Abstract
Hematopoietic stem and progenitors cells (HSPCs) are activated through toll-like receptor 4 (TLR4) in vitro. However, it remains unclear whether in vivo TLR4 sensing by HSPCs occurs directly or via other cell intermediates. Here, we examine the cellular mechanisms underlying murine hematopoietic stem cell (HSC) expansion and common lymphoid progenitor (CLP) depletion in a model of chronic low-dose lipopolysaccharide (LPS). Using adoptive transfer approaches, we show that HSC and CLP sensitivity to chronic LPS depends on hematopoietic-derived, cell subset-autonomous TLR4. Like murine progenitors, human HSPCs are activated by TLR4 in vitro. Using humanized mice, a pre-clinical model relevant to human physiology, we show that persistent endotoxin increases the frequency of Ki-67+ HSCs, and severely depletes CLPs and B precursors. Together, our findings show that murine HSPCs directly respond to endotoxin in vivo and that persistent LPS, a feature of several diseases of global health significance, impairs human lymphopoiesis.
Introduction
Bone marrow hematopoietic stem and progenitor cells (HSPCs) express Pattern Recognition Receptors (PRRs) such as toll-like receptors (TLRs) that detect microbial products including bacterial endotoxin (1, 2). While purified HSPCs can respond directly to the endotoxin LPS and other TLR ligands ex vivo (2, 3), the contribution of direct sensing mechanisms by HSPCs in vivo is considered relatively minor (4). LPS-mediated emergency myelopoiesis is independent of hematopoietic-derived TLR4 and instead requires non-hematopoietic TLR4 (5). Likewise, HSPC expansion following polymicrobial sepsis is independent of the TLR4 signaling adaptors MyD88/TRIF (6). Emerging evidence reveals that persistent exposure to endotoxin is a feature of clinically significant conditions including chronic infection, obesity and HIV/AIDS (7–13). A murine model of chronic LPS exposure demonstrated significant changes to the bone marrow compartment including HSPC expansion and reduced lymphoid potential (1). Moreover, hematopoietic stem cell (HSCs) from these LPS-exposed mice had poor self-renewal following serial adoptive transfer, indicating functional impairments. The relative importance of direct versus indirect TLR4-sensing mechanisms in the chronic LPS setting remains unknown.
In this study, we examine the cell-mediated mechanisms underlying murine HSPC dysfunction following chronic low-dose LPS, including the tissue source (hematopoietic versus nonhematopoietic) and cellular site (direct versus indirect) of TLR4 signaling. We use an established model of persistent low-dose LPS exposure (1, 2, 9, 14), and employ adoptive transfer strategies to determine the effects of chronic endotoxin on HSPC activity. We then assess the biological consequences of persistent low-dose LPS to human hematopoiesis using a pre-clinical experimental model. Human HSPCs are activated by TLR ligands in vitro (15–17) but the in vivo impact has been understudied. Together, our findings demonstrate that murine HSPCs can directly sense chronic LPS in vivo, and that chronic low-dose LPS perturbs human HSPCs and B lineage progenitors.
Materials and Methods
Mice
C57BL/6, TLR4-deficient and NSG (NOD scid gamma, NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ) mice were purchased from The Jackson Laboratory. Mice were bred or maintained in accordance with IACUC policies at the University of Pittsburgh School.
Human cord blood
De-identified cord blood cells were obtained with approval from the University of Pittsburgh School of Medicine Institutional Review Board. Purified CD34+ cord blood cells were purchased from AllCells, or cord blood obtained from Loma Linda University was enriched for CD34+lin− cells by magnetic separation.
Murine reconstitution chimeras and humanized mice
Wild type mice irradiated with 900 rads were subsequently engrafted with 2 × 106 wild type or TLR4-deficient BM cells i.v. via tail vein. Donor and host were distinguished with CD45 alleles: WT (CD45.1/2 or CD45.1) and TLR4 KO donor (CD45.2). At 12 weeks post-engraftment, mice were treated with 6 μg LPS or vehicle i.p. every other day for 4–6 weeks. NSG mice irradiated with 200 rads were engrafted with 2 × 105 human CD34+ cord blood cells infused i.v. via tail vein. At 16 weeks post-engraftment, mice were treated with LPS or vehicle for 4 weeks as above. Mice were immediately sacrificed after the last LPS treatment.
Flow cytometry, BrdU labeling and cell cycle analysis
Hematopoietic progenitors were isolated and stained for surface and intracellular markers including Ki-67 as we have reported (18).
Cell culture
For serum-free liquid culture assays, sorted HSCs, LKSneg or CLP subsets were cultured with X-VIVO 15 medium supplemented with recombinant flt3 ligand, stem cell factor, thrombopoietin, IL-7 or M-CSF as we have done (1, 18). Sorted HSCs were cultured with 10 μg/ml LPS. At harvest at the time points indicated each figure legend, cells were stained with antibodies to B220, CD19, CD11b, Gr-1 or BrdU.
Statistics
Statistical significance of differences between group means (p<0.05) was established using the two-sample t-test if comparisons were made between two independent groups or paired t-test if comparisons were made on paired samples, as indicated in each figure legend.
Results & Discussion
Hematopoietic-derived TLR4 directs HSC expansion and CLP reduction in a model of chronic LPS
TLR ligation can affect a wide variety of hematopoietic and non-hematopoietic cells. For example, activation of BM monocyte emigration by single, low-dose LPS exposure depends on TLR4+ BM reticular cells (19). To establish the relative importance of hematopoietic- versus nonhematopoietic-derived TLR4 in HSC expansion and CLP depletion following persistent LPS, we used reciprocal chimeras. We then examined the requirement for cell stage-autonomous TLR4 using mixed BM chimeras.
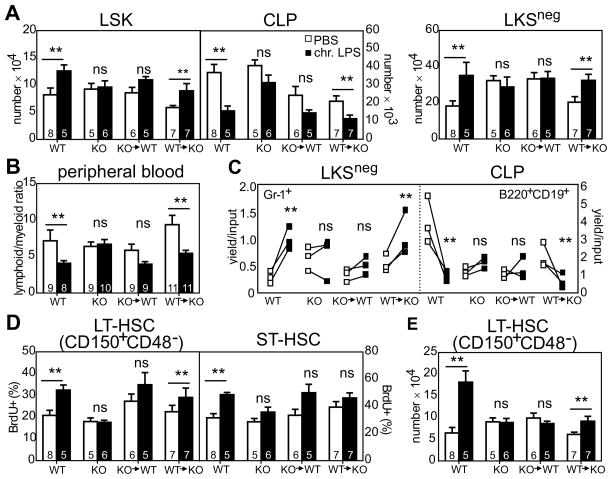
First, we generated chimeric mice by transferring TLR4KO BM into WT hosts (TLR4KO→WT) and vice versa (WT→TLR4KO), with donor and host distinguished by CD45 alleles. At 12 weeks post-engraftment, peripheral blood donor chimerism was 95% (not shown) and chimeric and control mice were subsequently exposed to low-dose LPS. We found that chronic activation of multipotent HSPCs as well as self-renewing LT-HSCs depends on TLR4-expressing hematopoietic cells. Following persistent LPS exposure, LSK and LKSneg cells from WT and WT→TLR4KO mice had a 20–40% increase in cellularity relative to PBS baseline for each cohort while CLPs were reduced ~50% relative to baseline (Figure 1A; phenotypic details in Figure S1A and Table S1). By contrast, in TLR4KO and TLR4KO→WT mice, numbers of LSK, LKSneg and CLP subsets were statistically unchanged relative to baseline (p>0.05), indicating that the effects of LPS on HSPC cellularity is independent of non-hematopoietic cell-mediated mechanisms. These findings suggest a major role for hematopoietic-derived TLR4 in the chronic model of LPS exposure, and a comparatively minor role for nonhematopoietic-derived TLR4.
Figure 1. LPS-mediated HSC proliferation and skewed HSPC differentiation requires hematopoietic-derived TLR4.
(A) WT, TLR4 KO, or the indicated reciprocal chimeric mice were exposed to 6 μg LPS or vehicle every other day i.p. for 4–6 weeks after which the indicated BM subsets were enumerated. n=5–8 mice/group. (B) Relative proportion of lymphoid (B220 + CD3) and myeloid cells (CD11b) in peripheral blood. (C) Following in vivo LPS exposure, mice were immediately sacrificed and LKSneg cells or CLPs sorted into cytokine-supplemented serum-free medium in the absence of any additional LPS. After 8 days of culture, cells were harvested and stained with antibodies to Gr-1 or B220. n=3, each symbol is a different animal. Paired sorts performed on the same day are connected by a line. (D–E) HSC proliferation and cellularity. Mice were injected twice daily with BrdU 48 hours prior to sacrifice. BM cells stained to detect HSC subsets were subsequently permeabilized for intracellular BrdU staining. Data are pooled from three separate adoptive transfers using different donors. The number of mice (n) is detailed within each column. Data (avg ± SEM) were analyzed by unpaired t-test. **p<0.05; ns, not significant.
LKSneg cells are enriched for myeloid precursors while CLPs are enriched for lymphoid precursors. Consistent with our observations of increased numbers of LKSneg cells and decreased CLPs in mice chronically exposed to LPS in vivo, peripheral blood from intact WT and WT→TLR4KO but not TLR4KO and TLR4KO→WT mice was myeloid skewed at the expense of lymphocytes in these animals (Figure 1B). These findings were additionally confirmed following examination of differentiation of each fraction in defined serum-free conditions ex vivo. LKSneg cells sorted from LPS-exposed WT and WT→TLR4KO had a 3-fold increase in myeloid lineage production as assessed using either the Mac-1 or Gr-1 differentiation markers while no enhancement of myeloid outgrowth was detectable in LKSneg cells derived from LPS exposed TLR4KO and TLR4KO→WT chimeras (Figure 1C and data not shown). CLPs exhibited a similar pattern of sensitivity with LPS-mediated depletion apparent in WT and WT→TLR4KO mice but not TLR4KO and TLR4KO→WT mice cohorts.
We examined whether LT-HSC numbers and proliferative activity also require hematopoietic-derived TLR4. LT-HSCs from LPS-exposed WT and WT→TLR4KO mice had a 30% increase in the frequency of proliferating BrdU+ cells and a 1.5–3-fold increase in cellularity relative to baseline for each group (Figure 1D–E; phenotypic gating in S1A). Similar to past studies showing increased basal proliferation of TLR4KO HSCs following adoptive transfer (20), we detected increased baseline proliferation of HSCs from PBS treated TLR4KO→WT mice as compared to PBS-treated WT or TLR4KO controls (Figure 1D, left). This effect appears specific to the LT-HSC subset as baseline ST-HSC proliferation in these same animals was similar to controls (Figure 1D, right). In contrast to TLR4-sufficient hematopoietic cells which were elevated following chronic LPS, numbers and proliferation status of LT-HSCs from LPS-exposed TLR4KO and TLR4KO→WT were unchanged relative to vehicle controls (Figure 1D–E). In these experiments, LT-HSCs were identified as CD150+CD48− LSK; exclusion of CD48 minimizes concerns about potential contamination of the gated HSC subset by CD150+ myeloid cells that may have upregulated Sca-1 during inflammation (1). Together, these studies with reciprocal chimeras emphasize a major role for hematopoietic-specific TLR4 in HSPC expansion and myeloid>lymphoid bias following persistent LPS.
HSPCs directly sense TLR4 ligand in vivo
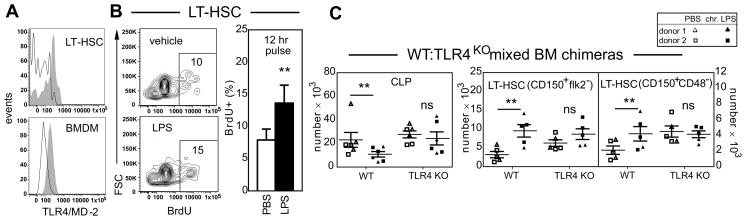
Like CLPs (2), LT-HSCs bear surface TLR4 and are capable of directly responding to LPS in vitro. Figure 2A depicts cell surface TLR4 expression on CD150+CD48− HSCs. The TLR4 co-receptor CD14 is also detectable (data not shown). We examined the ability of sort purified LT-HSCs to proliferate in response to LPS, using a dose and time point that maximizes BrdU detection sensitivity and limits cell differentiation, thereby minimizing potential concerns about LPS effects on the immediate downstream progeny of cultured HSCs. Sort purified LT-HSCs had a 50% increase in BrdU uptake following 12 hr LPS pulse (Figure 2B). To empirically establish whether LPS acts directly on HSPCs in vivo we generated mixed chimeras in which WT BM (CD45.1/2 or CD45.1) was mixed with TLR4KO BM (CD45.2) and engrafted into WT hosts (CD45.1 or CD45.1/2); data from two independent experiments using different allelic combinations were similar and are pooled. Since WT BM has a repopulation disadvantage against TLR4KO BM (20, 21), we used a 60:40 ratio (WT:KO) to ensure robust representation of both partners in the long-term chimeras. At twelve weeks post reconstitution, chimerism was ~50:50% (not shown) and mice were then chronically exposed to LPS or PBS. While numbers of TLR4-sufficient CLPs were reduced 50% in LPS- versus PBS-treated mice, cellularity was preserved in TLR4-deficient CLPs demonstrating a requirement for cell-intrinsic TLR4 (Figure 2C, left).
Figure 2. HSCs directly respond to LPS in vitro and in vivo.
(A) TLR4 expression on CD150+CD48− LSK LT-HSCs by flow cytometry. Bone marrow-derived macrophages (BMDM) are a positive control. Open histogram, isotype control; shaded histogram, TLR4 staining. Data are representative of 3 independent experiments. (B) Sort purified LT-HSCs from WT or TLR4 KO mice were pulsed with 10 μg/ul LPS for 12 hours in serum free medium and then stained with antibodies to BrdU. n=3 independent cell sorts. (C) Mixed WT:TLR4KO BM chimeras were exposed to LPS or vehicle every other for 4–6 weeks after which BM LT-HSCs and CLPs were enumerated. Two separate adoptive transfers were performed in which competitive donors were engrafted into six total mice in each experiment. Each donor is a different symbol. CLPs are depicted for all mice; one set of HSC samples was lost due to flow cytometry clogging. Data (avg ± SEM) were analyzed by paired t-test. **p<0.05; ns, not significant.
In the LT-HSC compartment, TLR4-sufficient HSC numbers increased 3-fold following LPS treatment while TLR4-deficient HSC numbers were unchanged as compared to baseline PBS for each group (Figure 2C, right). Our findings in this low-dose LPS experimental model suggest that HSCs and CLPs directly sense TLR4 stimuli in vivo.
Persistent TLR4 stimulation perturbs human HSPCs in vivo
Human HSPCs respond to multiple TLR ligands including TLR4 in vitro but the in vivo implications are understudied, a major gap in our knowledge given that persistent TLR4 stimulation is a feature of multiple diseases with broad clinical impact (7, 9–11, 13). We transferred human CD34+ cord blood (CB) into NSG immune deficient mice, rested animals for 4 months to attain stable engraftment (28.0% ± 16.1 hCD45+ cells across 23 total mice engrafted by 3 independent CB donors), and then exposed animals to chronic LPS. Mirroring the myeloid skewing observed in intact WT mice following LPS exposure (Figure 1), the relative proportion of hCD45+CD19+ B cells was diminished and hCD45+CD33+ myeloid cells enhanced following low-dose LPS treatment of humanized mice (Figure 3A, left; phenotypic gating in Figure S1A). Human CD45+ myeloid>B lymphoid skewing was also apparent in peripheral blood and spleen (Figure 3B, and data not shown). NK cells are capable of homeostatic expansion in lymphopenic environments, and it will be useful in future studies to determine whether hCD45+CD56+CD3− NK cellularity reflects de novo differentiation or homeostatic expansion of a small number of mature NK cells. Within the hCD45+ BM compartment, CLPs, pro-B and immature B subsets were virtually ablated while GMPs were increased in frequency, and MEP and CMP were comparable to controls (Figure 3A, right). There was also a 2-fold increase in the frequency of CD34+lin− HSPCs positive for the Ki-67 proliferation antigen accompanied by myeloid>lymphoid skewing (Figure 3C). Unlike the WT mouse model in which total cellularity in blood and BM is relatively unaffected by LPS, humanized mice chronically exposed to LPS had a significant reduction in hCD45+ chimerism in both blood and BM as compared to their PBS counterparts (Figure 3D, and data not shown). While we note that while xenogeneic studies should be interpreted with caution, collectively these data show that the xenotransplant model captures major aspects of chronic endotoxin exposure including HSPC activation and disproportionate loss of the B lymphoid lineage. This approach may advance the evaluation of therapeutic strategies in a pre-clinical setting relevant to human physiology.
Figure 3. Persistent low-dose LPS disrupts human hematopoiesis in vivo.
Human cord blood engrafted NSG mice were exposed to 6 μg LPS or vehicle i.p. for 4–6 weeks after which hematopoietic tissues were harvested and the indicated progenitors subsets resolved by flow cytometry. (A) Relative frequencies of human CD19+ B cells, CD56+ NK cells, and CD33+ myeloid cells in peripheral blood (left) or precursor subsets in BM (right). Each human donor is a different symbol, 2–3 mice per treatment group. Within the hCD45+mCD45− BM gate, phenotypic definitions were: CLPs (lin−CD34+CD45RA+CD10+), pro-B (CD19+CD10+CD34+), immature B (CD19+CD10+CD34−), GMP (lin−CD34+CD38+IL-3RαloCD45RA+), MEP (lin−CD34+CD38+IL-3Rα− CD45RA−), CMP (lin−CD34+CD38+IL-3RαloCD45RA−). (B) Human lymphoid (CD19) to myeloid (CD33) ratio in peripheral blood (avg ± SEM). (C) Cell cycling activity in hCD45+lin−CD34+ BM HSPCs was assessed by flow cytometry analysis for the Ki-67 proliferation antigen. (D) Peripheral blood hCD45+ chimerism before and after LPS exposure. Data were analyzed by unpaired t-test. **p<0.05; ns, not significant.
In summary, this study provides mechanistic insight into how persistent TLR4 ligand, a notable feature of diseases of global significance including chronic infection, obesity and HIV/AIDS perturbs HSPC homeostasis. Our observations that cell-autonomous TLR4 is required for HSPC activation during chronic LPS exposure differ from observations in the acute LPS setting (4–6), thereby deepening our understanding of the distinct pathways of LPS detection in the acute versus chronic contexts. Recent studies demonstrate cooperation between cell-autonomous TLRs and the LPS-inducible inflammatory cytokine interferon-γ (IFNγ) in enhancing TLR-mediated signals as well as suppressing B cell fate (22). Increased levels of IFNγ protein are detectable within BM of mice exposed to chronic LPS, suggesting a potential local source of this cytokine (data not shown). Moreover, that low-dose LPS disrupts human hematopoiesis in patterns appreciably similar to that observed in mouse highlights the potential impact of our findings with respect to human health.
Supplementary Material
Acknowledgments
This work is supported by NIH AI079047, AI105846 and American Society of Hematology Bridge Grant Program (LB); NIH P20 MD006988 (KJP), LLU institutional grants from the Department of Pathology and Human Anatomy, the Department of Basic Sciences, the Center for Health Disparities and Molecular Medicine, and Grant to Promote Collaborative and Translational Research (KJP); Hillman Cancer Center Support Grant and P30 CA4790413 (YD).
We thank Dewayne Falkner for excellent cell sorting.
References
- 1.Esplin BL, Shimazu T, Welner RS, Garrett KP, Nie L, Zhang Q, Humphrey MB, Yang Q, Borghesi LA, Kincade PW. Chronic exposure to a TLR ligand injures hematopoietic stem cells. Journal of immunology. 2011;186:5367–5375. doi: 10.4049/jimmunol.1003438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagai Y, Garrett KP, Ohta S, Bahrun U, Kouro T, Akira S, Takatsu K, Kincade PW. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity. 2006;24:801–812. doi: 10.1016/j.immuni.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Massberg S, Schaerli P, Knezevic-Maramica I, Kollnberger M, Tubo N, Moseman EA, Huff IV, Junt T, Wagers AJ, Mazo IB, von Andrian UH. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 2007;131:994–1008. doi: 10.1016/j.cell.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manz MG, Boettcher S. Emergency granulopoiesis. Nat Rev Immunol. 2014;14:302–314. doi: 10.1038/nri3660. [DOI] [PubMed] [Google Scholar]
- 5.Boettcher S, Ziegler P, Schmid MA, Takizawa H, van Rooijen N, Kopf M, Heikenwalder M, Manz MG. Cutting edge: LPS-induced emergency myelopoiesis depends on TLR4-expressing nonhematopoietic cells. Journal of immunology. 2012;188:5824–5828. doi: 10.4049/jimmunol.1103253. [DOI] [PubMed] [Google Scholar]
- 6.Scumpia PO, Kelly-Scumpia KM, Delano MJ, Weinstein JS, Cuenca AG, Al-Quran S, Bovio I, Akira S, Kumagai Y, Moldawer LL. Cutting edge: bacterial infection induces hematopoietic stem and progenitor cell expansion in the absence of TLR signaling. Journal of immunology. 2010;184:2247–2251. doi: 10.4049/jimmunol.0903652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amar J, Chabo C, Waget A, Klopp P, Vachoux C, Bermudez-Humaran LG, Smirnova N, Berge M, Sulpice T, Lahtinen S, Ouwehand A, Langella P, Rautonen N, Sansonetti PJ, Burcelin R. Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: molecular mechanisms and probiotic treatment. EMBO Mol Med. 2011;3:559–572. doi: 10.1002/emmm.201100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ara T, Kurata K, Hirai K, Uchihashi T, Uematsu T, Imamura Y, Furusawa K, Kurihara S, Wang PL. Human gingival fibroblasts are critical in sustaining inflammation in periodontal disease. J Periodontal Res. 2009;44:21–27. doi: 10.1111/j.1600-0765.2007.01041.x. [DOI] [PubMed] [Google Scholar]
- 9.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmee E, Cousin B, Sulpice T, Chamontin B, Ferrieres J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 10.Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, Rottier O, Geurts L, Naslain D, Neyrinck A, Lambert DM, Muccioli GG, Delzenne NM. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58:1091–1103. doi: 10.1136/gut.2008.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang W, Lederman MM, Hunt P, Sieg SF, Haley K, Rodriguez B, Landay A, Martin J, Sinclair E, Asher AI, Deeks SG, Douek DC, Brenchley JM. Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. J Infect Dis. 2009;199:1177–1185. doi: 10.1086/597476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pussinen PJ, Vilkuna-Rautiainen T, Alfthan G, Palosuo T, Jauhiainen M, Sundvall J, Vesanen M, Mattila K, Asikainen S. Severe periodontitis enhances macrophage activation via increased serum lipopolysaccharide. Arterioscler Thromb Vasc Biol. 2004;24:2174–2180. doi: 10.1161/01.ATV.0000145979.82184.9f. [DOI] [PubMed] [Google Scholar]
- 13.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. The Journal of clinical investigation. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao JL, Rao DS, O’Connell RM, Garcia-Flores Y, Baltimore D. MicroRNA-146a acts as a guardian of the quality and longevity of hematopoietic stem cells in mice. Elife. 2013;2:e00537. doi: 10.7554/eLife.00537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Luca K, Frances-Duvert V, Asensio MJ, Ihsani R, Debien E, Taillardet M, Verhoeyen E, Bella C, Lantheaume S, Genestier L, Defrance T. The TLR1/2 agonist PAM(3)CSK(4) instructs commitment of human hematopoietic stem cells to a myeloid cell fate. Leukemia. 2009;23:2063–2074. doi: 10.1038/leu.2009.155. [DOI] [PubMed] [Google Scholar]
- 16.Reece P, Thanendran A, Crawford L, Tulic MK, Thabane L, Prescott SL, Sehmi R, Denburg JA. Maternal allergy modulates cord blood hematopoietic progenitor Toll-like receptor expression and function. J Allergy Clin Immunol. 2011;127:447–453. doi: 10.1016/j.jaci.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Sioud M, Floisand Y. TLR agonists induce the differentiation of human bone marrow CD34+ progenitors into CD11c+ CD80/86+ DC capable of inducing a Th1-type response. Eur J Immunol. 2007;37:2834–2846. doi: 10.1002/eji.200737112. [DOI] [PubMed] [Google Scholar]
- 18.Yang Q, Kardava L, St Leger A, Martincic K, Varnum-Finney B, Bernstein ID, Milcarek C, Borghesi L. E47 controls the developmental integrity and cell cycle quiescence of multipotential hematopoietic progenitors. Journal of immunology. 2008;181:5885–5894. doi: 10.4049/jimmunol.181.9.5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi C, Jia T, Mendez-Ferrer S, Hohl TM, Serbina NV, Lipuma L, Leiner I, Li MO, Frenette PS, Pamer EG. Bone marrow mesenchymal stem and progenitor cells induce monocyte emigration in response to circulating toll-like receptor ligands. Immunity. 2011;34:590–601. doi: 10.1016/j.immuni.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schuettpelz LG, Borgerding JN, Christopher MJ, Gopalan PK, Romine MP, Herman AC, Woloszynek JR, Greenbaum AM, Link DC. G-CSF regulates hematopoietic stem cell activity, in part, through activation of Toll-like receptor signaling. Leukemia. 2014;28:1851–1860. doi: 10.1038/leu.2014.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ichii M, Shimazu T, Welner RS, Garrett KP, Zhang Q, Esplin BL, Kincade PW. Functional diversity of stem and progenitor cells with B-lymphopoietic potential. Immunol Rev. 2010;237:10–21. doi: 10.1111/j.1600-065X.2010.00933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baratono SR, Chu N, Richman LP, Behrens EM. Toll-like receptor 9 and interferon-γ receptor signaling suppress the B-cell fate of uncommitted progenitors in mice. Eur J Immunol. 2015 doi: 10.1002/eji.201445319. Ebpub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.