Abstract
Background
Lung is a common organ of metastases in patients with primary breast cancer. Pulmonary metastasis of primary breast cancer is usually considered as a systemic disease, however, the systemic approaches have achieved little progress in terms of prolonging survival time. In contrast, some studies revealed a probable survival benefit of pulmonary metastasectomy for such patients. However, the prognostic factor for pulmonary metastasectomy in breast cancer patients is still a controversial issue. The aim of this study was to conduct a systematic review and meta-analysis of cohort studies to assess the pooled 5-year overall survival (OS) rate and the prognostic factors for pulmonary metastasectomy from breast cancer.
Methods
An electronic search in MEDLINE (via PubMed), EMBASE (via OVID), CENTRAL (via Cochrane Library), and Chinese BioMedical Literature Database (CBM) complemented by manual searches in article references were conducted to identify eligible studies. All cohort studies in which survival and/or prognostic factors for pulmonary metastasectomy from breast cancer were reported were included in the analysis. We calculated the pooled 5-year survival rates, identified the prognostic factors for OS and combined the hazard ratios (HRs) of the identified prognostic factors.
Results
Sixteen studies with a total of 1937 patients were included in this meta-analysis. The pooled 5-year survival rates after pulmonary metastasectomy was 46% [95% confidence interval (95% CI): 43-49%]. The poor prognostic factors were disease-free interval (DFI) (<3 years) with HR =1.70 (95% CI: 1.37-2.10), resection of metastases (incomplete) with HR =2.06 (95% CI: 1.63-2.62), No. of pulmonary metastases (>1) with HR =1.31 (95% CI: 1.13-1.50) and the hormone receptor status of metastases (negative) with HR =2.30 (95% CI: 1.43-3.70).
Conclusions
Surgery with a relatively high 5-year OS rate after pulmonary metastasectomy (46%), may be a promising treatment for pulmonary metastases in the breast cancer patients with a good performance status and limited disease. The main poor prognostic factors were DFI (<3 years), resection of metastases (incomplete), No. of pulmonary metastasis (>1) and hormone receptor status of metastases (negative). And prospective randomized trials will be needed to address these issues in the future.
Keywords: Breast cancer, pulmonary metastases, prognostic factors, systematic review, meta-analysis
Introduction
Breast cancer is the most frequently diagnosed cancer and the leading cause of cancer death among females, accounting for 23% of the total cancer cases and 14% of the cancer deaths (1). In Asia, one in every three women faces the risk of breast cancer in their lifetime as per report of WHO 2012. Distant metastases appear in 20% of women who have primary breast cancer (2). And lung is a common organ of metastases in patients with primary breast cancer, with approximately 12% of the breast cancer patients found to have metastatic lesions in the lung (3).
Pulmonary metastasis of primary breast cancer is usually considered as a systemic disease, so most medical oncologists disapprove of surgical procedures for metastatic breast cancer or consider them just as palliative strategies (4). Only systemic treatments are routinely considered for such patients. Yet recently, the systemic approaches even with taxanes and/or anthracycline have achieved little progress in terms of prolonging survival time (5,6). High-dose chemotherapy with stem-cell transplantation has also failed to prolong life expectancy (7). The median survival after chemotherapy remains at about 24 months, and the cure rate is very low (8). Thus, metastatic breast cancer is still regarded as an incurable disease, and the treatment is usually just palliative (9). However, several studies revealed a probable survival benefit of pulmonary metastasectomy for such patients. In the study by Staren et al., which compared the medical and surgical management of pulmonary metastatic disease, the mean 5-year survival of the medically treated group was only 11% as comparing to the surgical group, whose mean 5-year survival was 36% (10). A significantly increased survival for the patients operated on comparing with the patients treated conservatively was also demonstrated by Meimarakis et al. (11) and Yhim et al. (12) in their respective studies. At the same time, with the technological advances in cardiothoracic surgery and anesthesia, the pulmonary metastasectomy is associated with a low perioperative morbidity and lethality now (11,13). Consequently, pulmonary metastasectomy may be a promising treatment for lung metastases of breast cancer. However, the prognostic factor of pulmonary metastasectomy in breast cancer patients is still a controversial issue.
In the current study, we conducted a systematic review and meta-analysis of cohort studies to assess the pooled 5-year overall survival (OS) rate and the prognostic factors for survival in pulmonary metastasectomy from breast cancer, which may help the clinicians to evaluate the potential surgical benefits of the patients with different prognostic factors and make clinical decisions.
Materials and methods
This systematic review and meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement protocol (14).
Search strategy
We searched MEDLINE (via PubMed), EMBASE (via OVID), CENTRAL (via Cochrane Library), and Chinese BioMedical Literature Database (CBM) to October 2014 to identify studies relevant to this review. Our search strategy included the following subject headings and/or key words variably combined by ‘‘breast neoplasm’’, ‘‘lung metastasis’’, and ‘‘surgery’’. The detailed search strategy of PubMed is listed here: ((((“breast neoplasms”[MeSH Terms]) OR breast cancer*) OR breast tumor*) OR breast carcinoma*) AND (("lung neoplasms/secondary"[MeSH Terms]) OR lung metastas*) AND ((“surgical procedures, operative”[MeSH Terms]) OR resect*). In addition, reference lists of the articles initially detected were searched by hand to identify additional relevant reports. The eligibility of references retrieved by the search was assessed independently by two of the authors (Jun Fan and Dali Chen), and the review authors resolved differences of opinion by discussion or by appeal to a third review author (Heng Du) when necessary. The full text of the remaining articles, including the references, was examined to determine whether the articles contained relevant information.
Inclusion and exclusion criteria
Studies were considered eligible if they met all of the following inclusion criteria: (I) studies on surgery for pulmonary metastases from breast cancer; (II) enrolled patients whose primary breast tumors had been resected completely proven by histopathology and metastatic disease was limited to the lung proven by imaging techniques; (III) 5-year survival rate and prognostic factors for patients after pulmonary metastasectomy were reported in the individual studies. Studies were excluded based on any of the following criteria: (I) the following article types were excluded: reviews, letters, laboratory researches, animal experiments; (II) the language is not English or Chinese; (III) the articles studied on the lung nodules(not only the lung metastases) in breast cancer patients.
Quality assessment
Quality assessment of individual studies was performed independently by two of the authors (Jun Fan and Dali Chen), using the Newcastle-Ottawa Scale for cohort studies. The scale allocates stars (maximum of 9) for quality of selection, comparability and outcome of study participants (15). Any discrepancies were addressed by joint reevaluation of the Original Article.
Data extraction
Data were extracted from the selected studies independently by two of the authors (Jun Fan and Dali Chen), using a predefined standardized form and resolved disagreements by discussion between two review authors or by appeal to a third review author (Cheng Shen). We extracted data of the eligible articles’ basic characteristics, including first author (year), country, study period, type of study, No. of patients, No. of patients evaluated survival and prognostic factors after pulmonary resection, median follow-up, median/mean age at pulmonary metastasectomy, median disease-free interval (DFI) which is the interval between surgery for the primary breast cancer and the appearance of pulmonary metastases, median OS time after pulmonary metastasectomy, 5-year survival rate and prognostic factors. Moreover, the original data included the Kaplan-Meier (K-M) survival curves or hazard ratio (HR) and 95% confidence interval (95% CI) of survival outcomes. The K-M survival curves’ data was extracted by Engauge Digitizer 4.1 (http://sourceforge.net) and the HR, which couldn’t be gotten directly from the individual studies, was estimated by the methods of Tierney et al. (16).
Outcome assessment
The analysis focused on assessing the pooled 5-year survival and the prognostic factors for OS in the patients who underwent pulmonary metastasectomy from breast cancer.
Data analysis and statistical analysis
Prognostic factors associated with outcome were extracted from all cohorts. A prognostic association was considered significant if the reported P value was less than 0.05, if the article reported that an association was significant, or if the 95% CI around a rate ratio or similar statistic did not cross 1. Only prognostic factors that were assessed via univariate analyses (log-rank test) are presented, this decision was taken due to the different statistical techniques and choice of covariates used in the individual multivariate models. We decided that the collective interpretation of factors drawn from different multivariate models may be potentially misleading.
To assess the pooled 5-year survival rate, proportions were Log transformed to satisfy the normality. And then we calculated the pooled 5-year survival rate with 95% CI by the DerSimonian & Laird method. When the 5-year survival rate and/or 95% CI was not available, these values were estimated according to data obtained from the K-M curve, using an actuarial method. We also carried out meta-analysis on the prognostic factors for OS using HR as a statistic. Heterogeneity was quantified using a chi-square heterogeneity statistic and by means of an I2 statistic for each analysis. Heterogeneity was defined as P<0.10 or I2>70%.When homogeneity was fine (P≥0.10; I2≤70%), a fixed effect model was used to combine effective sizes or else, a random effect model was used. Meta-regression was used to assess the impact of publish year and the prognostic factors on the 5-year survival rate. The potential publication bias was evaluated by the symmetry of funnel and Egger’s test, with P>0.05 indicating no potential publication bias (17). All analyses were performed using R software and the metafor package (18). P values less than 0.05 were considered significant.
Results
Reference retrieval
After primary retrieval, a total of 2,207 potentially relevant studies were incorporated into our initial study, including 746 in Medline, 1,369 in Embase, 47 in CBM, 40 in CENTRAL and 5 by reference list. A total of 403 were excluded for duplicates and 1,756 were excluded by title/abstract screening. Full texts were retrieved for the remaining 48 studies, and 16 of them met all the criteria for inclusion in the analysis, which included 1,937 patients (Figure 1, Table 1). All of the patients were female except two, which were excluded from this analysis.
Figure 1.
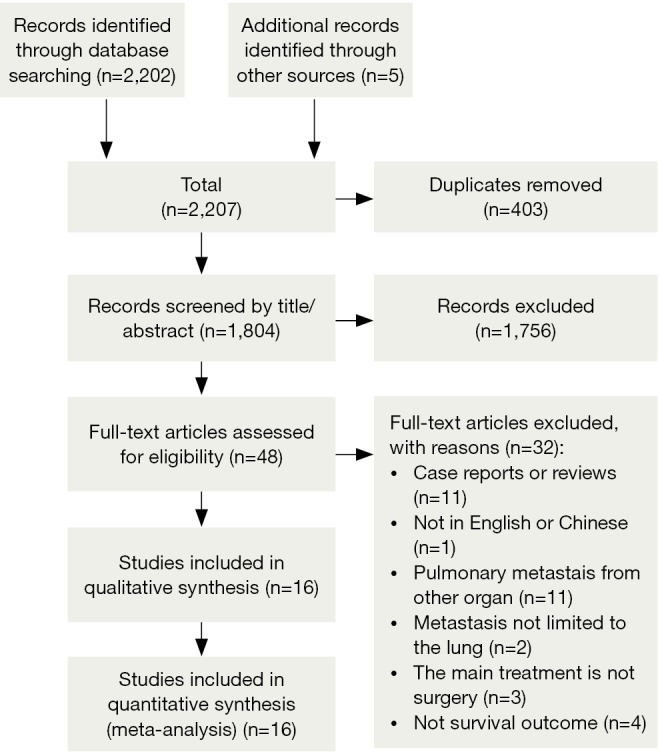
PRISMA Flow Chart of Reference Retrieval.
Table 1. Basic characteristics of included studies.
| Author (reference) | Country | Study period | Type of study | N | n | Age [year] | Follow-up [months] | DFI [months] | Median OS (months) | 5-year OS rate (%) | Prognostic factors, No. (%) |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DFI (<3 y) | Resection of meta* (incomplete) | No. of meta (>1) | HR of meta (negative) | |||||||||||
| Chen et al., 2009 (19) | Japan | 1991-2007 | Retrospective | 41 | 41 | 55 [35-81] | 25 [2-142] | 55 [0-192] | NR | 51 | 15 (36.6) | 0 (0) | 21 (51.2) | NR |
| Ludwig et al., 2003 (20) | Germany | 1989-1998 | Retrospective | 25 | 21 | 54 [35.3-77.3] | NR | 99.2 [13.4-165.8] | 96.9 | 53 | NR | 0 (0) | 9 (42.9) | NR |
| Yoshimoto et al., 2008 (21) | Japan | 1960-2000 | Retrospective | 90 | 90 | 55.1 [32-77] | 79.2 | 67.2 [0-244.8] | 75.6 | 54 | 33 (36.6) | 10 (11.1) | 12 (13.3) | NR |
| Lanza et al., 1992 (22) | USA | 1981-1990 | Retrospective | 44 | 37 | 55 [32-79] | NR | NR | 47 | 49.50 | NR | 0 (0) | 10 (27.0) | 15 (51.7) |
| Yhim et al., 2010 (12) | Korea | 1997-2007 | Retrospective | 45 | 15 | 49 [36-65] | 50 [5.0-136] | NR | NR | 82.10 | NR | 0 (0) | 4 (26.7) | 10 (66.7) |
| Meimarakis et al., 2013 (23) | Germany | 1982-2007 | Prospective | 81 | 81 | 58.2 [28.3-76.3] | NR | NR | NR | 59.60 | NR | 15 (18.5) | 30 (37.0) | 14 (31.1) |
| Welter et al., 2008 (24) | Germany | 1998-2007 | Retrospective | 47 | 47 | 55.0 [34.7-80] | 20.6 [3.2-110] | 44.4 [0-309.6] | 32 | 36 | 18 (38.3) | 14 (29.8) | 18 (38.3) | 25 (56.8) |
| Staren et al., 1992 (10) | USA | 1972-1990 | Retrospective | 63 | 33 | 50 | NR | 48 | 55 | 36 | NR | NR | 6 (18.2) | NR |
| McDonald et al., 1994 (25) | USA | 1982-1992 | Retrospective | 60 | 59 | 58 [21-81] | 42 [1-128.4] | 26.4 [1-247.2] | 42 | 37.80 | NR | 20 (33.9) | 28 (47.5) | NR |
| Simpson et al., 1997 (26) | Australia | 1984-1996 | Retrospective | 17 | 17 | 59 [40-74] | NR | 61.2 [8-218.4] | NR | 62 | 8 (47.1) | NR | 3 (18.8) | NR |
| Friedel et al., 2002 (27) | International | 1960-1994 | Retrospective | 467 | 467 | 53 [21-87] | 34 [0-240] | 43 | 35 | 35 | 181 (39.0) | 75 (16.1) | 148 (32.5) | NR |
| Tanaka et al., 2005 (28) | Japan | 1992-2001 | Retrospective | 52 | 39 | 53.7 [35-75] | NR | 66.8 [11.5-104.8] | 32 | 30.80 | NR | NR | 24 (61.5) | NR |
| Kycler and Laski, 2012 (29) | Poland | 1994-2002 | Retrospective | 33 | 33 | 53.4 [33-75] | 71.3 [0-232] | 51.9 | 73.2 | 54.50 | 16 (48.5) | 18(54.5) | 19 (57.6) | 20 (60.6) |
| Rena et al., 2007 (30) | Italy | 1990-2003 | Retrospective | 79 | 25 | 63 [42-82] | 43 [3-190] | NR | NR | 38 | 10 (40.0) | NR | NR | NR |
| Friedel et al., 1994 (31) | USA | 1979-1992 | Retrospective | 91 | 89 | 53 [23-78] | NR [2-144] | NR | 31 | 27 | NR | 21(23.6) | 28 (41.2) | 19 (57.6) |
| Planchard et al., 2004 (32) | France | 1972-1998 | Retrospective | 162 | 125 | 53 [30-82] | 102 [1-264] | 36 [2-252] | 50.4 | 45 | 65 (52.0) | 29(23.2) | 35 (28.0) | 25 (52.1) |
N, No. of patients; n, No. of analyzing survival and prognostic factors after pulmonary resection; median OS, median overall survival time after pulmonary metastasectomy; 5-year OS rate, 5-year overall survival rate; DFI, disease-free interval, between surgery for the primary tumor and the appearance of pulmonary metastases; meta, metastases; HR, hormone receptor, which includes estrogen receptor (ER) and progesterone receptor (PR), and only when both of them are negative, HR is negative; NR, no refer; *, complete resection was defined as no tumor cells at the surgical margin of the resected lung, which were examined macroscopically and histologically.
Characteristics and qualities of the included studies
Basic characteristics of included studies were summarized in Table 1. All of the 16 studies were cohort studies, in which only one study (23) was prospective, and the other 15 studies were retrospective. Eleven studies were published after 2000, and five studies were published in the 1990s. Nine studies analyzed survival and prognostic factors after pulmonary resection in only part of the enrolled patients, for the reasons of loss to follow-up or the patients with non-metastatic pulmonary nodules (benign pulmonary nodules or primary lung cancer). The median/mean age at pulmonary metastasectomy of included studies ranged from 49 to 63 years old. Reported median survival in these series was 31-96.9 months. Most of the median follow-up of the involved studies were short, and only in three studies, the median follow-up was more than 5 years. And there were seven studies which were conducted with a sample size less than 50 patients.
Quality assessments of individual studies were shown in Table 2. We used the Newcastle-Ottawa Scale (NOS) for cohort studies to assess included studies, which included three aspects (selection, comparability and outcome) and eight items. Fifteen studies scored not less than 6.
Table 2. Quality assessment of individual studies using the Newcastle-Ottawa Scale (NOS) for cohort studies.
| Study | Selection |
Comparability |
Outcome |
Score | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a |
b |
c |
d |
e |
f |
g |
h |
|||||||||||||||||||||
| a1* | a2* | a3 | a4 | b1* | b2 | b3 | c1* | c2* | c3 | c4 | d1* | d2 | e1* | e2* | f1* | f2* | f3 | f4 | g1* | g2 | h1* | h2* | h3 | h4 | ||||
| Chen et al., 2009 (19) | * | * | * | * | * | * | * | 7 | ||||||||||||||||||||
| Ludwig et al., 2003 (20) | * | * | * | * | * | * | 6 | |||||||||||||||||||||
| Yoshimoto et al., 2008 (21) | * | * | * | * | * | * | * | 7 | ||||||||||||||||||||
| Lanza et al., 1992 (22) | * | * | * | * | * | * | 6 | |||||||||||||||||||||
| Yhim et al., 2010 (12) | * | * | * | * | * | * | * | 7 | ||||||||||||||||||||
| Meimarakis et al., 2013 (23) | * | * | * | * | * | * | * | * | 8 | |||||||||||||||||||
| Welter et al., 2008 (24) | * | * | * | * | * | * | 6 | |||||||||||||||||||||
| Staren et al., 1992 (10) | * | * | * | * | * | * | 6 | |||||||||||||||||||||
| McDonald et al., 1994 (25) | * | * | * | * | * | * | 6 | |||||||||||||||||||||
| Simpson et al., 1997 (26) | * | * | * | * | * | * | * | 7 | ||||||||||||||||||||
| Friedel et al., 2002 (27) | * | * | * | * | * | * | * | 7 | ||||||||||||||||||||
| Tanaka et al., 2005 (28) | * | * | * | * | * | 5 | ||||||||||||||||||||||
| Kycler and Laski, 2012 (29) | * | * | * | * | * | * | * | 7 | ||||||||||||||||||||
| Rena et al., 2007 (30) | * | * | * | * | * | * | 6 | |||||||||||||||||||||
| Friedel et al., 1994 (31) | * | * | * | * | * | * | * | 7 | ||||||||||||||||||||
| Planchard et al., 2004 (32) | * | * | * | * | * | * | * | 7 | ||||||||||||||||||||
a, representativeness of the exposed cohort: a1, truly representative; a2, somewhat representative; a3, selected group of users; a4, no description of the derivation of the cohort. b, selection of the non-exposed cohort: b1, drawn from the same community as the exposed cohort; b2, drawn from a different source; b3, no description of the derivation of the non-exposed cohort. c, ascertainment of exposure: c1, secure record; c2, structured interview; c3, written self-report; c4, no description. d, demonstration that outcome of interest was not present at start of study: d1, yes; d2, no. e, comparability of cohorts on the basis of the design or analysis: e1, study controls for the most important factor; e2, study controls for any additional factor. f, assessment of outcome: f1, independent blind assessment; f2, record linkage; f3, self-report; f4, no description. g, was follow-up long enough for outcomes to occur: g1, yes; g2, no. h, adequacy of follow up of cohorts: h1, complete follow up—all subjects accounted for; h2, subjects lost to follow up unlikely to introduce bias—small number lost >80% follow-up, or description provided of those lost; h3, follow-up rate <80% and no description of those lost; h4, no statement.
Prognostic factors for OS
There were fifteen prognostic factors which were summarized in Table 3. The most frequently reported significant prognostic factors were DFI (nine studies), resection of metastases (four studies), No. of pulmonary metastases (three studies) and the hormone receptor status of metastases (three studies). And the number and proportion of the patients with the four main prognostic factors in respective studies were shown in Table 1. None of the involved studies reported there was a significant association between the OS and the following prognostic factors, which included additional adjuvant therapy, age at metastasectomy, type of surgical procedures, nodal status of primary tumor, other recurrence site before lung surgery, histologic grade of breast cancer. Among all of the fifteen prognostic factors, three prognostic factors (Stage of breast cancer at breast surgery, Nodal status of primary tumor, Histologic grade of breast cancer) were related to the primary breast cancer. However, there is only one study which reported that there was a significant association between the stage of breast cancer at breast surgery and survival.
Table 3. Prognostic factors for which univariate analyses were reported in at least two cohorts.
| Prognostic factors | Number/significant* |
|---|---|
| DFI | 15/9 (12,19,21,22,26,27,29,30,32) |
| <5 years | 1/0 |
| <3 years | 8/7 (19,21,26,27,29,30,32) |
| <2 years | 5/1 (12) |
| <1 year | 1/1 (22) |
| Resection of metastases (incomplete vs. complete) | 8/4 (23,27,29,31) |
| No. of pulmonary metastases (multiple vs. solitary) | 14/3 (19,23,25) |
| The HR status of metastases (negative vs. positive) | 9/3 (12,23,24) |
| Size of the largest metastasis (≥2 vs. <2 cm) | 6/2 (23,32) |
| Site of metastases (bilateral vs. unilateral) | 6/2 (19,29) |
| Stage of breast cancer at breast surgery | 5/1 (21) |
| No. of metastasectomies (redo vs. primary) | 3/1 (29) |
| Pleural invasion (yes vs. no) | 2/1 (23) |
| Additional adjuvant therapy | 6/0 |
| Age at metastasectomy | 6/0 |
| Type of surgical procedures | 4/0 |
| Nodal status of primary tumor | 4/0 |
| Other recurrence site before lung surgery | 2/0 |
| Histologic grade of breast cancer | 2/0 |
*, number of cohorts in which the factor was reported/number of cohorts in which a significant association with poor outcome was reported (log-rank test, α<0.05). DFI, disease-free interval.
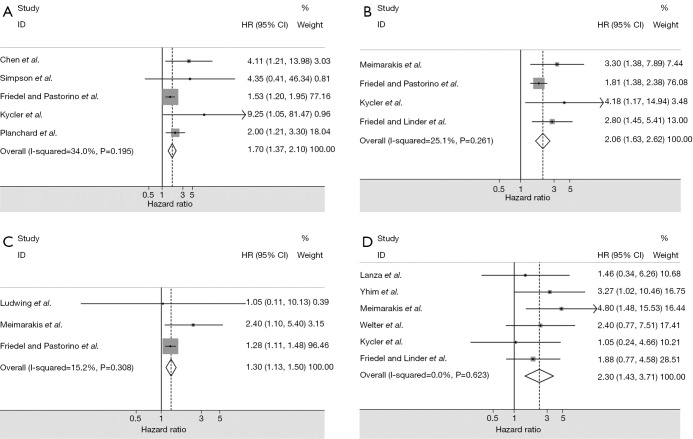
The four main prognostic factors for OS and the pooled HRs were shown in Table 4 and Figure 2. All of the four main prognostic factors had significant associations with the OS and the pooled HR values ranged from 1.31 to 2.30. And there were no significant heterogeneities found in all of the four meta-analyses, so we choose the fixed effect model to do the meta-analyses.
Table 4. Main prognostic factors for survival and the pooled hazard ratios.
| Prognostic factors | N | Patients | Heterogeneity (I-squared, P) | Model | Hazard ratio (95% CI) | P value |
|---|---|---|---|---|---|---|
| DFI (<3 years) | 5 | 683 | 34.0%, 0.195 | Fixed | 1.70 (1.37-2.10) | 0.000 |
| Resection of metastases (incomplete) | 4 | 670 | 25.1%, 0.261 | Fixed | 2.06 (1.63-2.62) | 0.000 |
| No. of pulmonary metastases (>1) | 3 | 569 | 15.2%, 0.308 | Fixed | 1.31 (1.13-1.50) | 0.000 |
| The hormone receptor status of metastases (−) | 6 | 302 | 0.0%, 0.623 | Fixed | 2.30 (1.43-3.70) | 0.001 |
N, reference count; DFI, disease-free interval.
Figure 2.
Evaluated hazard ratios summary for: (A) DFI (<3 years) for overall survival; (B) resection of metastases (incomplete) for overall survival; (C) No. of pulmonary metastases (>1) for overall survival; (D) hormone receptor status of metastases (negative) for overall survival. DFI, disease-free interval.
5-year OS rate
The pooled 5-year survival rate was 46% (95% CI: 43-49%) (Figure 3). And the test for heterogeneity revealed significant heterogeneity (I2=78.5%, P<0.001). Therefore, we assessed whether the study year and the four main prognostic factors could explain the heterogeneity by meta-regression. We found that there was a significant association between the study year and the 5-year survival rate shown by univariable meta-regression analysis (P=0.0323). And no significant associations were found between the four main prognostic factors and 5-year survival rate (Table 5).
Figure 3.
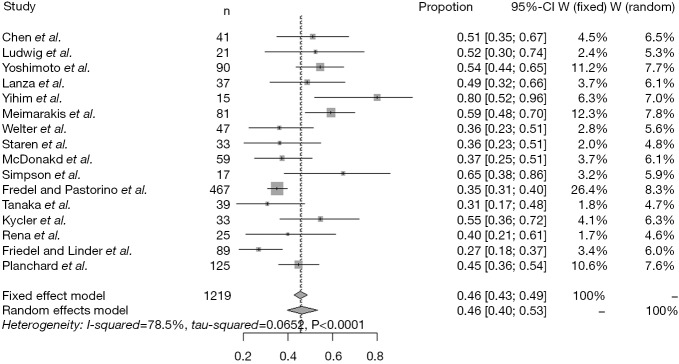
Forest plots showing 5-year survival in each study. Each square represents an individual survival, with the size of the square being proportional to the weight given to the study. The dotted and dashed vertical lines represent combined survival for the whole population.
Table 5. Relationship between the four main prognostic factors and 5-year survival.
| Characteristics | 5-year survival |
|
|---|---|---|
| Coefficienta | P valueb | |
| Study year | 0.0204 | 0.0323 |
| DFI (<3 years) | 0.8933 | 0.5399 |
| Resection of metastases (incomplete) | −0.6099 | 0.2474 |
| No. of pulmonary metastases (>1) | −0.6493 | 0.2471 |
| The hormone receptor status of metastases (−) | −0.0381 | 0.9772 |
a, coefficient represents a linear relationship between each prognostic factor and the survival outcome by a univariable meta-regression model. The sign of the coefficient indicates the positivity or negativity of the relationship. b, the P value (<0.1) of each coefficient indicates whether the linear correlation was significant or not.
Assessment of publication bias
We assessed the publication bias of the meta-analysis in 5-year survival rate. The funnel plot (Figure 4) was drawn with log of proportions along the horizontal axis and standard error along the vertical axis. We didn’t find a significant asymmetry in the funnel plot by Egger’s test (P=0.7879). So there is no publication bias for meta-analysis in 5-year survival rate.
Figure 4.
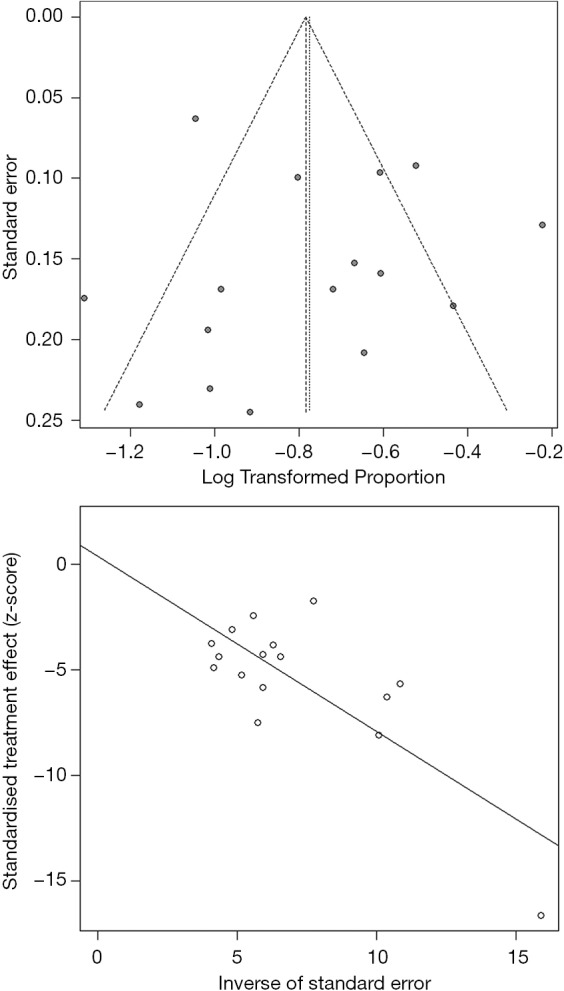
Funnel plot and Egger’s test for evaluation of the publication bias for 5-year survival rate.
Discussion
To our knowledge, it is the first time that a comprehensive and detailed systematic review and meta-analysis is performed to evaluate the survival and prognostic factors for resection of isolated pulmonary metastases in breast cancer patients. We discussed the prognostic factors and their associations with the OS. At the same time, we analyzed the pooled 5-year survival rate and the association between the prognostic factors and 5-year survival.
Pulmonary metastasectomy has become a standard therapy for various metastatic malignancies of the lungs. It has been reported that surgery of lung metastases of almost all solid tumors provided good long-term results (33). And the International Registry of Lung Metastases reports on a 5-year survival of 36% in 4,572 patients having resection of lung metastases (34). However, it is a controversial issue for lung resection in breast cancer. In the current analysis, the pooled 5-year OS rate after pulmonary metastasectomy in the breast cancer patients is 46%. In contrast, the 5-year survival was 16% in a case series of patients with breast cancer metastases limited to the lungs and treated with chemotherapy only (35). This result implies that surgery is a promising treatment for the pulmonary metastases in breast cancer patients. The potential survival benefit may be gotten from the reduction of the tumor burden and tumor heterogeneity by resection of pulmonary metastases. However, it should be emphasized that surgical candidates represented a highly selected population with a good performance status and limited disease. Furthermore, in addition to the primary surgical treatment, almost all of these patients in respective studies more or less received some chemotherapy or/and endocrine therapies. So the interpretation of the result should be prudent. In spite of that, at least this result could imply a trend that some selected breast cancer patients may get some survival benefits from the resection of pulmonary metastases.
Previous researches provided inconsistent findings as to the prognostic factors in the patients undergoing pulmonary metastasectomy from the breast cancer. In the present analysis, we extracted all of the prognostic factors from the sixteen involved studies, which univariate analyses were reported in at least two studies, and counted the number of times that a significant association was reported (Table 3). We found that DFI >3 years, complete resection of metastases, solitary pulmonary metastasis and positive hormone receptor status of metastases were most frequently reported as the significant good prognostic factors. So patients with these good prognostic factors may survive longer after pulmonary metastasectomy. And then we analyzed the magnitude of the association between the four main prognostic factors and survival by the pooled HRs (Table 4). We found that the hormone receptor status of metastases with the pooled HR 2.30 is the strongest prognostic factors among the four main factors. It may be because that the positive hormone receptor represents a slow-growing tumor biology with less aggressive clinical feature and these patients had a better response to the endocrine treatment. It is interesting that the DFI (<3 years), which was reported as a significant prognostic factor in 7 of 8 studies, didn’t have a very strong association with survival (the pooled HR 1.70). Consequently, although there were so many cohorts reporting DFI (<3 years) as a significant factor, the cut-off of DFI, 3 years, maybe was not adequate, for there were some studies choosing 1 year, 2 years or 5 years as their cut-offs. A further study would be needed to address this issue. Another interesting thing we found was that none of the four main prognostic factors would significantly affect the 5-year survival rate. However, it should be noted that there were some missing values in this analysis, which maybe also led to this result. So we should interpret this result cautiously.
With the development of imaging techniques, such as multidetector-row computed tomography (MDCT) and positron emission tomography (PET), we can detect earlier and smaller lung nodules in the breast cancer patients. So more and more breast cancer patients were detected with a lung nodules after mastectomy. However, the histological types of the lung nodules may be pulmonary metastases, primary lung cancers or benign lesions (28,30). The diagnosis of the histological types of lung nodules is crucial to the following therapies. Imaging examinations may be sometimes only indicative but not diagnostic. Fine-needle aspiration biopsy using CT-guidance or trans-bronchial needle aspiration biopsy is available to diagnose the lung nodules. However, when the lung nodule is small or its location is not favourable for diagnostic sampling, the biopsy may fail to diagnose. In that case, surgery, especially video-assisted thoracic surgery, is a useful modality to confirm pathologic diagnosis of pulmonary nodules in breast cancer patients and guide the clinicians to choose the following therapies.
Our study has several limitations. The findings of a meta-analysis depend on the qualities of the individual studies, as their potential problems and biases may affect the pooled effects. According to the quality assessment by NOS, fourteen of the sixteen involved studies scored 6 or 7, which indicated the qualities of most of the studies were only moderate. And there were seven individual studies conducted with a sample less than 50 patients and fifteen of sixteen cohorts were retrospective studies. At the same time, most studies confirmed the diagnosis of lung metastasis only according to the inpatient medical records or the records in clinical cases database. Meanwhile, some prognostic factors were assessed in only a few studies, which may also weaken the role of meta-analysis. And we didn’t search unpublished and grey literature database, which may lead to a potential publication bias. Maybe there is also a language bias, for we only screened the literature in English and Chinese.
In conclusion, surgery with a relatively high 5-year OS rate after pulmonary metastasectomy (46%), may be a promising treatment for the breast cancer patients with a good performance status and limited disease, who were diagnosed as isolated pulmonary metastases. For the breast cancer patients with pulmonary nodules, who failed to be diagnosed by biopsy, surgery is an available method to confirm the pathologic diagnosis. At the same time, the main poor prognostic factors for resection of isolated pulmonary metastases in breast cancer patients were DFI (<3 years), resection of metastases (incomplete), No. of pulmonary metastasis (>1) and hormone receptor status of metastases (negative). And prospective randomized trials will be needed to address these issues in the future.
Acknowledgements
We acknowledge Jing Bai, of the Department of Cardiovascular Medicine of the First Affiliated Hospital of Zhengzhou University for her careful revision of the manuscript.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [DOI] [PubMed] [Google Scholar]
- 2.Chlebowski RT, Kuller LH, Prentice RL, et al. Breast cancer after use of estrogen plus progestin in postmenopausal women. N Engl J Med 2009;360:573-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kreisman H, Wolkove N, Finkelstein HS, et al. Breast cancer and thoracic metastases: review of 119 patients. Thorax 1983;38:175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cardoso F, Harbeck N, Fallowfield L, et al. Locally recurrent or metastatic breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012;23 Suppl 7:vii11-9. [DOI] [PubMed] [Google Scholar]
- 5.Sledge GW, Jr. Should we dream the impossible dream? The meaning of long-term survival in metastatic breast cancer. J Clin Oncol 1996;14:2191-3. [DOI] [PubMed] [Google Scholar]
- 6.Giordano SH, Buzdar AU, Smith TL, et al. Is breast cancer survival improving? Cancer 2004;100:44-52. [DOI] [PubMed] [Google Scholar]
- 7.Gerrero RM, Stein S, Stadtmauer EA. High-dose chemotherapy and stem cell support for breast cancer: where are we now? Drugs Aging 2002;19:475-85. [DOI] [PubMed] [Google Scholar]
- 8.Greenberg PA, Hortobagyi GN, Smith TL, et al. Long-term follow-up of patients with complete remission following combination chemotherapy for metastatic breast cancer. J Clin Oncol 1996;14:2197-205. [DOI] [PubMed] [Google Scholar]
- 9.Cabuk D, Basaran G, Teomete M, et al. Clinical outcome of Turkish metastatic breast cancer patients with currently available treatment modalities--single center experience. Asian Pac J Cancer Prev 2014;15:117-22. [DOI] [PubMed] [Google Scholar]
- 10.Staren ED, Salerno C, Rongione A, et al. Pulmonary resection for metastatic breast cancer. Arch Surg 1992;127:1282-4. [DOI] [PubMed] [Google Scholar]
- 11.Meimarakis G, Angele M, Staehler M, et al. Evaluation of a new prognostic score (Munich score) to predict long-term survival after resection of pulmonary renal cell carcinoma metastases. Am J Surg 2011;202:158-67. [DOI] [PubMed] [Google Scholar]
- 12.Yhim HY, Han SW, Oh DY, et al. Prognostic factors for recurrent breast cancer patients with an isolated, limited number of lung metastases and implications for pulmonary metastasectomy. Cancer 2010;116:2890-901. [DOI] [PubMed] [Google Scholar]
- 13.Monteiro A, Arce N, Bernardo J, et al. Surgical resection of lung metastases from epithelial tumors. Ann Thorac Surg 2004;77:431-7. [DOI] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010;8:336-41. [DOI] [PubMed] [Google Scholar]
- 15.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. In: 2008. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 16.Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088-101. [PubMed] [Google Scholar]
- 18.Viechtbauer W. Conducting Meta-Analyses in R with the metafor Package. J Stat Software 2010;36:1-45. Available online: http://www.jstatsoft.org/v36/i03/paper [Google Scholar]
- 19.Chen F, Fujinaga T, Sato K, et al. Clinical features of surgical resection for pulmonary metastasis from breast cancer. Eur J Surg Oncol 2009;35:393-7. [DOI] [PubMed] [Google Scholar]
- 20.Ludwig C, Stoelben E, Hasse J. Disease-free survival after resection of lung metastases in patients with breast cancer. Eur J Surg Oncol 2003;29:532-5. [DOI] [PubMed] [Google Scholar]
- 21.Yoshimoto M, Tada K, Nishimura S, et al. Favourable long-term results after surgical removal of lung metastases of breast cancer. Breast Cancer Res Treat 2008;110:485-91. [DOI] [PubMed] [Google Scholar]
- 22.Lanza LA, Natarajan G, Roth JA, et al. Long-term survival after resection of pulmonary metastases from carcinoma of the breast. Ann Thorac Surg 1992;54:244-7; discussion 248. [DOI] [PubMed] [Google Scholar]
- 23.Meimarakis G, Rüttinger D, Stemmler J, et al. Prolonged overall survival after pulmonary metastasectomy in patients with breast cancer. Ann Thorac Surg 2013;95:1170-80. [DOI] [PubMed] [Google Scholar]
- 24.Welter S, Jacobs J, Krbek T, et al. Pulmonary metastases of breast cancer. When is resection indicated? Eur J Cardiothorac Surg 2008;34:1228-34. [DOI] [PubMed] [Google Scholar]
- 25.McDonald ML, Deschamps C, Ilstrup DM, et al. Pulmonary resection for metastatic breast cancer. Ann Thorac Surg 1994;58:1599-602. [DOI] [PubMed] [Google Scholar]
- 26.Simpson R, Kennedy C, Carmalt H, et al. Pulmonary resection for metastatic breast cancer. Aust N Z J Surg 1997;67:717-9. [DOI] [PubMed] [Google Scholar]
- 27.Friedel G, Pastorino U, Ginsberg RJ, et al. Results of lung metastasectomy from breast cancer: prognostic criteria on the basis of 467 cases of the International Registry of Lung Metastases. Eur J Cardiothorac Surg 2002;22:335-44. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka F, Li M, Hanaoka N, et al. Surgery for pulmonary nodules in breast cancer patients. Ann Thorac Surg 2005;79:1711-4; discussion 1714-5. [DOI] [PubMed]
- 29.Kycler W, Laski P. Surgical approach to pulmonary metastases from breast cancer. Breast J 2012;18:52-7. [DOI] [PubMed] [Google Scholar]
- 30.Rena O, Papalia E, Ruffini E, et al. The role of surgery in the management of solitary pulmonary nodule in breast cancer patients. Eur J Surg Oncol 2007;33:546-50. [DOI] [PubMed] [Google Scholar]
- 31.Friedel G, Linder A, Toomes H. The significance of prognostic factors for the resection of pulmonary metastases of breast cancer. Thorac Cardiovasc Surg 1994;42:71-5. [DOI] [PubMed] [Google Scholar]
- 32.Planchard D, Soria JC, Michiels S, et al. Uncertain benefit from surgery in patients with lung metastases from breast carcinoma. Cancer 2004;100:28-35. [DOI] [PubMed] [Google Scholar]
- 33.Martini N, McCormack PM. Evolution of the surgical management of pulmonary metastases. Chest Surg Clin N Am 1998;8:13-27. [PubMed] [Google Scholar]
- 34.Pastorino U, Buyse M, Friedel G, et al. Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg 1997;113:37-49. [DOI] [PubMed] [Google Scholar]
- 35.Diaz-Canton EA, Valero V, Rahman Z, et al. Clinical course of breast cancer patients with metastases confined to the lungs treated with chemotherapy. The University of Texas M.D. Anderson Cancer Center experience and review of the literature. Ann Oncol 1998;9:413-8. [DOI] [PubMed] [Google Scholar]