As such, wide variations between studies are observed, including cytokine concentrations in the peritoneal fluid. Despite these issues, this study showing the significant decrease in the plasma concentration of IL-17A post laparoscopic removal of the endometriotic lesion, coupled with the ability of IL-17A to induce a myriad of cytokines from stromal, epithelial and endothelial cells strongly suggests its potential contribution to endometriosis pathogenesis. Further research is required to identify the source and location of IL-17A in endometriosis to advance our understanding of the specific role(s) IL-17A may play in the pathogenesis of endometriosis.
Acknowledgments
Funding:
This research was supported with funds from Canadian Institutes of Health Research (CIHR 394924, CT) and National Institutes of Health (NIH R01-067721, SLY and BAL).
Disclosures
Dr. Sukhbir S. Singh is a speaker with Actavis, Abbvie, Bayer, and Hologic, and has research grants from Abbvie, Activas, and Bayer.
References
- 1.Murphy AA. Clinical Aspects of Endometriosis. Ann N Y Acad Sci. 2002;955:1–10. doi: 10.1111/j.1749-6632.2002.tb02760.x. [DOI] [PubMed] [Google Scholar]
- 2.Vercellini P, Vigano P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol. 2014;10:261–275. doi: 10.1038/nrendo.2013.255. [DOI] [PubMed] [Google Scholar]
- 3.Edwards AK, Nakamura DS, Virani S, Wessels JM, Tayade C. Animal models for anti-angiogenic therapy in endometriosis. J Reprod Immunol. 2013;97:85–94. doi: 10.1016/j.jri.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 4.Laschke MW, Menger MD. Anti-angiogenic treatment strategies for the therapy of endometriosis. Hum Reprod Update. 2012;18:682–702. doi: 10.1093/humupd/dms026. [DOI] [PubMed] [Google Scholar]
- 5.Nap AW, Griffoen AW, Dunselman GA, Bouma-Ter Steege JC, Thijssen VL, Evers JL, Groothuis PG. Antiangiogenic therapy for endometriosis. J Clin Endocrinol Metab. 2004;89:1089–1095. doi: 10.1210/jc.2003-031406. [DOI] [PubMed] [Google Scholar]
- 6.Sampson JA. The development of the implantation theory for the origin of peritoneal endometriosis. Am J Obstet Gynec. 1940;40:549–557. [Google Scholar]
- 7.Herington JL, Bruner-Tran KL, Lucas JA, Osteen KG. Immune interactions in endometriosis. Expert Rev Clin Immunol. 2011;7:611–626. doi: 10.1586/eci.11.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin Y-J, Lai M-D, Lei H-Y, Wing L-YC. Neutrophils and macrophages promote angiogenesis in the early stage of endometriosis in a mouse model. Endocrinology. 2006;147:1278–1286. doi: 10.1210/en.2005-0790. [DOI] [PubMed] [Google Scholar]
- 9.Hornung D, Bentzien F, Wallwiener D, Kiesel L, Taylor RN. Chemokine bioactivity of RANTES in endometriotic and normal endometrial stromal cells and peritoneal fluid. Mol Hum Reprod. 2001;7:163–168. doi: 10.1093/molehr/7.2.163. [DOI] [PubMed] [Google Scholar]
- 10.Mclaren J, Prentice A, Charnock-Jones DS, Millican SA, Muller KH, Sharkey AM, Smith SK. Vascular endothelial growth factor is produced by peritoneal fluid macrophages in endometriosis and is regulated by ovarian steroids. J Clin Invest. 1996;98:482–489. doi: 10.1172/JCI118815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schulke L, Berbic M, Manconi F, Tokushige N, Markham R, Fraser IS. Dendritic cell population in the eutopic and ectopic endometrium of women with endometriosis. Hum Reprod. 2009;24:1695–1703. doi: 10.1093/humrep/dep071. [DOI] [PubMed] [Google Scholar]
- 12.Hirata T, Osuga Y, Takamura M, Kodama A, Hirota Y, Koga K, Yoshino O, Harada M, Takemura Y, Yano T, Taketani Y. Recruitment of CCR6-expressing Th17 cells by CCL20 secreted from IL-1beta-, TNF-alpha, and IL-17A stimulated endometriotic stromal cells. Endocrinology. 2010;151:5468–5476. doi: 10.1210/en.2010-0398. [DOI] [PubMed] [Google Scholar]
- 13.Gazvani R, Templeton A. Peritoneal environment, cytokines and angiogenesis in the pathophysiology of endometrioisis. Reproduction. 2002;123:217–226. doi: 10.1530/rep.0.1230217. [DOI] [PubMed] [Google Scholar]
- 14.Berbic M, Fraser IS. Regulatory T cells and other leukocytes in the pathogenesis of endometriosis. J Reprod Immunol. 2011;88:149–155. doi: 10.1016/j.jri.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C, Pin JJ, Garrone P, Garcia E, Saeland S, Blanchard D, Gaillard C, Das Mahapatra B, Rouvier E, Golstein P, Banchereau J, Lebecque S. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183(6):2593–2603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song X, Qian Y. IL-17 family cytokines mediated signaling in the pathogenesis of inflammatory diseases. Cell Signal. 2013;25:2335–2347. doi: 10.1016/j.cellsig.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 17.Zou W, Restifo NP. Th17 cells in tumour immunity and immunotherapy. Nat Rev Immunol. 2011;10:248–256. doi: 10.1038/nri2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toy D, Kugler D, Wolfson M, Vanden Bos T, Gurgel J, Derry J, Tocker J, Peschon J. Cutting edge: interleukin 17 signals through a heteromeric receptor complex. J Immunol. 2006;177:36–39. doi: 10.4049/jimmunol.177.1.36. [DOI] [PubMed] [Google Scholar]
- 19.Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17 producing γδT cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31:321–330. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 20.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from γδT cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 21.Rei M, Goncalves-Sousa N, Lanca T, Thompson RG, Mensurado S, Balkwill FR, Kulbe H, Pennington DJ, Silva-Santos B. Murine CD27(−) Vγ6(+) γδ T cells producing IL-17A promote ovarian cancer growth via mobilization of protumor small peritoneal macrophages. Proc Natl Acad Sci U S A. 2014;111:E3562–E3570. doi: 10.1073/pnas.1403424111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Passos ST, Silver JS, O’Hara AC, Sehy D, Stumhofer JS, Hunter CA. IL-6 promotes NK cell production of IL-17 during toxoplasmosis. J Immunol. 2010;184:1776–1783. doi: 10.4049/jimmunol.0901843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le Jan S, Plee J, Vallerand D, Dupont A, Delanez E, Durlach A, Jackson PL, Blalock JE, Bernard P, Antonicelli F. Innate immune cell-produced IL-17 sustains inflammation in bullous Pemphigoid. J Invest Dermatol. 2014;134:2908–2917. doi: 10.1038/jid.2014.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heuber AJ, Asquith DL, Miller AM, Reilly J, Kerr S, Leipe J, Melendez AJ, McInnes IB. Mast cells express IL-17A in rheumatoid arthritis synovium. J Immunol. 2010;184:3336–3340. doi: 10.4049/jimmunol.0903566. [DOI] [PubMed] [Google Scholar]
- 25.Miossec P, Kolls JK. Targeting IL-17 and Th17 cells in chronic inflammation. Nat Rev Drug Discov. 2012;11:763–776. doi: 10.1038/nrd3794. [DOI] [PubMed] [Google Scholar]
- 26.Lu S, Hequan H, Gao R, Gao X, Xu F, Wang Q, Lu G, Xia D, Zhou J. IL-17A, but not IL-17F, is indispensable for airway vascular remodeling induced by exaggerated Th17 cell responses in prolonged ovalbumin-challenged mice. J Immunol. 2015;194:3557–3566. doi: 10.4049/jimmunol.1400829. [DOI] [PubMed] [Google Scholar]
- 27.Chung AS, Wu X, Zhuang G, Ngu H, Kasman I, Zhang J, Vernes JM, Jiang Z, Meng YG, Peale FV, Ouyang W, Ferrara N. An interleukin-17 mediated paracrine network promotes tumor resistance to anti-angiogenic therapy. Nat Med. 2013;19:1114–1123. doi: 10.1038/nm.3291. [DOI] [PubMed] [Google Scholar]
- 28.Hirata T, Osuga Y, Hamasaki K, Yoshino O, Ito M, Hasegawa A, Takemura Y, Hirota Y, Nose E, Morimoto C, Harada M, Koga K, Tajima T, Saito S, Yano T, Taketani Y. Interleukin (IL)-17A stimulates IL-8 secretion, cyclooxygenase-2 expression, and cell proliferation of endometriotic stromal cells. Endocrinology. 2008;149:1260–1267. doi: 10.1210/en.2007-0749. [DOI] [PubMed] [Google Scholar]
- 29.Zhang X, Xu H, Lin J, Qian Y, Deng L. BJOG peritoneal fluid concentration of IL-17 correlate with the severity of endometriosis and infertility of this disorder. BJOG. 2005;112:1153–1155. doi: 10.1111/j.1471-0528.2005.00639.x. [DOI] [PubMed] [Google Scholar]
- 30.Donnez J, Smoes P, Gillerot S, Casanas-Roux F, Nisolle M. Vascular endothelial growth factor (VEGF) in endometriosis. Human Repro. 1998;12:1686–1690. doi: 10.1093/humrep/13.6.1686. [DOI] [PubMed] [Google Scholar]
- 31.Viranis S, Edwards AK, Thomas R, Childs T, Tayade C. Blocking of stromal cell-derived factor-1 reduces neoangiogenesis in human endometriosis lesions in a mouse model. Am J Reprod Immunol. 2013;70:386–397. doi: 10.1111/aji.12134. [DOI] [PubMed] [Google Scholar]
- 32.Laschke MW, Giebels C, Nickels RM, Sheuber C, Menger MD. Endotheilal progenitor cells contribute to the vascularization of endometriotic lesions. Am J Pathol. 2011;178:442–450. doi: 10.1016/j.ajpath.2010.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roy LD, Sahraei M, Schettini JL, Gruber HE, Besmer DM, Mukherjee P. Systemic neutralization of IL-17A significantly reduces breast cancer associated metastasis in arthritic mice in reducing CXCL12/SDF-1 expression in the metastatic niches. BMC Cancer. 2014;13:225. doi: 10.1186/1471-2407-14-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fossiez F, Banchereau J, Murray R, Van Kooten C, Garrone P, Lebecque S. Interleukin-17. Int Rev Immunol. 1998;16(5–6):541–551. doi: 10.3109/08830189809043008. [DOI] [PubMed] [Google Scholar]
- 35.Yao Z, Spriggs MK, Derry JM, Strockbine L, Park LS, VandenBos T, Zappone JD, Painter SL, Armitage RJ. Molecular characterization of the human interleukin (IL)-17 receptor. Cytokine. 1997;9:794–800. doi: 10.1006/cyto.1997.0240. [DOI] [PubMed] [Google Scholar]
- 36.Jin DK, Shido K, Kopp HG, Petit I, Shmelkov SV, Young LM, Hooper AT, Amano H, Avecilla ST, Heissig B, Hattori K, Zhang F, Hicklin DJ, Wu Y, Zhu Z, Dunn A, Salari H, Werb Z, Hackett NR, Crystal RG, Lyden D, Rafii S. Cytokine mediated deployment of SDF-1 induces revascularization through recruitment of CXCR4+ hemangiocytes. Nat Med. 2006;12:557–567. doi: 10.1038/nm1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Imaizumi T, Yoshida H, Satoh K. Regulation of CX3CL1/Fractalkine expression in endothelial cells. J Atheroscler Throm. 2003;11:15–21. doi: 10.5551/jat.11.15. [DOI] [PubMed] [Google Scholar]
- 38.Volin MV, Huynh N, Klosowska K, Reyes RD, Woods JM. Fractalkine-induced endothelial cell migration requires MAP Kinase signaling. Pathobiology. 2008;77:7–16. doi: 10.1159/000272949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Imaizumi T, Matsumiya T, Fujimono K, Okamoto K, Cui XF, Ohtaki U, Yoshida H, Satoh K. Interferon-γ stimulates the expression of CX3CL1/fractalkine in cultured human endothelial cells. Tohoku J Exp Med. 2000;192:127–139. doi: 10.1620/tjem.192.127. [DOI] [PubMed] [Google Scholar]
- 40.Pickens SR, Violin MV, Mandeline AM, II, Kolls JK, Pope RM, Shahrara S. IL-17 contributes to angiogenesis in rheumatoid arthritis. J. Immunol. 2010;184:3233–3241. doi: 10.4049/jimmunol.0903271. [DOI] [PMC free article] [PubMed] [Google Scholar]