Abstract
Purpose
To develop and test a time-efficient, free-breathing, whole-heart T2 mapping technique at 3.0T.
Methods
ECG-triggered three-dimensional images were acquired with different T2 preparations at 3.0T during free breathing. Respiratory motion was corrected with a navigator-guided motion correction framework at near perfect efficiency. Image intensities were fit to a mono-exponential function to derive myocardial T2 maps. The proposed approach (3D-FB-MoCo) was studied in ex-vivo hearts and kidneys, healthy volunteers and canines with acute myocardial infarction (AMI).
Results
Ex-vivo T2 values from proposed 3D T2-prep GRE was not different from 2D SE (p=0.7) and T2-prep bSSFP (p=0.7). In volunteers, compared to the proposed approach (3D-FB-MoCo) and breath-held 2D T2-prep bSSFP (2D-BH), non-motion-corrected (3D-FB-Non-MoCo) myocardial T2 was longer, had larger coefficient-of-variation (COV) and lower image quality (IQ) score (T2=40.3 ms, COV=38% and IQ=2.3, all p<0.05). Conversely, the mean and COV and IQ of 3D-FB-MoCo (T2=37.7 ms, COV=17% and IQ=3.5.) and 2D-BH (T2=38.0 ms, COV=15% and IQ=3.8) were not different (p=0.99, p=0.74, and p=0.14, respectively). In AMI, T2 values and edema volumes from 3D-FB-MoCo and 2D-BH approaches were closely correlated (R2=0.88 and 0.96, respectively).
Conclusion
The proposed whole-heart T2 mapping approach can be performed within 5 minutes with similar accuracy to 2D-BH T2 mapping approach.
Keywords: fast imaging, cardiac T2 mapping, myocardial edema, cardiac BOLD MRI
INTRODUCTION
Over the past decade, T2-based cardiac MRI has evolved as an important tool for characterizing myocardial tissue. To date, numerous clinical and preclinical studies have shown that hyperintensity in T2 cardiac MRI is associated with myocardial edema, which can be used to stage myocardial infarction (1), detect myocarditis (2), acute ischemia (3) and early transplant rejection (4). Conversely, hypointensity in T2 MRI has been instrumental in examining intramyocardial hemorrhage (5), chronic iron overloading from ferritin cardiomyopathies (6) (7) and Blood-Oxygen-Level-Dependent (BOLD) MRI (8) (9).
Early approaches typically relied on T2-weighted imaging (T2-STIR(1), T2-prep-SSFP (10,11), etc.), but these approaches have well recognized image artifacts from motion, stagnant/slow moving blood and coil bias (12). As T2 mapping techniques with high SNR readouts have overcome many of the limitations (3,13,14) and an interest in quantitative tissue characterization became viable and of clinical interest, T2 mapping has become the preferred means for examining myocardial T2 changes. (15,16). Nonetheless, to date, T2 mapping is typically prescribed as 2D breath-held acquisitions to cover the full left ventricle (LV), which takes approximately 10–15 minutes. While respiratory navigator-gated approaches have overcome the discomfort associated with multiple breath holds, the concomitant reduction in scanning efficiency has been a limitation in decreasing the overall scan time to below 10 min (17,18).
In practice, long acquisition time and multiple breath holds impose significant limitations where whole-heart T2 mapping could be of great value. For instance, in cases where myocardial edema may serve as a marker of acute ischemia in the management of chest pain in the emergency department (19,20), target patients find it difficult to tolerate multiple breath holds and long acquisition times within the scanner. Moreover, in cases where contrast-agent free cardiac stress testing with myocardial BOLD MRI may be valuable, long acquisition times (> 6 minuts) do not permit whole heart T2 mapping within the standard duration of adenosine infusion (21). The growing interest in T2-based cardiac MRI for both of these applications could potentially be empowered by a fast, free-breathing T2 mapping strategy.
Moreover, most T2 mapping approaches are limited to 1.5T and prescribed with balanced steady-state free precession (bSSFP). Their robustness to image artifacts at 3.0T (banding, B1 in homogeneity, etc) continues to be problematic (22). A more pragmatic cardiac T2 mapping approach should be time efficient (preferably < 6 minutes) to accommodate cardiac stress testing with BOLD MRI; enable rapid edema assessment in a clinical environment without suspending breathing ; and should enable reliable acquisitions at 3T with minimal or no image artifacts, while providing full LV coverage. A T2 mapping technique that conforms to these desirable attributes could enhance rapid assimilation of T2 imaging as part of time-sensitive and time-efficient clinical cardiac MRI protocols.
We hypothesized that a robust, time-efficient, 3D free-breathing T2 mapping technique at 3.0T can be obtained through the combined use of (a) adiabatic RF pulses for B1 insensitive T2 preparation; (b) near 100% efficient navigator gating with affine motion correction to maximize efficiency of acquisition under free-breathing; and (c) stack-of-stars gradient-echo (GRE) readouts to eliminate banding artifacts associated with bSSFP readouts, without significant loss of SNR. We validated our approach in ex-vivo canine hearts and kidneys, in healthy human volunteers, and in canines subjected to reperfused acute myocardial infarction.
METHODS
Pulse sequence details and data acquisition, motion estimation and correction, and parameter estimation and heart rate corrections pertaining to the proposed imaging approach are described. The motion registration program was written in C using the Insight Toolkit. Image reconstruction and parameter fitting were performed offline using MATLAB (Mathworks, R2009a, MA, USA). All studies were performed on a 3.0 T clinical MRI system (MAGNETOM Verio®, Siemens Healthcare, Erlangen, Germany) and 32 channel matrix coils (16 anterior and 16 posterior) were used for data acquisition.
Pulse Sequence Details and Data Acquisition
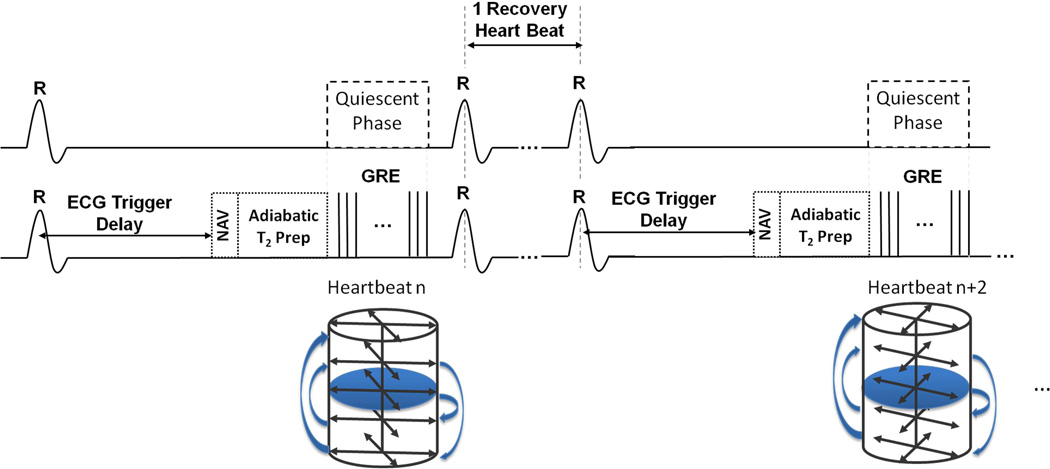
A T2 prepared GRE sequence was adapted for 3D T2 mapping at 3.0 T. To minimize image artifacts from increased B0 and B1 in homogeneities at 3T (23) and cardiac motion, an adiabatic T2 preparation (2× BIREF-1) (24) was used. A schematic of the timing diagram is shown in Fig. 1 and imaging parameters for each experiment are described in the following section. Images were acquired every other heart beat for longitudinal magnetization recovery. For the GRE readout, we used a stack of slices (along the long axis of the heart) with 144 evenly distributed radial lines to cover the 3D field of view. In each acquisition window (heart beat), a subset of 2–3 in-plane lines from all slices was acquired depending on the length of the quiescent period. In each subset, k-space lines were acquired in a slice-by-slice fashion. Centric reordering along the long axis of the heart was used to minimize T1 recovery following T2 preparation and the in-plane radial trajectory was ordered in an interleaved fashion to reduce the possibility of unevenly distributed data in each bin (details on bins below). For example, in a case where two in-plane radial lines A and B are acquired in each subset (A, B), the identity of the pair of radial lines conform to the sequence {(i, i+72)}, where i=1,72 and i is an integer. A representative acquisition scheme with 5 slices and 2 in-plane lines per heartbeat is shown in Fig. 1. GRE readout was trigged at the mid diastolic phase with appropriate trigger delay. A respiratory navigator signal at the lung-liver interface was continually acquired during the free breathing acquisitions without rejecting any data (25). The navigator module was played out before T2 preparation to (a) avoid potential errors in binning from T2 weighting of navigator signals at the different TEs; and (b) preserve the T2 weighting by minimizing T1 recovery prior to signal readout. In order to derive T2 maps, the sequence was prescribed with three different T2 preparation times (TE = 0, 24 and 55 ms).
Fig. 1. Pulse sequence timing diagram and k-space trajectory.
Details of the pulse sequence and the relative timing are presented in panels A and B. GRE readout is triggered during the quiescent period at mid diastole with a proper trigger delay from the R wave. Panel C shows an example of the stack-of-stars trajectory with 2 radial lines in each subset and 5 partitions.
Motion Estimation and Correction
Motion correction with 3D affine transforms, derived from the respiratory navigator signals, has been shown to reliably correct respiration-mediated motion of the heart (26,27). To maximize imaging efficiency (i.e., utilize all the data acquired during free breathing) the following steps were applied for motion-corrected T2 mapping:
-
◦Motion Estimation:
- Binning: k-space lines were sorted into 4 bins based on the diaphragm position in which the end expiratory bin was assigned as the reference and the others were labeled as the target bins.
- Data composition and respiratory phase-resolved image reconstruction: To reduce streaking artifact from undersampling of k-space, composite images were reconstructed by combining the data from all three TEs (within the same respiratory bin) followed by Gaussian smoothing. Representative images acquired at the different TEs for one of the respiratory bins and the effect of combining the images across the different TEs within the same bin are shown in Fig. 2A.
- Motion Registration: A contour isolating the heart from rest of the anatomy was drawn manually using the 3D image set from the reference bin. The contours were then converted into a mask and applied to images in all respiratory bins. Processed 3D images from each target bin were iteratively registered to the reference bin using Insight Tool Kit (ww.itk.org) (28). Affine transform matrices of each target bin were generated and stored for motion correction. Details of the registration process were previously described by Bhat et al (26).
-
◦
Motion Correction and Image Reconstruction: k-space data in each respiratory bin were sorted back into independent TEs. After gridding, affine transform matrices were applied to the corresponding images in each respiratory bin and motion-corrected images were reconstructed independently for each TE by combining complex images from all bins. Motion estimation, correction and image reconstruction steps are summarized in Fig. 2B.
Fig. 2. Image reconstruction steps.
Panel A shows a representative case of combining undersampled low-resolution images from a common respiratory phase from different TEs to arrive at composite data sets for each bin. This is shown for Bin 3 here. Similar steps are taken to arrive at data for Bins 1, 2 and reference bin (Ref Bin, end expiratory phase). Panel B shows the steps associated with motion correction (binning based on navigator signal, estimation of motion and image reconstruction) and parameter fitting. In essence, the k-space data is first combined with different TEs and separated into different respiratory bins based on the navigator signal. From each bin, a low-resolution composite image representing different respiratory position was reconstructed. On the basis of the reference bin, the affine motion parameters of all other (target) bins were estimated with ITK and stored for motion correction. Finally, k-space data with different T2 preparations were gridded independently and applied with corresponding affine transformation to yield motion-corrected images. Subsequently the pixel-wise fitting is performed using the images at different TEs to estimate T2.
Parameter Fitting and Heart Rate Correction
Pixel-wise fitting of signal intensities at the 3 TEs to a•exp(−b•TE) were performed with log-transformed linear least-squares fit, where a and b are fit parameters with a= M0 (which includes proton density, coil sensitivity etc.) and b = 1/T2. After the fitting for T2, a heart rate correction was applied to compensate for imperfect T1 recovery between magnetization preparations. To estimate true T2, the T2 estimates from mono-exponential fit (T2†) were multiplied by (1 + α) (Refer to Appendix). α was estimated from known values of T1 (1100 ms) and T2 (40 ms) of myocardium, data acquisition in every other heart beat, and ΔTE = 27.5 ms (the midpoint of TEs). Subsequently, heart rate corrected T2 was calculated as T2† · (1 + α). If we estimate α assuming T2 = 40 ms and T1 = 1200 ms at 3T, then the estimated errors in α and the corresponding T2 would be 0.08 ms and 3.2 ms (in cases of significant edema when the actual T2 = 60 ms and T1 = 1400 ms) (17,29).
Validation Studies
Ex-vivo Studies
Three freshly excised canine hearts and kidneys were immersed in saline solution and imaged with the proposed method, 2D spin-echo and 2D T2-prepared bSSFP sequences (with the same adiabatic T2 preparation as in the proposed method) to assess T2 accuracy. Proposed 3D sequence was prescribed covering the whole organ and 2D sequences were prescribed to match 3 separated partitions in the organs. Sequences were randomized with regard to order. Imaging parameters of the different sequences were as follows:
2D spin echo - TR = 5000 ms, TE = 6, 24, 58 ms, readout bandwidth (BW) = 300 Hz/pixel and voxel size = 2.0 × 2.0 × 6.0 mm3 interpolated to 1.0 × 1.0 × 3.0 mm3.
Proposed 3D T2 map - GRE readout with stack-of-stars trajectory, TR/TE = 3.4 ms/1.7 ms, flip angle (FA) = 15°, BW = 694 Hz/pixel, 40 lines per heartbeat (simulated heart rate = 60 beats/min), trigger pulse = 2, total projections = 144/slice, field-of-view (FOV) = 380 × 380 mm2, matrix size = 192 × 192 × 16, and voxel size = 2.0 × 2.0 × 6.0 mm3 interpolated to 1.0 × 1.0 × 3.0 mm3, T2 preparation (as TEs) = 0, 24, 55 ms. Gaussian apodization was used to increase SNR (30).
2D T2 maps with bSSFP readouts with Cartesian trajectory - TR/TE = 2.9 ms/1.1 ms, iPAT = 2, partial Fourier = 3/4, FA = 35°, BW = 1184 Hz/pixel, 86 lines per heartbeat (simulated rate 60/s), trigger pulse = 4, FOV = 288 × 360 mm2, matrix size = 154 × 192, and voxel size = 2.5 × 1.7 × 6.0 mm3, T2 preparation (as TEs) = 0, 24, 55 ms.
Healthy Volunteer Studies
Healthy volunteers (n=10, 3 men, average age 30± 5) were recruited in accordance with the protocol that was reviewed and approved by the Institutional Review Board. Every participant was competent and provided written informed consent and had no history of coronary artery disease, abnormal cardiac rhythm and rate, kidney/liver disease, and were not contraindicated for cardiac MR exams (completed a detailed cardiac MRI questionnaire). All studies were performed on the same clinical MRI system used for ex vivo studies with subjects placed on the supine position. After whole heart shimming and localization, proposed free-breathing 3D T2-prepared acquisitions were prescribed with whole LV coverage using 25% slice over-sampling and 32–48 radial lines per heartbeat triggered at approximately mid-diastole within a data acquisition window of 110–160 ms. A four-chamber cine scan was used to determine the quiescent period of the cardiac cycle. Navigator pulses were placed on the dome of the right hemidiaphragm. The data was reconstructed as described earlier to generate T2 maps. For comparison purposes, images were also reconstructed without motion correction. Also, the standard 2D breath-held T2-prepared bSSFP acquisitions were prescribed along representative short-axis slices that were matched to three slices centered at the level of mid ventricle within the 3D partitions. Imaging parameters and T2-preparation schemes were the same as those used for ex-vivo imaging. To minimize bias with respect to acquisition order, the acquisitions were randomized.
Canine Model of Acute Myocardial Infarction
Dogs (n= 10, 18–26 kg) were studied according to the protocols approved by the Institutional Animal Care and Use Committee. Animals were anesthetized, intubated and mechanically ventilated with 100% O2 mixed with isoflurane (1.0–2.5%). Subsequently, the dogs underwent left thoracotomies and the left anterior descending artery was ligated at 1.0–1.5 cm from the bifurcation of the left main coronary artery for 3 hours followed by reperfusion. The dogs were allowed to recover for 4 days before imaging studies.
Prior to imaging, animals were fasted, sedated, anesthetized, intubated, and transferred to the scanner table and were mechanically ventilated via a pneumatic anesthesia ventilator. Subsequently, they were placed on the scanner table in the feet-first right-lateral position. The imaging protocol (localizations, cardiac shimming, and T2-weighted acquisitions) and the scan parameters of proposed free-breathing 3D T2-prepared acquisitions were the same as those used for the human studies except for the FOV, number of slices acquired, and acquisition window (95–140 ms) reduced to adjust for differences in cardiac dimension and heart rate. 2D breath-held T2-prepared bSSFP acquisitions and phase-sensitive inversion recovery (PSIR) late-gadolinium-enhancement (LGE) acquisitions were prescribed with full left ventricle coverage comprising slices matched to the 3D partitions. PSIR LGE images were acquired 10 minutes after Gd-DTPA infusion (0.2 mmol/kg, gadoversetamide/Optimark, Mallinckrodt Inc., Hazelwood, MO), using non-selective inversion recovery preparation with GRE readout (TR/TE = 3.2/1.5 ms, FA = 20°, BW = 586 Hz/pixel, matrix = 96 × 192, in-plane resolution = 1.3 × 1.3 mm2; and slice thickness = 6.0 mm). A TI-scout sequence was used to find the optimal TI for nulling the healthy myocardium (240–270 ms).
Image Analysis and Statistics
All reconstructed T2 maps were analyzed in cvi42 (Circle Cardiovascular Imaging Inc., Calgary, AB, Canada). Images were imported into cvi42 and endo- and epi-cardial contours were drawn to segment the myocardium for further T2 analysis. In addition, all statistical analyses were performed in IBM SPSS (V21.0, NY, USA).
Ex vivo Studies
After segmenting the myocardium and parenchyma of the kidney, mean T2 values of the organs determined from images acquired using 2D spin echo, 2D T2-prepared bSSFP and 3D T2-prepared (proposed) sequences were compared. Repeated measurements ANOVA tests were performed to examine whether the T2 values were different between different sequences. Post-hoc comparison with Bonferroni correction was used if the null hypothesis was rejected.
Human Studies
The mean and coefficient-of-variation (COV) of myocardial T2, as well as overall image quality scores of the T2 maps generated using images from the (i) proposed 3D sequence with motion correction (3D FB MoCo), (ii) proposed 3D sequence without motion correction (3D FB Non-MoCo, using all respiratory phases), and (iii) 2D breath-held sequence (2D BH) were measured. Two independent, experienced observers who were blinded to the acquisition details evaluated image quality. All image quality assessments were based on the T2 maps. Mean myocardial T2 values were measured from the segmented myocardium and the COV of T2 was calculated as the quotient of the mean T2 and the corresponding standard deviation. Image quality was graded as 1, poor (non assessable); 2, fair (mild to moderate artifacts); 3, good (minimal to mild artifacts); 4, excellent (minimal or no artifacts). Repeated measurements ANOVA tests (mean T2 and COV in T2) and Friedman test (image quality scores) were performed to examine whether the measures were different between the three techniques. Post-hoc comparison with Bonferroni correction was used if the null hypothesis was rejected.
Canine Studies
Following segmentation of the myocardium (as described earlier), remote myocardium was defined as the region showing no hyperintensity on LGE images. A reference region of interest was drawn in remote myocardium on both LGE images and copied onto the corresponding slice positions of 2D and 3D T2 maps. Affected myocardium was defined as the hyperintense region with mean signal intensity at least 2 standard deviations (SDs) above that of the reference region of interest on both 2D and 3D T2 maps (13). Only LGE positive imaging slices were included in the analysis. Regression analysis was performed between slice-matched 2D and 3D acquisitions for (a) mean T2 values of remote and affected (edematous) myocardium; and (b) % edema volume, defined as the relative volume of affected myocardium to the total volume of the myocardium on a per-slice basis, for all animals. Bland-Altman analysis was performed to examine the limits of agreement and bias in mean T2 and % edema volume between the proposed 3D approach and the 2D approach.
RESULTS
Ex vivo Studies
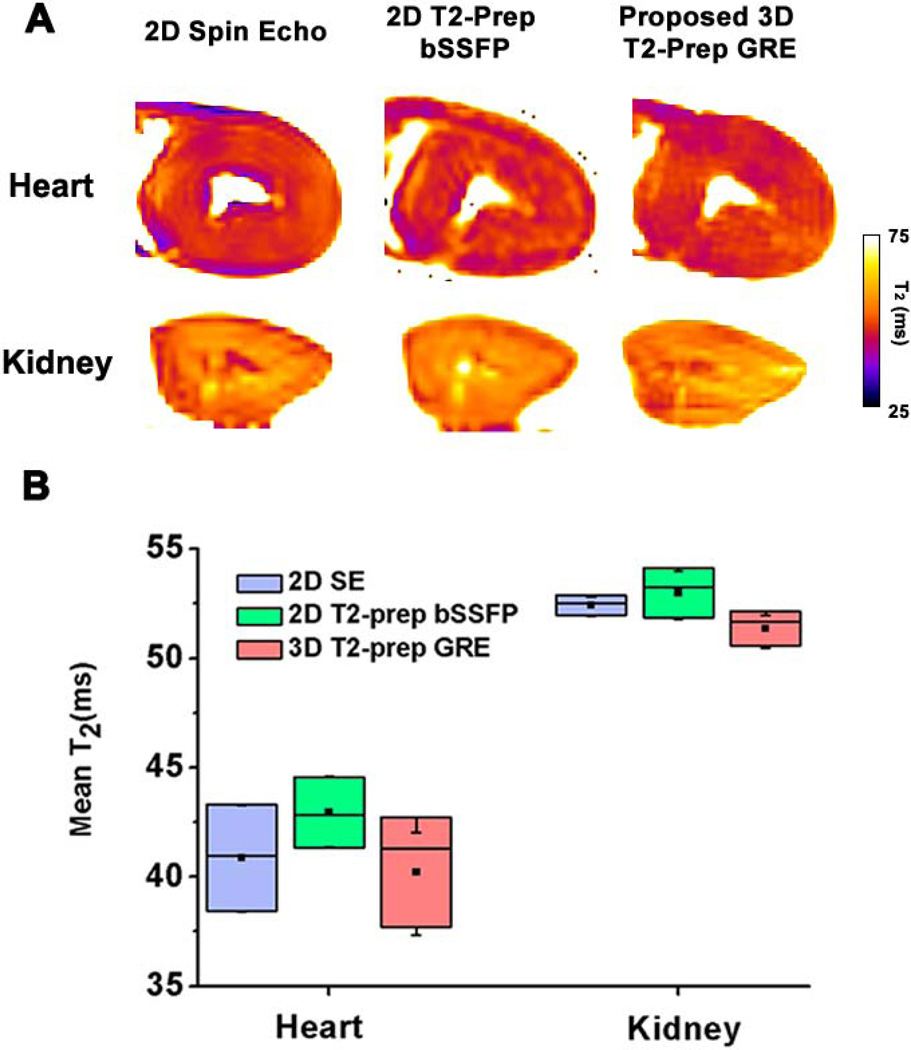
Representative T2 maps obtained using 2D spin-echo and T2-prep bSSFP acquisitions along with the proposed 3D acquisition (matched to the 2D slice) are shown in Fig. 3A. Mean T2 values derived from each sequences are shown in Fig. 3B (2D spin-echo T2 = 40.8±2.4 ms (heart), 52.4±0.5 ms (kidney) ms; 2D T2-prep bSSFP T2 = 42.4±1.6 (heart), 52.9±1.1 (kidney) ms; and proposed 3D sequence T2 = 40.2±2.5 (Heart), 51.3±0.7 (kidney) ms). Relative to the “gold standard” T2 values (2D spin-echo), T2 values obtained with 2D T2-prep bSSFP and 3D T2-prep GRE were not different (p = 0.7 and p=0.2, respectively).
Fig. 3. Representative short-axis T2 maps and box-plot of mean myocardial T2 from explanted dog hearts and kidneys obtained with 2D spin echo, 2D T2-prep SSFP and 3D T2-prep GRE acquisitions.
(A) Slice-matched T2 maps obtained at the level of mid ventricle from an explanted dog heart and mid slice of dog kidney using the different T2 approaches are shown. Mean T2 values (B) between the approaches were not different for heart and kidney.
Healthy Volunteers
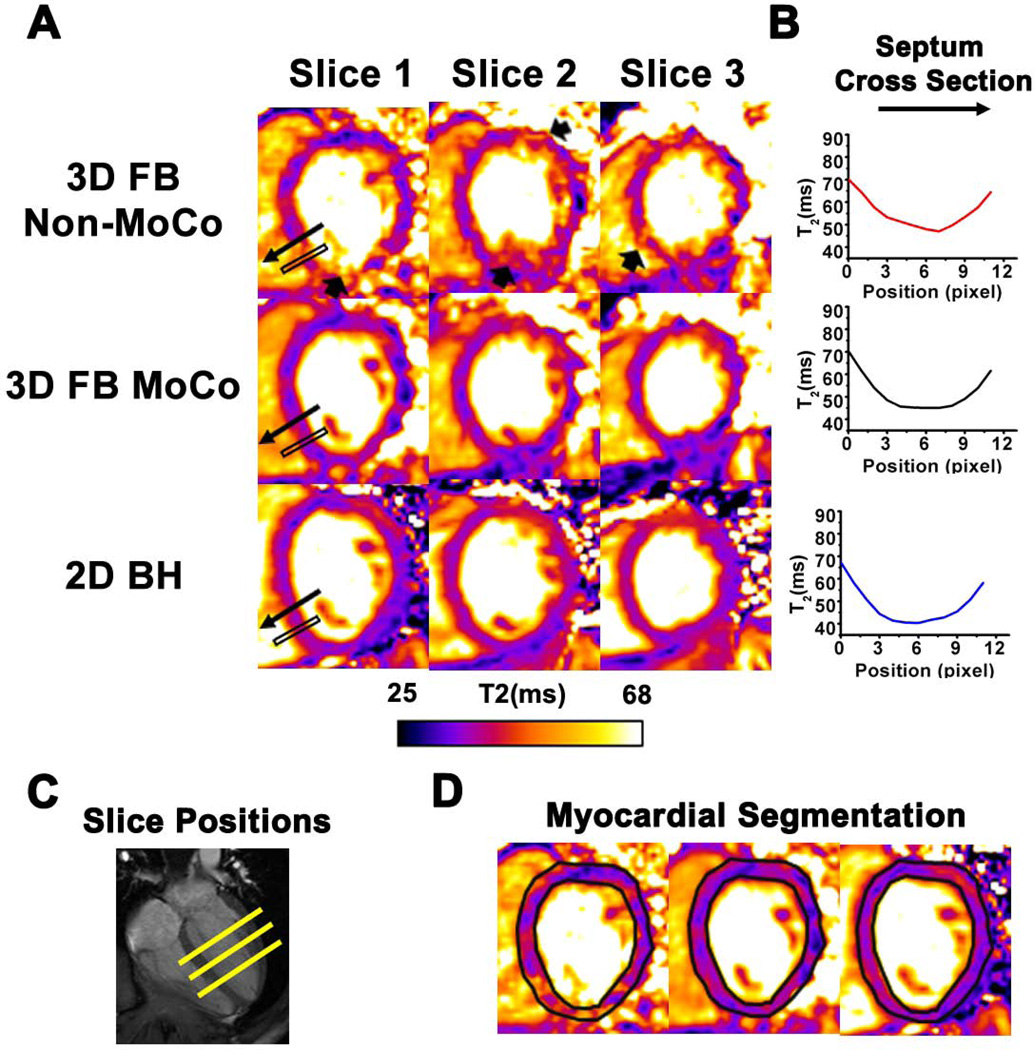
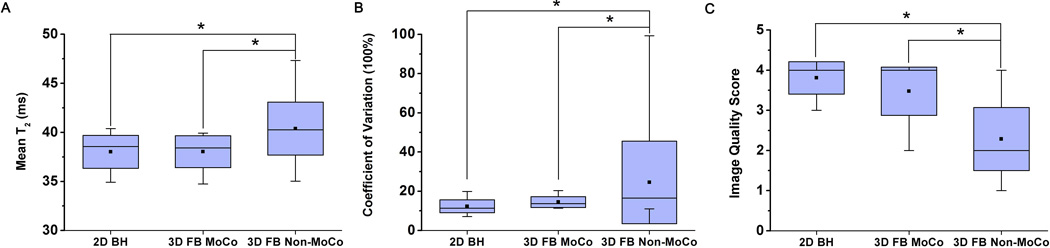
Figure 4 shows representative slice-matched T2-weighted images (Fig. 4A) and T2 maps (Fig. 4B) obtained using 3D FB MoCo, 3D FB Non-MoCo, and 2D BH approaches. 2D BH and 3D FB MoCo images at the different TEs appeared to have comparable image quality, while image blurring from respiratory motion was evident in 3D FB Non-MoCo images and maps (arrows). Quantitative measures of agreement between the methods are shown in Fig. 5 and collected in Table 1. 3D FB Non-MoCo showed significantly greater COV (p<0.05), longer T2 values (p<0.05), and lower IQ (p<0.05) compared to 3D FB MoCo and 2D BH images and maps. Conversely, 2D BH and 3D FB MoCo approaches did not show differences in mean T2 (p=0.99), COV T2 (p=0.74), and IQ (p=0.14).
Fig. 4. Representative short-axis T2 maps and T2 profile across a representative region in the septum acquired from a healthy volunteer using 2D BH, 3D FB MoCo and 3D FB Non-MoCo approaches.
T2 maps reconstructed using weighted images from the different TEs at the three short-axis positions are shown in panel A. Note the loss of detail and variation in signal intensity in 3D FB Non-MoCo images (arrows), which is absent in 3D FB MoCo and 2D BH T2 maps. In panel B, T2 profile within the black box containing the septal wall of slice 1 is shown. In the 3D FB Non-MoCo profile, T2 was significantly higher and showed larger deviation in the myocardial region relative to 3D FB MoCo and 2D BH. Corresponding slice positions of the short-axis images along the long axis of the heart (C) and representative myocardial segmentation contours (D) are also shown for reference.
Fig. 5. Quantitative measures of mean T2 (A), COV T2 (B) and Image Quality scores (C) between 2D BH, 3D FB MoCo and 3D FB Non-MoCo approaches obtained from healthy volunteers.
Across all measures, 2D BH and 3D FB MoCo approaches were not different but both were significantly different (* represents p<0.05) from 3D FB Non-MoCo approach.
Table 1.
Performance of 2D-BH, 3D-FB-MoCo and 3D-FB-Non-MoCo in humans
| Sequence | 2D-BH | 3D-FB-MoCo | 3D-FB-Non-MoCo |
|---|---|---|---|
| Subject | |||
| T2 | 38.0±1.7 ms | 37.7±2.0 ms | 40.3±2.7 ms* |
| COV | 15±4 % | 17.3±3 % | 38±55 %* |
| IQ | 3.8±0.4 | 3.5±0.6 | 2.3±0.8* |
(* denote p<0.05)
Canines with Reperfused Acute Myocardial Infarction
Representative slice-matched images (Fig. 6A) and T2-maps (Fig. 6B) obtained with the proposed 3D FB MoCo and 2D BH obtained on day 4 post-MI are shown. Corresponding LGE images are also shown for visual confirmation for the presence of infarction in the LAD territory. Edema (and infarction) was identified in 2–4 short-axis slices in each of the animals. Close correspondence between edematous territories in the slice-matched 2D BH and 3D FB T2 maps, and the enhanced territories in LGE images was observed. T2 values were significantly elevated in regions positive for LGE in both 2D BH and 3D FB T2 maps. Linear regression analysis showed that the T2 estimates (edematous and remote territories) and the % edema volume measured from 2D BH and 3D FB MoCo T2 maps were closely correlated (R2 = 0.88,and 0.96, respectively. Both p<0.05). Bland-Altman plots of mean T2 measures from edematous territories and remote myocardium and % Edema Volume were within the limits of agreement (bias in T2 = 0.4 ms; bias in % Edema Volume = 0.9%). The edema and LGE positive territories (on per slices basis) agreed well (R2 = 0.8, p<0.05) with the edema volume have a positive bias of 8% over LGE volume.
Fig. 6.
Representative short-axis images obtained from a canine on day 4 post-MI (A), the statistical relations between 2D BH and 3D MoCo for T2 and edema volume and the statistical relations between T2-based edematous and LGE volume across all animals (B–G). (A) Slice-matched T2 maps from 2D BH and 3D FB MoCo, as well as LGE images obtained from a canine at the basal, mid ventricle (Mid) and apical sections are shown. Contours delineating the edematous volumes are presented in the right column. Note the close correspondence between hyperintense regions identified on 2D BH and 3D FB MoCo T2 maps (black arrows) and their relation to hyperintense regions in LGE images (yellow arrows). Also note that the 2D BH images are blurrier due to cardiac motion (due to single-shot acquisitions in the presence of high heart rates in canines with recent infarction), which is not the case with the proposed approach. Linear regression analysis between T2 values (edematous territories and remote myocardium) of slice-matched 3D FB MoCo and 2D BH acquisitions and the corresponding Bland-Altman analysis are shown in panels B and C, respectively. Linear regression: y = 0.9 x − 3.4, where y = T2 from 3D FB MoCo and x = T2 from 2D BH acquisitions, with R2 = 0.88, p<0.05 and bias = 0.8 ms. Linear regression analysis between slice-matched 3D FB MoCo and 2D BH acquisitions for Edema volume and the corresponding Bland-Altman analysis are shown in panels D and E, respectively. Linear regression: y1 = 1.1 x1 −2.4, where y1 = Edema volume from 3D FB MoCo and x1 = Edema volume from 2D BH acquisitions, with R2 = 0.96, p<0.05 and bias = 0.6%. Linear regression analysis between slice-matched 3D FB MoCo Edema and LGE volume and the corresponding Bland-Altman analysis are shown in panels F and G, respectively. Linear regression: y2 = 0.7 x2 −3.4, where y1 = LGE volume and x1 = Edema volume from 3D FB MoCo acquisitions, with R2 = 0.8, p<0.05 and bias = 8%.
DISCUSSION
In this study we successfully developed and tested a motion-corrected, free-breathing 3D T2 mapping technique using GRE-based stack-of-stars acquisition at 3T that can be completed within 5 minutes. The accuracy of T2 measures from motion-corrected reconstruction was examined in ex-vivo canine hearts and kidneys, healthy human volunteers, and controlled canine model of acute myocardial infarction, respectively. The results showed that compared to the commonly used T2 mapping approach (2D acquisitions with multiple breath holds), the proposed 3D T2 mapping strategy can reliably estimate T2 values of the myocardium in health and disease. The proposed method is expected to be beneficial in patients who cannot tolerate suspension of breathing and in cases where the imaging exam needs to be completed with whole heart coverage within a short period of time. Hence the key features of the proposed approach (free-breathing, full LV coverage coverage and fast acquisitions) are expected to be particularly valuable in the management of chest pain in emergency department where myocardial edema may be used as a marker of ongoing ischemia (3) and for contrast-free cardiac stress testing using myocardial BOLD MRI (8,9).
While the benefits of fast, free-breathing T2 mapping of whole LV over the conventional multiple-breath-held T2 mapping are clear, it is useful to contrast the proposed approach against previously proposed 3D T2 mapping approaches. While navigator gated methods have shown low imaging efficiency and residual motion artifacts, different methods have been proposed to correct for respiratory motion and accelerate acquisition (17,31). In particular, the recent development by Heeswijk, et al. (31) has markedly reduced acquisition time. However, since their T2 mapping approach is founded upon 3D high-resolution isotropic imaging, acquisition time continues to be near 20 minutes, which imposes limitations on its use for time sensitive applications discussed above.
In this study we utilized a 3D stack-of-stars sampling scheme for T2 mapping, which lends some key benefits. First, at a heart rate of 60 beats/minute, with 48 projections acquired every other heartbeat, the total acquisition time for whole LV coverage is less than 5 minutes. Since the proposed approach does not restrict one to isotropic resolution, it provides the flexibility to control the resolution along the long axis, and hence imaging time, depending on the needs of the study. Second, the proposed approach allows for centric encoding, which reduces the T1 dependency in T2 maps (17). Finally, the proposed T2 mapping strategy offers robust image quality at 3T since the proposed approach uses adiabatic T2 preparation combined with GRE readouts to overcome image artifacts from B1 and B0 in homogeneities at 3T. Although bSSFP readouts can provide significant signal-to-noise benefits over GRE, previous studies have shown insignificant differences in myocardial T2 between the two readout sequences at 1.5T (32). Consistent with these studies, in the current study at 3T we observed comparable COV in myocardial T2 between bSSFP and GRE readout schemes. Nonetheless both readout schemes have their limitations in extreme cases - GRE acquisitions can suffer from lower SNR and have less homogeneous signal in large patients; and bSSFP readouts are prone to unavoidable banding artifacts.
The T2 values measured under conditions of health in this study were comparable to the values previously reported in humans. Specifically, we found that the T2 value of the healthy myocardium to be 37.7±2.0 ms vs. 38.5 ± 4.5 ms (17). Similar observations were made in animals with acute myocardial infarction as well (T2 in the infarct zone from our study was 55.2±7.1 ms vs. 58.1 ± 6.9 ms (33) and T2 in remote zone from our study was 36.7±3.5 ms vs. 41.1 ± 5.2 ms (33)). These favorable comparisons between our results and those reported in the literature further confirm that the proposed technique is a reliable method for measuring myocardial T2 under conditions of health and disease.
Although not explored here, we anticipate that further shortening of scan time may be possible using parallel imaging techniques (34,35) and/or compressed sensing (36) schemes. However, to avoid potential “smoothing” artifacts observed in certain accelerated approaches and also to strictly examine the capability of motion correction, we did not consider any imaging acceleration techniques in the current study. Additional studies are necessary to examine the limits of imaging acceleration strategies within the constraints of reliable measurements of myocardial T2.
There were some limitations in this study. First, while this study employed ex-vivo and in-vivo preparations of animals and healthy volunteers and showed that the proposed 3D approach can yield similar values of T2 as 2D breath-held acquisitions, the sample size of the study is limited. Additional studies are required to assess the robustness of the method under various physiological conditions, particularly in human subjects with disease. Second, based on the mono exponential model, 3 TEs are the minimum requirement for a least-squares linear-regression fitting of the logarithmically transformed data. To map the T2 of the remote and affected myocardium, we set the latest TE to be 55 ms, so as to achieve a reasonable dynamic range in signal intensities for the affected territory (which tends to have T2 ~ 60 ms) and still have sufficient in the remote myocardium (T2 ~ 40 ms). Although additional TEs may improve robustness of T2 fits (37), extra acquisition time associated with greater number of TEs can extend the study time beyond the demands of the key applications outlined earlier. Based on comparable results we obtained here in relation to past work (4,13,14,17,38), we expect that three TEs to be a good tradeoff between imaging speed and fidelity of myocardial T2 fits under conditions of health and disease. Third, in this study we assumed that the prescribed slice positions between 2D and 3D acquisition are the same, which may not be the case. To minimize this error, the data in the reference bin was always acquired at end expiration. Also, given that 2D slice thickness and 3D partitions are similar (6 mm), we anticipate that any contributions from potential differences to be small. Moreover, we used only 4 bins to derive and correct for respiration-induced motion. Previous studies that employed affine motion correction on the raw data (26,39) used 6 bins to estimate respiration-mediated motion for coronary MR angiography. Finally, we estimated the correction term (α) based on known T1 and T2 values of the myocardium at 3T in health. Since T1 and T2 of the edematous myocardium can be greater than 30% and 50% of the normal myocardium (17,29), respectively, it is conceivable that α may not be accurately estimated on the basis of healthy myocardial T1 and T2 values. However, our calculations showed that even in the presence of significant edema (T1 =1400 ms, T2=60 ms), the estimated error in α determined using healthy T1=1200 ms and T2=40 ms values is within 8%, which corresponds to a deviation in T2 of 3.2 ms, which is comparable to the standard deviation of myocardial T2.
Nonetheless, the results here suggest that the quality of motion correction based on the empirical binning at reduced resolution is adequate for reliable estimation of myocardial T2 values under conditions of health and disease.
CONCLUSION
We developed and tested a free-breathing 3D T2 mapping approach at 3T with respiratory motion correction, which can be performed within 5 minutes. The proposed approach yielded accurate estimates of myocardial T2 in explanted canine hearts and kidneys, healthy human volunteers, and canines with reperfused acute myocardial infarction. Clinical studies are needed to validate the robustness of the proposed approach in patients.
Acknowledgments
FUNDING INFORMATION
This work was supported in part by National Heart, Lung, and Blood Institute HL091989 (R.D.) and American Heart Association Grants 14SDG20480123 (B.S.) and 13PRE17210049 (A.K). The content is solely the responsibility of the authors and does not necessarily represent the official views of the American Heart Association or the National Heart, Lung, And Blood Institute or the National Institutes of Health.
APPENDIX
In the centric encoding scheme, the longitudinal magnetization immediately following T2 preparation of each heart beat can be written iteratively as
| (1) |
where MT2+ and MT2− are longitudinal magnetizations following the T2 preparations in the recent (MT2+) and previous (MT2−) heart beats, M0 is magnetization at thermal equilibrium, E1=exp(−RT/T1) with RT = recovery time, and E2TE= exp(−TE/T2). Assuming heart rate is constant throughout the acquisition, MT2 term will reach a steady state after a few heart beats (i.e. MT2+ = MT2− = MT2) and (1) can be rearranged to get
| (2) |
and the acquired signal can be expressed as
| (3) |
G accounts for readout scheme, coil sensitivity, etc.
When RT >> T1, E1 ~ 0, R2 (or 1/T2) can be derived with 2 measurements with different TEs using
| (4) |
where ΔTE = TE1 − TE2. However when RT is comparable to T1, E1 is not negligible and (2) has to be rewritten as
| (5) |
| (6) |
STE1, STE2, are Image intensities corresponding to the first and second TE and αR2 is the bias in R2 estimated from fitting to a mono-exponential under the assumption of perfect T1 recovery . Hence the appropriate correction factor to estimate true T2 is (1 + α); that is , where is .
REFERENCES
- 1.Abdel-Aty H, Zagrosek A, Schulz-Menger J, Taylor AJ, Messroghli D, Kumar A, Gross M, Dietz R, Friedrich MG. Delayed enhancement and T2-weighted cardiovascular magnetic resonance imaging differentiate acute from chronic myocardial infarction. Circulation. 2004;109(20):2411–2416. doi: 10.1161/01.CIR.0000127428.10985.C6. [DOI] [PubMed] [Google Scholar]
- 2.Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT, White JA, Abdel-Aty H, Gutberlet M, Prasad S, Aletras A, Laissy JP, Paterson I, Filipchuk NG, Kumar A, Pauschinger M, Liu P. International Consensus Group on Cardiovascular Magnetic Resonance in M. Cardiovascular magnetic resonance in myocarditis: A JACC White Paper. Journal of the American College of Cardiology. 2009;53(17):1475–1487. doi: 10.1016/j.jacc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verhaert D, Thavendiranathan P, Giri S, Mihai G, Rajagopalan S, Simonetti OP, Raman SV. Direct T2 quantification of myocardial edema in acute ischemic injury. JACC Cardiovascular imaging. 2011;4(3):269–278. doi: 10.1016/j.jcmg.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Usman AA, Taimen K, Wasielewski M, McDonald J, Shah S, Giri S, Cotts W, McGee E, Gordon R, Collins JD, Markl M, Carr JC. Cardiac magnetic resonance T2 mapping in the monitoring and follow-up of acute cardiac transplant rejection: a pilot study. Circulation Cardiovascular imaging. 2012;5(6):782–790. doi: 10.1161/CIRCIMAGING.111.971101. [DOI] [PubMed] [Google Scholar]
- 5.Payne AR, Casey M, McClure J, McGeoch R, Murphy A, Woodward R, Saul A, Bi X, Zuehlsdorff S, Oldroyd KG, Tzemos N, Berry C. Bright-blood T2-weighted MRI has higher diagnostic accuracy than dark-blood short tau inversion recovery MRI for detection of acute myocardial infarction and for assessment of the ischemic area at risk and myocardial salvage. Circulation Cardiovascular imaging. 2011;4(3):210–219. doi: 10.1161/CIRCIMAGING.110.960450. [DOI] [PubMed] [Google Scholar]
- 6.He T, Gatehouse PD, Smith GC, Mohiaddin RH, Pennell DJ, Firmin DN. Myocardial T2* measurements in iron-overloaded thalassemia: An in vivo study to investigate optimal methods of quantification. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 2008;60(5):1082–1089. doi: 10.1002/mrm.21744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papanikolaou N, Ghiatas A, Kattamis A, Ladis C, Kritikos N, Kattamis C. Non-invasive myocardial iron assessment in thalassaemic patients. T2 relaxometry and magnetization transfer ratio measurements. Acta radiologica. 2000;41(4):348–351. doi: 10.1080/028418500127345442. [DOI] [PubMed] [Google Scholar]
- 8.Fieno DS, Shea SM, Li Y, Harris KR, Finn JP, Li D. Myocardial perfusion imaging based on the blood oxygen level-dependent effect using T2-prepared steady-state free-precession magnetic resonance imaging. Circulation. 2004;110(10):1284–1290. doi: 10.1161/01.CIR.0000140673.13057.34. [DOI] [PubMed] [Google Scholar]
- 9.Arnold JR, Karamitsos TD, Bhamra-Ariza P, Francis JM, Searle N, Robson MD, Howells RK, Choudhury RP, Rimoldi OE, Camici PG, Banning AP, Neubauer S, Jerosch-Herold M, Selvanayagam JB. Myocardial oxygenation in coronary artery disease: insights from blood oxygen level-dependent magnetic resonance imaging at 3 tesla. Journal of the American College of Cardiology. 2012;59(22):1954–1964. doi: 10.1016/j.jacc.2012.01.055. [DOI] [PubMed] [Google Scholar]
- 10.Shea SM, Fieno DS, Schirf BE, Bi X, Huang J, Omary RA, Li D. T2-prepared steady-state free precession blood oxygen level-dependent MR imaging of myocardial perfusion in a dog stenosis model. Radiology. 2005;236(2):503–509. doi: 10.1148/radiol.2362040149. [DOI] [PubMed] [Google Scholar]
- 11.Kellman P, Aletras AH, Mancini C, McVeigh ER, Arai AE. T2-prepared SSFP improves diagnostic confidence in edema imaging in acute myocardial infarction compared to turbo spin echo. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 2007;57(5):891–897. doi: 10.1002/mrm.21215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdel-Aty H, Simonetti O, Friedrich MG. T2-weighted cardiovascular magnetic resonance imaging. Journal of magnetic resonance imaging : JMRI. 2007;26(3):452–459. doi: 10.1002/jmri.21028. [DOI] [PubMed] [Google Scholar]
- 13.Giri S, Chung YC, Merchant A, Mihai G, Rajagopalan S, Raman SV, Simonetti OP. T2 quantification for improved detection of myocardial edema. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance. 2009;11:56. doi: 10.1186/1532-429X-11-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thavendiranathan P, Walls M, Giri S, Verhaert D, Rajagopalan S, Moore S, Simonetti OP, Raman SV. Improved detection of myocardial involvement in acute inflammatory cardiomyopathies using T2 mapping. Circulation Cardiovascular imaging. 2012;5(1):102–110. doi: 10.1161/CIRCIMAGING.111.967836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He T, Gatehouse PD, Anderson LJ, Tanner M, Keegan J, Pennell DJ, Firmin DN. Development of a novel optimized breathhold technique for myocardial T2 measurement in thalassemia. Journal of magnetic resonance imaging : JMRI. 2006;24(3):580–585. doi: 10.1002/jmri.20681. [DOI] [PubMed] [Google Scholar]
- 16.Guo H, Au WY, Cheung JS, Kim D, Jensen JH, Khong PL, Chan Q, Chan KC, Tosti C, Tang H, Brown TR, Lam WW, Ha SY, Brittenham GM, Wu EX. Myocardial T2 quantitation in patients with iron overload at 3 Tesla. Journal of magnetic resonance imaging : JMRI. 2009;30(2):394–400. doi: 10.1002/jmri.21851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Heeswijk RB, Feliciano H, Bongard C, Bonanno G, Coppo S, Lauriers N, Locca D, Schwitter J, Stuber M. Free-breathing 3 T magnetic resonance T2-mapping of the heart. JACC Cardiovascular imaging. 2012;5(12):1231–1239. doi: 10.1016/j.jcmg.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 18.Giri S, Shah S, Xue H, Chung YC, Pennell ML, Guehring J, Zuehlsdorff S, Raman SV, Simonetti OP. Myocardial T(2) mapping with respiratory navigator and automatic nonrigid motion correction. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 2012;68(5):1570–1578. doi: 10.1002/mrm.24139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raman SV, Simonetti OP, Winner MW, 3rd, Dickerson JA, He X, Mazzaferri EL, Jr, Ambrosio G. Cardiac magnetic resonance with edema imaging identifies myocardium at risk and predicts worse outcome in patients with non-ST-segment elevation acute coronary syndrome. Journal of the American College of Cardiology. 2010;55(22):2480–2488. doi: 10.1016/j.jacc.2010.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abdel-Aty H, Cocker M, Meek C, Tyberg JV, Friedrich MG. Edema as a very early marker for acute myocardial ischemia: a cardiovascular magnetic resonance study. Journal of the American College of Cardiology. 2009;53(14):1194–1201. doi: 10.1016/j.jacc.2008.10.065. [DOI] [PubMed] [Google Scholar]
- 21.Gerber BL, Raman SV, Nayak K, Epstein FH, Ferreira P, Axel L, Kraitchman DL. Myocardial first-pass perfusion cardiovascular magnetic resonance: history, theory, and current state of the art. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance. 2008;10:18. doi: 10.1186/1532-429X-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schar M, Kozerke S, Fischer SE, Boesiger P. Cardiac SSFP imaging at 3 Tesla. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 2004;51(4):799–806. doi: 10.1002/mrm.20024. [DOI] [PubMed] [Google Scholar]
- 23.Nezafat R, Stuber M, Ouwerkerk R, Gharib AM, Desai MY, Pettigrew RI. B1-insensitive T2 preparation for improved coronary magnetic resonance angiography at 3 T. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 2006;55(4):858–864. doi: 10.1002/mrm.20835. [DOI] [PubMed] [Google Scholar]
- 24.Jenista ER, Rehwald WG, Chen EL, Kim HW, Klem I, Parker MA, Kim RJ. Motion and flow insensitive adiabatic T2 -preparation module for cardiac MR imaging at 3 Tesla. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 2013;70(5):1360–1368. doi: 10.1002/mrm.24564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stuber M, Botnar RM, Danias PG, Kissinger KV, Manning WJ. Submillimeter three-dimensional coronary MR angiography with real-time navigator correction: comparison of navigator locations. Radiology. 1999;212(2):579–587. doi: 10.1148/radiology.212.2.r99au50579. [DOI] [PubMed] [Google Scholar]
- 26.Bhat H, Ge L, Nielles-Vallespin S, Zuehlsdorff S, Li D. 3D radial sampling and 3D affine transform-based respiratory motion correction technique for free-breathing whole-heart coronary MRA with 100% imaging efficiency. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 2011;65(5):1269–1277. doi: 10.1002/mrm.22717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manke D, Nehrke K, Bornert P. Novel prospective respiratory motion correction approach for free-breathing coronary MR angiography using a patient-adapted affine motion model. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 2003;50(1):122–131. doi: 10.1002/mrm.10483. [DOI] [PubMed] [Google Scholar]
- 28.Luis ISW, Ng L, Cates J the Insight Software Consortium, ( www.itk.org) The ITK Software Guide. 2nd Ed. 2005. Updated for ITK version 2.4. 2.4; [Google Scholar]
- 29.Kali A, Cokic I, Tang RL, Yang HJ, Sharif B, Marban E, Li D, Berman DS, Dharmakumar R. Determination of location, size, and transmurality of chronic myocardial infarction without exogenous contrast media by using cardiac magnetic resonance imaging at 3 T. Circulation Cardiovascular imaging. 2014;7(3):471–481. doi: 10.1161/CIRCIMAGING.113.001541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharif B, Dharmakumar R, Arsanjani R, Thomson L, Bairey Merz CN, Berman DS, Li D. Non-ECG-gated myocardial perfusion MRI using continuous magnetization-driven radial sampling. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 2014 doi: 10.1002/mrm.25074. 10.100/mrm25074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Heeswijk RB, Piccini D, Feliciano H, Hullin R, Schwitter J, Stuber M. Self-navigated isotropic three-dimensional cardiac T2 mapping. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 2014 doi: 10.1002/mrm.25258. 10.1002/mrm.25258. [DOI] [PubMed] [Google Scholar]
- 32.Wassmuth R, Prothmann M, Utz W, Dieringer M, von Knobelsdorff-Brenkenhoff F, Greiser A, Schulz-Menger J. Variability and homogeneity of cardiovascular magnetic resonance myocardial T2-mapping in volunteers compared to patients with edema. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance. 2013;15:27. doi: 10.1186/1532-429X-15-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghugre NR, Pop M, Barry J, Connelly KA, Wright GA. Quantitative magnetic resonance imaging can distinguish remodeling mechanisms after acute myocardial infarction based on the severity of ischemic insult. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 2013;70(4):1095–1105. doi: 10.1002/mrm.24531. [DOI] [PubMed] [Google Scholar]
- 34.Pang J, Sharif B, Arsanjani R, Bi X, Fan Z, Yang Q, Li K, Berman DS, Li D. Accelerated whole-heart coronary MRA using motion-corrected sensitivity encoding with three-dimensional projection reconstruction. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 2014;71(1):67–74. doi: 10.1002/mrm.25097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wright KL, Hamilton JI, Griswold MA, Gulani V, Seiberlich N. Non-Cartesian parallel imaging reconstruction. Journal of magnetic resonance imaging : JMRI. 2014 Nov;40(5):1022–1040. doi: 10.1002/jmri.24521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Doneva M, Bornert P, Eggers H, Stehning C, Senegas J, Mertins A. Compressed sensing reconstruction for magnetic resonance parameter mapping. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 2010;64(4):1114–1120. doi: 10.1002/mrm.22483. [DOI] [PubMed] [Google Scholar]
- 37.MacFall JR, Riederer SJ, Wang HZ. An analysis of noise propagation in computed T2, pseudodensity, and synthetic spin-echo images. Medical physics. 1986;13(3):285–292. doi: 10.1118/1.595956. [DOI] [PubMed] [Google Scholar]
- 38.von Knobelsdorff-Brenkenhoff F, Prothmann M, Dieringer MA, Wassmuth R, Greiser A, Schwenke C, Niendorf T, Schulz-Menger J. Myocardial T1 and T2 mapping at 3 T: reference values, influencing factors and implications. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance. 2013;15:53. doi: 10.1186/1532-429X-15-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pang J, Bhat H, Sharif B, Fan Z, Thomson LE, LaBounty T, Friedman JD, Min J, Berman DS, Li D. Whole-heart coronary MRA with 100% respiratory gating efficiency: self-navigated three-dimensional retrospective image-based motion correction (TRIM) Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 2014;71(1):67–74. doi: 10.1002/mrm.24628. [DOI] [PMC free article] [PubMed] [Google Scholar]