Abstract
Clinical improvement during pregnancy in multiple sclerosis (MS) patients suggests that sex hormones exert potent regulatory effects on immune function. Our previous studies demonstrated that estrogen-(17β-estradiol; E2) mediated protection against experimental autoimmune encephalomyelitis (EAE), a mouse model for MS, hinges on the B cells, leading to elevated numbers of IL-10 secreting CD1dhiCD5+ B regulatory cells (Bregs) in wild type mice. Our data show that co-administration of E2 and IL-10+ B cells ameliorates EAE disease severity and limits CNS infiltrating leukocytes in B cell deficient mice. Additionally, treatment with E2 and Bregs reduces demyelination and dramatically decreases the proportion of CD11b+CD45hi activated microglia/macrophages found in the CNS of immunized animals compared to vehicle, E2 or Breg cells alone. Furthermore, mice given E2 and Bregs exhibit increased numbers of peripheral programmed death-1 positive CD4+Foxp3+ regulatory T cells (Tregs) and up-regulation of programmed death receptor-ligand-1 and CD80 expression on monocytes. Our study suggests IL-10 producing Bregs have powerful therapeutic potential as an agent against EAE when augmented with E2 treatment.
Keywords: IL-10, Regulatory B cell, Estrogen, EAE, Regulatory T cell, B cell deficiency
Introduction
Multiple sclerosis (MS) (Abramsky) is an autoimmune inflammatory disease of the brain and spinal cord characterized by demyelination and chronic neurodegeneration (Compston and Coles 2008). Although the incidence of MS is higher in women, it shows clinical improvement during pregnancy, followed by temporary post-partum exacerbations (Abramsky 1994; Confavreux et al. 1998). It is widely accepted that sex hormones have immune-regulatory activity and may prevent relapses in multiple sclerosis during pregnancy. Indeed, treatment with pregnancy levels of estrogen reduced disease severity and CNS lesions (Offner and Polanczyk 2006) in experimental autoimmune encephalomyelitis (EAE), an induced autoimmune disease of mice that mimics MS in susceptibility and histopathology (Maugh 1977),.
Previous studies have demonstrated that B cells may contribute to EAE pathogenesis. Although not essential for EAE induction, myelin oligodendrocyte glycoprotein (MOG)-specific antibodies enhance demyelination and inflammation in EAE (Linington et al. 1988; Lyons et al. 1999). In addition, MOG-specific B cells have been shown to function as antigen presenting cells (APCs) during the initiation of EAE (Bettelli et al. 2006). However, despite their demonstrably inflammatory activity, B cells may have a more nuanced role in EAE pathogenesis.
There is accumulating evidence that regulatory B lymphocytes (Bregs) play an important role in limiting disease progression by suppressing inflammation in the CNS and reducing the number of infiltrating pro-inflammatory cells (Fillatreau et al. 2002; Wolf et al. 1996). Studies addressing this phenomenon have identified phenotypically distinct subsets of IL-10 producing regulatory B cells including CD19+CD21hiIgMhiCD23hi, CD19+CD1dhiCD5+, CD19+Tim-1+ and B220+CD138+CD44hi that exert immunosuppressive activity in vivo as well as in vitro (Evans et al. 2007; Matsumoto et al. 2014; Matsushita et al. 2008; Mauri and Bosma 2012). Pivotal to regulatory B cell function is IL-10, which inhibits production of pro-inflammatory cytokines by leukocytes and supports the differentiation and activation of CD4+Foxp3+ regulatory T cells (Tregs) (Weber et al. 2007).
Our previous studies suggested that the protection induced by 17β-estradiol (E2) against EAE in the absence of Tregs included the induction of CD1dhiCD5+ regulatory B cells (Bregs). In addition, we have shown that programmed death receptor-1 (PD-1) expression is increased on Tregs in B cell replenished, E2 treated B cell-deficient (µMT−/−) mice with EAE (Bodhankar et al. 2012; Subramanian et al. 2011). These findings pointed to Bregs as key players in potentiating additional Treg mediated neuroprotection during EAE. Furthermore, we recently demonstrated that E2 associated protection was mitigated in B cell deficient mice with EAE, but could be restored by replenishment of splenic B cells. (Bodhankar et al. 2011). However, the protective effect of B cell transfers from immunized wild type (WT) mice was short-lived and the disease progressed in recipients from day 21 after immunization onwards (Bodhankar, S. 2012, 137(4):282-93). Parallel studies from our lab have also shown that IL-10 producing regulatory B cells limit CNS inflammation following experimental stroke (Bodhankar et al. 2013a). While the role of Bregs in down-regulating inflammatory reactions has been suggested in autoimmune diseases such as MS and Systemic Lupus Erythematosus (Mohrs et al.) (Blair et al. 2010; Duddy et al. 2007; Mauri and Bosma 2012), it remained unclear what part they play in E2-confered protection against EAE. Our present findings demonstrate that IL-10+ B cells (Bregs) are indispensable to E2-dependent amelioration of EAE neuro-inflammation, facilitating the recruitment of Tregs to the inflamed CNS and upregulating expression of PD-1/PD-L1 signaling molecules.
Materials and Methods
Animals
B cell deficient (µMT−/−) mice were obtained from Jackson Laboratories (Bar Harbor, ME) and bred at the Animal Resource Facility at the VA Portland Health Care System (VAPHCS). Briefly, the µMT−/− strain was generated though targeted disruption of the membrane exon of the immunoglobulin µ chain gene, leading to the absence of mature B cells, and is maintained on a C57BL/6 background. 7–8 week old females were used for this study.
IL-10 transcriptional reporter mice were obtained from Dr. Christopher Karp, Division of Molecular Immunology, University of Cincinnati College of Medicine, Cincinnati, Ohio. The generation and characterization of these mice has been described (Madan et al. 2009). The IL10-GFP reporter mice have a floxed neomycin-IRES eGFP cassette inserted between the endogenous stop site and the poly (A) site of Il10 to help track IL-10 producing cells in vivo. The mice (designated as Vert-X) are homozygous, develop normally and are viable and fertile without any obvious abnormal phenotype (Mohrs et al. 2001).
All animals were housed in the Animal Resource Facility at the VAPHCS in accordance with institutional guidelines. This study was conducted in accordance with National Institutes of Health guidelines for the use of experimental animals and the VAPHCS Animal Care and Use Committee approved all protocols.
Hormone treatment and induction of EAE
Female uMT−/− mice were implanted subcutaneously with 2.5mg/60-day release 17β-estradiol pellets (E2, Innovative Research of America, Sarasota, FL) or sham-treated control) one week prior to immunization with 200µg mouse (m) MOG-35-55 peptide (PolyPeptide Laboratories, San Diego, CA) in 200µg Complete Freund’s adjuvant (Incomplete Freund’s adjuvant (IFA, Sigma-Adrich, St. Louis, MO) complemented with heat-killed Mycobacterium tuberculosis (Mtb, Difco, Detroit, MI). Mice received pertussis toxin through intraperitoneal injection (Ptx, List Biologicals, Campbell, CA) on the day of immunization (75ng) and 2 days later (200ng).
All mice were monitored daily for clinical signs of disease and scored using the following scale: 0=normal; 1=limp tail or mild hind limb weakness; 2=moderate hind limb weakness or mild ataxia; 3=moderately severe hind limb weakness; 4=severe hind limb weakness or mild forelimb weakness or moderate ataxia; 5=paraplegia with no more than moderate forelimb weakness; and 6=paraplegia with severe forelimb weakness or severe ataxia or moribund condition. Mice were scored daily and were evaluated for incidence, day of onset, day of maximal clinical signs (peak) and for total disease score over the course of the experiment (Cumulative Disease Index, CDI). Mean ± SEM were calculated for these parameters for each experimental group.
Histopathology
Intact spinal columns removed from mice at the end of the study (i.e. Day 24 post-immunization) were fixed in 4% paraformaldehyde (PFA, eBioscience, San Diego, CA) then extricated from surrounding bone tissue. Dissected spinal cords were embedded in paraffin before sectioning. Ten µm thick slices were stained with hematoxylin and eosin to evaluate inflammatory lesions or stained using a Luxol Fast Blue (LFB) protocol to assess demyelination. Slides were imaged by light microscopy.
Isolation of leukocytes from spleen, spinal cord, and brain
Immediately prior to transcardial perfusion with Roswell Park Memorial Institute (RPMI, Corning Cellgro, Manassas, VA) 1640, spleens were removed from animals under sterile conditions and single cell suspensions of leukocytes were prepared by disaggregation of the tissue through a 100µm nylon mesh (BD Falcon, Bedford, MA). Cells were washed once with RPMI 1640 supplemented with 10% heat-inactivated FBS (FBS; Thermo Scientific, Waltham, MA), then incubated with RBC Lysis Buffer (eBioscience) for 1 min to remove red cells, and washed in RPMI 1640+FBS. Cells were enumerated in a 1:1 dilution with 0.2% trypan blue stain (Life Technologies, Grand Island, NY) using a Cellometer Auto T4 Cell Viability Counter (Nexcelom, Lawrence, MA), washed, and resuspended at 107 cells/mL in stimulation medium (RPMI 1640) containing 10% FBS, 1% sodium pyruvate (Life Technologies), 1% L-glutamine (Thermo Scientific), and 0.4% β-mercaptoethanol (ME)(Sigma-Aldrich)).
Brains and spinal cords were passed through 100µm mesh screens and washed as above. Cells were resuspended in 80% Percoll (GE Healthcare, Pittsburgh, PA) then overlaid with 40% Percoll to establish a density gradient and centrifuged at 1,600 rpm for 30min following a method previously described (Campanella et al. 2002). Leukocytes were collected from the resultant interface, counted, and resuspended in stimulation media for assay.
Flow cytometry
Single Cell suspensions were prepared as above then washed and resuspended in staining medium (phosphate buffered saline (PBS, Fisher Scientific, Fair Lawn, NJ), 0.5% bovine serum albumin (BSA, Sigma-Aldrich), and 0.02% sodium azide (Sigma-Aldrich). Leukocytes were labeled with a combination of the following antibodies obtained from BD Biosciences (San Jose, CA): PE-Cy7 CD4 (L3T4), APC CD8 (53-6.7), APC CD80 (16-10A1), PE I-Ab (AF6-120.1), PE PD-1 (J43), APC CXCR5 (2G8), APC CD19 (1D3), PE CD1d (1B1), PerCP CD5 (53–7.3), PE PD-L1 (MIH5), PerCP-Cy5.5 CD11b (M1/70), PE CD45 (30-F11), PE CD62L (MEL-14), APC CD44 (IM7), PE CD122 (TM-β1), APC IFN-γ (XMG1.2), PE IL-17 (TC11-18H10), PE IgG1,κ (R3-34), APC IgG1,κ (A85-1). Intracellular staining of Foxp3 (MF23) was completed following overnight incubation in fixation/permeabilization buffer (BD Biosciences). Data were acquired using a BD Accuri C6 (BD Biosciences) using BD’s proprietary C6 software.
Intracellular staining was visualized using a published immunofluorescence protocol (Subramanian et al. 2011). Briefly, 2×106 cells were incubated in 1mL of stimulation medium (as above) with PMA (50ng/mL, Sigma-Aldrich), ionomycin (500ng/mL, Sigma-Aldrich) and Brefeldin A (10µg/mL, BD Biosciences) at 37°C and 5% CO2. For intracellular IL-10 detection, 10µg/mL lipopolysaccharide from E. coli (055:B5, Sigma-Aldrich) was added to the above cocktail (Yanaba et al. 2008).
Following incubation, Fc receptors were blocked with anti-CD16/32 monoclonal antibodies (mAb) (2.3G2; BD Biosciences) before cell surface staining, fixed in 4% PFA (eBioscience), and permeabilized with Fixation/Permeabilization buffer (BD Biosciences) according to the manufacturer’s instructions. Permeabilized cells were washed with 1X Perm/Wash Buffer (BD Biosciences) and were stained with anti-IL-17, anti-TNFα, or anti-IFN-γ mAb. Isotype-matched mAb served as negative controls to demonstrate specificity and to establish background for the levels of IL-17, TNFα or IFN-γ expression.
Cell sorting and adoptive transfer of B cells
Age matched female IL-10 GFP+ reporter B cell donors were immunized with 200µg MOG35–55 peptide (PolyPeptide Laboratories) in 200µg CFA (as above). Ptx was not given to ensure the retention of MOG-primed cells in the spleens. Donor B cells were acquired on day 14 post immunization and splenic CD19+ B cells were purified using paramagnetic bead-conjugated antibodies (Abs) from the Miltenyi B cell isolation kit and subsequently separated by AutoMACS (both from Miltenyi Biotec, Auburn, CA).
The negative fraction of the cells thus separated were CD19+ B cells with a purity of ≥ 95%. CD19+ B cells were suspended in stimulation medium (described above) and cultured in the presence of 1 µg/mL LPS (from E. coli serotype 055:B5) (Sigma-Adrich) for 48hrs at 37°C, 5% CO2 and 95% humidity. After culture, B cells were harvested from culture plates, washed free of LPS, and viable cells were counted. The cells were then resuspended at 108 purified IL-10-GFP+ B cells/mL in 0.9% sodium chloride irrigation solution (saline, Baxter, Deerfield, IL ) and 100µL of either cell suspension (107 cells) or saline were transferred intravenously (i.v.) via tail vein into µMT−/− recipients on day 3 post MOG/CFA immunization.
Statistical analysis
Data were reported using GraphPad Prism (v5.0, San Diego, CA) and expressed as the mean ± SEM. Statistical significance for the disease course was calculated using the Mann-Whitney U test. The Student’s t-test and the Kruskal–Wallis Test (non-parametric analysis of variance) with Dunn’s multiple comparison of means post-test were used for analysis of the cumulative disease index. Statistical significance for flow cytometry data was calculated using One-way ANOVA (one way analysis of variance) and Newman-Keuls Multiple Comparison post-test. P-values ≤ 0.05 were considered to be significant (* p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001).
Results
Regulatory B cells require E2 to ameliorate clinical signs of EAE
We have reported previously that µMT−/− mice lost sustained clinical benefit from E2 during EAE, and that reconstitution with B cells from immunized donors restored E2-imparted protection (Bodhankar et al. 2012). In the present study, we confirmed that not only are B cells required to establish protection with E2, but that the IL-10+ regulatory B cells (Bregs) are also able to suppress clinical signs of EAE in the presence of E2. While mice given E2 in conjunction with either treatment displayed delayed onset of disease, clinical manifestations of EAE were present in only 30% (7 out of 23, Table 1) of E2-implanted µMT−/− mice that were the recipients of IL-10 producing B cells (E2/Bregs group) compared to 63% (12 out of 19, Table 1) of mice treated with E2 alone (E2/saline group) and 92% (11 out of 12, Table 1) of mice that received only Bregs (sham/Bregs group). Additionally, the clinical disease index (CDI) and disease peak of mice given E2 and Bregs were significantly lower than all other treatment groups (Table 1). Importantly, mice given Bregs but not E2 displayed no statistically significant differences in any of the clinical parameters evaluated compared to the control group. These data suggest that IL-10 producing Bregs are not sufficient to protect against EAE in µMT−/− recipients independent of E2 signaling.
Tab. 1. Clinical course of EAE in µMT−/− mice.
Statistical evaluation of EAE disease course for µMT−/− female mice treated with vehicle, 107 IL-10+ B cells, estradiol (E2), or 107 IL-10+ B cells in combination with E2, including the Cumulative Disease Index (CDI) through day 24 post-immunization for the 4 experiments (n = 3–6 mice/group/experiment) presented as mean ± SEM.
| Mice | Incidence | Onset | Peak | CDI | Mortality |
|---|---|---|---|---|---|
| Sham/Saline | 20/20 | 12.1 ± 0.4a | 5.0 ± 0.1a | 56.4 ± 2.3a | 1 |
| Sham/B cells | 11/12 | 11.0 ± 0.5b | 4.8 ± 0.4b | 51.3 ± 5.2b | 0 |
| E2/Saline | 12/19 | 20.3 ± 0.4 | 2.8 ± 0.5 | 10.4 ± 2.2 | 0 |
| E2/B cells | 7/23 | 12.1 ± 0.7 | 1.2 ± 0.4c | 4.4 ± 1.6c | 0 |
significant (P<0.0001) as Compared to E2 group
significant (P<0.0001) as Compared to E2/B cells group
significant (P<0.05) as Compared to E2/B cells group
IL-10 producing B cells in combination with E2 treatment inhibits infiltration of leukocytes into the CNS of µMT−/− mice
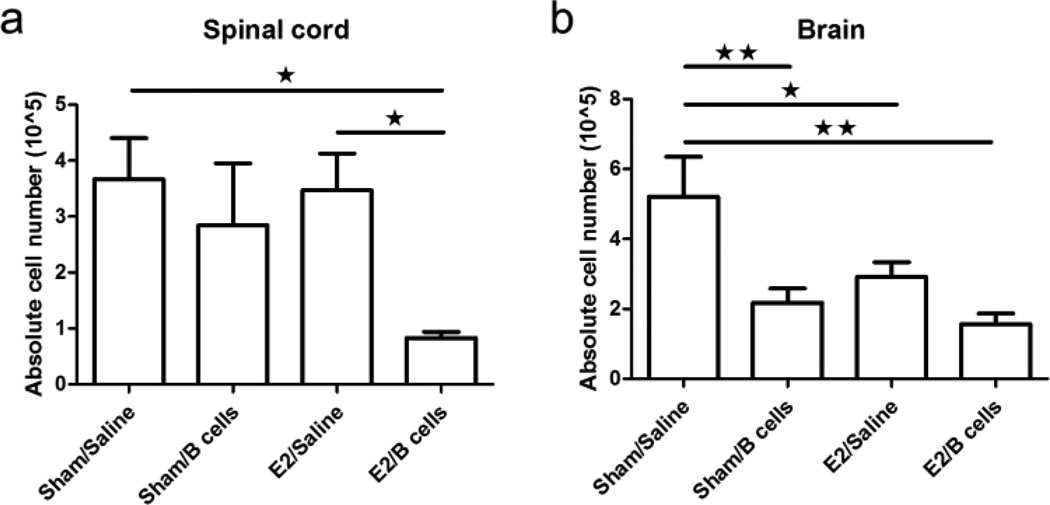
To evaluate the potential regulatory effects of Bregs on monocyte and T cell activation, we first enumerated the number of leukocytes present in the spinal cord, brain, and spleen. Treatment with Bregs in E2-implanted mice significantly reduced the number of infiltrating leukocytes in the spinal cord compared to the E2 only mice (Fig. 1a). Notably, treatment with E2, Bregs or both agents also significantly reduced the number of cells isolated from brains (Fig. 1b). These results suggest that IL-10 producing B cells exposed to E2 limit leukocyte infiltration into the CNS of µMT−/− mice during EAE.
Fig. 1. IL-10 producing B cells and E2 ameliorate EAE and decrease spinal cord inflammation in µMT−/− mice.
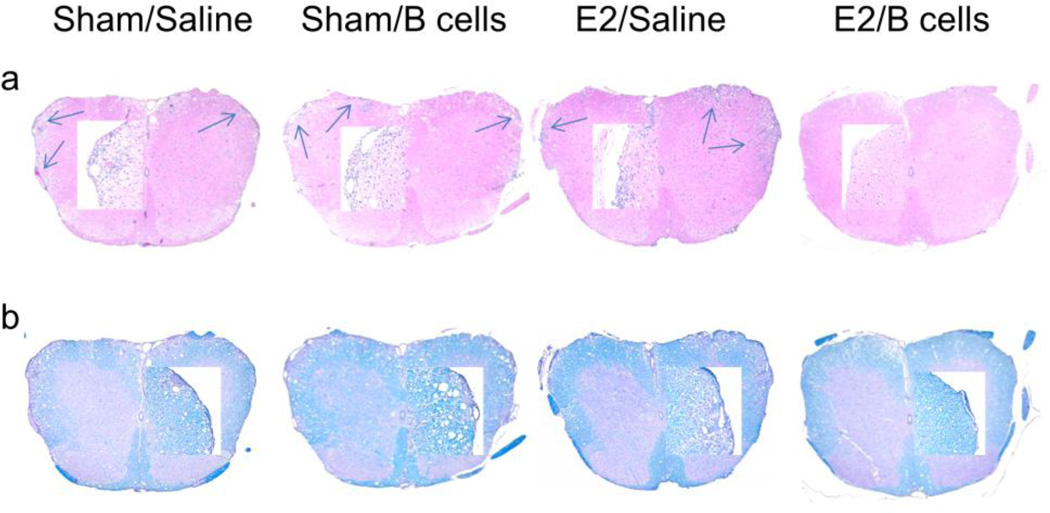
Spinal cords from each group were collected 24 days post-immunization, fixed in PFA, and embedded in paraffin for sectioning. Ten µm transverse sections from different regions of the spinal cord from each of the groups were stained with Hematoxylin & Eosin to enumerate infiltrating leukocytes (Fig. 1a) and with Luxol Fast Blue to visualize extent of demyelination (Fig. 1b). Arrows denote foci of inflammation. Magnification was 50× and 200× (inset). Sections are representative of 2 experiments (n = 3 mice/group/experiment).
These data were supported by histological examination of spinal cord sections from experimental animals. Staining was performed using hematoxylin & eosin to assess the distribution of infiltrating cells and Luxol Fast Blue (LFB) to visualize demyelination. Sections from mice given saline, Bregs or E2 alone demonstrated massive leukocyte infiltration with several foci of inflammation (indicated by arrows, Fig. 2a) accompanied by extensive demyelination (Fig. 2b). However, treatment with E2 together with Bregs noticeably reduced both infiltrating cells and demyelinated lesions compared to the other treatment groups. These observations were consistent with the amelioration of EAE signs seen in groups given E2 and Bregs and suggest that IL-10+ B cells arrest trafficking of leukocytes into the CNS in the presence of E2.
Fig. 2. IL-10 producing B cells and E2 treatment reduce infiltration of leukocytes into the CNS of µMT−/− mice.
Mononuclear cells were isolated from CNS tissues and total live cell numbers from spinal cords (Fig. 2a) and brains (Fig. 2b) were counted on a hemocytometer. Data shown are representative of 4 independent experiments consisting of 6 mice per group (mean ± SEM). Statistical analysis was performed with ANOVA followed by Newman-Kuels post hoc test. Significant differences between sample means are indicated (*p≤0.05; **p≤0.01; ***p≤0.001)
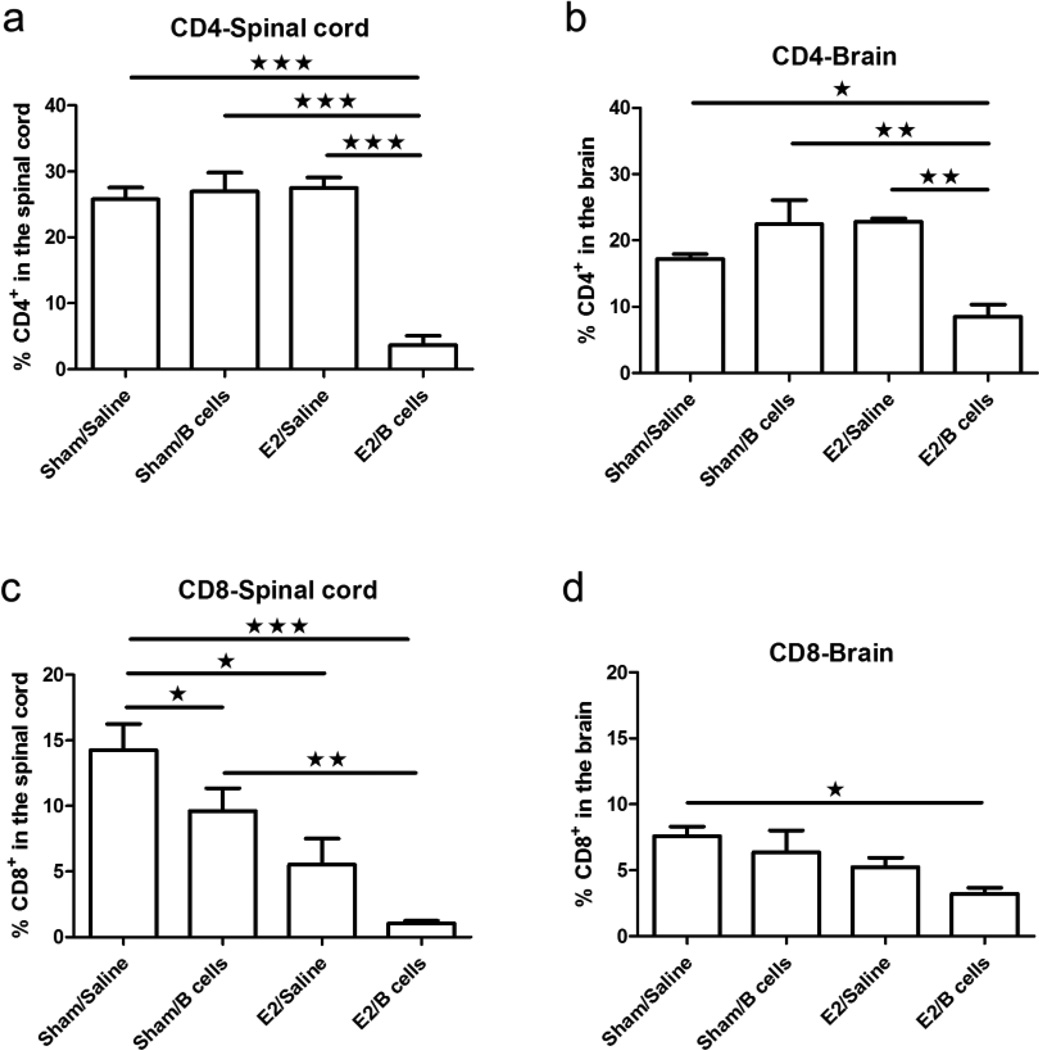
CNS infiltrating T lymphocytes are significantly reduced in µMT−/− mice treated with E2 and IL-10 producing B cells
Infiltrating leukocytes isolated from the spinal cords and brains of experimental animals were characterized in order to assess cells associated with clinical signs of disease. We observed a profound reduction in the number of infiltrating CD4+ T cells in the CNS of mice that received both E2 and IL-10+ B cells compared to either agent alone (Fig. 3a, 3b). CD8+ T cells were also significantly less frequent in the spinal cords of treated mice, but this effect was also observed after administration of Bregs or E2 alone (Fig. 3c). Interestingly, only therapy with E2 and Bregs caused a statistically significant decrease in the number of CD8+ T cells in the brains of mice with EAE (Fig. 3d).
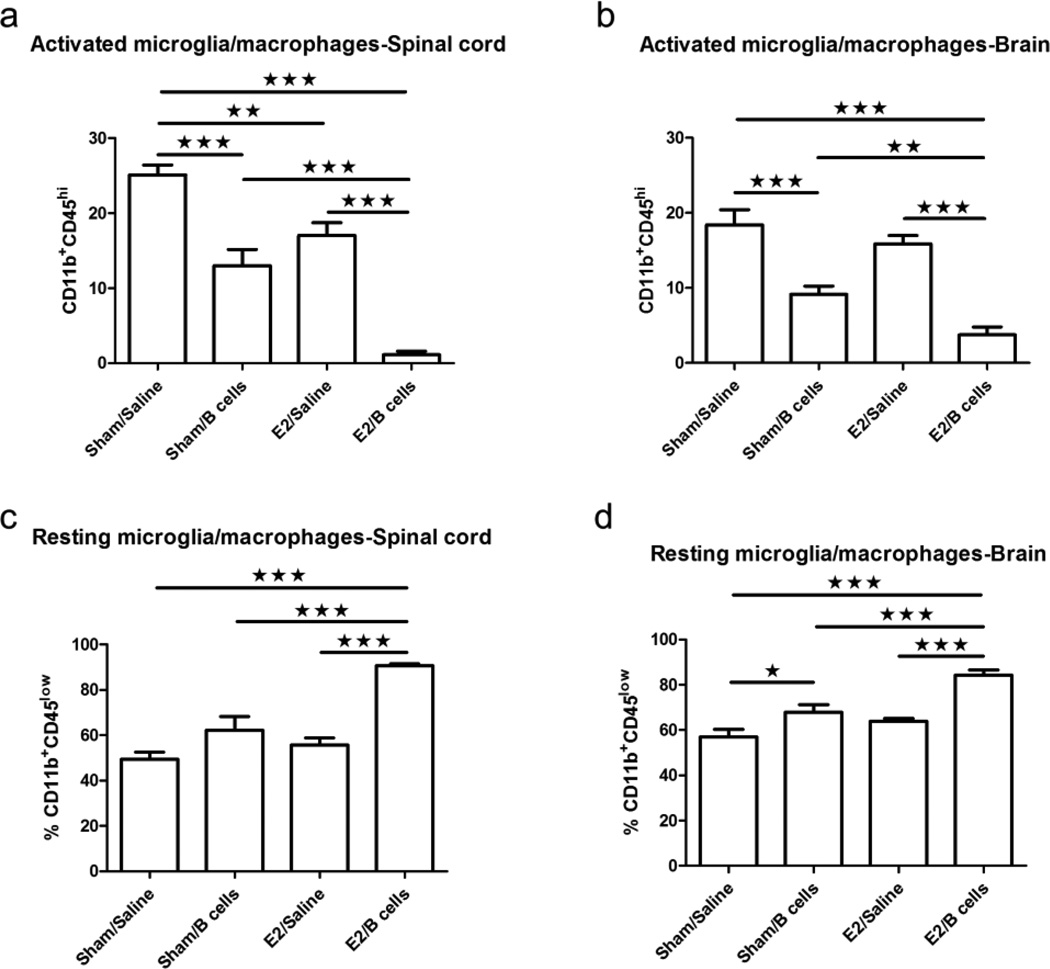
Fig. 3. Administration of IL-10 producing B cells in combination with E2 treatment decreased activation of microglia/macrophages in the CNS.
CD11b+CD45hi activated microglia/macrophages (Fig. 3a, 3b) and CD11b+CD45low resting microglia/macrophages (Fig. 3c, 3d) were determined in individual spinal cords and brains. Data shown are representative of 4 independent experiments consisting of 6 mice per group (mean ± SEM). Statistical analysis was performed with ANOVA followed by Newman-Kuels post hoc test. Significant differences between sample means are indicated (*p≤0.05; **p≤0.01; ***p≤0.001)
Previous reports indicated that activated CD4+ T cells, especially IL-17 producing CD4+ T cells, play a pathogenic role in the development of EAE. Here we identify the activation states and inflammatory cytokine production by peripheral CD4+ T cells. We found that there were no significant differences in the percentages of total splenic CD4+ T cells (Sup. 1a), Naïve CD4+ (CD62LhiCD44low) T cells (Sup. 1b) or effector memory CD62LlowCD44hiCD4+ T cells (Sup. 1c) in any of the treated groups. Interestingly, the percentage of central memory CD62LhiCD44hiCD4+ T cells was significantly increased in E2 treated mice given IL-10+ B cells compared to controls (Sup. 1d). This suggests that E2 signaling in the presence of IL-10 producing B cells may involve the induction, and proliferation of central memory CD4+ T cells. Evaluation of inflammatory cytokines produced by peripheral CD4+ T cells revealed no significant differences in either IL-17 (Sup. 1e) or IFN-γ among groups (Sup. 1f).
Due to the lack of a difference in peripheral cytokine production among treatment groups, we examined the spinal cords and brains of experimental animals for IL-17 production. Because of the limited numbers of cells available from CNS tissue, we pooled leukocytes from brains or spinal cords of sham/saline, sham/Bregs, E2/saline, and E2/Bregs groups, respectively, for intracellular staining of IL-17 by flow cytometry. Our results indicated that the percentage of IL-17+CD4+ T cells in the spinal cords of E2/B Breg recipients was markedly decreased compared to controls (3.2% E2/Bregs vs. 13.3% sham/saline, data not shown). In contrast, the percentage of IL-17+CD4+ T cells in the brains of E2/ Breg- treated recipients were not different compared to controls (10.8% E2/Bregs vs. 9.5% sham/saline, data not shown). Taken together, these results suggest that IL-10 producing B cells require E2 to reduce the infiltration of pathogenic immune cells into the CNS during EAE.
IL-10+ B cells induce microglial quiescence in the presence of E2 during EAE
Microglia are the primary immune effector cells in the CNS, which are capable of responding to a vast array of challenges and undergo phenotypic or morphological transformations upon encountering infection or injury. They are involved in almost all neuropathological conditions such as degenerative diseases, stroke, tumors, and traumatic brain injury (Nayak et al. 2014). Reactive microglia/macrophages make up the majority of cells in multiple sclerosis plaques (Carson 2002) and act as APCs by expressing MHC class I, MHC class II and co-stimulatory molecules (Becher and Antel 1996).
In the present study, we found that treatment with E2 or Bregs individually caused a limited but significant reduction in the number of CD11b+CD45hi activated microglia/macrophages in the CNS of EAE-induced mice (Fig. 4a, 4b). However, concurrent administration of Bregs and E2 led to profound suppression in the percentage of activated microglia/macrophages compared to control (sham/saline), E2 only (E2/saline), and Bregs only (sham/Bregs) treated groups (Fig. 4a, 4b). Conversely, the proportion of resting microglia/macrophages (CD11b+CD45low) was significantly increased in the E2/Bregs treated group (Fig. 4c, 4d), further supporting a role for E2-affected Bregs in suppressing microglial activation during EAE.
Fig. 4. Lower infiltration of proinflammatory immune cells in the CNS with IL-10 producing B cells plus E2 treatment.
Frequencies of CD4+ (Fig. 4a, 4b) and CD8+ T cells (Fig. 4c, 4d) were determined in individual spinal cords and brains and indicate the percentages of total gated live leukocytes (n=6). Data are representative of 4 independent experiments consisting of 6 mice per group (mean ± SEM). Statistical analysis was performed with ANOVA followed by Newman-Kuels post hoc test. Significant differences between sample means are indicated (*p≤0.05; **p≤0.01; ***p≤0.001)
Activation of peripheral monocytes is increased following transfer of IL-10+ B cells in E2 treated mice
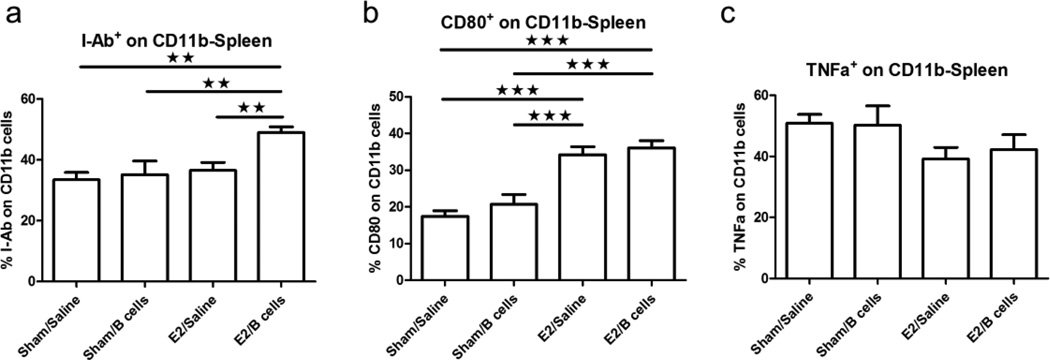
Previous studies have determined that CD11b+MHCII+ monocytes direct differentiation of TH2 cells and CD4+CD25+Foxp3+ regulatory T cells independently of antigen specificity (Weber et al. 2007). Additionally, CD80 expression by CD11b+ monocytes has been shown to play a critical part in the suppression of antigen-specific immune responses (Yang et al. 2006).
Our results showed that treatment with Bregs and E2 significantly increased the expression of I-Ab on CD11b+ monocytes in the spleen when compared to other groups (Fig. 5a). We also found that monocyte expression of CD80 was significantly increased in E2 treated groups with or without B cells (Fig. 5b). Further evaluation of the expression of the pro-inflammatory cytokine, TNF-α, in splenic monocytes indicated no significant differences in the number of TNFα+CD11b+ cells in the spleen amongst experimental groups (Fig. 5c).
Fig. 5. Activation and pro-inflammatory state of CD11b+ monocytes.
Expression of the monocyte activation markers I-Ab (Fig. 5a) and CD80 (Fig. 5b) were determined on CD11b+ splenocytes from the various treatment groups. Fig. 5c: TNF-α producing CD11b+ monocytes. Data are representative of 4 independent experiments consisting of 6 mice per group (mean ± SEM). Statistical analysis was performed with ANOVA followed by Newman-Kuels post hoc test. Significant differences between sample means are indicated (*p≤0.05; **p≤0.01; ***p≤0.001)
IL-10+ B cells in combination with E2 treatment promotes proliferation of peripheral CD4+Foxp3+ regulatory T cells and activates the PD1/PD-L1 pathway
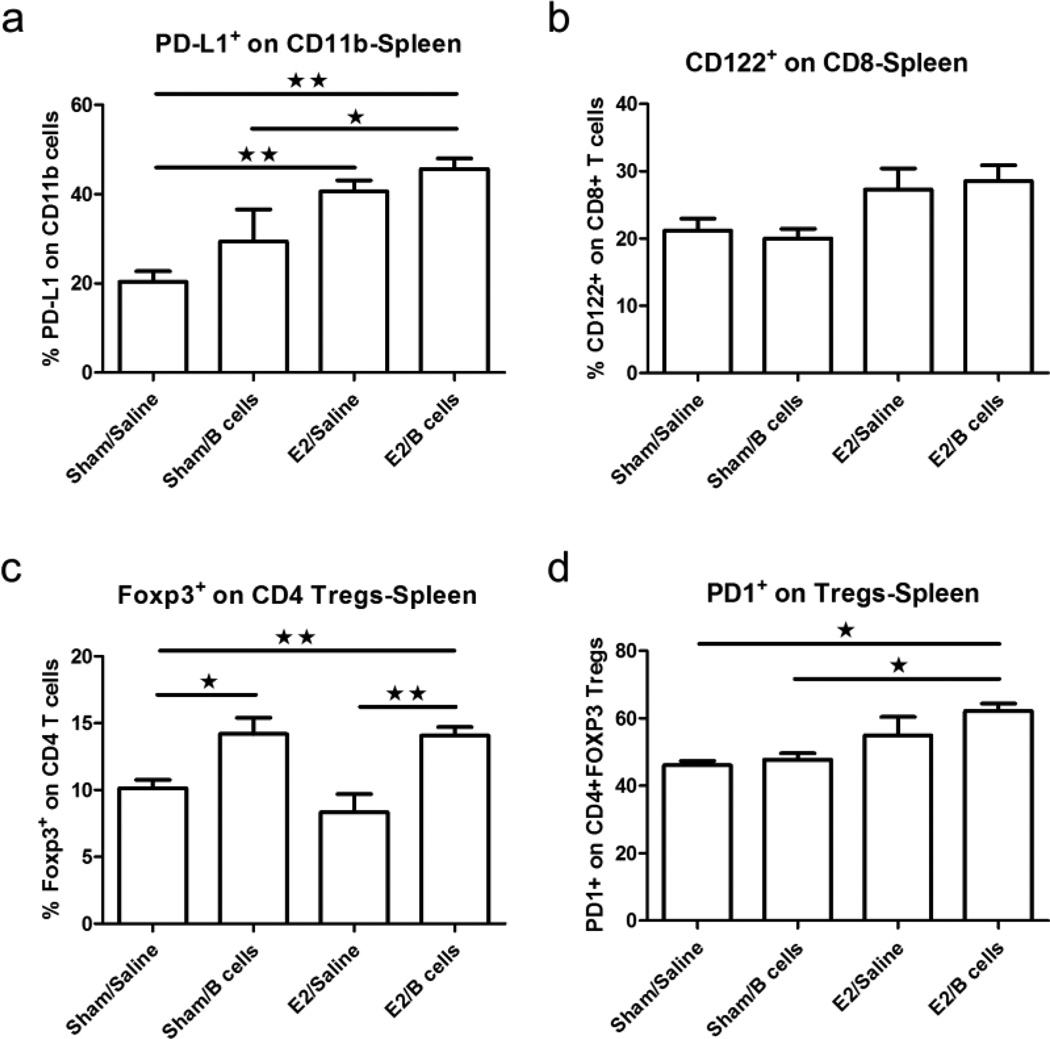
Replenishment of B cell populations in µMT−/− mice exposed to E2 has previously been shown to significantly increase total numbers of PD-L1 expressing cells and PD-1+CD4+CD25+Foxp3+ regulatory T cells (Tregs) (Bodhankar et al. 2012). Furthermore, E2 treatment did not protect against EAE in mice lacking PD-L1 (PD-L1−/−), which developed an EAE disease course equivalent to that of WT and PD-L1−/− controls (Bodhankar et al. 2013b; Bodhankar et al. 2011). These results suggested that PD-L1 is a critical co-inhibitory molecule in E2-mediated protection against EAE.
Our current study explored whether the protection induced by E2 and IL-10+ B cells was mediated in part by regulatory T cells and the PD-1/PD-L1 pathway. Our data confirm that PD-L1+CD11b+ monocytes were enriched in the spleen of E2/Breg treated recipients versus control mice and Breg alone treated mice (Fig. 6a). In contrast, there was no significant difference in the expression of PD-L2+CD11b+ monocytes (data not shown). This finding is consistent with our previously published studies (Bodhankar et al. 2013b).
Fig. 6. PD-1/PD-L1 pathway molecules are up-regulated in µMT−/− mice that receive IL-10 producing B cells and E2.
PD-L1 expression was assessed on splenic CD11b+ monocytes (Fig. 6a); CD122, on splenic CD8+ T cells (Fig. 6b); Foxp3, on splenic CD4+ T cells (Fig. 6c) and PD-1, on splenic CD4+Foxp3+ regulatory T cells (Fig. 6d). Data are representative from 4 independent experiments consisting of 6 mice per group (mean ± SEM). Statistical analysis was performed with ANOVA followed by Newman-Kuels post hoc test. Significant differences between sample means are indicated (*p≤0.05; **p≤0.01; ***p≤0.001)
Recently, CD8+CD122+ regulatory T cells have been identified (Rifa'i et al. 2004; Rifa'i et al. 2008) that effectively suppress the proliferation and IFN-γ production in both CD8+ and CD4+ T cells through production of IL-10. Our results suggest that CD8+CD122+ are increased in the periphery of mice exposed to E2, although not in a statistically significant way (Fig. 6b). In contrast, CD4+Foxp3+ T cells were significantly enriched in the spleens of mice treated with IL-10+ B cells (Fig. 6c). Interestingly, this effect appeared to be independent of E2 treatment. Examining PD-1+ Tregs from each group, we found that only Tregs from E2/Breg treated mice exhibited significantly higher levels of PD-1 compared to control groups (Fig. 6d), suggesting a role for this cell type in E2/PD-L1 mediated protection.
Cumulatively, these data indicate that IL-10 producing B cells in combination with E2 treatment increases the proportion of CD4+Foxp3+ regulatory T cells and emphasizes the PD-1/PD-L1 signaling pathway’s involvement in protection against EAE in B-cell deficient µMT−/− mice.
Discussion
Multiple sclerosis is a debilitating autoimmune disease of the central nervous system. Activation of auto-reactive T cells and increased secretion of inflammatory and neurotoxic factors by microglia leads to demyelination in the CNS (Chabot and Yong 2000; Malpass 2012; Steinman 2001) and subsequent disability. EAE is an induced autoimmune disease in mice that mimics a portion of the clinical signs of MS, resulting in inflammatory demyelination of the CNS by encephalitogenic CD4+ T cells specific for CNS autoantigens (Williams et al. 1994). Women with MS often experience clinical improvement during pregnancy, indicating that sex hormones may hold therapeutic potential in the treatment of MS. Indeed, it has been shown by numerous laboratories that E2 confers potent protection against clinical and histopathological signs of EAE (Daumer et al. 2012).
Previous studies suggest that B cell deficient mice develop a severe, non-remitting form of EAE (Fillatreau et al. 2002; Wolf et al. 1996). Supportive of this finding, B cell depletion with anti-CD20 antibody before EAE induction resulted in exacerbation of clinical signs of disease and increased encephalitogenic T cell influx into the CNS (Matsushita et al. 2008). These studies led to the identification of IL-10 producing 12 CD19+CD1dhiCD5+ regulatory B cells, which, when depleted, resulted in increased disease severity. Furthermore, adoptive transfer of wild-type B-cells but not B cells from IL-10 deficient (IL-10−/−) mice restored typical EAE in µMT−/− mice (Matsushita et al. 2008; Yanaba et al. 2008). Parallel studies from our group have demonstrated a role for IL-10+ Bregs in limiting CNS inflammation subsequent to experimental stroke (Bodhankar et al. 2013a), indicating a role for Bregs in attenuating a range of inflammatory responses.
Our previous work demonstrated that E2-implanted µMT−/− mice developed only transient E2-mediated protection, but reconstitution with wild-type B cells provided significant protection from EAE compared with sham-treated recipients (Bodhankar et al. 2011). However, the protection gained through B cell transfers from naïve WT mice was short-lived, with recipients losing protection after day 21 post-EAE induction. In the present study, in order to dissect the interaction of IL-10+ Bregs and E2 in neuroprotection, we assessed sham controls, IL-10+ B cell administration alone, E2 implantation alone and the combination of these agents for preventing clinical signs of EAE and reducing CNS inflammation. Additionally, using CNS antigen specific B cells purified from MOG35-55-immunized donors and stimulated with LPS to augment IL-10 production, we observed enhanced protection against EAE with delayed or less aggressive onset of disease.
The regulatory function of B cells is considered to be mainly determined by the secretion of IL-10. However, various B cell subsets are capable of IL-10 secretion (Iwata et al. 2011; Kalampokis et al. 2013; Yang et al. 2010). In order to identify the diversity in the phenotype of IL-10 producing regulatory B cells, evaluation of stimulated B cells used for adoptive transfer showed that approximately 30% of B cells yielded measurable quantities of IL-10-eGFP conjugate (data not shown). Furthermore, regulatory B cell subset phenotypes such as CD19+CD1dhiCD5+ (B10), CD19+CD21hiIgMhiCD23hi (T2-MZ), CD1d-CD5+ (B1a) have been shown to be evenly distributed in IL-10 secretion (Bodhankar et al. 2013a). This finding suggests that these cells are exerting an overall immunosuppressive effect in their recipients. Future studies will delineate the contributions to E2 mediated protection of each of these regulatory B cell subsets in ameliorating EAE signs.
Regulatory T cells and the co-inhibitory PD-1/PD-L1 signaling pathway have previously been shown to be involved in the E2-conferred protection against EAE in µMT−/− mice (Bodhankar et al. 2013b; Bodhankar et al. 2012). Our data indicate that the significant increase in percentages of CD4+Foxp3+PD-1+ regulatory T cells and up-regulation of PD-1/PD-L1 pathway molecules is a result of co-administration of IL-10+ B cells acting in concert with E2.
Persistent activation of microglia is associated with infiltration of inflammatory cells and neuronal dysfunction in the CNS during EAE (Marques et al. 2006; Rasmussen et al. 2007). Thus, we analyzed the phenotypes of microglia/macrophages found in the CNS of EAE-afflicted µMT−/− mice. Our findings indicate that regulatory B cells in the presence of E2 dramatically attenuate activation of microglia/macrophages in the CNS during EAE. Whether this effect is due to direct interaction between these cell types or relies on intermediate cellular signaling remains unclear. In vitro examinations of how these populations interact alone and in the presence of powerful endocrine signals such as E2 may be warranted to untangle the involvement of Bregs in reducing microglial activation during EAE.
Regulatory B cells modify immune activity by acting as APCs to induce immune tolerance as well as by secreting IL-10 (Bodhankar et al. 2012; Yang et al. 2010). Our past findings have shown that MOG-specific B cells obtained from WT donors are not sufficient to sustain E2 mediated protection against EAE long-term in µMT−/− mice (Bodhankar et al. 2012; Bodhankar et al. 2011). Other reports show that regulatory B cells inhibit EAE initiation in mice while pathogenic B cells promote disease progression (Matsushita et al. 2008; Ray and Basu 2014). Our current study demonstrates that a small sub-population of B cells enriched in producing IL-10 conferred peripheral and CNS-localized anti-inflammatory effects in mice exposed to pregnancy levels of E2. Hallmarks of these effects include the suppression of microglia/macrophage activation, arrest of leukocyte infiltration into the CNS, activation & differentiation of CD4+Foxp3+PD-1+ Tregs, and activation of the co-inhibitory PD-1/PD-L1 pathway. Understanding the mechanisms that drive the potent suppressive effects observed after E2/IL-10+ B cell co-administration may expose new targets for pharmaceutical or cell therapy based interventions in multiple sclerosis and other neurodegenerative or neuro-inflammatory disorders.
Supplementary Material
Total CD4+ T cells (a), naïve CD4+ T cells (b), effector memory CD4+ T cells (c) central memory CD4+ T cells (d) and IL-17 (e) and IFN-γ (f) were assessed in the spleen. Data are representative from 4 independent experiments consisting of 6 mice per group (mean ± SEM). Statistical analysis was performed with ANOVA followed by Newman-Kuels post hoc test. Significant differences between sample means are indicated (*p≤0.05; **p≤0.01; ***p≤0.001)
Acknowledgements
This work was supported by National Institutes of Health/National Institute of Neurological Disorders and Stroke grant RO1 NS080890. The authors wish to thank Gail Kent for assistance with manuscript preparation and OHSU research cores for histopathology. This material is the result of work supported with resources and the use of facilities at the VA Portland Health Care System, Portland, OR. The contents do not represent the views of the Department of Veterans Affairs or the US government.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Abramsky O. Pregnancy and multiple sclerosis. Annals of neurology. 1994;36(Suppl):S38–S41. doi: 10.1002/ana.410360712. [DOI] [PubMed] [Google Scholar]
- Becher B, Antel JP. Comparison of phenotypic and functional properties of immediately ex vivo and cultured human adult microglia. Glia. 1996;18:1–10. doi: 10.1002/(SICI)1098-1136(199609)18:1<1::AID-GLIA1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Baeten D, Jager A, Sobel RA, Kuchroo VK. Myelin oligodendrocyte glycoprotein-specific T and B cells cooperate to induce a Devic-like disease in mice. The Journal of clinical investigation. 2006;116:2393–2402. doi: 10.1172/JCI28334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair PA, Norena LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, Mauri C. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus. Erythematosus patients Immunity. 2010;32:129–140. doi: 10.1016/j.immuni.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Bodhankar S, Chen Y, Vandenbark AA, Murphy SJ, Offner H. IL-10-producing B-cells limit CNS inflammation and infarct volume in experimental stroke. Metabolic brain disease. 2013a;28:375–386. doi: 10.1007/s11011-013-9413-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodhankar S, Galipeau D, Vandenbark AA, Offner H. PD-1 Interaction with PD-L1 but not PD-L2 on B-cells Mediates Protective Effects of Estrogen against EAE. Journal of clinical & cellular immunology. 2013b;4:143. doi: 10.4172/2155-9899.1000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodhankar S, Vandenbark AA, Offner H. Oestrogen treatment of experimental autoimmune encephalomyelitis requires 17beta-oestradiol-receptor-positive B cells that up-regulate PD-1 on CD4+ Foxp3+ regulatory T cells. Immunology. 2012;137:282–293. doi: 10.1111/imm.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodhankar S, Wang C, Vandenbark AA, Offner H. Estrogen-induced protection against experimental autoimmune encephalomyelitis is abrogated in the absence of B cells. European journal of immunology. 2011;41:1165–1175. doi: 10.1002/eji.201040992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanella M, Sciorati C, Tarozzo G, Beltramo M. Flow cytometric analysis of inflammatory cells in ischemic rat brain. Stroke; a journal of cerebral circulation. 2002;33:586–592. doi: 10.1161/hs0202.103399. [DOI] [PubMed] [Google Scholar]
- Carson MJ. Microglia as liaisons between the immune and central nervous systems: functional implications for multiple sclerosis. Glia. 2002;40:218–231. doi: 10.1002/glia.10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabot S, Yong VW. Interferon beta-1b increases interleukin-10 in a model of T cell-microglia interaction: relevance to MS. Neurology. 2000;55:1497–1505. doi: 10.1212/wnl.55.10.1497. [DOI] [PubMed] [Google Scholar]
- Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- Confavreux C, Hutchinson M, Hours MM, Cortinovis-Tourniaire P, Moreau T. Rate of pregnancy-related relapse in multiple sclerosis. Pregnancy in Multiple Sclerosis Group. The New England journal of medicine. 1998;339:285–291. doi: 10.1056/NEJM199807303390501. [DOI] [PubMed] [Google Scholar]
- Daumer M, Weinshenker BG, Voskuhl R. Pregnancy: a "modifiable" risk factor in MS? Neurology. 2012;78:846–848. doi: 10.1212/WNL.0b013e31824d1858. [DOI] [PubMed] [Google Scholar]
- Duddy M, et al. Distinct effector cytokine profiles of memory and naive human B cell subsets and implication in multiple sclerosis. Journal of immunology. 2007;178:6092–6099. doi: 10.4049/jimmunol.178.10.6092. [DOI] [PubMed] [Google Scholar]
- Evans JG, Chavez-Rueda KA, Eddaoudi A, Meyer-Bahlburg A, Rawlings DJ, Ehrenstein MR, Mauri C. Novel suppressive function of transitional 2 B cells in experimental arthritis. Journal of immunology. 2007;178:7868–7878. doi: 10.4049/jimmunol.178.12.7868. [DOI] [PubMed] [Google Scholar]
- Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nature immunology. 2002;3:944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- Iwata Y, et al. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood. 2011;117:530–541. doi: 10.1182/blood-2010-07-294249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalampokis I, Yoshizaki A, Tedder TF. IL-10-producing regulatory B cells (B10 cells) in autoimmune disease. Arthritis research & therapy. 2013;15(Suppl 1):S1. doi: 10.1186/ar3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linington C, Bradl M, Lassmann H, Brunner C, Vass K. Augmentation of demyelination in rat acute allergic encephalomyelitis by circulating mouse monoclonal antibodies directed against a myelin/oligodendrocyte glycoprotein. The American journal of pathology. 1988;130:443–454. [PMC free article] [PubMed] [Google Scholar]
- Lyons JA, San M, Happ MP, Cross AH. B cells are critical to induction of experimental allergic encephalomyelitis by protein but not by a short encephalitogenic peptide. European journal of immunology. 1999;29:3432–3439. doi: 10.1002/(SICI)1521-4141(199911)29:11<3432::AID-IMMU3432>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Madan R, et al. Nonredundant roles for B cell-derived IL-10 in immune counter-regulation. Journal of immunology. 2009;183:2312–2320. doi: 10.4049/jimmunol.0900185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malpass K. Multiple sclerosis: 'Outside-in' demyelination in MS. Nature reviews Neurology. 2012;8:61. doi: 10.1038/nrneurol.2011.217. [DOI] [PubMed] [Google Scholar]
- Marques KB, Santos LM, Oliveira AL. Spinal motoneuron synaptic plasticity during the course of an animal model of multiple sclerosis. The European journal of neuroscience. 2006;24:3053–3062. doi: 10.1111/j.1460-9568.2006.05184.x. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, et al. Interleukin-10-producing plasmablasts exert regulatory function in autoimmune inflammation. Immunity. 2014;41:1040–1051. doi: 10.1016/j.immuni.2014.10.016. [DOI] [PubMed] [Google Scholar]
- Matsushita T, Yanaba K, Bouaziz JD, Fujimoto M, Tedder TF. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. The Journal of clinical investigation. 2008;118:3420–3430. doi: 10.1172/JCI36030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maugh TH., 2nd The EAE Model: A Tentative Connection to Multiple Sclerosis. Science. 1977;195:969–971. doi: 10.1126/science.195.4282.969. [DOI] [PubMed] [Google Scholar]
- Mauri C, Bosma A. Immune regulatory function of B cells. Annual review of immunology. 2012;30:221–241. doi: 10.1146/annurev-immunol-020711-074934. [DOI] [PubMed] [Google Scholar]
- Mohrs M, Shinkai K, Mohrs K, Locksley RM. Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity. 2001;15:303–311. doi: 10.1016/s1074-7613(01)00186-8. [DOI] [PubMed] [Google Scholar]
- Nayak D, Roth TL, McGavern DB. Microglia development and function. Annual review of immunology. 2014;32:367–402. doi: 10.1146/annurev-immunol-032713-120240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offner H, Polanczyk M. A potential role for estrogen in experimental autoimmune encephalomyelitis and multiple sclerosis. Annals of the New York Academy of Sciences. 2006;1089:343–372. doi: 10.1196/annals.1386.021. [DOI] [PubMed] [Google Scholar]
- Rasmussen S, Wang Y, Kivisakk P, Bronson RT, Meyer M, Imitola J, Khoury SJ. Persistent activation of microglia is associated with neuronal dysfunction of callosal projecting pathways and multiple sclerosis-like lesions in relapsing--remitting experimental autoimmune encephalomyelitis. Brain : a journal of neurology. 2007;130:2816–2829. doi: 10.1093/brain/awm219. [DOI] [PubMed] [Google Scholar]
- Ray A, Basu S. Regulatory B cells in experimental autoimmune encephalomyelitis (EAE) Methods in molecular biology. 2014;1190:243–255. doi: 10.1007/978-1-4939-1161-5_17. [DOI] [PubMed] [Google Scholar]
- Rifa'i M, Kawamoto Y, Nakashima I, Suzuki H. Essential roles of CD8+CD122+ regulatory T cells in the maintenance of T cell homeostasis. The Journal of experimental medicine. 2004;200:1123–1134. doi: 10.1084/jem.20040395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifa'i M, Shi Z, Zhang SY, Lee YH, Shiku H, Isobe K, Suzuki H. CD8+CD122+ regulatory T cells recognize activated T cells via conventional MHC class I-alphabetaTCR interaction and become IL-10-producing active regulatory cells. International immunology. 2008;20:937–947. doi: 10.1093/intimm/dxn052. [DOI] [PubMed] [Google Scholar]
- Steinman L. Multiple sclerosis: a two-stage disease. Nature immunology. 2001;2:762–764. doi: 10.1038/ni0901-762. [DOI] [PubMed] [Google Scholar]
- Subramanian S, Yates M, Vandenbark AA, Offner H. Oestrogen-mediated protection of experimental autoimmune encephalomyelitis in the absence of Foxp3+ regulatory T cells implicates compensatory pathways including regulatory B cells. Immunology. 2011;132:340–347. doi: 10.1111/j.1365-2567.2010.03380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber MS, et al. Type II monocytes modulate T cell-mediated central nervous system autoimmune disease. Nature medicine. 2007;13:935–943. doi: 10.1038/nm1620. [DOI] [PubMed] [Google Scholar]
- Williams KC, Ulvestad E, Hickey WF. Immunology of multiple sclerosis. Clinical neuroscience. 1994;2:229–245. [PubMed] [Google Scholar]
- Wolf SD, Dittel BN, Hardardottir F, Janeway CA., Jr Experimental autoimmune encephalomyelitis induction in genetically B cell-deficient mice. The Journal of experimental medicine. 1996;184:2271–2278. doi: 10.1084/jem.184.6.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28:639–650. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Yang M, et al. Novel function of B cell-activating factor in the induction of IL-10-producing regulatory B cells. Journal of immunology. 2010;184:3321–3325. doi: 10.4049/jimmunol.0902551. [DOI] [PubMed] [Google Scholar]
- Yang R, Cai Z, Zhang Y, Yutzy WHt, Roby KF, Roden RB. CD80 in immune suppression by mouse ovarian carcinoma-associated Gr-1+CD11b+ myeloid cells. Cancer research. 2006;66:6807–6815. doi: 10.1158/0008-5472.CAN-05-3755. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Total CD4+ T cells (a), naïve CD4+ T cells (b), effector memory CD4+ T cells (c) central memory CD4+ T cells (d) and IL-17 (e) and IFN-γ (f) were assessed in the spleen. Data are representative from 4 independent experiments consisting of 6 mice per group (mean ± SEM). Statistical analysis was performed with ANOVA followed by Newman-Kuels post hoc test. Significant differences between sample means are indicated (*p≤0.05; **p≤0.01; ***p≤0.001)