Abstract
Sleep disordered breathing (SDB) comprises a number of breathing disturbances occurring during sleep including snoring, the obstructive sleep apnoea/hypopnea syndrome (OSAHS), central sleep apnoea (CSA) and hypoventilation syndromes. This review focuses on sleep disordered breathing and diagnostic approaches in adults, in particular clinical assessment and overnight assessment during sleep. Although diagnostic approaches to respiratory sleep disorders are reasonably straightforward, they do require a degree of clinical acumen when it comes to assessing severity and management options. Diagnosing respiratory sleep disorders on clinical features alone has limitations. Monitoring and measuring respiration during sleep has undergone many advances in the last 40 years in respect of quality and validity, largely regarding OSAHS. Despite the improvement in our diagnostic standards and recognition of sleep disordered breathing, many limitations still need to be overcome. Apart from assessing the individual patient, population screening for sleep disorders continues to preoccupy health professionals and policy makers in many countries. Research in the field is pushing current boundaries in terms of simplifying diagnosis and enhancing screening for sleep disordered breathing in large populations. At present, a number of these newer approaches require further validation.
Keywords: Clinical assessment, diagnosis, objective assessment, population screening, respiratory sleep disorders, sleep apnoea, sleep disordered breathing (SDB)
Introduction
Sleep disordered breathing (SDB) comprises a number of sleep related breathing disturbances: snoring, the obstructive sleep apnoea/hypopnea syndrome (OSAHS), central sleep apnoea (CSA), hypoventilation syndromes and other rarer forms of disturbed breathing (1).
The commonest form of sleep disordered breathing within industrialised communities is the OSAHS affecting at least 2-4% of the middle aged population (2). The definition of OSAHS is made on the basis of symptoms of daytime sleepiness and objective measures of disordered breathing during sleep. Recurrent upper airway (UA) obstruction during sleep, resulting in repetitive apnoeas accompanied by oxygen desaturation and arousal from sleep is the chief diagnostic characteristic of OSAHS (2).
OSAHS leads to widespread physiological changes which may contribute to the development of both cardiovascular and cerebrovascular morbidity (2), in addition to diurnal sleepiness and cognitive impairment. Currently, the standard treatment for moderate to severe OSAHS is in the form of splinting the airways mechanically using compressed air delivered through a nasal or full face mask worn during sleep [continuous positive airway pressure (CPAP)] (2).
Table 1 lists the current classification of breathing disturbances during sleep according to the International Classification of Sleep Disorders 3rd edition (ICSD-3), published in 2014 (1). The clinical presentation and diagnostic criteria of abnormal breathing in sleep are different for adult and paediatric cases. In this review the focus will be on sleep disordered breathing in adults, in particular, OSAHS, CSA and sleep related hypoventilation syndromes.
Table 1. Classification of sleep related breathing disorders according to the ICSD-3 classification of sleep disorders manual (1).
| Disorder | ICD-10-CM code |
|---|---|
| Obstructive sleep apnea disorders | |
| Obstructive sleep apnea, adult | G47.33 |
| Obstructive sleep apnea, pediatric | G47.33 |
| Central sleep apnea syndromes | |
| Central sleep apnea with Cheyne-Stokes breathing | RO6.3 |
| Central apnea due to a medical disorder | |
| without Cheyne-Stokes breathing | G47.37 |
| Central sleep apnea due to high altitude periodic breathing | G47.32 |
| Central sleep apnea due to a medication or substance | G47.39 |
| Primary central sleep apnea | G47.31 |
| Primary central sleep apnea of infancy | P28.3 |
| Primary central sleep apnea of prematurity | P28.4 |
| Treatment-emergent central sleep apnea | G47.39 |
| Sleep related hypoventilation disorders | |
| Obesity hypoventilation syndrome | E66.2 |
| Congenital central alveolar hypoventilation syndrome | G47.35 |
| Late-Onset central hypoventilation with hypothalamic Dysfunction | G47.36 |
| Idiopathic central alveolar hypoventilation | G47.34 |
| Sleep related hypoventilation due to a medication of Substance | G47.36 |
| Sleep related hypoventilation due to a medical disorder | G47.36 |
| Sleep related hypoxaemia disorder | |
| Sleep related hypoxaemia | G47.36 |
| Isolated symptoms and normal variants | |
| Snoring | R06.83 |
| Catathrenia |
ICSD, International Classification of Sleep Disorders
Clinical assessment of sleep related breathing disorders
The obstructive sleep apnoea/hypopnoea syndrome
The pathophysiology of OSAHS is based in UA occlusion during sleep (3). Occlusion of the UA is termed an apnoea if complete and hypopnoea if partial (see Figure 1). An obstructive apnoea is defined as a cessation of airflow with continued effort for at least 10 seconds. Apnoeas/hypopnoeas are often, associated with an electroencephalographic (EEG) arousal at their termination and with a drop in oxygen saturation (4-7). The standardisation of hypopnoea definitions has fluctuated over time. However, the definition adopted by the American Academy of Sleep Medicine (AASM) in 2007 is a 30-50% reduction in thoraco-abdominal movement for at least 10 seconds from the preceding stable baseline with either an accompanying 3% or 4% desaturation or an arousal (5). In reality, a number of definitions are likely to be in current use, some idiosyncratic to a given sleep laboratory and potentially encompassing older definitions of hypopnoeas, including the ‘Chicago Criteria’ and a definition provided by the 2001 AASM position paper (8). Thus, the effect on the overall apnoea/hypopnoea index (AHI) which is the metric employed to define OSAHS severity, is highly variable and several studies have demonstrated that diagnosis can differ as a result (9,10). The American Academy of Sleep Medicine Task Force in 1999 (4) defined OSAHS severity on the basis of two components: severity of daytime sleepiness and the AHI. Sleepiness and breathing events are rated separately. Sleep-related obstructive breathing events are rated as mild (5-15 events per hour of sleep), moderate (15-30 events per hour of sleep) or severe (greater than 30 events per hour of sleep) (4). This largely constitutes a good general working definition of the disorder but does not account for age- or gender-related changes in sleepiness and sleep-disordered breathing. It is important to note, that there are very few normative data for either the general population, let alone more specific populations e.g., the elderly, people with intellectual disability (3).
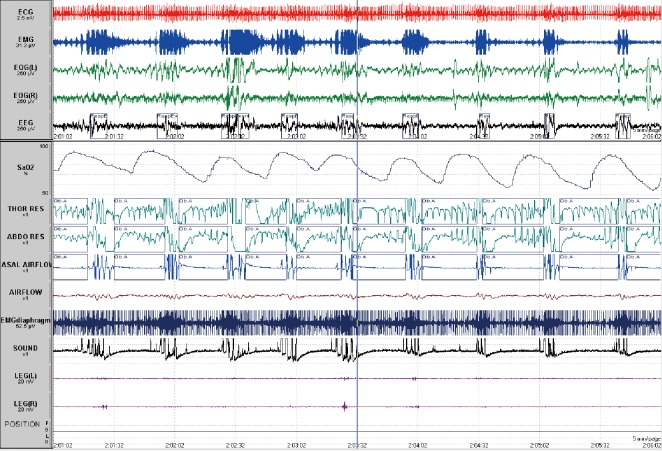
Figure 1.
Five-minute page from a polysomnogram showing obstructive apnoeas (Ob.A) recorded on thoracic, abdominal bands and airflow associated with desaturation and arousals at the termination of the events on electroencephalogram. ECG, electrocardiogram; EMG, electromyogram; EOG, electro-oculogram; EEG, electroencephalogram; SaO2, pulse oximetry saturation; Thor Res, thoracic band; Abdo res, abdominal band.
Presenting symptoms of OSAHS can be divided into those that manifest themselves during sleep and those which are present during wakefulness (Table 2). The most common complaint is excessive day time sleepiness (EDS). A multi-centre cohort study published in 2008 suggested that apnoea and sleep disruption were not the primary determinants of EDS, although patients with EDS had longer sleep duration, increased slow wave sleep and sleep fragmentation (11).
Table 2. Symptoms of the obstructive sleep apnoea/hypopnoea syndrome.
| During sleep | Awake |
|---|---|
| Non-restorative sleep | Daytime sleepiness |
| Witnessed apnoeas by bed partner | Lack of concentration |
| Awakening with choking | Cognitive deficits |
| Nocturnal restlessness | Changes in mood |
| Vivid dreams | Morning headaches |
| Gastroesophageal reflux | Dry mouth |
| Insomnia with frequent awakenings | Impotence or decreased libido |
| Nocturia | |
| Hypersalivation | |
| Diaphoresis |
EDS can be a symptom of many different pathologies. Important differential diagnoses for EDS include: sleep deprivation, metabolic disorders and depression (Table 3). Nocturnal symptoms of OSAHS are sometimes apparent to the patient but generally are reported by the bed partner. Most commonly reported are snoring, snorting, choking attacks terminating a snore and apnoeas. An absence of snoring does not exclude a diagnosis of OSAHS, but virtually all patients snore. Apnoeic episodes are reported by approximately 75% of bed partners. Many patients report waking up with choking or gasping but remain unaware of their disordered breathing. Those who are aware of these events sometimes choose to delay sleep onset and can present with ‘paradoxical insomnia’. A number of clinical features are associated with OSAHS; many occur in combination and may also be subtle (Table 4). The predictive value of any single feature is limited (12).
Table 3. Differential diagnosis of excessive daytime sleepiness.
| Obstructive sleep apnea/hypopnea syndrome (OSAHS) |
| Upper airway resistance syndrome (UARS) |
| Narcolepsy—central nervous system hypersomnolence |
| Periodic leg movements during sleep |
| Insufficient sleep syndrome |
| Alcohol, medication and drug use |
| Schedule disorders/shift work |
| Chronic pain and discomfort |
| Delayed sleep phase syndrome |
| Severe lung disease |
| Neuromuscular disease |
| Metabolic abnormalities |
| Vitamin and trace element deficiencies (if profound) |
| Depression |
Table 4. Commonly encountered clinical features of obstructive sleep apnoea/hypopnoea syndrome.
| Obesity (particularly central, BMI >30 kg/m2) |
| Large neck circumference (>40 cm) |
| Narrow mandible, narrow maxilla |
| Retrognathia |
| Dental malocclusion, overbite |
| Reduced nasal patency |
| High and narrow hard palate |
| Elongated and low-lying uvula |
| Enlarged tonsils and adenoids |
| Macroglossia |
When considering the clinical presentation of OSAHS, it should be borne in mind that the bulk of the scientific literature has to date focussed on middle-aged men who have for the most part been overweight. The female phenotype is less clearly defined and male bed partners are less likely to report snoring and apnoeas to a health professional. Female OSAHS patients may present with less typical and more diffuse signs such as headache, insomnia and mood disturbances (13). Age at presentation will also determine clinical symptoms. Younger patients may have behavioural and cognitive disturbances, rather than EDS as a primary presenting feature. In the elderly, co-morbidities and lifestyle changes (e.g., diurnal napping) may cloud symptomatology (14). Patients with intellectual disability may have presenting features more akin to children with OSAHS (15).
Clinical examination of a patient presenting with a history of obstructive respiratory events during sleep should include cardio-respiratory auscultation, blood pressure measurement, examination of the oral cavity, noting the presence of teeth and dentures. It is also highly recommended to comment on tonsil size, tongue size and architecture of the hard palate and faucial pillars. A simple way of recording this information is by using the Mallampati score, first devised for assessing ease of intubation of patients for purposes of anaesthesia (16). There have been several studies now which have shown an independent association between Mallampati score and presence or severity of OSAHS (17). Another score which is also useful is the Friedman score, used more frequently within Ear Nose and Throat practice (18).
Since OSAHS is an independent risk factor for hypertension and may contribute to and co-exist with a number of co-morbidities, it is now increasingly being recommended, that these should be examined for and addressed as well. A suggested approach would include screening for at least the following: hypertension, cardiac hypertrophy and atrial fibrillation, evidence of atherosclerosis (coronary or peripheral), COPD, depression, obesity (waist/hip ratio; plasma lipids, hepatic enzymes, liver ultrasound), Type II diabetes mellitus, insulin resistance and metabolic syndrome.
History and clinical examination alone, including blood pressure and body mass index (BMI) measurement can predict the presence of OSAHS in about 50% of patients attending a specialised sleep clinic (12). Further, definitive diagnosis requires additional monitoring during sleep.
Central sleep apnoea (CSA)
Overall, 10% of breathing disturbances during sleep are secondary to CSA (1). CSA is commoner in the elderly population and in males (19). The reason for this observation may lie in the fact that women have a lower apnoeic threshold which stabilises respiration (20).
CSA is defined by recurrent cessation of airflow with simultaneous cessation of respiratory effort (5,6,7). The primary pathophysiology is related to a reduction in or complete lack of ventilator drive/impulse generation. Table 5 lists the commonest causes of CSA, including hypoventilation syndromes (discussed below).
Table 5. Physiological classification of CSA based on wakefulness CO2 levels (some overlap) [Based on reference (21)].
| Hypercapnic CSA |
| “Won’t breathe” |
| Brainstem lesions |
| Congenital central hypoventilation |
| Opioids |
| Obesity hypoventilation |
| “Can’t breathe” |
| Neuromuscular disorders |
| Chest wall syndromes |
| Nonhypercapnic/Eucapnic |
| Cheyne-Stokes breathing |
CSA, central sleep apnoea.
The term CSA includes a number of different disorders characterised by either hypo- or hyperventilation and sensitivity to carbon dioxide (CO2). These are primary CSA, Cheyne-Stokes respiration (CSR), CSA secondary to high-altitude (periodic breathing), CSA secondary to brain-stem lesions and also secondary to drugs, most commonly opioids (1).
Clinical presentation of patients with CSA will also vary in respect of the underlying disorder, including heart failure, atrial fibrillation (22) and stroke (23), chronic opioid use (24), family history, neurological disorder, or immediate environmental situation e.g., living at high altitude.
Generally, the symptoms of CSA are often subtle and nonspecific and include frequent awakenings during sleep, paroxysmal nocturnal dyspnoea in association with heart failure, fatigue and EDS. The latter symptom is often less marked than in obstructive sleep apnoea. Snoring is not a feature of this condition unless UAs obstruction is also present (1).
In clinical practice, the most common form of CSA occurs secondary to poorly controlled heart failure (25). In this disorder CSR is the primary diagnostic sign (see Figure 2). CSR is characterised by recurrent apnoeas and hypopnoeas occurring in a crescendo-decrescendo pattern of flow and effort. In CSR, cycle-length averages about 60-90 seconds, compared to other forms of CSA when it is shorter (26).
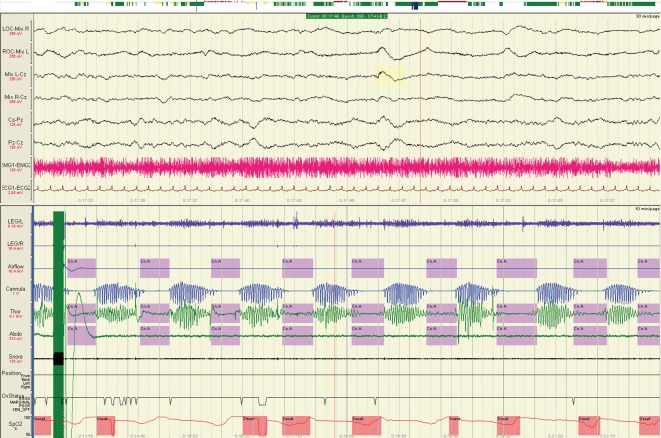
Figure 2.
Cheyne-Stokes respiration on a 10-minute page from a polysomnogram during N2 sleep. EMG, electromyogram; ECG, electrocardiogram; Thor, thoracic band; Abdo, abdominal band.
In CSA, arterial blood gas examination is a useful investigative tool. Results can assist with a physiological classification based on wakefulness levels of CO2, although there is some overlap between the different types of disorders. The hypercapnic CSA patients are those who “won’t breathe” and include people with brain stem lesions, congenital central hypoventilation, opioids and obesity hypoventilation. Those who “can’t breathe” are more likely to be those with neuromuscular disorders and chest wall syndromes. Non-hypercapnic or eucapnic forms of CSA include CSR and idiopathic CSA (see Table 5).
Hypoventilation syndromes
The primary pathophysiological trait of hypoventilation syndromes is a PaCO2 >45 mmHg on arterial blood gas during sleep, which eventually manifests as abnormally elevated PaCO2 during wakefulness (1). Arterial oxygen desaturations less than 90% for longer than 5 minutes during the sleep period with a nadir of less than 85% also characterise hypoventilation (27). Hypoventilation first becomes apparent in REM-sleep and as it progresses, will occur throughout all sleep stages.
Obesity hypoventilation syndrome (OHS)
OHS deserves special consideration as it is increasingly common with a prevalence of patients presenting to sleep clinics in industrialised countries ranging from 10% to 20% (1). Fifty percent of hospitalised patients with a BMI of greater 50 kg/m2 are likely to have OHS. An overall estimate has suggested that 0.15-0.4% of the population has OHS with the incidence increasing as obesity increases in the general population. The diagnosis is important in that those with OHS have a 23% mortality at 18 months, which falls to 3% with appropriate treatment (1,28).
OHS is defined by a BMI greater than 30 kg/m2, a PaCO2 >45 mmHg and a FEV1/VC ratio >60% on respiratory function testing (1,28). The triad of obesity, hypersomnolence and awake hypercapnia in the absence of an alternative neuromuscular, mechanical or metabolic explanation for hypoventilation are the cornerstones of this diagnosis. Eighty to 90% of people with OHS also have UAs obstruction (1).
Generally, patients will report EDS. Other complaints include morning headache and loss of appetite in the morning which lifts throughout the day, mood disturbances and neurocognitive dysfunction (1,28).
Clinical examination is as above for OSAHS, but in severe cases, additional clinical signs should be noted including generalised plethora, scleral injection, peripheral oedema and signs of right heart failure and pulmonary hypertension (1).
Apart from arterial blood gases, other investigations should include assessment for polycythaemia, performing electrocardiogram (ECG) and echocardiography, looking for evidence of right ventricular dysfunction or failure, pulmonary hypertension and right ventricular hypertrophy.
Unfortunately, even severe OHS can go undetected for long periods of time until a catastrophic event such as myocardial infarction or a severe infective illness tips the balance into acute-on-chronic respiratory failure requiring urgent hospitalisation and high-level care (1,28).
Objective assessment of respiratory sleep disorders
Definitive diagnosis of most respiratory sleep disorders requires objective recording and measurement of sleep and breathing during sleep, in addition to a measure of daytime sleepiness (either objective or subjective) and symptoms of sleepiness.
Assessing day time sleepiness
The assessment of sleepiness is complex. Sleepiness per se is difficult to define objectively, but can be classified using a number of methods: behavioural measures (e.g., observation of yawning frequency, actigraphy, facial expression), performance tests (e.g., a driving simulator, psychomotor vigilant tests and reaction time tests), self-evaluation by rating scales [e.g., the Stanford Sleepiness Scale (SSS), Epworth Sleepiness Scale (ESS)] or direct electro-physiological measures (e.g., multiple sleep latency testing and multiple wakefulness testing, pupillometry and cerebral evoked potentials) (29).
In clinical practice, the most commonly used and best validated scale for assessing day time sleepiness is the ESS (30). An ESS score greater than 11 out of 24 (maximum score) is generally indicative of abnormal levels of daytime sleepiness, irrespective of age. The ESS can be applied in all disorders of sleepiness, including disorders of central hypersomnolence as well as circadian rhythm disorders.
The ESS aims to measure the general level of diurnal sleepiness as a stable individual characteristic and has satisfactory test/retest reliability. However, it can be misinterpreted and the questionnaire itself filled out inappropriately by patients, sometimes to minimise symptomatology when a sleep disorder diagnosis might potentially threaten livelihood, e.g., a sleepy truck driver who then needs annual monitoring of adequate treatment of OSAHS to fulfil driving license regulations. A pictorial Epworth Sleepiness Score published recently has potentially made interpretation of the questions easier (31). However, the population in which this version of the ESS is applied may not necessarily make this a more workable tool.
Objective tests of daytime sleepiness include the multiple sleep latency test (MSLT) and the maintenance of wakefulness test (MWT) (32). The MSLT, originally designed to facilitate the diagnosis of narcolepsy, is also used in the assessment of disorders presenting with EDS. The central premise of the MSLT is that the sleepier a subject is, the more quickly he/she will fall asleep (32). In respect of routine use in respiratory sleep disorders, it is not recommended but can be useful if there are ongoing issues with EDS which have not been addressed through adequate treatment or when co-pathologies are suspected. The MWT measures the ability to stay awake for a defined period of time in a laboratory-controlled, stimulus-free environment (32). Again, this test is not useful in the routine management of respiratory sleep disorders, but can be utilised in the context of specific clinical questions e.g., has the patient responded sufficiently to treatment?
A number of additional computer-based assessments of visual attention and reaction times such as the OSLER test are also available (33). It is important to note, that despite their diagnostic usefulness under certain circumstances, no test of sleepiness can reliably predict sleepiness and performance in real-life situations with certainty.
Recording and measurement of sleep and breathing
Overnight polysomnography (PSG) is the most widely used method for the diagnosis of sleep related respiratory disorders. Currently it is considered the “gold standard” by which newer developments in the measurement of breathing during sleep have been assessed. Whether this is strictly appropriate is debatable. The PSG is designed to simultaneously monitor the following physiological recordings: nasal and/or oral airflow; thoracoabdominal movement; snoring; EEG; electro-oculogram (EOG); electromyogram (EMG); oxygen saturation and occasionally transcutaneous CO2 (4,6,7).
The recording of any abnormal movements by additionally using video may help identify changes in airflow or desaturations. Signal collection and interpretation are usually computerised, but manual scoring of the trace should still be performed using guidelines for interpretation of the EEG (4,6,7). The scoring of respiratory and other events is likewise subject to guidelines. There have been a number of changes in guidelines over the last decade. Initially, the 1968 Rechtschaffen and Kales (34) rules for the scoring of EEG remained unchallenged until 2007 when the AASM revised the criteria and in some respects simplified EEG montage and scoring (6). There have been additional overviews of scoring since then (7). The standardisation of respiratory scoring using PSG has been the primary improvement, but variation still remains. Additionally, night to night variability in PSG makes it possible for a single study to underestimate the severity of OSAHS. A negative PSG should be viewed with scepticism if the clinical suspicion of OSAHS remains high (35).
Split-night studies in which the first half of the study night is used for diagnosis and the second for monitoring treatment response using CPAP are also used. Split-night studies are considered accurate and cost-effective when criteria for conducting them are met (36).
More recent introductions to the assessment of sleep disordered breathing have been cardio-respiratory monitoring devices or polygraphy (PG). These devices are also known as Type 3 devices using the AASM classification (Table 6) (37). PG comprises the measurement of airflow, respiratory effort, oxygen saturation and heart rate, to the exclusion of EEG. The great advantages of these systems are price, portability and convenience to the patient (38). Although automated scoring algorithms exist, manual scoring and overview should be undertaken. The results are expressed as A + H per time in bed as it is impossible to determine for sure without EEG how long the patient has been asleep. This can lead to both under- and overestimation of the actual severity of sleep disordered breathing. There are no clear clinical guidelines for the scoring of respiratory events using PG. The AASM guidelines (4-7), have a definition for the scoring of obstructive apnoeas and hypopneas as well as central events. However, these are based on the presence of EEG. Additionally, accuracy of Type III equipment can differ, depending on the manufacturer. There are currently two possibilities for scoring respiratory events utilising Type III systems: (I) Ignore any events that do not have a desaturation; (II) score all events with or without desaturation ‘in the hope’ that there may be an associated arousal with events without desaturation.
Table 6. American College of Chest Physicians and the American Sleep Disorders Association classification of sleep monitoring devices [Based on reference (37)].
| Type 1: full attended PSG (≥7 channels) in a laboratory setting |
| Type 2: full unattended PSG (≥7 channels) |
| Type 3: limited channel devices (4-7 channels) |
| Type 4: 1 or 2 channels usually using oximetry as one of the parameters |
PSG, polysomnography.
Even more simplified systems have been utilised in screening and diagnosis of sleep disordered breathing, most commonly overnight oximetry. However, single channel oximetry should never be seen as a substitute for either in-lab PSG or PG. There are limitations to the technique, in particular, failure to detect apnoeas/hypopnoeas although the pattern may be suggestive (39,40). Furthermore, oxygen desaturation is not necessarily a marker of apnoeic episodes and may be the results of other disorders including hypoventilation without UAs obstruction. Oximetry can be used to monitor patients who have been appropriately diagnosed with a respiratory sleep disorder in respect of progression of disease or treatment response.
Other forms of single-channel devices comprise measurement of nasal flow to characterise apnoeas and hypopnoeas. A recent multi-centre study testing a home single-channel nasal pressure device in over 700 patients with sleep disordered breathing showed very good diagnostic accuracy and cost-effectiveness compared to PSG and PG, particularly when the traces were manually scored (41).
There have been newer additions to the diagnostic armamentarium and approach to the physical space where diagnosis is undertaken. These include not only in-lab studies or home-based monitoring, but also telemonitoring via remote control and remote monitoring of diagnostic studies. Currently, there are few well-conducted trials reported, but preliminary results suggest that this is a promising area which may further increase convenience to patients and decrease the costs to a diagnostic service running these studies (42,43).
PSG is highly useful in diagnosing CSA and hypoventilation syndromes, including monitoring response to treatment and titrating bilevel or positive airway pressure therapies. PG is less validated in these disorders and is not considered particularly useful for the screening or diagnosis of CSA (44). Therefore, any measurement technique less complex than PSG cannot be recommended for an indubitable diagnosis of any respiratory sleep disorder other than OSAHS at the present time.
Screening adult populations for respiratory sleep disorders
The definition of sleep disordered breathing depends on the AHI and ranges from 9% to 24% in the general adult population. The prevalence will change with age and differs between men and women (2).
EDS which occurs at least 3 days a week has been found to occur in 4-20.6% of the population. Severe EDS occurs in 5% of the population and men and women are equally affected (45).
History and clinical assessment are extremely important in attaining this diagnosis but require objective verification through overnight studies.
With regard to OSAHS, the commonest respiratory sleep disorder, several algorithms have been developed to predict its presence in patients presenting with suggestive complaints. These include utilising a combination of clinical variables such as BMI, neck circumference, jaw structure, snoring, reports of nocturnal breathing disturbances and the presence of hypertension. The sensitivity of these approaches is high (78-95%) but the specificity tends to be low (41-63%) (46).
Screening questionnaires for OSAHS have been utilised for some time. A recent meta-analysis of these questionnaires included 10 studies with a total of 1,484 subjects (47). Four studies utilised the Berlin questionnaire (48), two studies the Wisconsin Sleep Questionnaire (49), one study the STOP questionnaire (50) and one the STOP-BANG questionnaire (51). Four studies on referred patients gave a pooled sensitivity of 73% (95% CI, 66-78%) for the diagnosis of OSAHS and a pooled specificity of 61% (95% CI, 55-67%). Six studies on patients without a history of sleep disorders revealed a pooled sensitivity of 77% (95% CI, 73-80%) for detecting OSAHS and a pooled specificity of 53% (95% CI, 50-57%) (47).
Additional questionnaires have been devised over the last two decades to evaluate both sleepiness and quality of life in OSAHS. Whether they are applicable to any of the other respiratory sleep disorders discussed has not been explored. These include the Functional Outcomes of Sleep Questionnaire (52), the Calgary Sleep Apnoea Quality of Life Index (SAQLI) (53), the short SAQLI (54), the SSS (55) and the ESS (30) as discussed above.
In terms of objective assessment and screening of large populations, Type III and IV device studies most likely represent the way forward. PG or Type IV device studies should always be conducted in conjunction with a comprehensive sleep evaluation. Results are improved if there is a high pre-test probability of sleep disordered breathing. At present, limited device studies (i.e., without EEG and out of hospital) are not indicated if the patient has significant co-morbidities, other associated sleep disorders, e.g., disorders of central hypersomnolence or an inability to use equipment despite education (56). Raw data requires manual scoring or editing, particularly if automated scoring is also available. Diagnostic accuracy can be questionable at times. Currently there are issues with scoring respiratory events using PG and even with home nasal pressure traces, as discussed above. The AASM criteria for PSG are not applicable to scoring respiratory events on PG.
Pulse oximetry is useful but presents some difficulties. At the present time there is no internationally standardised technical specification or standardisation of signal processing in oximeters with the minimum standard criteria set by the AASM comprising a sample rate of 25 Hz with an average of three values (6) and a resolution of 0.1% (57). The current desaturation definitions usually revolve around a decrease of >4% from baseline Sp02. However, there is no unanimity in terms of what constitutes a normal or abnormal desaturation index. Additional limitations of pulse oximetry include potential problems with blood flow and haemoglobinopathies, tissue optics in the very obese and an inability to detect other forms of sleep disordered breathing. Movement artefact needs to be taken into account and there can be significant measurement inaccuracies of ±2% in Sp02 (58).
Currently there is no data to suggest that oximetry is a useful tool in screening for CSA or disorders of hypoventilation.
Summary
Diagnostic approaches to respiratory sleep disorders are reasonably straightforward, but do require a combination of subjective and objective sleep assessment as well as a degree of clinical acumen when it comes to assessing severity and management options.
Monitoring and measuring respiration during sleep has undergone many advances in the last 40 years in respect of quality and validity, largely with respect to OSAHS. The technology has allowed for the recognition of other disorders of respiration during sleep including CSA and hypoventilation syndromes. Diagnosing respiratory sleep disorders on clinical features alone is limited. Despite the improvement in our diagnostic standards and recognition of sleep disordered breathing, many limitations still need to be overcome. Many researchers in the field continue to push the boundaries in respect of simplifying diagnosis and enhancing screening for sleep disordered breathing in large populations. At present, these approaches require further validation.
The most common disorder of respiration during sleep, OSAHS, can potentially be easily suspected and diagnosed in general practice with referral in difficult cases to secondary or tertiary specialised units. Since OSAHS affects 5% of the population in industrialised countries, it is as common as asthma (59) and if all forms of sleep disordered breathing are considered together they are as common as COPD (60). Just as the diagnosis and management of hypertension has devolved into general practice, then both subjective and objective assessments of OSAHS have the potential of being devolved into primary care.
This is unlikely to be true of the other sleep related breathing disorders at the present time. Unfortunately, there is still some way to go with respect to validating and ensuring that diagnoses are appropriate by “non-experts” prior to initiation of treatment. However, this is not an insurmountable task. The cost-effectiveness of simplified diagnostic tools with good sensitivity and specificity may eventually drive this approach and help to determine policy at governmental levels.
Respiratory sleep disorders are important, common conditions within our community. They pose a significant health burden and are frequently associated with other resource draining co-morbidities. Sleep disordered breathing can be diagnosed reasonably readily although management is not always straightforward.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- 1.American Academy of Sleep Medicine. International Classification of Sleep Disorders, 3rd ed. Darien, IL: American Academy of Sleep Medicine, 2014. [Google Scholar]
- 2.Jennum P, Riha RL. Epidemiology of sleep apnoea/hypopnoea syndrome and sleep-disordered breathing. Eur Respir J 2009;33:907-14. [DOI] [PubMed] [Google Scholar]
- 3.Eckert DJ, Malhotra A. Pathophysiology of adult obstructive sleep apnea. Proc Am Thorac Soc 2008;5:144-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep 1999;22:667-89. [PubMed] [Google Scholar]
- 5.Iber C, Ancoli-Israel S, Chesson A, et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Westchester, IL: American Academy of Sleep Medicine, 2007. [Google Scholar]
- 6.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med 2012;8:597-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berry RB, Brooks R, Gamaldo CE, et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, Version 2.1. Darien, IL: American Academy of Sleep Medicine, 2014. [Google Scholar]
- 8.Meoli AL, Casey KR, Clark RW, et al. Hypopnea in sleep-disordered breathing in adults. Sleep 2001;24:469-70. [PubMed] [Google Scholar]
- 9.Ruehland WR, Rochford PD, O'Donoghue FJ, et al. The new AASM criteria for scoring hypopneas: impact on the apnea hypopnea index. Sleep 2009;32:150-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Redline S, Budhiraja R, Kapur V, et al. The scoring of respiratory events in sleep: reliability and validity. J Clin Sleep Med 2007;3:169-200. [PubMed] [Google Scholar]
- 11.Roure N, Gomez S, Mediano O, et al. Daytime sleepiness and polysomnography in obstructive sleep apnea patients. Sleep Med 2008;9:727-31. [DOI] [PubMed] [Google Scholar]
- 12.Riha RL. Clinical assessment of the obstructive sleep apnoea/hypopnoea syndrome. Ther Adv Respir Dis 2010;4:83-91. [DOI] [PubMed] [Google Scholar]
- 13.Ye L, Pien GW, Weaver TE. Gender differences in the clinical manifestation of obstructive sleep apnea. Sleep Med 2009;10:1075-84. [DOI] [PubMed] [Google Scholar]
- 14.McMillan A, Bratton DJ, Faria R, et al. Continuous positive airway pressure in older people with obstructive sleep apnoea syndrome (PREDICT): a 12-month, multicentre, randomised trial. Lancet Respir Med 2014;2:804-12. [DOI] [PubMed] [Google Scholar]
- 15.Malt EA, Dahl RC, Haugsand TM, et al. Health and disease in adults with Down syndrome. Tidsskr Nor Laegeforen 2013;133:290-4. [DOI] [PubMed] [Google Scholar]
- 16.Mallampati SR. Clinical sign to predict difficult tracheal intubation (hypothesis). Can Anaesth Soc J 1983;30:316-7. [DOI] [PubMed] [Google Scholar]
- 17.Nuckton TJ, Glidden DV, Browner WS, et al. Physical examination: Mallampati score as an independent predictor of obstructive sleep apnea. Sleep 2006;29:903-8. [DOI] [PubMed] [Google Scholar]
- 18.Friedman M, Hamilton C, Samuelson CG, et al. Diagnostic value of the Friedman tongue position and Mallampati classification for obstructive sleep apnea: a meta-analysis. Otolaryngol Head Neck Surg 2013;148:540-7. [DOI] [PubMed] [Google Scholar]
- 19.Bixler EO, Vgontzas AN, Lin HM, et al. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med 2001;163:608-13. [DOI] [PubMed] [Google Scholar]
- 20.Skatrud JB, Berssenbrugge AD. Effect of sleep state and chemical stimuli on breathing. Prog Clin Biol Res 1983;136:87-95. [PubMed] [Google Scholar]
- 21.Eckert DJ, Jordan AS, Merchia P, et al. Central sleep apnea: Pathophysiology and treatment. Chest 2007;131:595-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Solin P, Roebuck T, Swieca J, et al. Effects of cardiac dysfunction on non-hypercapnic central sleep apnea. Chest 1998;113:104-10. [DOI] [PubMed] [Google Scholar]
- 23.Parra O, Arboix A, Bechich S, et al. Time course of sleep-related breathing disorders in first-ever stroke or transient ischemic attack. Am J Respir Crit Care Med 2000;161:375-80. [DOI] [PubMed] [Google Scholar]
- 24.Wang D, Teichtahl H. Opioids, sleep architecture and sleep-disordered breathing. Sleep Med Rev 2007;11:35-46. [DOI] [PubMed] [Google Scholar]
- 25.Sin DD, Fitzgerald F, Parker JD, et al. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med 1999;160:1101-6. [DOI] [PubMed] [Google Scholar]
- 26.Tkacova R, Wang H, Bradley TD. Night-to-night alterations in sleep apnea type in patients with heart failure. J Sleep Res 2006;15:321-8. [DOI] [PubMed] [Google Scholar]
- 27.Caples SM, Wolk R, Somers VK. Influence of cardiac function and failure on sleep-disordered breathing: evidence for a causative role. J Appl Physiol (1985) 2005;99:2433-9. [DOI] [PubMed] [Google Scholar]
- 28.Pépin JL, Borel JC, Janssens JP. Obesity hypoventilation syndrome: an underdiagnosed and undertreated condition. Am J Respir Crit Care Med 2012;186:1205-7. [DOI] [PubMed] [Google Scholar]
- 29.Cluydts R, De Valck E, Verstraeten E, et al. Daytime sleepiness and its evaluation. Sleep Med Rev 2002;6:83-96. [DOI] [PubMed] [Google Scholar]
- 30.Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep 1992;15:376-81. [DOI] [PubMed] [Google Scholar]
- 31.Ghiassi R, Murphy K, Cummin AR, et al. Developing a pictorial Epworth Sleepiness Scale. Thorax 2011;66:97-100. [DOI] [PubMed] [Google Scholar]
- 32.Littner MR, Kushida C, Wise M, et al. Practice parameters for clinical use of the multiple sleep latency test and the maintenance of wakefulness test. Sleep 2005;28:113-21. [DOI] [PubMed] [Google Scholar]
- 33.Bennett LS, Stradling JR, Davies RJ. A behavioural test to assess daytime sleepiness in obstructive sleep apnoea. J Sleep Res 1997;6:142-5. [DOI] [PubMed] [Google Scholar]
- 34.Rechtschaffen A, Kales A, editors. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Bethesda, Md: U.S. National Institute of Neurological Diseases and Blindness, Neurological Information Network, 1968. [Google Scholar]
- 35.Meyer TJ, Eveloff SE, Kline LR, et al. One negative polysomnogram does not exclude obstructive sleep apnea. Chest 1993;103:756-60. [DOI] [PubMed] [Google Scholar]
- 36.Kapur VK, Sullivan SD. More isn't always better: cost-effectiveness analysis and the case for using a split-night protocol. J Clin Sleep Med 2006;2:154-5. [PubMed] [Google Scholar]
- 37.Practice parameters for the use of portable recording in the assessment of obstructive sleep apnea. Standards of Practice Committee of the American Sleep Disorders Association. Sleep 1994;17:372-7. [PubMed] [Google Scholar]
- 38.Flemons WW, Littner MR, Rowley JA, et al. Home diagnosis of sleep apnea: a systematic review of the literature. An evidence review cosponsored by the American Academy of Sleep Medicine, the American College of Chest Physicians, and the American Thoracic Society. Chest 2003;124:1543-79. [DOI] [PubMed] [Google Scholar]
- 39.Collop NA, Anderson WM, Boehlecke B, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med 2007;3:737-47. [PMC free article] [PubMed] [Google Scholar]
- 40.Ramsey R, Mehra R, Strohl KP. Variations in physician interpretation of overnight pulse oximetry monitoring. Chest 2007;132:852-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Masa JF, Duran-Cantolla J, Capote F, et al. Effectiveness of home single-channel nasal pressure for sleep apnea diagnosis. Sleep 2014;37:1953-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coma-Del-Corral MJ, Alonso-Álvarez ML, Allende M, et al. Reliability of telemedicine in the diagnosis and treatment of sleep apnea syndrome. Telemed J E Health 2013;19:7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sparrow D, Aloia M, Demolles DA, et al. A telemedicine intervention to improve adherence to continuous positive airway pressure: a randomised controlled trial. Thorax 2010;65:1061-6. [DOI] [PubMed] [Google Scholar]
- 44.Smith LA, Chong DW, Vennelle M, et al. Diagnosis of sleep-disordered breathing in patients with chronic heart failure: evaluation of a portable limited sleep study system. J Sleep Res 2007;16:428-35. [DOI] [PubMed] [Google Scholar]
- 45.Ohayon MM. From wakefulness to excessive sleepiness: what we know and still need to know. Sleep Med Rev 2008;12:129-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rowley JA, Aboussouan LS, Badr MS. The use of clinical prediction formulas in the evaluation of obstructive sleep apnea. Sleep 2000;23:929-38. [DOI] [PubMed] [Google Scholar]
- 47.Abrishami A, Khajehdehi A, Chung F. A systematic review of screening questionnaires for obstructive sleep apnea. Can J Anaesth 2010;57:423-38. [DOI] [PubMed] [Google Scholar]
- 48.Netzer NC, Stoohs RA, Netzer CM, et al. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med 1999;131:485-91. [DOI] [PubMed] [Google Scholar]
- 49.Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993;328:1230-5. [DOI] [PubMed] [Google Scholar]
- 50.Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008;108:812-21. [DOI] [PubMed] [Google Scholar]
- 51.Chung F, Subramanyam R, Liao P, et al. High STOP-Bang score indicates a high probability of obstructive sleep apnoea. Br J Anaesth 2012;108:768-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weaver TE, Laizner AM, Evans LK, et al. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep 1997;20:835-43. [PubMed] [Google Scholar]
- 53.Flemons WW, Reimer MA. Development of a disease-specific health-related quality of life questionnaire for sleep apnea. Am J Respir Crit Care Med 1998;158:494-503. [DOI] [PubMed] [Google Scholar]
- 54.Flemons WW, Reimer MA. Measurement properties of the calgary sleep apnea quality of life index. Am J Respir Crit Care Med 2002;165:159-64. [DOI] [PubMed] [Google Scholar]
- 55.Hoddes E, Zarcone V, Smythe H, et al. Quantification of sleepiness: a new approach. Psychophysiology 1973;10:431-6. [DOI] [PubMed] [Google Scholar]
- 56.Collop NA, Tracy SL, Kapur V, et al. Obstructive sleep apnea devices for out-of-center (OOC) testing: technology evaluation. J Clin Sleep Med 2011;7:531-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Böhning N, Schultheiss B, Eilers S, et al. Comparability of pulse oximeters used in sleep medicine for the screening of OSA. Physiol Meas 2010;31:875-88. [DOI] [PubMed] [Google Scholar]
- 58.Netzer N, Eliasson AH, Netzer C, et al. Overnight pulse oximetry for sleep-disordered breathing in adults: a review. Chest 2001;120:625-33. [DOI] [PubMed] [Google Scholar]
- 59.Anandan C, Nurmatov U, van Schayck OC, et al. Is the prevalence of asthma declining? Systematic review of epidemiological studies. Allergy 2010;65:152-67. [DOI] [PubMed] [Google Scholar]
- 60.Tilert T, Dillon C, Paulose-Ram R, et al. Estimating the U.S. prevalence of chronic obstructive pulmonary disease using pre- and post-bronchodilator spirometry: the National Health and Nutrition Examination Survey (NHANES) 2007-2010. Respir Res 2013;14:103. [DOI] [PMC free article] [PubMed] [Google Scholar]