Abstract
Aims
AUGMENT-HF was an international, multi-centre, prospective, randomized, controlled trial to evaluate the benefits and safety of a novel method of left ventricular (LV) modification with alginate-hydrogel.
Methods
Alginate-hydrogel is an inert permanent implant that is directly injected into LV heart muscle and serves as a prosthetic scaffold to modify the shape and size of the dilated LV. Patients with advanced chronic heart failure (HF) were randomized (1 : 1) to alginate-hydrogel (n = 40) in combination with standard medical therapy or standard medical therapy alone (Control, n = 38). The primary endpoint of AUGMENT-HF was the change in peak VO2 from baseline to 6 months. Secondary endpoints included changes in 6-min walk test (6MWT) distance and New York Heart Association (NYHA) functional class, as well as assessments of procedural safety.
Results
Enrolled patients were 63 ± 10 years old, 74% in NYHA functional class III, had a LV ejection fraction of 26 ± 5% and a mean peak VO2 of 12.2 ± 1.8 mL/kg/min. Thirty-five patients were successfully treated with alginate-hydrogel injections through a limited left thoracotomy approach without device-related complications; the 30-day surgical mortality was 8.6% (3 deaths). Alginate-hydrogel treatment was associated with improved peak VO2 at 6 months—treatment effect vs. Control: +1.24 mL/kg/min (95% confidence interval 0.26–2.23, P = 0.014). Also 6MWT distance and NYHA functional class improved in alginate-hydrogel-treated patients vs. Control (both P < 0.001).
Conclusion
Alginate-hydrogel in addition to standard medical therapy for patients with advanced chronic HF was more effective than standard medical therapy alone for improving exercise capacity and symptoms. The results of AUGMENT-HF provide proof of concept for a pivotal trial.
Trial Registration Number
Keywords: Advanced chronic heart failure, Alginate-hydrogel, Exercise capacity, Symptoms, Safety
See page 2276 for the editorial comment on this article (doi:10.1093/eurheartj/ehv283)
Introduction
Heart failure (HF) is a major health problem worldwide with 5-year mortality rates that exceed 50%.1 As HF progresses, the heart undergoes progressive left ventricular (LV) remodelling. According to the principle of Laplace's law, as the LV dilates and LV wall thins, and wall stress increases, resulting in continued myocardial damage. Unless an intervention can break this deleterious spiral of events, HF will continue to progress with worsening LV dilation. Left ventricular remodelling is maladaptive and its progression contributes to worsening of clinical symptoms, marked exercise intolerance, and congestion, all of which culminate in hospitalizations due to HF decompensation and premature death of the afflicted patient.
Despite recent advances in therapy, morbidity and mortality resulting from HF remain unacceptably high.2 The use of tissue engineering principles to improve myocardial functionality has shown encouraging preclinical results.3–5 The concept of LV modification or restoration with the intra-myocardial injection of an alginate-based polymer results in increased wall thickness and a change of LV geometry.6 In a canine model of HF, LV injection/implantation with an alginate-based formulation led to improvement in indexes of LV systolic function without negatively impacting LV relaxation or filling.7 In a prior clinical study evaluating the safety and feasibility of alginate-hydrogel administered at the time of cardiac bypass surgery, there were observations of an increase in LV wall thickness, reduction of end-diastolic volume, and end-systolic volume as well as decreases in myocardial wall stress at end-diastole and end-systole over 3–6 months.8
The AUGMENT-HF clinical trial was designed to evaluate the benefits and safety of treatment with alginate-hydrogel in patients with advanced chronic HF.
Methods
Study design and protocol
AUGMENT-HF was an international, multi-centre, prospective, randomized, controlled clinical trial of alginate-hydrogel in patients with advanced chronic HF. Eligible patients were randomly allocated to receive either alginate-hydrogel in addition to standard medical therapy or standard medical therapy alone (Control). Randomization was stratified for two variables: aetiology of cardiomyopathy (ischaemic vs. non-ischaemic) and baseline peak VO2 (greater than or ≤12.5 mL/kg/min). The study included patients aged 18–79 years, who had a peak VO2 of 9.0–14.5 mL/kg/min, a left ventricular ejection fraction (LVEF) ≤35%, a left ventricular end-diastolic dimension indexed to body surface area of 30–40 mm/m2, and were required to be on stable, evidence-based therapy for HF. Primary exclusion criteria were an LV wall thickness <0.8 cm (mid-ventricular level), a serum creatinine >2.5 mg/dL, clinically significant liver enzyme abnormalities, Q-wave myocardial infarction (MI) within the last 30 days, or a history of stroke within 60 days.
Core laboratories, blinded to treatment assignment, conducted the evaluation of key measures of cardiopulmonary exercise (CPX) testing (peak VO2), cardiac imaging (echocardiography), Holter monitors, and laboratory evaluations. Blinded endpoint assessment was used to compensate partially for the fact that the trial could not be truly double blind. Sham thoracotomy and intra-myocardial injection (with no prospect of improvement) in the control patients were not considered ethically acceptable. We used, as the basis for the primary endpoint peak VO2, which is objective in as much as it measures an objective physiological variable, recognizing it remains subject to bias in the form of differential effort during exercise testing, in that the patient knows whether or not he/she had undergone surgery. We measured peak achieved respiratory exchange rate (RER) as a measure of exercise effort, to assess if the randomized groups would demonstrate any differential effort in follow-up exercise tests. An independent clinical events committee (CEC) adjudicated all events suggestive of study endpoints, including major adverse cardiac events (MACE), whilst blinded to treatment group allocation. Major adverse cardiac events were defined as cardiac death, cardiac arrest, MI, sustained ventricular arrhythmias, pulmonary oedema, acute HF, unstable angina, and major bleeding. A Data Safety Management Board provided an independent ongoing assessment of safety.
The study was conducted at 14 centres in Australia, Germany, Italy, the Netherlands, and Romania. The protocol was approved by the regulatory authorities in each country and the local Ethics Committees. The study was conducted in accordance with the principles of the Declaration of Helsinki, the International Conference on Harmonization Good Clinical Practice guidelines, and local and national regulations. Written informed consent was provided by all patients.
The trial protocol defined an initial study report on efficacy (exercise tolerance) to be generated after every patient had been followed for a minimum of 6 months and a final ‘extended follow-up phase’ study report focusing on long-term safety after 24 months follow-up. First enrolment occurred in May 2012, and enrolment was completed in April 2014. This paper provides the report on the primary results analyses for 6 months of follow-up.
Investigations
After signing informed consent, each patient underwent a baseline screening assessment including CPX testing and assessment of 6-min walk test (6MWT) distance. In addition, at specific visits echocardiography assessments were performed, quality-of-life questionnaires were completed, and safety blood samples were taken. To investigate for potential ventricular ectopy, 24-h-Holter monitoring was performed at screening as well as 3 and 6 months. Holter recordings were digitally stored and assessed by central blinded review (BioClinica Cardiovascular Safety Services).
Patients were required to perform two CPX tests within 30 days of randomization and performed at least 20 h apart that differed by no >15% in the observed value for peak VO2 (as per Core laboratory report). Cardiopulmonary exercise could be repeated a third time, if needed. Patients meeting the entry criteria (based on the mean of the last two assessments) were randomized to alginate-hydrogel or Control.
Training on standardized procedures for conduct of CPX testing and validation testing were required for each clinic prior to study initiation. Revalidation was required every 6 months. Cardiopulmonary exercise data were uploaded to the blinded core lab for analysis and the core laboratory provided rapid feedback on test quality for each test. Analyses of CPX measures were determined from averaged 10 s gas exchange data from the start to the end of exercise. Patients returned to the clinic at 3 and 6 months post-discharge for follow-up evaluations. Patients were required to complete one CPX test at 3 months, and two CPX tests at the 6 months (with the average value for these two tests being employed in analyses).
Therapy
Patients randomized to the investigational device group had alginate-hydrogel (calcium-alginate-hydrogel) administered during a surgical procedure as previously described.8 The approximate locations of alginate-hydrogel injections and technical details are presented in Figure 1.
Figure 1.
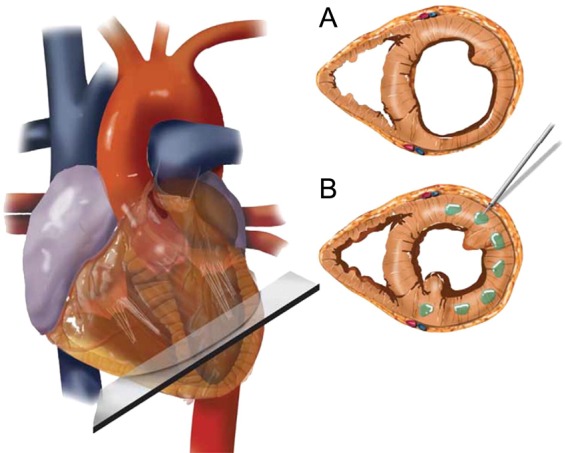
Algisyl-LVR implantation. Standardization of the placement of the implants at the mid-ventricular level identified as the halfway point between the left ventricular base (defined as the atrioventricular groove) and apex (A). The implants are equally spaced in the mid-myocardium starting at the antero-septal groove and ending at the postero-septal groove (B). Note: alginate-hydrogel (LoneStar Heart, Inc., Laguna Hills, CA, USA) is a proprietary calcium-alginate-hydrogel that consists of two components: an Na+-alginate component supplied as a sterile aqueous solution with 4.6% mannitol and a Ca2+-alginate component consisting of water insoluble particles suspended in a sterile 4.6% mannitol solution. These two components are mixed immediately before use, and then combined in one syringe for delivery as intra-myocardial injections. Alginate-hydrogel is administered during a surgical procedure. A left thoracotomy is performed to expose the heart and the pericardium. The left ventricular free wall was identified and the injection sites marked with a surgical marker. In total, 10–19 intra-myocardial injections were made on the beating heart, circumferentially, at the left ventricular mid-ventricular level halfway between the left ventricular apex and base starting at the antero-septal groove and ending at the postero-septal groove. Left ventricular wall thickness must not have been <8 mm at the locations where the alginate-hydrogel was to be injected. The total number of injections administered for an individual patient depended on the size of the heart (amount of space available on the LV-free wall). All injections were made along or within ∼1 cm of a single mid-ventricular line, being careful to avoid any visible coronary vasculature. Standardization of the placement of the implants was performed via the identification of standard anatomical features (atrioventricular groove and apex). Injections of 0.3 mL of alginate-hydrogel were separated by ∼0.5–1 cm and made at the mid-wall depth of the myocardium.
Statistical methods
The primary efficacy endpoint in this study was the change in peak VO2 from baseline to 6 months. The primary safety objective was to estimate the 30-day mortality associated with the implantation of alginate-hydrogel. Six-minute walk test distance, quality of life as measured by Kansas City Cardiomyopathy Questionnaire (KCCQ), patient global assessment (PGA), New York Heart Association (NYHA) functional class, CPX measures of peak watts and total exercise time, and measures of echocardiographic imaging were all pre-specified secondary endpoints. Statistical significance was attached to P-values of <0.05 (SAS version 9.3; SAS institute).
Data analysis were performed according to intention to treat. The primary and secondary efficacy analyses were performed on the modified intention-to-treat (mITT) analysis population, which included all randomized patients (Control group) and for the alginate-hydrogel group, all patients randomized to the alginate-hydrogel group for whom the surgery to implant the alginate-hydrogel device was started. The safety analysis dataset comprised all randomized patients. No imputation was performed for missing data.
To test the group differences for the primary outcome, a repeated-measures mixed model was used with an unstructured covariance matrix to model the within-patient variability. The same model was used to test the group differences for echocardiographic imaging results and KCCQ data. It was pre-specified that non-normally distributed data were to be analysed using non-parametric testing, which was the case for 6MWT distance. The treatment effect for NYHA functional class at 6 months was compared by means of logistic regression with ordinal polytomous response adjusted for baseline.
Cumulative survival curves for the time-to-event analyses were constructed according to the Kaplan–Meier method and differences were examined by the log-rank statistic. The Cox proportional hazards regression (SAS proc phreg procedure) was used to estimate the hazard ratios with treatment as the only covariate. Event rates were expressed as the percentage of events per 100 patient-years of follow-up, taking into account the censoring of follow-up data. The repeated-measures analysis for categorical variables was done using the SAS proc logistic procedure including terms for treatment and baseline value.
The primary safety endpoint for the study was 30-day all-cause mortality in patients randomized to alginate-hydrogel for whom the alginate-hydrogel device was implanted. The starting point for the 30-day count was the start of surgery. The 30-day all-cause mortality associated with the implantation of the alginate-hydrogel device was to be qualitatively compared with the observed rate of 5%, estimated based upon three recently completed clinical trials that investigated similar patient populations and evaluated surgical ventricular reconstruction or the CorCap device.9,10 Assuming the 30-day mortality rate is consistent with the estimated 5% rate and a sample size of N = 38, the estimate was that the observed mortality in this study would be between 0.1 and 17.4% with 95% confidence (calculations performed using PASS 2008 and the Exact (Clopper Pearson) confidence interval formula). Based on the binomial distribution, a sample size of 38 patients, and a probability of post-surgical mortality equal to 5%, the probability of observing four or fewer deaths was 96.0%. The probability of observing five or more deaths was 4.0%.
Results
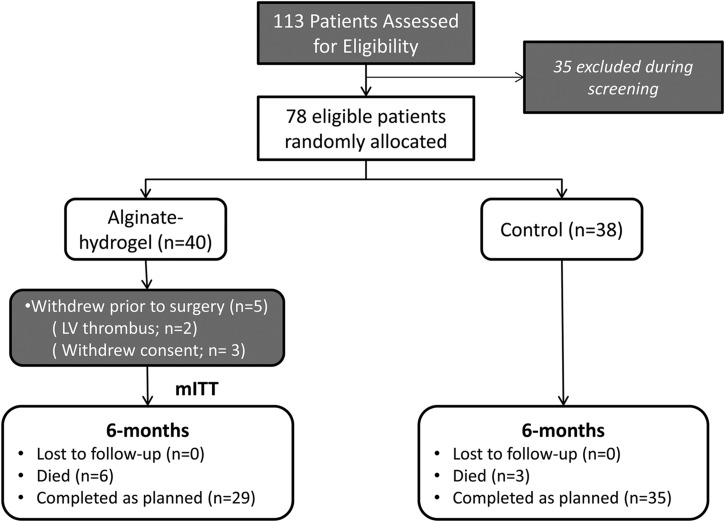
In total, 113 patients were screened for the study, and 35 patients were found to be ineligible or declined participation at screening. A total of 78 patients were randomized (1 : 1) to alginate-hydrogel (n = 40) and Control (n = 38). The procedure to implant the alginate-hydrogel device was not performed in five patients randomized to this treatment group. Two of these patients were found to have a LV thrombus (echocardiography) on the day prior to the planned surgical procedure and deemed therefore ineligible. Three patients withdrew consent after randomization to the alginate-hydrogel group and prior to the surgical procedure. All other patients randomized to the alginate-hydrogel (n = 35) underwent a successful surgical procedure to implant the device; there were no failures to implant the device.
Demographics and baseline characteristics for the mITT population are summarized in Table 1. All baseline characteristics were comparable between groups. The patients enrolled showed significant LV dysfunction and significantly reduced functional capacity with a mean LVEF of 26 ± 5%, and a mean peak VO2 of 12.2 ± 1.8 mL/kg/min. Baseline concomitant HF medications are summarized in Table 2.
Table 1.
Baseline demographics
| Alginate-hydrogel (n = 35)a | Control (n = 38) | |
|---|---|---|
| Age (years) | 63.1 ± 10.1 | 62.1 ± 9.2 |
| Male | 27 (77%) | 34 (90%) |
| Ethnicity (white) | 35 (100%) | 38 (100%) |
| Ischaemic HF | 20 (57%) | 22 (58%) |
| Non-ischaemic HF | 15 (42.9%) | 16 (42.1%) |
| NYHA functional class (mean) | 2.9 ± 0.4 | 2.8 ± 0.5 |
| Class II/III/IV | 5/28/2 | 9/26/3 |
| LVEF (%) | 25.4 ± 5.3 | 25.6 ± 5.0 |
| Peak VO2 (mL/kg/min) | 12.1 ± 1.8 | 12.2 ± 1.8 |
| 6MWT distance (m) | 275 ± 86 | 310 ± 80 |
| Mitral regurgitation ≥3+ | 15 (43%) | 22 (58%) |
| Hypertension | 20 (57%) | 23 (61%) |
| Diabetes | 12 (34%) | 17 (45%) |
| Atrial fibrillation | 16 (40.0%) | 37 (47.4%) |
| Stroke (CVA) | 4 (11%) | 5 (13%) |
| Prior myocardial infarction | 22 (55.0%) | 39 (50.0%) |
| Previous PCI or CABG | 9 (26%) | 11 (29%) |
| CRT | 5 (14%) | 5 (13%) |
| ICD | 10 (29%) | 9 (24%) |
Control, standard medical therapy alone; n, number of patients; 6MWT, 6-min walk test; HF, heart failure; CVA, cardio vascular accident; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting.
aModified intention-to-treat population; data are mean ± SD or patients (%).
Table 2.
Concomitant heart failure and cardiac medications at baseline
| Alginate-hydrogel (n = 35) | Control (n = 38) | |
|---|---|---|
| Diuretics | 34 (97%) | 38 (100%) |
| β-Blockers | 33 (94%) | 37 (97%) |
| ARB/ACEi | 30 (86%) | 35 (92%) |
| Mineralocorticoid receptor antagonists | 26 (74%) | 25 (66%) |
| Anti-thrombotics or anti-platelet agentsa | 34 (97%) | 38 (100%) |
| Anti-platelet aggregation agentsa | 27 (77%) | 21 (55%) |
| Anti-coagulanta | 17 (49%) | 24 (63%) |
| Lipid lowering | 25 (71%) | 27 (71%) |
Data are number of patients.
Control, standard medical therapy alone; n, number of patients.
aNot mutually exclusive.
For the patients undergoing the alginate-hydrogel procedure, the mean procedure duration was 81 ± 25 min, ranging from 50 to 160 min. The median ICU length of stay (LOS) was 2 days and median hospital LOS was 15 days. The mean number of intra-myocardial implants/injections for patients undergoing the alginate-hydrogel procedure was 16 ± 2, ranging from 11 to 19 (Table 3).
Table 3.
Summary details of operative procedure and index hospitalization
| Alginate-hydrogel (n = 35)a | |
|---|---|
| Procedure duration (min) | |
| Mean (SD) | 80.5 (24.9) |
| Median (min–max) | 78 (50–160) |
| Anesthesia duration (min) | |
| Mean (SD) | 190 (29) |
| Median (min–max) | 184 (120–240) |
| Total number of alginate-hydrogel implants (injections) | |
| Mean (SD) | 15.5 (2.0) |
| Median (min–max) | 15 (11–19) |
| Total volume administered (mL) | |
| Mean (SD) | 4.6 (0.6) |
| Median (min–max) | 4.5 (3.3–5.7) |
| ICU length of stay (days) | |
| Mean (SD) | 4.3 (7.3) |
| Median (min–max) | 2 (1–43) |
| Hospital length of stay (days) | |
| Mean (SD) | 18.4 (13.0) |
| Median (min–max) | 15 (7–65) |
Control, standard medical therapy alone; n, number of patients; SD, standard deviation; min, minimum; max, maximum; perc, percentile; ICU, intensive care unit; Index surgical procedure, procedure to implant the alginate-hydrogel device.
aModified intention-to-treat population.
Cardiopulmonary exercise testing
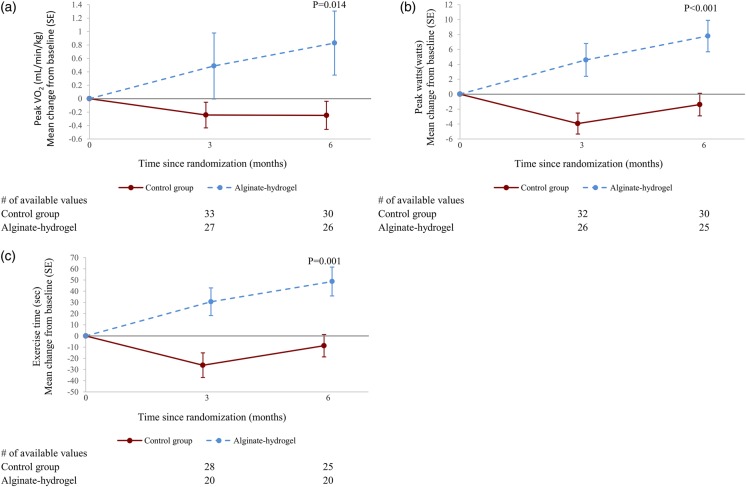
Mean peak VO2 gradually increased over time in patients in the alginate-hydrogel group while it remained unchanged in the Control group (Figure 2A). Alginate-hydrogel treatment was associated with improvement in peak VO2 compared with Control treatment; the mean treatment effect was an increase of 1.24 mL/kg/min (95% confidence interval, CI 0.26–2.23, P = 0.014).
Figure 2.
(A) Peak VO2 at 6 months: mean change (from baseline). (B) Peak watts at 6 months: mean change (from baseline). (C) Maximum exercise time at 6 months: mean change (from baseline). Control, standard medical therapy; SE, standard error; P-value, (adjusted) mean product effect.
The improvement in peak VO2 was accompanied by a 1.0 min (P = 0.001) improvement in total treadmill exercise time (Figure 2B) and a 10 W improvement in maximum workload (P < 0.001). The peak exercise RER remained unchanged over time from baseline (1.02 ± 0.09) to 3 months (1.03 ± 0.12) and 6 months (1.02 ± 0.11).
In subgroup analyses, neither aetiology of HF (ischaemic vs. non-ischaemic; P-value for interaction 0.066) nor baseline peak VO2 (greater than or ≤12.5 mL/kg/min; P-value for interaction 0.23) had a significant interaction for treatment effect. Also for 15 pre-specified subgroups analysed, there were no significant treatment-by-subgroup interactions for the primary endpoint with the exception of the subgroups of patients split by median 6MWT distance at baseline (P-value for interaction 0.014). Regional analysis did not find a significant interaction for treatment effect; results were the same for patients from Romania (n = 45) compared with patients from all other countries (n = 28).
Six-minute walk test distance
Mean 6MWT distance increased over time in patients in the alginate-hydrogel group while mean 6MWT distance remained unchanged or declined for patients in the Control group (P < 0.001, change in median 6MWT distance +141 m, Figure 3).
Figure 3.
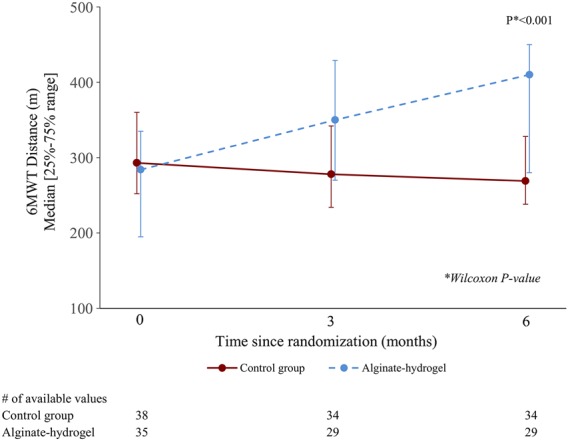
Six-minute walk test distance at 6 months. Control, standard medical therapy; 6MWT, 6-min walk test.
New York Heart Association assessment
The NYHA functional class at 6 months was improved in the Algisyl group, with 84% having an NYHA functional class I or II, when compared with 26% in the Control group (odds ratio for improvement by one class: 30.2; 95% CI 5.7–160.5, P < 0.001, Figure 4).
Figure 4.
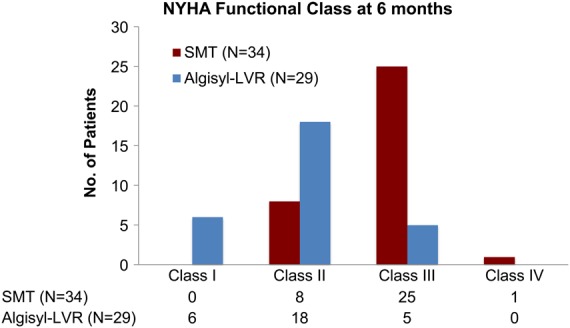
New York Heart Association functional class at 6 months. Control, standard medical therapy; n, number of available values.
Quality of life
There were no statistically significant differences between groups for any of the 10 KCCQ domain scores (Table 4). The self-reported PGA at 6 months was improved in the Algisyl group with >55% of patients reporting that they were much or moderately improved, when compared with 28% of patients in the placebo group (odds ratio for being in a better rank, 3.2; 95% CI 1.2–8.6, P = 0.019).
Table 4.
Mean (Kansas City Cardiomyopathy Questionnaire) scores and changes from screening values
| KCCQ domain | Visit | Alginate-hydrogel |
Control |
P-value between groups | ||
|---|---|---|---|---|---|---|
| n | Mean ± SD | n | Mean ± SD | |||
| Overall summary | Baseline | 35 | 47 ± 21 | 37 | 49 ± 22 | |
| 6-month | 29 | 65 ± 25 | 34 | 60 ± 23 | 0.12 | |
| Clinical summary | Baseline | 35 | 52 ± 21 | 37 | 55 ± 22 | |
| 6-month | 29 | 69 ± 26 | 34 | 64 ± 21 | 0.14 | |
| Quality of life | Baseline | 35 | 39 ± 27 | 37 | 42 ± 26 | |
| 6-month | 29 | 63 ± 27 | 34 | 54 ± 26 | 0.066 | |
| Total symptom | Baseline | 35 | 58 ± 22 | 37 | 61 ± 25 | |
| 6-month | 29 | 74 ± 26 | 34 | 68 ± 22 | 0.11 | |
| Social limitation | Baseline | 35 | 45 ± 27 | 36 | 45 ± 28 | |
| 6-month | 28 | 60 ± 32 | 33 | 58 ± 28 | 0.46 | |
| Self-efficacy | Baseline | 35 | 71 ± 25 | 37 | 78 ± 18 | |
| 6-month | 28 | 80 ± 21 | 34 | 79 ± 18 | 0.16 | |
| Symptom burden | Baseline | 35 | 60 ± 23 | 37 | 61 ± 25 | |
| 6-month | 29 | 74 ± 26 | 34 | 68 ± 22 | 0.22 | |
| Symptom frequency | Baseline | 35 | 55 ± 24 | 37 | 62 ± 25 | |
| 6-month | 29 | 74 ± 28 | 34 | 67 ± 24 | 0.079 | |
| Symptom stability | Baseline | 34 | 52 ± 21 | 37 | 52 ± 27 | |
| 6-month | 29 | 61 ± 22 | 34 | 48 ± 25 | 0.072 | |
| Physical limitation | Baseline | 35 | 47 ± 21 | 37 | 48 ± 25 | |
| 6-month | 29 | 64 ± 27 | 34 | 61 ± 24 | 0.31 | |
Control, standard medical therapy alone; n, number of available values; SD, standard deviation.
Echocardiographic findings
Echocardiograms were performed at baseline and again at 3 and 6 months. While a majority of patients had an interpretable pre- and post-study, there was a significant amount of missing data due to various technical limitations for many studies. There were no statistically significant differences between treatment groups for any of the echocardiographic measures (Table 5).
Table 5.
Transthoracic echocardiogram mean (standard deviation) values and changes from screening values for selected parameters
| Visit | Alginate-hydrogel |
Control |
P-value between groups | |||
|---|---|---|---|---|---|---|
| n | Mean ± SD | n | Mean ± SD | |||
| LVEDD (cm) | Baseline | 33 | 6.3 ± 0.40 | 34 | 6.4 ± 0.50 | |
| 6-month | 26 | 6.0 ± 0.42 | 33 | 6.2 ± 0.47 | 0.17 | |
| LVESD (cm) | Baseline | 33 | 5.5 ± 0.52 | 34 | 5.7 ± 0.56 | |
| 6-month | 26 | 5.2 ± 0.65 | 34 | 5.4 ± 0.59 | 0.091 | |
| LVEF (%) | Baseline | 34 | 25 ± 5 | 36 | 26 ± 5 | |
| 6-month | 28 | 28 ± 5 | 34 | 28 ± 6 | 0.61 | |
| LV mass (g) | Baseline | 33 | 296 ± 59 | 34 | 317 ± 59 | |
| 6-month | 25 | 275 ± 63 | 33 | 300 ± 56 | 0.44 | |
Control, standard medical therapy alone; n, number of available values; SD, standard deviation.
Safety profile
In total, three deaths occurred during the 30-day period after the surgical procedure to implant the alginate-hydrogel device. The 30-day absolute mortality rate was 8.57% (95% CI: 1.80–23.06%). The 30-day absolute mortality rate in the Control group (no surgery) was 0%. The major surgical complication rate for the alginate-hydrogel device procedure was 25% (safety population).
The overall incidence of serious adverse events (SAEs) through 6 months of follow-up was not significantly different between the alginate-hydrogel and Control group (Table 6). There were also no statistically significant differences between treatment and control for any of the categories of SAEs by body system or preferred term. The incidence of SAEs of Cardiac Disorders was generally reported to be more common among patients in the Control group compared with patients in the alginate-hydrogel group.
Table 6.
Summary of adverse events
| Alginate-hydrogel (n = 35) |
Control (n = 38) |
HR (95% CI) | P | |||
|---|---|---|---|---|---|---|
| Total no. of events | No. of patients with events (incidence per 100 patient-years at risk) | Total no. of events | No. of patients with events (incidence per 100 patient-years at risk) | |||
| All adverse events | 115 | 31 (489.1) | 63 | 17 (119.1) | 3.41 (1.87–6.22) | <0.001 |
| Serious adverse events | 33 | 16 (135.4) | 26 | 10 (63.0) | 2.08 (0.94–4.60) | 0.063 |
Control, standard medical therapy alone; n, number of patients; HR, hazard ratio; CI, confidence interval. P, log-rank P-value.
Overall, MACE, excluding the index hospitalization, were lower for patients receiving alginate-hydrogel and appears to be due to substantially lower rates of worsening HF and sustained ventricular arrhythmias in patients receiving the alginate-hydrogel device. The incidence of CEC adjudicated secondary safety endpoints and MACE are summarized in Tables 7 and 8. The study was not powered to detect differences in either overall MACE or specific categories of events. The listing of causes for non-heart failure hospitalizations is provided in Table 10.
Table 7.
Major adverse cardiac events excluding index hospitalization
| Event | Modified ITT |
HR (95% CI) | P-value | ITT |
HR (95% CI) | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alginate-hydrogel (n = 35) |
Control (n = 38) |
Alginate-hydrogel (n = 40) |
Control (n = 38) |
|||||||||
| Total no. of events | No. of patients with events (incidence per 100 patient-years at risk) | Total no. of events | No. of patients with events (incidence per 100 patient-years at risk) | Total no. of events | No. of patients with events (incidence per 100 patient-years at risk) | Total no. of events | No. of patients with events (incidence per 100 patient-years at risk) | |||||
| All-cause death | 6 | 6 (38.4) | 3 | 3 (16.6) | 2.32 (0.58–9.26) | 0.22 | 6 | 6 (37.3) | 3 | 3 (16.6) | 2.26 (0.57–9.05) | 0.24 |
| Cardiovascular death | 5 | 5 (32.0) | 3 | 3 (16.6) | 1.94 (0.46–8.11) | 0.36 | 5 | 5 (31.1) | 3 | 3 (16.6) | 1.89 (0.45–7.93) | 0.37 |
| MACE events (excluding index hospitalization) | 9 | 7 (47.2) | 22 | 10 (61.3) | 0.78 (0.30–2.04) | 0.61 | 9 | 7 (45.8) | 22 | 10 (61.3) | 0.76 (0.29–2.00) | 0.58 |
| All-cause hospitalization | 18 | 10 (76.4) | 27 | 12 (75.9) | 1.01 (0.44–2.34) | 0.98 | 18 | 10 (73.9) | 27 | 12 (75.9) | 0.98 (0.42–2.27) | 0.96 |
| Heart failure hospitalization | 5 | 4 (26.8) | 14 | 8 (48.4) | 0.56 (0.17–1.85) | 0.33 | 5 | 4 (26.0) | 14 | 8 (48.4) | 0.54 (0.16–1.80) | 0.31 |
| All-cause hospitalization or CV death | 23 | 13 (99.6) | 30 | 12 (75.9) | 1.30 (0.59–2.85) | 0.51 | 23 | 13 (96.2) | 30 | 12 (75.9) | 1.26 (0.57–2.76) | 0.56 |
| HF hospitalization or CV death | 10 | 9 (60.7) | 17 | 8 (48.4) | 1.25 (0.48–3.25) | 0.64 | 10 | 9 (58.9) | 17 | 8 (48.4) | 1.22 (0.47–3.17) | 0.68 |
Major adverse cardiac event are defined as cardiac death, cardiac arrest, myocardial infarction, sustained ventricular arrhythmias, pulmonary oedema, acute HF, unstable angina, and major bleeding.
P-value, log-rank P-value; MACE, major adverse cardiac events; HF, heart failure; CV, cardiovascular; N, number of patients; HR, hazard ratio; CI, confidence interval.
Table 8.
Overall major adverse cardiac events
| Event | Modified ITT |
ITT |
||||||
|---|---|---|---|---|---|---|---|---|
| Alginate-hydrogel (n = 35) |
Control (n = 38) |
Alginate-hydrogel (n = 40) |
Control (n = 38) |
|||||
| Total no. of events | No. of patients (%) | Total no. of events | No. of patients (%) | Total no. of events | No. of patients (%) | Total no. of events | No. of patients (%) | |
| Overall MACE | 18 | 11 (31.4%) | 22 | 10 (26.3%) | 18 | 11 (27.5%) | 22 | 10 (26.3%) |
| MACE during index hospitalization | 9 | 6 (17.0%) | NA | 9 | 6 (15.0%) | NA | ||
| Cardiovascular death | 2 | 2 (5.7%) | NA | 2 | 2 (5.0%) | NA | ||
| Cardiac arrest | 2 | 2 (5.7%) | NA | 2 | 2 (5.0%) | NA | ||
| Worsening HF | 1 | 1 (2.9%) | NA | 1 | 1 (2.5%) | NA | ||
| Major bleeding | 3 | 3 (8.6%) | NA | 3 | 3 (7.5%) | NA | ||
| Sustained ventricular arrhythmias | 1 | 1 (2.9%) | NA | 1 | 1 (2.5%) | NA | ||
Major adverse cardiac events are defined as cardiac death, cardiac arrest, myocardial infarction, sustained ventricular arrhythmias, pulmonary oedema, acute HF, unstable angina, and major bleeding.
Overall, MACE include all MACE from baseline to 6 months (including index hospitalization).
MACE, major adverse cardiac events; N, number of patients.
Table 10.
Listing of causes for non-heart failure hospitalizations
| Patient ID | Study group | Hospitalization | Investigator event term | Fatal outcome | CEC adjudicated |
|---|---|---|---|---|---|
| AU-38-0010 | Alginate-hydrogel | 1 | Central chest pain | No | NA |
| 2 | Continuation of atypical centralized chest pain | ||||
| AU-38-0070 | Alginate-hydrogel | 3 | Acute injury of chronic renal failure | No | NA |
| Worsening bilateral peripheral oedema | |||||
| IT-20-0016 | Alginate-hydrogel | 4 | Lobar right pneumonia | No | NA |
| IT-20-0094 | Alginate-hydrogel | 5 | Erysipelas bilateral lower limbs | No | NA |
| 6 | Worsening HF | ||||
| Septic shock due to legionella pneumonia | |||||
| Acute cholangitis | |||||
| IT-20-0109 | Alginate-hydrogel | 7 | Hemisyndrome facio-brachio-crural right | No | NA |
| 8 | Hypertensive crisis associated with pulmonary oedema | ||||
| 9 | Respiratory infection | ||||
| 10 | Syndrome of Dressler | ||||
| NL-32-0026 | Alginate-hydrogel | 11 | Sepsis | Yes | CV |
| Haematoma at location of surgery wound | |||||
| Critical illness polyneuropathy syndrome secondary to sepsis | |||||
| RO-44-0019 | Alginate-hydrogel | 12 | Right-sided pneumonia with poliresistant Klebsiella | Yes | Non-CV |
| RO-44-0069 | Alginate-hydrogel | 13 | Cardiac transplant | Yes | CV |
| DE-16-0096 | Control | 14 | Worsening of mitral function | No | NA |
| 15 | Symptomatic persistent atrial fibrillation | ||||
| IT-18-0057 | Control | 16 | Symptomatic tachycardia | No | NA |
| IT-49-0048 | Control | 17 | Supraventricular tachycardia provoking two ICD shocks | No | NA |
| RO-44-0055 | Control | 18 | Syncope | No | NA |
| RO-44-0071* | Control | 19 | Worsening of HF | No | NA |
| RO-47-0054 | Control | 20 | Sustained ventricular tachycardia | No | NA |
| RO-48-0046 | Control | 21 | Interstitial pneumonia | No | NA |
| Planned hospitalization for assessment for cardiac transplantation | |||||
| 22 | Worsening of HF | ||||
| 23 | Planned hospitalization for cardiac catheterism | ||||
| 24 | Junctional rhythm | ||||
| 25 | Planned hospitalization for investigation for cardiac transplantation |
Analysis of 24-h-Holter monitors demonstrated no statistically significant differences between groups for measures of supraventricular or ventricular ectopy. There was no increase in ventricular arrhythmias observed for patients receiving alginate-hydrogel.
Discussion
A large number of patients have symptomatic HF, despite the use of all available guideline recommended treatments.11 The therapeutic options for patients with advanced HF who have become refractory to the currently available medical therapies (pharmacologic and device) are limited;12 there is an unmet need for new therapeutic options for this growing patient population.13
The AUGMENT-HF trial provides the first evidence that surgical treatment with alginate-hydrogel in addition to standard medical therapy was more effective than standard medical therapy alone for improving exercise capacity and symptoms in patients with advanced HF. The results of AUGMENT-HF provide proof of concept that intracardiac injection of alginate-hydrogel leads to beneficial effects in patients with advanced chronic HF and hence warrant further studies to validate the observed effects and to extend them in greater and possibly broader study populations with longer follow-up.
The baseline demographics for the study population represent a group of patients with advanced chronic HF that is very well treated. It is rare in clinical trials of ambulatory HF patients, that the patients studied show a mean peak VO2 at baseline that is as low as 12.2 mL/kg/min, with all patients having values of 14.5 mL/kg/min or less. These results are based on the average of two qualifying maximum exercise tests, and hence are not the result of a possible under-performance during the learning curve experience of patients.
We consider the treatment effect of 1.24 mL/kg/min for changes in peak VO2 over 6 months as an important observation for this patient population. It represents a 10.2% improvement over baseline. Sarullo et al. reported that clinically stable HF patients with a peak VO2 <12.2 mL/kg/min had a 1-year cardiovascular mortality of 66% and a 1-year cardiovascular hospitalization rate of 63%, while patients with a peak VO2 >12.2 mL/kg/min had rates of only 34 and 37%, respectively.14 Swank et al. reported that for every 6% increase in peak VO2 there was an 8% reduction in cardiovascular mortality or HF hospitalization and a 7% reduction in all-cause mortality.15 In this context, it is interesting that a secondary per-protocol analysis found that alginate-hydrogel provided an improvement in peak VO2 compared with patients in the Control group with a mean treatment effect of 1.59 mL/kg/min (P = 0.002).
It is an interesting observation that the mean 6MWT distance in the alginate-hydrogel group at baseline was substantially below the 300 m threshold and was observed to increase above the 300 m threshold at the 3- and 6-month follow-up visits. Several studies have demonstrated that a 6MWT distance of <300 m is strongly prognostic of subsequent mortality and hospital admission in stable chronic HF16,17 and in patients with advanced HF.18 Many consider that an improvement of >50 m in 6MWT distance is clinically very meaningful,19,20 and changes between 30 and 50 m for 6MWT distance are also often considered clinically relevant.21 The improvement of 6MWT distance for alginate-hydrogel-treated patients in AUGMENT-HF was >100 m, and hence was an interesting finding. Still, this finding needs to be validated.
The improvements in peak VO2, 6MWT, and NYHA class in AUGMENT-HF provide a demonstration of a possible clinical benefit for the alginate-hydrogel device treatment. The results provide proof of concept for this new treatment approach. The results require validation and extension in larger patient cohorts that are followed for longer periods of times. A pivotal trial to this end is in the planning phase.
The alginate-hydrogel implant acts as a permanent prosthetic scaffold that aims to reduce wall stress, and prevent further LV enlargement based on the physical principles described in Laplace law. Previously, surgical ventricular restoration (SVR) and devices employing LV reshaping strategies were evaluated in clinical studies such as the STICH trial25 the ACORN trial9 of the CorCap device (Acorn) and the PEERLESS-HF trial22 of the HeartNet device (Paracor Medical Inc.). The goals of SVR were to reduce the increased radius of curvature present in a dilated heart, and both the CorCap and HeartNet devices aimed to restrict and reverse LV dilatation; however, neither of these devices were approved for clinical use and the large multi-centre STICH trial failed to demonstrate a clinical benefit of SVR combined with CABG compared with CABG alone. More recently, other LV reshaping approaches include the Parachute device (Cardikinetix)23 to reduce the size of the LV cavity with an insert and the Revivent device (Bioventrix)24 to isolate big dysfunctional regions of the LV. Results from controlled randomized intervention trials are not yet available for these.
The procedural success of the alginate-hydrogel injection approach was 100% in this trial. The mean operative procedure time of 80.5 min demonstrated that in most patients the alginate-hydrogel device is implanted with relative ease in the context of a limited surgical procedure. These procedure metrics compare favourably with many current commonly performed ‘non-surgical’ procedures performed in patients with advanced structural heart disease. This is an important consideration, since both prolonged operative time and anaesthesia time are associated with increased rates of complications. The overall hospital LOS was longer than expected with a median of 15 days. However, there was no suggestion that this was a result of complications or a complicated post-operative course in general. Excluding all 22 patients who experienced an adverse event during the index hospitalization (within 30 days after surgery) results in a median LOS of 12.5 days, i.e. it did not significantly change overall hospital LOS. Hence, the longer than expected LOS appears to be a reflection of a cautious approach by investigators to discharge patients home following this initial experience with a novel device and procedure.
The 30-day all-cause mortality associated with the implantation of the alginate-hydrogel device was the primary safety endpoint for this study. In total, three deaths occurred during the 30-day period after the surgical procedure to implant the alginate-hydrogel device (8.57% in the mITT population, 95% CI 1.80–23.06%) (Table 9). This met the primary safety endpoint for the study, and it is comparable with mortality rates observed in prior reports of surgical device-based therapies for HF. For example, Mann et al.9 reported a 30-day mortality of 7.8% for advanced HF patients receiving the Acorn CorCap device, and Grossi et al.10 reported a 30-day mortality of 8.1% for advanced HF patients receiving the Myocor Coapsys device.
Table 9.
Listing of cause of death
| Patient ID | Study group | CEC adjudication |
Days post-randomization | |
|---|---|---|---|---|
| RO-48-0063 | Alginate-hydrogel | Cardiovascular | Intra-cranial haemorrhage | 28 |
| RO-44-0095 | Alginate-hydrogel | Cardiovascular | Sudden death witnessed | 32 |
| RO-47-0083 | Alginate-hydrogel | Cardiovascular | Non-haemorrhagic stroke | 33 |
| RO-44-0071 | Control | Cardiovascular | Cardiac arrest | 38 |
| RO-44-0017 | Control | Cardiovascular | Congestive HF/cardiogenic shock | 39 |
| RO-44-0019 | Alginate-hydrogel | Non-cardiovascular | Infection (including sepsis) | 69 |
| NL-32-0026 | Alginate-hydrogel | Cardiovascular | Congestive HF/cardiogenic shock | 85 |
| RO-44-0069 | Alginate-hydrogel | Cardiovascular | Other vascular: 10 days following cardiac transplant | 165 |
| IT-21-0035 | Control | Cardiovascular | Congestive HF/cardiogenic shock | 165 |
The major surgical complication rate for the alginate-hydrogel device procedure was 25%. Overall, 6-month mortality was higher for patients receiving the alginate-hydrogel device with six deaths compared with three in the Control group. The 30-day MACE rate during the index hospitalization was 15%. The 6-month MACE rate excluding the index hospitalization was lower for patients receiving alginate-hydrogel and mostly appears to have been attributed to lower rates of hospitalizations due to worsening HF. Conclusions from this information are not possible, as power is limited and none of the differences were significant.
An important consideration for assessing the risks of the alginate-hydrogel therapy will be observations of long-term mortality and longer term clinical benefits from longer follow-up and future studies. These data will provide important context on whether the early risks associated with surgery can be offset by later benefits.
A theoretical concern of intra-myocardial injections/implants is that they could be a basis for sustained ventricular arrhythmias as seen with the prior experience of myoblast therapy. Therefore, the absence of any increase in ventricular arrhythmia or ventricular ectopy in alginate-hydrogel patients is reassuring.
Thus the results of the AUGMENT-HF trial suggest that alginate-hydrogel can be administered safely in patients with advanced HF.
Limitations
A primary limitation of this current study is the lack of blinding for the assignment of patients to surgical device therapy. Endpoints such as exercise testing (which depend on patient effort) and subjective endpoints such as NYHA functional class can be subject to bias in a non-blinded trial. We used blinded core laboratory assessments wherever possible to generate valid data as much as possible. Additionally, the limited sample size (35 patients undergoing the surgical device procedure) requires some caution when drawing conclusions and a larger trial experience will be needed to validate the findings.
The mean RERs for the study cohort were <1.05 at all-time points and in both treatment groups. However, the 25th to 75th percentile of RER was 0.95–1.08 and the 25th to 75th percentile were also essentially unchanged across the visits. While an RER >1.05 or 1.10 is often used as a criterion to judge presence of a maximum effort during CPX, prior reports have observed that a very large percentage of chronic HF patients are unable to achieve an RER of >1.00 when performing the CPX test.28 The consistency of the RER over the course of the study and the duplication of exercise tests at important time points suggest that the changes in peak VO2 over time were not a result of changes in effort and support the consistency and reproducibility of the test results.
None of the echocardiographic measures in this study reached statistical significance for the group comparisons. This is not surprising given the small size with respect to measures of echocardiography. Additionally, many HF therapies require relatively long time periods to reach a maximal effect or demonstrate reverse remodelling. It is possible that a longer observation period is needed to realize the full impact of alginate-hydrogel treatment on measures LV remodelling. Future studies need to study cardiac function in more detail.
Conclusion
The AUGMENT-HF trial documents that surgical treatment with alginate-hydrogel was effective in improving exercise capacity and symptoms in patients with advanced chronic HF. The safety profile of this device treatment is acceptable. Further clinical trial experience is needed to validate these promising results.
Funding
This study was supported by research grants from LoneStar Heart, USA, the manufacturer of Algisyl-LVR. Funding to pay the Open Access publication charges for this article was provided by Lonestar Heart.
Conflict of interest: S.D.A., A.J.S.C., R.J.L., D.L.M., R.D., and H.N.S. have received consulting fees from LoneStar Heart for services provided as Scientific Advisory Board members. S.D.S. has received research funding from LoneStar Heart for service as the Echocardiography Core Laboratory. B.-A.K. has received research funding from LoneStar Heart for Data Management and Statistical Analysis services. F.A. and A.H. are employees of LoneStar Heart.
Acknowledgements
Clinical trial registration information: ClinicalTrials.gov NCT01311791 https://www.clinicaltrials.gov/ct2/show/NCT01311791. The authors thank Dr Fabio Miraldi and Dr Norman Tarazona for their invaluable support and training during the surgical procedures. Additionally, the authors thank Linda Clark and Karen Stephens for providing valuable clinical support to the research staff, physicians, and patients.
Appendix 1
Steering committee
Stefan D. Anker (chair), Douglas L. Mann (co-chair), Andrew J.S. Coats, Robert Dowling, and Hani N. Sabbah
Clinical events committee
Nicolas Danchin (Chair), Gerasimos Filippatos, and Jean-Louis Mas
Data safety monitoring committee
Piotr Ponikowski (Chair), Sidney Goldstein, Tim Clayton, and Arjang Ruhparwar
Investigators/centers
The study was performed in centers of 5 countries (Table 11).
Australia: Prof. Tony Dart, Heart Center at the Alfred, Melbourne
Germany: Prof. Robert Bauernschmitt, Universitätsklinikum Ulm, Ulm; Dr Hakim-Maibodi, Herz- und Diabeteszentrum Nordrhein Westfalen, Bad Oeynhausen; Prof. Klaus Matschke, Herzzentrum Dresden Universitätsklinik, Dresden
Italy: Dr Ottavio Alfieri, Instituto Scientifico Univ. San Raffaele, Milan; Prof. Antonello Gavazzi, Azienda Ospedaliera Ospedali Riuniti di Bergamo, Bergamo; Prof. Fabio Miraldi, Policlinico Umberto I Rome, Rome; Dr Andrea Mortara, Policlinico di Monza, Monza; Dr Enrico Pusineri, IRCCS Policlinico San Donato, San Donato; Prof. Pirelli Salvatore, Istituti Ospitalieri di Cremona, Cremona; Prof. Maurizio Volterrani, IRCCS San Raffaele, Rome
The Netherlands: Dr Benno Rensing, St. Antonius Ziekenhuis Nieuwegein, Nieuwegein
Romania: Prof. Garbiel Cristian, Military Hospital, Bucharest; Dr Dinu Dragomir, Spitalul Clinic De Urgenta MAI, Bucharest; Dr Sorin Stamate, Clinica de Cardiologie Spitalul Clinic Urgenta, Bucharest
Table 11.
Assignment of patients by country
| Informed consent | Screening failure | Randomized (% of randomized) |
Surgery (% of randomized to Algisyl-LVR) |
|||
|---|---|---|---|---|---|---|
| Control | Alginate-hydrogel | Did not perform surgery | Alginate-hydrogel implanted | |||
| All patients | 113 | 35 (31.0%) | 38 (48.7%) | 40 (51.3%) | 5 (12.5%) | 35 (87.5%) |
| Australia | 3 | 1 (33.3%) | 0 (0.0%) | 2 (100.0%) | 0 (0.0%) | 2 (100.0%) |
| Germany | 7 | 4 (57.1%) | 2 (66.7%) | 1 (33.3%) | 1 (100.0%) | 0 (0.0%) |
| Italy | 45 | 21 (46.7%) | 10 (41.7%) | 14 (58.3%) | 2 (14.3%) | 12 (85.7%) |
| The Netherlands | 5 | 3 (60.0%) | 1 (50.0%) | 1 (50.0%) | 0 (0.0%) | 1 (100.0%) |
| Romania | 52 | 5 (9.6%) | 25 (53.2%) | 22 (46.8%) | 2 (9.1%) | 20 (90.9%) |
Data are number of patients (%).
Appendix 2
Figure A1
Figure A1.
Consort diagram.
References
- 1.McMurray JJ, Stewart S. Heart failure: epidemiology, aetiology, and prognosis of heart failure. Heart 2000;83:596–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen J, Normand SL, Wang Y, Krumholz HM. National and regional trends in heart failure hospitalization and mortality rates for medicare beneficiaries, 1998–2008. JAMA 2011;306:1669–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mihardja SS, Gonzales JA, Gao D, Sievers RE, Fang Q, Stillson CA, Yu J, Peng M, Lee RJ. The effect of a peptide-modified thermo-reversible methylcellulose on wound healing and LV function in a chronic myocardial infarction rodent model. Biomaterials 2013;34:8869–8877. [DOI] [PubMed] [Google Scholar]
- 4.Song M, Jang H, Lee J, Kim JH, Kim SH, Sun K, Park Y. Regeneration of chronic myocardial infarction by injectable hydrogels containing stem cell homing factor SDF-1 and angiogenic peptide Ac-SDKP. Biomaterials 2014;35:2436–2445. [DOI] [PubMed] [Google Scholar]
- 5.Ungerleider JL, Christman KL. Concise review: injectable biomaterials for the treatment of myocardial infarction and peripheral artery disease: translational challenges and progress. Stem Cells Transl Med 2014;3:1090–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu J, Christman KL, Chin E, Sievers RE, Saeed M, Lee RJ. Restoration of left ventricular geometry and improvement of left ventricular function in a rodent model of chronic ischemic cardiomyopathy. J Thorac Cardiovasc Surg 2009;137:180–187. [DOI] [PubMed] [Google Scholar]
- 7.Sabbah HN, Wang M, Gupta RC, Rastogi S, Ilsar I, Sabbah MS, Kohli S, Helgerson S, Lee RJ. Augmentation of left ventricular wall thickness with alginate hydrogel implants improves left ventricular function and prevents progressive remodeling in dogs with chronic heart failure. JACC Heart Fail 2013;1:252–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee LC, Wall ST, Klepach D, Ge L, Zhang Z, Lee RJ, Hinson A, Gorman JH, III, Gorman RC, Guccione JM. Algisyl-LVR™ with coronary artery bypass grafting reduces left ventricular wall stress and improves function in the failing human heart. Int J Cardiol 2013;168:2022–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mann DL, Acker MA, Jessup M, Sabbah HN, Starling RC, Kubo SH, Acorn Trial Principal Investigators and Study Coordinators. Clinical evaluation of the CorCap cardiac support device in patients with dilated cardiomyopathy. Ann Thorac Surg 2007;84:1226–1235. [DOI] [PubMed] [Google Scholar]
- 10.Grossi EA, Patel N, Woo YJ, Goldberg JD, Schwartz CF, Subramanian V, Feldman T, Bourge R, Baumgartner N, Genco C, Goldman S, Zenati M, Wolfe JA, Mishra YK, Trehan N, Mittal S, Shang S, Mortier TJ, Schweich CJ, Jr, RESTOR-MV Study Group. Outcomes of the RESTOR-MV trial (randomized evaluation of a surgical treatment for off-pump repair of the mitral valve). J Am Coll Cardiol 2010;56:1984–1993. [DOI] [PubMed] [Google Scholar]
- 11.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Køber L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Rønnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, ESC Committee for Practice Guidelines. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European society of cardiology. Developed in collaboration with the heart failure association (HFA) of the ESC. Eur Heart J 2012;33:1787–1847. [DOI] [PubMed] [Google Scholar]
- 12.Cowie MR, Anker SD, Cleland JG, Felker GM, Filippatos F, Jaarsma T, Jourdain P, Knight E, Massie B, Ponikowski P, López-Sendón J. Improving care for patients with acute heart failure: before, during and after hospitalization. ESC Heart Failure 2014;1:110–145. [DOI] [PubMed] [Google Scholar]
- 13.Ponikowski P, Anker SD, AlHabib KF, Cowie MR, Force TL, Hu S, Jaarsma T, Krum H, Rastogi V, Rohde LE, Samal UC, Shimokawa H, Siswanto BB, Sliwa K, Filippatos G. Heart failure: preventing disease and death worldwide. ESC Heart Failure 2014;1:4–25. [DOI] [PubMed] [Google Scholar]
- 14.Sarullo FM, Fazio G, Brusca I, Fasullo S, Paterna S, Licata P, Novo G, Novo S, Di Pasquale P. Cardiopulmonary exercise testing in patients with chronic heart failure: prognostic comparison from peak VO2 and VE/VCO2 slope. Open Cardiovasc Med J 2010;4:127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swank AM, Horton J, Fleg JL, Fonarow GC, Keteyian S, Goldberg L, Wolfel G, Handberg EM, Bensimhon D, Illiou MC, Vest M, Ewald G, Blackburn G, Leifer E, Cooper L, Kraus WE; HF-ACTION Investigators. Modest increase in peak VO2 is related to better clinical outcomes in chronic heart failure patients: results from heart failure and a controlled trial to investigate outcomes of exercise training. Circ Heart Fail 2012;5:579–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cahalin L, Mathier M, Semigran M, Dec G, DiSalvo T. The six-minute walk test predicts peak oxygen uptake and survival in patients with advanced heart failure. Chest 1996;110:325–332. [DOI] [PubMed] [Google Scholar]
- 17.Roul G, Germain P, Bareiss P. Does the 6-minute walk test predict the prognosis in patients with NYHA class II or III chronic heart failure? Am Heart J 1998;136:449–457. [DOI] [PubMed] [Google Scholar]
- 18.Shah MR, Hasselblad V, Gheorghiade M, Adams KF, Swedberg K, Califf RM, O'Connor CM. Prognostic usefulness of the six-minute walk in patients with advanced congestive heart failure secondary to ischemic or nonischemic cardiomyopathy. Am J Cardiol 2001;88:987e93. [DOI] [PubMed] [Google Scholar]
- 19.Perera S, Mody S, Woodman R, Studenski S. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatrics Soc 2006;54:743–749. [DOI] [PubMed] [Google Scholar]
- 20.Ingle L, Shelton RJ, Rigby AS, Nabb S, Clark AL, Cleland JG. The reproducibility and sensitivity of the 6-min walk test in elderly patients with chronic heart failure. Eur Heart J 2005;26:1742–1751. [DOI] [PubMed] [Google Scholar]
- 21.Ponikowski P, van Veldhuisen DJ, Comin-Colet J, Ertl G, Komajda M, Mareev V, McDonagh T, Parkhomenko A, Tavazzi L, Levesque V, Mori C, Roubert B, Filippatos G, Ruschitzka F, Anker SD, CONFIRM-HF Investigators. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur Heart J 2015;36:657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Costanzo MR, Ivanhoe RJ, Kao A, Anand IS, Bank A, Boehmer J, Demarco T, Hergert CM, Holcomb RG, Maybaum S, Sun B, Vassiliades TA, Jr, Rayburn BK, Abraham WT. Prospective evaluation of elastic restraint to lessen the effects of heart failure (PEERLESS-HF) trial. J Card Fail 2012;18:446–458. [DOI] [PubMed] [Google Scholar]
- 23.Cardiokinetics Parachute Mazzaferri EL, Jr, Gradinac S, Sagic D, Otasevic P, Hasan AK, Goff TL, Sievert H, Wunderlich N, Nikolic SD, Abraham WT. Percutaneous left ventricular partitioning in patients with chronic heart failure and a prior anterior myocardial infarction: results of the PercutAneous Ventricular RestorAtion in Chronic Heart failure Patients Trial. Am Heart J 2012;163:812–820. [DOI] [PubMed] [Google Scholar]
- 24.Cheng Y, Aboodi MS, Wechsler AS, Kaluza GL, Granada JF, Van Bladel K, Annest LS, Yi GH. Epicardial catheter-based ventricular reconstruction: a novel therapy for ischaemic heart failure with anteroapical aneurysm. Interact Cardiovasc Thorac Surg 2013;17:915–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones RH, Velazquez EJ, Michler RE, Sopko G, Oh JK, O'Connor CM, Hill JA, Menicanti L, Sadowski Z, Desvigne-Nickens P, Rouleau JL, Lee KL, STICH Hypothesis 2 Investigators. Coronary bypass surgery with or without surgical ventricular reconstruction. N Engl J Med 2009;360:1705–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]