Abstract
Background
Cediranib (AZD2171), an oral, pan VEGF inhibitor, was evaluated in this phase I study to determine its toxicity profile, dose-limiting toxicities (DLTs), maximum-tolerated dose (MTD), pharmacokinetics, and pharmacodynamics, in children and adolescents with recurrent or refractory primary central nervous system (CNS) tumors.
Methods
Children and adolescents <22 years were enrolled into one of two strata: Stratum I: those not receiving enzyme inducing anticonvulsant drugs (EIACD) and Stratum II: those receiving EIACDs. Dose level selection was based on the Continual Reassessment Method (CRM).
Results
Thirty-six eligible patients, median age 12.7 years, (range, 5.4–21.7 years) in stratum I (24 males) and twelve patients (7 males) in stratum II median age 13.4 years (range, 8.9–19.5 years) were initially assessed over a 4-week DLT evaluation period, modified to 6 weeks during the study. An MTD of 32 mg/m2/day was declared; however, excessive toxicities (transaminitis, proteinuria, diarrhea, hemorrhage, palmer-planter syndrome, reversible posterior leukoencephalopathy) in the expansion cohort treated at this dose suggested that it might not be tolerated over a longer time period. An expansion cohort at 20 mg/m2/day also demonstrated poor longer-term tolerability. Diffusion and perfusion MRI and PET imaging variables as well as biomarker analysis were performed and correlated with outcome. At 20 mg/m2/day, the median plasma area-under the concentration-time curve at steady-state was lower than that observed in adults at similar dosages.
Conclusions
While the MTD of once daily oral cediranib in children with recurrent or progressive CNS tumors was initially defined as 32 mg/m2/day, this dose and 20 mg/m2/day, were not considered tolerable over a protracted time period.
Keywords: AZD2171, Recentin, Cediranib, antiangiogenesis, Pediatric Brain Tumor
INTRODUCTION
Cediranib (Recentin, AZD2171, AstraZeneca Pharmaceuticals, Wilmington, DE) is an orally available, highly potent inhibitor of the VEGF receptor tyrosine kinases (VEGFR1–3)(1) on vascular endothelium. This molecule also inhibits signaling through c-kit, which has been implicated in the pathogenesis of certain central nervous system (CNS) tumors(2). Preclinical models have demonstrated activity in suppression of tumor growth(3), inhibition of brain metastases(4), and reduction of tumor associated edema(5). The role of VEGF in malignant gliomas has been well-documented and vascular proliferation is incorporated into the classification of malignant astrocytic tumors(6). VEGF blockade has been extensively studied in preclinical models of both primary CNS tumors as well as in metastatic models to the CNS(4). Preclinical tests of cediranib in pediatric cancer showed improved activity when combined with multiple agents in some, but not all, models(7–9).
Phase I adult clinical trials have identified once daily administration at 20–45mg as the MTD using a 4-week dose limiting toxicity (DLT) evaluation period, although frequent dose reductions and/or drug holidays were required(10). In a phase II study in adults with progressive glioblastoma multiforme, treatment with cediranib resulted in radiographic response in 57% of patients, an encouraging 6-month progression-free survival of 26%, and a steroid-sparing effect in patients with manageable toxicity(10, 11). While therapy was initiated at 45mg, the majority of patients required either a dose reduction and/or drug holiday. Similar issues have been reported in other cancers treated with cediranib(12). In a pediatric solid tumor phase I trial of cediranib, which excluded CNS tumors, the MTD was assessed over 4 weeks. The toxicities included nausea, vomiting, fatigue occurring in the first patient and hypertension and prolonged corrected QT interval in the second patient treated at 12mg/m2/day. Six additional patients demonstrated only one additional DLT (diarrhea). At 17mg/m2/day, two of 4 patients had DLTs with nausea and fatigue and, with the MTD pre-defined as <34% of patients with a DLT, 12 mg/m2/day was declared the MTD. This MTD is similar to the 20 mg dose identified in some adult trials. One additional patient < 12 years was accrued to the 12mg/m2/day dose for PK analysis in this younger age group(13). Toxicities between the adult and pediatric studies were similar and included fatigue, diarrhea, nausea, hypertension, headache, vomiting and anorexia(14, 15). An adult study of cediranib resulted in hypertension in 87% of participants, 67% of whom developed the hypertension within 3 days of starting cediranib. Almost 50% of patients developed proteinuria(16). The pharmacokinetics of cediranib appeared similar between adults and children although with significant interpatient variability. The median Cmax and AUC(0-inf) at the pediatric MTD of 12mg/m2/day were 67ng/mL and 900ng.h/mL, respectively. Toxicities were associated with a higher AUC. Responses have been observed in a number of adult tumor types and at a variety of doses. In pediatric solid tumor patients, two partial responses were observed, one each in Ewing’s sarcoma (8mg/m2/day) and synovial sarcoma (17mg/m2/day) while two patients experienced minor responses (one each for synovial sarcoma and osteosarcoma). Two patients had prolonged stable disease, one lasting >14 cycles (alveolar soft part sarcoma) and one fibrolamellar hepatocellular carcinoma (8 cycles). A recent randomized phase II trial in adults with recurrent platinum-sensitive ovarian cancer demonstrated a significant improvement in progression-free survival in those treated with cediranib and olaparib versus olaparib alone (17). In this trial, cediranib was used at 30mg and the expected toxicities of fatigue, hypertension and diarrhea were observed. Based on these results, a phase III trial of cediranib is now under development.
Based on the promising preclinical and early clinical results, we conducted a phase I study of cediranib in children with recurrent or progressive CNS tumors after standard therapy.
MATERIAL AND METHODS
Patient Population
Pediatric patients less than 22 years of age with recurrent, progressive, or refractory (considered residual tumor after therapy) primary CNS tumor were eligible. Patients had to be able to swallow intact tablets and present with a Karnofsky (age ≥16 years) or Lansky (age <16 years) ≥60% (70% if compromised left ventricular ejection fraction). Patients had to have recovered from the toxic effects of prior therapy and be at least 3 weeks from chemotherapy (6 weeks for a nitrosourea), 1 week from biologic therapies with half-lives <48 hours or greater than 5 half-lives for those greater than 48 hours, greater than 4 weeks for limited-field radiation or >3 months for craniospinal radiation, greater than 3 months for autologous or 6 months for allogeneic stem-cell transplantation, and off colony forming growth factor(s) for at least one week (2 weeks for Neulasta). Patients had to be on stable or decreasing doses of corticosteroids for at least one week. Patients were required to have the following within 2 weeks of registration and within 7 days of start of therapy: an absolute neutrophil count ≥1000/ul, platelets ≥75,000/ul, hemoglobin ≥8g/dl (can be transfused), serum creatinine ≤1.5 times the upper limit of institutional normal for age (ULNFA) or GFR ≥70ml/min/1.73m2, bilirubin ≤1.5 times ULNFA, SGPT (ALT) ≤2.5 times ULNFA, urine dipstick or urinalysis with <1+ protein, albumin ≥3g/dl, no overt renal, hepatic, cardiac or pulmonary disease. Female patients of childbearing potential required a negative pregnancy test, could not be pregnant or breast feeding and both males and females of childbearing potential were required to use an accepted form of birth control. All patients or legal guardians were required to provide institutional approved consent (and assent where appropriate) to the study treatment and pharmacokinetics.
Exclusion criteria included any clinically significant unrelated systemic illness (serious infections or significant cardiac, pulmonary, hepatic or other organ dysfunction), that would likely interfere with the study procedures or results. Patients receiving other anticancer therapy, patients with uncontrolled hypertension, those previously treated with cediranib, and those unable to return for follow-up or complete the required studies were excluded from participation.
Drug Supply and Administration
Cediranib (AZD2171) was supplied as 2.5, 10, 15 and 20mg tablets by AstraZeneca Pharmaceuticals, Wilmington, DE and was administered orally, once daily, continuously rounded to the nearest 2.5mg. Patients were stratified according to those not receiving enzyme inducing anticonvulsant drugs (EIACD) (Stratum I) or those receiving EIACD (Stratum II) to assess for the interaction, if any, between cediranib and EIACD. The starting dose for patients, which varied by strata, was 20mg/m2 for stratum I (which is 80% of what was the adult MTD at the time of 45mgs or 25mg/m2) with planned escalations of 25, 32 and 40mg/m2 and 15mg/m2 for stratum II with planned dose escalations of 20, 25 and 32mg/m2. Dose escalations were performed separately in stratum I and II and intrapatient dose escalation was not permitted. Doses could not exceed 60 mg (the maximal dose tested in adult patients). Treatment cycles were repeated every 28 days with no break between cycles. Patient diaries were used to monitor adherence.
A modified version of the CRM (18, 19) was used to estimate the MTD with a target DLT probability of 25%. For the planned expansion cohort an attempt was made to have 6 of 12 patients treated at the MTD be below the age of 12. Patient evaluability was initially determined during the first four weeks of therapy; however, the DLT observation period was extended to the first six weeks of therapy part way through the trial due to concerns about delayed toxicities.
Toxicity Assessment
Monitoring for cediranib-related toxicities included weekly physical examination, serum chemistries, CBCs and urine protein over the first 8 weeks of therapy. Thyroid-stimulating hormone was monitored before each cycle, and electrocardiograms (EKGs) were performed monthly for the first two cycles and then as clinically indicated. Clinical and laboratory adverse events, except hypertension, were initially graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 3 and then switched to CTCAEv4 when this became available. Blood pressure (BP) was measured twice daily for the first two months and then every two weeks or more often as clinically indicated. Age- and sex-specific normal values were used to determine whether BP was elevated(20). An algorithm for management of cediranib-related hypertension was used (appendix Fig 1).
Definition of Dose-Limiting Toxicities (DLT)
Hematologic DLT was defined as a single episode of grade 4 neutropenia (ANC <500/ul) or thrombocytopenia (<50,000/ul). Patients with grade 3 hypertension that could not be controlled by therapeutic intervention (appendix Fig 1) warranting treatment interruption of greater than 14 days were considered to have a dose-limiting toxicity. Other non-hematologic DLTs included any grade 3 or 4 toxicity related to cediranib except for grade 3 nausea and vomiting responsive primarily to non-parenteral antiemetics, grade 3 fever or infection, grade 3 diarrhea responsive to optimal use of antidiarrheals, grade 3 or 4 hypokalemia, hypophosphatemia, or hypomagnesemia, which resolved to ≤ grade 2 by supplementation, within 7 days. DLTs also included any grade 2 non-hematological toxicity that persisted for more than 7 days and was considered sufficiently medically significant or sufficiently intolerable by patients as to warrant treatment interruption and/or dose reduction as well as proteinuria >2gms/24 hours or 1–2gms/24 hours that persisted for 7 days.
Pharmacokinetic Methods
Serial whole blood samples (4.5ml) were collected for steady-state pharmacokinetic assessment of cediranib on day 28 of course 1 at pre-dose and 1, 2, 4, 6, and 8 hours post dosing and assayed by a validated bioanalytical method using solid phase extraction followed by reverse phase chromatography with detection by tandem mass spectroscopy, as previously described(15). Concentration-time data were analyzed using non-compartmental pharmacokinetic techniques with nominal sampling times. The maximal observed plasma concentration was defined as Css,max, with tmax defined as the time to reach this peak concentration. The pre-dose trough concentration was defined as Css,min. The area under the plasma concentration-time curve from time zero to the last collected sample (AUC0—tlast) was calculated by the trapezoidal rule. The duration of pharmacokinetic sampling window precluded estimation of apparent oral clearance or half-life.
Pharmacodynamic Methods
Plasma VEGF, bFGF, endostatin and IL8 and urine MMPs were quantified prior to course 1, once between days 7–14 of course 1 at the time of the optional MRI scan, and then at the time of each MRI scan, as well as at the discontinuation (with the exception of patient withdrawal) or completion of treatment using human immunoassays (Quantikine, R&D Systems, Minneapolis, MN). An attempt to assess circulating endothelial cell precursors was discontinued early due to poor accrual.
Tumor Response Assessment
Conventional standard MRI, MR diffusion, T1 permeability perfusion and T2* perfusion studies were obtained within 2 weeks prior to registration, once between days 7–14 of course 1 (optional), and every 2–3 months (i.e., after courses 2, 4, 6, 9 and 12) thereafter. FDG-PET scans were obtained at baseline and prior to course 3 in consenting patients. The optional MRI scan in consenting patients obtained between days 7–14 after the start of cediranib was requested to evaluate dynamic contrast enhanced (DCE) sequences for early changes in perfusion of the tumor. Details of the imaging methods, definitions of response and statistics are included in the supplemental methods file.
Statistical Methods for Correlative Data Analyses
A number of correlations of biomarkers to response and outcome were evaluated and considered exploratory. To investigate the changes of parameter values across time points “change ratios”, i.e. fold changes, were used. The “change ratio” was calculated by dividing the marker value at the later time point by the marker value at the earlier time point. Paired Wilcoxon signed rank tests were used to test whether biomarkers changed significantly between baseline and course 1 (day 7–14); between baseline and end of course 2; and between course 1 (day 7–14) baseline and end of course 2. Additional details of the statistics are included in the supplemental methods file.
RESULTS
Patient Characteristics
Thirty-six eligible patients were enrolled onto Stratum I (non-EIACDs) and twelve eligible patients were enrolled onto Stratum II (EIACDs), of whom 29 and 11 patients were evaluable respectively. Of the seven patients not evaluable for toxicity assessment in stratum I, five patients had rapid tumor progression and two patients had early protocol withdrawal. Patient enrollment occurred between November 2006 and November 2011 and their characteristics are listed in Table 1.
Table 1.
Patient demographics
| Stratum 1 (N=36) | Stratum 2 (N=12) | |||
|---|---|---|---|---|
| AGE (Years) | at Diagnosis | at Study Entry | at Diagnosis | at Study Entry |
| Median | 9.4 | 12.7 | 10.2 | 13.4 |
| Minimum | 1.0 | 5.4 | 1.7 | 8.9 |
| Maximum | 20.0 | 21.7 | 17.0 | 19.5 |
| Number | Percentage | Number | Percentage | |
| SEX | ||||
| Males | 24 | 66.7 | 7 | 58.3 |
| Females | 12 | 33.3 | 5 | 41.7 |
| ETHNICITY | ||||
| Hispanic or Latino | 3 | 8.3 | 0 | 0.0 |
| Non-Hispanic | 31 | 86.1 | 11 | 91.7 |
| Unknown | 2 | 5.6 | 1 | 8.3 |
| RACE | ||||
| Asian | 4 | 11.1 | 2 | 16.7 |
| Black | 4 | 11.1 | 1 | 8.3 |
| Unknown | 1 | 2.8 | 0 | 0.0 |
| White, Non-Hispanic | 27 | 75.0 | 9 | 75.0 |
| DIAGNOSIS | ||||
| Astroblastoma | 1 | 2.8 | 0 | 0.0 |
| Astrocytoma, Anaplastic | 3 | 8.3 | 6 | 41.7 |
| Astrocytoma, LGG | 4 | 11.1 | 0 | 0.0 |
| Atypical Teratoid/Rhabdoid Tumor | 1 | 2.8 | 0 | 0.0 |
| Brain Stem Glioma | 6 | 16.7 | 1 | 8.3 |
| Choroid Plexus Papilloma, Malignant | 1 | 2.8 | 0 | 0.0 |
| Ependymoma, Anaplastic | 4 | 11.1 | 1 | 8.3 |
| Ependymoma, Nos | 3 | 8.3 | 0 | 0.0 |
| Glioblastoma Multiforme | 6 | 16.7 | 0 | 0.0 |
| Glioblastoma, Nos | 0 | 0.0 | 1 | 8.3 |
| Glioma, Malignant | 1 | 2.8 | 2 | 16.7 |
| 2 | ||||
| Medulloblastoma, Nos | 1 | 2.8 | 0 | 0.0 |
| Pilocytic Astrocytoma | 5 | 13.9 | 1 | 8.3 |
Toxicities and MTD
For Stratum I, two evaluable patients were treated at 20mg/m2 without toxicity and the dose was escalated to 25mg/m2. Two new evaluable patients were treated with no DLTs in the first 4 weeks, allowing for a further dose escalation to 32mg/m2 where three of three evaluable patients tolerated therapy without DLTs. At 40mg/m2, 1 evaluable patient was originally classified as a DLT (grade 4 hemorrhage) but the toxicity on re-assessment was reclassified as grade 2 and attributable to disease progression and thus did not qualify as a DLT. This patient was subsequently classified as inevaluable since the patient was taken off therapy prior to the end of the DLT evaluation period. By the time this was recognized, the dose evaluation period had been arbitrarily extended from 4 to 6 weeks based on PBTC Data Safety Monitoring Board (DSMB)’s recommendation to account for possible late occurring toxicities. For this reason, one patient at the 25mg/m2 (grade 3 transaminitis) and one patient at the 32mg/m2 dose level (grade 3 headache, confusion, dehydration and vision change in the context of progressive disease in week 5 of therapy) were redefined as having had DLTs in the 5th or 6th week of therapy. Thus, the dose assessment level was dropped to 25mg/m2. Three additional patients accrued at the 25mg/m2 dose level tolerated the drug without a DLT and so the dose was re-escalated to 32mg/m2. Three additional patients at 32mg/m2 resulted in a single DLT (grade 2 proteinuria) and based on the CRM model, this dose was declared the MTD. Per protocol, accrual was expanded at the MTD level of 32mg/m2 by enrolling 6 more patients to better define the tolerability of cediranib. Of 6 additional patients, one was inevaluable and three had toxicities that would have been considered DLTs had this occurred within the DLT assessment phase of the protocol (these toxicities occurred in the expansion cohort after the MTD had already been declared to be 32mg/m2). Hence based on the combined results from the initial dose escalation and the expansion phase where 5/11 patients experienced toxicities, the 32mg/m2/day dose level was considered too toxic to be recommended as a phase II dose.
The first two patients on stratum 2 treated at 15mg/m2 tolerated therapy without difficulty and dose escalation proceeded to 20mg/m2. While the first patient tolerated the therapy, the second and third patients treated at 20mg/m2 were classified as having had DLTs although both had significant confounding factors. The first patient had grade 2 intolerable diarrhea without proper loperamide prophylaxis (as required). The second patient had proteinuria and had just completed bevacizumab, an expected toxicity of this drug, placing the attribution of the toxicity in question. A decision to count both of these as DLTs but decrease their weight in the CRM model by 40% was made by the PBTC toxicity monitoring committee. Two additional patients were therefore treated and tolerated the 20mg/m2 dose level.
Upon reviewing the toxicities for the entire trial and taking into account the toxicities observed during the expansion phase at the initial estimate of the MTD of 32mg/m2, this dose was not considered sustainable without dose reductions or drug holidays (which were not allowed in this trial). A similar excessive toxicity pattern has been observed in adults at the equivalent of 32mg/m2 and, in consultation with the sponsor and CTEP, the study opted to focus on evaluating the safety of 20mg/m2 in children. Accrual at this dose level was restarted with a goal of enrolling 12 evaluable patients in Stratum I. Of the 12 patients enrolled in Stratum I, 3 had DLTs (grade 2 palmar-plantar erythrodysesthesia syndrome, grade 2 CNS hemorrhage and grade 3 reversible posterior leukoencephalopathy). In stratum 2, of 9 evaluable patients enrolled at the 20mg/m2 dose, a grade 4 transaminitis elevation and the two 60% weighted toxicities discussed above were identified. A summary of all DLTs observed in each stratum are provided in Table 2. Table 3 provides a list of all toxicities observed during the trial in both strata.
Table 2.
DLT Summary for First 6 Weeks of Treatment
| Stratum I | ||||
|---|---|---|---|---|
| Dose level | Number of eligible patients | Number of evaluable Patients | Number of patients with DLTs | Description of DLTs |
| 20mg/m2 | 14 | 12 | 3 | Grade 2 Palmar-plantar erythrodysesthesia syndrome (n=1) Grade 2 Intracranial hemorrhage (n=1) Grade 3 Reversible posterior leukoencephalopathy syndrome (n=1) |
| 25mg/m2 | 6 | 5 | 1 | Grade 3 ALT, SGPT(n=1)* Grade 2 AST, SGOT(n=1)* |
| 32mg/m2 | 13 | 11 | 5 | Grade 3 Dehydration(n=1)* Grade 3 Confusion (n=1)* Grade 3 Ocular/Visual (n=1)* Grade 3 Pain (n=1)* Grade 2 Proteinuria(n=1) Grade 3 palmar-plantar erythrodysesthesia syndrome (n=1) Grade 3 ALT, SGPT(n=1) Grade 3 AST, SGOT(n=1) Grade 3 Fatigue (lethargy, malaise, asthenia) (n=1) |
| 40mg/m2 | 3 | 1 | 0 | |
| Stratum II | ||||
|---|---|---|---|---|
| Dose level | Number of eligible patients | Number of evaluable Patients | Number of patients with DLTs | Description of DLTs |
| 15mg/m2 | 3 | 2 | 0 | |
| 20mg/m2 | 9 | 9 | 3 | Grade 2 Diarrhea (n=1) Grade 3 Proteinuria (n=1) Grade 4 Alanine aminotransferase increased(n=1) Grade 4 Aspartate aminotransferase increased (n=1) |
DLTs marked with an * occurred during weeks 5–6 of treatment.
Table 3.
Type and Grade of Toxicities (attributed to Cediranib) and Adverse Events (regardless of Attribution) observed in all patients treated in Strata I and II across all treatment courses
Cells Contain the Number of Events (Number of Patients) Sorted in Order of Decreasing Frequency of the Total Number of Toxicities
| ADVERSE EVENTS (AE Version=CTCAEVer3 AEs were mapped to CTCAEVer4 AEs) | GRADE | TOTAL | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||||||
| TOX | AE | TOX | AE | TOX | AE | TOX | AE | TOX | AE | |
| Alanine aminotransferase increased | 41(22) | 44(25) | 17(9) | 18(10) | 6(5) | 7(6) | 1(1) | 1(1) | 65(28) | 70(30) |
| Diarrhea | 43(30) | 44(30) | 16(11) | 16(11) | 2(2) | 3(3) | 61(32) | 63(32) | ||
| Hypertension | 19(13) | 21(14) | 28(28) | 28(28) | 10(10) | 10(10) | 57(37) | 59(38) | ||
| Aspartate aminotransferase increased | 37(19) | 38(20) | 4(4) | 4(4) | 1(1) | 2(2) | 1(1) | 1(1) | 43(21) | 45(23) |
| Proteinuria | 20(13) | 22(14) | 13(12) | 15(14) | 3(3) | 3(3) | 36(20) | 40(21) | ||
| Vomiting | 23(12) | 33(19) | 3(3) | 6(6) | 1(1) | 2(2) | 27(13) | 41(21) | ||
| Hypoalbuminemia | 20(13) | 25(16) | 6(4) | 9(7) | 26(13) | 34(17) | ||||
| Platelet count decreased | 22(17) | 23(17) | 3(3) | 3(3) | 25(18) | 26(18) | ||||
| Lymphocyte count decreased | 11(8) | 13(10) | 7(5) | 12(8) | 5(3) | 5(3) | 1(1) | 1(1) | 24(10) | 31(13) |
| Hypothyroidism | 10(10) | 10(10) | 10(10) | 10(10) | 1(1) | 1(1) | 21(15) | 21(15) | ||
| Neutrophil count decreased | 11(8) | 13(8) | 7(4) | 8(5) | 2(2) | 2(2) | 20(10) | 23(11) | ||
| Nausea | 15(10) | 18(13) | 3(3) | 4(4) | 1(1) | 3(3) | 19(10) | 25(16) | ||
| Fatigue | 7(7) | 11(10) | 9(9) | 10(10) | 2(2) | 4(4) | 18(16) | 25(20) | ||
| White blood cell decreased | 13(11) | 17(14) | 4(2) | 5(3) | 1(1) | 1(1) | 18(11) | 23(14) | ||
| Hypocalcemia | 16(8) | 23(14) | 1(1) | 3(3) | 17(8) | 26(16) | ||||
| Palmar-plantar erythrodysesthesia syndrome | 4(4) | 4(4) | 10(7) | 10(7) | 3(3) | 3(3) | 17(7) | 17(7) | ||
| Hypokalemia | 14(11) | 20(16) | 2(1) | 3(2) | 16(12) | 23(18) | ||||
| Hypophosphatemia | 10(8) | 15(12) | 4(2) | 6(4) | 14(8) | 21(13) | ||||
| Blood bilirubin increased | 13(7) | 17(10) | 1(1) | 13(7) | 18(10) | |||||
| Anorexia | 8(8) | 9(9) | 5(5) | 6(6) | 13(10) | 15(12) | ||||
| Hyponatremia | 9(5) | 23(12) | 3(3) | 5(5) | 1(1) | 12(6) | 29(13) | |||
| Headache | 5(4) | 21(12) | 3(3) | 10(7) | 2(2) | 7(5) | 10(7) | 38(19) | ||
| Abdominal pain | 5(5) | 5(5) | 5(5) | 5(5) | 1(1) | 10(10) | 11(11) | |||
| Hyperglycemia | 7(6) | 19(14) | 2(2) | 3(3) | 9(8) | 22(16) | ||||
| Hypermagnesemia | 7(5) | 14(9) | 1(1) | 7(5) | 15(9) | |||||
| Hypomagnesemia | 6(4) | 8(6) | 1(1) | 1(1) | 7(5) | 9(7) | ||||
| Hemoglobin increased | 6(2) | 11(6) | 1(1) | 6(2) | 12(6) | |||||
| Constipation | 5(2) | 7(4) | 1(1) | 2(2) | 6(2) | 9(4) | ||||
| Hypernatremia | 5(4) | 8(5) | 4(1) | 2(1) | 2(1) | 5(4) | 16(5) | |||
| Epistaxis | 5(4) | 6(5) | 5(4) | 6(5) | ||||||
| Rash maculo-papular | 5(5) | 6(5) | 5(5) | 6(5) | ||||||
| Dizziness | 3(3) | 3(3) | 2(1) | 2(1) | 5(3) | 5(3) | ||||
| Hematuria | 5(3) | 5(3) | 5(3) | 5(3) | ||||||
| Pain in extremity | 3(3) | 6(5) | 1(1) | 3(3) | 4(4) | 9(7) | ||||
| Dehydration | 1(1) | 2(2) | 1(1) | 2(2) | 2(2) | 4(4) | 4(4) | 8(7) | ||
| Intracranial hemorrhage | 4(4) | 4(4) | 4(4) | 4(4) | ||||||
| Anemia | 2(2) | 4(4) | 1(1) | 1(1) | 2(2) | 3(3) | 7(6) | |||
| Hypercalcemia | 3(2) | 6(3) | 3(2) | 6(3) | ||||||
| Hypoglycemia | 2(2) | 5(5) | 1(1) | 1(1) | 3(3) | 6(5) | ||||
| Weight loss | 1(1) | 2(2) | 1(1) | 2(2) | 1(1) | 1(1) | 3(3) | 5(5) | ||
| Dyspepsia | 3(3) | 3(3) | 1(1) | 3(3) | 4(4) | |||||
| Mucositis oral | 1(1) | 1(1) | 2(2) | 2(2) | 1(1) | 3(3) | 4(4) | |||
| Flushing | 3(1) | 3(1) | 3(1) | 3(1) | ||||||
| Back pain | 1(1) | 4(3) | 1(1) | 1(1) | 2(2) | 5(4) | ||||
| Alkaline phosphatase increased | 2(2) | 4(3) | 2(2) | 4(3) | ||||||
| Hyperkalemia | 2(2) | 3(3) | 1(1) | 2(2) | 4(4) | |||||
| Oral pain | 2(2) | 2(2) | 2(2) | 2(2) | 4(4) | |||||
| Bone pain | 1(1) | 1(1) | 1(1) | 2(2) | 2(1) | 3(2) | ||||
| Malaise | 2(1) | 3(1) | 2(1) | 3(1) | ||||||
| Myalgia | 1(1) | 1(1) | 1(1) | 2(2) | 2(2) | 3(3) | ||||
| Stomach pain | 2(2) | 3(3) | 2(2) | 3(3) | ||||||
| Gastrointestinal disorders - Other, specify | 1(1) | 1(1) | 1(1) | 1(1) | 2(2) | 2(2) | ||||
| Hyperthyroidism | 2(2) | 2(2) | 2(2) | 2(2) | ||||||
| Investigations - Other, specify | 2(2) | 2(2) | 2(2) | 2(2) | ||||||
| Lipase increased | 1(1) | 1(1) | 1(1) | 1(1) | 2(1) | 2(1) | ||||
| Rash acneiform | 2(2) | 2(2) | 2(2) | 2(2) | ||||||
| Reversible posterior leukoencephalopathy syndrome | 1(1) | 1(1) | 1(1) | 1(1) | 2(1) | 2(1) | ||||
| Vascular disorders - Other, specify | 2(2) | 2(2) | 2(2) | 2(2) | ||||||
| Voice alteration | 2(2) | 2(2) | 2(2) | 2(2) | ||||||
| Acidosis | 1(1) | 7(5) | 1(1) | 7(5) | ||||||
| Seizure | 1(1) | 3(3) | 3(3) | 1(1) | 1(1) | 7(6) | ||||
| Cough | 1(1) | 4(4) | 1(1) | 1(1) | 5(4) | |||||
| Creatinine increased | 1(1) | 3(2) | 2(2) | 1(1) | 5(3) | |||||
| Hydrocephalus | 2(2) | 1(1) | 2(2) | 1(1) | 4(3) | |||||
| Nervous system disorders - Other, specify | 2(2) | 1(1) | 1(1) | 1(1) | 1(1) | 4(4) | ||||
| Pain | 3(3) | 1(1) | 1(1) | 1(1) | 4(4) | |||||
| Peripheral motor neuropathy | 1(1) | 2(2) | 2(2) | 1(1) | 4(4) | |||||
| Depression | 1(1) | 3(3) | 1(1) | 3(3) | ||||||
| Dysphasia | 1(1) | 1(1) | 2(2) | 1(1) | 3(3) | |||||
| Fever | 1(1) | 2(2) | 1(1) | 1(1) | 3(3) | |||||
| Non-cardiac chest pain | 1(1) | 2(2) | 1(1) | 1(1) | 3(3) | |||||
| Urinary retention | 1(1) | 2(2) | 1(1) | 1(1) | 3(3) | |||||
| Chest wall pain | 1(1) | 1(1) | 1(1) | 1(1) | 2(2) | |||||
| Confusion | 1(1) | 1(1) | 1(1) | 1(1) | 2(2) | |||||
| Depressed level of consciousness | 1(1) | 2(2) | 1(1) | 2(2) | ||||||
| Edema limbs | 1(1) | 2(2) | 1(1) | 2(2) | ||||||
| Endocrine disorders - Other, specify | 1(1) | 1(1) | 1(1) | 1(1) | 2(2) | |||||
| Eye disorders - Other, specify | 1(1) | 1(1) | 1(1) | 1(1) | 2(2) | |||||
| Fecal incontinence | 1(1) | 2(2) | 1(1) | 2(2) | ||||||
| Gastritis | 1(1) | 2(2) | 1(1) | 2(2) | ||||||
| Respiratory, thoracic and mediastinal disorders - Other, specify | 1(1) | 1(1) | 1(1) | 1(1) | 2(2) | |||||
| Skin and subcutaneous tissue disorders - Other, specify | 1(1) | 1(1) | 1(1) | 1(1) | 2(2) | |||||
| Skin ulceration | 1(1) | 2(2) | 1(1) | 2(2) | ||||||
| Urinary frequency | 1(1) | 2(2) | 1(1) | 2(2) | ||||||
| Anal mucositis | 1(1) | 1(1) | 1(1) | 1(1) | ||||||
| Anal pain | 1(1) | 1(1) | 1(1) | 1(1) | ||||||
| Arthralgia | 1(1) | 1(1) | 1(1) | 1(1) | ||||||
| Blurred vision | 1(1) | 1(1) | 1(1) | 1(1) | ||||||
| Capillary leak syndrome | 1(1) | 1(1) | 1(1) | 1(1) | ||||||
| Cardiac troponin I increased | 1(1) | 1(1) | 1(1) | 1(1) | ||||||
| Cholesterol high | 1(1) | 1(1) | 1(1) | 1(1) | ||||||
| Chronic kidney disease | 1(1) | 1(1) | 1(1) | 1(1) | ||||||
| Dry mouth | 1(1) | 1(1) | 1(1) | 1(1) | ||||||
| Dry skin | 1(1) | 1(1) | 1(1) | 1(1) | ||||||
| Edema face | 1(1) | 1(1) | 1(1) | 1(1) | ||||||
| Enterocolitis | 1(1) | 1(1) | 1(1) | 1(1) | ||||||
| GGT increased | 1(1) | 1(1) | 1(1) | 1(1) | ||||||
| Hoarseness | 1(1) | 1(1) | 1(1) | 1(1) | ||||||
| Hypertriglyceridemia | 1(1) | 1(1) | 1(1) | 1(1) | ||||||
| Hypoxia | 1(1) | 1(1) | 1(1) | 1(1) | ||||||
| INR increased | 1(1) | 1(1) | 1(1) | 1(1) | ||||||
| Joint effusion | 1(1) | 1(1) | 1(1) | 1(1) | ||||||
| Leukoencephalopathy | 1(1) | 1(1) | 1(1) | 1(1) | ||||||
| Papilledema | 1(1) | 1(1) | 1(1) | 1(1) | ||||||
| Pericardial effusion | 1(1) | 1(1) | 1(1) | 1(1) | ||||||
| Pleural effusion | 1(1) | 1(1) | 1(1) | 1(1) | ||||||
| Rectal pain | 1(1) | 1(1) | 1(1) | 1(1) | ||||||
| Renal and urinary disorders - Other, specify | 1(1) | 1(1) | 1(1) | 1(1) | ||||||
| Serum amylase increased | 1(1) | 1(1) | 1(1) | 1(1) | ||||||
Toxicities associated with cediranib included hypertension in 37 of 48 (77%) eligible patients, 10 of whom reached grade 3 and required multiagent antihypertensive therapy (Table 3 and Supplemental Table). Hypothyroidism was seen in 15 (31%) patients, proteinuria in 20 (42%) patients (two of which were considered DLTs; one each in stratum I and stratum II). Thirty-two patients had diarrhea, all but 2 were grade 1 or 2 and in one of the 2 patients with grade 3 diarrhea, this was associated with sub-therapeutic loperamide use (and was considered a DLT). Fatigue was reported in 16 patients (1 of whom was considered a DLT) while palmar-plantar erythrodysesthesia syndrome was reported in 7 patients (1 considered a DLT). Two episodes of reversible posterior leukoencephalopathy syndrome (RPLS) were reported in the same patient, one of which was considered a DLT. These are similar to the side effects observed in multiple other trials with this compound. No left ventricular dysfunction was identified in any of the patients, a finding which differs from the solid tumor pediatric phase I where these abnormalities occurred in 5 of 16 patients(13). Effect on bone growth was assessed and no bone-related AEs were identified although this may be influenced by the short duration of drug exposure in the majority of patients. One patient was noted to have widening of the distal femoral growth plate of unclear significance.
Pharmacokinetics
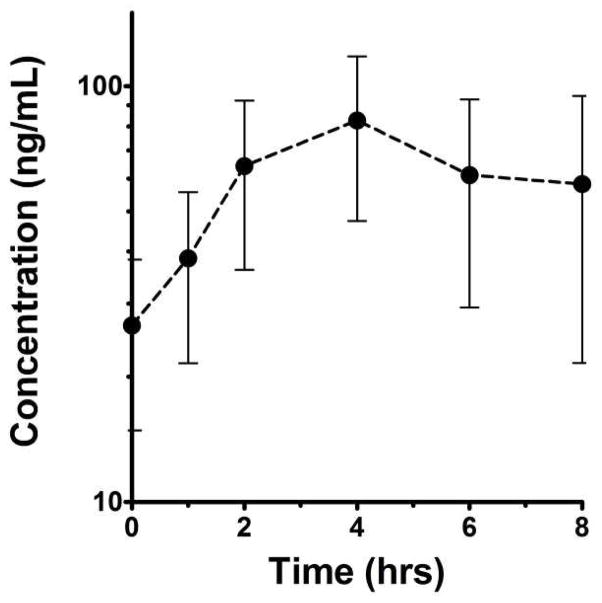
Plasma samples for cediranib pharmacokinetic studies were collected from 11 consenting patients with a median age of 11.8 (range: 5.4–21.6) including eight at the 20 mg/m2 level. The day 28 median (range) Css,max was 48.0ng/mL (14.8 to 311ng/mL) and tmax was measured at between 2 hr and 6 hr post dosing (overall median 4 hr). The median (range) Css,min at 20mg/m2 QD was 11.5ng/mL (2.67 to 96.4ng/mL). Figure 1 shows the average day 28 concentration-time profile for patients studied after receiving 20mg/m2 QD. The median AUC0—tlast for this regimen was 239ng/mL×hr (range: 94.0 to 1,870ng/mL×hr), and no age dependency in AUC0—tlast was observed in these eight patients. For comparison, the 32mg/m2 QD regimen steady-state AUC0—tlast was 892ng/mL×hr (range: 522 to 1,260ng/mL×hr; n=2), and 770ng/mL×hr (n=1) for the 40mg/m2 QD regimen. No formal statistical analysis was performed to evaluate dose proportionality of the cediranib exposure because of inadequate numbers of patients with PK data treated at the different dosages.
Figure 1.
Mean (± SEM) day 28 steady-state concentration-time profile of cediranib for eight patients studied on the 20 mg/m2 QD regimen.
Pharmacodynamics
In the combined cohort (both strata, all dose levels), significant increases between course 1 day 7–14 values and baseline were observed for PIGF (median fold change=1.32, p<0.0001), TSP (median fold change =1.11, p=0.0466), VEGF (median fold change =1.09, p=0.0258), whereas significant decreases were noted for Tek Tie Receptor (median fold change =0.97, p=0.04), and MMP 2 (median fold change =0.95, p=0.0129). In contrast, when we compared fold changes between course 2 day 28 values and baseline, the only significant change was observed for PIGF (median fold change=1.54, p<0.0034). When fold changes were compared between end of course 2 values and course 1 day 7–14, the only significant change was observed for VEGF (median fold change=1.12, p<0.0313). See supplemental data. No significant changes in MMP-9, MMP-9 Dimers or MMP-9/NGAL were observed between baseline and day 7–14 of cycle 1 or between baseline and end of cycle 2.
Tumor Response
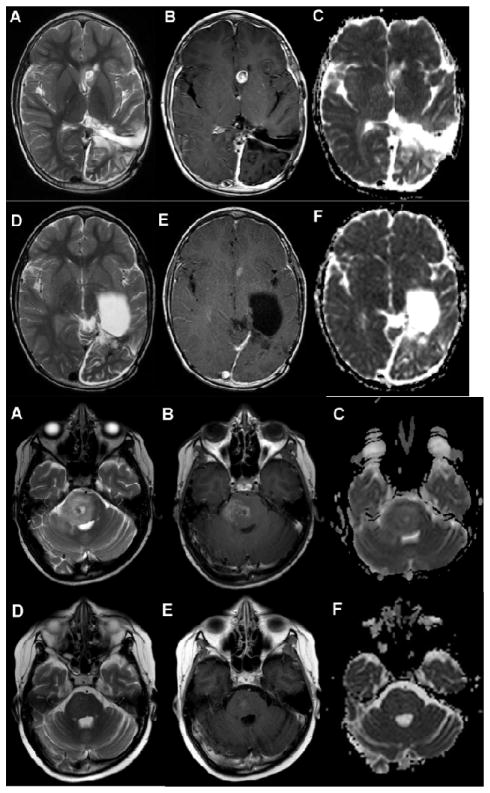
Two partial responses with greater than 50% reduction in tumor volume and maintained for at least 6 weeks were observed in a patient with choroid plexus carcinoma (observed in course 4 and maintained for 13 courses – Figure 2a) and pontine glioma (observed after course 2 and maintained after course 4 - Figure 2b). An additional patient with a recurrent high-grade glioma who initiated therapy after a gross total resection, completed two years of cediranib in continuous CR.
Figure 2. Radiographic response.
Figure 2a Recurrent choroid plexus tumor.
A–C. Axial T2(A), Axial T1 post contrast(B) and axial apparent diffusion coefficient map(C) images of the brain demonstrate a T2 hyperintense, enhancing nodule with increased diffusion in the left caudate/anterior horn of the lateral ventricle. There is a surgical cavity in the left parietal lobe from previous surgery. D–F. Axial T2(D), Axial T1 post contrast (E) and axial apparent diffusion coefficient map(F) images demonstrate decrease in size of enhancing nodule in the region of the left caudate 4 months later.
Figure 2b Recurrent brainstem glioma.
A–C. Axial T2(A), axial T1 postcontrast(B) and axial apparent diffusion coefficient map(C) images of the brain demonstrate a T2 hyperintense lesion in the pons with mass effect on the fourth ventricle and heterogeneous enhancement and diffusion characteristics. D–F Axial T2(D), axial T2 post contrast (E) and axial diffusion coefficient map (F)images of the brain demonstrate marked decrease in size of pontine lesion with minimal patchy enhancement and decreased diffusion 2 months later.
Correlation Between Biomarkers, Imaging and PFS
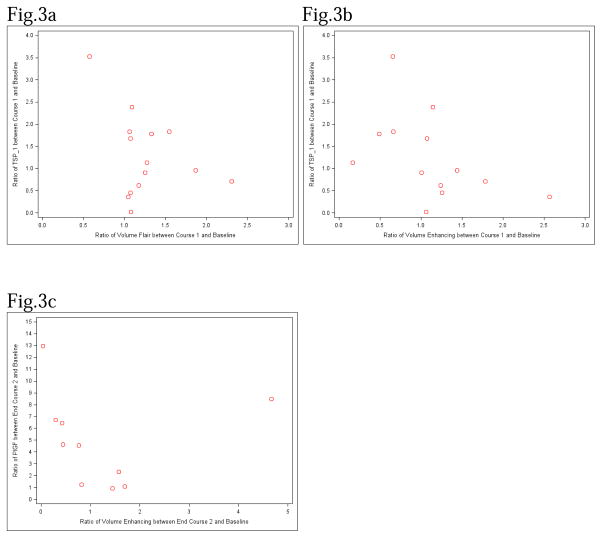
There was an association of the imaging variables of tumor volume on FLAIR and PFS (HR:0.628, p-value=0.003) as well as two and three dimensional mean permeability values and PFS, (HR:3.341, p=0.0458 and HR:2.907, p=0.0240 respectively) between day 7–14 compared to the baseline suggesting that larger tumor volumes and permeability values were associated with shorter PFS. A decrease in the diffusion ratio between baseline and course 1 day 7–14 was detected (median fold change=0.8668, p=0.0107). No significant associations were found between the biomarkers and MRI parameters at baseline. Negative significant associations were detected between TSP levels and FLAIR volume (r=−0.53, p=0.0187, Figure 3a) as well as between TSP and enhancing volume (r=−0.68, p=0.0021, Figure 3b) for the fold changes between baseline and course 1 day 7–14. These findings suggest that as FLAIR volume or enhancing volume increases between baseline and course 1 day 7–14, the TSP value tends to decrease and vice versa. A significant negative association was also detected between PIGF and enhancing volume (r=−0.64, p=0.0388, Figure 3c) for the fold change between baseline and end of course 2. See supplemental data.
Figure 3. Correlation Between Biomarkers and Imaging.
Fig 3a. Ratio of TSP-1 vs. Ratio of Flair volume between baseline and course 1.
Fig 3b. Ratio of TSP-1 vs. Ratio of enhancing volume between baseline and course 1.
The change in the two dimensional mean permeability values was significant (p=0.0469) between baseline and course 1 day 7–14 and suggested a decrease from baseline. There were also significant changes in the values of 2D permeability (p=0.0078) and 3D permeability values (p=0.0078) between baseline and end of course 2 both of which suggested a decrease from baseline.
For PET imaging, no significant associations of baseline PET uptake with PFS were detected. The Spearman rank correlation showed that there was positive association of tumor vs. white matter (TM/WM) with uniformity at baseline (r=0.43, p=0.0312). Further Wilcoxon rank sum test suggested a statistically significant difference in tumor vs. gray matter (TM/GM) ratios among levels of uniformity at baseline (p=0.0420). More specifically the TM/GM uptake in the tumors with low uniformity (0 and 1 combined) was significantly less than that of uniformity 2 (p=0.0424) and uniformity 3 (p=0.0067), respectively. We also observed statistically significant associations between tumor PET uptake with diffusion ratio and perfusion ratio at baseline. There was a negative association between TM/WM and diffusion ratio (r=−0.53, p=0.0086) as well as between TM/GM and diffusion ratio; (r=−0.50, p=0.0158) and a positive association between TM/WM and perfusion ratio (r=0.60, p=0.0246).
DISCUSSION
The maximum-tolerated dose of daily, oral, continuously administered cediranib was initially identified to be 32mg/m2 in pediatric patients with CNS tumors not receiving EIACDs (an MTD was not achieved for patients receiving EIACD), although this dose is not considered the recommended dose based on excessive toxicities observed in patients treated as part of the expansion cohort at this MTD. The doses studied in this trial for both strata are similar to the MTD in adults but exceed the MTD identified for pediatric solid tumor patients which was 12mg/m2/dose, orally, daily, on a continuous administration schedule. The toxicity profile identified in this study was similar to both pediatric and adult patients and included elevated blood pressure, proteinuria, fatigue, GI toxicity (diarrhea, nausea, vomiting, anorexia, abdominal pain), and elevated thyroid stimulating hormone. Growth plate volume was increased in one patient receiving multiple treatment cycles. Cardiovascular toxicity, in particular elevated blood pressure, is thought to be an on target effect of VEGF inhibition and was identified at all dose levels in 37 of the 48 patients but was not more evident at the higher dose levels. Similar to the pediatric phase I solid tumor study of cediranib, we utilized a hypertension management algorithm for elevation in either diastolic and/or systolic BP. No dose-limiting decreases in left ventricular function were observed, which contrasts with the pediatric solid tumor phase I trial where this was a significant finding and may be related to the use of anthracylines and chest radiation therapy in that population.
Pharmacokinetic data from two adult trials reported that the time to maximum cediranib concentrations are achieved between 1 to 8 hours after oral administration, and concentrations declined with a terminal phase half life of 12.5 to 35.4 hours (14, 15). The time to maximum cediranib concentrations observed in the present study was similar to that reported in the adult trial, although because of study design the terminal half-life could not be calculated. The results of the present study revealed that, when treated at 20mg/m2/day, the median steady-state cediranib systemic exposure in children and adolescents with CNS tumors (239ng/ml×hr) is similar to that observed in adults treated with a 5 mg flat dose (226ng/ml×hr)(15). Based on this information, children and adolescents may be less susceptible to the pharmacologic effect (e.g., toxicity and antitumor effect) than adults due to the lower exposure achieved at equivalent doses.
Radiographic assessment was performed using standard MR, diffusion and perfusion techniques. There was an association of tumor FLAIR volumes and permeability values with PFS with larger tumors and higher permeability values associated with shorter progression free survival. There was a significant decrease in Kps values and diffusion values between baseline and course 1 day 7–14 which correlated with the drug mechanism of action and was previously described in the adult cediranib trial (11, 21) and in other antiangiogenesis pediatric clinical trials (22–24). Larger tumors demonstrated increased permeability in our study.
PET variables were not associated with PFS. Intensity of FDG PET tumor uptake correlated with the percentage of the tumor (as delineated on the FLAIR MR) demonstrating FDG uptake. Increased uptake in the tumors was associated with decreased diffusion values and lower cellularity. This association has been described in previous work(25) in children with brainstem glioma and in adult brain tumor patients(26, 27). Higher PET uptake is likely associated with a greater number of viable tumor cells with increased cellularity. There was a significant correlation of PET uptake with perfusion values as assessed on T2* perfusion images. A recent study in children also noted this association likely related to a similar mechanism of radiotracer uptake and association with tumor grade(28).
In spite of the defined role of VEGF in malignant gliomas, assessment of this marker has not correlated to activity, response or outcome in a number of targeted VEGF therapy trials. Biomarker analysis in this study was designed to mimic those in the adult malignant glioma trial of cediranib(10, 11). Differences in results between the studies may have resulted from a number of factors such as differences between adult and pediatric patients and the heterogeneous pediatric population being studied in this protocol compared to the more uniform GBM patient population on which results were previously reported (10, 11). It is of interest that elevated thrombospondin levels, which when elevated, have correlated with improved response to anti-angiogenic therapies(29–33), were also identified in this group of patients.
The design of this clinical trial raises a number of important issues in the identification of appropriate doses that are tolerated and suitable for both phase II studies as single agents as well as in combination with other therapies. The recognition of late-occurring toxicities is an important factor in the determination of the tolerable dose level for clinical study. While a 4-week DLT evaluation period has been used in the majority of clinical studies, including both the adult and pediatric trials of cediranib, the extended assessment period in this study documented the additional toxicity that would have made a dose level intolerable in the intermediate term. This suggests that either a lower dose of drug is needed for development in phase II efficacy trials, or a dose reduction schema is required from the outset. Dose reduction and drug holidays have been routinely used in adults receiving cediranib. The optimum biologic effect of a drug may well be impacted by these factors and it remains unknown what the required schedule and dosing of cediranib is. This study highlights the critical importance of the phase I trial design in achieving a useful determination of the phase II dose and the toxicity profile associated with its use. Since each of the three studies of cediranib (adult brain tumor, pediatric solid tumor and the current study) used different toxicity assessment periods, different toxicity thresholds and different toxicity grading systems, the identification of different MTDs is not surprising. Mandating uniformity in such cases will be important in future studies of experimental agents, especially biologic targeted drugs where classic myelosuppression is not the expected major toxicity.
The CRM model was used in this study over the more standard 3+3 design (used for both the adult and pediatric solid tumor phase I studies) with the goal of reducing the periods of study closure due to toxicity assessment. While for oral medications, discrete pill sizes could introduce significant variability from the target dose based on the per meter squared calculation which could have been adjusted for by the CRM method, the availability of 2.5mg tablets and requirement that all patients be old enough to swallow pills negated the need to account for this design parameter in this trial. For pediatric clinical trials where small dose forms are not available, these issues may be of much greater importance. The adult brain tumor, pediatric solid tumor and current study all arrived at different declared MTDs for cediranib. Such differences in the estimated MTD are not necessarily uncommon and could arise from a variety of reasons including differences in patient populations studied as well as reliance on small sample sizes to estimate the MTD. Further it is well known that different designs used for dose escalation can also influence the chosen MTD(34). The definition of the MTD was also different between our study and the pediatric solid tumor study. In our study <25% was the targeted toxicity level for the MTD in the CRM. Later when the safety of the 20mg/m2 dose was being studied a threshold of no more than 2 DLTs in 12 patients was set as the acceptable toxicity boundary. In contrast, in the pediatric solid-tumor study 3 of 8 patients or 37% with a DLT was considered the MTD. The evaluation period of 6 rather than 4 weeks and the transition from CTCAE3.0 to CTCAE4.0 part way through the trial also likely impacted the MTD estimate. The determination of acceptable toxicity (definitions of what was considered a DLT) also differed between the studies, as did the ability to have dose holidays or reductions. A final factor that might influence the toxicity assessment in this report was age of the patients. In stratum 1, 8 DLTs were identified in 24 children ≥10 years old while only one in the 12 children <10 years of age had a DLT.
Although the single agent activity of anti-angiogenic agents appears to be less than was initially predicted based on animal models, very few biologic or traditional chemotherapy drugs have broad single agent activity. Combination therapy has become the mainstay of effective treatment in oncology and it appears that this will likely be true for antiangiogenic drugs as well. The encouraging results of a recent phase II randomized trial of a combination including cediranib(17), reflects that of other VEGF inhibitors(35).
In summary, cediranib at 32mg/m2/d daily was initially declared as the MTD for children and adolescents with CNS tumors not on EIACD. Expansion at this dose indicated that treatment at this dose level was not tolerated in a sufficient number of patients without permitting drug holidays or dose reduction. In response, additional patients were treated at 20mg/m2 in an effort to study the safety of this dose however 3 out of 11 patients treated at this dose level experienced toxicities which exceeded the pre-set safety threshold. An MTD was not achieved for the cohort on EIACD and the maximum dose tested was 20mg/m2/d. Objective responses were observed.
Supplementary Material
Acknowledgments
We thank Stacye Richardson of the Operations and Biostatistics Center of the Pediatric Brain Tumor Consortium for outstanding administrative and data management support throughout the development and conduct of this trial and Nathan Pinches and William Fletcher for excellent technical assistance.
Grant support: This work was supported in part by NIH grant U01 CA81457 for the Pediatric Brain Tumor Consortium, American Lebanese Syrian Associated Charities and the Stop&Shop Pediatric Brain Tumor Program at the Dana-Farber Cancer Institute and Boston Children’s Hospital.
Footnotes
Conflict of interest: None
References
- 1.Sahade M, Caparelli F, Hoff PM. Cediranib: a VEGF receptor tyrosine kinase inhibitor. Future Oncol. 2012;8:775–81. doi: 10.2217/fon.12.73. [DOI] [PubMed] [Google Scholar]
- 2.Haberler C, Gelpi E, Marosi C, et al. Immunohistochemical analysis of platelet-derived growth factor receptor-alpha, -beta, c-kit, c-abl, and arg proteins in glioblastoma: possible implications for patient selection for imatinib mesylate therapy. J Neurooncol. 2006;76:105–9. doi: 10.1007/s11060-005-4570-9. [DOI] [PubMed] [Google Scholar]
- 3.Wedge SR, Kendrew J, Hennequin LF, et al. AZD2171: a highly potent, orally bioavailable, vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor for the treatment of cancer. Cancer Res. 2005;65:4389–400. doi: 10.1158/0008-5472.CAN-04-4409. [DOI] [PubMed] [Google Scholar]
- 4.Yin JJ, Zhang L, Munasinghe J, Linnoila RI, Kelly K. Cediranib/AZD2171 inhibits bone and brain metastasis in a preclinical model of advanced prostate cancer. Cancer Res. 2010;70:8662–73. doi: 10.1158/0008-5472.CAN-10-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamoun WS, Ley CD, Farrar CT, et al. Edema control by cediranib, a vascular endothelial growth factor receptor-targeted kinase inhibitor, prolongs survival despite persistent brain tumor growth in mice. J Clin Oncol. 2009;27:2542–52. doi: 10.1200/JCO.2008.19.9356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, editors. WHO Classification of Tumours of the Central Nervous System. Lyon: International Agency for Research on Cancer; 2007. [Google Scholar]
- 7.Maris JM, Courtright J, Houghton PJ, et al. Initial testing of the VEGFR inhibitor AZD2171 by the pediatric preclinical testing program. Pediatr Blood Cancer. 2008;50:581–7. doi: 10.1002/pbc.21232. [DOI] [PubMed] [Google Scholar]
- 8.Kendrew J, Odedra R, Logie A, et al. Anti-tumour and anti-vascular effects of cediranib (AZD2171) alone and in combination with other anti-tumour therapies. Cancer Chemother Pharmacol. 2013;71:1021–32. doi: 10.1007/s00280-013-2097-x. [DOI] [PubMed] [Google Scholar]
- 9.Morton CL, Maris JM, Keir ST, et al. Combination testing of cediranib (AZD2171) against childhood cancer models by the pediatric preclinical testing program. Pediatr Blood Cancer. 2011;58:566–71. doi: 10.1002/pbc.23159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Batchelor TT, Sorensen AG, di Tomaso E, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Batchelor TT, Duda DG, di Tomaso E, et al. Phase II study of cediranib, an oral pan-vascular endothelial growth factor receptor tyrosine kinase inhibitor, in patients with recurrent glioblastoma. J Clin Oncol. 2010;28:2817–23. doi: 10.1200/JCO.2009.26.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sridhar SS, Mackenzie MJ, Hotte SJ, Mukherjee SD, Tannock IF, Murray N, Kollmannsberger C, Haider MA, Chen EX, Halford R, Wang L, Ivy SP, Moore MJ. A phase II study of cediranib (AZD 2171) in treatment naive patients with progressive unresectable recurrent or metastatic renal cell carcinoma. A trial of the PMH phase 2 consortium. Invest New Drugs. 2013 Aug;31(4):1008–15. doi: 10.1007/s10637-013-9931-1. Epub 2013 Jan 26. [DOI] [PubMed] [Google Scholar]
- 13.Fox E, Aplenc R, Bagatell R, et al. A phase 1 trial and pharmacokinetic study of cediranib, an orally bioavailable pan-vascular endothelial growth factor receptor inhibitor, in children and adolescents with refractory solid tumors. J Clin Oncol. 2010;28:5174–81. doi: 10.1200/JCO.2010.30.9674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ryan CJ, Stadler WM, Roth B, et al. Phase I dose escalation and pharmacokinetic study of AZD2171, an inhibitor of the vascular endothelial growth factor receptor tyrosine kinase, in patients with hormone refractory prostate cancer (HRPC) Invest New Drugs. 2007;25:445–51. doi: 10.1007/s10637-007-9050-y. [DOI] [PubMed] [Google Scholar]
- 15.Drevs J, Siegert P, Medinger M, et al. Phase I clinical study of AZD2171, an oral vascular endothelial growth factor signaling inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2007;25:3045–54. doi: 10.1200/JCO.2006.07.2066. [DOI] [PubMed] [Google Scholar]
- 16.Robinson ES, Matulonis UA, Ivy P, et al. Rapid development of hypertension and proteinuria with cediranib, an oral vascular endothelial growth factor receptor inhibitor. Clin J Am Soc Nephrol. 2010;5:477–83. doi: 10.2215/CJN.08111109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu JF, Barry WT, Birrer M, et al. Combination cediranib and olaparib versus olaparib alone for women with recurrent platinum-sensitive ovarian cancer: a randomised phase 2 study. Lancet Oncol. 2014;15:1207–14. doi: 10.1016/S1470-2045(14)70391-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piantadosi S, Fisher JD, Grossman S. Practical implementation of a modified continual reassessment method for dose-finding trials. Cancer Chemother Pharmacol. 1998;41:429–36. doi: 10.1007/s002800050763. [DOI] [PubMed] [Google Scholar]
- 19.Onar A, Kocak M, Boyett JM. Continual reassessment method vs. traditional empirically based design: modifications motivated by Phase I trials in pediatric oncology by the Pediatric Brain Tumor Consortium. J Biopharm Stat. 2009;19:437–55. doi: 10.1080/10543400902800486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Update on the 1987 Task Force Report on High Blood Pressure in Children and Adolescents: a working group report from the National High Blood Pressure Education Program. National High Blood Pressure Education Program Working Group on Hypertension Control in Children and Adolescents. Pediatrics. 1996;98:649–58. [PubMed] [Google Scholar]
- 21.Sorensen AG, Batchelor TT, Zhang WT, et al. A “vascular normalization index” as potential mechanistic biomarker to predict survival after a single dose of cediranib in recurrent glioblastoma patients. Cancer Res. 2009;69:5296–300. doi: 10.1158/0008-5472.CAN-09-0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gururangan S, Chi SN, Young Poussaint T, et al. Lack of efficacy of bevacizumab plus irinotecan in children with recurrent malignant glioma and diffuse brainstem glioma: a Pediatric Brain Tumor Consortium study. J Clin Oncol. 2010;28:3069–75. doi: 10.1200/JCO.2009.26.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gururangan S, Fangusaro J, Poussaint TY, et al. Efficacy of bevacizumab plus irinotecan in children with recurrent low-grade gliomas--a Pediatric Brain Tumor Consortium study. Neuro Oncol. 2013 doi: 10.1093/neuonc/not154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gururangan S, Fangusaro J, Young Poussaint T, et al. Lack of efficacy of bevacizumab + irinotecan in cases of pediatric recurrent ependymoma--a Pediatric Brain Tumor Consortium study. Neuro Oncol. 2012;14:1404–12. doi: 10.1093/neuonc/nos213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zukotynski KA, Fahey FH, Kocak M, et al. Evaluation of 18F-FDG PET and MRI associations in pediatric diffuse intrinsic brain stem glioma: a report from the Pediatric Brain Tumor Consortium. J Nucl Med. 2011;52:188–95. doi: 10.2967/jnumed.110.081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holodny AI, Makeyev S, Beattie BJ, Riad S, Blasberg RG. Apparent diffusion coefficient of glial neoplasms: correlation with fluorodeoxyglucose-positron-emission tomography and gadolinium-enhanced MR imaging. AJNR Am J Neuroradiol. 2010;31:1042–8. doi: 10.3174/ajnr.A1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palumbo B, Angotti F, Marano GD. Relationship between PET-FDG and MRI apparent diffusion coefficients in brain tumors. Q J Nucl Med Mol Imaging. 2009;53:17–22. [PubMed] [Google Scholar]
- 28.Zukotynski KA, Fahey FH, Vajapeyam S, et al. Exploratory evaluation of MR permeability with 18F-FDG PET mapping in pediatric brain tumors: a report from the Pediatric Brain Tumor Consortium. J Nucl Med. 2013;54:1237–43. doi: 10.2967/jnumed.112.115782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kieran MW, Turner CD, Rubin JB, et al. A feasibility trial of antiangiogenic (metronomic) chemotherapy in pediatric patients with recurrent or progressive cancer. J Pediatr Hematol Oncol. 2005;27:573–81. doi: 10.1097/01.mph.0000183863.10792.d4. [DOI] [PubMed] [Google Scholar]
- 30.Matsuki K, Tanabe A, Hongo A, et al. Anti-angiogenesis effect of 3'-sulfoquinovosyl-1'-monoacylglycerol via upregulation of thrombospondin 1. Cancer Sci. 2012;103:1546–52. doi: 10.1111/j.1349-7006.2012.02333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bocci G, Francia G, Man S, Lawler J, Kerbel RS. Thrombospondin 1, a mediator of the antiangiogenic effects of low-dose metronomic chemotherapy. Proc Natl Acad Sci U S A. 2003;100:12917–22. doi: 10.1073/pnas.2135406100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamano Y, Sugimoto H, Soubasakos MA, et al. Thrombospondin-1 associated with tumor microenvironment contributes to low-dose cyclophosphamide-mediated endothelial cell apoptosis and tumor growth suppression. Cancer Res. 2004;64:1570–4. doi: 10.1158/0008-5472.can-03-3126. [DOI] [PubMed] [Google Scholar]
- 33.Robison NJ, Campigotto F, Chi SN, Manley PE, Turner CD, Zimmerman MA, Chordas CA, Werger AM, Allen JC, Goldman S, Rubin JB, Isakoff MS, Pan WJ, Khatib ZA, Comito MA, Bendel AE, Pietrantonio JB, Kondrat L, Hubbs SM, Neuberg DS, Kieran MW. A phase II trial of a multiagent oral antiangiogenic (metronomic) regimen in children with recurrent or progressive cancer. Pediatr Blood Cancer. 2014 Apr;61(4):636–42. doi: 10.1002/pbc.24794. Epub 2013 Oct 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Onar-Thomas A, Xiong Z. A simulation-based comparison of the traditional method, Rolling-6 design and a frequentist version of the continual reassessment method with special attention to trial duration in pediatric Phase I oncology trials. Contemp Clin Trials. 2010;31:259–70. doi: 10.1016/j.cct.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saltz LB, Clarke S, Diaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013–9. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.