Abstract
Objectives
Stimuli that activate the sympathetic nervous system, such as acute psychological stress, rapidly invoke a robust mobilization of lymphocytes into the circulation. Experimental animal studies suggest that bone marrow-derived progenitor cells (PCs) also mobilize in response to sympathetic stimulation. Here we tested the effects of acute psychological stress and brief pharmacological β-adrenergic (βAR) stimulation on peripheral PC numbers in humans.
Methods
In two studies, we investigated PC mobilization in response to an acute speech task (n=26) and βAR-agonist (isoproterenol) infusion (n=20). A subset of 8 participants also underwent the infusion protocol with concomitant administration of the βAR-antagonist propranolol. Flow cytometry was used to enumerate lymphocyte subsets, total progenitor cells, total haematopoietic stem cells (HSC), early HSC (multi-lineage potential), late HSC (lineage committed), and endothelial PCs (EPCs).
Results
Both psychological stress and βAR-agonist infusion caused the expected mobilization of total monocytes and lymphocytes and CD8+ T lymphocytes. Psychological stress also induced a modest, but significant, increase in total PCs, HSCs, and EPC numbers in peripheral blood. However, infusion of a βAR-agonist did not result in a significant change in circulating PCs.
Conclusion
PCs are rapidly mobilized by psychological stress via mechanisms independent of βAR-stimulation, although the findings do not exclude βAR-stimulation as a possible cofactor. Considering the clinical and physiological relevance, further research into the mechanisms involved in stress-induced PC mobilization seems warranted.
1. Introduction
Progenitor cells (PCs) comprise an heterogeneous population uniquely capable of both self-renewal and multi-lineage differentiation (Weissman 2000). They replenish specialized somatic cells and maintain the normal turnover of regenerative tissues and organs, such as the blood and skin. PCs generally reside in the bone marrow, with a small number continually migrating into the circulation and tissues (Mazo, Massberg et al. 2011). Enhanced mobilization of endothelial PCs (EPCs) into the blood has been associated with improved endothelial function and repair (Foresta, De Toni et al. 2010). Conversely, low circulating PC number and reduced PC function are associated with cardiovascular disease and mortality. Likewise, successful reconstitution of the obliterated haematopoietic system in chemotherapeutic or radiation treated patients is critically dependent on mobilizing at least 2×106 HSCs per kg body mass from the donor (Winkler and Levesque 2006). Thus, there is clinical potential for methods that could aid mobilization of stem cells.
The bone marrow receives dense sympathetic innervation (Elenkov, Wilder et al. 2000). Animal studies show that sympathetic nervous system activation induces the release of PCs into the blood, that this mobilization can be replicated by administration of a β2AR-agonist, and that increased circulating PC numbers correlate with circadian sympathetic oscillations (Katayama, Battista et al. 2006, Spiegel, Shivtiel et al. 2007, Mendez-Ferrer, Lucas et al. 2008, Dar, Schajnovitz et al. 2011). Further, both murine and human PCs express functional adrenergic receptors (Muthu, Iyer et al. 2007, Spiegel, Shivtiel et al. 2007). In humans the number of circulating PCs is increased by sympathetic stimuli such as exercise and acute myocardial infarction, and can be reduced by treatment with βAR-antagonist (Barrett, Longhurst et al. 1978, Shintani, Murohara et al. 2001, Bible, Pasupuleti et al. 2014).
The above observations are remarkably similar to those reported for lymphocytes, which are likewise mobilized during sympathetic activation via stimulation of β2AR expressed on these cells (Benschop, Rodriguez-Feuerhahn et al. 1996, Dimitrov, Lange et al. 2010). Stress and beta-adrenergic induced lymphocytosis is a rapid response, observable within minutes. Considering the strong resemblance between observations and mechanisms of PC and lymphocyte mobilization, the present study tested if acute stress and brief infusion of the βAR agonist isoproterenol may promote rapid mobilization of HSC and EPC into peripheral blood in humans.
2. Methods
2.1 Participants
The stress study was performed at the University of Birmingham (UoB) and the infusion study at the University of California San Diego (UCSD). Methods and procedures were rigorously standardized to ensure comparability between results obtained between each site (further detailed below). All participants reported to be in good health and were non-medicated with exception of the contraceptive pill. Volunteers were instructed not to engage in strenuous physical exercise, to refrain from consuming alcohol or non-prescription drugs 24 hours before their experimental session, and to abstain from smoking and caffeine on the day of the experiment. Participants provided informed consent and study protocols were approved by the appropriate institutional review boards (UoB or UCSD).
2.2 Psychological stress study
2.2.1 Procedure
Twenty-six volunteers (mean age 31.5 years, SD ±8.0; 12 female) gave informed consent and had: (1) electrodes for electrocardiography (ECG) and impedance cardiography (ICG) were placed; (2) an intravenous cannula (Becton-Dickinson, Oxford, UK) inserted; and, (3) an occluding cuff placed for systolic (SBP) and diastolic (DBP) blood pressure measurements. While seated in a comfortable upright position, participants filled out questionnaires and engaged in leisure reading. After 20 minutes, a baseline blood sample was obtained and the laboratory stressor was initiated.
2.2.2 Public Speaking Task
Participants performed two back-to-back speeches as previously described (Bosch, de Geus et al. 2009). A blood sample was obtained 13 minutes into the stress task. A final blood sample was obtained after 15 minutes of recovery.
2.2.3 Cardiovascular assessment
Cardiac sympathetic and vagal control were assessed as previously described (Bosch, Berntson et al. 2003). ECG and ICG signals were assessed using six Ag-AgClspot-electrodes (AMI type 1650-005, Medtronic) and the Vrije Universiteit Ambulatory Monitoring System (VU-AMS) device (Willemsen, De Geus et al. 1996). Average heart rate (HR) and cardiac pre-ejection period (PEP) over 6 minutes was computed during baseline, both tasks, and during the recovery period. PEP was used to index changes in cardiac sympathetic drive, whereas heart rate variability, computed as Root Mean Square of Successive Difference (RMSSD), was used to index changes in cardiac vagal tone.
2.3 βAR-agonist infusion study
2.3.1 Procedures
Twenty volunteers (Mean age 35.9 years, SD±9.3; 8 female) were infused with the βAR-agonist isoproterenol according to a standardized protocol (Mills, Goebel et al. 2000). Body surface area (BSA) was used to standardize the βAR-agonist infusion rate. Participants rested in a semi-supine position for 15 minutes following placement of: 1) two intravenous cannulas (Becton-Dickinson); 2) three spot ECG electrodes; and, 3) an occlusion cuff.
The βAR-agonist was infused at incremental rates (0.1μg, 0.5μg and 1μg/minute/1.73 m2 BSA for 5 minutes each) for 15 minutes until the participant's heart rate had increased by ∼20 beats per minute (bpm), to correspond with the level of cardiovascular activity induced by the speaking task. The final infusion rate was maintained for 10 minutes. On average, the maximal dose reached 1μg/minute/1.73 m2 BSA. Blood was taken immediately prior to infusion (‘baseline’) and during the final minutes of the infusion. ECG, heart rate and blood pressure were monitored throughout the procedure.
2.3.2 βAR-antagonist
A sub-group of 8 participants (Mean age 34.6, SD±11.5 years, 2 female) underwent the infusion twice following a 5-day course of either of 80mg of the non-selective βAR- antagonist propranolol or placebo. Condition was counter-balanced, separated by at least 7 days and single-blinded.
2.4 Questionnaires
Affective responses to the speaking task were assessed using the short-form of the Profile of Mood States (POMS) (McNair, Lorr et al. 1992). Participants completed the POMS at baseline, immediately post-task and at 15-minutes recovery.
2.5 Flow cytometry
The following PC populations were identified on the basis of cell surface protein expression using multi-parameter flow cytometry; total PCs, HSCs (Sutherland, Keating et al. 1994), early and late HSCs (Terstappen, Huang et al. 1991), and two EPC populations (Timmermans, Plum et al. 2009) (Figure 2). In brief, 9ml of ammonium chloride lysis solution (0.15M NH4Cl, 10mM KHCO3, 0.1mM EDTA) was added to 1ml of EDTA whole blood (10 mins, RT). Phosphate-buffered saline (PBS, 5ml) was added, samples were centrifuged (283× g, 7 mins, RT), and the supernatants removed prior to incubation with monoclonal antibodies: CD34-FITC, CD133-PE, IgG Isotype-PE, CD3-PerCP, CD45-PerCP and CD8-APC-Cy7 (Becton-Dickinson, Oxford, UK), and CD38-PE-Cy7 (eBioscience, Insight Biotechnology Ltd, Middlesex, UK). Cell were washed, re-suspended in PBS/paraformaldehyde (1-2%), and stored in the dark (4°C) until acquisition. Cells were read using a FACS-Canto II (Becton Dickinson, Oxfordshire UK). At least 1×106 gated lymphocytes and monocytes were acquired. Data were analysed using Flowjo 7.4 (Treestar Inc, Ashland, OR, USA). Complete white blood cell counts were obtained using a Haematology analyser (Coulter ACTdiff, Beckman Coulter, High Wycombe, UK or Coulter GEN-S haematology analyser, Beckman-Coulter, Miami, USA). Numbers of PCs were then calculated using standard dual platform methods. Analyses was also adjusted for stress-induced changes in heamoconcentration as previously described (Bosch, Berntson et al. 2005). Total PC, HSC, early HSC, late HSC, EPC and CD8+ T lymphocyte numbers were examined in both studies. An additional EPC marker, CD133, was assessed in the psychological stress study.
Figure 2.
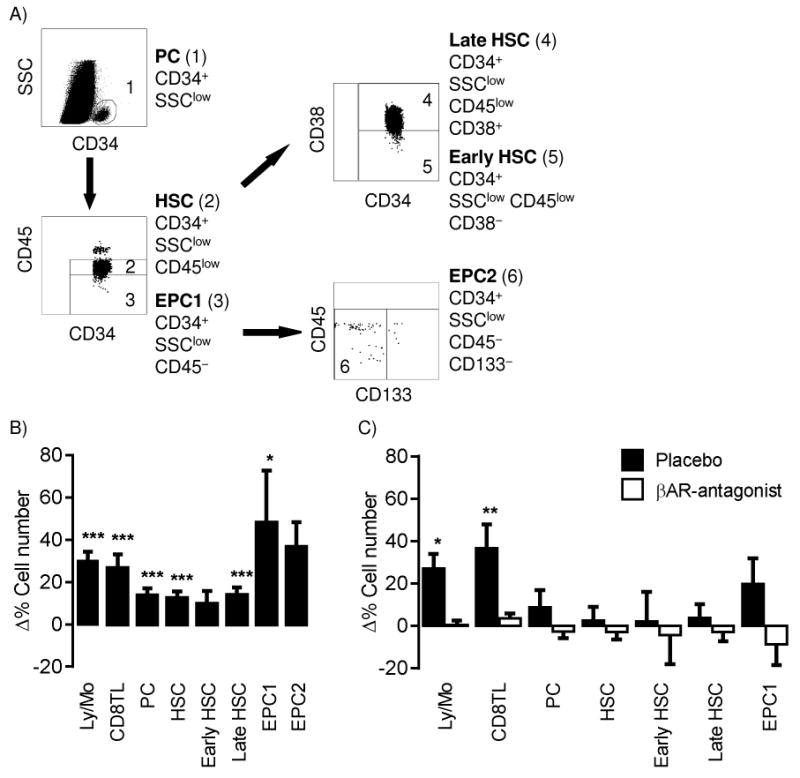
Progenitor cell analyses and mobilization during acute psychological stress and βAR-agonist infusion. (A) Progenitor cells and subsets were identifies by flow cytometry using the following gating strategy: CD34 expression and low SSC identified progenitor cells PC, which were further separated into haematopoietic stem cells (HSC) and endothelial progenitor cells definition 1 (EPC1) based on CD45 expression; early (CD38 negative) and late HSC (CD38 positive) were gated from the HSC population and; EPC definition 2 (EPC2) were gated from the EPC1 population based on negative CD133 expression. Relative change (Δ%) in cell number (counts) from baseline to stress (B) or baseline to infusion (C) for total lymphocyte and monocyte (Ly/Mo), CD8+ T cells (CD8TL), and PC populations. p <.05*, <.01** and <.001*** indicates a significant increase in cell number from baseline value (Paired T-test on Ln+1 transformed data).
2.6 Quality control and procedure standardisation
Laboratory protocols were standardized by using a BD FACS Canto II with comparable laser settings and identical protocols and materials, including fluorescent antibodies from the same lot, at each site. Further, sample preparation and analyses were done by the same person. The same brand haematology analyser was used at each site and no difference was observed between the resting numbers of lymphocytes and monocytes between participants at the two sites (Figure 1). Participants at each site were matched in age, gender, BMI, and resting HR, SBP, and DBP. The infusion dose and effects of stress were comparable as confirmed in post-hoc analyses of HR responses, and total monocyte and lymphocyte and CD8 T cell mobilization (Figure 1). SBP and DBP changes are partly under alpha-adrenergic mechanisms, and thus could not be expected to be affected in a similar fashion by isoproterenol versus stress. A within subject study design was used to further minimize between-site differences, i.e., the participants baseline sample acted as the reference value to determine the effect of the intervention.
Figure 1.
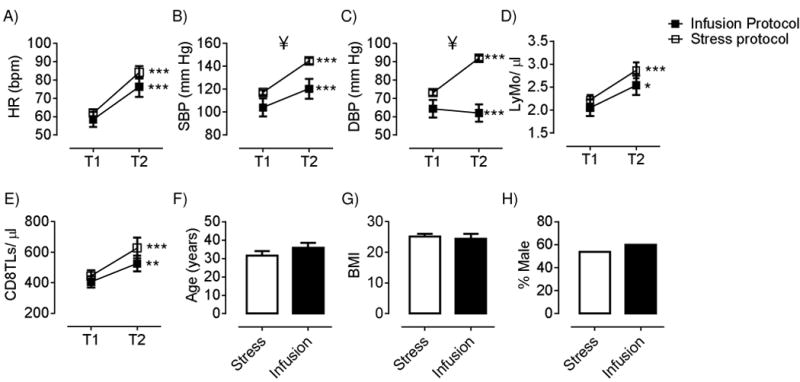
Comparison of cardiovascular and immunological responses, baseline values, and participant characteristics for the stress and infusion protocol. A-E; heart rate (HR), systolic and diastolic blood pressure (SBP and DBP), and combined lymphocytes and monocytes and CD8TL numbers at baseline (T1) and during intervention (T2). F-H; comparison of participant age, BMI and gender. * represent significant differences from T1 to T2, ¥ represents significant different scores between the two protocols. Results from independent T-test.
2.7 Statistical analysis
The Kolmogorov–Smirnov test indicated that immunological data significantly deviated from a normal distribution, and thus Ln+1 of cell numbers was calculated and the transformed data were used for all further statistical analyses. Ln+1 immunological data, and cardiovascular and psychological data was analysed using repeated measures ANOVA. For post hoc analysis, Paired T-tests were used. Non-parametric analyses (Friedman test and post hoc Wilcoxon signed rank test) was also performed on the EPC2 subset as the Ln+1 EPC2 numbers were not normally distributed. The results were unaltered by the inclusion of gender, BMI and age as covariates. Variations in degrees of freedom reflect occasional missing data. Data were analysed using IBM-SPSS 20 for windows (SPSS Inc, Chicago, Illinois).
3. Results
3.1 Psychological stress study
3.1.1 Anxiety and cardiovascular responses
Increases in the tension-anxiety POMS subscale confirmed that the speech tasks were perceived as stressful (Mean +8.2 (SEM 0.8); F(2,48) = 102.3, p<.001). A physiological stress response was confirmed by significant increases in HR, SBP and DBP (Figure 1, all p<.001). These cardiovascular changes appeared driven by an increase in sympathetic cardiac drive, as reflected by a decrease in PEP (Mean – 12.1 ms (SEM 2.3); F(2,26) = 14.9, p<.001), and a vagal withdrawal, as evidenced by a decrease in RMSSD (Mean –24.5 ms (SEM 5.5); F(2,26) = 15.1, p<.001). At 15-minutes post-stress, all cardiovascular and autonomic measures had returned to baseline values.
3.1.2 Mobilization of PC subsets during psychological stress
As shown in Figures 1 and 2 and Supplementary Table 1, a significant effect of time was found for total lymphocytes and monocytes, CD8+ T lymphocytes, PC, HSC, late HSC and EPC1. Post hoc analyses revealed that these effects were largely driven by an increase in cell number from baseline to stress, and that all PC numbers returned to baseline value at post-15 minute recovery. The added total of lymphocytes and monocytes and CD8+ T lymphocytes remained modestly increased at recovery. EPC2 populations also showed a modest increase, which only reached significance in non-parametric analyses (Friedman test and post hoc Wilcoxon signed rank test)] (X 2 (15) = 7.18, p=.028).
The results were essentially unaltered by adjusting for changes in blood volume and haemoconcentration (p's <.05) (Bosch et al., 2005, NK cell paper), with the exception of the EPC1 subset which no longer significantly increased during the task (F(2,40)= 2.69, p=.08).
3.2 βAR-agonist infusion study
3.2.1 Cardiovascular responses
Significant increases were found in HR and SBP, whilst DBP significantly decreased during the infusion (Figure 1, all p<.001). At 15-minutes post-infusion, all cardiovascular measures had returned to baseline values.
3.2.2 βAR-agonist infusion did not induce PC mobilization
βAR-agonist infusion did replicate the stressor by increasing the total number of lymphocytes and monocytes and CD8+ T lymphocytes (Figures 1 and 2 and Supplementary table 2) with approximately similar effect sizes (Supplementary data). However, in contrast to the stressor, βAR-agonist infusion had no effect on PC recruitment (Figure 2 and Supplementary Table 2). Adjustment for effects of haemoconcentration and blood volume did not alter the results.
Administration of the βAR-antagonist abrogated the effects of βAR-agonist infusion on lymphocyte and monocyte number, CD8+ T lymphocyte numbers, and cardiovascular responses as expected. The βAR-agonist also had no effect on SC mobilization (Figure 2 and Supplementary Table 2).
4. Discussion
The present study demonstrated, for the first time, that PC subsets can be mobilized rapidly during acute psychological stress in humans. Unexpectedly, this effect was not replicated by infusion of the βAR-agonist isoproterenol, although infusion effectively replicated the robust effects of stress on lymphocytes and monocytes as well as CD8+ T lymphocytes, similar to previous findings (Anane, Edwards et al. 2009). Further, the percentage increase in HR seen during the stress study and infusion study were highly comparable (both effect sizes were ηP2 >.77, indicating that the infusion dosage invoked adequate β-adrenergic receptor stimulation. Together these findings suggest that the rapid PC mobilization during acute stress involves a different mechanism than the simultaneous β-adrenergic dependent lymphocytosis.
It is possible that other adrenergic mechanisms may induce PC mobilization during acute stress. HSC, for example, also express alpha-adrenergic receptors (αARs) (Muthu, Iyer et al. 2007). In support of αAR-involvement is the finding that impairment of PC mobilization is more profound in mice chemically sympathectomised than it is in mice injected with βAR-antagonist (Katayama, Battista et al. 2006). Further, administration of a β2AR-agonist only partially restores the PC mobilization defect in mice unable to synthesise norepinephrine (Chen, Cao et al. 2013).
In addition to norepinephrine and epinephrine, several other factors released during stressors have a demonstrated ability to induce PC mobilization such as granulocyte colony-stimulating factor (G-CSF), nitric oxide synthase 3 (NOS3) and nitric oxide (NO) (Suzuki, Yamada et al. 2000, Aicher, Heeschen et al. 2004, Winkler and Levesque 2006, Yang, Wang et al. 2007). Of these, only NOS3 and NO are up-regulated within minutes of stressor onset in line with the speed of mobilization seen in the present study. Recent evidence suggests that βAR mechanisms may have a role, but only if associated with another co-mediator that is also released during psychological stress. For example, norepinephrine does not elicit circulating PCs without co-stimulation with G-CSF in animals (Chen, Cao et al. 2013). Second, reduced NO bioavailability in humans decreases PC mobilization during exercise (Cubbon, Murgatroyd et al. 2010). Clearly, this idea would need further confirmation in future research.
A limitation of the current study is that PCs were identified using cell-surface markers and not culture assays that might provide a more direct measure of subset lineage potential (Timmermans, Plum et al. 2009). However, the identification methods used in the present study have been validated (see methods for references) suggesting that the phenotypic pattern of PC mobilization would largely be replicated when using culturing methods. Secondly, a recovery blood sample was not taken following the cessation of βAR-agonist infusion, and we cannot exclude a delayed onset of PC mobilization similar to what is observed in animal studies (Katayama, Battista et al. 2006). However, the purpose of the present short infusion protocol was to verify if βAR-mechanisms accounted for the rapid increase in peripheral PC numbers seen within 15 min of stressor onset. If similar mechanisms play a role it would be expected that βAR-agonist infusion would induce PC mobilization within the same time frame, as we indeed confirmed for lymphocyte mobilisation. A third limitation is the absence of a βAR-antagonist condition in the psychological stress study. As speculated above, it is possible that βAR stimulation is effective only in conjunction with another mediator or mediators; in that case infusion would have no effects, as indeed is seen in the present study, but beta-blockade would still effectively abrogate the stress-induced effects. Without such blockade data we are not able to rule out the involvement of βAR-mechanisms as a co-mediator.
In conclusion, the present study demonstrated that acute psychological stress, but not βAR-infusion, can induce PC mobilization within minutes. These findings do not match the observations for stress lymphocytosis, which is clearly βAR-mediated, and thus suggest that stress-induced mobilization of PC and lymphocytes is governed by different mechanisms. Understanding the mechanisms allowing for PCs mobilization during acute stress may have clinical utility. As such, future studies examining potential mediators of rapid PC mobilization are warranted.
Supplementary Material
Highlights.
Methods that raise circulating PC numbers have great clinical utility.
Here we show that psychological stress induces such increases.
This was not replicated by infusion of a β-adrenergic receptor agonist in humans.
Acknowledgments
We would like to thank the volunteers, nurses at UCSD GCRC, and Charlotte Dennis, Fay Kitchen, Gareth Price, Katy Wilson, Merrly Spencer and Sara Cunliff for their help in running the study. This work was supported by grants from the NIH (HL-073355 to P. J. Mills), Wellcome Trust (VIP to J. A. Bosch), BSI (Travel Award to N. E. Riddell), and JEB (Travelling Fellowship to N. E. Riddell).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aicher A, Heeschen C, Dimmeler S. The role of NOS3 in stem cell mobilization. Trends Mol Med. 2004;10(9):421–425. doi: 10.1016/j.molmed.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Anane LH, Edwards KM, Burns VE, Drayson MT, Riddell NE, van Zanten JJ, Wallace G, Mills PJ, Bosch JA. Mobilization of gammadelta T lymphocytes in response to psychological stress, exercise, and beta-agonist infusion. Brain Behav Immun. 2009 doi: 10.1016/j.bbi.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Barrett AJ, Longhurst P, Sneath P, Watson JG. Mobilization of CFU-C by exercise and ACTH induced stress in man. Exp Hematol. 1978;6(7):590–594. [PubMed] [Google Scholar]
- Benschop RJ, Rodriguez-Feuerhahn M, Schedlowski M. Catecholamine-induced leukocytosis: early observations, current research, and future directions. Brain Behav Immun. 1996;10(2):77–91. doi: 10.1006/brbi.1996.0009. [DOI] [PubMed] [Google Scholar]
- Bible LE, Pasupuleti LV, Alzate WD, Gore AV, Song KJ, Sifri ZC, Livingston DH, Mohr AM. Early propranolol administration to severely injured patients can improve bone marrow dysfunction. J Trauma Acute Care Surg. 2014;77(1):54–60. doi: 10.1097/TA.0000000000000264. discussion 59-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch JA, Berntson GG, Cacioppo JT, Dhabhar FS, Marucha PT. Acute stress evokes selective mobilization of T cells that differ in chemokine receptor expression: a potential pathway linking immunologic reactivity to cardiovascular disease. Brain Behav Immun. 2003;17(4):251–259. doi: 10.1016/s0889-1591(03)00054-0. [DOI] [PubMed] [Google Scholar]
- Bosch JA, Berntson GG, Cacioppo JT, Marucha PT. Differential mobilization of functionally distinct natural killer subsets during acute psychologic stress. Psychosom Med. 2005;67(3):366–375. doi: 10.1097/01.psy.0000160469.00312.8e. [DOI] [PubMed] [Google Scholar]
- Bosch JA, de Geus EJ, Carroll D, Goedhart AD, Anane LA, van Zanten JJ, Helmerhorst EJ, Edwards KM. A general enhancement of autonomic and cortisol responses during social evaluative threat. Psychosom Med. 2009;71(8):877–885. doi: 10.1097/PSY.0b013e3181baef05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Cao J, Song X, Zeng L, Li Z, Li Y, Xu K. Adrenaline administration promotes the efficiency of granulocyte colony stimulating factor-mediated hematopoietic stem and progenitor cell mobilization in mice. Int J Hematol. 2013;97(1):50–57. doi: 10.1007/s12185-012-1228-1. [DOI] [PubMed] [Google Scholar]
- Cubbon RM, Murgatroyd SR, Ferguson C, Bowen TS, Rakobowchuk M, Baliga V, Cannon D, Rajwani A, Abbas A, Kahn M, Birch KM, Porter KE, Wheatcroft SB, Rossiter HB, Kearney MT. Human exercise-induced circulating progenitor cell mobilization is nitric oxide-dependent and is blunted in South Asian men. Arterioscler Thromb Vasc Biol. 2010;30(4):878–884. doi: 10.1161/ATVBAHA.109.201012. [DOI] [PubMed] [Google Scholar]
- Dar A, Schajnovitz A, Lapid K, Kalinkovich A, Itkin T, Ludin A, Kao WM, Battista M, Tesio M, Kollet O, Cohen NN, Margalit R, Buss EC, Baleux F, Oishi S, Fujii N, Larochelle A, Dunbar CE, Broxmeyer HE, Frenette PS, Lapidot T. Rapid mobilization of hematopoietic progenitors by AMD3100 and catecholamines is mediated by CXCR4-dependent SDF-1 release from bone marrow stromal cells. Leukemia. 2011;25(8):1286–1296. doi: 10.1038/leu.2011.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov S, Lange T, Born J. Selective mobilization of cytotoxic leukocytes by epinephrine. J Immunol. 2010;184(1):503–511. doi: 10.4049/jimmunol.0902189. [DOI] [PubMed] [Google Scholar]
- Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve--an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000;52(4):595–638. [PubMed] [Google Scholar]
- Foresta C, De Toni L, Ferlin A, Di Mambro A. Clinical implication of endothelial progenitor cells. Expert Rev Mol Diagn. 2010;10(1):89–105. doi: 10.1586/erm.09.80. [DOI] [PubMed] [Google Scholar]
- Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, Frenette PS. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124(2):407–421. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- Mazo IB, Massberg S, von Andrian UH. Hematopoietic stem and progenitor cell trafficking. Trends Immunol. 2011;3210:493–503. doi: 10.1016/j.it.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. Manual for the Profile of Mood States. San Diego: Educational and Industrial Testing Service; 1992. [Google Scholar]
- Mendez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452(7186):442–447. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- Mills PJ, Goebel M, Rehman J, Irwin MR, Maisel AS. Leukocyte adhesion molecule expression and T cell naive/memory status following isoproterenol infusion. J Neuroimmunol. 2000;102(2):137–144. doi: 10.1016/s0165-5728(99)00180-0. [DOI] [PubMed] [Google Scholar]
- Muthu K, Iyer S, He LK, Szilagyi A, Gamelli RL, Shankar R, Jones SB. Murine hematopoietic stem cells and progenitors express adrenergic receptors. J Neuroimmunol. 2007;186(1-2):27–36. doi: 10.1016/j.jneuroim.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintani S, Murohara T, Ikeda H, Ueno T, Honma T, Katoh A, Sasaki K, Shimada T, Oike Y, Imaizumi T. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation. 2001;103(23):2776–2779. doi: 10.1161/hc2301.092122. [DOI] [PubMed] [Google Scholar]
- Spiegel A, Shivtiel S, Kalinkovich A, Ludin A, Netzer N, Goichberg P, Azaria Y, Resnick I, Hardan I, Ben-Hur H, Nagler A, Rubinstein M, Lapidot T. Catecholaminergic neurotransmitters regulate migration and repopulation of immature human CD34+ cells through Wnt signaling. Nat Immunol. 2007;8(10):1123–1131. doi: 10.1038/ni1509. [DOI] [PubMed] [Google Scholar]
- Sutherland DR, Keating A, Nayar R, Anania S, Stewart AK. Sensitive detection and enumeration of CD34+ cells in peripheral and cord blood by flow cytometry. Exp Hematol. 1994;22(10):1003–1010. [PubMed] [Google Scholar]
- Suzuki K, Yamada M, Kurakake S, Okamura N, Yamaya K, Liu Q, Kudoh S, Kowatari K, Nakaji S, Sugawara K. Circulating cytokines and hormones with immunosuppressive but neutrophil-priming potentials rise after endurance exercise in humans. Eur J Appl Physiol. 2000;81(4):281–287. doi: 10.1007/s004210050044. [DOI] [PubMed] [Google Scholar]
- Terstappen LW, Huang S, Safford M, Lansdorp PM, Loken MR. Sequential generations of hematopoietic colonies derived from single nonlineage-committed CD34+CD38-progenitor cells. Blood. 1991;77(6):1218–1227. [PubMed] [Google Scholar]
- Timmermans F, Plum J, Yoder MC, Ingram DA, Vandekerckhove B, Case J. Endothelial progenitor cells: identity defined. J Cell Mol Med. 2009;13(1):87–102. doi: 10.1111/j.1582-4934.2008.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman IL. Stem cells: units of development, units of regeneration, and units in evolution. Cell. 2000;100(1):157–168. doi: 10.1016/s0092-8674(00)81692-x. [DOI] [PubMed] [Google Scholar]
- Willemsen GH, De Geus EJ, Klaver CH, Van Doornen LJ, Carroll D. Ambulatory monitoring of the impedance cardiogram. Psychophysiology. 1996;33(2):184–193. doi: 10.1111/j.1469-8986.1996.tb02122.x. [DOI] [PubMed] [Google Scholar]
- Winkler IG, Levesque JP. Mechanisms of hematopoietic stem cell mobilization: when innate immunity assails the cells that make blood and bone. Exp Hematol. 2006;34(8):996–1009. doi: 10.1016/j.exphem.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Yang Z, Wang JM, Chen L, Luo CF, Tang AL, Tao J. Acute exercise-induced nitric oxide production contributes to upregulation of circulating endothelial progenitor cells in healthy subjects. J Hum Hypertens. 2007;21(6):452–460. doi: 10.1038/sj.jhh.1002171. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


