Summary
Fragile X mental retardation 1 (FMR1) premutation associated phenotypes have been explored extensively since the molecular mechanism emerged involving elevated FMR1 messenger ribonucleic acid (mRNA) levels. Lowered fragile X mental retardation protein (FMRP) can also occur which may have an additive effect to the high levels of mRNA leading to neurodevelopmental problems and psychopathology. This paper was aimed to review psychosis and catatonia in premutation carriers, express the role of elevated FMR1 mRNA and lowered FMRP in the phenotype of carriers and present a case of psychosis and catatonia in a carrier. This case also demonstrates additional genetic and environmental factors which may also affect the phenotype. We review the literature and report an exemplary case of a 25 year old male premutation carrier with elevated FMR1 mRNA, low FMRP, a cytochrome P450 family 2 subfamily D polypeptide 6 (CYP2D6)*2xN mutation and a perinatal insult. This patient developed an autism spectrum disorder, psychosis, catatonia with subsequent cognitive decline after electro-convulsive therapy (ECT) for his catatonia. He had a premutation of 72 CGG repeat in FMR1, FMR1 mRNA level that was over 2.4 times normal and FMRP level at 18% of normal, and additionally, a CYP2D6 allelic variant which leads to ultrarapid metabolism (UM) of medication. There is an overlapping pathophysiological mechanism of catatonia and fragile X-associated premutation phenotypes including autism and psychosis. This case demonstrates the shared phenotype and the overlap of the pathophysiological mechanisms that can influence the intervention. Multiple genetic and environmental hits can lead to more significant involvement in premutation carriers.
Keywords: Catatonia, fragile X syndrome, premutation, psychosis
1. Introduction
Fragile X-associated premutation disorders represent a wide spectrum of clinical manifestations including neurodevelopmental disorder, autism spectrum disorder (ASD), attention deficit hyperactivity disorder (ADHD); a neurodegenerative disorder, fragile X-associated tremor ataxia syndrome (FXTAS); neuropsychiatric disorders (depression, anxiety); and reproductive disorders (fragile X-associated premature ovarian insufficiency (FXPOI)) (1–5). The prevalence of the premutation is high in the general population and estimated approximately at 1:200 in females and 1:450 in males (6,7). In premutation carriers, in whom the expansion of CGG repeats in the promoter region of FMR1 gene is 55–200, the FMR1 gene remains active and demonstrates an increase in transcriptional activity, thus leading to increased FMR1 mRNA levels up to 8-fold higher than in the normal range with 5–44 CGG repeats (8–10). The elevated FMR1 mRNA causes toxicity because the hairpin formation in the CGG expansion sequesters important proteins needed for normal cellular function (11). In those with FXTAS there is inclusion formation in both neurons and astroglial cells in the brain and in the peripheral nervous system and these inclusions have the excess FMR1 mRNA, fragile X mental retardation protein (FMRP) and many other proteins and neurofilaments (11–13).
Decreased levels of FMRP are observed in some premutation carriers due to reduced translational efficiency of FMR1 mRNA containing the expanded CGG repeat (14–16). Lowered FMRP in the premutation range will often lead to neurodevelopmental problems including intellectual disability (ID), ASD and ADHD (1), although additional environmental or medical problems such as seizures, trauma, toxins or additional genetic mutations may also cause further developmental problems in these carriers (17–19).
FMRP is a mRNA-binding protein that regulates hundreds of mRNA targets at the synapse and it inhibits protein translation that is stimulated by metabotropic glutamate receptors (mGluRs) (20,21). The lack of FMRP will cause down-regulation of the gamma-amino butyric acid (GABA) A and B receptors and up-regulation of metabotropic glutamate receptor 5 (mGluR5) throughout the brain (22–24). FMRP is highly expressed in neurons and FMRP is critical for synaptic plasticity (25,26). Accordingly, the absence of this key protein in the synapse irreversibly alters neuronal connectivity to produce significant behavioral disorders including ID, ASD schizophrenia, bipolar disorder, and major depression (27–29). Therefore, the role of FMRP in the synapse affects not only fragile X-associated disorders but other neuropsychiatric disorders such as schizophrenia in those without an FMR1 mutation (30,31). Moreover, Aziz et al. (32) reported that FMR1 expanded CGG repeat of premutation and gray zone alleles (45–54 repeats), may demonstrate some clinical features of fragile X syndrome (FXS) in those who presented clinically. It is uncertain if the gray zone allele has FMRP deficits but the premutation demonstrates FMRP deficits that increase according to the CGG repeat number increases (33).
Catatonia is a neuropsychiatric syndrome characterized by abnormalities of movement, speech, functional skills and behavior and most commonly associated with mood disorders, psychotic disorders, ASD or other medical conditions, in the absence of psychiatric illness (34–37). Its historical association with schizophrenia is now widely regarded as an erroneous tradition, and has been revised in the most recent version of the Diagnostic and Statistical Manual (DSM) for psychiatric disorders, 5th edition (38). The central symptom of catatonia is disturbance of motor activity (overall increased activity, reduced activity or mixed) and a variety of other abnormal movements (e.g. reduced eye blink rate, grimacing, sudden cessation of movements or immobility) (39). Clinical diagnosis of catatonia requires at least 3 of the following symptoms: stupor (no psychomotor activity), catalepsy (maintaining a passively induced posture), waxy flexibility (slight, even resistance to positioning by the examiner), mutism, negativism (opposition or no response to instructions or external stimuli), posturing (spontaneous and active maintenance of a posture against gravity), mannerisms, stereotypies, agitation, grimacing, echolalia or echopraxia (38).
There are three neurochemical alterations that basically underlie the mechanism of catatonia i.e. dopamine hypoactivity, GABA hypoactivity, and glutamate hyperactivity (40,41). The second and the third alteration are similar to the FMRP deficient phenotype that is seen in the majority of FXS and the minority of fragile X-associated disorders individuals. However, dopamine dysfunction also occurs in those with FXS leading to ADHD in childhood (42). Aging individuals with FXS and those with FXTAS often have Parkinsonian symptoms related to dopamine dysfunction (43,44). Catatonia is a heterogeneous condition with discriminate subtypes of pathophysiological mechanisms, consequently, multiple agents may be required to treat acute catatonia, maintain or prevent the reoccurrence of chronic catatonia (41). However, at this stage benzodiazepines and electroconvulsive therapy (ECT) remain the most effective treatments of catatonia (45).
Psychiatric spectrum disorders have been discovered associated with fragile X-associated disorders include FXS and fragile X premutation (FXPM) since the 1990s ranging from mild to severe, such as hypersensitivity to stimuli, hyperarousal, inattention, hyperactivity, explosive and aggressive behavior, ASD, social anxiety, depression, mood/bipolar disorders, and psychosis (46–49). The neurobiology of fragile X syndrome is relatively well defined, while scientists have struggled to understand the consistent neurobiology of ASD, the most common neurodevelopmental psychiatric disorder. In FXS, decreased levels of FMRP will result in the FXS phenotype due to impaired synaptic plasticity leading to the cognitive impairment, relatively constant behavioral abnormalities and ASD in the majority of patients (50,51). Psychosis is seen in less than 10% of those with FXS (49) and some cases suggest that those with mosaicism or a lack of methylation so that there is both lowered FMRP and elevated mRNA, often called a double hit, have a higher rate of psychosis (52–57). Psychosis combined with catatonia has not previously been described in those with FXS or in those with the premutation. Below is a case of a premutation male with both psychosis and catatonia.
2. Case Report
2.1. Subject and setting
A 25-year old male with the fragile X premutation, who had been diagnosed with ADHD, ASD, bipolar disorder, obsessive compulsive disorder (OCD) and Tourette syndrome presented initially at age 20 years old to the Medical Investigation of Neurodevelopmental Disorders (MIND) Institute at UC Davis Medical Center. His CGG repeat size was 72, FMR1 mRNA was 2.4 (± 0.23) times normal and his FMRP level was 18.3 (± 0.2) which is severely deficient. He was born at 32.5 weeks gestation (premature) and delivered by C-section. He was a second twin born; he weighed 4lb 8oz whereas his fraternal twin sister (fragile X negative) weighed 4lbs 2oz. He had respiratory distress, was intubated and was in the intensive care unit for 40 days. He suffered an intraventricular hemorrhage (IVH) that affected his frontal lobes, although subsequent MRIs in childhood were read as normal.
Although he was a good eater, he was developmentally delayed with sitting at 13.5 months, crawling at 14 months, walking at 16 months, saying words at 12 months and phrases at about 30 months. He received speech and language therapy at age 2 because of language delay. He had a variety of autistic features such as memorizing names, phone numbers, neighborhood license plate numbers, poor eye contact, stereotypies and was diagnosed with a pervasive developmental disorder not other specified (PDD NOS). His psychiatrist diagnosed Tourette syndrome and obsessive compulsive disorder (OCD) because verbal and motor tics and obsessive symptoms developed when he was approximately 6 years old. He was also diagnosed with Bipolar Disorder when he was 10 years old. He had staring spells from 19 months through the ninth grade that were initially thought to be seizures. His electroencephalography (EEG) showed mild abnormalities and he was treated with valproate for both possible seizures and mood stabilization. His EEG was normal at age 18. Because of mood instability he was treated with lithium beginning in mid-adolescence, although he had an episode of lithium toxicity related to dehydration. He was also tried on multiple antipsychotics, risperidone, olanzapine, ziprasidone, asenapine, and eventually clozapine with very little benefit and many side-effects so they were all discontinued.
He had multiple psychiatric hospitalizations during his teenage and young adult life mainly related to behavior and emotional problems including mood instability, aggression, agitation and subsequently catatonia diagnosed at age 21 years old. At the time of his presentation with catatonia, some symptoms had been present for the past one year and included markedly increased motor activity with incessant pacing up to 5 or 6 hours each day, other abnormal movements (stereotyped finger movements, change in posture, episodic cessation of motor activity/freezing and grimacing), reduced speech, sudden and relatively unprovoked physical aggression, increased anxiety and obsessional preoccupations, diminished awareness of surroundings for personal safety, decline in skill level including inability to perform previously attained skills, and a delusional belief that his “father was John Lennon”. There were no hallucinations noted and the delusion was reported to be associated with starting an antipsychotic agent. There was progressive weight loss with his catatonia with a total loss of 50 lbs over a one year period.
Pharmacogenetics examination found that he had a CYP2D6*2xN (duplication) indicating he was an ultrarapid metabolizer (UM) of medications, and developed akathisia (inner restlessness secondary to antipsychotics) and then he became mute. He received high doses of benzodiazepines (lorazepam) with partial improvement, followed by weekly ECT beginning at age 20, leading to gradual improvement of his catatonic symptoms. He received 21 bilateral treatments using the Monitored Electro Convulsive Therapy (MECTA) device (MECTA Corporation, Tualatin, Ore). After this treatment and improvement of his catatonia his ECT was gradually decreased and stopped for a year and restarted when his catatonic symptoms gradually reoccurred.
2.2. Assessments, follow-up, and interventions
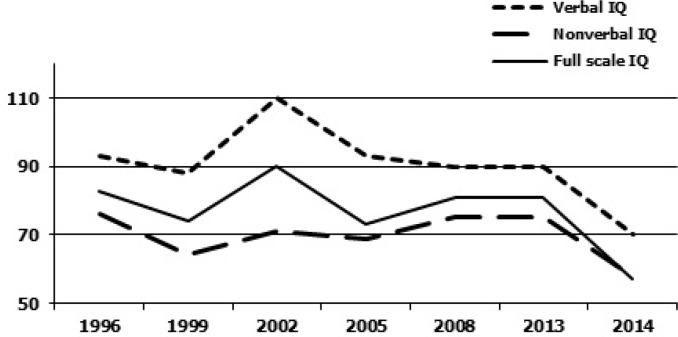
He was examined at the MIND Institute, UC Davis Medical Center at age 20 and then subsequently at age 25 years old. He is a tall young man with a long face but his ears are not prominent, although his palate is high arched and his jaw is mildly prominent. He also has large testicles (40 to 45 ml bilaterally). He does not have tremor nor ataxia but he appears mildly sedated on his medications. He demonstrates poor eye contact and he speaks in a slow monotone voice. The Autism Diagnostic Observation Scale (ADOS) module 4 score falls in the autism range at age 25 with a significant worsening of his autism score since age 20. The patient's mother provided previous cognitive test results from outside assessments, which are included in Figure 1. Figure 1 gives an overview of the trajectory of his cognitive results.
Figure 1.
Overview of cognitive testing results. Trajectory of IQ testing 1996–2014, X-Axis shows the year the testing was done, the Y-axis shows the IQ score range (IQ scale: mean 100 and SD 15).
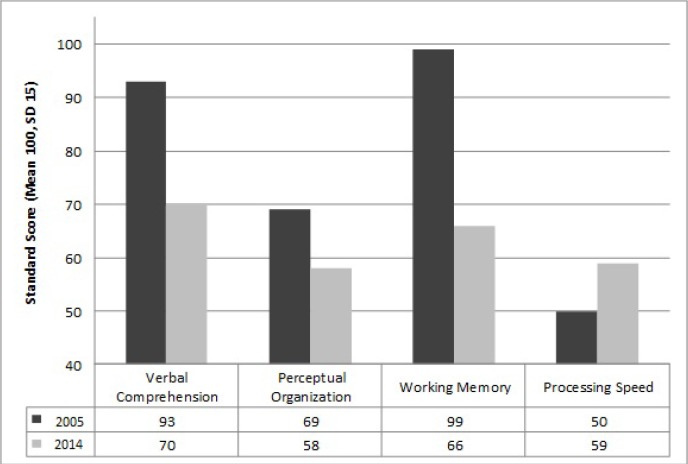
The patient shows a significant decline in both verbal and nonverbal intellectual quotient (IQ) domains since age 20 (full scale IQ 81) compared to his current testing (full scale IQ 57) at age 25 (Figure 1). There is a recent significant drop in the full scale IQ score that is related to a decline in working memory capacity. From the literature, generally a decline in processing speed is reported during ECT (58), which could not be seen in our patient. The cognitive decline may or may not be directly related to the ECT, but his high level of benzodiazepine medication and sedation are likely contributing factors. Table 1 and Figure 2 give a more detailed overview of the different sub domains in the cognitive assessments. The patient was assessed with the Wechsler Intelligence Scale for Children-IV (WISC-IV) at age 15, and the WAIS-IV at age 25, both age-appropriate cognitive assessments with similar test-structure.
Table 1. Comparison of cognitive assessment sub-domains from 2005 and 2014.
| Items | 2005 WISC-IV | 2014 WAIS-IV |
|---|---|---|
| Verbal Comprehensiona | 93 | 70 |
| Vocabulary | 10 | 6 |
| Similarities | 9 | 3 |
| Arithmetic | 5 | 4b |
| Information | 6 | 5 |
| Comprehension | 11 | 6 |
| Perceptual Organizationa | 69 | 58 |
| Picture completion | 8 | 4 |
| Block Design | 6 | 6 |
| Matrix Reasoning | 7 | 1 |
| Picture Arrangement | 5 | N/A |
| Visual Puzzles | N/A | 2 |
| Working Memorya | 99 | 66 |
| Digit span | 9 | 4 |
| Letter-Number Sequencing | Not reported | 2 |
| Processing Speeda | 50 | 59 |
| Digit Symbol Coding | 4 | 1 |
| Symbol search | Not reported | 4 |
Results given in Standard Score (Mean 100, SD 15), all other scores given in Scaled Scores (Mean 10, SD 3),
part of working memory in Wechsler Intelligence Scale for Children-IV (WAIS-IV).
Figure 2.
Comparison of IQ sub-domains from 2005 and 2014. The decreasing of three IQ sub-domains (verbal comprehension, perceptual organization, working memory) trajectory in 9 years.
The psychiatric evaluation was based on the Structured Clinical Interview (SCI) for Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision (DSM-IV-TR) (SCID) (59) and conducted with the patient and his mother. The SCID confirmed the previously established diagnoses of Bipolar Disorder type II (age of onset 10 years old), psychotic symptoms and OCD. Per mother, the patient's obsessions and compulsions started at age 2 (as previously described), with an exacerbation around age 14, when he exhibited contamination fears, excessive hand washing, and watching favorite movies over and over. His psychotic symptoms became apparent around age 14 and consisted of misidentification delusions (believing that familiar people are famous persons), grandiose delusions (believing he will win a large amount of money or that he will become famous), and nihilistic delusions (thinking that everybody in the world will die, with him being the sole survivor). His magnetic resonance imaging (MRI) is normal except that his corpus callosum is somewhat narrowed.
He is currently receiving maintenance ECT treatment at age 25, with the goal of decreasing frequency as tolerated. Lorazepam was switched to clonazepam (1 mg three times a day and 0.5 mg at 8 pm). He is currently also taking lithium 300 mg three times daily and thioridazine 42.5 mg three times a day (because of his psychotic thinking and he has not tolerated all other antipsychotics), in addition to melatonin 3 mg at bedtime.
3. Discussion
The patient presented here is an individual with the fragile X premutation with both elevated FMR1 mRNA levels and a significant deficit of FMRP, termed a double hit. He has features of FXS including a long face, high narrow palate and macroorchidism which are seen with more significant developmental problems or cognitive deficits in carriers (60). He had a history of questionable seizures which is also associated with ASD in our previous studies of carriers (17). His birth history included hypoxia which lowers FMRP levels and an IVH with damage to the frontal lobes that could add additional problems including executive dysfunction to the premutation condition. In addition, he has significant psychosis which is occasionally seen in those with low FMRP and high mRNA, a double hit FMRP (52).
FMRP deficits are not only associated with the severity of ID in FXS (29,61–64), but are also seen in other neuropsychiatric disorders without an FMR1 mutation including schizophrenia, ASD, OCD, mood disorders, major depressive disorder and bipolar disorder (27,30,31). There may be many proteins/micro RNAs (miRNAs) that regulate the expression of FMRP in those without an FMR1 mutation. Kovacs et al. (31) found that the age of onset of schizophrenia and the IQ correlates with the level of FMRP in blood in psychotic patients that do not have an FMR1 mutation. It is likely that those with the premutation may be even more vulnerable to the effects of lowered FMRP since their neurons already die earlier in culture related to the RNA toxicity of elevated FMR1 mRNA (65). Further studies of psychotic thinking in those with the premutation and in those with the full mutation are warranted especially when it is associated with catatonia.
Individuals with autism are at an increased risk to develop catatonia, which occurs in 17% of adolescents and young adults with ASD (36,66). Age appears to be the risk factor of catatonia in addition to stressful events, passivity in social situations, and impairment of expressive language skills (66). Catatonia is a severe neuropsychiatric syndrome with a 2.5% risk of developing malignant catatonia (label used when no documented exposure to antipsychotic agents) and Neuroleptic Malignant Syndrome (NMS; label used when known exposure to antipsychotics). There is a considerable risk of mortality in individuals who develop malignant catatonia/NMS which is known to present with severe functional impairment, autonomic and cardiovascular instability, reduced food and fluid intake resulting in dehydration, weight loss, multi-organ failure and other medical complications (41). The neurochemical pathology of catatonia includes decreased GABA activity and up-regulation of the glutamate system, both of which occur in those with a premutation and a full mutation (22,67,68).
His treatment history was complicated by the pharmacogenetic result of CYP2D6*2P/2P variant which cause UM of CYP2D6 metabolized drugs. Prevalence rates of the UM phenotype in American Caucasians is reported to be low at 4.3% compared to those in Ethiopians (30%) and in Saudi Arabian (20%) (69–71). Acknowledged UM allelic variants are CYP2D6*1, *2, *35, and *41 duplicated and multiduplicated, among those, CYP2D6*2 and *41 are the most frequent variants of CYP2D6 gene that cause extremely increased enzyme activity, wherein the lack of drug response/treatment failure is the most common clinical consequences (72). He has homozygous CYP2D6*2P/2P (CYP2D*2xN) promoter polymorphism (two copies of the gene), which may explain why he had failed multiple drug treatments. The failure of treatment may also be associated with various behavioral and psychiatric problems including catatonia, Bipolar Disorder type II with severe mood lability, aggression and psychotic thinking.
The patient's catatonia did not respond completely to benzodiazepines alone but he had a robust response to ECT. ECT is a well-established treatment for catatonia across the age span including children and adolescents (73,74). This is the first report of catatonia and ECT therapy in a premutation carrier, although the neurochemical changes that occur in both FXS and in premutation carriers (lower GABA and elevated glutamate, specifically mGluR5 up-regulation because of an FMRP deficit) is likely to predispose to catatonia (40). We would suggest testing for the FMR1 mutation or at least checking FMRP levels when they become clinically available for those who experience catatonia.
Although this patient responded well to ECT therapy it is of great concern that his IQ declined over time. The etiology of his cognitive decline is unclear. Generally a decline in processing speed reported during ECT (58), but not decline in IQ. Cognitive functions are known to recover once ECT is completed and the recovery appears to be irrespective of the age of the patient (75). A recent review by the Food Drug Administration (FDA) found that cognitive function recovery following ECT may take up to six months after the completion of ECT (76). Therefore, measuring cognitive functions during ongoing ECT is likely to identify deficits, which are expected to recover upon completion of the treatment while a global score such as intellectual functioning will likely be influenced by deficits in language, fluency, spatial orientation and memory. However, other causes of cognitive decline, such as chronic catatonia should also be considered as contributing factors (77). We know that seizures can worsen cognitive and behavioral aspects of FXS and seizures are associated with ASD in premutation carriers (17). In addition his relatively high dose of benzodiazepines and thioridazine may have deleterious cognitive effects and could lead to intermittent sedation and a lack of stimulation in his environment especially since he is not in school currently. We have recommended cognitive stimulation with digital programs and vocational rehabilitation. His history of IVH might contribute to his cognitive deficits, although it would not explain the recent decline.
Recent work by the Benke laboratory at the University of Colorado has demonstrated that seizures in early life in rats without an FMR1 mutation will disrupt the FMRP/Akt complex causing FMRP to pull away from the dendrites and move to the cell body, thereby disrupting the development of synaptic plasticity (78). Of concern is what ECT therapy will do to FMRP levels in those at risk for lowered FMRP levels particularly those with an FMR1 mutation, such as the patient presented here (77). Studies of animal models that undergo ECT will help to evaluate this concern. In addition further work is needed to understand the relationship between premutation involvement, psychosis, ASD and catatonia and the most optimal treatments for these problems.
Acknowledgements
This work was supported by National Institute of Health funding HD 036071 and the MIND Institute Intellectual and Developmental Disabilities Research Center (U54 HD079125).We thank Prof. Flora Tassone from Department of Biochemistry and Molecular Medicine, School of Medicine, UC Davis, Davis, CA, for providing the molecular data and the family who participated to this study.
References
- 1. Farzin F, Perry H, Hessl D, Loesch D, Cohen J, Bacalman S, Gane L, Tassone F, Hagerman P, Hagerman R. Autism spectrum disorders and attention-deficit/hyperactivity disorder in boys with the fragile X premutation. J Dev Behav Pediatr. 2006; 27(2 Suppl):S137-S144. [DOI] [PubMed] [Google Scholar]
- 2. Hagerman RJ, Greco C, Chudley A, Leehey M, Tassone F, Grigsby J, Hills J, Wilson R, Harris SW, Hagerman PJ. Neuropathology and neurodegenerative features in some older male premutation carriers of fragile X syndrome. Am J Hum Genet. 2001; A8 (Suppl 69):177. [Google Scholar]
- 3. Hunter JE, Leslie M, Novak G, Hamilton D, Shubeck L, Charen K, Abramowitz A, Epstein MP, Lori A, Binder E, Cubells JF, Sherman SL. Depression and anxiety symptoms among women who carry the FMR1 premutation: Impact of raising a child with fragile X syndrome is moderated by CRHR1 polymorphisms. Am J Med Genet B Neuropsychiatr Genet. 2012; 159B:549-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sherman SL. Premature ovarian failure in the fragile X syndrome. Am J Med Genet. 2000; 97:189-194. [DOI] [PubMed] [Google Scholar]
- 5. Hagerman R, Hagerman P. Advances in clinical and molecular understanding of the FMR1 premutation and fragile X-associated tremor/ataxia syndrome. Lancet Neurol. 2013; 12:786-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hantash FM, Goos DM, Crossley B, Anderson B, Zhang K, Sun W, Strom CM. FMR1 premutation carrier frequency in patients undergoing routine population-based carrier screening: Insights into the prevalence of fragile X syndrome, fragile X-associated tremor/ataxia syndrome, and fragile X-associated primary ovarian insufficiency in the United States. Genet Med. 2011; 13:39-45. [DOI] [PubMed] [Google Scholar]
- 7. Tassone F, Iong KP, Tong TH, Lo J, Gane LW, Berry-Kravis E, Nguyen D, Mu LY, Laffin J, Bailey DB, Hagerman RJ. FMR1 CGG allele size and prevalence ascertained through newborn screening in the United States. Genome Med. 2012; 4:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tassone F, Hagerman RJ, Taylor AK, Gane LW, Godfrey TE, Hagerman PJ. Elevated levels of FMR1 mRNA in carrier males: A new mechanism of involvement in the fragile-X syndrome. Am J Hum Genet. 2000; 66:6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tassone F, Beilina A, Carosi C, Albertosi S, Bagni C, Li L, Glover K, Bentley D, Hagerman PJ. Elevated FMR1 mRNA in premutation carriers is due to increased transcription. RNA. 2007; 13:555-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tassone F, Adams J, Berry-Kravis EM, Cohen SS, Brusco A, Leehey MA, Li L, Hagerman RJ, Hagerman PJ. CGG repeat length correlates with age of onset of motor signs of the fragile X-associated tremor/ataxia syndrome (FXTAS). Am J Med Genet B Neuropsychiatr Genet. 2007; 144B:566-569. [DOI] [PubMed] [Google Scholar]
- 11. Hagerman P. Fragile X-associated tremor/ataxia syndrome (FXTAS): Pathology and mechanisms. Acta Neuropathol. 2013; 126:1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Greco CM, Hagerman RJ, Tassone F, Chudley AE, Del Bigio MR, Jacquemont S, Leehey M, Hagerman PJ. Neuronal intranuclear inclusions in a new cerebellar tremor/ataxia syndrome among fragile X carriers. Brain. 2002; 125:1760-1771. [DOI] [PubMed] [Google Scholar]
- 13. Hunsaker MR, Greco CM, Spath MA, Smits AP, Navarro CS, Tassone F, Kros JM, Severijnen LA, Berry-Kravis EM, Berman RF, Hagerman PJ, Willemsen R, Hagerman RJ, Hukema RK. Widespread non-central nervous system organ pathology in fragile X premutation carriers with fragile X-associated tremor/ataxia syndrome and CGG knock-in mice. Acta Neuropathol. 2011; 122:467-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kenneson A, Zhang F, Hagedorn CH, Warren ST. Reduced FMRP and increased FMR1 transcription is proportionally associated with CGG repeat number in intermediate-length and premutation carriers. Hum Mol Genet. 2001; 10:1449-1454. [DOI] [PubMed] [Google Scholar]
- 15. Primerano B, Tassone F, Hagerman RJ, Hagerman PJ, Amaldi F, Bagni C. Reduced FMR1 mRNA translation efficiency in Fragile X patients with premutations. RNA. 2002; 8:1482-1488. [PMC free article] [PubMed] [Google Scholar]
- 16. Tassone F, De Rubeis S, Carosi C, La Fata G, Serpa G, Raske C, Willemsen R, Hagerman PJ, Bagni C. Differential usage of transcriptional start sites and polyadenylation sites in FMR1 premutation alleles. Nucleic Acids Res. 2011; 39:6172-6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chonchaiya W, Au J, Schneider A, Hessl D, Harris SW, Laird M, Mu Y, Tassone F, Nguyen DV, Hagerman RJ. Increased prevalence of seizures in boys who were probands with the FMR1 premutation and co-morbid autism spectrum disorder. Hum Genet. 2012; 131:581-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Paul R, Pessah IN, Gane L, Ono M, Hagerman PJ, Brunberg JA, Tassone F, Bourgeois JA, Adams PE, Nguyen DV, Hagerman R. Early onset of neurological symptoms in fragile X premutation carriers exposed to neurotoxins. Neurotoxicology. 2010; 31:399-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lozano R, Hagerman RJ, Duyzend M, Budiminovic DB, Eichler EE, Tassone F. Genomic Studies in Fragile X Premutation Carriers. J Neurodev Disord. 2014; 6:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li Z, Zhang Y, Ku L, Wilkinson KD, Warren ST, Feng Y. The fragile X mental retardation protein inhibits translation via interacting with mRNA. Nucleic Acids Res. 2001; 29:2276-2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Laggerbauer B, Ostareck D, Keidel EM, Ostareck-Lederer A, Fisher U. Evidence that fragile X mental retardation protein is a negative regulator of translation. Hum Mol Genet. 2001; 10:329-338. [DOI] [PubMed] [Google Scholar]
- 22. Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004; 27:370-377. [DOI] [PubMed] [Google Scholar]
- 23. Paluszkiewicz SM, Martin BS, Huntsman MM. Fragile X syndrome: The GABAergic system and circuit dysfunction. Dev Neurosci. 2011; 33:349-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Heulens I, D'Hulst C, Van Dam D, De Deyn PP, Kooy RF. Pharmacological treatment of fragile X syndrome with GABAergic drugs in a knockout mouse model. Behav Brain Res. 2012; 229:244-249. [DOI] [PubMed] [Google Scholar]
- 25. Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci U S A. 2002; 99:7746-7750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sidorov MS, Auerbach BD, Bear MF. Fragile X mental retardation protein and synaptic plasticity. Mol Brain. 2013; 6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fernandez E, Rajan N, Bagni C. The FMRP regulon: From targets to disease convergence. Front Neurosci. 2013; 7:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fatemi SH, Folsom TD, Kneeland RE, Liesch SB. Metabotropic glutamate receptor 5 upregulation in children with autism is associated with underexpression of both Fragile X mental retardation protein and GABAA receptor beta 3 in adults with autism. Anat Rec (Hoboken). 2011; 294:1635-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Loesch DZ, Huggins RM, Bui QM, Epstein JL, Taylor AK, Hagerman RJ. Effect of the deficits of fragile x mental retardation protein on cognitive status of fragile x males and females assessed by robust pedigree analysis. J Dev Behav Pediatr. 2002; 23:416-423. [DOI] [PubMed] [Google Scholar]
- 30. Fatemi SH, Folsom TD. The role of fragile X mental retardation protein in major mental disorders. Neuropharmacology. 2011; 60:1221-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kovacs T, Kelemen O, Keri S. Decreased fragile X mental retardation protein (FMRP) is associated with lower IQ and earlier illness onset in patients with schizophrenia. Psychiatry Res. 2013; 210:690-693. [DOI] [PubMed] [Google Scholar]
- 32. Aziz M, Stathopulu E, Callias M, Taylor C, Turk J, Oostra B, Willemsen R, Patton M. Clinical features of boys with fragile X premutations and intermediate alleles. Am J Med Genet B Neuropsychiatr Genet. 2003; 121B:119-127. [DOI] [PubMed] [Google Scholar]
- 33. Pretto DI, Mendoza-Morales G, Lo J, Cao R, Hadd A, Latham GJ, Durbin-Johnson B, Hagerman R, Tassone F. CGG allele size somatic mosaicism and methylation in FMR1 premutation alleles. J Med Genet. 2014; 51:309-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dhossche DM, Shah A, Wing L. Blueprints for the assessment, treatment, and future study of catatonia in autism spectrum disorders. Int Rev Neurobiol. 2006; 72:267-284. [DOI] [PubMed] [Google Scholar]
- 35. Chalasani P, Healy D, Morriss R. Presentation and frequency of catatonia in new admissions to two acute psychiatric admission units in India and Wales. Psychol Med. 2005; 35:1667-1675. [DOI] [PubMed] [Google Scholar]
- 36. Mazzone L, Postorino V, Valeri G, Vicari S. Catatonia in patients with autism: Prevalence and management. CNS drugs. 2014; 28:205-215. [DOI] [PubMed] [Google Scholar]
- 37. Jaimes-Albornoz W, Serra-Mestres J. Prevalence and clinical correlations of catatonia in older adults referred to a liaison psychiatry service in a general hospital. Gen Hosp Psychiatry. 2013; 35:512-516. [DOI] [PubMed] [Google Scholar]
- 38. American Psychiatric Association. Desk reference to the diagnostic criteria from DSM-5. American Psychiatric Publishing, Washington DC, USA, 2013; pp.18-45. [Google Scholar]
- 39. Ghaziuddin N, Dhossche D, Marcotte K. Retrospective chart review of catatonia in child and adolescent psychiatric patients. Acta Psychiatr Scand. 2012; 125:33-38. [DOI] [PubMed] [Google Scholar]
- 40. Northoff G. What catatonia can tell us about “top-down modulation”: A neuropsychiatric hypothesis. Behav Brain Sci. 2002; 25:555-577. [DOI] [PubMed] [Google Scholar]
- 41. Carroll BT, Lee JWY, Appiani F, Thomas C. The pharmacotherapy of catatonia. Prim Psychiatry. 2010; 17:41-47. [Google Scholar]
- 42. Wang H, Kim SS, Zhuo M. Roles of Fragile X Mental Retardation Protein in Dopaminergic Stimulation-induced Synapse-associated Protein Synthesis and Subsequent α-Amino-3-hydroxyl-5-methyl-4-isoxazole-4-propionate (AMPA) Receptor Internalization. J Biol Chem. 2010; 285:21888-21901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Utari A, Adams E, Berry-Kravis E, Chavez A, Scaggs F, Ngotran L, Boyd A, Hessl D, Gane LW, Tassone F, Tartaglia N, Leehey MA, Hagerman RJ. Aging in fragile X syndrome. J Neurodev Disord. 2010; 2:70-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jacquemont S, Hagerman RJ, Leehey M, et al. Fragile X premutation tremor/ataxia syndrome: Molecular, clinical, and neuroimaging correlates. Am J Hum Genet. 2003; 72:869-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rosebush PI, Mazurek MF. Catatonia and its treatment. Schizophr Bull. 2010; 36:239-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tsiouris JA, Brown WT. Neuropsychiatric symptoms of fragile X syndrome: Pathophysiology and pharmacotherapy. CNS drugs. 2004; 18:687-703. [DOI] [PubMed] [Google Scholar]
- 47. Jeffries FM, Reiss AL, Brown WT, Meyers DA, Glicksman AC, Bandyopadhyay S. Bipolar spectrum disorder and fragile X syndrome: A family study. Biol Psychiatry. 1993; 33:213-216. [DOI] [PubMed] [Google Scholar]
- 48. Reiss AL, Hagerman RJ, Vinogradov S, Abrams M, King RJ. Psychiatric disability in female carriers of the fragile X chromosome. Arch Gen Psychiatry. 1988; 45:25-30. [DOI] [PubMed] [Google Scholar]
- 49. Hagerman RJ. Physical and behavioral phenotype. In: Fragile X Syndrome: Diagnosis, Treatment, and Research (Hagerman RJ, Hagerman PJ, eds.). Johns Hopkins University Press, Baltimore, Maryland, 2002; pp. 3-109. [Google Scholar]
- 50. Zalfa F, Giorgi M, Primerano B, Moro A, Di Penta A, Reis S, Oostra B, Bagni C. The fragile X syndrome protein FMRP associates with BC1 RNA and regulates the translation of specific mRNAs at synapses. Cell. 2003; 112:317-327. [DOI] [PubMed] [Google Scholar]
- 51. McKinney RA. Physiological roles of spine motility: Development, plasticity and disorders. Biochem Soc Trans. 2005; 33:1299-1302. [DOI] [PubMed] [Google Scholar]
- 52. Schneider A, Seritan A, Tassone F, Rivera SM, Hagerman R, Hessl D. Psychiatric features in high-functioning adult brothers with fragile x spectrum disorders. Prim Care Companion CNS Disord. 2013; 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bourgeois JA, Coffey SM, Rivera SM, Hessl D, Gane LW, Tassone F, Greco C, Finucane B, Nelson L, Berry-Kravis E, Grigsby J, Hagerman PJ, Hagerman RJ. A review of fragile X premutation disorders: Expanding the psychiatric perspective. J Clin Psychiatry. 2009; 70:852-862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wheeler AC, Bailey DB, Jr, Berry-Kravis E, Greenberg J, Losh M, Mailick M, Milà M, Olichney JM, Rodriguez-Revenga L, Sherman S, Smith L, Summers S, Yang JC, Hagerman R. Associated features in females with an FMR1 premutation. J Neurodev Disord. 2014; 6:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hessl D, Tassone F, Loesch DZ, et al. Abnormal elevation of FMR1 mRNA is associated with psychological symptoms in individuals with the fragile X premutation. Am J Med Genet B Neuropsychiatr Genet. 2005; 139B:115-121. [DOI] [PubMed] [Google Scholar]
- 56. Bourgeois JA, Seritan AL, Casillas EM, Hessl D, Schneider A, Yang Y, Kaur I, Cogswell JB, Nguyen DV, Hagerman RJ. Lifetime prevalence of mood and anxiety disorders in fragile X premutation carriers. J Clin Psychiatry 2011; 72:175-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hessl D, Wang JM, Schneider A, Koldewyn K, Le L, Iwahashi C, Cheung K, Tassone F, Hagerman PJ, Rivera SM. Decreased fragile X mental retardation protein expression underlies amygdala dysfunction in carriers of the fragile X premutation. Biol Psychiatry. 2011; 70:859-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tsourtos G, Spong J, Stough C. The effects of electro-convulsive therapy on the speed of information processing in major depression. J Affect Disord. 2007; 103:263-266. [DOI] [PubMed] [Google Scholar]
- 59. First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P). New York: Biometrics Research, New York State Psychiatric Institute, New York, USA, 2002. [Google Scholar]
- 60. Lozano R, Summers S, Lozano C, Mu Y, Hessl D, Nguyen D, Tassone F, Hagerman R. Association Between Macroorchidism and Intelligence in FMR1 Premutation Carriers. Am J Med Genet A. 2014; 164A:2206-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hatton DD, Sideris J, Skinner M, Mankowski J, Bailey DB, Jr, Roberts J, Mirrett P. Autistic behavior in children with fragile X syndrome: Prevalence, stability, and the impact of FMRP. Am J Med Genet A. 2006; 140A:1804-1813. [DOI] [PubMed] [Google Scholar]
- 62. Kaufmann WE, Abrams MT, Chen W, Reiss AL. Genotype, molecular phenotype, and cognitive phenotype: Correlations in fragile X syndrome. Am J Med Genet. 1999; 83:286-295. [PubMed] [Google Scholar]
- 63. Loesch DZ, Huggins RM, Hagerman RJ. Phenotypic variation and FMRP levels in fragile X. Ment Retard Dev Disabil Res Rev. 2004; 10:31-41. [DOI] [PubMed] [Google Scholar]
- 64. Tassone F, Hagerman RJ, Taylor AK, Mills JB, Harris SW, Gane LW, Hagerman PJ. Clinical involvement and protein expression in individuals with the FMR1 premutation. Am J Med Genet. 2000; 91:144-152. [DOI] [PubMed] [Google Scholar]
- 65. Chen Y, Tassone F, Berman RF, Hagerman PJ, Hagerman RJ, Willemsen R, Pessah IN. Murine hippocampal neurons expressing Fmr1 gene premutations show early developmental deficits and late degeneration. Hum Mol Genet. 2010; 19:196-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wing L, Shah A. Catatonia in autistic spectrum disorders. Br J Psychiatry. 2000; 176:357-362. [DOI] [PubMed] [Google Scholar]
- 67. D'Hulst C, Kooy RF. The GABAA receptor: A novel target for treatment of fragile X? Trends Neurosci. 2007; 30:425-431. [DOI] [PubMed] [Google Scholar]
- 68. Braat S, D'Hulst C, Heulens I, De Rubeis S, Mientjes E, Nelson DL, Willemsen R, Bagni C, Van Dam D, De Deyn PP, Kooy RF. The GABAA receptor is an FMRP target with therapeutic potential in fragile X syndrome. Cell Cycle. 2015. (DOI: 10.4161/15384101.2014.989114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. London SJ, Daly AK, Leathart JB, Navidi WC, Carpenter CC, Idle JR. Genetic polymorphism of CYP2D6 and lung cancer risk in African-Americans and Caucasians in Los Angeles County. Carcinogenesis. 1997; 18:1203-1214. [DOI] [PubMed] [Google Scholar]
- 70. Aklillu E, Persson I, Bertilsson L, Johansson I, Rodrigues F, Ingelman-Sundberg M. Frequent distribution of ultrarapid metabolizers of debrisoquine in an ethiopian population carrying duplicated and multiduplicated functional CYP2D6 alleles. J Pharmacol Exp Ther. 1996; 278:441-446. [PubMed] [Google Scholar]
- 71. McLellan RA, Oscarson M, Seidegaård J, Price Evans DA, Ingelman-Sundberg M. Frequent occurrence of CYP2D6 gene duplication in Saudi Arabians. Pharmacogenetics. 1997; 7:187-191. [DOI] [PubMed] [Google Scholar]
- 72. Zanger UM, Raimundo S, Eichelbaum M. Cytochrome P450 2D6: Overview and update on pharmacology, genetics, biochemistry. Naunyn Schmiedebergs Arch Pharmacol. 2004; 369:23-37. [DOI] [PubMed] [Google Scholar]
- 73. Consoli A, Benmiloud M, Wachtel L, Dhossche D, Cohen D, Bonnot O. Electroconvulsive therapy in adolescents with the catatonia syndrome: Efficacy and ethics. J ECT. 2010; 26:259-265. [DOI] [PubMed] [Google Scholar]
- 74. Bush G, Fink M, Petrides G, Dowling F, Francis A. Catatonia. II. Treatment with lorazepam and electroconvulsive therapy. Acta Psychiatr Scand. 1996; 93:137-143. [DOI] [PubMed] [Google Scholar]
- 75. Cohen D, Taieb O, Flament M, Benoit N, Chevret S, Corcos M, Fossati P, Jeammet P, Allilaire JF, Basquin M. Absence of cognitive impairment at long-term follow-up in adolescents treated with ECT for severe mood disorder. Am J Psychiatry. 2000; 157:460-462. [DOI] [PubMed] [Google Scholar]
- 76. Jenkins JK. CDER New Drug Review: 2011 update. http://www.fda.gov/downloads/AboutFDA/CentersOffices/CDER/UCM282984.pdf (accessed July 25, 2015).
- 77. Baker IW, Jackson M, Bass C. Catatonia causing permanent cognitive impairment: A case study. Cogn Behav Neurol. 2005; 18:141-143. [DOI] [PubMed] [Google Scholar]
- 78. Bernard PB, Castano AM, O'Leary H, Simpson K, Browning MD, Benke TA. Phosphorylation of FMRP and alterations of FMRP complex underlie enhanced mLTD in adult rats triggered by early life seizures. Neurobiol Dis. 2013; 59:1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]