Abstract
Background
Radiation therapy is used increasingly as a component of multidisciplinary treatment for many solid tumors. One complication of such treatment is the development of radiation-associated sarcoma (RAS). Undifferentiated pleomorphic sarcoma (UPS), previously termed “malignant fibrous histiocytoma” (MFH) is the most common histologic subtype of RAS. This study investigated the clinical outcomes for patients with radiation-associated UPS (RA-UPS/MFH).
Methods
The study identified 1068 patients with UPS/MFH treated at the authors’ institution. Patient and tumor factors were collected and compared. Regression analysis was performed to identify independent predictors of survival. A matched-cohort survival and recurrence analysis was performed for radiation-associated and sporadic UPS/MFH.
Results
The findings showed that RA-UPS/MFH comprised 5.1 % of the UPS population. The median latency to the development of RA-UPS/MFH was 9.3 years. The 5-year disease-specific survival (DSS) was 52.2 % for patients identified with RA-UPS/MFH (n = 55) compared with 76.4 % for patients with unmatched sporadic UPS/MFH (n = 1,013; p < 0.001). A matched-cohort analysis also demonstrated that the 5-year DSS was significantly worse for RA-UPS/MFH (52.2 vs 73.4 %; p = 0.002). Furthermore, higher local recurrence rates were observed for patients with RA-UPS/MFH than for patients with sporadic lesions (54.5 vs 23.5 %; p < 0.001). Radiation-associated status and incomplete resection were identified as independent predictors of local recurrence.
Conclusion
This study demonstrated worse clinical outcomes for patients with RA-UPS/MFH than for patients with sporadic UPS/MFH. Local recurrence was significantly higher for patients with RA-UPS/MFH, suggesting a unique tumor biology for this challenging disease.
Radiation therapy (RT) is increasingly used in the multidisciplinary treatment of solid tumors, with approximately 50–60 % of all cancer patients receiving RT during their treatment.1–3 When applied properly, radiation improves local control and reduces the risk of recurrence. Unfortunately, adverse events can occur after treatment with radiation, including the development of a second malignancy.4–6 Sarcoma is one such cancer, with radiation-associated soft tissue sarcomas (RAS) occurring in approximately 0.03–0.8 % of patients after radiation.3,7 Findings show that RAS accounts for approximately 1–3 % of all patients with a diagnosis of sarcoma.8–10
The diagnosis of RAS generally is made clinically according to guidelines initially developed in 1948 and revised in 1971.11,12 The diagnosis requires that a patient had prior radiation exposure, subsequent development of sarcoma within radiated tissue (i.e., in or near the radiation field), and a histologic diagnosis of sarcoma distinct from the original primary malignancy.
The latency period between the administration of radiation and the development of sarcoma was initially thought to be several years.7,12 However, more recent evidence suggests that this period may be much shorter for soft tissue sarcoma (STS).9,11 Whereas the latency period for bone sarcoma is reported to be as long as 15 years,13 the latency period for STS is commonly reported to be 7–16 years.8–10,14,15
Radiation-associated sarcoma is thought to carry a worse disease-specific survival (DSS) than sporadic lesions,8,9 and local recurrence rates of approximately 50 % have been reported.14,16
Most reports in the literature describe a heterogeneous group of histologic sarcoma subtypes. Therefore, we focused our study on the most common RAS, undifferentiated pleomorphic sarcoma (UPS/MFH), which represents 23–37 % of RAS in various reports.3,8,9,16 Our study aimed to determine whether radiation-associated UPS (RA-UPS/MFH) is associated with a worse prognosis than sporadic UPS/MFH.
METHODS
All studies were conducted with approval of the Institutional Review Board (IRB) of the University of Texas MD Anderson Cancer Center (UTMDACC).
Clinical Database
A clinical database was compiled by collecting all patients with UPS/MFH from 1990 to 2012. We excluded patients with sarcomas originating in bone because evidence indicates that they have a different biology and survival than patients with STS.13 A retrospective chart review was performed to extract clinical and pathologic information including patient age, date of sarcoma diagnosis, date of sarcoma resection, size of sarcoma, date of recurrence, and recurrence type (local/distant). For patients with RA-UPS/MFH, date of diagnosis for initial cancer, date of radiation, dose of radiation, and use of chemotherapy for primary malignancy were recorded. A diagnosis of UPS was confirmed by two sarcoma pathologists (A.L. and W.W.) at MDACCC. Tumor size was determined by the maximum dimension on either imaging or pathology reports.
Overall survival (OS) was determined from the date of diagnosis to death from any cause. DSS was defined as the time from the date of sarcoma diagnosis to death due to sarcoma. Local recurrence-free survival (LRFS) was defined from time from surgery to recurrence within the affected region. Distant recurrence-free survival (DRFS) was defined as time from surgery for sarcoma to the time of disease present outside the initial resection field. Patients who survived or were lost to follow-up evaluation were censored on the date of last contact.
Statistical Analysis
Survival was compared using Kaplan–Meier analysis with the log-rank test.17 Multivariate analysis was performed using Cox proportional hazard analysis.18 Significance was set at a p value lower than 0.05. Statistical analysis was performed using SPSS version 21 (IBM Corporation, Armonk, NY, USA). A subset of patients who had sporadic UPS/MFH with primary and resectable disease was selected using R 3.0.2 Matching v(4.8–3.4) software (R Foundation for Statistical Computing, Vienna, Austria) to match the RA-UPS/MFH patients on the basis of age, sex, and tumor size, site, and margin status. All statistical analyses were performed with R 3.0.2 (R Core Team, 2013) using the following R packages: survival v(2.37–4), lattice v(0.20–29), MASS v(7.3–29), and Matching v(4.8–3.4).19
RESULTS
Patient and Tumor Characteristics
We identified 1068 patients with undifferentiated pleomorphic sarcoma (UPS/MFH) treated at MDACC between the years 1990 and 2012. The tumor and demographic characteristics are shown in Table 1. A total of 55 patients had RA-UPS/MFH, comprising 5.1 % of the cohort. The characteristics of the patients in both groups (RA-UPS/MFH and sporadic UPS/MFH) also are shown in Table 1. The most common primary cancers for which patients received radiation were breast cancer and lymphoma (n = 16, 28.6 % for each, data not shown). The median latency period between radiation and the development of RA-UPS/MFH was 9.33 years (range 1–40 years) (Table 1). Within 5 years after receiving radiation, 15 patients (25.5 %) experienced RA-UPS/MFH, 7 of whom experienced RA-UPS/MFH within 3 years (12.7 %). The median dose of radiation received was 50 ± 5.2 Gy.
TABLE 1.
Clinical and pathologic features of 1068 patients with undifferentiated pleomorphic sarcoma/malignant fibrous histiocytoma (UPS/MFH)
| Factor | Entire cohort (n = 1,068) | Sporadic (n = 1,013) | RA-UPS/MFH (n = 55) | p valueb |
|---|---|---|---|---|
| Tumor size (cm)a | 0.154 | |||
| Median | 6.0 | 6.1 | 6.0 | |
| Range | 0.25–36.0 | 0.25–36.0 | 0.9–20 | |
| Size ≥5 cm | 554 (51.9) | 525 (51.9) | 29 (52.7) | 0.348 |
| Median age at sarcoma diagnosis | 61 | 62 | 59 | 0.281 |
| Female | 443 (41.5) | 410 (40.5) | 33 (60.0) | 0.005 |
| Locationb | < 0.001 | |||
| Head & neck | 62 (5.8) | 52 (5.1) | 10 (18.2) | |
| Trunkc | 262 (24.5) | 234 (23.1) | 28 (50.9) | |
| Extremity | 620 (58.1) | 611 (60.3) | 9 (16.4) | |
| Retroperitoneal | 122 (11.4) | 114 (11.3) | 8 (14.5) | |
| Radiation dose (Gy) | N/A | N/A | ||
| Median ± SEM | 50.0 ± 2.3 | |||
| Range | 32–90 | |||
| Latency (years) | N/A | N/A | ||
| Median ± SEM | 9.33 ± 1.31 | |||
| Range | 1.2–39.7 | |||
| Chemotherapy for primary cancer | N/A | N/A | 26 (47.3) | |
| Age at diagnosis of first primary | N/A | N/A | ||
| Median ± SEM | 45 ± 2.6 | |||
| Range | 10–83 |
RA, radiation-associated; N/A, not available; SEM, standard error of the mean
Specific size available for 860 patients
Sporadic lesions versus RA-UPS/MFH
Chest and abdominal wall
Survival Analysis: Entire Cohort
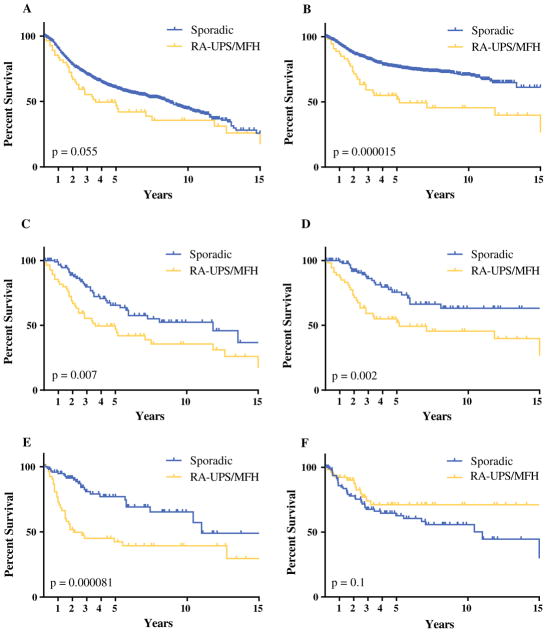
Analysis of the entire cohort demonstrated improved OS and DSS in the sporadic group (n = 1,013) compared with the RA-UPS/MFH group (n = 55) (Fig. 1a and b). The median OS was 8.5 years for sporadic UPS/MFH and 3.6 years for RA-UPS/MFH (p = 0.055). The median DSS was 3.6 years in the RA-UPS/MFH group, which was significantly worse than for the DSS patients with sporadic UPS/MFH, for whom the median DSS was not met. Uni-and multivariate analyses were performed with the entire cohort, showing that radiation-associated status, age older than 60 years, and size larger than 5 cm were associated with worse prognosis (Table 2).
FIG. 1.
Survival analysis of sporadic lesions and radiation-associated undifferentiated pleomorphic sarcoma (RA-UPS)/malignant fibrous histiocytoma (MFH). a Overall survival (OS) of 1069 patients with UPS. The 1-, 3-, and 5-year OS rates were respectively 90.1, 70.2 and 60.5 % for sporadic lesions versus 83.5, 55.4, and 47.0 % for RA-UPS. b Disease-specific survival (DSS) was similarly worse for the RA-UPS patients. The 1-, 3-, and 5-year DSS rates were respectively 94.1, 82.7 and 76.4 % for sporadic lesions versus 86.9, 59.2, and 52.2 % for RA-UPS. c In this study, 98 sporadic patients were matched with 55 RA-UPS patients based on tumor size, age, and anatomic distribution. The OS was worse in the sporadic group (median survival, 11.8 years for sporadic lesions vs 3.6 years for RA-UPS). d The DSS also was worse for the RA-UPS group (median DSS, 22 years vs 5.2 years in the RA-UPS group). e The 1-, 3-, and 5-year local recurrence-free survival rates were respectively 94.7, 80.9, and 74.5 % for sporadic lesions versus 76.7, 45.1, and 42.4 % for RA-UPS. f The 1-, 3-, and 5-year distant recurrence-free survival rates were respectively 84.8, 67.6, and 62.6 % for sporadic lesions versus 92.3, 74.1, and 71.1 % for RA-UPS (statistically nonsignificant difference)
TABLE 2.
Disease-specific survival analysis of 1068 patients with undifferentiated pleomorphic sarcoma (UPS)
| Factor | Patients | Univariate p value | Multivariate p value | HR (95 % CI) |
|---|---|---|---|---|
| Age (years) | 0.020 | 0.002 | 1.62 (1.19–2.22) | |
| ≥60 | 568 | |||
| < 60 | 479 | |||
| Gender | 0.377 | |||
| Female | 436 | |||
| Male | 611 | |||
| Size (cm) | < 0.001 | < 0.001 | 2.84 (1.95–4.14) | |
| < 5 | 306 | |||
| ≥5 | 550 | |||
| RAS | < 0.001 | < 0.001 | 3.27 (2.09–5.12) | |
| Yes | 55 | |||
| No | 1,013 | |||
| Location | < 0.001 | 0.1 | ||
| Head & neck | 58 | |||
| Trunk | 258 | |||
| Extremity | 609 | |||
| Retroperitoneal | 120 |
HR, hazard ratio; CI, confidence interval; RAS, radiation-associated sarcoma
Matched Cohort Analysis
In an effort to characterize patients better, particularly with regard to surgical characteristics, a subset of sporadic UPS/MFH patients were matched with the RA-UPS/MFH patients based on age, tumor location, and size. The demographic information is presented in Table 3 and shows that in addition to the aforementioned variables, both groups were similar with respect to depth of sarcoma, resection margin, gender, and use of chemotherapy.
TABLE 3.
Clinical and pathologic factors of matched-patient cohort
| Factor | Sporadic (n = 98) | RA-UPS/MFH (n = 55) | p value |
|---|---|---|---|
| Sarcoma location | 0.129 | ||
| Head & neck | 10 (10.3) | 10 (18.2) | |
| Trunk | 54 (55.1) | 28 (49.1) | |
| Extremity | 27 (27.6) | 9 (16.4) | |
| Retroperitoneal | 7 (7.1) | 8 (14.5) | |
| Female | 55 (56.1) | 33 (60.0) | 0.642 |
| Median size (cm) | 6.0 | 6.0 | 0.460 |
| Deep | 68 (72.3) | 44 (81.5) | 0.212 |
| Median age (years) | 62.2 | 57.46 | 0.540 |
| Size ≥5 cm | 59 (60.8) | 29 (59.2) | 0.848 |
| Resection score | 0.532 | ||
| R0 | 76 (79.2) | 44 (80.0) | |
| R1 | 14 (14.6) | 8 (14.5) | |
| R2 | 3 (3.1) | 3 (5..5) | |
| Received chemotherapya | 22 (24.2) | 16 (29.1) | 0.547 |
| Received radiationb | 69 (71.1) | 12 (22.3) | < 0.001 |
RA, radiation-associated; UPS, undifferentiated pleomorphic sarcoma; MFH, malignant fibrous histiocytoma
Either adjuvant or neoadjuvant chemotherapy for sarcoma
Either pre- or postoperative radiation for sarcoma
All the patients in the matched-cohort analysis underwent surgical resection. The margin status was R0 for 79.2 % of the sporadic lesions and 80 % for RA-UPS/MFH (nonsignificant difference) (Table 3). Chemotherapy (pre-or postoperative) use was similar between the groups (24.2 % of the sporadic cases and 29.1 % of the RA-UPS/MFH cases; p = 0.547). As might have been anticipated, the use of radiation differed significantly, with 71.1 % of the patients in the sporadic cohort receiving radiation compared with only 22.3 % in the RA-UPS/MFH group (p < 0.001).
After adjustment for the potential confounding variables of age, size, depth, location, and margin status, RA-UPS/MFH remained associated with a worse OS and DSS than sporadic UPS/MFH (Fig. 1c, d). Local recurrence occurred for 30 (54.5 %) of the RA-UPS/MFH patients compared with 23 (23.5 %) of the sporadic UPS/MFH patients (p < 0.001). The 5-year LRFS for the RA-UPS/MFH group was 42.4 versus 74.5 % for the sporadic UPS/MFH group (p < 0.0001), (Fig. 1e). No difference in DRFS was found between the groups (Fig. 1f). Uni- and multivariate analyses demonstrated that radiation-associated status was an independent predictor of worse DSS. Radiation-associated status and incomplete resection margin also independently predicted worse LRFS (Table 4).
TABLE 4.
Matched-cohort analysis of factors affecting disease-specific survival (DSS) and local recurrence-free survival (LRFS)
| Factor | DSS
|
LRFS
|
||||||
|---|---|---|---|---|---|---|---|---|
| Patients | Univariate p value | Multivariate p value | HR (95 % CI) | Patients | Univariate p value | Multivariate p value | HR (95 % CI) | |
| Age (years) | 0.735 | 0.540 | ||||||
| ≥60 | 74 | 79 | ||||||
| < 60 | 79 | 74 | ||||||
| Gender | 0.606 | 0.914 | ||||||
| Female | 88 | 88 | ||||||
| Male | 65 | 65 | ||||||
| Size (cm) | 0.167 | 0.789 | ||||||
| < 5 | 58 | 58 | ||||||
| ≥5 | 88 | 88 | ||||||
| Deep | 0.096 | 0.241 | 1.591 (0.732–3.46) | 0.803 | ||||
| Yes | 112 | 112 | ||||||
| No | 36 | 36 | ||||||
| RAS | 0.002 | 0.004 | 2.328 (1.32–4.1) | 0.000081 | 0.000118 | 4.282 (2.042–8.979) | ||
| Yes | 55 | 55 | ||||||
| No | 98 | 98 | ||||||
| Location | 0.048 | 0.308 | 1.194 (0.848–1.682) | 0.008 | 0.374 | 1.176 (0.82–1.68) | ||
| Head & neck | 21 | 20 | ||||||
| Trunk | 82 | 82 | ||||||
| Extremity | 36 | 36 | ||||||
| Retroperitoneal | 15 | 15 | ||||||
| Margin status | 0.501 | 0.019 | 0.032 | 2.086 (1.066–4.085) | ||||
| R0 | 120 | 120 | ||||||
| R1/R2 | 28 | 28 | ||||||
| Chemotherapy for sarcoma | 0.465 | 0.372 | ||||||
| Yes | 38 | 38 | ||||||
| No | 106 | 106 | ||||||
| Radiation for sarcoma | 0.168 | 0.070 | 0.467 | 1.316 (0.628–2.758) | ||||
| Yes | 81 | 81 | ||||||
| No | 69 | 69 | ||||||
HR, hazard ratio; CI, confidence interval; RAS, radiation-associated sarcoma
DISCUSSION
Radiation-associated sarcoma was previously reported to be associated with worse outcomes, but few studies have focused on a single histologic subtype of RAS. The most common subtype of RAS is UPS/MFH. To our knowledge, our study is the first to focus specifically on RA-UPS/MFH, allowing for a more accurate, subtype-specific comparison of sporadic lesions and RAS. This study demonstrated that RA-UPS/MFH is associated with worse OS and DSS than sporadic UPS/MFH. Moreover, our data confirm that a significant percentage (25.5 %) of RA-UPS/MFH develops within a short latency period (<5 years). Finally, our results suggest that local recurrence rates are much higher for patients with RA-UPS/MFH than for those with sporadic UPS/MFH lesions.
Our data are unique in that we focused on a specific subtype of sarcoma, namely UPS. However, other studies that included all RAS subtypes have reported consistent results. Gladdy et al.9 reported a series of 130 primary RAS cases treated at Memorial Sloan-Kettering Cancer Center, the largest series of RAS cases reported to date. These authors demonstrated that radiation-associated status carried a hazard ratio of 1.7 compared with sporadic UPS/MFH. A total of 34 patients (26 %) had a diagnosis of MFH, making it the most common of the RAS histologic subtypes. Analysis of these patients with RA-MFH versus sporadic MFH showed a trend toward inferior survival for the RAS group, although this trend did not reach statistical significance (the 5-year DSS for the sporadic group was 66 vs 44 % for the RAS-MFH group).9
Our results are consistent but more definitive in that we demonstrated a statistically significant difference in 5-year DSS between sporadic lesions and RA-UPS/MFH (76.4 % for sporadic lesions vs 52.2 % for RA-UPS/MFH). Additionally, we demonstrated that the local recurrence rate for patients with RA-UPS/MFH is significantly higher than for those with sporadic lesions. To our knowledge, our study is the first to compare the rate of local recurrence directly between sporadic UPS/MFH and RA-UPS/MFH. The local recurrence rate for RA-UPS/MFH was 54.5 %, higher than that previously reported in a study of all RAS cases.10 However, this reflects the importance of a subtype-specific approach in that our data reflect the true recurrence rate for RA-UPS. Cha et al.8 reported that local recurrence was associated with an overall worse prognosis, which we demonstrated to be true for RA-UPS.
Our median latency from the time of radiation to the diagnosis of RAS was 9.33 years. Additionally, we identified seven patients who experienced RA-UPS/MFH within 3 years after radiotherapy (12.7 %). Our results are consistent with other published reports demonstrating relatively short latency periods.9,10,14 Torres et al.14 demonstrated that the latency of radiation-associated angiosarcoma was 7 years. In a study of 123 patients with mixed RAS, Cha et al.8 demonstrated a latency of 8.4 years. Gladdy et al.9 showed eight patients who experienced RAS within 3 years (6.2 %). However, three of these were MFH cases, comprising 8.8 % of the RA-MFH patients. Thus, our results support a modification to the definition of radiation-associated sarcoma that includes a latency period shorter than 3 years.
The limitations of the study include its retrospective nature. The location of RA-UPS is primarily truncal, whereas sporadic UPS is found predominantly in extremities. Our data suggest that location is not an important prognostic factor. However, we also attempted to control for this with our matched-cohort analysis. Our number of patients with RA-UPS was relatively small but does represent the largest subtype-specific analysis of RAS to our knowledge.
In our study, only 24 % of the patients with RA-UPS/MFH underwent re-irradiation compared with 71 % of the patients in the sporadic group (matched cohort). Similarly, 22 % of the patients reported by Gladdy et al.9 underwent re-irradiation. We found that re-irradiation did not reduce the rate of local recurrence.
In contrast, Riad et al.10 reported on 42 patients with mixed-histology RAS who underwent surgical resection. Of these 42 patients, 13 (31 %) received adjuvant radiation, and only one patient experienced local recurrence.10 However, re-irradiation was associated with a morbidity rate of approximately 50 %. It should be noted that the location of RAS in the study by Riad et al.10 was primarily in an extremity (80 %), in contrast to our cohort, half of whose tumors developed in the trunk.10 Although location was not identified to be an independent prognostic factor, an extremity location may affect the decision to re-irradiate, although this is speculative.
The biologic differences between sporadic lesions and RAS remain unclear. Our matched-cohort analysis was fairly homogeneous in that all the patients had high-grade tumors of a single histology that were similar in size and anatomic distribution. However, the difference in behavior, as evidenced by the increased local recurrence rates and worse DSS, suggests that the underlying biologic processes are not the same. Some recent studies are beginning to elucidate the mechanisms mediating these differences. For example, a high rate of TP53 mutation has been reported in RAS compared with sporadic lesions.20,21 Consistent with this, patients with hereditary retinoblastoma are at high risk for the development of radiation-associated sarcoma, specifically MFH.22,23 Additionally, recent data have demonstrated a transcriptome panel of 135 genes that was able to distinguish RAS from sporadic lesions.24 The genes involved in discrimination between the two suggest that chronic oxidative stress is a characteristic of RAS.24 The full array of differences remains to be elucidated, but the reported information may help to identify novel treatment strategies.
In conclusion, we report on a large cohort of single-histology RAS. We show that DSS is worse for patients treated for RA-UPS/MFH. Additionally, we report a high rate of local failure and a worse LRFS, which suggests differences in tumor biology. As the number of cancer survivors who have received radiation increases, RAS will continue to be a significant postradiation complication and should be considered when the risks and benefits of radiation treatment are assessed. The mainstay of treatment continues to be aggressive surgical management.
Acknowledgments
Funding for this research was provided in part by NIH/NCI K08CA160443 (to K.T.), the Sally M. Kingsbury Sarcoma Research Foundation (to K.T.), the Amschwand Foundation (supporting C.M), and NIH/NCI 2P30CA016672-38 (supporting S.Z.).
Footnotes
DISCLOSURE
There are no conflicts of interest.
The data in this report were previously presented in an oral presentation at the SSO 2014 Annual Cancer Symposium, Phoenix, AZ on 15 March 2014.
References
- 1.Beyzadeoglu M, Ozyigit G, Ebruli C. Basic Radiation Oncology. Heidelberg: Springer; 2010. [Google Scholar]
- 2.Thariat J, Hannoun-Levi JM, Sun Myint A, Vuong T, Gerard JP. Past, present, and future of radiotherapy for the benefit of patients. Nat Rev Clin Oncol. 2013;10:52–60. doi: 10.1038/nrclinonc.2012.203. [DOI] [PubMed] [Google Scholar]
- 3.Thariat J, Italiano A, Collin F, Iannessi A, Marcy PY, Lacout A, Birtwisle-Peyrottes I, Thyss A, Lagrange JL. Not all sarcomas developed in irradiated tissue are necessarily radiation-induced–spectrum of disease and treatment characteristics. Crit Rev Oncol Hematol. 2012;83(3):393–406. doi: 10.1016/j.critrevonc.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Berrington de Gonzalez A, Curtis RE, Kry SF, Gilbert E, Lamart S, Berg CD, Stovall M, Ron E. Proportion of second cancers attributable to radiotherapy treatment in adults: a cohort study in the US SEER cancer registries. Lancet Oncol. 2011;12:353–60. doi: 10.1016/S1470-2045(11)70061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berrington de Gonzalez A, Kutsenko A, Rajaraman P. Sarcoma risk after radiation exposure. Clin Sarcoma Res. 2012;2:18–26. doi: 10.1186/2045-3329-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morton LM, Dores GM, Curtis RE, Lynch CF, Stovall M, Hall P, Gilbert ES, Hodgson DC, Storm HH, Johannesen TB, et al. Stomach cancer risk after treatment for Hodgkin lymphoma. J Clin Oncol. 2013;31:3369–77. doi: 10.1200/JCO.2013.50.6832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mark RJ, Poen J, Tran LM, Fu YS, Selch MT, Parker RG. Postirradiation sarcomas: a single-institution study and review of the literature. Cancer. 1994;73:2653–62. doi: 10.1002/1097-0142(19940515)73:10<2653::aid-cncr2820731030>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 8.Cha C, Antonescu CR, Quan ML, Maru S, Brennan MF. Long-term results with resection of radiation-induced soft tissue sarcomas. Ann Surg. 2004;239:903–10. doi: 10.1097/01.sla.0000128686.51815.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gladdy RA, Qin LX, Moraco N, Edgar MA, Antonescu CR, Alektiar KM, Brennan MF, Singer S. Do radiation-associated soft tissue sarcomas have the same prognosis as sporadic soft tissue sarcomas? J Clin Oncol. 2010;28:2064–9. doi: 10.1200/JCO.2009.25.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riad S, Biau D, Holt GE, Werier J, Turcotte RE, Ferguson PC, Griffin AM, Dickie CI, Chung PW, Catton CN, et al. The clinical and functional outcome for patients with radiation-induced soft tissue sarcoma. Cancer. 2012;118:2682–92. doi: 10.1002/cncr.26543. [DOI] [PubMed] [Google Scholar]
- 11.Arlen M, Higinbotham NL, Huvos AG, Marcove RC, Miller T, Shah IC. Radiation-induced sarcoma of bone. Cancer. 1971;28:1087–99. doi: 10.1002/1097-0142(1971)28:5<1087::aid-cncr2820280502>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 12.Cahan WG, Woodard HQ, et al. Sarcoma arising in irradiated bone: report of 11 cases. Cancer. 1948;1:3–29. doi: 10.1002/1097-0142(194805)1:1<3::aid-cncr2820010103>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 13.Mavrogenis AF, Pala E, Guerra G, Ruggieri P. Postradiation sarcomas: clinical outcome of 52 patients. J Surg Oncol. 2012;105:570–6. doi: 10.1002/jso.22122. [DOI] [PubMed] [Google Scholar]
- 14.Torres KE, Ravi V, Kin K, Yi M, Guadagnolo BA, May CD, Arun BK, Hunt KK, Lam R, Lahat G, et al. Long-term outcomes in patients with radiation-associated angiosarcomas of the breast following surgery and radiotherapy for breast cancer. Ann Surg Oncol. 2012;20:1267–74. doi: 10.1245/s10434-012-2755-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiklund TA, Blomqvist CP, Raty J, Elomaa I, Rissanen P, Miettinen M. Postirradiation sarcoma: analysis of a nationwide cancer registry material. Cancer. 1991;68:524–31. doi: 10.1002/1097-0142(19910801)68:3<524::aid-cncr2820680313>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 16.Thijssens KM, van Ginkel RJ, Suurmeijer AJ, Pras E, van der Graaf WT, Hollander M, Hoekstra HJ. Radiation-induced sarcoma: a challenge for the surgeon. Ann Surg Oncol. 2005;12:237–45. doi: 10.1245/ASO.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 18.Cox DR. Regression models and life tables. J R Stat Soc B. 1972;34:197–220. [Google Scholar]
- 19.R Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2014. http://www.R-project.org/ [Google Scholar]
- 20.Nakanishi H, Tomita Y, Myoui A, Yoshikawa H, Sakai K, Kato Y, Ochi T, Aozasa K. Mutation of the p53 gene in postradiation sarcoma. Lab Invest. 1998;78:727–33. [PubMed] [Google Scholar]
- 21.Gonin-Laurent N, Gibaud A, Huygue M, Lefevre SH, Le Bras M, Chauveinc L, Sastre-Garau X, Doz F, Lumbroso L, Chevillard S, et al. Specific TP53 mutation pattern in radiation-induced sarcomas. Carcinogenesis. 2006;27:1266–72. doi: 10.1093/carcin/bgi356. [DOI] [PubMed] [Google Scholar]
- 22.Kleinerman RA, Tucker MA, Tarone RE, Abramson DH, Seddon JM, Stovall M, Li FP, Fraumeni JF., Jr Risk of new cancers after radiotherapy in long-term survivors of retinoblastoma: an extended follow-up. J Clin Oncol. 2005;23:2272–9. doi: 10.1200/JCO.2005.05.054. [DOI] [PubMed] [Google Scholar]
- 23.Kleinerman RA, Tucker MA, Abramson DH, Seddon JM, Tarone RE, Fraumeni JF., Jr Risk of soft tissue sarcomas by individual subtype in survivors of hereditary retinoblastoma. J Natl Cancer Inst. 2007;99:24–31. doi: 10.1093/jnci/djk002. [DOI] [PubMed] [Google Scholar]
- 24.Hadj-Hamou NS, Ugolin N, Ory C, Britzen-Laurent N, Sastre-Garau X, Chevillard S, Malfoy B. A transcriptome signature distinguished sporadic from postradiotherapy radiation-induced sarcomas. Carcinogenesis. 2011;32:929–34. doi: 10.1093/carcin/bgr064. [DOI] [PMC free article] [PubMed] [Google Scholar]