Abstract
B cells have both antibody-dependent and antibody-independent functions in systemic autoimmune diseases, including systemic lupus erythematosus (SLE). Antibody-independent functions are known to be important, since mice with B cells but no secreted immunoglobulin have severe disease. These functions could include roles in lymphoid development, cytokine secretion, and antigen presentation; however, these possibilities have not been directly tested in SLE models. Here we show by lineage-specific ablation of MHC class II (MHCII) that B cell antigen presentation plays a non-redundant role in CD4+ T cell activation and effector differentiation in the MRL.Faslpr mouse model of SLE. MHCII-mediated interactions between B cells and T cells further promote B cell proliferation and differentiation and, in fact, inefficient MHCII deletion on B cells led to strong selection of “escaped” cells in activated and plasmablast compartments, further underscoring the central role of B cell antigen presentation. Despite the “leakiness” in the system, B cell-specific MHCII deletion resulted in substantially ameliorated clinical disease. Hence, B cell antigen presentation is critical for T and B cell activation and differentiation, as well as target organ damage.
Keywords: autoimmunity, systemic lupus erythematosus, B cells, antigen presentation/processing
Introduction
SLE is a chronic autoimmune disease with multiple immunologic and clinical manifestations. A hallmark of SLE is the presence of autoantibodies to ubiquitous self-antigens. Antibody deposition in kidneys of lupus patients underpins the long-held notion that autoantibodies play a major part in disease pathogenesis. Indeed, B cells have been shown to play a central role in SLE, with the first direct evidence coming from genetic ablation in lupus-prone MRL.Faslpr mice (1). In the absence of B cells, there was a complete amelioration of glomerulonephritis. Strikingly, in these mice there was no development of interstitial nephritis, which is largely comprised of a T cell infiltrate. Further, there was a marked reduction in CD4 and CD8 T cell activation as well as lymphadenopathy and splenomegaly, suggesting direct effects of B cells on T cells and that these effects contributed to end organ damage. These effects were antibody-independent, as demonstrated by MRL.Faslpr mice engineered to have B cells that do not secrete immunoglobulin. Such mice still developed many features of SLE, including extensive T cell activation and renal disease (2). Together, these experiments indicated that B cells have both antibody-dependent and -independent functions in murine SLE.
Though B cells can present antigen to T cells, the importance of this function in lupus has not been directly demonstrated. In particular, it remains controversial whether B cells can initiate responses by presenting to naïve T cells. Classically, dendritic cells (DCs) are considered primary antigen-presenting cells and are arguably essential for initiating adaptive immune responses. However, DC-deficient MRL.Faslpr mice (3) had relatively minimal alterations in the activation, expansion, and differentiation of peripheral T cells. Instead, they appeared to be critical for local T cell expansion and differentiation in target organs, as these DC-deficient mice had significantly fewer renal infiltrates and improved kidney function. These findings might suggest that other APCs are more important in initial activation of autoreactive T cells, and DCs play a critical role in downstream events leading to disease pathology. However, results from DC-deficient mice do not exclude that B cells normally play only a secondary and redundant role, but that B cells are sufficient when DCs are absent. Given the strong paradigm that DCs must be the primary APC to initiate an immune response, this is an important question that remains to be addressed.
The potential importance of B cell APC function in promoting autoimmunity is highlighted by recent findings that B cells specific for self- antigens that contain Toll-like receptor (TLR) 7 or TLR9 ligands can be activated by co-engagement of their B cell receptor (BCR) and TLRs (4, 5), bypassing, in part, the need for T cell help (6, 7). This type of autonomous activation also suggests that, once activated by BCR and TLR signals alone, B cells may be the initial APCs to break tolerance in the T cell compartment at the outset of the anti-self response (8–10). Notably, when T cells are present in vivo they do amplify this BCR/TLR driven activation, which is evidence of productive B-T interactions. Furthermore, B cells are likely to be particularly relevant APCs in an autoimmune response due to their ability to concentrate very small amounts of antigen though selective uptake of the BCR – endowing them with the potential to active low affinity autoreactive T cells (11–14).
Nonetheless, despite theories that B cell APC function is critical in systemic autoimmunity (1, 2, 15, 16), this has never been directly demonstrated. Nor is it known whether such APC function is non-redundant and whether it is, at least in part, upstream of DC-dependent T cell activation. In the current studies, we sought to formally address whether B cell APC function is in fact important in both disease and T cell activation by specifically deleting MHCII on B cells in MRL.Faslpr mice.
Materials and Methods
Mice
CD19-Cre and MHCIIfl/fl mice (17) were backcrossed ten generations onto the Fas-deficient, lupus prone MRL-MpJ-Faslpr/J strain (Jackson, referred to as MRL.Faslpr). These mice were intercrossed to generate CD19-Cre+/− MHCIIfl/fl MRL.Faslpr mice. CD19-Cre+/− MHCIIfl/fl MRL.Faslpr mice and MHCIIfl/fl MRL.Faslpr mice were bred together to generated experimental mice. All mice were analyzed at 12 weeks of age. For BrdU labeling, mice were given an i.p. injection of 1mg BrdU in sterile PBS one hour before sacrifice. All animals were maintained under specific-pathogen-free conditions and handled according to protocols approved by the Institutional Animal Care and Use Committee at Yale and the University of Pittsburgh.
Evaluation of Renal Disease
Kidneys were formalin-fixed, paraffin-embedded, and stained with H&E. Glomerular and interstitial nephritis were scored blindly by a pathologist (M.K.) as described (18). Proteinuria was assessed with Bayer Albustix stips.
Flow Cytometry
All surface staining was performed in ice-cold PBS with 3% calf serum and FcR blocking antibody, 24G.2, except for CXCR4 staining which was done at room temperature. Antibody clones used for surface staining were: anti-CD19 (1D3), anti-CD22 (Cy34.1), anti-CD138 (281-2), anti-CD44 (1M7), anti-IE/IA (M5/114), anti-TCRβ (H57–597), anti-CD4 (GK1.5), anti-CD8 (TIA 105), anti-CD62L (Mel-14), anti-PSGL-1 (2PH1), anti-CXCR4 (2B11), anti-CD11c (N418), anti-CD11b (M1/70), anti-F4/80 (BM8), anti-BST2 (927), anti-Siglec H (eBio440c), anti-Ly6G/Ly6C (RB6-8C5), anti-Ly6C (HK1.4), anti-PNA (Vector labs), anti-CD38 (90). Bcl6 staining used the eBioscience FoxP3 staining buffer set and the K112-91 clone. All other intracellular staining used the BD Cytofix/Cytoperm and Permwash buffers. Plasmablasts were detected with intracellular anti-κ (187.1) and anti-IgM (B7–6). For intracellular cytokine staining, 4×106 splenocytes were stimulated with PMA (20ng/mL) and ionomycin (750ng/mL) for 4 to 6 hr at 37°C; Brefeldin A (10ug/mL) was added after the first 2 hrs. IFN-γ was detected with clone XMG1.2; IL-21 was detected with a recombinant mouse IL-21R subunit/human IgG1 Fc chimera (R&D systems) and goat anti-human Fcγ conjugated to PE (Jackson ImmunoResearch). Ethidium monoazide (EMA) was used for live-dead discrimination (Invitrogen); the cells were incubated under foil for ten minutes and under a bright fluorescent light for ten minutes. BrdU incorporation was detected as previously described (19).
qPCR
To quantitate the deletion efficiency of MHCII, genomic DNA was extracted from FACS-purified cells. For each sample, the ΔCT was calculated by comparing MHCII and the unaffected gene, IL-10 (20). The ΔΔCT was calculated by comparing each sorted subset (naïve, activated, and plasmablasts) from CD19-Cre mice to control mice. IL-10 was amplified with forward primer, (5'−3') GCTCTTACTGACTGGCATGAG and reverse primer, CGCAGCTCTAGGAGCATGTG. MHCII was amplified with forward primer, CCTGGTGACTGCCATTACCT, and reverse primer, AGGGTCCCTCAGAACACGAC.
Cytokine message in FACs-purified DCs was measured as previously described (20). qRT-PCR was performed with the Agilent Brilliant II SYBR Green QPCR kit on a Stratagene Mx3000P instrument.
ELIspot, ELISA and Luminex
AFCs were analyzed by ELISPOT as previously described (20). Anti-nucleosome (21), anti-RNA (18) and anti-Sm (22) serum titers were determined by specific ELISAs as previously described. The total serum immunoglobulin of IgM, IgG2a, IgG1, and IgA was analyzed using Luminex assay (Millipore) according to the manufacturer's instructions.
Results
Effects of selective deletion of MHCII in B cells
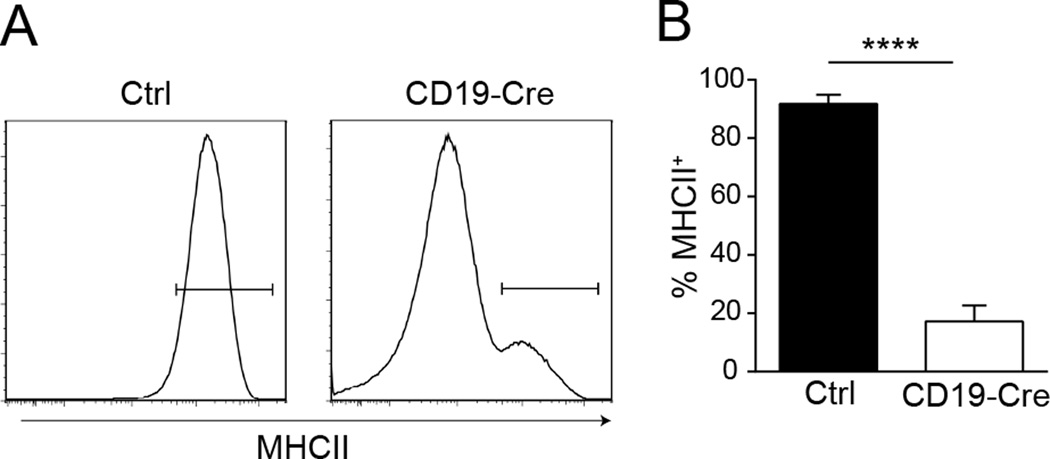
To study the role of antigen presentation by B cells in lupus, we generated mice carrying both CD19-Cre and homozygous loxP-flanked MHCII (MHCIIfl/fl) alleles, hereafter called CD19-Cre mice, on the MRL.Faslpr background. The mice were aged to 12 weeks and compared to MHCIIfl/fl littermate controls. In CD19-Cre mice, an average of 85% of the B cell population had undetectable surface MHCII expression (Fig. 1). Negligible loss of MHCII expression was observed in cDCs, plasmacytoid DCs, macrophages, and neutrophils (data not shown). Interestingly, there was an increase in the total number of cDCs in the CD19-Cre mice, and this population had an increase in surface expression of MHCII (Supplemental Fig. 1A and B). However, there was a decrease in CD86 expression (Supplemental Fig. 1C) and no detectable differences in cytokine message for IL-1b, IL-6, p35, or p40 by qPCR (data not shown) – indicating the cDCs were not in a more activated state.
Figure 1. Deletion of MHCII in B cells.
(A) Representative histograms of MHCII staining of splenic B cells from control, MHCIIfl/fl, and CD19-Cre MHCIIfl/fl mice. Cells were first gated as EMA− TCRβ−, then CD19HiCD138−.
(B) Frequency of MHCII+ splenic B cells, as identified as in (A), in the experimental cohorts. Data are pooled from 6 independent cohorts of 12 week old mice; control n=30 and CD19-Cre n=32. Data are represented as mean +/− SEM. Statistics were calculated by two-tailed Mann-Whitney test. ****p < 0.0001.
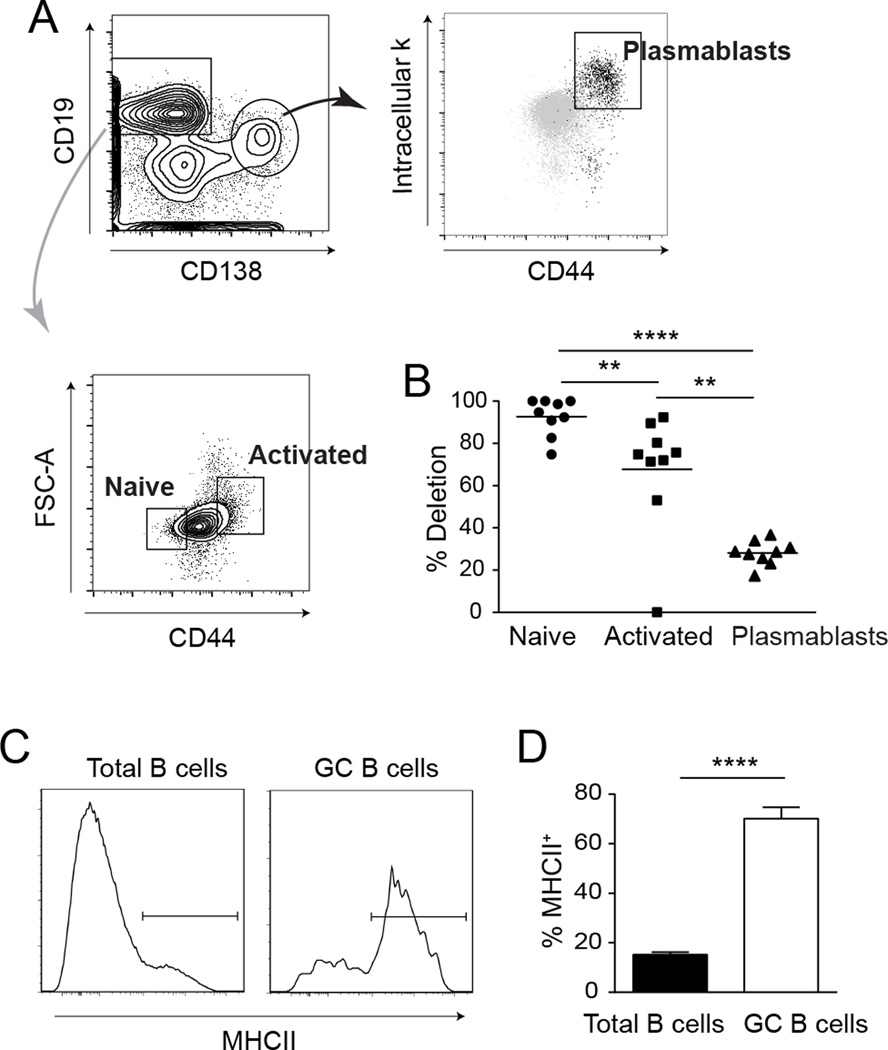
To directly assess the effect of MHCII expression on B cell activation and differentiation, we sorted naive B cells, activated B cells, and plasmablasts from the same CD19-Cre animals (Fig. 2A). Since MHCII is downregulated as B cells differentiate into antibody-forming cells, we used qPCR to determine the amount of deletion within each population. Deletion efficiency among naive B cells was 95%. In stark contrast, only 29% of alleles were deleted in sorted plasmablasts (Fig. 2B). Activated B cells exhibited an intermediate genotype with 75% deletion. Since germinal center (GC) B cells normally express MHCII, we could assess deletion efficiency in this population by flow cytometry (Fig 2C). In CD19-Cre mice, approximately 75% of GC B cells expressed MHCII (Fig. 2D). Thus, although 95% of naïve B cells and the majority of activated B cells lacked MHCII expression, the B cells that were able to form antigen-specific interactions with T cells had a significant advantage in further differentiation into both plasmablasts and GC B cells. The presence of these activated MHCII+ B cells in the CD19-Cre mice, however, does limit the impact of genetic deletion of MHCII in the CD19-Cre mice. Because there is only a partial loss of MHCII expression and that such loss is further attenuated upon differentiation, any observed phenotypes would be minimal approximations of what would be achieved by complete deletion of MHCII on all B cells.
Figure 2. Selective differentiation of residual MHCII+ B cells in CD19-Cre mice.
(A) FACS strategy to identify naive B cells, activated B cells, and plasmablasts. The cells were first gated as EMA− TCRβ− CD11c− CD11b−. (B) Deletion efficiency of MHCII in the three sorted cell populations was determined by qRT-PCR. Deletion efficiency was calculated with the equation (1-residual MHCII) × 100. Residual MHCII was calculated as 2−ΔΔCt. Data represent a total of 9 CD19-Cre mice from 2 independent experiments; each dot is an individual mouse. Horizontal bars mark the mean. (C) Representative staining plots of MHCII staining on total B cells (EMA− TCRβ−CD22+) and GC B cells (EMA− TCRβ−CD22+ CD38Int PNA+) from one CD19-Cre mouse. (D) Summary data are represented as mean +/− SEM. Data are pooled from 2 independent cohorts of 12 week old mice; control n=16 and CD19-Cre n=16. Statistics were calculated by two-tailed Mann-Whitney test. **p < 0.01; ****p < 0.0001.
MHCII-positive B cells have a significant proliferation advantage
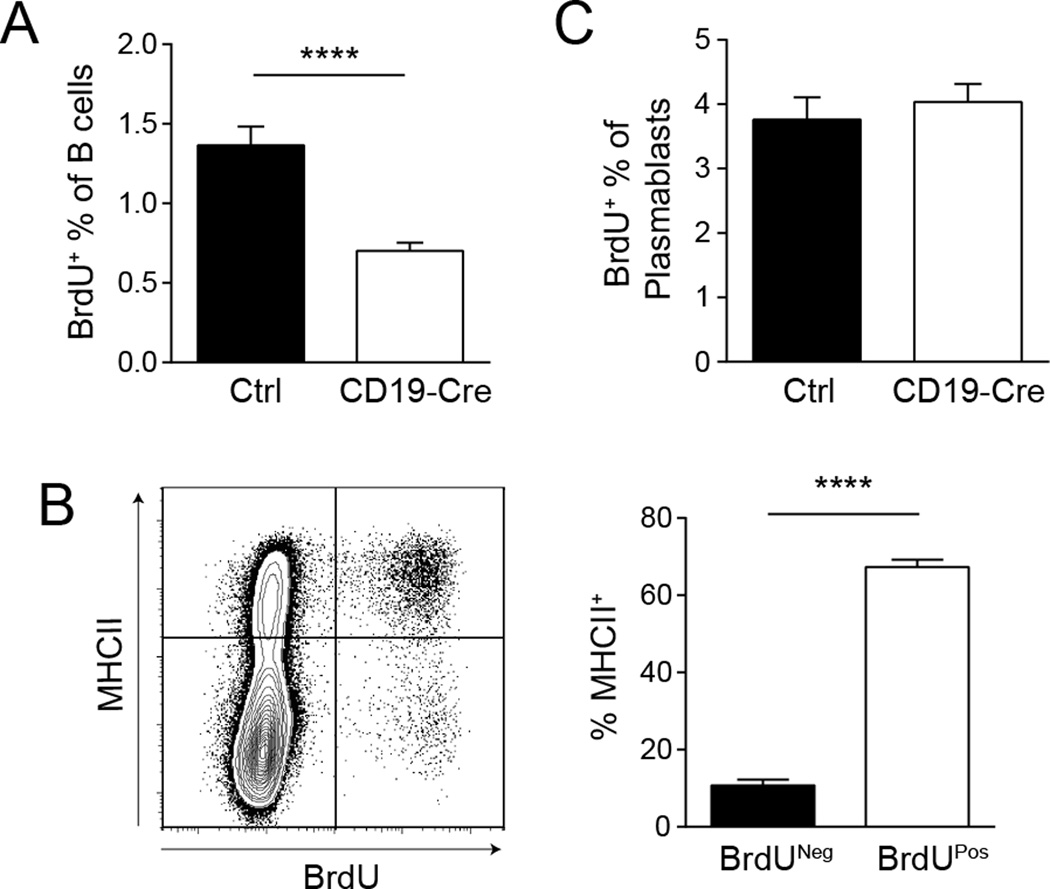
To assess the dependence of B cell proliferation on MHCII expression, we performed a one-hour BrdU pulse, which labels cells in S phase. There were about 50% fewer splenic BrdU+ B cells in the CD19-Cre mice compared to littermate controls (Fig. 3A). Strikingly, within the BrdU+ population in CD19-Cre mice, 65% of the cells were MHCII positive. In contrast, only 10% of the BrdU− cells expressed MHCII (Fig. 3B). On the other hand, there was no difference in the percentage of BrdU+ cells within the plasmablast compartment between CD19-Cre and control animals (Fig. 3C), likely reflective of the fact that essentially all plasmablasts must harbor at least one intact MHCII allele (given the approximately 25% deletion frequency, Fig. 2B) and were thus able to engage T cell help at some point during their evolution.
Figure 3. MHCII+ B cells have a significant proliferation advantage.
One hour before sacrifice, mice were injected with 1mg of BrdU i.p. (A) Splenic B cells (EMA− TCRβ−CD22+) were evaluated for BrdU incorporation by flow cytometry. Data are represented as mean +/− SEM. (B) Representative staining plot of MHCII expression and BrdU incorporation of B cells from a CD19-Cre mouse. The bar graph represents the percent of MHCII+ B cells within the BrdU− and BrdU+ B cell populations as the mean +/− SEM. (C) Plasmablasts (EMA− TCRβ−CD22IntCD138+) were evaluated for BrdU incorporation by flow cytometry. Data are represented as mean +/− SEM. Data are pooled from 2 independent experiments; control n=21, CD19-Cre n=20. Statistics were calculated by two-tailed Mann-Whitney test. ****p < 0.0001.
Expression of MHCII is critical for B cell differentiation
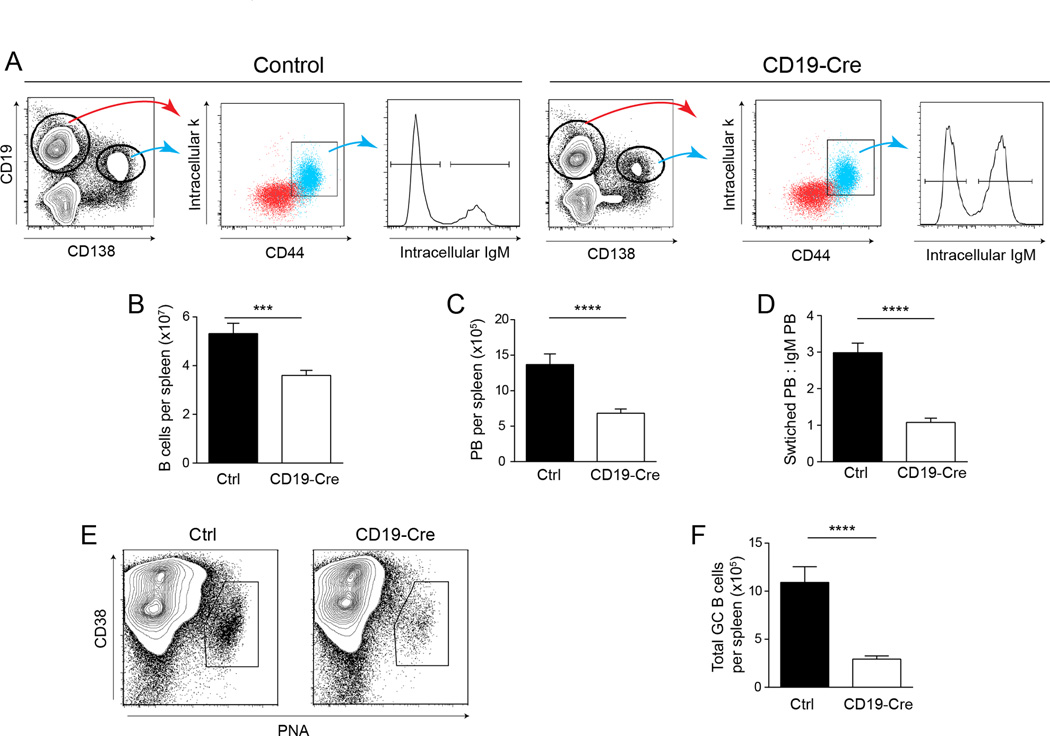
Although there was clearly strong selection for cells that escaped deletion of MHCII during the progression of B cell activation, in the CD19-Cre mice there were nevertheless fewer total CD19HiCD138− B cells, a population that likely included a combination of activated, naïve, and memory B cells (Figs. 4A and B). This was probably, at least in part, due to the observed difference in proliferation (Fig. 3A). There were also decreases in the transitional and marginal zone B cell compartments, but no alterations were found in the bone marrow (data not shown). The total number of plasmablasts (CD19IntCD138+CD44Hiintracellular-κHi) per spleen was also reduced in CD19-Cre mice compared to littermate controls (Figs. 4A and C), probably due to decreased differentiation, as proliferation within the plasmablast compartment was similar between genotypes (Fig. 3C). Furthermore, the ratio of isotype-switched to non-switched plasmablasts was significantly reduced in CD19-Cre mice (Fig. 4A and D). Similarly, GC B cells were also drastically reduced in CD19-Cre mice (Fig. 4E and F).
Figure 4. Expression of MHCII is critical for plasmablast and GC B cell differentiation.
(A) Representative staining plots of plasmablast and B cell gating. Cells were first gated as EMA− TCRβ−. (B) Total number of B cells and (C) plasmablasts per spleen determined by flow cytometry. Data are represented as mean +/− SEM. (D) Isotype switched plasmablasts (IgM−) were normalized to the non-switched (IgM+). Data are represented as mean +/− SEM. Data are pooled from 6 independent cohorts of 12 week old mice; control n=30 and CD19-Cre n=32. (E) Representative staining plots of GC B cell gating from one control mouse and one CD19-Cre mouse. Cells were first gated on EMA− TCRβ−CD22+. (F) Total number of GC B cells per spleen identified as in (E). Data are represented as mean +/− SEM. Data are pooled from 2 independent cohorts of 12 week old mice; control n=26 and CD19-Cre n=16. Statistics were calculated by two-tailed Mann-Whitney test. **p < 0.01; ***p < 0.001; ****p < 0.0001.
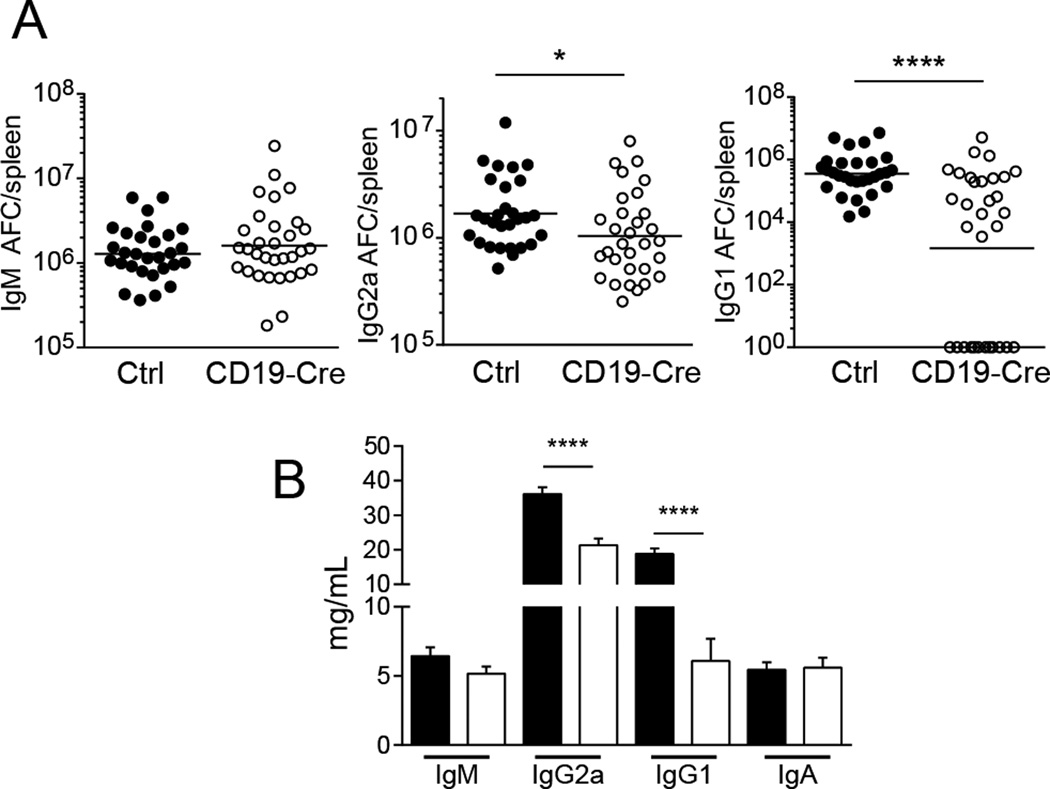
CD19-Cre mice did not have significantly reduced IgM antibody-forming cell (AFC) numbers as measured by ELISpot, but, commensurate with flow cytometry data on plasmablasts, IgG2a and IgG1 AFCs were decreased (Fig. 5A). These differences were reflected in the total serum immunoglobulin titers (Fig. 5B). Total serum IgG2a and IgG1 correlated with the percent of residual MHCII+ B cells (Supplemental Fig. 2A and B). These findings agree with previous data that RF B cell AFC differentiation in T cell deficient hosts showed a strong effect on isotype switched AFCs but no effect on IgM AFCs (7). The results support a critical role for cognate interactions with MHCII-restricted T cells in B cell differentiation and isotype switch.
Figure 5. Isotype switch in antibody-forming cells is dependent on MHCII.
(A) Total numbers of AFCs per spleen were determined by ELISpot for IgM, IgG2a, and IgG1. Each dot is an individual mouse. Bars show geometric means. Data are pooled from 6 independent cohorts of 12 week old mice; control n=29 and CD19-Cre n=32. (B) Serum immunoglobulin concentrations determined by multiplex ELISAs. Data are represented as mean +/− SEM. Data are pooled from 7 independent cohorts of 12 week old mice; control n=35 and CD19-Cre n=32. Statistics were calculated by two-tailed Mann-Whitney test. *p < 0.05; ****p < 0.0001.
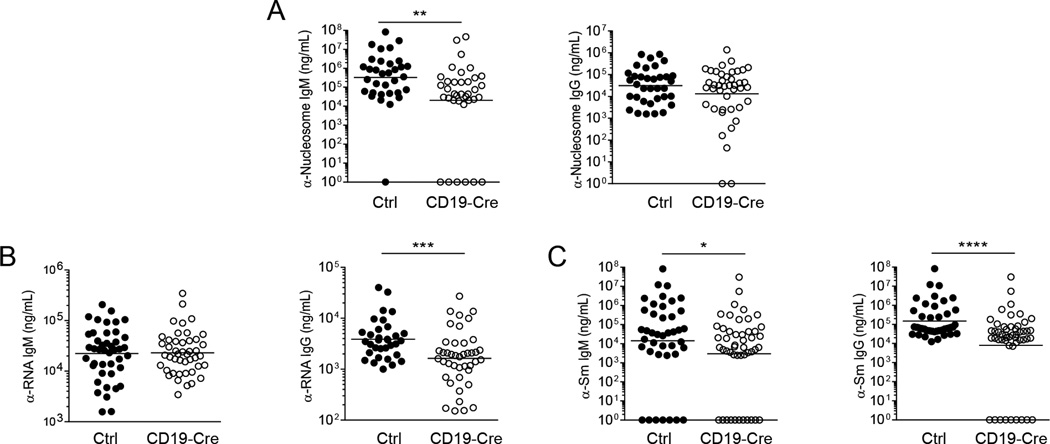
Specific autoantibodies differ in their dependence on B cell MHCII expression
While serum IgG is generally thought to be generated by GC-derived long-lived plasma cells, at least some autoantibodies are largely produced by short-lived plasmablasts, which may be less dependent on MHCII expression and T cell help (23, 24). To assess the effects of B cell MHCII expression on the titers of serum autoantibodies, we performed specific ELISAs for anti-nucleosome, anti-RNA, and anti-Sm. Anti-nucleosome IgM titers were significantly reduced in CD19-Cre mice; however, there was not a statistically significant difference between CD19-Cre and control mice in isotype-switched anti-nucleosome titers (Fig. 6A). Anti-RNA autoantibodies exhibited the opposite pattern: no difference in the IgM titer, but lower titers of anti-RNA IgG (Fig. 6B) in CD19-Cre mice which correlated with the percent of residual MHCII+ B cells (Supplemental Fig. 2C). Anti-Sm exhibited the greatest dependence on B cell MHCII expression; both IgM and IgG anti-Sm titers were lower in the CD19-Cre mice (Fig. 6C); IgG correlated with the percent of residual MHCII+ B cells (Supplemental Fig. 2D). These results are likely a reflection of the differential abilities of the particular self-antigens to induce autonomous B cell activation without the initial need for T cells. In particular, B cells specific for antigens with strong BCR and TLR ligand activities may not depend as greatly on T cell help for activation and differentiation.
Figure 6. Specific autoantibodies differ in their dependence on B cell MHCII expression.
(A) Serum concentration of anti-nucleosome IgM and IgG, (B) anti-RNA IgM and IgG, and (C) anti-Sm IgM and IgG were determined by ELISA. Bars show geometric means. Data are pooled from 7 independent cohorts of 12 week old mice; control n=35 and CD19-Cre n=39. Statistics were calculated by two-tailed Mann-Whitney test. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
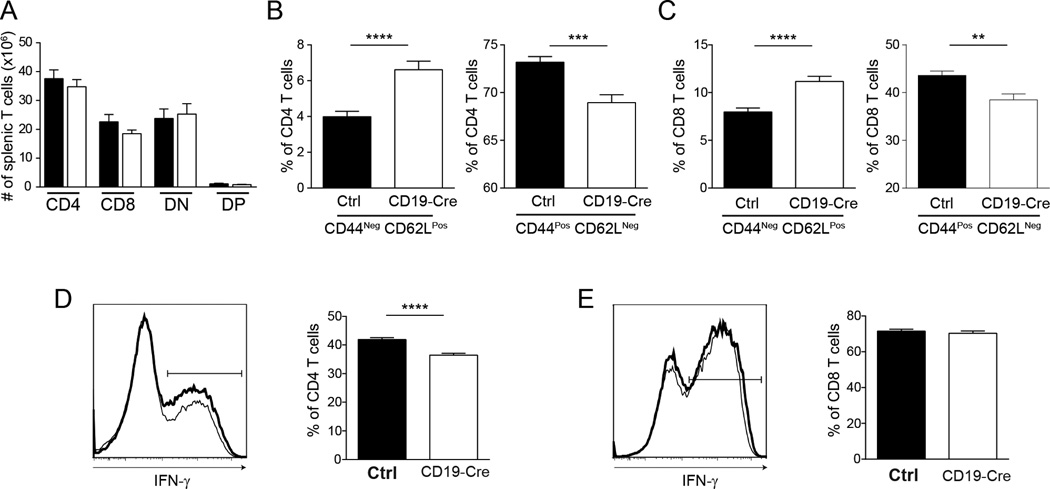
B cell antigen presentation plays a non-redundant role in T cell activation and differentiation
The greater reduction in numbers of activated and memory phenotype T cells previously reported in B cell-deficient vs. DC-deficient MRL.Faslpr mice suggests that B cells may be more important in initial antigen presentation and activation (1, 3, 25). We assessed the T cell compartment in CD19-Cre mice to directly test whether B cell APC function is needed for optimal T cell activation and expansion. CD19-Cre mice had no reduction in the total number of splenic T cells (Fig. 7A). However, the percentage of activated T cells in CD19-Cre mice was significantly lower in both the CD4 and CD8 populations (Figs. 7B and C). There was a concurrent increase in naive phenotype cells which correlated with the percent of residual MHCII+ B cells (Figs. 7B and C; Supplemental Figs. 2 E and F). There was a consistent decrease in frequencies of IFN-γ+ CD4 T cells, but not in IFN-γ+ CD8 T cells, upon PMA/ionomycin restimulation in vitro which also correlated with the percent of residual MHCII+ B cells (Figs. 7D and E; Supplemental Fig. 2G). Hence, there is a non-redundant role for B cell-MHCII expression in the activation and subsequent differentiation of T cells in lupus. As discussed above, the incomplete deletion and preferential activation of escaped MHCII+ B cells could have led to the large number of activated effectors observed in the CD19-Cre mice. A more complete deletion almost certainly would have resulted in a more profound effect in the T cell compartment, possibly approaching that seen in the total B cell knockout. It is also possible that other professional antigen presenting cells could have compensated in the absence of B cell-expressed MHCII.
Figure 7. B cell antigen presentation plays a non-redundant role in T cell activation and differentation.
(A) Total numbers of CD4, CD8, DN, and T cells enumerated by flow cytometry. Frequency of naive (CD44−CD62L+) and activated (CD44+CD62L−) (B) CD4, and (C) CD8 T cells determined by flow cytometry. Representative intracellular IFN-γ staining histograms and numbers of (D) CD4 and (E) CD8 T cells stimulated with PMA and ionomycin. Control mice are shown in bold. Data are represented as mean +/− SEM. Data are pooled from 6 independent cohorts of 12 week old mice; control n=30 and CD19-Cre n=32. Statistics were calculated by two-tailed Mann-Whitney test. **p < 0.01; ***p < 0.001; ****p < 0.0001.
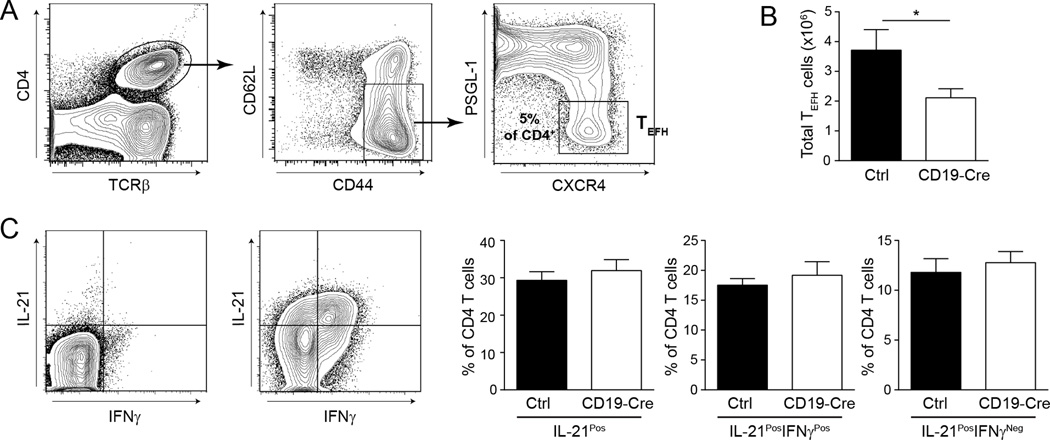
Decrease in T helper cells in CD19-Cre mice
T extrafollicular helper cells (TEFH) comprise in both lupus-prone and normal mice a Bcl6-expressing B-helper CD4 T cell subset that is present extrafollicularly, presumably as a result of CXCR4, rather than CXCR5, expression (26–30). Work from our lab has found a near complete loss of these cells in B cell-deficient MRL.Faslpr mice (31). There was a significant decrease in the number of splenic TEFH cells in the CD19-Cre mice (Figs. 8A and B). However, the frequency of IL-21-producing cells within the CD4 T cell compartment, which include TEFH as well as other more numerous effector populations, was similar between CD19-Cre and control mice (Fig. 8C).
Figure 8. T extrafollicular helper cells are decreased in CD19-Cre mice.
(A) Representative staining plots of TEFH gating. Cells are first gated on EMA− CD19−. Percentage is the mean of TEFH of CD4 T cells in CD19-Cre mice. (B) The number of TEFH cells per spleen as identified in (A). Data are pooled from 2 independent cohorts; control n=9, CD19-Cre n=12. (C) Representative intracellular IL-21 staining plots of CD4 T cells unstimulated (left) and stimulated with PMA and ionomycin (right). The bar graphs represent numbers. Data are pooled from 2 independent cohorts of 12 week old mice; control n=15 and CD19-Cre n=10. Statistics were calculated by two-tailed Mann-Whitney test. *p < 0.05.
MHCII on B cells significantly affects clinical disease
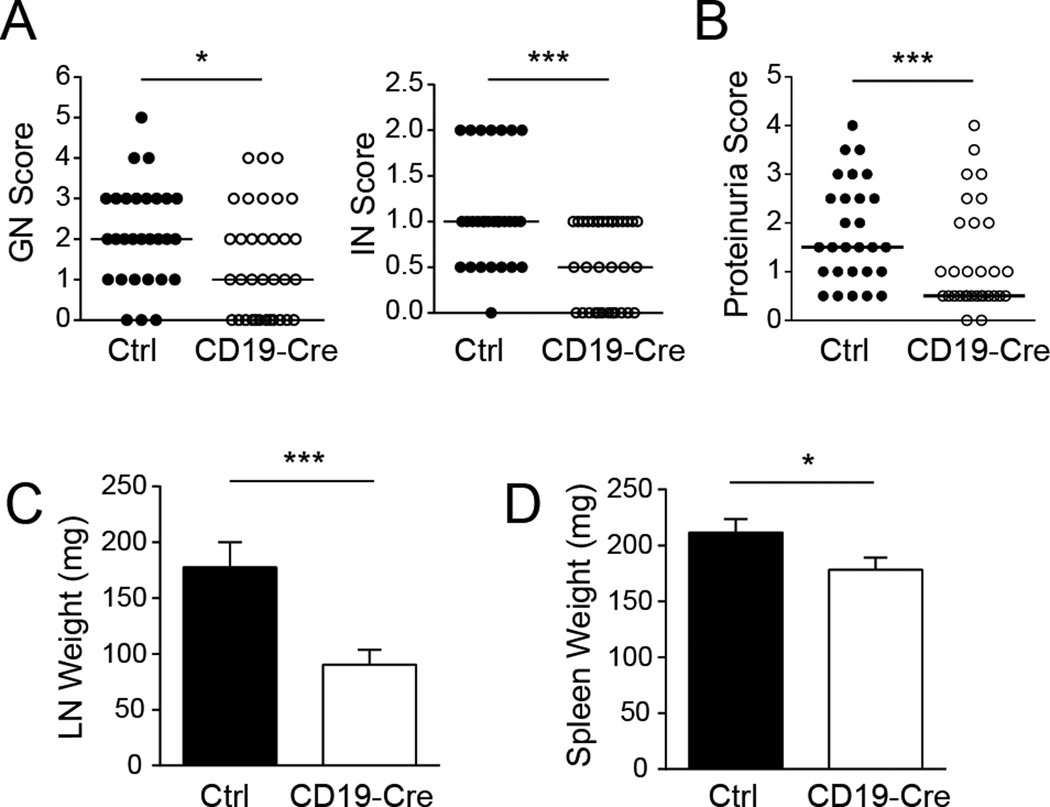
Perhaps the most important aspect of our studies was the opportunity to directly test whether the development of clinical disease would depend on the ability of B cells to present antigen to CD4 T cells. Critically, and despite inefficient B cell MHCII deletion, CD19-Cre mice had significantly less interstitial and glomerular nephritis than littermate controls (Fig. 9A). These histological differences were reflected in the improved kidney function as measured by proteinuria (Fig. 9B). CD19-Cre mice had substantially reduced lymph node weight and a small but significant decrease in spleen weight compared to controls (Figs. 9C and D). These findings demonstrate a non-redundant role for antigen presentation by B cells in end-organ damage and overall pathogenesis.
Figure 9. Deletion of MHCII on B cells ameliorates clinical disease.
(A) Glomerular and interstitial nephritis (GN and IN) were scored from 0 to 6. (B) Proteinuria was scored from 0 to 5. Each dot represents an individual mouse. Horizontal lines represent the medians. (C) Weights of combined axillary lymph nodes and (D) spleen were measured. Data are represented as median +/− SEM. Data are pooled from 6 independent cohorts of 12 week old mice; control n=30 and CD19-Cre n=32. Statistics were calculated by two-tailed Mann-Whitney test. *p < 0.05; **p < 0.01; ***p < 0.001.
Discussion
B cells play central and multiple roles in systemic autoimmune diseases (32). While autoantibody production was originally thought to be the main contribution of B cells, accumulating data support that B cells have important antibody-independent functions. It is critical to distinguish putative functions that were suggested or inferred in discussions of data derived from complete B cell deletion or receptor restriction from those that have been directly tested and proven (33–36). Subsequent work has addressed these various functions in turn. Antibody-independent roles in autoimmunity have been demonstrated by the observation of persistent disease in mice with B cells that cannot secrete antibodies (2). Potential roles in lymphoid tissue development were addressed by depletion of B cells in adult mice, which did partially ameliorate disease, demonstrating that at least some aspects by which B cells promote disease are independent of developmental issues (37, 38). However, though a role for antigen presentation may have been inferred, in our view it could not have been addressed without directly impairing antigen presentation on B cells, which in turn would be most convincingly achieved by cell-specific deletion of MHCII. An important role for B cells as APCs has been implicated in other autoimmune diseases. A role for B cell-specific antigen presentation was recently shown in MOG-induced EAE, in which heterologous antigen given in CFA induces a transient T cell autoimmune syndrome (39). In the NOD mice, MHCII expression on B cells was necessary for the development of spontaneous destructive diabetes (40). To our knowledge the importance of B cell presentation has yet to be directly tested in any model of SLE. Here we report the outcome in mice carrying CD19-Cre and MHCIIfl/fl genotypes that were fully backcrossed onto the lupus-prone MRL.Faslpr background. These results provide direct evidence that antigen presentation by B cells does have a non-redundant role in T cell activation, Th1 differentiation, and T helper cell differentiation in a murine model of lupus. We also found that the cognate, MHCII-mediated interactions between B cells and T cells are important for B cell activation, proliferation, and differentiation.
Given the robust response of the escapee B cells in our system, it is notable that there was a clear biologically and statistically significant effect on clinical disease—including kidney disease, lymphadenopathy and splenomegaly—indicating a requisite role for B cell APC function in these processes. Complete deletion of MHCII in the B cell compartment almost certainly would have led to an even more profound effect. These results, in combination with previous studies, formally demonstrate that B cells are key APCs in systemic autoimmune disease and highlight the importance of antigen-specific B cell-T cell interactions even in a TLR-driven response that can occur outside of GCs.
The incomplete deletion of MHCII on B cells in the CD19-Cre MHCIIfl/fl mice, though not ideal, did enable us to compare the fate of MHCII+ and MHCII− B cells within the same animal. MHCII+ B cells had a substantial advantage in almost every parameter we examined: proliferation, differentiation into PB or GC, and isotype switching. The dramatic expansion of B cells that retained an MHCII allele during progression through expansion and differentiation underscores the importance of B-T cognate interactions in all of these processes. A similar escape phenomenon was observed in a study using the same Cre-LoxP system and a traditional T-dependent model antigen. When CD19-Cre MHCIIfl/fl C57BL/6 mice were immunized with NP-CGG, as few as 2% of residual MHCII+ B cells were able to expand, with the response eventually reaching similar numbers of GC B cells to the MHCII-intact animals (41).
Despite the importance of T-B cognate interactions, our experiments do show that expression of MHCII is not strictly required for any aspect of the B cell response. Indeed, the majority of activated B cells still had fully deleted MHCII. This indicates that initial activation is mostly T-independent, as we have previously found for the TLR-dependent RF B cell response in the AM14 model system (6, 7). However, further differentiation into plasmablasts or GC B cells is substantially affected by the loss of cognate T cell help. Although the differentiation into plasmablasts was significantly decreased in the CD19-Cre MHCIIfl/fl mice, only isotype-switched AFC and resulting serum IgG were affected. This greater dependence on T cell help for isotype-switched AFCs is also consistent with our previous studies with the AM14 B cells and also studies of T cell-deficient MRL.Faslpr mice (6, 7, 42, 43). It is interesting that the large number of activated and cytokine-producing CD4 T cells that were present in CD19-Cre mice could not compensate for the lack of antigen-specific interactions. The necessity for cognate interactions to promote full-blown B cell-mediated autoimmunity was also suggested by a previous study showing reduced disease in TCR transgenic MRL.Faslpr mice with a restricted repertoire (44).
The partial T-independence of B cell activation as shown here and also as demonstrated for in vivo activation of RF B cells, raises the question of how important T-B interactions are in an immune response that is driven by B cell-intrinsic TLR signaling (6). B cell-specific deletion of MyD88 in otherwise intact MRL.Faslpr mice demonstrated that this molecule is not only critical for intrinsic activation but also promotes disease including kidney infiltration by T cells (20). This in turn indicates that certain types of autoreactive B cells that can initially be activated via BCR and TLR dual signals are subsequently important for broader T cell activation and disease pathology. Autoreactive T cells are believed to escape central deletion because they have very low affinity for self antigens. Cognate autoreactive B cells could concentrate and present self antigen at levels high enough to induce activation even in very low affinity T cells. Conversely, DCs are not needed for the vast majority of peripheral T cell activation in this lupus model, suggesting that at best they play a redundant role in this process (3). While these findings are consistent with the notion that B cells have non-redundant roles in antigen presentation, the lack of requirement for DCs in peripheral T cell activation by no means proved that B cells are required. This key fact is something that only B cell-intrinsic deletion of MHCII could demonstrate.
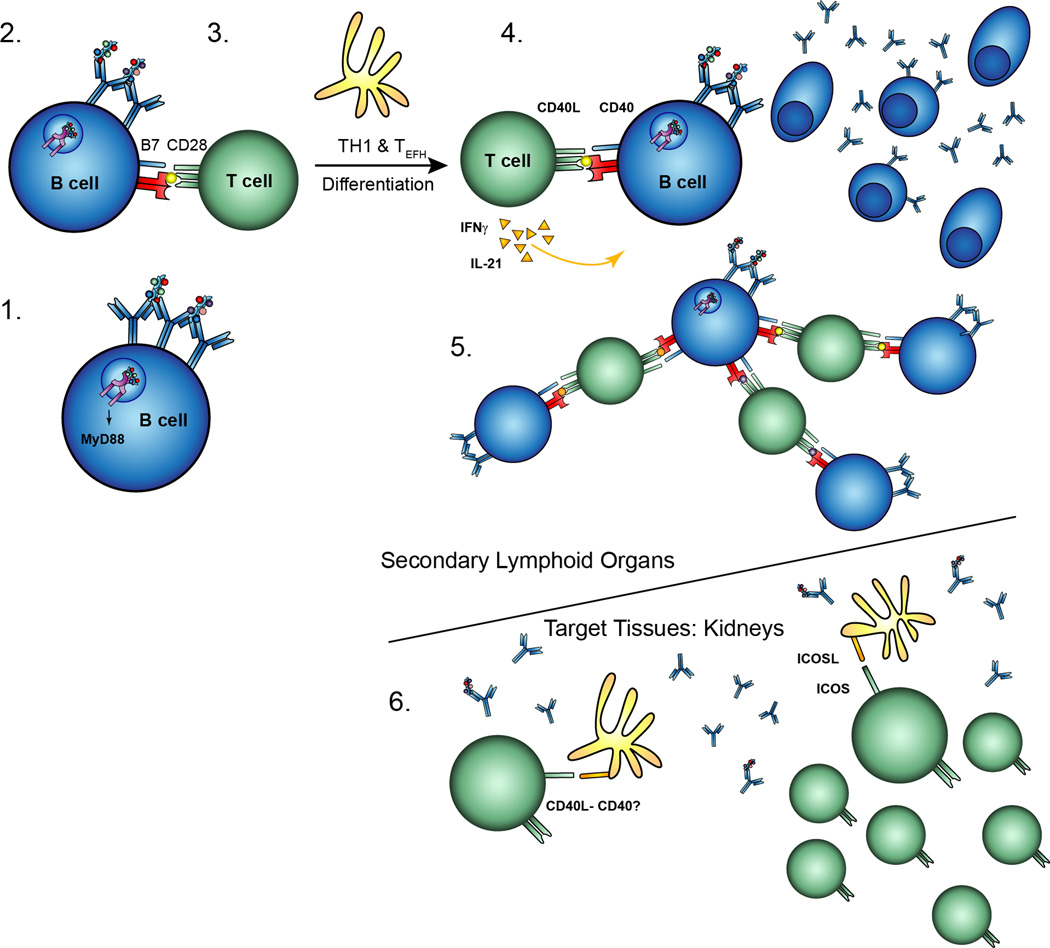
By incorporating present and prior studies, we present a model (Fig. 10) in which B cells are the initial APCs for the activation of autoreactive T cells, something that occurs after T cell-independent/TLR-dependent activation of the B cell. Subsequent B cell and T cell expansion and differentiation — both of which result in target organ damage — depends on B cell-expressed MHCII. Once there has been substantial T cell activation and epitope spreading, T cells that infiltrate organs will be sustained by CD11c+ cells in an ICOSL-dependent, MyD88-independent fashion (20, 31). In target organs, we propose that previously activated infiltrating T cells can provide CD40L signals to DCs in order to induce optimal costimulatory signals from DCs. This is consistent with the fact that MyD88-dependent signals are dispensable in DCs with respect to kidney disease. The next step in defining this schema is to better understand the nature and regulation of the autoreactive T cells that help B cells and promote tissue damage.
Figure 10. Activation and pathogenesis in systemic autoimmunity.
(1) Autoreactive B cells, such as those specific for DNA, become activated through engagement of their BCR and TLRs in a MyD88-dependent manner. (2) Activated autoreactive B cells present antigen on MHCII and upregulate costimulatory molecules, resulting in the activation of cognate autoreactive CD4 T cells. (3) B cells and DCs promote subsequent differentation into TH1 and TEF cells. (4) Cognate activated T cells provide help such as CD40 ligation and cytokines, resulting in significant expansion and isotype switch of the plasmablasts. (5) Activated autoreactive B cells promote epitope spreading and expansion of the anti-self response. B cells have the potential to present anything that is endocytosed through their BCR, not only the protein sequences recognized by the CDR3 region. (6) Activated T cells migrate and infiltrate target tissues, such as the kidneys, in a DC-dependent manner. These T cells may activate resident or migratory DCs through CD40-CD40L ligation which induces ICOSL expression on the DCs. DC-expressed ICOSL promotes kidney damage.
Supplementary Material
Acknowledgements
We thank Jaime Cullen for excellent technical assistance. We thank the technicians of the Yale Animal Resources Center and Division of Laboratory Animal Resources at the University of Pittsburgh for excellent work in animal husbandry.
Abbreviations used in this work
- DC
dendritic cells
- GC
germinal center
- MHCII
major histocompatibility complex class II
- SLE
systemic lupus erythematosus
- TEFH
T extrafollicular helper cells
Footnotes
This work was supported by the National Institutes of Health grant R01-AR044077 (to MJS) and T32-AI07019 (for JRG).
References
- 1.Chan O, Shlomchik MJ. A New Role for B Cells in Systemic Autoimmunity: B Cells Promote Spontaneous T Cell Activation in MRL-lpr/lpr Mice. J Immunol. 1998;160:51–59. [PubMed] [Google Scholar]
- 2.Chan OTM, Hannum LG, Haberman AM, Madaio MP, Shlomchik MJ. A Novel Mouse with B Cells but Lacking Serum Antibody Reveals an Antibody-independent Role for B Cells in Murine Lupus. J. Exp. Med. 1999;189:1639–1648. doi: 10.1084/jem.189.10.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teichmann LL, Ols ML, Kashgarian M, Reizis B, Kaplan DH, Shlomchik MJ. Dendritic Cells in Lupus Are Not Required for Activation of T and B Cells but Promote Their Expansion, Resulting in Tissue Damage. Immunity. 2010;33:967–978. doi: 10.1016/j.immuni.2010.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–607. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 5.Lau CM, Broughton C, Tabor AS, Akira S, Flavell RA, Mamula MJ, Christensen SR, Shlomchik MJ, Viglianti GA, Rifkin IR, Marshak-Rothstein A. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J Exp Med. 2005;202:1171–1177. doi: 10.1084/jem.20050630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herlands RA, Christensen SR, Sweet RA, Hershberg U, Shlomchik MJ. T Cell-Independent and Toll-like Receptor-Dependent Antigen-Driven Activation of Autoreactive B Cells. Immunity. 2008;29:249–260. doi: 10.1016/j.immuni.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sweet RA, Ols ML, Cullen JL, Milam AV, Yagita H, Shlomchik MJ. Facultative role for T cells in extrafollicular Toll-like receptor-dependent autoreactive B-cell responses in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:7932–7937. doi: 10.1073/pnas.1018571108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodríguez-Pinto D. B cells as antigen presenting cells. Cellular Immunology. 2005;238:67–75. doi: 10.1016/j.cellimm.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Rodríguez-Pinto D, Moreno J. B cells can prime naive CD4+ T cells in vivo in the absence of other professional antigen-presenting cells in a CD154-CD40-dependent manner. European Journal of Immunology. 2005;35:1097–1105. doi: 10.1002/eji.200425732. [DOI] [PubMed] [Google Scholar]
- 10.Yan J, Harvey BP, Gee RJ, Shlomchik MJ, Mamula MJ. B cells drive early T cell autoimmunity in vivo prior to dendritic cell-mediated autoantigen presentation. J Immunol. 2006;177:4481–4487. doi: 10.4049/jimmunol.177.7.4481. [DOI] [PubMed] [Google Scholar]
- 11.Kurt-Jones EA, Liano D, HayGlass KA, Benacerraf B, Sy MS, Abbas AK. The role of antigen-presenting B cells in T cell priming in vivo. Studies of B cell-deficient mice. J Immunol. 1988;140:3773–3778. [PubMed] [Google Scholar]
- 12.Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature. 1985;314:537–539. doi: 10.1038/314537a0. [DOI] [PubMed] [Google Scholar]
- 13.Lanzavecchia A. Receptor-mediated antigen uptake and its effect on antigen presentation to class II-restricted T lymphocytes. Annual Review of Immunology. 1990;8:773–793. doi: 10.1146/annurev.iy.08.040190.004013. [DOI] [PubMed] [Google Scholar]
- 14.Tony HP, Phillips NE, Parker DC. Role of membrane immunoglobulin (Ig) crosslinking in membrane Ig-mediated, major histocompatibility-restricted T cell-B cell cooperation. The Journal of Experimental Medicine. 1985;162:1695–1708. doi: 10.1084/jem.162.5.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mamula MJ, Fatenejad S, Craft J. B cells process and present lupus autoantigens that initiate autoimmune T cell responses. J Immunol. 1994;152:1453–1461. [PubMed] [Google Scholar]
- 16.Yan J, Harvey BP, Gee RJ, Shlomchik MJ, Mamula MJ. B Cells Drive Early T Cell Autoimmunity In Vivo prior to Dendritic Cell-Mediated Autoantigen Presentation. The Journal of Immunology. 2006;177:4481–4487. doi: 10.4049/jimmunol.177.7.4481. [DOI] [PubMed] [Google Scholar]
- 17.Hashimoto K, Joshi SK, Koni PA. A conditional null allele of the major histocompatibility IA-beta chain gene. Genesis. 2002;32:152–153. doi: 10.1002/gene.10056. [DOI] [PubMed] [Google Scholar]
- 18.Campbell AM, Kashgarian M, Shlomchik MJ. NADPH Oxidase Inhibits the Pathogenesis of Systemic Lupus Erythematosus. Science Translational Medicine. 2012;4:157ra141. doi: 10.1126/scitranslmed.3004801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nickerson KM, Cullen JL, Kashgarian M, Shlomchik MJ. Exacerbated Autoimmunity in the Absence of TLR9 in MRL.Faslpr Mice Depends on Ifnar1. The Journal of Immunology. 2013;190:3889–3894. doi: 10.4049/jimmunol.1203525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teichmann Lino L, Schenten D, Medzhitov R, Kashgarian M, Shlomchik Mark J. Signals via the Adaptor MyD88 in B Cells and DCs Make Distinct and Synergistic Contributions to Immune Activation and Tissue Damage in Lupus. Immunity. 2013;38:528–540. doi: 10.1016/j.immuni.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nickerson KM, Christensen SR, Shupe J, Kashgarian M, Kim D, Elkon K, Shlomchik MJ. TLR9 Regulates TLR7-and MyD88-Dependent Autoantibody Production and Disease in a Murine Model of Lupus. Journal of Immunology. 2010;184:1840–1848. doi: 10.4049/jimmunol.0902592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Christensen SR, Kashgarian M, Alexopoulou L, Flavell RA, Akira S, Shlomchik MJ. Toll-like receptor 9 controls anti-DNA autoantibody production in murine lupus. J. Exp. Med. 2005;202:321–331. doi: 10.1084/jem.20050338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.William J, Euler C, Shlomchik MJ. Short-Lived Plasmablasts Dominate the Early Spontaneous Rheumatoid Factor Response: Differentiation Pathways, Hypermutating Cell Types, and Affinity Maturation Outside the Germinal Center. J Immunol. 2005;174:6879–6887. doi: 10.4049/jimmunol.174.11.6879. [DOI] [PubMed] [Google Scholar]
- 24.Hoyer BF, Moser K, Hauser AE, Peddinghaus A, Voigt C, Eilat D, Radbruch A, Hiepe F, Manz RA. Short-lived Plasmablasts and Long-lived Plasma Cells Contribute to Chronic Humoral Autoimmunity in NZB/W Mice. The Journal of Experimental Medicine. 2004;199:1577–1584. doi: 10.1084/jem.20040168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan OTM, Madaio MP, Shlomchik MJ. B Cells Are Required for Lupus Nephritis in the Polygenic, Fas-Intact MRL Model of Systemic Autoimmunity. J Immunol. 1999;163:3592–3596. [PubMed] [Google Scholar]
- 26.Odegard JM, Marks BR, DiPlacido LD, Poholek AC, Kono DH, Dong C, Flavell RA, Craft J. ICOS-dependent extrafollicular helper T cells elicit IgG production via IL-21 in systemic autoimmunity. Journal of Experimental Medicine. 2008;205:U2873–U2102. doi: 10.1084/jem.20080840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weinstein JS, Hernandez SG, Craft J. T cells that promote B-Cell maturation in systemic autoimmunity. Immunological Reviews. 2012;247:160–171. doi: 10.1111/j.1600-065X.2012.01122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu D, Tan AH, Hu X, Athanasopoulos V, Simpson N, Silva DG, Hutloff A, Giles KM, Leedman PJ, Lam KP, Goodnow CC, Vinuesa CG. Roquin represses autoimmunity by limiting inducible T-cell co-stimulator messenger RNA. Nature. 2007;450:299–303. doi: 10.1038/nature06253. [DOI] [PubMed] [Google Scholar]
- 29.Linterman MA, Rigby RJ, Wong RK, Yu D, Brink R, Cannons JL, Schwartzberg PL, Cook MC, Walters GD, Vinuesa CG. Follicular helper T cells are required for systemic autoimmunity. Journal of Experimental Medicine. 2009;206:561–576. doi: 10.1084/jem.20081886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee SK, Rigby RJ, Zotos D, Tsai LM, Kawamoto S, Marshall JL, Ramiscal RR, Chan TD, Gatto D, Brink R, Yu D, Fagarasan S, Tarlinton DM, Cunningham AF, Vinuesa CG. B cell priming for extrafollicular antibody responses requires Bcl-6 expression by T cells. The Journal of Experimental Medicine. 2011;208:1377–1388. doi: 10.1084/jem.20102065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teichmann LL, Cullen JL, Kashgarian M, Dong C, Craft J, Shlomchik MJ. Local triggering of the ICOS coreceptor by CD11c+ myeloid cells drives organ inflammation in lupus. Immunity. 2015;42:552–565. doi: 10.1016/j.immuni.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chan OTM, Madaio MP, Schlomchik MJ. Immunological Reviews. Blackwell Publishing Limited; 1999. The central and multiple roles of B cells in lupus pathogenesis; pp. 107–121. [DOI] [PubMed] [Google Scholar]
- 33.Noorchashm H, Noorchashm N, Kern J, Rostami SY, Barker CF, Naji A. B-Cells Are Required for the Initiation of Insulitis and Sialitis in Nonobese Diabetic Mice. Diabetes. 1997;46:941–946. doi: 10.2337/diab.46.6.941. [DOI] [PubMed] [Google Scholar]
- 34.Akashi M, Nagafuchi S, Anzai K, Kondo S, Kitamura D, Wakana S, Ono J, Kikuchi M, Niho Y, Watanabe T. Direct evidence for the contribution of B cells to the progression of insulitis and the development of diabetes in non-obese diabetic mice. International Immunology. 1997;9:1159–1164. doi: 10.1093/intimm/9.8.1159. [DOI] [PubMed] [Google Scholar]
- 35.Serreze DV, Chapman HD, Varnum DS, Hanson MS, Reifsnyder PC, Richard SD, Fleming SA, Leiter EH, Shultz LD. B lymphocytes are essential for the initiation of T cell-mediated autoimmune diabetes: Analysis of a new 'speed congenic' stock of NOD.Igµ(null) mice. Journal of Experimental Medicine. 1996;184:2049–2053. doi: 10.1084/jem.184.5.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Serreze DV, Fleming SA, Chapman HD, Richard SD, Leiter EH, Tisch RM. B Lymphocytes Are Critical Antigen-Presenting Cells for the Initiation of T Cell-Mediated Autoimmune Diabetes in Nonobese Diabetic Mice. The Journal of Immunology. 1998;161:3912–3918. [PubMed] [Google Scholar]
- 37.Ahuja A, Shupe J, Dunn R, Kashgarian M, Kehry MR, Shlomchik MJ. Depletion of B cells in murine lupus: Efficacy and resistance. Journal of Immunology. 2007;179:3351–3361. doi: 10.4049/jimmunol.179.5.3351. [DOI] [PubMed] [Google Scholar]
- 38.Bekar KW, Owen T, Dunn R, Ichikawa T, Wang W, Wang R, Barnard J, Brady S, Nevarez S, Goldman BI, Kehry M, Anolik JH. Prolonged effects of short-term anti-CD20 B cell depletion therapy in murine systemic lupus erythematosus. Arthritis & Rheumatism. 2010;62:2443–2457. doi: 10.1002/art.27515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Molnarfi N, Schulze-Topphoff U, Weber MS, Patarroyo JC, Prod’homme T, Varrin-Doyer M, Shetty A, Linington C, Slavin AJ, Hidalgo J, Jenne DE, Wekerle H, Sobel RA, Bernard CCA, Shlomchik MJ, Zamvil SS. MHC class II–dependent B cell APC function is required for induction of CNS autoimmunity independent of myelin-specific antibodies. The Journal of Experimental Medicine. 2013;210:2921–2937. doi: 10.1084/jem.20130699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Noorchashm H, Lieu YK, Noorchashm N, Rostami SY, Greeley SAS, Schlachterman A, Song HK, Noto LE, Jevnikar AM, Barker CF, Naji A. I-Ag7-Mediated Antigen Presentation by B Lymphocytes Is Critical in Overcoming a Checkpoint in T Cell Tolerance to Islet β Cells of Nonobese Diabetic Mice. The Journal of Immunology. 1999;163:743–750. [PubMed] [Google Scholar]
- 41.Shimoda M, Li T, Pihkala JPS, Koni PA. Role of MHC class II on memory B cells in post-germinal center B cell homeostasis and memory response. Journal of Immunology. 2006;176:2122–2133. doi: 10.4049/jimmunol.176.4.2122. [DOI] [PubMed] [Google Scholar]
- 42.Jevnikar AM, Grusby MJ, Glimcher LH. Prevention of nephritis in major histocompatibility complex class II-deficient MRL-lpr mice. J Exp Med. 1994;179:1137–1143. doi: 10.1084/jem.179.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wofsy D, Ledbetter J, Hendler P, Seaman W. Treatment of murine lupus with monoclonal anti-T cell antibody. J Immunol. 1985;134:852–857. [PubMed] [Google Scholar]
- 44.Peng SL, Fatenejad S, Craft J. Induction of Nonpathologic, Humoral Autoimmunity in Lupus-Prone Mice by a Class II-Restricted, Transgenic αβ T Cell: Separation of Autoantigen-Specific and -Nonspecific Help. Journal of Immunology. 1996;157:5225–5230. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.