Summary>
Reported here is the case of a 55-year-old man who had tarry stools for 3 days before he was seen at this Department. The man had weight loss and an intermittent fever for 3 months prior. Histopathology revealed an inflammatory pseudotumor of the liver. This case is reported here along with a review of the literature. Nine days after surgery, the patient passed bright red blood (150 mL) in the stool with no clear trigger. A colonoscopy a month later revealed no abnormalities. This is a rare report of an inflammatory pseudotumor featuring intractable bleeding. An inflammatory pseudotumor of the liver is a rare condition, and differentiating this pseudotumor from hepatic space-occupying lesions is crucial. An inflammatory pseudotumor of the liver may spontaneously regress and mimic other liver tumors. The treatment of choice for this pseudotumor is still surgical resection, and this is especially true for patients with severe symptoms or an indeterminate diagnosis.
Keywords: Inflammatory pseudotumor (IPT), liver, bleeding
1. Introduction
An inflammatory pseudotumor (IPT) is a rare condition that was first described in the lung in 1939 (1). IPT most commonly occurs in the lung, but it can be found in other locations including the central nervous system, major salivary glands, the kidneys, the liver, the omentum, the ovaries, the larynx, the urinary bladder, the breasts, the pancreas, the spleen, lymph nodes, skin, soft tissues, and the orbit of the eye (2). An IPT of the liver (IPTL) is a rare benign lesion characterized by chronic infiltration of inflammatory cells and an area of fibrosis that sometimes mimics a malignant tumor (3,4). An IPTL was first reported by Pack and Baker in 1953 (5).
The etiology and pathogenesis of IPTL remain unknown. Biologically, there are no specific symptoms or laboratory or radiologic findings that are useful at diagnosing IPTL. Differentiating between IPTs and other focal hepatic lesions remains a major problem. The treatment of choice is still surgical resection, and this is especially true for patients with severe symptoms or an indeterminate diagnosis (6,7).
2. Case Report
This case involved a 55-year-old man who had an unremarkable medical history and tarry stools for 3 days before he was seen at this Department. The man also had weight loss (15 kg) and an intermittent fever for 3 months prior. He had no abdominal pain or night sweats and he presented without jaundice. On admission, a physical examination revealed no signs (“stigmataC”) of chronic liver disease or hepatomegaly. A previous computed tomography (CT) scan showed a well-defined heterogeneous mass 4.0 cm × 4.0 cm in size situated in the left hepatic lobe (Figure 1). On CT, the lesion featured central necrosis, a hyper-dense rim, and mild enhancement starting in the arterial phase, thus corresponding to a hepatic abscess.
Figure 1.
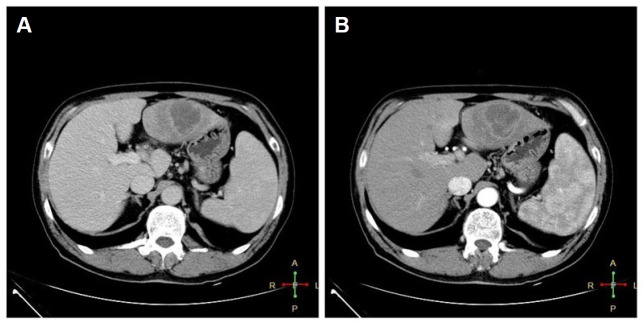
Computed tomography images of the abdomen. (A) A well-defined heterogeneous mass 4.0 cm × 4.0 cm in size was situated in the left hepatic lobe. (B) The lesion featured central necrosis, a hyper-dense rim, and mild enhancement starting in the arterial phase.
Laboratory results revealed an aspartate amino-transferase level of 122 IU/L (normal, 5–40 IU/L), an alanine aminotransferase level of 89 IU/L (normal, 8–40 IU/L), an alkaline phosphatase (ALP) level of 162 IU/L (normal, 40–140 IU/L), a gamma-glutamyl transpeptidase (GGT) level of 159 U/L (normal, 7–40 U/L), an erythrocyte sedimentation rate (ESR) of 45 mm/h (normal, < 7 mm/h), a CEA level of 1.34 ng/mL (normal, < 3.4 ng/mL), AFP level of 3.41 ng/mL (normal, < 7.02 ng/mL), and a CA 19-9 level of 6.64 U/mL (normal, < 39 U/mL). In addition, the patient had negative serologic results for hepatotropic viruses, cytomegalovirus, Epstein-Barr virus (EBV), and HIV 1 and 2. Standardized immunohistochemistry (Xijing Hospital, Fourth Military Medical University, Xi'an, China) showed monoclonal antibodies against broad-spectrum keratin AE/AE3(+), CD138(+), CK19(+), LCA(+), and Hep(−) (Dako, Glostrup, Denmark). Both antibodies reacted positively to cells from the lesion. An ultrasound-guided percutaneous liver biopsy with a tru-cut needle was performed and revealed hepatic tissue with proliferation of spindle-shaped cells mixed with an inflammatory infiltrate of histiocytes, suggesting a diagnosis of IPTL.
After surgery, a specimen of the liver parenchyma was examined histopathologically. The tumor had been completely removed. Microscopy revealed a host of benign cells, including numerous inflammatory cells, mature plasma cells, lymphocytes, eosinophils, and macrophages; most of these cells had xanthomatous changes (Figure 2). The patient received no further treatment. Nine days after surgery, the patient passed bright red blood (150 mL) in the stool with no clear trigger. A colonoscopy a month later revealed no abnormalities. Two months later, the patient's condition was still satisfactory.
Figure 2.
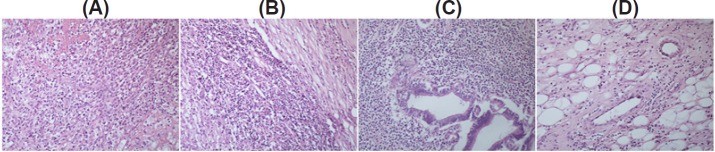
Histology of a surgical specimen. (A) Numerous lymphocytes and plasma cells are evident. (B) Cells mainly consist of inflammatory cells and eosinophils. (C) Shown are lymphocytes and macrophages. (D) Shown are inflammatory and xanthomatous changes. (hematoxylin and eosin, × 200).
3. Discussion
IPT is also known as an inflammatory myofibroblastic tumor or plasma cell granuloma, a xanthomatous pseudotumor, and inflammatory fibrosarcoma (8). Someren classified IPTs into 3 groups according to histology: xanthogranuloma-type pseudotumors, plasma cell granuloma-type pseudotumors, and sclerosing pseudotumors (9). Macroscopically, the lesion may mimic a malignancy and may be alone or several lesions may be present. The lesion may be as large as 25 cm. Microscopically, IPT is characterized by spindle-shaped cells, myofibroblasts, and mixed inflammatory cells (plasma cells, lymphocytes, and sporadic histiocytes). IPTL most often occurs in childhood and early adulthood (10). In adults, the ratio of males to females affected ranges from 1:1 to 3.5:1 (11). IPTL appear to be more common in non-European populations (12). These IPTs are most likely inflammatory or infectious in origin. The lesions often appear to develop from a healing abscess or an inflammatory condition (11). The most common symptoms of IPTL are abdominal pain, a fever, and weight loss. IPTL frequently resolves spontaneously with a good prognosis (13).
IPTL is quite rare and accounts for 8% of extrapulmonary IPTs. The incidence of IPTL is reported to be around 0.7% according to recent studies (14,15). The etiology and pathogenesis of IPTL remain unknown since a variety of tumorous inflammatory lesions lack the features of other IPTs, but IPTL is thought to involve an inflammatory reaction (16). Infectious agents that have been hypothesized to cause IPTL include infections, trauma, vascular causes, and autoimmune disorders (17). Numerous studies have noted that the microorganisms responsible for IPTL include Bacteroides caccae, Actinomyces, Klebsiella, Escherichia coli, Gram-positive cocci, and b-hemolytic Streptococcus (9). Other studies reported that hepatopancreatobiliary autoimmune diseases, such as IgG4 sclerosing cholangitis, could also cause IPTL (18).
The diagnosis of liver pseudotumors is obviously difficult. Ultrasound and CT scans are not specific, revealing variable patterns of echogenicity or a liver mass mimicking hepatocellular cancer or an abscess (19,20). A CT scan usually reveals lesions with variable contrast enhancement. IPTs may display a hypovascular pattern because of fibrosis and also display delayed enhancement, similar to metastatic liver tumors and cholangio-carcinomas (21). MRI may produce low signal intensity (hypointensity) on T1-weighted images with moderate to high signal intensity (hyperintensity) on a T2 sequence (16,21). In general, differentiating IPTs from malignant tumors with radiographic studies is difficult. A definitive diagnosis of IPT can be made based on needle biopsy findings and, occasionally, in-needle aspiration, as long as the pathologist is aware of this possibility.
Biologically, there are no specific symptoms or laboratory or radiologic findings that are useful at diagnosing IPTL. Despite recent increases in the diagnostic capability of radiologic studies, differentiating IPTL from other focal hepatic lesions remains a major problem. Unfortunately, clinical and radiologic features of IPTL can mimic other liver tumors like lymphoma, malignant fibrous histiocytoma, hepatocellular carcinoma, metastatic tumor, tuberculosis, and sarcoidosis and thus lead to surgery (16). If an atypical solid mass is found in the liver, IPTL should be considered as a potential diagnosis, particularly if the mass is accompanied by clinical evidence of an inflammatory process: a recent history of asthenia, malaise, vague upper abdominal discomfort, and/or an intermittent fever; the presence of stigmata of chronic liver disease or splenomegaly; abnormal liver function test results; and a lack of specific imaging findings.
Although liver biopsy indisputably has a role in the investigation and management of liver metastases of unknown origin, its role is more contentious and possibly dangerous in cases of a solitary hepatic mass that is likely to be malignant (22). The main histopathological findings in all cases are the presence of myofibroblastic spindle cells, plasma cells, macrophages, and lymphocytes without cellular atypia or atypical mitotic figures (23). A biopsy of the tumor is not necessary when planning a surgical intervention for the liver.
Furthermore, an optimal treatment for IPTL and a method of determining its prognosis have yet to be established (24,25). Due to its diagnostic ambiguity, the lesion completely resolved in some patients that received antibiotics and/or corticosteroids, but some of these lesions recurred (25). In contrast, numerous studies have reported performing a hepatic resection, mainly due to evidence that the tumor is malignant according to preoperative radiography, after which IPTL never recurred (26).
Even though IPTL may spontaneously regress or regress following antibiotic treatment, the treatment of choice is still surgical resection, and this is especially true for patients with severe symptoms or an indeterminate diagnosis (6,27). Hepatectomy has become a safer option for non-cirrhotic patients over the past 20 years, with mortality converging to 0%. Therefore, the treatment of choice should be surgical resection in such cases (28). This approach is preferable because it minimizes the risk of a biopsy-related complication (dissemination in cases of malignancy) and because it eliminates the possibility of IPT recurring.
In conclusion, IPTL is a rare condition, and differentiating this pseudotumor from hepatic space-occupying lesions is crucial. IPTL may regress spontaneously and it may mimic other liver tumors. The treatment of choice is still surgical resection, and this is especially true for patients with severe symptoms or an indeterminate diagnosis.
References
- 1. Copin MC, Gosselin BH, Ribet ME. Plasma cell granuloma of the lung: Difficulties in diagnosis and prognosis. Ann Thorac Surg. 1996; 61:1477-1482. [DOI] [PubMed] [Google Scholar]
- 2. Hsieh SC, Wu CH, Chan WP. Diagnosis of inflammatory pseudotumour of the urinary bladder aided by dynamic computed tomography. Acta Clin Belg. 2010; 65:360. [DOI] [PubMed] [Google Scholar]
- 3. Faraj W, Ajouz H, Mukherji D, Kealy G, Shamseddine A, Khalife M. Inflammatory pseudo-tumor of the liver: A rare pathological entity. World J Surg Oncol. 2011; 9:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Torzilli G, Inoue K, Midorikawa Y, Hui AM, Takayama T, Makuuchi M. Inflammatory pseudotumors of the liver: Prevalence and clinical impact in surgical patients. Hepatogastroenterology. 2001; 48:1118-1123. [PubMed] [Google Scholar]
- 5. Pack GT, Baker HW. Total right hepatic lobectomy: Report of a case. Ann Surg. 1953; 138:253-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nagarajan S, Jayabose S, McBride W, Prasadh I, Tanjavur V, Marvin MR, Rodriguez-Davalos MI. Inflammatory myofibroblastic tumor of the liver in children. J Pediatr Gastroenterol Nutr. 2013; 57:277-280. [DOI] [PubMed] [Google Scholar]
- 7. Huang YH, Zhong DJ, Tang J, Han JJ, Yu JD, Wang J, Wan YL. Inflammatory myofibroblastic tumor of the liver following renal transplantation. Ren Fail. 2012; 34:789-791. [DOI] [PubMed] [Google Scholar]
- 8. Fragoso AC, Eloy C, Estevão-Costa J, Campos M, Farinha N, Lopes JM. Abdominal inflammatory myofibroblastic tumor a clinicopathologic study with reappraisal of biologic behavior. J Pediatr Surg. 2011; 46:2076-2082. [DOI] [PubMed] [Google Scholar]
- 9. Ntinas A, Kardassis D, Miliaras D, Tsinoglou K, Dimitriades A, Vrochides D. Inflammatory pseudotumor of the liver: A case report and review of the literature. J Med Case Rep. 2011; 5:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Levy S, Sauvanet A, Diebold MD, Marcus C, Da Costa N, Thiefin G. Spontaneous regression of an inflammatory pseudotumor of the liver presenting as an obstructing malignant biliary tumor. Gastrointest Endosc. 2001; 53:371–374.. [DOI] [PubMed] [Google Scholar]
- 11. Agaimy A, Markl B. Inflammatory angiomyolipoma of the liver: An unusual case suggesting relationship to IgG4-related pseudotumor. Int J Clin Exp Pathol. 2013; 6:771-779. [PMC free article] [PubMed] [Google Scholar]
- 12. Schmid A, Janig D, Bohuszlavizki A, Henne-Bruns D. Inflammatory pseudotumor of the liver presenting as incidentaloma: Report of a case and review of the literature. Hepatogastroenterology. 1996; 43:1009-1014. [PubMed] [Google Scholar]
- 13. Yamaguchi J, Sakamoto Y, Sano T, Shimada K, Kosuge T. Spontaneous regression of inflammatory pseudotumor of the liver: Report of three cases. Surg Today. 2007; 37:525-529. [DOI] [PubMed] [Google Scholar]
- 14. Tang L, Lai EC, Cong WM, Li AJ, Fu SY, Pan ZY, Zhou WP, Lau WY, Wu MC. Inflammatory myofibroblastic tumor of the liver: A cohort study. World J Surg. 2010; 34:309-313. [DOI] [PubMed] [Google Scholar]
- 15. Park JY, Choi MS, Lim YS, Park JW, Kim SU, Min YW, Gwak GY, Paik YH, Lee JH, Koh KC, Paik SW, Yoo BC. Clinical features, image findings, and prognosis of inflammatory pseudotumor of the liver: A multicenter experience of 45 cases. Gut Liver. 2014; 8:58-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goldsmith PJ, Loganathan A, Jacob M, Ahmad N, Toogood GJ, Lodge JP, Prasad KR. Inflammatory pseudotumours of the liver: A spectrum of presentation and management options. Eur J Surg Oncol. 2009; 35:1295-1298. [DOI] [PubMed] [Google Scholar]
- 17. Tsou YK, Lin CJ, Liu NJ, Lin CC, Lin CH, Lin SM. Inflammatory pseudotumor of the liver: Report of eight cases, including three unusual cases, and a literature review. J Gastroenterol Hepatol. 2007; 22:2143-2147. [DOI] [PubMed] [Google Scholar]
- 18. Kamisawa T, Okamoto A. IgG4-related sclerosing disease. World J Gastroenterol. 2008; 14:3948-3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Caramella T, Novellas S, Fournol M, Saint-Paul MC, Bruneton JN, Chevallier P. Imaging of inflammatory pseudotumors of the liver. J Radiol. 2007; 88:882-888. [DOI] [PubMed] [Google Scholar]
- 20. Herek D, Karabulut N. Education and imaging. Hepatobiliary and pancreatic: inflammatory pseudotumors of the liver. J Gastroenterol Hepatol. 2011; 26:1217. [DOI] [PubMed] [Google Scholar]
- 21. Brunello F, Caremani M, Marcarino C, Benci A, Menchetti D, Emanuelli G. Inflammatory pseudotumour of the liver: Diagnosis by fine needle biopsy in two cases and a review of the literature. Ital J Gastroenterol. 1994; 26:151-153. [PubMed] [Google Scholar]
- 22. Vicuña-González RM, Rivera-Salgado MI, Garcia-Velarde PM, de León-Bojorge B, Ortiz-Hidalgo C. Multicentric inflammatory pseudotumor with asynchronic presentation in meninges, liver, spleen and lymph nodes in a patient with seronegative spondiloarthropathy. Case report and review of the literature. Rev Neurol. 2008; 47:464-468 (in Spanish) [PubMed] [Google Scholar]
- 23. Iguchi H, Yamazaki H, Tsunoda H, Takahashi Y, Yokomori H. A case of inflammatory pseudotumor of the liver mimicking hepatocellular carcinoma on EOB-MRI and PET. Case Rep Med. 2013; 2013:594254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Di Vita G, Soresi M, Patti R, Carroccio A, Leo P, Franco V, Montalto G. Concomitant inflammatory pseudotumor of the liver and spleen. Liver. 2001; 21:217-222. [DOI] [PubMed] [Google Scholar]
- 25. Salakos C, Nikolakopoulou NM, De Verney Y, Tsamandas AC, Ziambaras T, Petsas T, Papanastasiou DA. Anaplastic lymphoma kinase (ALK) positive inflammatory pseudotumor of the liver: Conservative treatment and long-term follow-up. Eur J Pediatr Surg. 2010; 20:278-280. [DOI] [PubMed] [Google Scholar]
- 26. Koea JB, Broadhurst GW, Rodgers MS, McCall JL. Inflammatory pseudotumor of the liver: Demographics, diagnosis, and the case for nonoperative management. J Am Coll Surg. 2003; 196:226-235. [DOI] [PubMed] [Google Scholar]
- 27. Chablé-Montero F, Angeles-Ángeles A, Albores-Saavedra J. Inflammatory myofibroblastic tumor of the liver. Ann Hepatol. 2012; 11:708-709. [PubMed] [Google Scholar]
- 28. Imamura H, Seyama Y, Kokudo N, Maema A, Sugawara Y, Sano K, Takayama T, Makuuchi M. One thousand fifty-six hepatectomies without mortality in 8 years. Arch Surg. 2003; 138:1198-1206 discussion 1206. [DOI] [PubMed] [Google Scholar]


