Abstract
DNA methylation is an epigenetic mechanism that modulates gene expression in mammalian cells including T cells. Memory T cells are heterogeneous populations. Human effector memory (EM) CD8+ T cells in peripheral blood contain two cell subsets with distinct traits that express low and high levels of the IL-7 receptor alpha chain (IL-7Rα). However, epigenetic mechanisms involved in defining such cellular traits are largely unknown. Here we employ genome-wide DNA methylation and individual gene expression to show the possible role of DNA methylation in conferring distinct traits of chemotaxis and inflammatory responses in human IL-7Rαlow and high EM CD8+ T cells. In particular, IL-7Rαlow EM CD8+ T cells had increased expression of CX3C receptor 1 (CX3CR1) along with decreased DNA methylation in the CX3CR1 gene promoter compared to IL-7Rαhigh EM CD8+ T cells. Altering the DNA methylation status of the CX3CR1 gene promoter changed its activity and gene expression. IL-7Rαlow EM CD8+ T cells had an increased migratory capacity to the CX3CR1 ligand fractalkine compared to IL-7Rαhigh EM CD8+ T cells, suggesting an important biological outcome of the differential expression of CX3CR1. Moreover, IL-7Rαlow EM CD8+ T cells induced fractalkine expression on endothelial cells by producing IFN-γ and TNF-α, forming an autocrine amplification loop. Overall, our study shows the role of DNA methylation in generating unique cellular traits in human IL-7Rαlow and high EM CD8+ T cells, including differential expression of CX3CR1, as well as potential biological implications of this differential expression.
Introduction
DNA methylation is a type of epigenetic mechanism that can be maintained during cell division and propagated to daughter cells (1–3). DNA methylation can affect the accessibility of DNA to transcription factors and RNA polymerases, leading to the modulation of gene expression (2, 3). In mammals, DNA methylation is found at cytosines within CpG dinucleotides. DNA methyltransferases (Dnmts) regulate this process by adding methyl groups to cytosines (4). In general, DNA hypomethylation is associated with active gene expression while DNA hypermethylation is related to decreased gene expression (4). DNA methylation has an important role in the differentiation of CD4+ T cell subsets. Hypomethylation of the IFNG gene was found in human CD4+ T helper (Th) 1 cells with the capacity to produce IFN-γ (5). Similarly, hypothmethylation of the il4 gene occurred during the development of mouse Th2 cells that produced high levels of IL-4 (6) while the ifng gene became rapidly demethylated in memory, but not naive CD8+ T cells, upon activation in mice (7). Also, DNA methylation has been implicated in globally regulating antigen-specific effector CD8+ T cell function following acute lymphocytic choriomeningitis virus infection (8).
IL-7, a member of the common cytokine-receptor γ-chain family of cytokines, is produced by multiple stromal cells, including epithelial cells in the thymus and bone marrow (9). IL-7 is essential in the development and maintenance (homeostasis) of naïve and memory CD8+ T cells by promoting cell survival (10–13). The effect of IL-7 on T cells is controlled by the expression of the specific receptor (R) for IL-7 that is composed of two chains: the high affinity IL-7Rα chain (CD127) and the common cytokine γ chain (γC) (CD132) (10, 11). In mice, compared to cells with low levels of IL-7Rα expression, effector CD8+ T cells with high levels of IL-7Rα expression survived better and became memory CD8+ T cells during microbial infections (10, 11)
Previously, we reported two unique subsets of effector memory (CCR7−, EM) CD8+ T cells that expressed low and high levels of IL-7Rα (IL-7Rαlow and high) in human peripheral blood (14). Compared to IL-7Rαhigh EM CD8+ T cells, IL-7Rαlow EM CD8+ T cells were highly antigen-experienced cells with limited T cell receptor (TCR) repertoire and decreased expression of the co-stimulatory molecules CD27 and CD28 (14). Also, IL-7Rαlow EM CD8+ T cells had increased expression levels of perforin, a cytotoxic molecule. The differential expression of IL-7Rα by EM CD8+ T cells was associated with different levels of DNA methylation in the IL7RA gene promoter (15). Expansion of the IL-7Rαlow EM CD8+ T cells was found in older adults and patients with systemic lupus erythematosus (SLE), suggesting a potential association of this cell subset with immunosenescence and inflammation (14, 16). However, the exact functional characteristics of IL-7Rαlow and high EM CD8+ T cells and the mechanism(s) defining such characteristics are still largely unknown. Here we show the possible role of DNA methylation in conferring the distinct traits of human IL-7Rαlow and high EM CD8+ T cells, including the differential expression of CX3CR1, as well as the biological relevance of such differential expression.
Materials and Methods
Human subjects
Healthy adult subjects who were not taking immunosuppressive drugs and did not have a disease affecting the immune system were recruited (14, 17). Informed consent was obtained from all subjects. This work was approved by the institutional review committee of Yale University.
Cells and flow cytometry
Mononuclear cells were prepared from peripheral blood on FicollPAQUE (GE Healthcare, Piscataway, NJ) gradients. Peripheral blood mononuclear cells (PBMCs) were labeled with antibodies to APC-cyanin (Cy)-CD3, Pacific Blue–CD8α, PE–Cy5–CD45RA, PE-Cy7-CCR7 (all from BD Biosciences, San Diego, CA), IL-7Rα antibodies (R&D Systems, Minneapolis, MN) and PE-CX3CR1, PE-CXCR1 or PE-CXCR6 (BioLegend, San Diego, CA). Unconjugated IL-7Rα antibodies were labeled with FITC conjugated anti-goat IgG (Santa Cruz Biotechnology, Santa Cruz, CA) secondary antibodies. Some cells were fixed, permeabilized using Cytofix/Cytoperm solution (BD Biosciences), and additionally labeled with antibodies to PE-CCL3, PE-CCL4 or PE-CCL20 (all from R&D Systems). Labeled cells were analyzed using an LSRII® flow cytometer (BD Biosciences) and FlowJo software (TreeStar, Ashland, OR).
Cell sorting
Freshly isolated PBMCs were stained with antibodies to APC-Cy7-CD16, Pacific Blue–CD8α, PE–cyanin 5 (Cy5)–CD45RA, PE-Cy7-CCR7 and IL-7Rα antibodies followed by labeling with FITC-conjugated anti-goat IgG antibodies to detect unconjugated IL-7Rα antibodies. Labeled cells were sorted into IL-7Rαlow and high EM (CD45RA+/− CCR7−) CD8+ T cells using a FACSAria® (BD Biosciences). The purity of cells was greater than 97%.
Identification of differentially methylated sites
The Infinium Human Methylation27 array (Illumina Inc.) was used to analyze bisulfite treated DNA from IL-7Rαlow and high EM (CD45RA− CCR7+/−) CD8+ T cells. The methylation array data were submitted to the Gene Expression Ommibus (accession number: GSE67816, http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=mtctwsmolxifjcz&acc=GSE67816). The standard protocol from Illumina Inc. was used for DNA purification and bisulfite conversion. Prior to computing the methylation signal, probes with ≥ 0.05 “Detection P value” were removed from analysis. To determine the differentially methylated sites between IL-7Rαlow and high EM CD8+ T cells, we performed an integrative statistical method which involves the integration of the t-test and the median fold-change test (18). For this analysis, we used the M-value method rather than the Beta-value method to compute methylation signal. The M value is approximately homoscedastic in the entire methylation range, providing a better performance of the statistical test in the low and high methylation ranges whereas the Beta value method has a bounded range, violating the normality assumption underlying the t-test and median fold-change test (19). The M-values were computed as the log2 ratio of the signals of the methylation-specific probe over the unmethylation-specific probe (20) and were normalized using the quantile method (21). With the normalized M values, we conducted the t-test and the median fold-change test. Individual P values from the two tests were integrated into the false discovery rate (FDR) using Stouffer’s method (18). In selecting differentially methylated sites, we applied an FDR cutoff of 0.05 and a constant fold change threshold of M values of 1.8 to increase true positives of differentially methylated probes. To determine the fold-change threshold, we estimated an empirical null distribution by permuting the samples, and calculated the 0.5 percentiles (α = 0.01) of the fold changes of M values using the null distribution, resulting the cutoff as 1.8. To explore cellular processes enriched by genes with differentially methylated sites, functional enrichment analysis was performed using the Database for Annotation, Visualization and Integrated Discovery (DAVID) software (22). Finally, we identified the annotation clusters comprised of significantly enriched cellular processes and pathways and genes involved in the annotation clusters using the functional annotation cluster report functionality in the DAVID.
Quantitative RT-PCR (RT-qPCR)
Total RNA was isolated from sorted cells using the RNeasy Plus Micro kit (Qiagen, Valencia, CA) and cDNA was synthesized. Real-time quantitative reverse-transcription–polymerase chain reaction (RT-qPCR) was performed on an Mx3005P QPCR system (Stratagene, Santa Clara, CA) using the 2x Brilliant SYBR green master mix (Stratagene). Primers were designed using PrimerBank (http://pga.mgh.harvard.edu/primerbank/index.html). Primers used for RT-qPCR are as follows: CX3CR1 (forward, 5′-TTGCCCTCACCAACAGCAAG-3′; reverse, 5′-AAGGCGGTAGTGAATTTGCAC-3′), fractalkine (forward, 5′-CCCGGAGCTGTGGTAGTAAT-3′; reverse, 5′-AAGGTGGAGAATGGTCAAGG-3′) and ACTINB, (forward, 5′-CGTGGACATCCGCAAAGA-3′; reverse, 5′-TGCATCCTGTCGGCAATG-3′). The levels of gene expression were normalized to the expression of ACTINB. The comparative CT method (ΔΔCT) was used for the quantification of gene expression.
Bisulfite genomic DNA sequencing
To determine the methylation status of CpG sequences in the CX3CR1 gene promoter, bisulfite genomic DNA sequencing was performed. Bisulfite modification was performed using EpiTect Bisulfite Kits (Qiagen) according to the manufacturer’s instructions. The Bisulfite modified-genomic DNA was amplified using bisulfite-specific PCR primers designed by MethPrimer (http://www.urogene.org/cgi-bin/methprimer/methprimer.cgi). The sequences of the primers are listed in Supplemental Figure 1. The PCR products were cloned into pCR2.1 using a TA cloning kit (Invitrogen, Grand Island, NY) and approximately 10 individual colonies were isolated and sequenced per experiment.
Promoter assay
To generate the CX3CR1 gene promoter construct (pGL3-CX3CR1), the CX3CR1 gene promoter region was amplified from genomic DNA using high-fidelity DNA polymerase (Invitrogen Life Technologies), cloned into pGL3-basic vector (Promega, Madison, WI) and verified by sequencing. In methylating the CX3CR1 promoter construct, the pGL3-CX3CR1 construct and pGL3-basic vector were incubated for 1 hour at 37°C in the presence or absence of SssI methylase (New England Biolabs, Beverly, MA) with 80 μM S-adenosylmethionine (New England Biolabs). Methylated and unmethylated constructs were purified using a Qiagen PCR purification kit (Qiagen) and transfected into 293 T cells using lipofectamine (Invitrogen Life Technologies) according to the manufacturer’s instructions. The pRL-TK (Renilla luciferase control reporter vector, Promega) was co-transfected as an internal control for transfection efficiency. Transfected cells were harvested after 24 hours and analyzed according to the manufacturer’s instructions for luciferase and Renilla activities.
In vitro migration assay
PBMCs were seeded at a density of 1 × 106 cells in 50 μL of RPMI 1640 supplemented with 0.2% bovine serum albumin (BSA) per well in the upper chambers of a Corning® HTS Transwell 96-well Permeable Support with the 5 μm pore polycarbonate membrane (Corning Inc., Chicago, IL). The lower chambers were filled with 2% FBS RPMI containing fractalkine (100 ng/mL, R&D Systems). The cells were incubated for 4 hours at 37°C in a CO2 humid incubator. Cells in the lower chambers were collected and labeled with antibodies to APC-Cy7-CD3, Pacific Blue–CD8α, PE–Cy5–CD45RA, PE-Cy7-CCR7 and IL-7Rα antibodies followed by labeling with FITC conjugated anti-goat IgG (Santa Cruz Biotechnology) secondary antibodies. Labeled cells were analyzed on an LSRII® flow cytometer.
Stimulation of primary human umbilical vein endothelial cells (HUVECs)
Primary HUVECs (passages 2–3) purchased from the Yale Stem Cell Center were seeded in 48-well plates at a cell density of 3 × 104 in Medium 200 containing low serum growth supplement (GIBCO) per well and incubated for 24 hours. The cells were incubated for 8 hours with the supernatant (10% final concentration) of FACS-sorted 7Rαlow or high EM CD8+ T cells that were stimulated for 18 hours in RPMI 1640 media supplemented with 10% FBS in the presence of anti-CD3 antibodies (OKT3). In some experiments, supernatants were pretreated for 10 min with anti-human IFN-γ (1μg/mL) and anti-TNF-α (1μg/mL) antibodies (R&D Systems). The cells were harvested, fixed, permeabilized and stained with PE-conjugated anti-human fractalkine mouse antibodies (R&D Systems). Stained cells were analyzed using an LSRII® flow cytometer (BD Biosciences) and FlowJo software. Fractalkine gene expression in HUVECs was analyzed by RT-qPCR.
Statistical analysis
Results were statistically analyzed by the matched or unmatched Student’s t-test or ANOVA as appropriate. P values less than 0.05 were considered statistically significant.
Results
A set of genes, including chemotaxis-related genes, is differentially methylated in human IL-7Rαlow and high EM CD8+ T cells
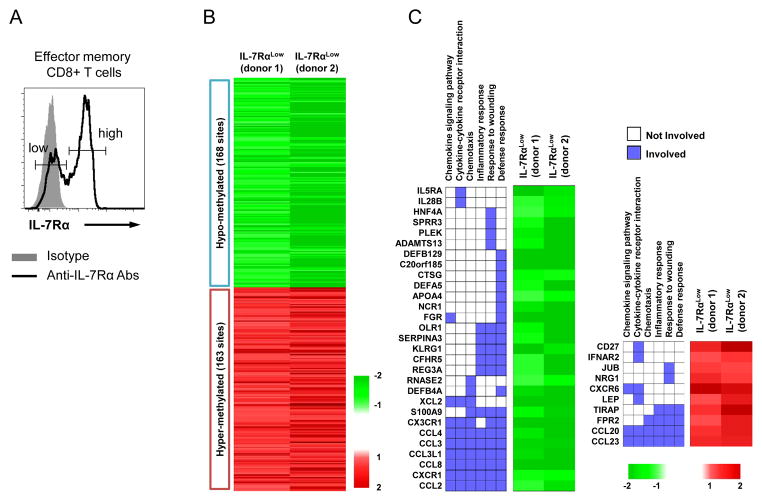
IL-7Rα can define different subsets of CD8+ T cells with distinct cellular characteristics (11, 14). Although DNA methylation is an important gene regulatory mechanism, whether this mechanism is associated with the distinct traits in human effector memory (EM) CD8+ T cell subsets with low and high levels of IL-7Rα expression is largely unknown. To address this question, we first analyzed DNA methylation in FACS-purified human IL-7Rαlow and high EM CD8+ T cells (Fig 1A) using Infinium Human Methylation27 array. We identified 331 differentially methylated CpG sites between IL-7Rαlow and high EM CD8+ T cells with FDR < 0.05 with fold change of M value ≥ 1.8 (Fig 1B). Out of the 331 CpG sites, methylation of 168 sites from 159 genes and 163 sites from 147 genes decreased and increased in IL-7Rαlow EM CD8+ T cells, respectively, compared to IL-7Rαhigh EM CD8+ T cells (Fig. 1B). We next performed functional annotation clustering analysis using the DAVID software to identify significantly enriched cellular processes and pathways. Notably, chemokine signaling pathway, chemotaxis, cell adhesion, cytokine-cytokine receptor interaction, inflammatory response, response to wounding, and defense response were identified as a significantly enriched annotation cluster by the genes with differentially methylated CpG sites (Fig. 1C and Table I). Among the enriched cellular processes and pathways in the annotation cluster, chemokine- and cytokine-related terms were more significantly enriched (P = 4.93E-6) than others (Table I), and an array of chemokines and chemokine-receptors including CX3CR1, CXCR1, CXCR6, CCL2, 3, 4, 8, 20, 23 and CCL3L1 belonged to the annotation cluster (Fig 1C). These findings suggest that low and high levels of IL-7Rα expression by EM CD8+ T cells can be associated with their distinct chemotactic functions through differential CpG methylation of the genes related to chemokines and their receptors.
Figure 1. Human IL-7Rαlow and high effector memory CD8+ T cells have differential DNA methylation.
IL-7Rαlow and high effector memory (CCR7−, EM) CD8+ T cells were purified from fresh PBMCs of 2 healthy donors using a FACSAria®. DNA were purified from sorted cells and analyzed using an Illumina human DNA methylation array chip (see details in materials and methods). (A) Representative histograms showing IL-7Rαlow and high cells in human peripheral EM CD8+ T cells. (B) Heatmap showing the pattern of differentially methylated CpG sites between IL-7Rαlow and high EM CD8+ T cells. The red and green colors indicate increased and decreased methylation of CpG sites, respectively. (C) The most significantly enriched annotation cluster by the genes with differentially methylated sites in IL-7Rαlow and high EM CD8+ T cells. Two-dimensional map displays the genes that are involved in the annotation cluster. Heatmap display the representative differential methylation patterns of the genes in IL-7Rαlow EM CD8+ T cells compared to IL-7Rαhigh EM CD8+ T cells. Left and right panels are for the genes with decreased and increased methylation, respectively.
Table I.
Enriched Cellular Process of Differentially Methylated Genes in IL-7Rαlow and high EM CD8+ T cells.
| Category | Enriched Cellular Process | P Value |
|---|---|---|
| GOBP* | Chemotaxis | 0.00000493 |
| Cell adhesion | 0.000103 | |
| Cell-cell adhesion | 0.0035712 | |
| Positive regulation of MAPKKK cascade | 0.0098789 | |
| Cell proliferation | 0.01034598 | |
| Positive regulation of JNK cascade | 0.03514993 | |
|
| ||
| KEGG** | Cytokine-cytokine receptor interaction | 4.20E-05 |
| Chemokine signaling pathway | 0.001006 | |
| Systemic lupus erythematosus | 0.01279013 | |
| Graft-versus-host disease | 0.04046789 | |
Gene Ontology Biological Process
Kyoto Encyclopedia of Genes and Genomes
IL-7Rαlow EM CD8+ T cells have increased levels of the CX3CR1 gene and protein expression
To determine the consequences of differential DNA methylation on transcription in these cells, we measured the gene expression of the chemokine receptors and chemokines that showed different levels of DNA methylation in IL-7Rαlow and high EM CD8+ T cells on the DNA methylation array. We detected increased levels of CX3CR1, CXCR1, CCL3 and CCL4 in IL-7Rαlow EM CD8+ T cells while the levels of CXCR6 and CCL20 genes were higher in IL-7Rαhigh EM CD8+ T cells (Fig 2A and Supplemental Fig 2A). The expression levels of other chemokine genes were below detection limits (CCL2, CCL8) or similar (CCL23, CCL3LI) between the two cell subsets (data not shown). We analyzed the protein expression of CX3CR1, CXCR1, CCL3, CCL4, CXCR6 and CCL20 by IL-7Rαlow and high EM CD8+ T cells. In accordance with gene expression, CX3CR1 and CXCR1 were highly expressed by IL-7Rαlow EM CD8+ T cells, which occurred in both CD45RA+ and − EM CD8+ T cells (Fig 2B–C and Supplemental Fig 2B–C). However, the expression levels of other molecules were not different as measured by flow cytometry (Supplemental Fig 2B).
Figure 2. Human IL-7Rαlow and high effector memory CD8+ T cells have different levels of CX3CR1 gene and protein expression.
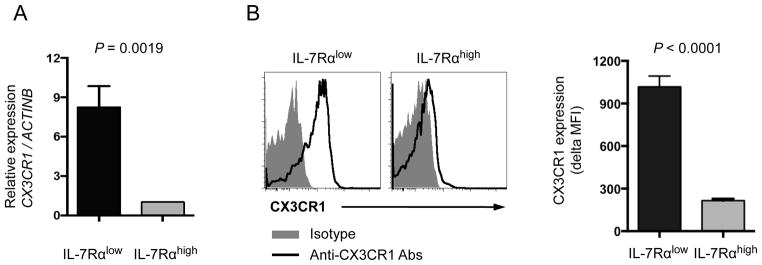
(A) RT-qPCR analysis of CX3CR1 gene expression in FACS-sorted IL-7Rαlow and high effector memory (EM) CD8+ T cells of healthy individuals (n = 5) (B–C) Flow cytometric analysis of CX3CR1 expression on IL-7Rαlow and high EM CD8+ T cells in peripheral blood of healthy people (n = 39). (B) Representative histograms of CX3CR1 and isotype control staining. (C) Mean fluorescent intensity (MFI) of CX3CR1 expression by IL-7Rαlow and high EM CD8+ T cells. Delta MFI of CX3CR1 expression was obtained by subtracting MFI of isotype control staining from MFI of CX3CR1 staining. Bars and error bars indicate mean and standard error of the mean (SEM), respectively.
IL-7Rαlow and high EM CD8+ T cells have different levels of DNA methylation in the CX3CR1 gene promoter region
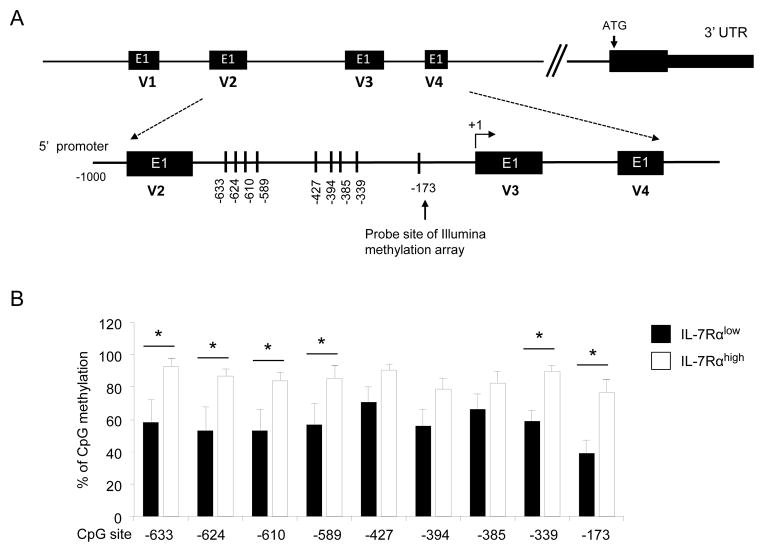
The DNA methylation array covers only a limited number of CpG sites in human genes. Thus, we analyzed the DNA methylation status in the CpG site included in the array chip and other CpG sites of the CX3CR1 gene promoter. We focused our analysis on the CX3CR1 gene for several reasons. Despite the important role of the CX3CR1 gene in regulating the migration of inflammatory cells in normal and pathologic conditions, the molecular mechanism(s) involved in regulating its expression is largely unknown. Also, CX3CR1 was expressed at high levels by IL-7Rαlow EM CD8+ T cells which are known to express increased levels of effector molecules including perforin, granzyme B and IFN-γ (14, 23). Of note, several variants of CX3CR1 transcripts were reported in human cells (24). According to NCBI Gene, there are four transcript variants of human CX3CR1 including NM_001171174.1 (variant 1), NM_001171171.1 (variant 2), NM_001171172.1 (variant 3) and NM_001337.3 (variant 4) (Fig 3A). The differentially methylated probes of the CX3CR1 CpG site included in the array chip were for the cytosine of −173 position from the first nucleotide of the first exon of transcript variant 3 (+1) (Fig 3A). We first analyzed DNA methylation status at the −173 position in IL-7Rαlow and high EM CD8+ T cells by sequencing DNA after bisulfite treatment. IL-7Rαlow EM CD8+ T cells had lower levels of DNA methylation at −173 than IL-7Rαhigh EM CD8+ T cells (Fig 3B). We also measured DNA methylation status in additional CpG sites in the region upstream to −173, corresponding to the positions −633, −624 −610, −589, −427, −394, −385, and −339 from the first exon of transcript variant 3. Notably, IL-7Rαlow EM CD8+ T cells had lower levels of DNA methylation than IL-7Rαhigh EM CD8+ T cells at 5 of the additional 8 sites that were analyzed (Fig 3B). A similar trend was noticed in other CpG sites without reaching the levels of statistical significance. Consistent with the measurement of the total CX3CR1 gene transcript, the transcript variants 1, 3 and 4 were more highly expressed in IL-7Rαlow EM CD8+ T cells than in IL-7Rαhigh EM CD8+ T cells (Supplemental Fig 3). Both cell subsets barely expressed the transcript variant 2. We also analyzed the CX3CR1 gene and protein expression as well as the DNA methylation status in the CX3CR1 promoter in naïve CD8+ T cells. The gene and protein expression levels of this molecule were lower in naïve CD8+ T cells than in IL-7Rαlow EM CD8+ T cells (Supplemental Fig 4 and Fig 6A). The CpG sites indicated in Fig 3A were methylated at levels greater than 90% in naïve CD8+ T cells (data not shown).
Figure 3. DNA methylation profiles in the CX3CR1 gene promoter are different in IL-7Rαlow and high effector memory CD8+ T cells.
(A) Illustration of the promoter regions of four CX3CR1 transcript variants (V1, V2, V3 and V4) and detailed map of analyzed CpG sites. The first exons (E1) of individual variants are indicated in black boxes. The CpG site (−173) included in the original DNA methylation array chip and additional CpG sites (0 to −1 KB) are indicated with vertical lines and numbers. The latter numbers were given in respect to the first nucleotide of the first exon of variant 3 (+1). (B) Methylation status of CpG sites in the CX3CR1 gene promoter region depicted in (A) among IL-7Rαlow and high effector memory (EM) CD8+ T cells. Details for bisulfite treatment and DNA methylation analysis are stated in Materials and Methods. Bars and error bars indicate the mean and SEM for individual CpG sites in IL-7Rαlow and high EM CD8+ T cells, respectively, (n = 7 donors). *P < 0.05
Figure 6. IL-7Rαlow EM CD8+ T cells have increased migration capacity in response to fractalkine.
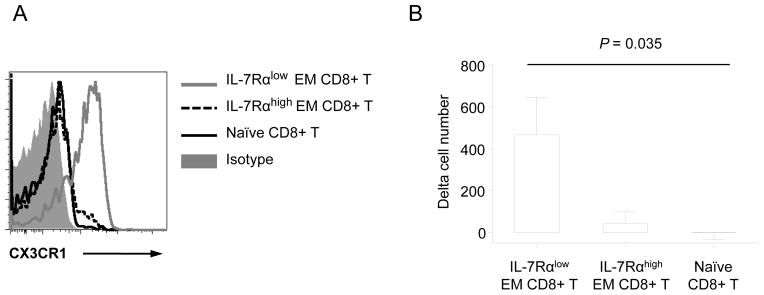
(A) Flow cytometric analysis of CX3CR1 expression on IL-7Rαlow, high EM and naïve human CD8+ T cells. Representative data from more than 10 independent experiments. (B) PBMCs at 1 × 106 cells purified from the peripheral blood of healthy adult donors were seeded into the upper chambers of a transwell migration culture unit. Tissue culture media containing with or without fractalkine (100 ng/ml) was added to the lower chambers. After 4 hours of incubation, cells in the lower chambers were collected and labeled with antibodies to CD3, CD8, CD45RA, CCR7 and IL-7Rα. Labeled cells were analyzed using flow cytometry to analyze IL-7Rαlow, high EM and naïve CD8+ T cells. Numbers on the Y-axis indicate the difference of cell numbers (delta cell numbers) between the lower chambers containing with and without fractalkine. Bars and error bars indicate the mean and SEM, respectively (n = 8).
Altering DNA methylation affects CX3CR1 expression and its gene promoter activity
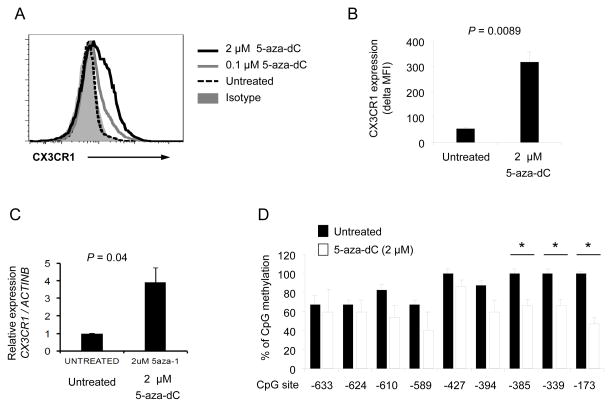
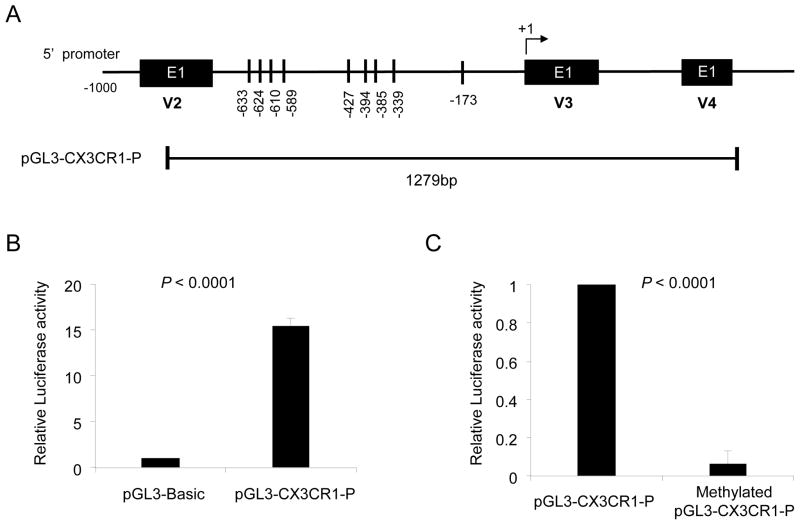
DNA methylation can be inhibited by 5-aza-2′-deoxycytidine (5-aza-dC), a nucleoside analog of cytidine, which traps DNA methyltransferase 1 during cell replication (25, 26). We noticed that Jurkat cells, a leukemic T cell line, have low levels of CX3CR1 gene and protein expression with high levels of DNA methylation at several CpG sites, including the one at −173, in the CX3CR1 gene promoter (Fig 4 and Supplemental Fig 4). Since Jurkat T cells undergo spontaneous replication, which is required for the effect of 5-aza-dC, we incubated these cells with or without 5-aza-dC to further explore the role of DNA methylation in regulating CX3CR1 expression. Indeed, 5-aza-dC lowered DNA methylation and enhanced the expression of both CX3CR1 gene and protein by Jurkat cells (Fig 4B–D). We next determined the effect of DNA methylation on the promoter activity of the CX3CR1 gene. We cloned a region containing the CpG sites of the CX3CR1 gene that was analyzed for DNA methylation status (Fig 5A). The promoter activity of this region was validated by inserting it into the pGL3 vector (pGL3-CX3CR1-P) and measuring luciferase activity in transfected 293T cells (Fig 5B). Methylating the pGL3-CX3CR1-P reduced the luciferase activity (Fig 5C), supporting the role of DNA methylation in regulating expression of CX3CR1.
Figure 4. 5-aza-dC up-regulates the expression of CX3CR1 by Jurkat T cells.
Jurkat T cells were cultured for 4 days with or without 5-aza-dC (0, 0.1 and 2 μM). (A–B) Flow cytometric analysis of CX3CR1 expression on untreated and 5-aza-dC-treated Jurkat T cells. (A) Representative histograms of CX3CR1 and isotype control staining. (B) Delta MFI of CX3CR1 expression obtained by subtracting MFI values of isotype controls from MFI values of CX3CR1 staining. (C) RT-qPCR analysis of CX3CR1 gene expression in untreated and 5-aza-dC-treated Jurkat T cells. (D) The methylation status of individual CpG sites in the CX3CR1 gene promoter in untreated and 5-aza-dC-treated Jurkat T cells at day 4. Bars and error bars indicate the mean and SEM, respectively, (n = 3 (B), 7 (C) and 3 (D)). *P < 0.05
Figure 5. CX3CR1 gene promoter activity is affected by DNA methylation.
(A) Schematic presentation of the CX3CR1 gene promoter region inserted into the luciferase reporter gene construct (pGL3-CX3CR1-P). (B) The pGL3-CX3CR1-P and pGL3-basic vector were transiently transfected into 293 T cells with the pRL-TK vector (Renilla luciferase control reporter vector) as an internal control. Promoter activity is expressed as relative light units (RLU) to the pGL3-basic vector of which was set as 1. (C) pGL3-CX3CR1-P was methylated using SssI methylase (see details in Materials and Methods). Unmethylaed and methylated pGL3-CX3CR1-P were transfected into 293T cells with pRL-TK vector. Promoter activity is expressed as RLU to the unmethylated pGL3-CX3CR1-P of which was set as 1. Bars and error bars indicate the mean and SD, respectively, (n = 6 independent experiments performed in triplicates) (B, C).
IL-7Rαlow EM CD8+ T cells have increased migration capacity in response to fractalkine
The natural ligand for CX3CR1 is the chemokine fractalkine (CX3CL1) (27). We found an increased expression of CX3CR1 on IL-7Rαlow EM CD8+ T cells compared to IL-7Rαhigh EM and naïve CD8+ T cells (Fig 6A). We thus determined whether IL-7Rαlow EM CD8+ T cells had an increased migration capacity in response to fractalkine compared to other CD8+ T cell subsets with lower levels of CX3CR1 expression using an in vitro trans-well migration assay. While the migration of cells varied among donors, the number of IL-7Rαlow EM CD8+ T cells that migrated to the lower chamber in the presence of fractalkine was higher than the number of IL-7Rαhigh EM and naïve CD8+ T cells that migrated to the same chamber (Fig 6B). These findings suggest that IL-7Rαlow EM CD8+ T cells have increased migration capacity in response to fractalkine in association with higher expression of CX3CR1 compared to other CD8+ T cell subsets.
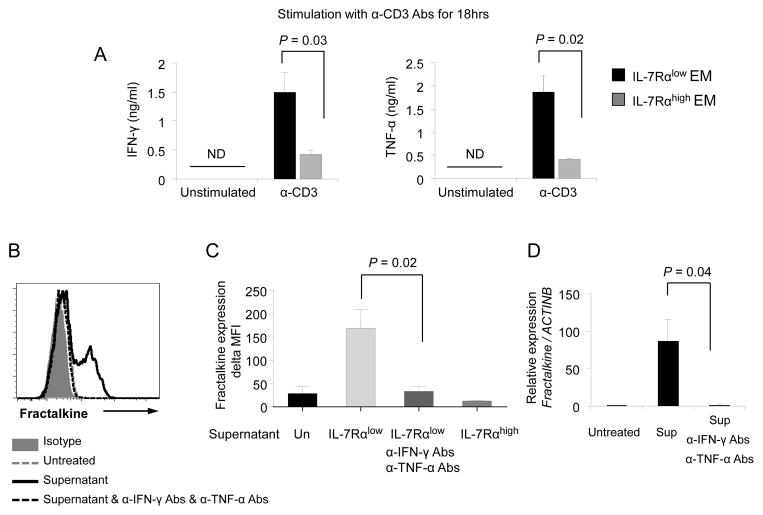
IL-7Rαlow EM CD8+ T cells produce high levels of IFN-γ and TNF-α that can up-regulate fractalkine expression by endothelial cells
We next determined whether IL-7Rαlow EM CD8+ T cells could induce the expression of fractalkine, possibly forming an autocrine amplification loop. Fractalkine is expressed by cells including endothelial cells, lymphocytes and neurons and has been associated with pathologic conditions such as atherosclerosis, asthma, rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), Crohn’s disease, cancers and fibrogenesis (28, 29). It is known that IFN-γ and TNF-α can induce fractalkine on endothelial cells (29). Indeed, IL-7Rαlow EM CD8+ T cells rapidly produced these cytokines at high levels upon T cell receptor triggering (Fig 7A). Incubating human umbilical vein endothelial cells (HUVECs) with the culture supernatant of anti-CD3 antibody-stimulated IL-7Rαlow EM CD8+ T cells induced fractalkine expression by HUVECs as measured by RT-qPCR and flow cytometry (Fig 7B–D). However, this finding was not observed when HUVECs were incubated with the culture supernatant of anti-CD3 antibody-stimulated IL-7Rαhigh EM CD8+ T cells (Fig 7C). The expression of fractalkine on HUVECs by the culture supernatant of IL-7Rαlow EM CD8+ T cells was reduced by adding neutralizing antibodies (anti-IFN-γ and -TNF-α) to the culture supernatant during the incubation (Fig 7C–D). These findings suggest that IL-7Rαlow EM CD8+ T cells not only have a potent migration response to fractalkine but also have the capacity to induce the same chemokine, forming an autocrine amplification loop.
Figure 7. IL-7Rαlow effector memory CD8+ T cells produce high levels of IFN-γ and TNF-α, leading to up-regulation of fractalkine expression by HUVECs.
(A) ELISA of IFN-γ and TNF-α in culture supernatants of FACS-sorted IL-7Rαlow and high effector memory (EM) CD8+ T cells that were incubated for 18 hours with or without anti-CD3 antibodies. (B–D) HUVECs were incubated for 8 hours in the culture supernatants (Sup, 10% final concentration) of anti-CD3 antibody-stimulated IL-7Rαlow or high EM CD8+ T cells from (A) in the presence or absence of human anti-IFN-γ and TNF-α neutralizing antibodies (1μg/mL for both). Un: not treated with supernatant. (B–C) Flow cytometric analysis of fractalkine by HUVECs. (B) Representative histograms of the flow cytometric analysis. (C) Delta MFI values of fractalkine expression were obtained by subtracting MFI values of isotype control staining from MFI values of fractalkine staining. (D) RT-qPCR analysis of fractalkine gene expression in HUVECs. Bars and error bars indicate the mean and SEM, respectively (n = 3 (A), 2–5 (C–D)). ND indicates not detected.
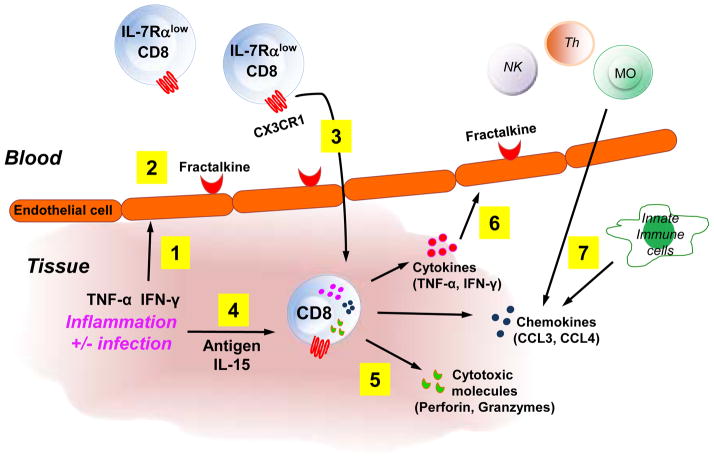
A proposed model for the role of IL-7Rαlow EM CD8+ T cells in inflammation
The primary role of CD8+ T cells is to kill infected or tumor-transformed cells by producing cytotoxic molecules (30). CD8+ T cells also are found in the affected tissues of an array of inflammatory disorders including atherosclerosis, autoimmune and respiratory diseases (31–36). In fact, CD8+ T cells can produce molecules such as cytokines and chemokines, contributing to inflammation (30). IL-7Rαlow EM CD8+ T cells express high levels of the cytotoxic molecules perforin and granzyme B (14, 23) as well as the inflammatory cytokines IFN-γ and TNF-α. Thus, it is conceivable that the inflammatory cytokines IFN-γ and TNF-α produced from innate immune cells like NK cells and macrophages in an infected or damaged site can induce fractalkine on endothelial cells, leading to the attraction of IL-7Rαlow EM CD8+ T cells with high levels of CX3CR1 expression to the infected or damaged site. Activating the effector functions of the recruited IL-7Rαlow EM CD8+ T cells would lead to cytotoxicity and the release of cytokines and chemokines. The released molecules would further recruit immune cells directly or indirectly by inducing additional chemokines like fractalkine. Figure 8 shows this model proposing the possible role of IL-7Rαlow EM CD8+ T cells in defending the host against infections or tumors as well as in contributing to inflammation.
Figure 8. A proposed model for the role of IL-7Rαlow effector memory CD8+ T cells in promoting inflammation by forming an amplification loop of CX3CR1 and fractalkine.
In response to an infection or tissue damage at a peripheral site, IFN-γ and TNF-α are secreted from innate immune cells like natural killer (NK) cells and macrophages (1), leading to inducing the expression of fractalkine by endothelial cells (2). In the bloodstream, CX3CR1-expressing IL-7Rαlow EM CD8+ T cells and other immune cells (not shown) migrate to the endothelial cells in response to fractalkine and move into the infected or damaged peripheral site (3) where activated IL-7Rαlow EM CD8+ T cells promote inflammation by producing chemokines (CCL3, CCL4), cytokines (IFN-γ, TNF-α) and cytotoxic molecules (perforin, granzymes) with further immune cell recruitment, activation and tissue damage (5–7). Also, the secreted IFN-γ and TNF-α further fractalkine expression on the endothelial cells, resulting in additional recruitment of CX3CR1-espressing IL-7Rαlow EM CD8+ T and other cells by forming an amplification loop of IL-7Rαlow EM CD8+ T cells, CX3CR1 and fractalkine that augments inflammation (6).
Discussion
Memory T cells are heterogeneous populations with distinct cellular traits. Cell surface molecules like cytokine receptors can define such cell subsets (11, 14, 37). Previously, we reported the presence of cell subsets expressing low and high levels of IL-7Rα in human peripheral EM CD8+ T cells that have different phenotypic and functional traits (14). Here we showed the possible role of DNA methylation, an epigenetic gene regulatory mechanism, in conferring these characteristics, especially the ones associated with chemotaxis and inflammatory response. In particular, IL-7Rαlow EM CD8+ T cells had an increased expression of CX3CR1 along with decreased DNA methylation in the CX3CR1 gene promoter compared to IL-7Rαhigh EM CD8+ T cells. Also, altering the DNA methylation status of the CX3CR1 gene promoter changed the promoter activity and expression of CX3CR1, further supporting the role of DNA methylation in regulating the gene expression of CX3CR1 in human CD8+ T cells. IL-7Rαlow EM CD8+ T cells potently migrated to the CX3CR1 ligand fractalkine, suggesting the biological significance of increased expression of CX3CR1. Moreover, IL-7Rαlow EM CD8+ T cells induced fractalkine on HUVECs by producing high levels of IFN-γ and TNF-α, possibly forming an autocrine amplification loop. Overall, our study shows the involvement of DNA methylation in generating unique cellular traits of human IL-7Rαlow and high EM CD8+ T cells, especially the differential expression of CX3CR1, as well as the possible biological implication of such differential expression.
DNA Methylation is one of the mechanisms that mediate epigenetic regulation of gene expression (1–3). Genome-wide DNA methylation studies indicate that the patterns of DNA methylation are different between blood cell populations including T and B cells, which can account in part for unique cellular characteristics of individual cell populations (38). Also, global DNA methylation changes were found during the differentiation of naïve T cells to effector and memory T cells in mice upon viral or antigenic challenge (8, 39). In fact, effector molecules such as IFN-γ, IL-2 and IL-4 can be differentially expressed in T cells through the regulation of DNA methylation of respective genes (5–7, 40). Similarly, the role of DNA methylation in controlling the expressions of FOXP3 and IL-7Rα in humans was reported in naturally occurring regulatory T cells and resting CD8+ T cells, respectively (15, 41). These findings indicate that DNA methylation is involved in defining cellular traits of resting immune cells as well as in programming the function of effector cells during the development of immune responses. In concordance with this notion, our study showed that IL-7Rαlow and high EM CD8+ T cells have distinct DNA methylation profiles in more than 300 genes including particularly those related to chemotaxis, chemokine signaling pathway, cell adhesion, cytokine-cytokine receptor interaction and inflammatory response. In addition, we found the role of DNA methylation in differentially regulating CX3CR1 expression on IL-7Rαlow and high EM CD8+ T cells. Our finding is supported by a recent study reporting that increased DNA methylation of the CX3CR1 gene with decreased gene expression occurred during the in vitro differentiation of human monocytes to macrophages, although this was demonstrated for only a single CpG site and not confirmed by analyzing the effect of DNA methylation on gene expression (42). Overall, DNA methylation likely serves as an important mechanism for regulating the expression of CX3CR1 in multiple immune cells including CD8+ T cells and myeloid cells.
CX3CR1 is the receptor for fractalkine with adhesive and chemoattractant functions (27). CX3CR1 is mainly found on immune cells including T cells, monocytes and natural killer (NK) cells while fractalkine is widely expressed by neurons, epithelial cells, dendritic cells (DCs) and macrophages (27, 29). Also, endothelial cells up-regulate fractalkine upon stimulation with inflammatory cytokines (43–45). Studies reported increased levels of CX3CR1 expression by CD8+ T cells that express the replication senescent molecule CD57 as well as the cytotoxic molecules perforin and granzyme B (46, 47) although the underlying molecular mechanism for this finding was unknown. Of interest, IL-7Rαlow EM CD8+ T cells that have increased expression of CX3CR1 also highly express CD57, perforin and grazyme B compared to other CD8+ T cell subsets including IL-7Rαhigh EM CD8+ T cells (14, 23). Such differential expression of CX3CR1 on IL-7Rαlow EM CD8+ T cells, which is mediated by DNA methylation, appears to have biological implications given the enhanced migratory capacity of this cell subset in response to fractalkine as well as potent inflammatory and cytotoxic properties of IL-7Rαlow EM CD8+ T cells. The membrane bound form of fractalkine, such as the one expressed on endothelial cells, can promote the retention of leukocytes as an adhesion molecule while the soluble form of fractalkine attracts immune cells, including T cells, having a potent chemotactic activity(27, 48, 49). Thus, it is possible that CX3CR1 expressed on circulating IL-7Rαlow EM CD8+ T cells interacts with the membrane bound form of fractalkine and further directs chemotactic migration to the inflamed tissue sites where the soluble form is present (28, 29). IL-7Rαlow EM CD8+ T cells produce high levels of IFN-γ and TNF-α that can up-regulate fractalkine by endothelial cells, suggesting the possible dual roles of this cell subset in CX3CR1/fractalkine-mediated inflammation. Increased expression of fractalkine and/or CX3CR1 has been reported in affected tissues of inflammatory diseases such as atherosclerosis, RA, SLE and systemic sclerosis (27, 29, 50, 51). Inhibiting fractalkine or CX3CR1 reduced disease activity in mouse models of RA, SLE and atherosclerosis (52–54). The expansion of IL-7Rαlow EM CD8+ T cells and increased expression of CX3CR1 on CD8+ T cells were reported in the peripheral blood of patients with SLE and RA, respectively (16, 51). These observations warrant further studies investigating the specific role of CX3CR1 expression by CD8+ T cells in inflammatory diseases.
In summary, our results show the involvement of DNA methylation in defining the unique cellular characteristics of human EM CD8+ T cell subsets that include IL-7Rαlow and high EM CD8+ T cells. In particular, these two cell subsets with distinct traits have different levels of DNA methylation and expression in the genes related to chemotaxis, chemokine signaling pathway and inflammatory response, suggesting a coordinated regulation of such gene expression by DNA methylation. Furthermore, this is the first demonstration of the role of DNA methylation in regulating the expression of the CX3CR1 gene by CD8+ T cells and the potential biological relevance of increased expression of CX3CR1 by IL-7Rαlow EM CD8+ T cells in humans.
Supplementary Material
Acknowledgments
This work was supported in part by grants from the National Institutes of Health (AG028069, AG030834 to IK; U19 AI089992 and contract 272201100019C-3-0-1 to R.R.M. and A.C.S; and K24 AG042489 to A.C.S); and Institute for Basic Science (IBS-R013-G1-2014-a00) funded by Korean Ministry of Science, ICT, and Future Planning.
We thank Ms. Laurie Kramer and Yale Center for Clinical Investigation (UL1 RR024139 from the NCRR) for assisting in the recruitment of human subjects.
References
- 1.Wilson CB, Makar KW, Shnyreva M, Fitzpatrick DR. DNA methylation and the expanding epigenetics of T cell lineage commitment. Semin Immunol. 2005;17:105–119. doi: 10.1016/j.smim.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Egger G, Liang G, Aparicio A, Jones PA. Epigenetics in human disease and prospects for epigenetic therapy. Nature. 2004;429:457–463. doi: 10.1038/nature02625. [DOI] [PubMed] [Google Scholar]
- 3.Fuks F. DNA methylation and histone modifications: teaming up to silence genes. Curr Opin Genet Dev. 2005;15:490–495. doi: 10.1016/j.gde.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Bird AP, Wolffe AP. Methylation-induced repression--belts, braces, and chromatin. Cell. 1999;99:451–454. doi: 10.1016/s0092-8674(00)81532-9. [DOI] [PubMed] [Google Scholar]
- 5.Yano S, Ghosh P, Kusaba H, Buchholz M, Longo DL. Effect of promoter methylation on the regulation of IFN-gamma gene during in vitro differentiation of human peripheral blood T cells into a Th2 population. J Immunol. 2003;171:2510–2516. doi: 10.4049/jimmunol.171.5.2510. [DOI] [PubMed] [Google Scholar]
- 6.Makar KW, Perez-Melgosa M, Shnyreva M, Weaver WM, Fitzpatrick DR, Wilson CB. Active recruitment of DNA methyltransferases regulates interleukin 4 in thymocytes and T cells. Nat Immunol. 2003;4:1183–1190. doi: 10.1038/ni1004. [DOI] [PubMed] [Google Scholar]
- 7.Kersh EN, Fitzpatrick DR, Murali-Krishna K, Shires J, Speck SH, Boss JM, Ahmed R. Rapid Demethylation of the IFN-{gamma} Gene Occurs in Memory but Not Naive CD8 T Cells. J Immunol. 2006;176:4083–4093. doi: 10.4049/jimmunol.176.7.4083. [DOI] [PubMed] [Google Scholar]
- 8.Scharer CD, Barwick BG, Youngblood BA, Ahmed R, Boss JM. Global DNA methylation remodeling accompanies CD8 T cell effector function. J Immunol. 2013;191:3419–3429. doi: 10.4049/jimmunol.1301395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fry TJ, Mackall CL. Interleukin-7: from bench to clinic. Blood. 2002;99:3892–3904. doi: 10.1182/blood.v99.11.3892. [DOI] [PubMed] [Google Scholar]
- 10.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 11.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 12.Schluns KS, Lefrancois L. Cytokine control of memory T-cell development and survival. Nat Rev Immunol. 2003;3:269–279. doi: 10.1038/nri1052. [DOI] [PubMed] [Google Scholar]
- 13.Marrack P, Kappler J. Control of T cell viability. Annu Rev Immunol. 2004;22:765–787. doi: 10.1146/annurev.immunol.22.012703.104554. [DOI] [PubMed] [Google Scholar]
- 14.Kim HR, Hong MS, Dan JM, Kang I. Altered IL-7R{alpha} expression with aging and the potential implications of IL-7 therapy on CD8+ T-cell immune responses. Blood. 2006;107:2855–2862. doi: 10.1182/blood-2005-09-3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim HR, Hwang KA, Kim KC, Kang I. Down-regulation of IL-7Ralpha expression in human T cells via DNA methylation. J Immunol. 2007;178:5473–5479. doi: 10.4049/jimmunol.178.9.5473. [DOI] [PubMed] [Google Scholar]
- 16.Kim JS, Cho BA, Sim JH, Shah K, Woo CM, Lee EB, Lee DS, Kang JS, Lee WJ, Park CG, Craft J, Kang I, Kim HR. IL-7Ralphalow memory CD8+ T cells are significantly elevated in patients with systemic lupus erythematosus. Rheumatology (Oxford) 2012;51:1587–1594. doi: 10.1093/rheumatology/kes100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simpson JL, Scott RJ, Boyle MJ, Gibson PG. Differential proteolytic enzyme activity in eosinophilic and neutrophilic asthma. Am J Respir Crit Care Med. 2005;172:559–565. doi: 10.1164/rccm.200503-369OC. [DOI] [PubMed] [Google Scholar]
- 18.Hwang D, Rust AG, Ramsey S, Smith JJ, Leslie DM, Weston AD, de Atauri P, Aitchison JD, Hood L, Siegel AF, Bolouri H. A data integration methodology for systems biology. Proc Natl Acad Sci U S A. 2005;102:17296–17301. doi: 10.1073/pnas.0508647102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Du P, Zhang X, Huang CC, Jafari N, Kibbe WA, Hou L, Lin SM. Comparison of Beta-value and M-value methods for quantifying methylation levels by microarray analysis. BMC Bioinformatics. 2010;11:587. doi: 10.1186/1471-2105-11-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Irizarry RA, Ladd-Acosta C, Carvalho B, Wu H, Brandenburg SA, Jeddeloh JA, Wen B, Feinberg AP. Comprehensive high-throughput arrays for relative methylation (CHARM) Genome Res. 2008;18:780–790. doi: 10.1101/gr.7301508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19:185–193. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- 22.da Huang W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 23.Lee N, You S, Shin MS, Lee WW, Kang KS, Kim SH, Kim WU, Homer RJ, Kang MJ, Montgomery RR, Dela Cruz CS, Shaw AC, Lee PJ, Chupp GL, Hwang D, Kang I. IL-6 Receptor alpha Defines Effector Memory CD8(+) T Cells Producing Th2 Cytokines and Expanding in Asthma. Am J Respir Crit Care Med. 2014;190:1383–1394. doi: 10.1164/rccm.201403-0601OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garin A, Pellet P, Deterre P, Debre P, Combadiere C. Cloning and functional characterization of the human fractalkine receptor promoter regions. Biochem J. 2002;368:753–760. doi: 10.1042/BJ20020951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agarwal S, Rao A. Modulation of chromatin structure regulates cytokine gene expression during T cell differentiation. Immunity. 1998;9:765–775. doi: 10.1016/s1074-7613(00)80642-1. [DOI] [PubMed] [Google Scholar]
- 26.Lu Q, Wu A, Ray D, Deng C, Attwood J, Hanash S, Pipkin M, Lichtenheld M, Richardson B. DNA methylation and chromatin structure regulate T cell perforin gene expression. J Immunol. 2003;170:5124–5132. doi: 10.4049/jimmunol.170.10.5124. [DOI] [PubMed] [Google Scholar]
- 27.Liu H, Jiang D. Fractalkine/CX3CR1 and atherosclerosis. Clin Chim Acta. 2011;412:1180–1186. doi: 10.1016/j.cca.2011.03.036. [DOI] [PubMed] [Google Scholar]
- 28.D’Haese JG, Friess H, Ceyhan GO. Therapeutic potential of the chemokine-receptor duo fractalkine/CX3CR1: an update. Expert Opin Ther Targets. 2012;16:613–618. doi: 10.1517/14728222.2012.682574. [DOI] [PubMed] [Google Scholar]
- 29.Jones B, Koch AE, Ahmed S. Pathological role of fractalkine/CX3CL1 in rheumatic diseases: a unique chemokine with multiple functions. Front Immunol. 2012;2:82. doi: 10.3389/fimmu.2011.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DiSpirito JR, Shen H. Quick to remember, slow to forget: rapid recall responses of memory CD8+ T cells. Cell Res. 2010;20:13–23. doi: 10.1038/cr.2009.140. [DOI] [PubMed] [Google Scholar]
- 31.Weber C, Zernecke A, Libby P. The multifaceted contributions of leukocyte subsets to atherosclerosis: lessons from mouse models. Nat Rev Immunol. 2008;8:802–815. doi: 10.1038/nri2415. [DOI] [PubMed] [Google Scholar]
- 32.Cho BA, Sim JH, Park JA, Kim HW, Yoo WH, Lee SH, Lee DS, Kang JS, Hwang YI, Lee WJ, Kang I, Lee EB, Kim HR. Characterization of effector memory CD8+ T cells in the synovial fluid of rheumatoid arthritis. J Clin Immunol. 2012;32:709–720. doi: 10.1007/s10875-012-9674-3. [DOI] [PubMed] [Google Scholar]
- 33.Winchester R, Wiesendanger M, Zhang HZ, Steshenko V, Peterson K, Geraldino-Pardilla L, Ruiz-Vazquez E, D’Agati V. Immunologic characteristics of intrarenal T cells: trafficking of expanded CD8+ T cell beta-chain clonotypes in progressive lupus nephritis. Arthritis Rheum. 2012;64:1589–1600. doi: 10.1002/art.33488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Rensen EL, Sont JK, Evertse CE, Willems LN, Mauad T, Hiemstra PS, Sterk PJ. Bronchial CD8 cell infiltrate and lung function decline in asthma. Am J Respir Crit Care Med. 2005;172:837–841. doi: 10.1164/rccm.200504-619OC. [DOI] [PubMed] [Google Scholar]
- 35.Rovina N, Koutsoukou A, Koulouris NG. Inflammation and immune response in COPD: where do we stand? Mediators Inflamm. 2013;2013:413735. doi: 10.1155/2013/413735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huseby ES, Huseby PG, Shah S, Smith R, Stadinski BD. Pathogenic CD8 T cells in multiple sclerosis and its experimental models. Front Immunol. 2012;3:64. doi: 10.3389/fimmu.2012.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee WW, Kang SW, Choi J, Lee SH, Shah K, Eynon EE, Flavell RA, Kang I. Regulating human Th17 cells via differential expression of IL-1 receptor. Blood. 2010;115:530–540. doi: 10.1182/blood-2009-08-236521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Glossop JR, Nixon NB, Emes RD, Haworth KE, Packham JC, Dawes PT, Fryer AA, Mattey DL, Farrell WE. Epigenome-wide profiling identifies significant differences in DNA methylation between matched-pairs of T- and B-lymphocytes from healthy individuals. Epigenetics. 2013;8:1188–1197. doi: 10.4161/epi.26265. [DOI] [PubMed] [Google Scholar]
- 39.Hashimoto S, Ogoshi K, Sasaki A, Abe J, Qu W, Nakatani Y, Ahsan B, Oshima K, Shand FH, Ametani A, Suzuki Y, Kaneko S, Wada T, Hattori M, Sugano S, Morishita S, Matsushima K. Coordinated changes in DNA methylation in antigen-specific memory CD4 T cells. J Immunol. 2013;190:4076–4091. doi: 10.4049/jimmunol.1202267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sawalha AH. Epigenetics and T-cell immunity. Autoimmunity. 2008;41:245–252. doi: 10.1080/08916930802024145. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Y, Maksimovic J, Naselli G, Qian J, Chopin M, Blewitt ME, Oshlack A, Harrison LC. Genome-wide DNA methylation analysis identifies hypomethylated genes regulated by FOXP3 in human regulatory T cells. Blood. 2013;122:2823–2836. doi: 10.1182/blood-2013-02-481788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vento-Tormo R, Alvarez-Errico D, Rodriguez-Ubreva J, Ballestar E. Gains of DNA methylation in myeloid terminal differentiation are dispensable for gene silencing but influence the differentiated phenotype. Febs J. 2014 doi: 10.1111/febs.13045. [DOI] [PubMed] [Google Scholar]
- 43.Matsumiya T, Ota K, Imaizumi T, Yoshida H, Kimura H, Satoh K. Characterization of synergistic induction of CX3CL1/fractalkine by TNF-alpha and IFN-gamma in vascular endothelial cells: an essential role for TNF-alpha in post-transcriptional regulation of CX3CL1. J Immunol. 2010;184:4205–4214. doi: 10.4049/jimmunol.0903212. [DOI] [PubMed] [Google Scholar]
- 44.Moon SO, Kim W, Sung MJ, Lee S, Kang KP, Kim DH, Lee SY, So JN, Park SK. Resveratrol suppresses tumor necrosis factor-alpha-induced fractalkine expression in endothelial cells. Mol Pharmacol. 2006;70:112–119. doi: 10.1124/mol.106.022392. [DOI] [PubMed] [Google Scholar]
- 45.Garcia GE, Xia Y, Chen S, Wang Y, Ye RD, Harrison JK, Bacon KB, Zerwes HG, Feng L. NF-kappaB-dependent fractalkine induction in rat aortic endothelial cells stimulated by IL-1beta, TNF-alpha, and LPS. J Leukoc Biol. 2000;67:577–584. doi: 10.1002/jlb.67.4.577. [DOI] [PubMed] [Google Scholar]
- 46.Le Priol Y, Puthier D, Lecureuil C, Combadiere C, Debre P, Nguyen C, Combadiere B. High cytotoxic and specific migratory potencies of senescent CD8+ CD57+ cells in HIV-infected and uninfected individuals. J Immunol. 2006;177:5145–5154. doi: 10.4049/jimmunol.177.8.5145. [DOI] [PubMed] [Google Scholar]
- 47.Nishimura M, Umehara H, Nakayama T, Yoneda O, Hieshima K, Kakizaki M, Dohmae N, Yoshie O, Imai T. Dual functions of fractalkine/CX3C ligand 1 in trafficking of perforin+/granzyme B+ cytotoxic effector lymphocytes that are defined by CX3CR1 expression. J Immunol. 2002;168:6173–6180. doi: 10.4049/jimmunol.168.12.6173. [DOI] [PubMed] [Google Scholar]
- 48.Fong AM, Robinson LA, Steeber DA, Tedder TF, Yoshie O, Imai T, Patel DD. Fractalkine and CX3CR1 mediate a novel mechanism of leukocyte capture, firm adhesion, and activation under physiologic flow. J Exp Med. 1998;188:1413–1419. doi: 10.1084/jem.188.8.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bazan JF, Bacon KB, Hardiman G, Wang W, Soo K, Rossi D, Greaves DR, Zlotnik A, Schall TJ. A new class of membrane-bound chemokine with a CX3C motif. Nature. 1997;385:640–644. doi: 10.1038/385640a0. [DOI] [PubMed] [Google Scholar]
- 50.Wong BW, Wong D, McManus BM. Characterization of fractalkine (CX3CL1) and CX3CR1 in human coronary arteries with native atherosclerosis, diabetes mellitus, and transplant vascular disease. Cardiovasc Pathol. 2002;11:332–338. doi: 10.1016/s1054-8807(02)00111-4. [DOI] [PubMed] [Google Scholar]
- 51.Nanki T, Imai T, Nagasaka K, Urasaki Y, Nonomura Y, Taniguchi K, Hayashida K, Hasegawa J, Yoshie O, Miyasaka N. Migration of CX3CR1-positive T cells producing type 1 cytokines and cytotoxic molecules into the synovium of patients with rheumatoid arthritis. Arthritis Rheum. 2002;46:2878–2883. doi: 10.1002/art.10622. [DOI] [PubMed] [Google Scholar]
- 52.Nanki T, Urasaki Y, Imai T, Nishimura M, Muramoto K, Kubota T, Miyasaka N. Inhibition of fractalkine ameliorates murine collagen-induced arthritis. J Immunol. 2004;173:7010–7016. doi: 10.4049/jimmunol.173.11.7010. [DOI] [PubMed] [Google Scholar]
- 53.Inoue A, Hasegawa H, Kohno M, Ito MR, Terada M, Imai T, Yoshie O, Nose M, Fujita S. Antagonist of fractalkine (CX3CL1) delays the initiation and ameliorates the progression of lupus nephritis in MRL/lpr mice. Arthritis Rheum. 2005;52:1522–1533. doi: 10.1002/art.21007. [DOI] [PubMed] [Google Scholar]
- 54.Poupel L, Boissonnas A, Hermand P, Dorgham K, Guyon E, Auvynet C, Charles FS, Lesnik P, Deterre P, Combadiere C. Pharmacological inhibition of the chemokine receptor, CX3CR1, reduces atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2013;33:2297–2305. doi: 10.1161/ATVBAHA.112.300930. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.