Abstract
Background
Ontario spends about 9% of its health budget on care for people at the end of life (EoL), most of whom die from chronic, prolonged conditions. For many people, patient care planning discussions (PCPDs) can improve the quality and reduce the cost of care.
Objectives
This evidence-based analysis aimed to examine the effectiveness of PCPDs in achieving better patient-centred outcomes for people at the EoL.
Data Sources
A systematic literature search was conducted in MEDLINE, Embase, CINAHL, and EBM Reviews to identify relevant literature published between January 1, 2004, and October 9, 2013.
Review Methods
Peer-reviewed reports from randomized controlled trials (RCTs) and observational studies were examined. Outcomes included quality of life (QoL), satisfaction, concordance, advance care planning (ACP), and health care use. Quality of evidence was assessed using GRADE.
Results
While the effects of PCPDs on QoL are unclear, single-provider PCPDs were associated with family members being very satisfied with EoL care (odds ratio [OR]: 5.17 [95% CI: 1.52, 17.58]), improved concordance between patients’ and families’ wishes (OR: 4.32, P < 0.001), fewer episodes of hospital care (mean difference [MD]: −0.21, P = 0.04), spending fewer days in hospital (MD: −1.8, P = 0.03), and receiving hospice care (OR: 5.17 [95% CI: 2.03, 13.17]). Team-based PCPDs were associated with greater patient satisfaction (standardized mean difference [SMD]: 0.39 [95% CI: 0.17, 0.60]) and fewer outpatient visits (MD: −5.20 [95% CI: −9.70, −0.70]). Overall, PCPDs were associated with more ACP and more optimal health care use.
Limitations
Most of the RCTs were unblinded, intervention was measured or described inadequately in some studies, and the term “usual care” was often undefined.
Conclusions
Patients at the EoL and their families benefited from PCPDs. Furthermore, PCPDs occurring earlier in the course of illness were associated with better outcomes than those occurring later.
Plain Language Summary
In 2009–2010, about 88,000 people in Ontario were near the end of their lives; nearly all of these people were adults who died from chronic illnesses such as cancer, heart disease, stroke, diabetes, and Alzheimer's disease. Providing better-quality health care for people at the end of life has become a priority and research suggests that the quality of care can be improved by patient care planning discussions among health care providers, patients, and families. These discussions focus on designing care for a particular patient on the basis of disease progress, treatment options, preferences, goals, values, and other related considerations. This review was conducted to examine the effects of patient care planning discussions on the quality of care provided and the use of health care resources at the end of life.
This review found that patient care planning discussions with a team of providers from multiple professions were beneficial for patients and their families. High-quality evidence indicates that discussions with a single provider can improve families’ satisfaction with care at the end of a loved one's life and increase agreement between the wishes of the patient and his or her family. These discussions can also reduce the likelihood that patients will need care in hospital and reduce the number of days a patient spends in hospital. Finally, discussions with a single provider increased care planning and the use of hospice services. Moderate- to high-quality evidence shows that patient care planning discussions with a team of providers from multiple professions led to increases in care planning, fewer days in intensive care, and fewer visits for outpatient services.
Background
In July 2013, the Evidence Development and Standards (EDS) branch of Health Quality Ontario (HQO) began work on developing an evidentiary framework for end of life care. The focus was on adults with advanced disease who are not expected to recover from their condition. This project emerged from a request by the Ministry of Health and Long-Term Care that HQO provide them with an evidentiary platform on strategies to optimize the care for patients with advanced disease, their caregivers (including family members), and providers.
After an initial review of research on end-of-life care, consultation with experts, and presentation to the Ontario Health Technology Advisory Committee (OHTAC), the evidentiary framework was produced to focus on quality of care in both the inpatient and the outpatient (community) settings to reflect the reality that the best end-of-life care setting will differ with the circumstances and preferences of each client. HQO identified the following topics for analysis: determinants of place of death, patient care planning discussions, cardiopulmonary resuscitation, patient, informal caregiver and healthcare provider education, and team-based models of care. Evidence-based analyses were prepared for each of these topics.
HQO partnered with the Toronto Health Economics and Technology Assessment (THETA) Collaborative to evaluate the cost-effectiveness of the selected interventions in Ontario populations. The economic models used administrative data to identify an end-of-life population and estimate costs and savings for interventions with significant estimates of effect. For more information on the economic analysis, please contact Murray Krahn at murray.krahn@theta.utoronto.ca.
The End-of-Life mega-analysis series is made up of the following reports, which can be publicly accessed at http://www.hqontario.ca/evidence/publications-and-ohtac-recommendations/ohtas-reports-and-ohtac-recommendations.
-
▸
End-of-Life Health Care in Ontario: OHTAC Recommendation
-
▸
Health Care for People Approaching the End of Life: An Evidentiary Framework
-
▸
Effect of Supportive Interventions on Informal Caregivers of People at the End of Life: A Rapid Review
-
▸
Cardiopulmonary Resuscitation in Patients with Terminal Illness: An Evidence-Based Analysis
-
▸
The Determinants of Place of Death: An Evidence-Based Analysis
-
▸
Educational Intervention in End-of-Life Care: An Evidence-Based Analysis
-
▸
End-of-Life Care Interventions: An Economic Analysis
-
▸
Patient Care Planning Discussions for Patients at the End of Life: An Evidence-Based Analysis
-
▸
Team-Based Models for End-of-Life Care: An Evidence-Based Analysis
Objective of Analysis
This evidence-based analysis (EBA) aimed to examine the effectiveness of patient care planning discussions (PCPDs) in achieving better patient-centred outcomes for people at the end of life (EoL). This EBA is particularly focused on the effectiveness of having a discussion that includes a single health care provider, having a discussion that includes an interprofessional team of health care providers, and the timing of discussions about EoL care. This EBA is part of a mega-analysis focused on EoL care.
Clinical Need and Target Population
End-of-Life Population
It is difficult to know in advance when patients are definitely at the EoL; hence, EoL is defined on the basis of assumptions about a person's risk of dying. These assumptions tend to be related: the period in which the person is expected to die; eligibility for certain health services (e.g., hospice care, palliative care); or the part of life in which patients, family members, informal caregivers, and health care providers struggle with the implications of a chronic illness that has progressed to advanced stages. (1)
Because each of the commonly used definitions of EoL is quite narrow, this EBA and the mega-analysis aimed to be inclusive by defining EoL broadly. Thus, the EoL population was defined as adult patients with advanced disease not expected to recover from their condition or stabilize. This population included people who were seriously or terminally ill, those whom clinicians believed were at the EoL, and those receiving palliative care. This definition aims to incorporate the 3 approaches to identifying patients at the EoL.
From 2007 to 2009, the 3 most recent years for which complete data were available, 264,503 persons died in Ontario. Of these, 261,135 persons (98.7%) were adults aged 18 and older, and chronic, prolonged conditions accounted for most deaths in this group. In Ontario, the top 10 causes of death in 2009 were cancer (29.5%), diseases of the heart (20.9%), cerebrovascular diseases (6.2%), accidents (4.4%), chronic lower respiratory illnesses (4.2%), diabetes mellitus (3.2%), Alzheimer's disease (2.5%), influenza and pneumonia (2.4%), kidney-related diseases (1.4%), and suicide (1.4%). (2) Most of these are advanced chronic conditions, so the above definition is appropriate for identifying the EoL population in Ontario.
Quality of End-of-Life Care and Communication
Quality of EoL care is a domain of the multidimensional quality of dying and death construct (QODD). The QODD consists of 7 broad and overlapping domains: physical, psychological, social, spiritual or existential, nature of health care, life course and death preparation, and circumstances of death. The quality of EoL care depends on the extent to which the health care patients receive affects outcomes in those domains. (3) Additionally, good-quality EoL care must be informed by the best available evidence when appropriate. (4) The health care received should thus be related to a plan of care; the patient's and family's goals, values, needs, and so forth; receiving services that are consistent with the care plan; and applying the best available evidence, among other things. (4)
Open and honest communication between patients, families, and health care providers is essential for achieving good-quality EoL care. Communication exchanges information between patients and providers, (5) and it can help to inform patients about their condition, prognosis, and treatment options as well as elicit patients’ goals, values, and preferences. Given that PCPDs include advance care planning (ACP) and goals of care discussions, this type of intervention can help to improve communication about EoL care. They are important for obtaining informed consent and for sharing decision-making.
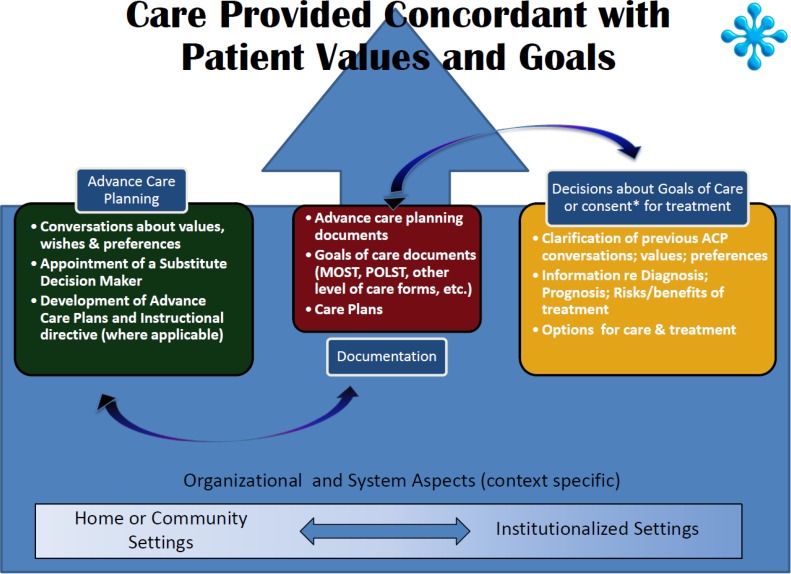
Ontario law specifies that, even when an advanced care plan or do-not-resuscitate (DNR) order is in place, decisions about treatments cannot be made without informed consent, which requires health care providers to discuss care options with patients or their substitute decision makers. (6) Patient care planning discussions are thus a necessary and important component of decision-making in health care. Advance care plans and DNR orders are outputs from the PCPDs, and they should be updated if a patient's wishes, values, or beliefs related to care change in any way. More recent expressions of care preferences take precedence over older ones, even if the older ones are written and the more recent ones are verbal. Communication about EoL care has thus become an area of focus for the Canadian Researchers at the End of Life Network (CARENET), a group focused on improving palliative and EoL care. Their conceptual model for improving communication about EoL care is presented in Figure 1.
Figure 1: CARENET's Model for Improving Communication about End-of-Life Care.
Abbreviations: ACP, advance care planning; CARENET, Canadian Researchers at the End of Life Network; MOST, medical orders on scope of treatment; POLST, physician orders for life-sustaining treatment.
Results from recent systematic reviews show that these discussions can be beneficial. For instance, family meetings are associated with lower health care use, and palliative care teams are associated with better use of health care. (7) Palliative care consultations and conferences are associated with increased family satisfaction, improved decision making, and reduced health care use. (8) Ethics consultations also show benefits, because they lead to improved health care use. (7;8) Last, ACP interventions are associated with an increase in ACP documentation, a reduction in the use of health care services, and an increase in hospice use. (9) This evidence indicates PCPDs have the potential to improve the quality of EoL care and reduce health care costs for the province.
Ontario Context
No population-based estimates for the prevalence of PCPDs in Ontario were located, but estimates from 2 hospital-based studies conducted in Canada might provide some insight. A study published in 2013 was conducted with a convenience sample of hospital inpatients in Ontario and 3 other provinces. All patients in the sample had chronic diseases or were aged 80 years or older. Within the sample, 20.1% of 278 patients and 33.2% of 224 family members indicated that they had ever discussed the patient's prognosis and remaining time to live with their physicians. Further, 47.9% of patients and 52.2% of family members indicated that they or their loved one had written documents specifying their treatment preferences; and only 11.9% of patients and 14.7% of family members preferred to receive aggressive care at the EoL. (10) In another study published in 2009, 25.5% (24/108 in Kingston and 13/37 in Toronto) of Ontarians in the sample reported having had a discussion about their prognosis with a physician. This was a multicentre study with a convenience sample of 412 Canadians in 5 tertiary care teaching hospitals. Within the sample as a whole, 18.0% (74/412) of participants had reported having these discussions. The study also showed that people who had prognostic discussions with physicians had higher scores for overall satisfaction and satisfaction with communication and decision making. Their families reported higher overall satisfaction and higher satisfaction with communication and decision making and social support. (11) Although these estimates are based on convenience samples, they are consistent in suggesting that fewer than 30% of Canadians, including Ontarians, are having PCPDs.
Patient care planning discussions also have the potential to meaningfully reduce health care costs in Ontario. Care at the EoL is quite costly for Ontarians, and these costs are expected in increase as the population ages. In 2009–2010, Ontario's health budget was approximately $44.8 billion. The province spends an estimated 9% of its health budget on EoL care. (Ba’ Pham, personal communication, March 19, 2014) Additionally, a report from the Canadian Institute for Health Information states that care for seniors older than 65 years accounted for 44.0% of average health care spending at the national level in 2000 and 45.0% in 2011. This was because of the high cost of EoL care and because health care use for chronic conditions increases with age. (12)
Technology/Technique
“Patient care planning discussions” is an umbrella term used to describe discussions that usually lead to a written medical and nursing care program specifically designed for a particular patient. It encompasses advance care planning or goals of care conversations (i.e., discussions with patients and/or their substitute decision makers about the goals and desired direction of their care). (13) There are many interventions for patient care planning, but this EBA focuses on discussion interventions for reasons described above.
Discussions are complex interventions, because their multiple components can affect their efficacy. Although they can take different forms depending on the context, they adhere to an underlying structure. (14) These interventions can vary by setting, health care personnel, frequency, topics discussed, intensity, structure, and so forth, and therefore must be broken into their constituent parts when they are being evaluated, and each part must be assessed separately. This EBA has deconstructed PCPDs to assess the number of providers involved and the timing of discussions.
Evidence-Based Analysis
Research Question
Which approaches to patient care planning discussions (PCPDs) optimize the quality of end-of-life (EoL) care for patients with advanced disease, informal caregivers, and providers?
Research Methods
Literature Search Strategy
A literature search was performed on October 9, 2013, using Ovid MEDLINE, Ovid MEDLINE In-Process and Other Non-Indexed Citations, Ovid Embase, EBSCO Cumulative Index to Nursing & Allied Health Literature (CINAHL), and EBM Reviews, for studies published from January 1, 2004, to October 9, 2013. (Appendix 1 provides details of the search strategies.) Abstracts were reviewed by a single reviewer and, for those studies meeting the eligibility criteria, full-text articles were obtained. Reference lists were also examined for any additional relevant studies not identified through the search.
Inclusion Criteria
English-language full-text publications
published between January 1, 2004, and October 9, 2013
randomized controlled trials (RCTs), systematic reviews (SRs), meta-analyses, and observational studies
adult patients (aged 18 and older) with advanced disease or who are seriously ill and whose health is likely to continue to deteriorate
adult patients at the EoL
communication between providers and patients
communication between providers and families
communication between patients and their families
goals of care or PCPDs, conferences, conversations, or consultations
Exclusion Criteria
related to sudden or violent death
non-SRs, case reports, editorials, letters, comments, conference abstracts
include children only (younger than 18 years)
most participants are children
Outcomes of Interest
primary outcome is quality of life (QoL)
-
secondary outcomes are the following:
patient satisfaction
family satisfaction
concordance between patient's wishes and care received
concordance between patient's wishes and family's or substitute decision maker's wishes
completion of advance care planning processes or documentation
health care use
Statistical Analysis
Data Extraction
Data were extracted from the studies with a standardized data form. The form collected information about the following:
Source (i.e., citation information, contact details, study type);
Methods (i.e., study design, study duration and years, participant allocation, allocation sequence concealment, blinding, reporting of missing data, reporting of outcomes, and whether or not the study compared 2 or more groups);
Outcomes (i.e., outcomes measured, number of participants for each outcome, number of participants missing for each outcome, outcome definition and source of information, unit of measurement, upper and lower limits [for scales], and time points at which the outcome was assessed);
Participants (i.e., number of participants, population, diseases and conditions represented, setting, country, age, sex, proportion of racial minorities, proportion of immigrants, proportion of Canadian Aboriginals, marital status, education level, and income or poverty status);
Intervention (number of intervention groups, type of intervention, individuals and professional groups present for the discussions, number of participants in each arm of the study, initiator of the discussion, use of a communication tool to facilitate the discussion, structure of the discussion, timing of the discussion, content of the discussion, and frequency of discussions);
Results (i.e., summary data for each intervention group, effect estimates, and confidence intervals or P values for each effect estimate); and
Other information (i.e., funding source, key conclusions, other information to guide the review, and whether the study's authors needed to be contacted).
Authors of the studies were contacted to provide unpublished data when required for comparisons and meta-analysis.
Assessment of Risk of Bias in Included Primary Studies
The risk of bias assessment was guided by a modified version of the tool in the Cochrane Handbook for Systematic Reviews of Interventions. (15) For randomized controlled trials, bias assessment considered selection bias (i.e., allocation concealment), performance bias (i.e., blinding of participants and health care providers), attrition bias (i.e., incomplete outcome data), reporting bias (i.e., selective outcome reporting), and other limitations (e.g., related to study design). For observational studies, the tool considered selection bias (i.e., appropriate eligibility criteria, adequate control for confounding), measurement bias (i.e., appropriate measurement of exposures and outcomes), and attrition bias (i.e., incomplete follow-up). The results of bias assessment are presented in Appendix 2 and were used to assess the overall quality of evidence for each outcome.
Assessment of Publication Bias
Funnel plots were used to assess potential publication biases, and the results of these assessments were used to rate the quality of the evidence for each outcome (Appendix 2).
Data Synthesis
The studies were divided into 2 subgroups on the basis of whether or not they included patient care planning discussions (PCPDs) with one provider (single-provider) or an interprofessional team of providers working in a coordinated way (team-based). Within the subgroups, studies were pooled if they employed the same study design and used (or did not use) a tool to facilitate discussions.
The EoL population was defined broadly, and this systematic review is concerned with the average effectiveness of PCPDs on EoL care, so a great deal of heterogeneity in the results was expected given the diversity in the EoL population. Hence, while the I2 statistic was used to assess heterogeneity in the meta-analyses, it was not used to determine whether or not the results should be pooled. According to the Cochrane Handbook for Systematic Reviews of Interventions, heterogeneity of 0–40% may not be important, heterogeneity between 30% and 60% is moderate, heterogeneity from 50% to 90% is substantial, and heterogeneity between 75% and 100% is considerable. (15) Notably, the importance of I2 in assessing heterogeneity depends on the magnitude and direction of the effect estimates and the strength of the evidence for heterogeneity. Given the diversity in the population, certain judgments needed to be made about heterogeneity.
Results were pooled only when the confidence intervals for their effect estimates overlapped. If the I2 statistic was greater than 50% and the confidence intervals overlapped, then a random effects model was used to pool the estimates. If the I2 statistic was greater than 50% and the confidence intervals did not overlap, the estimates were not pooled. Additional, steps were taken to explain potential reasons for heterogeneity in the studies. For instance, the diseases and conditions in the population, the country and setting, the outcomes measured, and the demographic characteristics of the samples were considered.
Quality of Evidence
The quality of the body of evidence for each outcome was examined according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Working Group criteria. (16) The overall quality was determined to be high, moderate, low, or very low using a step-wise, structural methodology.
Study design was the first consideration; the starting assumption was that randomized controlled trials (RCTs) are high quality, whereas observational studies are low quality. Five additional factors—risk of bias, inconsistency, indirectness, imprecision, and publication bias—were then taken into account. Limitations in these areas resulted in downgrading the quality of evidence. Finally, 3 main factors that can raise the quality of evidence from observation studies were considered: large magnitude of effect, dose-response gradient, and accounting for all residual confounding factors. (16) For more detailed information, please refer to the latest series of GRADE articles. (16)
As stated by the GRADE Working Group, the final quality score can be interpreted using the following definitions:
| High | High confidence in the effect estimate—the true effect lies close to the estimate of the effect |
| Moderate | Moderate confidence in the effect estimate—the true effect is likely to be close to the estimate of the effect, but may be substantially different |
| Low | Low confidence in the effect estimate—the true effect may be substantially different from the estimate of the effect |
| Very Low | Very low confidence in the effect estimate—the true effect is likely to be substantially different from the estimate of effect |
Results of Evidence-Based Analysis
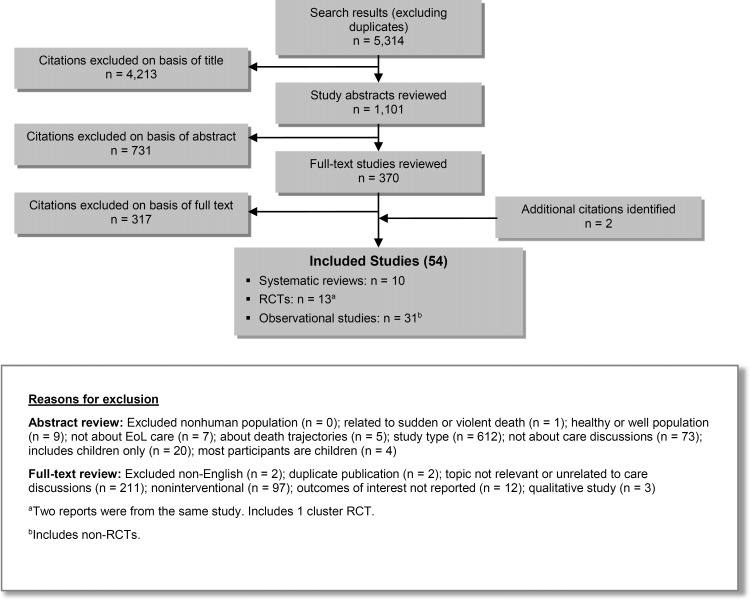
The database search yielded 5,314 citations published between January 1, 2004, and October 9, 2013 (with duplicates removed). Articles were excluded on the basis of information in the title and abstract. The full texts of potentially relevant articles were obtained for further assessment. Figure 2 shows the breakdown of when and for what reason citations were excluded from the analysis.
Figure 2: Citation Flow Chart.
Abbreviations: EoL, end of life; RCT, randomized controlled trial.
Fifty-two studies (10 systematic reviews, 13 RCTs, and 29 observational studies) met the inclusion criteria. The reference lists of these studies were hand-searched to identify other relevant studies, and 2 additional citations (2 observational studies) were included, for a total of 54.
For each included study, the study design was identified and is summarized below in Table 1, a modified version of a hierarchy of study design by Goodman. (17)
Table 1:
Body of Evidence Examined According to Study Design
| Study Design | Number of Eligible Studies | |
|---|---|---|
| RCTs | ||
| Systematic review of RCTs | 1 | |
| Large RCT | 10a | |
| Small RCT | 3 | |
| Observational Studies | ||
| Systematic review of non-RCTs with contemporaneous controls | 3 | |
| Non-RCT with contemporaneous controls | 11 | |
| Systematic review of non-RCTs with historical controls | 6 | |
| Non-RCT with historical controls | 3 | |
| Database, registry, or cross-sectional study | 3 | |
| Case series | ||
| Retrospective review, modelling | 14 | |
| Studies presented at international conference | ||
| Expert opinion | ||
| Total | 54 | |
Abbreviation: RCT, randomized controlled trial.
Two reports were from the same study. Includes one cluster RCT.
Systematic reviews and observational studies with low-quality designs were excluded from the analyses for this EBA. As mentioned previously, the search for this EBA identified 10 systematic reviews. While these systematic reviews were used to provide background information about the topic, they were not included in the analyses, because none included the populations, (7;8) interventions, (9;18–22) and outcomes (23) that were the focus of this EBA. Further, 1 of the systematic reviews was an overview of other systematic reviews. (24) Studies with the lowest-quality designs, as assessed through Goodman's hierarchy in Table 1, were excluded from further analyses. Some of these studies were retrospective and based on data collected from chart reviews; (25–34) the rest did not provide unadjusted estimates. (35–38)
Description of Included Studies
The analyses for this EBA used data from 30 studies, and details about their characteristics and participants are presented in Table 2. Nine of the 13 RCTs had more than 50 participants in each intervention arm. (39–47) Eight of the RCTs were conducted in the United States, (40–43;46–50) 2 were conducted in the United Kingdom, (51;52) 2 were conducted in Australia, (39;44) and 1 was conducted in France. (45) They all included older adults who had a variety of diseases and conditions, such as cancer, chronic heart failure, chronic obstructive pulmonary disease, and dementia. The RCTs included men and women, and although most participants were white, they included ethno-racial minorities as well.
Table 2:
Description of Included Studies
| Author, Year | Country, Setting | Study Design | Length of Study | Sample Size, Intervention/Control | Diseases and Conditions | Age, Intervention/Control | Gender, Intervention/Control | Ethnicity, Intervention/Control |
|---|---|---|---|---|---|---|---|---|
| RCTs | ||||||||
|
Kirchhoff et al, 2012; (40) Kirchhoff et al, 2010 (41) |
United States, multiple outpatient clinics | Multicentre RCT | 3 years, 7 months |
Patients 160/153 Surrogates 160/153 |
CHF, ESRD | Mean (SD): Patients 71.4 (12.2)/70.6 (11.6) Surrogates 59.5 (13.9)/57.4 (13.6) |
Patients 96 (60%) male/89 (58%) male Surrogates 43 (27%) male/41 (27%) male |
Patients 149 (93%) white/146 (95%) white Surrogates 153 (96%) white/147 (96%) white |
| Au et al, 2012 (42) | United States, hospital | Clustered RCT | 3 years, 11 months | 194/182 | COPD | Mean (SD): 69.4 (10.0)/69.4 (10.0) | 98% male/96% malea | 85% white/87% whitea |
| Sampson et al, 2011 (51) | United Kingdom, palliative care unit in hospital | RCT | 6 months |
Patients 22/10 Carers 22/10 |
Dementia | Mean (SD): Patients 87 (6.1)/85 (6.6) Carers 60 (13.0)/57 (12.0) |
Patients 5 (23%) male/1 (10%) male Carers 14 (64%) male/3 (30%) male |
Patients 20 (91%) white/7 (78%) white Carers 20 (91%) white/8 (80%) white |
| Detering et al, 2010 (39) | Australia, multiple medical units in hospital | RCT | 8 months | 154/155 | Mixed | Median (IQR): 85 (82–88)/84 (81–87) | 83 (54%) male/64 (41%) male | Includes minoritiesa |
| Gade et al, 2008 (43) | United States, hospitals | Multicentre RCT | 1 year, 7 months | 275/237 | Mixed | Mean (SD): 73.6 (12.6)/73.1 (13.2) | 113 (41%) male/116 (49%) male | NA |
| Clayton et al, 2007 (44) | Australia, multiple out-patient palliative care clinics | Multicentre RCT | 1 year, 11 months | 92/82 | Cancer | Mean (SD): 65.5 (12.6)/64.6 (14.1) | 56 (61%) male/49 (60%) male | NA |
| Lautrette et al, 2007 (45) | France, ICU in hospital | RCT | 9 months |
Patients 63/63 Surrogates 63/63 |
Mixed | Median (IQR): Patients 74 (56–80)/68 (56–76) Surrogates 54 (47–58)/54 (46–64) |
Patients 33 (52%) male/37 (59%) male Surrogates 17 (30%) male/12 (23%) male |
Patients French descent: 58 (92%)/56 (89%) Surrogates French descent: 48 (86%)/46 (88%) |
| Gilmer et al, 2005 (46) | United States, ICUs in multiple hospitals | Multicentre RCT | 2 years, 2 months | 252/247 | Mixed | Mean (SD): 67.7 (17.5)/68.5 (17.3) | 133 (53%) male/135 (55%) male | 155 (62%) white/157 (62%) white |
| Casarett et al, 2005 (48) | United States, nursing home | RCT | 1 year, 1 month |
Patients 107/98 Surrogates 88/85 |
Mixed | Mean (range): Patients 84 (66–102)/83 (54–101) Surrogates 59 (29–88)/57 (23–91) |
Patients 27 (25%) male/25 (26%) male Surrogates 22 (25%) male/25 (29%) male |
Patients 78 (73%) white/74 (76%) white Surrogates 64 (73%) white/59 (69%) white |
| Song et al, 2005 (49) | United States, cardiac surgery clinic | RCT | 1 year |
Patients 16/16 Surrogates 16/16 |
Cardiac disease | Mean (SD): Patients 69.8 (8.6)/68.0 (8.0) Surrogates 64.4 (11.6)b |
Patients 8 (50%) male/9 (56%) male Surrogates 34% maleb |
Patients 100% white/100% white Surrogates 100% white/100% white |
| Nicolasora et al, 2006 (47) | United States, medical unit in hospital | RCT | 3 months | 136/161 | Mixed | Median: 65/69 | 54% male/46% malea | 77% white/70% whitea |
| Dyar et al, 2012 (50) | United States, hospital | RCT | 9 months | 12/14 | Cancer | Mean (SD): 66.7 (16.3)/64.9 (7.5) | 25% male/36% male | NA |
| Jones et al, 2011 (52) | United States, multiple outpatient clinics | Multicentre RCT | 1 year, 9 months |
Preference cohort 21/14 Randomized cohort 22/20 |
Cancer | Mean (SD): Preference cohort 62.0 (11.0)/67.7 (7.9) Randomized cohort 58.6 (8.1)/60.2 (13.3) |
Preference cohort 48% male/50% malea Randomized cohort 57% male/50% male |
Preference cohort 95% white/93% whitea Randomized cohort 86% white/95% whitea |
| Observational with contemporaneous controls | ||||||||
| Evangelista et al, 2012 (54) | United States, hospital | Prospective case-control | 5 months | 36/36 | Mixed | Mean (SD): 53.9 (8.3)/53.3 (8.7) | 26 (72%) male/25 (69%) male | 22 (61%) white/22 (61%) white |
| Jacobsen et al, 2011 (55) | United States, hospital | Non-RCT | 7 months | 517/382 | NA | Mean: 62.9/63.5 | 308 (60%) male/217 (57%) male | NA |
| Engelhardt et al, 2009 (56) | United States, health insurance network | Multicentre non-RCT | NA |
Patients 198/205 Informal Caregivers 100/85 |
Mixed | Mean (SD): Patients 66.0 (12.1)/68.4 (12.1) Informal Caregivers, 59.9 (14.6)/63.8 (13.1) |
Patients 63 (32%) male/108 (53%) male Informal Caregivers 40 (41%) male/9 (12%) male |
Patients 167 (85%) white/183 (91%) white Informal Caregivers NA |
| Rabow et al, 2004 (57) | United States, home and community | Non-RCT | NA | 50/40 | Cancer, COPD, advanced CHF | Mean (SD): 67.9 (13.9)/69.4 (11.2) | 13 (26%) male/19 (47%) male | 22 (44%) white/26 (65%) white |
| Mack et al, 2012 (58) | United States, health insurance networks and Veterans Affairs health network | Multicentre prospective cohort | 2 years | 1,231b | Lung or colorectal cancer | Range: No. (%) 21–54: 172 (14%)b 55–59: 149 (12%)b 60–64: 157 (13%)b ≥65: 753 (61%)b | 766 (62%) maleb | 935 (76%) whiteb |
| Mack et al, 2010 (59) | United States, multiple hospital sites | Multicentre prospective cohort | 5 years | 332b | Cancer | Mean (SD): Stratified by raceb Black: 55.6 (11.1) White: 60.5 (11.9) | 185 (56%) maleb | 261 (79%) whiteb |
| Wright et al, 2008 (60) | United States, outpatient cancer clinics | Multicentre prospective cohort | 5 years, 6 months | 123/209 | Cancer | Mean (SD): 57.5 (12.0)/58.0 (12.3) | 66 (54%) male/117 (56%) male | 84 (68%) white/128 (61%) white |
| Casarett et al, 2008 (61) | United States, Veterans Affairs health network | Multicentre retrospective cohort | 10 months | 296/228 | Mixed | Mean (range): 72 (27–93)/72 (26–100) | 99% male/97% malea | 77% white/74% whitea |
| Morrison et al, 2005 (62) | United States, nursing home | Non-RCT | 1 year, 10 months | 43/96 | Mixed | Mean (range): 87 (75–100)/86 (65–102) | 16% male/16% malea | 67% white/77% whitea |
| Zhang et al, 2009 (53) | United States, multiple hospitals | Multicentre prospective cohort | 5 years, 4 months | 188/415 | Cancer | Mean (SD): 59.8 (12.9)/58.6 (13.2) | 51% male/51% malea | 74% white/70% whitea |
| Briggs et al, 2004 (63) | United States, multiple units in a hospital | Non-RCT | NA |
Patients 13/14 Surrogates 13/14 |
Heart failure, renal failure | Mean (SD): Patients 68.7 (9.2)b Surrogates 50 (14.8)b |
Patients 39% male/79% malea Surrogates 23% male/29% malea |
NA |
| Observational with historical controls | ||||||||
| Lamba et al, 2012 (64) | United States, surgical ICU in hospital | Prospective before and after | 1 year, 1 month; 1 year, 3 months | 31/21 | Mixed | Mean: 54/46 | 19 (61%) male/12 (57%) male | NA |
| Norton et al, 2007 (65) | United States, medical ICU in hospital | Prospective before and after | 1 year, 1 month | 126/65 | Mixed | Mean (SD): 66.3 (16.3)/68.8 (15.4) | 57 (45%) male/33 (51%) male | 95 (75%) white/51 (79%) white |
| Lindner et al, 2007 (66) | United States, nursing home | Prospective before and after | 6 months | 107/117 | Mixed | Mean (SD): 72 (12.2)/71 (12.3) | 91% male/89% malea | 70% white/57% whitea |
| Cross-sectional | ||||||||
| Leung et al, 2012 (67) | United States, Veterans Affairs health network | Multicentre cross-sectional | 2 years, 2 months | 55/321 | COPD | Mean: 70.9/69.1 | 52 (95%) male/313 (98%) male | 44 (80%) white/247 (77%) white |
| Mori et al, 2013 (68) | United States, hospital | Cross-sectional | 2 years | 20/29 | Cancer | Mean (SD): 60.1 (9.8)/67.5 (11.7) | 10 (50%) male/14 (48%) male | NA |
| Heyland et al, 2009 (11) | Canada, multiple hospitals | Multicentre cross-sectional | NA |
Patients 74/338 Family members 46/107 |
Mixed | Mean (SD): Patients 67.6 (7.2)/71.9 (9.4) Family members 56.6 (14.0)/57.4 (13.5) |
Patients 51% male/51% malea Family members 40% male/34% malea |
NA |
Abbreviations: CHF, chronic heart failure; COPD, chronic obstructive pulmonary disease; ESRD, end-stage renal disease; ICU, intensive care unit; IQR, interquartile range; NA, not available; RCT, randomized controlled trial; SD, standard deviation.
Disaggregated information was not provided.
Data for the intervention and control arms were not provided separately.
The EBA includes a total of 17 observational studies. Eleven of the observational studies had contemporaneous controls, (53–63) and like the RCTS, most of these studies had more than 50 participants in each intervention arm. (53;55;56;58–61) The participants in these observational studies also tended to be older, had a variety of diseases and conditions, and were ethno-racially diverse. All of these studies were conducted in the United States. Another 3 observational studies from the United States had historical controls, (64–66) and 2 had more than 50 participants in each intervention arm. (65;66) They all included older adults and had mixed disease populations, and most participants were white. Last, there were 3 cross-sectional studies, (11;67;68) 2 of which had more than 50 people in each intervention arm. (67;68) Two of the cross-sectional studies were disease-specific and from the United States, (67;68) but one was conducted in Canada with a mixed disease population. (11)
Description of Interventions
The interventions from each study are described in detail in Table 3. As the table shows, each intervention was unique. Only 7 of the interventions were team-based, (43;45;46;57;61;64;65) and 16 of the interventions included the use of a tool to facilitate patient care planning discussions (PCPDs). (39–49;51;55;56;62;63;66) These tools included, but were not limited to, automated forms, frameworks, models, question prompt lists, mnemonics, and structured questionnaires. In 7 of the studies, (11;53;58–60;67;68) the intervention was compared with having no discussions; and in the rest of the studies, (39–52;54–57;61–66) the intervention was compared with usual care.
Table 3:
Description of Interventions and Outcomes
| Author, Year | Interventions | Comparisons | Outcomes of Interest |
|---|---|---|---|
| Randomized controlled trials (RCTs) | |||
|
Kirchhoff et al, 2012; (40) Kirchhoff et al, 2010 (41) |
|
Usual care: standard AD counseling, assessment of AD on admission, question whether patient wanted more information, referrals according to institutional protocol |
|
| Au et al, 2012 (42) |
|
Usual carea | Completion of ACP documents and processes |
| Sampson et al, 2011 (51) |
|
Usual carea |
|
| Detering et al, 2010 (39) |
|
Usual care: no ACP unless specifically requested |
|
| Gade et al, 2008 (43) |
|
Usual carea |
|
| Clayton et al, 2007 (44) |
|
Usual care: routine consultation | Patient's satisfaction with care |
| Lautrette et al, 2007 (45) |
|
Usual care: EoL family conference | ICU LOS |
| Gilmer et al, 2005 (46) |
|
Usual care: no ethics consultation was offered |
|
| Casarett et al, 2005 (48) |
|
Usual care: assessment by research assistant |
|
| Song et al, 2005 (49) |
|
Usual care: information cards and a booklet if more information was desired | Concordance between patient's and surrogate's wishes |
| Nicolasora et al, 2006 (47) |
|
Usual care: not approached by physician | Completion of ACP documents and processes |
| Dyar et al, 2012 (50) |
|
Usual carea | Patient's QoL |
| Jones et al, 2011 (52) |
|
Usual carea | Patient's satisfaction with care |
| Observational with contemporaneous controls | |||
| Evangelista et al, 2012 (54) |
|
Usual care: no palliative care consultation | Patient's QoL |
| Jacobsen et al, 2011 (55) |
|
Usual carea | Completion of ACP documents and processes |
| Engelhardt et al, 2009 (56) |
|
Usual carea |
|
| Rabow et al, 2004 (57) |
|
Usual carea |
|
| Mack et al, 2012 (58) |
|
No discussiona |
|
| Mack et al, 2010 (59)a |
|
No discussiona |
|
| Wright et al, 2008 (60)a |
|
No discussiona |
|
| Casarett et al, 2008 (61) | Palliative care consultation
|
Usual carea | Patient's QoL |
| Morrison et al, 2005 (62) |
|
Usual carea | Concordance between patient's wishes and care received |
| Zhang et al, 2009 (53)a |
|
No discussiona |
|
| Briggs et al, 2004 (63) |
|
Usual care: ACP literature, referral to trained ACP facilitator | Concordance between patient's and surrogate's wishes |
| Observational with historical controls | |||
| Lamba et al, 2012 (64) |
|
Usual carea |
|
| Norton et al, 2007 (65) |
|
Usual care: physician makes a palliative care referral |
|
| Lindner et al, 2007 (66) |
|
Usual carea |
|
| Cross-sectional | |||
| Leung et al, 2012 (67)a |
|
No discussiona | Patient's satisfaction with care |
| Mori et al, 2013 (68)a |
|
No discussiona | Family's satisfaction with care |
| Heyland et al, 2009 (11)a |
|
No discussiona |
|
Abbreviations: ACP, advance care planning; AD, advance directive; CPR, cardiopulmonary resuscitation; EoL, end of life; ICU, intensive care unit; LOS, length of stay; NA, not available; QoL, quality of life; RCT, randomized controlled trial; STP, statement of treatment preferences; VALUE, value comments made by the family, acknowledge family emotions, listen, understand the patient as a person, elicit family questions.
Additional information was not provided.
The single-provider PCPD interventions were performed by a variety of providers. In some cases, the intervention was delivered by a provider from one of several specified professional groups; (40;41;52;54–56;63) in other cases, the intervention was delivered by a provider from only 1 specific professional group. Medical doctors were included in 10 single-provider PCPD interventions, (42;44;47;48;52–55;66;67) nurses were included in 8, (39–41;49;51;52;54;55;63) and social workers were included in 5. (40;41;55;56;62;63) Chaplains were included in 2, (40;41;63) and nurse practitioners (50) and health educators (56) were each included in 1 of the single-provider PCPD interventions. In 5 studies, the profession of the provider was not specified. (11;58–60;68)
The team-based PCPDs included at least 2 providers from various professional groups. Six of the PCPD teams included physicians, (43;45;57;61;64;65) 4 included nurses, (43;57;61;64) 4 included chaplains, (43;57;61;65) 3 included social workers, (43;57;61) 2 included psychologists, (57;65) and 2 included nurse practitioners. (61;65) Each of the following professions were included in only one intervention: ethicist, (46) pharmacist, (57) art therapist, (57) volunteer coordinator, (57) volunteer, (61) counsellor, (64) interfaith pastor, (64) music therapist, (65) and massage therapist. (65) Three studies did not specify which professional groups were included in their interventions. (45;46;61) Additionally, the sizes of the interprofessional teams varied. In one study, the intervention had 2 parts—the first part included 2 professional groups, and the second part included 4 professions. (64) Another intervention had a 4–member team, (43) and 3 interventions had 6-member teams. (57;61;65) In 2 studies, the size of the intervention team was not specified but was dependent on the patient's needs. (45;46)
In most of the studies, the length and frequency of the PCPDs were not specified. However, in studies that reported this information, the PCPDs lasted from 10 minutes to 3 hours and 20 minutes. (39–41;45;54;63) Most discussions lasted between 30 and 60 minutes, however. In most studies, the discussion took place once, but in some, (51;54;57) they took place over multiple sessions.
The discussions covered a variety of topics. The 4 most frequently covered topics were advance care planning (ACP), (39–41;43;47–49;51;52;54–56;58;62–64;66;67) treatment options (including resuscitation), (40;41;44;47–49;54–56;58;63;64;66) patients’ preferences, (40–42;46;47;50;51;53–55;63;66) and patients’ goals. (39;43;48;54;55;62;64;66) Other commonly covered topics included prognosis, physical or medical needs, patient and surrogate understanding and knowledge, social support and needs, palliative services, and patients’ values.
Quality of Life
Table 4 provides details about the relationship between PCPDs and quality of life (QoL) for patients at the EoL. Compared with the controls, single-provider PCPDs could have had a small effect on QoL (GRADE: very low). In 1 small randomized RCT that used 2 instruments to measure QoL, patients in the intervention arm reported higher QoL than those in the control arm, but the differences were not statistically significant. (50) One observational study showed that patients receiving single-provider PCPDs had higher QoL than control patients, (54) and another showed that the intervention had no effect on patients’ QoL. (56) In one of the original articles, the P value for the effect showed that the difference observed was significant. It is likely that the conservative estimate of the confidence interval in Table 4 produced the discrepancy between the statistical significance of the results. Similarly, the highest-quality evidence from a large RCT could not demonstrate that team-based PCPDs affected patients’ QoL, either (GRADE: moderate). (43)
Table 4:
Effect of Patient Care Planning Discussions on Patient's Quality of Life
| Author, Year Instrument Range | Study Design | Tool Used? | Results Intervention | Results Control | Difference in Means (95% CI) |
|---|---|---|---|---|---|
|
Single-Provider Discussion vs. Usual Care or No Discussion GRADE for highest-quality evidence: Very low | |||||
| Highest-quality evidence | |||||
|
Dyar et al, 2012 (50) FACT-G (0–108) |
Small RCT | No | Mean change in FACT-G score from baseline (SD): 1.2 (12.5) | Mean change in FACT-G score from baseline (SD): −3.9 (5.0) | 5.10 (−3.98, 14.18) SMD: 0.47 (−0.58, 1.52) |
|
Dyar et al, 2012 (50) LASA (12–120) |
Small RCT | No | Mean change in LASA score from baseline (SD): 2.0 (25.7) | Mean change in LASA score from baseline (SD): −8.8 (21.7) | 10.80 (−11.10, 32.70) SMD: 0.44 (−0.52, 1.37) |
|
Evangelista et al, 2012 (54) a MLHFQ (0–105) |
Obs-cont | No | Mean change in MLHFQ score from baseline: 9.9 (17.3)b | Mean change in MLHFQ score from baseline: 4.3 (15.6)b | 5.60 (−2.01, 13.21)b,c,d SMD: 0.34 (−0.13, 0.80)b |
|
Engelhardt et al, 2009 (56) McGill Quality of Life Questionnaire (0–10) |
Obs-cont | Yes | Mean change in score from baseline: 0.1 (1.2)b | Mean change in score from baseline: 0.1 (1.5)b | 0.01 (−0.25, 0.27)b SMD: 0.01 (−0.19, 0.20)b |
|
Team-Based Discussion vs. Usual Care or No Discussion GRADE for highest-quality evidence: Moderate GRADE for all evidence: Very low | |||||
| Highest-quality evidence | |||||
|
Gade et al, 2008 (43) 11-point Likert scale (0–10) |
Large RCT | Yes | Mean change in score from baseline: 2.3 (3.7)b | Mean change in score from baseline: 2.2 (3.5)b | 0.10 (−0.61, 0.81) SMD: 0.03 (−0.17, 0.23) |
| Lower-quality evidence | |||||
|
Rabow et al, 2004 (57) Multidimensional Quality of Life Scale—Cancer Version (0–100) |
Obs-cont | No | Mean change in score from baseline: −0.4 | Mean change in score from baseline: 2.3 | −2.7, P = 0.43e |
|
Casarett et al, 2008 (61) FATE (0–100) |
Obs-cont | No | Mean FATE score: 64 | Mean FATE score: 54 | 10, P < 0.001d,e |
Abbreviations: CI, confidence interval; FACT-G, Functional Assessment of Cancer Therapy-General; FATE, Family Assessment of Treatment at End-of-Life; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; LASA, Linear Analogue Self-Assessment Scale; MLHFQ, Minnesota Living with Heart Failure Questionnaire; Obs-cont, observational study with contemporaneous control; QoL, quality of life; RCT, randomized controlled trial; SD, standard deviation; SMD, standardized mean difference.
Statistically significant at P ≤ 0.05.
Lower scores indicated higher QoL in the original scale, so the results were multiplied by −1 to make the signs for the differences consistent with the results from other studies.
Standard deviations and CIs are conservatively estimated because information about the correlation between the estimates was not provided.
The effect estimate was statistically significant in the original article, but was not significant here because of the conservative estimation of the confidence interval.
Confidence interval could not be calculated given the information provided.
One observational study of a team-based PCPD found that, after adjusting for other covariates, earlier discussions were associated with greater well-being for patients (β = 0.003, P = 0.006; GRADE: low). (61) In that study, the timing of PCPDs had little effect on QoL. While the effect was statistically significant, it might not have been clinically significant. The study's report did not present unadjusted estimates of the effect.
One study, a small randomized controlled trial (RCT), assessed the effect of a single-provider PCPD on informal caregivers’ QoL. (51) The study suffered from high attrition, which resulted in only 11 informal caregivers completing the 6-month follow-up and 4 informal caregivers completing the post-bereavement follow-up. Because of the small sample size, statistical comparisons of the intervention and control arms were not performed. Overall, the evidence did not indicate whether or not the intervention was associated with greater QoL for informal caregivers (GRADE: very low). See Table 5 for details.
Table 5:
Effect of Patient Care Planning Discussions on Informal Caregiver's Quality of Life
| Author, Year Instrument Range | Study Design | Tool Used? | Results Intervention | Results Control | Difference in Means (95% CI) |
|---|---|---|---|---|---|
|
Single-Provider Discussion vs. Usual Care or No Discussion GRADE for all evidence: Very low | |||||
|
Sampson et al, 2011 (51)a EuroQoL EQ-5D (0–100) |
Small RCT | Yes | Score at baseline (SD): 69.8 (23.6) Score at 6 wk (SD): 73.6 (11.1) Score at 6 wk (SD): 73.6 (11.1) Score at 6 mo (SD): 80.0 (6.1) Score post-death (SD): 69.3 (24.7) |
Score at baseline (SD): 62.7 (37.5) Score at 6 wk (SD): 79.8 (12.2) Score at 6 wk (SD): 79.8 (12.2) Score at 6 mo (SD): 80.8 (13.2) Score post-death: 92.0 (1 person) |
NA |
Abbreviations: CI, confidence interval; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; QoL, quality of life; RCT, randomized controlled trial; SD, standard deviation.
No statistical comparisons were performed in this study.
Satisfaction with End-of-Life Care
Results pertaining to the patient's satisfaction with EoL care (Table 6) seem to suggest that PCPDs, whether with single providers or with teams, were associated with greater satisfaction with EoL care. Results from 3 RCTs showed no evidence that single-provider PCPDs affected patients’ overall satisfaction with care (GRADE: moderate). (39;44;52) However, results from 1 large RCT indicated that single-provider PCPDs were associated with patients being “very satisfied” with their EoL care. (39) One large RCT assessing a team-based PCPD found that the intervention was significantly associated with greater satisfaction with EoL care (GRADE: high). (43)
Table 6:
Effect of Patient Care Planning Discussions on Patient's Satisfaction with End-of-Life Care
| Author, Year Instrument Range | Study Design | Tool Used? | Results Intervention | Results Control | Effect Estimate (95% CI) |
|---|---|---|---|---|---|
|
Single-Provider Discussion vs. Usual Care or No Discussion GRADE for highest-quality evidence: Moderate GRADE for all evidence: Very low | |||||
| Highest-quality evidence | |||||
|
Detering et al, 2010 (39) 3-point Likert |
Large RCT | Yes | Very satisfied: 125/133 (94.0%) Satisfied or very satisfied: 131/133 (98.5%) |
Very satisfied: 91/139 (65.5%) Satisfied or very satisfied: 131/139 (94.2%) |
OR: 8.24 (3.72, 18.26)a OR: 4.00 (0.83, 19.19) |
|
Clayton et al, 2007 (44)b,c 25-item scale (25–125) |
Large RCT | Yes | Mean score: 110.1 | Mean score: 110.3 | MD: −0.2 (−3.4, 2.9) |
|
Jones et al, 2011 (52) 5-item scale (range not reported) |
Small RCT | No | Mean change in score from baseline (SD): 0.6 (1.5) | Mean change in score from baseline (SD): 1.9 (1.1) | MD: −1.3 (−2.09, −0.51)a SMD: −0.96 (−1.61, −0.32) |
| Lower-quality evidence | |||||
|
Zhang et al, 2009 (53)d 11-point Likert (0–10) |
Obs-cont | No | Mean score (SD): 6.3 (2.7) | Mean score (SD): 5.7 (3.3) | MD: 0.60 (−0.39, 1.59) SMD: 0.20 (−0.13, 0.53) |
|
Leung et al, 2012 (67) 5-point Likert dichotomized as very satisfied versus less satisfied |
Cross-sectional | No | NA | NA | OR: 2.02 (1.16, 3.50)a |
|
Heyland et al, 2009 (11) CARENET's Family Satisfaction Survey (1–100) |
Cross-sectional | No | Mean score (SD): 76.1 (9.7) | Mean score (SD): 73.1 (10.6) | MD: 3.00 (0.53, 5.47)a SMD: 0.29 (0.03, 0.54) |
|
Team-Based Discussion vs. Usual Care or No Discussion GRADE for highest-quality evidence: High GRADE for all evidence: Low | |||||
| Highest-quality evidence | |||||
|
Gade et al, 2008 (43)b 11-point Likert (0–10) |
Large RCT | Yes | Mean score (SD): 8.0 (1.4) | Mean score (SD): 7.4 (1.7) | MD: 0.60 (0.27, 0.93)a SMD: 0.39 (0.17, 0.60) |
| Lower-quality evidence | |||||
|
Rabow et al, 2004 (57) Group Health Association of American Consumer Satisfaction Survey (20–100) |
Obs-cont | No | Mean change in score between 6- and 12-month evaluations: 0.5e | Mean change in score between 6- and 12-month evaluations: −2.1e | MD: 2.6, P = 0.26f |
Abbreviations: CARENET, Canadian Researchers at the End of Life Network; CI, confidence interval; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; MD, difference in means; NA, not available; Obs-cont, observational study with contemporaneous controls; OR, odds ratio; RCT, randomized controlled trial; SD, standard deviation; SMD, standardized mean difference.
Statistically significant at P ≤ 0.05.
Assessed satisfaction with communication about care.
Standardized MD could not be computed from the information provided.
Assessed quality of death.
Adjusted for baseline values.
Confidence interval could not be calculated from the information provided.
Results from 3 RCTs showed that single-provider PCPDs were associated with greater satisfaction with EoL care among family members (GRADE: high). (39;48;51) As was seen when patients’ satisfaction with EoL care was assessed, family members in the intervention arm were more likely to report being “very satisfied” with care than those in the control arm (OR [95% CI]: 5.17 [1.52, 17.58]). (39) These results are presented in detail in Table 7.
Table 7:
Effect of Patient Care Planning Discussions on Family's Satisfaction with End-of-Life Care
| Author, Year Instrument Range | Study Design | Tool Used? | Results Intervention | Results Control | Effect Estimate (95% CI) |
|---|---|---|---|---|---|
|
Single-Provider Discussion vs. Usual Care or No Discussion GRADE for highest-quality evidence: High GRADE for all evidence: Moderate | |||||
| Highest-quality evidence | |||||
|
Detering et al, 2010 (39)a 3-point Likert |
Large RCT | Yes | Very satisfied: 24/29 (82.8%) Satisfied or very satisfied: 26/29 (89.7%) |
Very satisfied: 13/27 (48.1%) Satisfied or very satisfied: 21/27 (77.8%) |
OR: 5.17 (1.52, 17.58)b OR: 2.48 (0.55, 11.10) |
|
Casarett et al, 2005 (48)c Toolkit Afterdeath Survey (1–5) |
Large RCT | Yes | Mean score (SD): 4.3 (1.0) | Mean score (SD): 2.2 (1.5) | MD: 2.10 (1.75, 2.45)b SMD: 1.67 (1.35, 1.99) |
|
Sampson et al, 2011 (51) Satisfaction with End-of-Life Care in Advanced Dementia Scale (10–40) |
Small RCT | Yes | Mean score (SD): 27.6 (8.5) | Mean score (SD): 23.0 (1 person) | MD: 4.6c |
| Lower-quality evidence | |||||
|
Mori et al, 2013 (68)d Toolkit of Instruments to Measure End-of-Life Care (0–10) |
Cross-sectional | No | Mean score (SD): 9.7 (0.6) | Mean score (SD): 8.7 (1.4) | MD: 1.00 (0.42, 1.58)b SMD: 0.84 (0.24, 1.43) |
|
Heyland et al, 2009 (11) CARENET's Family Satisfaction Survey (1–100) |
Cross-sectional | No | Mean score (SD): 75.2 (13.3) | Mean score (SD): 70.4 (12.2) | MD: 4.89 (0.41, 9.37)b SMD: 0.39 (0.04, 0.74) |
| Pooled estimate (2 cross-sectional studies, FE), I2 = 39% | SMD: 0.50 (0.20, 0.80)b | ||||
Abbreviations: CARENET, Canadian Researchers at the End of Life Network; CI, confidence interval; FE, fixed effects; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; MD, difference in means; OR, odds ratio; RCT, randomized controlled trial; SD, standard deviation; SMD, standardized mean difference; RCT, randomized controlled trial.
Assessed satisfaction with quality of death.
Statistically significant at P ≤ 0.05.
CI cannot be calculated from information provided.
Assessed quality of care.
Concordance
Evidence from a large RCT suggests that single-provider PCPDs did not significantly increase concordance between patient's wishes and the care they received (OR [95% CI]: 1.73 [0.76, 3.90]; GRADE: high). (40;41) However, despite not being significant, the point estimate suggests that the intervention was associated with greater concordance, as shown in Table 8.
Table 8:
Effect of Patient Care Planning Discussions on Concordance Between Patient's Wishes and Care Received
| Author, Year | Study Design | Tool Used? | Results Intervention | Results Control | Odds Ratio (95% CI) |
|---|---|---|---|---|---|
|
Single-Provider Discussion vs. Usual Care or No Discussion GRADE for highest-quality evidence: High GRADE for all evidence: Moderate | |||||
| Highest-quality evidence | |||||
| Kirchhoff et al, 2012; (40) Kirchhoff et al, 2010 (41) | Large RCT | Yes | Concordant: 46/62 (74.2%) | Concordant: 30/48 (62.5%) | 1.73 (0.76, 3.90) |
| Lower-quality evidence | |||||
| Mack et al, 2010 (59)a | Obs-cont | No | Concordant: 87/113 (77.0%) | Concordant: 137/219 (62.6%) | 2.00 (1.19, 3.36)b |
| Morrison et al, 2005 (62) | Obs-cont | No | Concordant: 47/49 (95.9%) | Concordant: 79/96 (82.3%) | 5.06 (1.12, 22.87)b |
| Pooled estimate (2 obs-cont studies, FE), I2 = 24% | 2.28 (1.41, 3.70)b | ||||
| Lindner et al, 2007 (66) | Obs-hist | Yes | Concordant: 39/40 (97.5%) | Concordant: 38/44 (86.4%) | 6.16 (0.71, 53.59) |
Abbreviations: CI, confidence interval; FE, fixed effects; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; Obs-cont, observational study with contemporaneous controls; Obs-hist, observational study with historical controls; RCT, randomized controlled trial.
Raw numbers were not provided in the article and were therefore estimated using the odds ratio and algebraic formulas.
Statistically significant at P ≤ 0.05.
Also, Table 9 shows that single-provider PCPDs were associated with greater concordance between the patient's wishes and the family's wishes, and these effect estimates were fairly large and statistically significant (GRADE: high). (40;41;49)
Table 9:
Effect of Patient Care Planning Discussions on Concordance between Patient's and Family's Wishes
| Author, Year | Study Design | Tool Used? | Results Intervention | Results Control | Effect Estimate (P value) |
|---|---|---|---|---|---|
|
Single-Provider Discussion vs. Usual Care or No Discussion GRADE for highest-quality evidence: High GRADE for all evidence: Moderate | |||||
| Highest-quality evidence | |||||
| Kirchhoff et al, 2012; (40) Kirchhoff et al, 2010 (41) | Large RCT | Yes | Agreement on resuscitation, K (95% CI): 0.74 (0.65, 0.83) | Agreement on resuscitation, K (95% CI): 0.26 (0.14, 0.39) | OR: 4.32 (< 0.001)a |
| Song et al, 2005 (49) | Small RCT | Yes | Congruence, mean (SD): 2.8 (0.6) | Congruence, mean (SD): 1.4 (1.0) | MD: 1.4 (0.002)a |
| Lower-quality evidence | |||||
| Briggs et al, 2004 (63) | Obs-cont | Yes | Mean rank: 17.8 | Mean rank: 9.9 | Mann-Whitney U: 33.00 (< 0.01)a |
Abbreviations: CI, confidence interval; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; MD, difference in means; Obs-cont, observational study with contemporaneous controls; OR, odds ratio; RCT, randomized controlled trial; SD, standard deviation.
Statistically significant at P ≤ 0.05.
Completion of Advance Care Planning Documents and Processes
Evidence shows that both single-provider and team-based PCPDs were associated with greater completion of advance care planning (ACP) documents and processes. Based on evidence from 1 large cluster RCT and 2 large RCTs, single-provider PCPDs were associated with a 13% to 77% (95% CIs ranged from 5% to 83%) increase in completion of ACP documents and processes (GRADE: high). (39;42;47) Notably, the results from the 2 RCTs were not pooled because the CIs did not overlap, and the I2 was 99%. Both studies included mixed disease populations, so it is unlikely that the disease mix was the source of heterogeneity. The heterogeneity might have been related to the countries in which the studies were conducted (i.e., Australia versus the United States), the number of hospital units in which the studies were conducted (i.e., multiple versus one), the outcomes that were assessed (i.e., receiving ACP versus completing advance directives), and/or the age of patients included in the studies (i.e., older seniors versus younger seniors). (39;47) Results from a large RCT show that team-based PCPDs were associated with a 22% (95% CI: 15%, 30%) increase in the completion of ACP processes and documents (GRADE: high). (43) Table 10 provides further details about these results.
Table 10:
Effect of Patient Care Planning Discussions on Completion of Advance Care Planning Documents and Processes
| Author, Year | Study Design | Tool Used? | Results Intervention | Results Control | Difference in Proportions (95% CI) |
|---|---|---|---|---|---|
|
Single-Provider Discussion vs. Usual Care or No Discussion GRADE for highest-quality evidence: High GRADE for all evidence: Moderate | |||||
| Highest-quality evidence | |||||
| Au et al, 2012 (42) | Large cluster RCT | Yes | Change in proportion who discussed treatment preferences with clinician: 38.0% | Change in proportion who discussed treatment preferences with clinician: 13.4% | 25% (17, 33)a |
| Detering et al, 2010 (39) | Large RCT | Yes | Received ACP: 119/154 (77.3%) | Received ACP: 1/155 (0.6%) | 77% (70, 83)a |
| Nicolasora et al, 2006 (47) | Large RCT | Yes | Completed ADs: 13/102 (12.7%) | Completed ADs: 1/128 (0.8%) | 13% (5, 19)a |
| Lower-quality evidence | |||||
| Jacobsen et al, 2011 (55) | Obs-cont | Yes | ACP preferences discussed and documented: 175/517 (33.8%) | ACP preferences discussed and documented: 81/382 (21.2%) | 13% (7, 18)a |
| Engelhardt et al, 2009 (56) | Obs-cont | Yes | Completed Ads: 78/166 (47.0%) | Completed ADs: 41/194 (21.1%) | 26% (16, 35)a |
| Pooled estimate (2 obs-cont studies, RE), I2 = 82% | 19% (6, 32)a | ||||
| Lindner et al, 2007 (66) | Obs-hist | Yes | Physician completed AD note: 67/107 (62.6%) | Physician completed AD note: 5/117 (4.3%) | 59% (48, 68)a |
|
Team-Based Discussion vs. Usual Care or No Discussion GRADE for highest-quality evidence: High GRADE for all evidence: Low | |||||
| Highest-quality evidence | |||||
| Gade et al, 2008 (43) | Large RCT | Yes | Change in proportion who completed ADs: 37.7% | Change in proportion who completed ADs: 15.6% | 22% (15, 30)a |
| Lower-quality evidence | |||||
| Rabow et al, 2004 (57) | Obs-cont | No | Durable powers of attorney since baseline: 12/22 (54.5%) | Durable powers of attorney since baseline: 5/18 (27.8%) | 27% (−3, 56) |
| Lamba et al, 2012 (64) | Obs-hist | No | Completion of DNR status: 25/31 (80.6%) | Completion of DNR status: 11/21 (52.4%) | 29% (3, 54)a |
Abbreviations: ACP, advance care planning; AD, advance directive; CI, confidence interval; DNR, do not resuscitate; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; Obs-cont, observational study with contemporaneous controls; Obs-hist, observational study with historical controls; RCT, randomized controlled trial; RE, random effects.
Statistically significant at P ≤ 0.05.
Health Care Use
Chemotherapy
Pooled results from 3 observational studies with contemporaneous controls (Table 11) indicate that single-provider PCPDs were associated with a lower likelihood of receiving chemotherapy at the EoL (OR [95% CI]: 0.50 [0.35, 0.72]; GRADE: low). (53;58;60) All of the studies had similar point estimates indicating a 50% reduction in the receipt of chemotherapy, but only 1 study, the largest, showed a difference that was statistically significant. (58)
Table 11:
Effect of Patient Care Planning Discussions on Receiving Chemotherapy at End of Life
| Author, Year | Study Design | Tool Used? | Results Intervention | Results Control | Odds Ratio (95% CI) |
|---|---|---|---|---|---|
|
Single-Provider Discussion vs. Usual Care or No Discussion GRADE for all evidence: Low | |||||
| Mack et al, 2012 (58) | Obs-cont | No | Received chemotherapy: 158/1082 (14.6%) | Received chemotherapy: 39/149 (26.2%) | 0.48 (0.32, 0.72)a |
| Wright et al, 2008 (60) | Obs-cont | No | Received chemotherapy: 5/123 (4.1%) | Received chemotherapy: 14/209 (6.7%) | 0.59 (0.21, 1.68) |
| Zhang et al, 2009 (53) | Obs-cont | No | Received chemotherapy: 4/75 (5.3%) | Received chemotherapy: 7/70 (10.0%) | 0.51 (0.14, 1.81) |
| Pooled estimate (3 obs-cont studies, FE), I2 = 0% | 0.50 (0.35, 0.72)a | ||||
Abbreviations: CI, confidence interval; FE, fixed effects; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; Obs-cont, observational study with contemporaneous controls.
Statistically significant at P ≤ 0.05.
Table 12 presents evidence from a large observational study showing that patients who had single-provider PCPDs more than 30 days before their death were less likely to receive chemotherapy than patients who had these discussions within 30 days of death (χ2 statistic: 17.057, P < 0.001; GRADE: low). (58)
Table 12:
Effect of Timing of Patient Care Planning Discussions on Receiving Chemotherapy at End of Life
| Author, Year | Study Design | Tool Used? | Results |
|---|---|---|---|
|
Single-Provider Discussion vs. Usual Care or No Discussion GRADE for all evidence: Low | |||
| Mack et al, 2012 | Obs-cont | No | Proportion receiving chemotherapy in the last 14 days of life |
| Days between first discussion and death: ≤ 30: 65/311 (20.9%) 31–60: 19/186 (10.2%) 61–90: 9/108 (8.3%) > 90: 23/189 (12.2%) χ2: 17.057, df=3, P < 0.001a |
|||
Abbreviations: GRADE, Grading of Recommendations Assessment, Development, and Evaluation; Obs-cont, observational study with contemporaneous control.
Statistically significant at P ≤ 0.05.
Resuscitation
Pooled results from 2 observational studies presented in detail in Table 13 show that patients who received single-provider PCPDs were less likely to be resuscitated than those in the control arm (OR [95% CI]: 0.13 [0.03, 0.55]; GRADE: very low). (53;60) The effect was statistically significant, and its magnitude was large, which adds credibility to the result, despite the very low quality of the evidence.
Table 13:
Effect of Patient Care Planning Discussions on Resuscitation
| Author, Year | Study Design | Tool Used? | Results Intervention | Results Control | Odds Ratio (95% CI) |
|---|---|---|---|---|---|
|
Single-Provider Discussion vs. Usual Care or No Discussion GRADE for all evidence: Very low | |||||
| Wright et al, 2008 (60) | Obs-cont | No | 1/123 (0.8%) | 14/209 (6.7%) | 0.11 (0.01, 0.88)a |
| Zhang et al, 2009 (53) | Obs-cont | No | 1/75 (1.3%) | 6/70 (8.6%) | 0.14 (0.02, 1.23) |
| Pooled estimate (2 obs-cont studies, FE), I2 = 0% | 0.13 (0.03, 0.55)a | ||||
Abbreviations: CI, confidence interval; FE, fixed effects; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; Obs-cont, observational study with contemporaneous controls.
Statistically significant at P ≤ 0.05.
Hospital Care
Evidence from a large RCT (Table 14) shows that, on average, patients who received single-provider PCPDs had 0.21 fewer episodes of hospital care than those in the control arm (P = 0.04; GRADE: high). (48) On the other hand, evidence from an observational study with contemporaneous controls suggests that, on average, patients who received team-based PCPDs had 0.40 more episodes of hospital care than those in the study's control arm (95% CI: −0.24, 1.04; GRADE: low). (57)
Table 14:
Effect of Patient Care Planning Discussions on Receiving Hospital Care
| Author, Year | Study Design | Tool Used? | Results Intervention | Results Control | Effect Estimate (95% CI) |
|---|---|---|---|---|---|
|
Single-Provider Discussion vs. Usual Care or No Discussion GRADE for highest-quality evidence: High GRADE for all evidence: Moderate | |||||
| Highest-quality evidence | |||||
| Casarett et al, 2005 (48) | Large RCT | Yes | Mean number of acute care admissions (range): 0.3 (0-4) | Mean number of acute care admissions (range): 0.5 (0–4) | MD: −0.21, P = 0.04a |
| Lower-quality evidence | |||||
| Engelhardt et al, 2009 (56) | Obs-cont | Yes | Mean change in number of inpatient admissions from baseline (SD): 0.5 (6.1)b | Mean change in number of inpatient admissions from baseline (SD): 2.1 (17.3)b | MD: −1.67 (−3.82, 0.48)a,c |
| Mack et al, 2012 (58) | Obs-cont | No | Received acute care at EoL: 424/1082 (39.2%) | Received acute care at EoL: 72/149 (48.3%) | OR: 0.69 (0.49, 0.97)a |
|
Team-Based Discussion vs. Usual Care or No Discussion GRADE for all evidence: Low | |||||
| Rabow et al, 2004 (57) | Obs-cont | No | Mean (SD): 1.2 (2.0) | Mean (SD): 0.8 (1.0) | MD: 0.40 (−0.24, 1.04) |
Abbreviations: CI, confidence interval; EoL, end of life; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; MD, difference in means; Obs-cont, observational study with contemporaneous controls; OR, odds ratio; RCT, randomized controlled trial; SD, standard deviation.
Statistically significant at P ≤ 0.05.
Standard deviations are slightly larger because information about the correlation between estimates was not provided.
Significant difference was shown in the paper, but the difference was not significant in this estimate because the SDs for the MDs were estimated conservatively.
Furthermore, the evidence shows that earlier single-provider PCPDs were associated with a lower likelihood of receiving hospital care at the EoL (χ2 statistic: 55.906, P < 0.001; GRADE: moderate). (58) As shown in Table 15, there was an apparent gradient between the timing of PCPDs and the likelihood of receiving hospital care.
Table 15:
Effect of Timing of Patient Care Planning Discussions on Receiving Hospital Care
| Author, Year | Study Design | Tool Used? | Results |
|---|---|---|---|
|
Single-Provider Discussion vs. Usual Care or No Discussion GRADE for all evidence: Moderate | |||
| Mack et al, 2012 (58) | Obs-cont | No | Proportion receiving acute care in the last 30 days of life Days between first discussion and death: ≤ 30: 180/311 (57.9%) 31–60: 76/186 (40.9%) 61–90: 35/108 (32.4%) > 90: 49/189 (25.9%) χ2: 55.905, df = 3, P < 0.001a |
Abbreviations: GRADE, Grading of Recommendations Assessment, Development, and Evaluation; Obs-cont, observational study with contemporaneous control.
Statistically significant at P ≤ 0.05.
The results in Table 16 show that single-provider PCPDs were associated with spending 1.8 fewer days in hospital (P = 0.03; GRADE: high). (48) The evidence does not clearly indicate any relationship between team-based PCPDs and hospital length of stay, however. Results from 1 large multicentre RCT suggest that team-based PCPDs had no effect on the number of days spent in the hospital, (43) and results from another large multicentre RCT suggest that team-based PCPDs were associated with spending 3.00 fewer days in hospital (GRADE: low). (46) Both of these RCTs were conducted with mixed disease patient populations in hospitals in the United States, and the patients were of similar ages. However, 1 study focused on patients for whom death was imminent, (46) and the other did not. (43) Patients for whom death is imminent comprise a small but special EoL population. (Ba’ Pham, personal communication, February 10, 2014).
Table 16:
Effect of Patient Care Planning Discussions on Hospital Length of Stay
| Author, Year | Study Design | Tool Used? | Results Intervention | Results Control | Difference in Means (95% CI) |
|---|---|---|---|---|---|
|
Single-Provider Discussion vs. Usual Care or No Discussion GRADE for all evidence: High | |||||
| Casarett et al, 2005 (48) | Large RCT | Yes | Mean (range): 1.2 (0–18) | Mean (range): 3.0 (0–29) | −1.8, P = 0.03a |
|
Team-Based Discussion vs. Usual Care or No Discussion GRADE for highest-quality evidence: Low GRADE for all evidence: Low | |||||
| Highest-quality evidence | |||||
| Gade et al, 2008 (43) | Large RCT | Yes | Median (IQR): 7 (4–12) | Median (IQR): 7 (4–12) | 0 |
| Gilmer et al, 2005 (46) | Large RCT | Yes | Mean (SD): 8.3 (9.4) | Mean (SD): 11.3 (16.5) | −3.00 (−6.07, 0.07)a,b |
| Lower-quality evidence | |||||
| Rabow et al, 2004 (57) | Obs-cont | No | Mean (SD): 6.3 (12.4) | Mean (SD): 4.3 (9.0) | 2.00 (−2.43, 6.43) |
| Lamba et al, 2012 (64) | Obs-hist | No | Mean (SD): 22.2 (23.1) | Mean (SD): 31.1 (26.9) | −8.90 (−22.99, 5.19) |
| Norton et al, 2007 (65) | Obs-hist | No | Mean (SD): 35.8 (50.2) | Mean (SD): 41.4 (58.4) | −5.63 (−22.32, 11.06) |
| Pooled estimate (2 obs-hist studies, FE), I2 = 0% | −7.54 (−18.30, 3.23) | ||||
Abbreviations: CI, confidence interval; FE, fixed effects; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; IQR, interquartile range; Obs-cont, observational study with contemporaneous controls; Obs-hist, observational study with historical controls; RCT, randomized controlled trial; SD, standard deviation.
Statistically significant at P ≤ 0.05.
Significant difference was shown in the paper, but the difference was not significant in this estimate because the SDs for the mean differences were estimated conservatively.
Emergency Department Visits
The evidence from 1 observational study suggests that patients who received single-provider PCPDs tended to have fewer visits to the emergency department when compared with their controls (−1.27 [95% CI: −3.34, 0.80]; GRADE: low). (56) These results (Table 17) were not statistically significant, however. One observational study assessed the relationship between team-based PCPDs and emergency department visits. (57) That study did not provide evidence that team-based PCPDs were associated with the number of times a patient visits emergency departments (difference in means [95% CI]: −0.10 [−1.16, 0.96]; GRADE: moderate). Table 17 provides additional details about these results.
Table 17:
Effect of Patient Care Planning Discussions on Emergency Department Visits
| Author, Year | Study Design | Tool Used? | Results Intervention | Results Control | Difference in Means (95% CI) |
|---|---|---|---|---|---|
|
Single-Provider Discussion vs. Usual Care or No Discussion GRADE for all evidence: Low | |||||
| Engelhardt et al, 2009 (56) | Obs-cont | Yes | Mean change from baseline (SD): 1.7 (6.8)a | Mean change from baseline (SD): 3.0 (13.5)a | −1.27 (−3.34, 0.80) |
|
Team-Based Discussion vs. Usual Care or No Discussions GRADE for all evidence: Moderate | |||||
| Rabow et al, 2004 (57) | Obs-cont | No | Mean (SD): 1.6 (2.2) | Mean (SD): 1.7 (2.8) | −0.10 (−1.16, 0.96) |
Abbreviations: CI, confidence interval; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; Obs-cont, observational study with contemporaneous controls; SD, standard deviation.
Standard deviations are slightly larger because information about the correlation between estimates was not provided.
Care in Intensive Care Unit
The pooled estimate from 3 observational studies does not indicate that single-provider PCPDs were associated with the likelihood that a patient will receive care in the intensive care unit (ICU) (OR [95% CI]: 0.44 [0.13, 1.53]; GRADE: very low). (53;58;60) However, 2 of the 3 studies included in the pooled results showed that single-provider PCPDs were associated with less ICU care (Table 18). Notably, all 3 studies were multicentre prospective studies conducted in the United States with patients who had similar demographic characteristics. The patients were recruited from various settings, however.
Table 18:
Effect of Patient Care Planning Discussions on Care in an Intensive Care Unit
| Author, Year | Study Design | Tool Used? | Results Intervention | Results Control | Odds Ratio (95% CI) |
|---|---|---|---|---|---|
|
Single-Provider Discussion vs. Usual Care or No Discussion GRADE for all evidence: Very low | |||||
| Mack et al, 2012 (58) | Obs-cont | No | ICU care at EoL: 64/1082 (5.9%) | ICU care at EoL: 7/149 (4.7%) | 1.28 (0.57, 2.84) |
| Wright et al, 2008 (60) | Obs-cont | No | ICU admission: 5/123 (4.1%) | ICU admission: 26/209 (12.4%) | 0.30 (0.11, 0.80)a |
| Zhang et al, 2009 (53) | Obs-cont | No | ICU stay: 2/75 (2.7%) | ICU stay: 10/70 (14.3%) | 0.16 (0.03, 0.78)a |
| Pooled estimate (3 obs-cont studies, RE), I2 = 75% | 0.44 (0.13, 1.53) | ||||
Abbreviations: CI, confidence interval; EoL, end of life; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; ICU, intensive care unit; Obs-cont, observational study with contemporaneous control; RE, random effects.
Statistically significant at P ≤ 0.05.
Table 19 shows that earlier single-provider PCPDs were associated with a lower likelihood of receiving ICU care at EoL (χ2 statistic: 16.606, P < 0.001; GRADE: low). (58) The data were too sparse to indicate whether or not a gradient was present, but the results showed that people who had single-provider PCPDs more than 30 days before death were less likely to receive ICU care at the EoL.
Table 19:
Effect of Timing of Patient Care Planning Discussions on Care in an Intensive Care Unit
| Author, Year | Study Design | Tool Used? | Results |
|---|---|---|---|
|
Single-Provider Discussion vs. Usual Care or No Discussion GRADE for all evidence: Low | |||
| Mack et al, 2012 (58) | Obs-cont | No | Receiving ICU care in the last 30 days of life |
| Days between first discussion and death: ≤ 30: 37/311 (11.9%) 31–60: 9/186 (4.8%) 61–90: 6/108 (5.6%) > 90: 6/183 (3.3%) χ2: 16.606, df = 3, P < 0.001a |
|||
Abbreviations: GRADE, Grading of Recommendations Assessment, Development, and Evaluation; ICU, intensive care unit; Obs-cont, observational study with contemporaneous control.
Statistically significant at P ≤ 0.05.
As shown in Table 20, patients in 2 large RCTs who received team-based PCPDs appeared to spend fewer days in the ICU, but none of the differences was statistically significant (GRADE: high). (46;58) While point estimates from both studies suggest that team-based PCPDs are associated with spending fewer days in the ICU, these differences could have also resulted from chance.
Table 20:
Effect of Patient Care Planning Discussions on Length of Stay in Intensive Care Unit
| Author, Year | Study Design | Tool Used? | Results Intervention | Results Control | Difference in Means (95% CI) |
|---|---|---|---|---|---|
|
Team-Based Discussion vs. Usual Care or No Discussion GRADE for highest-quality evidence: High GRADE for all evidence: Moderate | |||||
| Highest-quality evidence | |||||
| Lautrette et al, 2007 (45) | Large RCT | Yes | Median (IQR): 7 (4–14) | Median (IQR): 9 (5–20) | −2, P = 0.54a |
| Gilmer et al, 2005 (46) | Large RCT | Yes | Mean (SD): 6.0 (9.4) | Mean (SD): 7.5 (10.3) | −1.50 (−3.48, 0.48) |
| Lower-quality evidence | |||||
| Lamba et al, 2012 (64) | Obs-hist | No | Mean (SD): 14.6 (21.3) | Mean (SD): 17.2 (18.7) | −2.60 (−13.56, 8.36) |
| Norton et al, 2007 (65) | Obs-hist | No | Mean (SD): 9.0 (9.3) | Mean (SD): 16.3 (16.5) | −7.32 (−11.65, −2.99)b |
| Pooled estimate (2 obs-hist, FE), I2 = 0% | −6.68 (−10.71, −2.65)b | ||||
Abbreviations: CI, confidence interval; FE, fixed effects; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; IQR, interquartile range; Obs-hist, observational study with historical controls; RCT, randomized controlled trial; SD, standard deviation.
Information for calculating CI was not provided.
Statistically significant at P ≤ 0.05.
Table 21:
Effect of Patient Care Planning Discussions on Home Health Visits
| Author, Year | Study Design | Tool Used? | Results Intervention | Results Control | Difference in Means (95% CI) |
|---|---|---|---|---|---|
|
Single-Provider Discussion vs. Usual Care or No Discussion GRADE for all evidence: Very low | |||||
| Engelhardt et al, 2009 (56) | Obs-cont | Yes | Mean change from baseline (SD): 1.8 (15.9)a | Mean change from baseline (SD): 1.3 (15.3)a | 0.49 (−2.57, 3.55) |
Abbreviations: CI, confidence interval; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; Obs-cont, observational study with contemporaneous controls; SD, standard deviation.
Standard deviations are slightly larger because information about the correlation between estimates was not provided.
Home Health Visits
Results from one observational study showed that patients who received single-provider PCPDs tended to receive more home health visits than patients in the control arm (difference in means [95% CI]: 0.49 [−2.57, 3.55]; GRADE: very low). (56) It must be noted that this difference was not statistically significant, however.
Urgent Care Visits
An observational study from the United States found that patients who received team-based PCPDs had fewer urgent care visits than those in the study's control arm (difference in means [95% CI]: −0.30 [−0.61, 0.01]; GRADE: moderate; Table 22). (57) As with some of the results discussed previously, the difference between the study's 2 arms was not statistically significant in the table below but was significant in the original study. It is worth noting that, in the United States, urgent care centres are similar to walk-in clinics that deliver ambulatory care. They are typically used for conditions that require urgent attention, but are not serious enough to warrant a visit to an emergency department.
Table 22:
Effect of Patient Care Planning Discussions on Urgent Care Visits
| Author, Year | Study Design | Tool Used? | Results Intervention | Results Control | Difference in Means (95% CI) |
|---|---|---|---|---|---|
|
Team-Based Discussion vs. Usual Care or No Discussion GRADE for all evidence: Moderate | |||||
| Rabow et al, 2004 (57) | Obs-cont | No | Mean (SD): 0.3 (0.5) | Mean (SD): 0.6 (0.9) | −0.30 (−0.61, 0.01)a,b |
Abbreviations: CI, confidence interval; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; Obs-cont, observational study with contemporaneous controls; SD, standard deviation.
Statistically significant at P ≤ 0.05.
Significant difference was shown in the paper, but the difference was not significant in this estimate.
Other Outpatient Visits
The results in Table 23 show that patients who received single-provider PCPDs tended to have more outpatient visits than those in the control group (difference in means [95% CI]: 2.4 [−4.2, 9.0]; GRADE: low), but the difference was not statistically significant. (56) The results also show that team-based PCPDs were significantly associated with having fewer outpatient visits (differences in means [95% CI]: −5.2 [−9.7, −0.7]; GRADE: moderate). (57) While the 2 sets of results may seem contradictory, they are not entirely surprising, because patients who receive team-based PCPDs may have all of their needs met by the team that is delivering the intervention. Furthermore, the study from which the latter result was taken featured a team-based PCPD with a team that included a psychologist, a pharmacist, a nurse, and 3 physicians.
Table 23:
Effect of Patient Care Planning Discussions on Other Outpatient Visits
| Author, Year | Study Design | Tool Used? | Results Intervention | Results Control | Difference in Means (95% CI) |
|---|---|---|---|---|---|
|
Single-Provider Discussion vs. Usual Care or No Discussion GRADE for all evidence: Low | |||||
| Engelhardt et al, 2009 (56) | Obs-cont | Yes | Outpatient visits | Outpatient visits | 2.39 (−4.18, 8.96) |
| Mean change from baseline (SD): −0.4 (32.5)a | Mean change from baseline (SD): −2.8 (34.8)a | ||||
|
Team-Based Discussion vs. Usual Care or No Discussion GRADE for all evidence: Moderate | |||||
| Rabow et al, 2004 (57) | Obs-cont | No | Clinic and specialist visits combined | Clinic and specialist visits combined | −5.20 (−9.70, −0.70)b |
| Mean (SD): 12.4 (9.5) | Mean (SD): 17.6 (11.8) | ||||
Abbreviations: CI, confidence interval; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; Obs-cont, observational study with contemporaneous controls; SD, standard deviation.
Standard deviations are slightly larger because information about the correlation between estimates was not provided.
Statistically significant at P ≤ 0.05.
Hospice Care
One large RCT showed that patients who received single-provider PCPDs were more likely to receive hospice care at the EoL when compared with their controls (OR [95% CI]: 5.17 [2.03, 13.17]; GRADE: high). (48) Further details about the results for this outcome are presented in Table 24.
Table 24:
Effect of Patient Care Planning Discussions on Receiving Hospice Care
| Author, Year | Study Design | Tool Used? | Results Intervention | Results Control | Odds Ratio (95% CI) |
|---|---|---|---|---|---|
|
Single-Provider Discussion vs. Usual Care or No Discussion GRADE for highest-quality evidence: High GRADE for all evidence: Moderate | |||||
| Highest-quality evidence | |||||
| Casarett et al, 2005 (48) | Large RCT | Yes | Hospice enrolment: 27/107 (25.2%) | Hospice enrolment: 6/98 (6.1%) | 5.17 (2.03, 13.17)a |
| Lower-quality evidence | |||||
| Mack et al, 2012 (58) | Obs-cont | No | Any hospice care: 688/1082 (63.6%) | Any hospice care: 30/149 (20.1%) | 6.93 (4.56, 10.53)a |
| Mack et al, 2010b (59) | Obs-cont | No | Hospice care for > 1 week: 79/113 (69.9%) |
Hospice care for > 1 week: 115/219 (52.5%) |
2.10 (1.30, 3.40)a |
| Wright et al, 2008 (60) | Obs-cont | No | Hospice care for > 1 week: 80/122 (65.6%) |
Hospice care for > 1 week: 93/209 (44.5%) |
2.38 (1.50, 3.77)a |
| Zhang et al, 2009 (53) | Obs-cont | No | Hospice care for > 1 week: 56/75 (74.7%) |
Hospice care for > 1 week: 36/70 (51.4%) |
2.78 (1.38, 5.61)a |
| Pooled estimate (3 obs-cont studies, FE), I2 = 0% | 2.33 (1.72, 3.15)a | ||||
Abbreviations: CI, confidence interval; FE, fixed effects; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; Obs-cont, observational study with contemporaneous controls; RCT, randomized controlled trial; SD, standard deviation.
Statistically significant at P ≤ 0.05.
Raw numbers were not provided in the article and were therefore estimated using the odds ratio and algebraic formulas.
As was seen with other results related to the timing of PCPDs, earlier PCPDs were associated with more optimal health service use at the EoL. An observational study with contemporaneous controls showed that earlier single-provider PCPDs were associated with a higher likelihood of receiving hospice care, as shown in Table 25 (χ2 statistic: 50.756, P 0.001; GRADE: moderate). (58)
Table 25:
Effect of Timing of Patient Care Planning Discussions on Receiving Hospice Care
| Author, Year | Study Design | Tool Used? | Results |
|---|---|---|---|
|
Single-Provider Discussion vs. Usual Care or No Discussion GRADE for all evidence: Moderate | |||
| Mack et al, 2012 (58) | Obs-cont | No | Receiving any hospice care |
| Days between first discussion and death: ≤ 30: 152/311 (48.9%) 31–60: 126/186 (67.7%) 61–90: 80/108 (74.1%) > 90: 146/189 (77.2%) χ2: 50.756, df = 3, P < 0.001a |
|||
Abbreviations: GRADE, Grading of Recommendations Assessment, Development, and Evaluation; Obs-cont, observational study with contemporaneous control.
Statistically significant at P ≤ 0.05.
Summary of Findings
The findings from the highest-quality evidence for each outcome assessed in this EBA are summarized in Tables 26 and 27. While PCPDs might not have been associated with patients’ QoL or satisfaction with care, they were associated with families’ satisfaction with the care their loved ones received and with greater concordance between patients’ wishes and those of their families. This concordance is one of the first steps in achieving optimal communication about EoL care, as is shown in CARENET's model (Figure 1). The next stage of the model focuses on the completion of ACP documents and processes; again, the results from the EBA indicate that PCPDs were associated with this stage of the process of communicating about EoL care. The last stage of the model focuses on the care patients receive. While the results from this EBA do not suggest that PCPDs improved concordance between patients’ wishes and the care they received, PCPDs were associated with receiving less aggressive care at EoL, a lower likelihood of being resuscitated, and a greater likelihood of receiving hospice care. These health care use outcomes are consistent with patients’ stated preference for less aggressive care at EoL. (10) Furthermore, the results from this analysis unequivocally show that earlier PCPDs lead to better outcomes than later discussions. Notably, however, all of the PCPDS in this study were provided to an EoL population (i.e., adults with life-threatening illnesses who are not expected to recover or stabilize).
Table 26:
Summary of the Highest-Quality Evidence on Outcomes of Patient Care Planning Discussions
| Outcome | Single-Provider Discussion vs. Usual Care or No Discussion | Team-Based Discussion vs. Usual Care or No Discussion | ||
|---|---|---|---|---|
| Effect Estimate (95% CI) | GRADE | Effect Estimate (95% CI) | GRADE | |
| Patient's QoL |
SMD 0.47 (−0.58, 1.52) 0.44 (−0.52, 1.37) 0.34 (−0.13, 0.80)a,b 0.01 (−0.19, 0.20) |
Very low |
SMD 0.03 (−0.17, 0.23) |
Moderate |
| Informal caregiver's QoL | Estimates NA | Very low | NA | |
| Patient's satisfaction with EoL care |
OR Very satisfied: 8.24 (3.72, 18.26)a Satisfied or very satisfied: 4.00 (0.83, 19.19) MD −0.2 (−3.4, 2.9) SMD −0.96 (−1.61, −0.32)a |
Moderate |
SMD 0.39 (0.17, 0.60)a |
High |
| Family's satisfaction with EoL care |
OR Very satisfied: 5.17 (1.52, 17.58)a Satisfied or very satisfied: 2.48 (0.55, 11.10) MD 2.10 (1.75, 2.45)a 4.6 |
High | NA | |
| Concordance between patient's wishes and care received |
OR 1.73 (0.76, 3.90) |
High | NA | |
| Concordance between patient's and family's wishes |
OR 4.32, P < 0.001a MD 1.4, P = 0.002a |
High | NA | |
| Completion of ACP documents and processes |
Differences in Proportions 25% (17, 33)a 77% (70, 83)a 13% (5, 19)a |
High |
Differences in Proportions 22% (15, 30)a |
High |
| Receiving chemotherapy at EoL |
OR 0.50 (0.35, 0.72)a |
Low | NA | |
| Resuscitation |
OR 0.13 (0.03, 0.55)a |
Very low | NA | |
| Receiving hospital care |
MD −0.21, P = 0.04a |
High |
MD 0.40 (−0.24, 1.04)b |
Low |
| Hospital LOS |
MD −1.8, P = 0.03a |
High |
MD 0 −3.00 (−6.07, 0.07)a,b |
Low |
| ED visits |
MD −1.27 (−3.34, 0.80) |
Low |
MD −0.10 (−1.16, 0.96) |
Moderate |
| ICU care |
OR 0.44 (0.13, 1.53) |
Very low | NA | |
| ICU LOS | NA |
MD −2 −1.50 (−3.48, 0.48) |
High | |
| Home health visits |
MD 0.49 (−2.57, 3.55) |
Very low | NA | |
| Urgent care visits | NA |
MD −0.30 (−0.61, 0.01)a,b |
Moderate | |
| Other outpatient visits |
MD 2.39 (−4.18, 8.96) |
Low |
MD −5.20 (−9.70, −0.70)a |
Moderate |
| Receiving hospice care |
OR 5.17 (2.03, 13.17)a |
High | NA | |
Abbreviations: ACP, advance care planning; CI, confidence interval; ED, emergency department; EoL, end of life; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; ICU, intensive care unit; LOS, length of stay; MD, difference in means; NA, not available; OR, odds ratio; QoL, quality of life; SMD, standardized mean difference.
Statistically significant at P ≤ 0.05.
Significant difference was shown in the paper, but the difference was not significant in this estimate because the standard deviations for the MDs were estimated conservatively.
Table 27:
Summary of the Highest-Quality Evidence on Timing of Patient Care Planning Discussions
| Outcome | Results | GRADE |
|---|---|---|
| Patient's QoL | Earlier discussions were associated with greater QoL adjusted β = 0.003, P = 0.006a |
Low |
| Receiving chemotherapy at EoL | Earlier discussions were associated with lower receipt of chemotherapy in the last 14 days of life χ2: 17.057, P < 0.001a |
Low |
| Receiving hospital care | Earlier discussions were associated with less hospital care in the last 30 days of life χ2: 55.905, P < 0.001a |
Moderate |
| ICU care | Earlier discussions were associated with less ICU care in the last 30 days of life χ2: 16.606, P < 0.001a |
Low |
| Receiving hospice care | Earlier discussions were associated with receiving hospice care χ2: 50.756, P < 0.001a |
Moderate |
Abbreviations: EoL, end of life; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; ICU, intensive care unit; QoL, quality of life.
Statistically significant at P ≤ 0.05.
Conclusions
Based on the best available evidence, it is clear that single-provider and team-based patient care planning discussions (PCPDs) provide benefits for patients at the end of life (EoL) and their families. These benefits can be observed in terms of patient-centred outcomes and the use of health care resources. Additionally, earlier PCPDs are associated with better patient-centred and utilization outcomes.
High-quality evidence with large magnitudes of effect lend moderate certainty to the conclusion that single-provider PCPDs:
Improve families’ satisfaction with EoL care and concordance between patients’ and families’ wishes.
Reduce the likelihood of receiving hospital care and the number of days spent in hospital.
Increase the completion of advance care planning (ACP) processes and documents and the likelihood of receiving hospice care.
Moderate- to high-quality evidence with large magnitudes of effect indicate, with moderate certainty, that team-based PCPDs:
Increase patient satisfaction and the completion of ACP documents and processes.
Reduce the number of days spent in intensive care and decrease the use of outpatient services.
Finally, moderate-quality evidence indicates that earlier PCPDs are associated with reduced hospital care and with increased hospice care.
The body of evidence used in this evidence-based analysis suffered from common limitations, described below.
Some of the randomized controlled trials were unblinded, and in some cases blinding was done inappropriately or not reported. This could have led to an overestimation of the effect estimates.
Most studies did not specify how missing data were handled. Because the patients included in the studies were at the EoL, and patients who died likely had a different EoL trajectory or prognosis than those who completed the studies, missing data could have introduced attrition bias. The direction in which this bias would have affected the results is unclear.
In some observational studies, exposure to the intervention was measured on the basis of a patient's or family member's response to a single question about discussing EoL care plans with a health care provider. Furthermore, the interventions were often not described in enough detail. As a result, it was impossible to compare multiple components of each intervention or assess how well the intervention was delivered.
Usual care was not defined in most studies, so it is possible that some effect estimates, especially those related to team-based PCPDs, were underestimated.
Studies that measured satisfaction with care and quality of life used a variety of instruments that did not consistently assess the same components of the construct being measured, so their results, as part of an overall body of evidence for those outcomes, should be interpreted cautiously.
Acknowledgements
Editorial Staff
Elizabeth Jean Betsch, ELS
Medical Information Services
Corinne Holubowich, BEd, MLIS
Kellee Kaulback, BA(H), MISt
Health Quality Ontario's Expert Advisory Panel on End-of-Life Care
| Panel Member | Affiliation(s) | Appointment(s) |
|---|---|---|
| Panel Co-Chairs | ||
| Dr Robert Fowler | Sunnybrook Research Institute University of Toronto |
Senior Scientist Associate Professor |
| Shirlee Sharkey | St. Elizabeth Health Care Centre | President and CEO |
| Professional Organizations Representation | ||
| Dr Scott Wooder | Ontario Medical Association | President |
| Health Care System Representation | ||
| Dr Douglas Manuel | Ottawa Hospital Research Institute University of Ottawa |
Senior Scientist Associate Professor |
| Primary/Palliative Care | ||
| Dr Russell Goldman | Mount Sinai Hospital, Tammy Latner Centre for Palliative Care | Director |
| Dr Sandy Buchman | Mount Sinai Hospital, Tammy Latner Centre for Palliative Care Cancer Care Ontario University of Toronto |
Educational Lead Clinical Lead QI Assistant Professor |
| Dr Mary Anne Huggins | Mississauga Halton Palliative Care Network; Dorothy Ley Hospice | Medical Director |
| Dr Cathy Faulds | London Family Health Team | Lead Physician |
| Dr José Pereira | The Ottawa Hospital University of Ottawa |
Professor, and Chief of the Palliative Care program at The Ottawa Hospital |
| Dean Walters | Central East Community Care Access Centre | Nurse Practitioner |
| Critical Care | ||
| Dr Daren Heyland | Clinical Evaluation Research Unit Kingston General Hospital |
Scientific Director |
| Oncology | ||
| Dr Craig Earle | Ontario Institute for Cancer Research Cancer Care Ontario |
Director of Health Services Research Program |
| Internal Medicine | ||
| Dr John You | McMaster University | Associate Professor |
| Geriatrics | ||
| Dr Daphna Grossman | Baycrest Health Sciences | Deputy Head Palliative Care |
| Social Work | ||
| Mary-Lou Kelley | School of Social Work and Northern Ontario School of Medicine Lakehead University |
Professor |
| Emergency Medicine | ||
| Dr Barry McLellan | Sunnybrook Health Sciences Centre | President and Chief Executive Officer |
| Bioethics | ||
| Robert Sibbald | London Health Sciences Centre University of Western Ontario |
Professor |
| Nursing | ||
| Vicki Lejambe | Saint Elizabeth Health Care | Advanced Practice Consultant |
| Tracey DasGupta | Sunnybrook Health Sciences Centre | Director, Interprofessional Practice |
| Mary Jane Esplen | De Souza Institute University of Toronto |
Director Clinician Scientist |
Abbreviations: CEO, Chief Executive Officer; QI, Quality Improvement.
Appendices
Appendix 1: Literature Search Strategies
Search date: October 9, 2013
Databases searched: Ovid MEDLINE, Ovid MEDLINE In-Process, Embase, All EBM Databases (see below), CINAHL
Database: EBM Reviews – Cochrane Database of Systematic Reviews 〈2005 to August 2013〉, EBM Reviews – ACP Journal Club 〈1991 to September 2013〉, EBM Reviews – Database of Abstracts of Reviews of Effects 〈3rd Quarter 2013〉, EBM Reviews – Cochrane Central Register of Controlled Trials 〈September 2013〉, EBM Reviews – Cochrane Methodology Register 〈3rd Quarter 2012〉, EBM Reviews – Health Technology Assessment 〈3rd Quarter 2013〉, EBM Reviews – NHS Economic Evaluation Database 〈3rd Quarter 2013〉, Embase 〈1980 to 2013 Week 40〉, Ovid MEDLINE(R) 〈1946 to September Week 4 2013〉, Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations 〈October 01, 2013
Search Strategy:
| # | Searches | Results |
|---|---|---|
| 1 | exp Terminal Care/ | 86347 |
| 2 | exp Palliative Care/ use mesz,acp,cctr,coch,clcmr,dare,clhta,cleed | 41169 |
| 3 | exp palliative therapy/ use emez | 61505 |
| 4 | exp Terminally Ill/ use mesz,acp,cctr,coch,clcmr,dare,clhta,cleed | 5628 |
| 5 | exp terminally ill patient/ use emez | 5936 |
| 6 | exp terminal disease/ use emez | 4501 |
| 7 | exp dying/ use emez | 5665 |
| 8 | ((End adj2 life adj2 care) or EOL care or (terminal∗ adj2 (care or caring or ill∗ or disease∗)) or palliat∗ or dying or (Advanced adj3 (disease∗ or illness∗)) or end stage∗).ti,ab. | 336689 |
| 9 | or/1–8 | 430311 |
| 10 | exp Patient Care Planning/ | 79710 |
| 11 | ((goal∗ adj2 care) or patient care plan∗ or advance care plan∗).ti,ab. | 5370 |
| 12 | ((communicat∗ or conversation∗ or conference∗ or discuss∗ or consult∗) adj2 (strateg∗ or plan∗ or model∗ or intervention∗ or framework∗ or care or program∗ or process∗)).ti,ab. | 83458 |
| 13 | or/10–12 | 165666 |
| 14 | 9 and 13 | 10891 |
| 15 | limit 14 to english language [Limit not valid in CDSR,ACP Journal Club,DARE,CCTR,CLCMR; records were retained] | 10171 |
| 16 | limit 15 to yr=“2004 -Current” [Limit not valid in DARE; records were retained] | 6293 |
| 17 | limit 16 to yr=“2009 -Current” [Limit not valid in DARE; records were retained] | 4023 |
| 18 | remove duplicates from 17 | 2853 |
| 19 | limit 16 to yr=“2004 – 2008” [Limit not valid in DARE; records were retained] | 2270 |
| 20 | remove duplicates from 19 | 1473 |
| 21 | 18 or 20 | 4326 |
CINAHL
| # | Query | Results |
|---|---|---|
| S1 | (MH “Terminal Care+”) | 38,947 |
| S2 | (MH “Palliative Care”) | 19,702 |
| S3 | (MH “Terminally Ill Patients+”) | 7,636 |
| S4 | ((End N2 life N2 care) or EOL care or (terminal∗ N2 (care or caring or ill∗ or disease∗)) or palliat∗ or dying or (advanced N3 (disease∗ or illness∗)) or end stage∗) | 52,178 |
| S5 | S1 OR S2 OR S3 OR S4 | 60,161 |
| S6 | (MH “Patient Care Plans+”) | 6,983 |
| S7 | ((goal∗ N2 care) or patient care plan∗ or advance care plan∗) | 6,438 |
| S8 | ((communicat∗ or conversation∗ or conference∗ or discuss∗ or consult∗) N2 (strateg∗ or plan∗ or model∗ or intervention∗ or framework∗ or care or program∗ or process∗)) | 19,731 |
| S9 | S6 OR S7 OR S8 | 29,515 |
| S10 | S5 AND S9 | 2,848 |
| S11 | S5 AND S9 Limiters – Published Date: 20040101-20131231; English Language | 2,237 |
Appendix 2: Evidence Quality Assessment
Table A1:
GRADE Evidence Profile for Comparison of Single-Provider Discussions and Usual Care or No Discussion
| Number of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| Patient's Quality of Life | |||||||
| 1 (RCT) | Serious limitations (−1)a | No serious limitations | No serious limitations | Serious limitations (−1)b | Undetected | Large magnitude of effect (+1) | ⊕ Very low |
| 2 (observational) | |||||||
| Informal Caregiver's Quality of Life | |||||||
| 1 (RCT) | Very serious limitations (−2)c | No serious limitations | No serious limitations | Serious limitations (−1)b | Undetected | None | ⊕ Very low |
| Patient's Satisfaction with End-of-Life Care | |||||||
| 3 (RCTs) | No serious limitations | Serious limitations (−1)d | No serious limitations | No serious limitations | Undetected | None | ⊕⊕⊕ Moderate |
| Family's Satisfaction with End-of-Life Care | |||||||
| 3 (RCTs) | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | None | ⊕⊕⊕⊕ High |
| Concordance Between Patient's Wishes and Care Received | |||||||
| 1 (RCT) | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | None | ⊕⊕⊕⊕ High |
| Concordance Between Patient's and Family's Wishes | |||||||
| 2 (RCTs) | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | None | ⊕⊕⊕⊕ High |
| Completion of Advance Care Planning Documents and Processes | |||||||
| 3 (RCTs) | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | None | ⊕⊕⊕⊕ High |
| Receiving Chemotherapy at End of Life | |||||||
| 3 (observational) | Serious limitations (−1)e,f | No serious limitations | No serious limitations | No serious limitations | Undetected | Large magnitude of effect (+1) | ⊕⊕ Low |
| Resuscitation | |||||||
| 2 (observational) | Serious limitations (−1)e,f | No serious limitations | No serious limitations | Serious limitations (−1)b | Undetected | Large magnitude of effect (+1) | ⊕ Very low |
| Receiving Hospital Care | |||||||
| 1 (RCT) | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | None | ⊕⊕⊕⊕ High |
| Hospital Length of Stay | |||||||
| 1 (RCT) | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | None | ⊕⊕⊕⊕ High |
| Emergency Department Visits | |||||||
| 1 (observational) | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | None | ⊕⊕ Low |
| Intensive Care Unit Care | |||||||
| 3 (observational) | Serious limitations (−1)e,f | Serious limitations (−1)d | No serious limitations | No serious limitations | Undetected | Large magnitude of effect (+1) | ⊕ Very low |
| Home Health Visits | |||||||
| 1 (observational) | No serious limitations | No serious limitations | No serious limitations | Serious limitations (−1)b | Undetected | None | ⊕ Very low |
| Other Outpatient Visits | |||||||
| 1 (observational) | No serious limitations | No serious limitations | No serious limitations | Serious limitations (−1)b | Undetected | Large magnitude of effect (+1) | ⊕⊕ Low |
| Receiving Hospice Care | |||||||
| 1 (RCT) | No serious limitations | No serious limitations | No serious limitations | Serious limitations (−1)b | Undetected | None | ⊕⊕⊕⊕ High |
Abbreviations: GRADE, Grading of Recommendations Assessment, Development, and Evaluation; RCT, randomized controlled trial.
Randomized controlled trial has serious risk of bias because of concerns about allocation concealment and blinding, and 1 observational study did not adequately account for confounding.
Wide confidence intervals.
Very high level of attrition and uncertainty about who was blinded or how blinding was achieved.
Results were inconsistent.
Observational studies had some limitations related to how the exposure was assessed.
Observational studies had limitations related to confounding.
Table A2:
GRADE Evidence Profile for Comparison of Team-Based Discussions and Usual Care or No Discussion
| Number of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| Patient's Quality of Life | |||||||
| 1 (RCT) | No serious limitations | No serious limitations | No serious limitations | Serious limitations (−1)a | Undetected | None | ⊕⊕⊕ Moderate |
| Patient's Satisfaction with End-of-Life Care | |||||||
| 1 (RCT) | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | None | ⊕⊕⊕⊕ High |
| Completion of Advance Care Planning Documents and Processes | |||||||
| 1 (RCT) | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | None | ⊕⊕⊕⊕ High |
| Receiving Hospital Care | |||||||
| 1 (observational) | No serious limitations | No serious limitations | No serious limitations | Serious limitations (−1)a | Undetected | All plausible confounding increases confidence in estimate (+1) | ⊕⊕ Low |
| Hospital Length of Stay | |||||||
| 2 (RCT) | Serious limitations (−1)b | Serious limitations (−1)c | No serious limitations | No serious limitations | Undetected | None | ⊕⊕ Low |
| Emergency Department Visits | |||||||
| 1 (observational) | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | All plausible confounding increases confidence in estimate (+1) | ⊕⊕⊕ Moderate |
| Intensive Care Unit Length of Stay | |||||||
| 2 (RCT) | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | None | ⊕⊕⊕⊕ High |
| Urgent Care Visits | |||||||
| 1 (observational) | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | All plausible confounding increases confidence in estimate (+1) | ⊕⊕⊕ Moderate |
| Other Outpatient Visits | |||||||
| 1 (observational) | No serious limitations | No serious limitations | No serious limitations | Serious limitations (−1)a | Undetected | Large magnitude of effect (+1) All plausible confounding increases confidence in estimate (+1) | ⊕⊕⊕ Moderate |
Abbreviations: GRADE, Grading of Recommendations Assessment, Development, and Evaluation; RCT, randomized controlled trial.
Wide confidence intervals.
RCTs suffer limitations because of blinding and allocation concealment.
One study showed that the intervention had beneficial effects and the other showed no effect.
Table A3:
GRADE Evidence Profile for Timing of Patient Care Planning Discussions
| Number of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| Patient's Quality of Life | |||||||
| 1 (observational) | Serious limitations (−1)a | No serious limitations | No serious limitations | No serious limitations | Undetected | Dose-response gradient (+1) | ⊕⊕ Low |
| Receiving Chemotherapy at End of Life | |||||||
| 1 (observational) | Serious limitations (−1)b | No serious limitations | No serious limitations | No serious limitations | Undetected | Large magnitude of effect (+1) | ⊕⊕ Low |
| Receiving Hospital Care | |||||||
| 1 (observational) | Serious limitations (−1)b | No serious limitations | No serious limitations | No serious limitations | Undetected | Large magnitude of effect (+1) Dose-response gradient (+1) | ⊕⊕⊕ Moderate |
| Intensive Care Unit Care | |||||||
| 1 (observational) | Serious limitations (−1)b | No serious limitations | No serious limitations | No serious limitations | Undetected | Large magnitude of effect (+1) | ⊕⊕ Low |
| Receiving Hospice Care | |||||||
| 1 (observational) | Serious limitations (−1)b | No serious limitations | No serious limitations | No serious limitations | Undetected | Large magnitude of effect (+1) Dose-response gradient (+1) | ⊕⊕⊕ Moderate |
Abbreviations: GRADE, Grading of Recommendations Assessment, Development, and Evaluation; RCT, randomized controlled trial.
Information about what variables were adjusted for in the multivariable model was missing.
Observational study suffered from limitations in how the exposure was measured and in confounding.
Table A4:
Risk of Bias Among Randomized Controlled Trials for Comparison of Single-Provider Discussions and Usual Care or No Discussion
| Author, Year | Allocation Concealment | Blinding | Complete Accounting of Patients and Outcome Events | Selective Reporting Bias | Other Limitations |
|---|---|---|---|---|---|
| Kirchhoff et al, 2012 (40); Kirchhoff et al 2010 (41) | No limitations | Limitationsa | Limitationsb | No limitations | No limitations |
| Au et al, 2012 (42) | No limitations | Limitationsc | No limitations | No limitations | Limitationsd |
| Sampson et al, 2011 (51) | No limitations | Unclear | Limitationsb | No limitations | Limitationse |
| Detering et al, 2010 (39) | No limitations | Limitationsf | Limitationsb | No limitations | No limitations |
| Clayton et al, 2007 (44) | No limitations | No limitations | Limitationsb | No limitations | No limitations |
| Casarett et al, 2005 (48) | No limitations | Limitationsg | Limitationsb | No limitations | No limitations |
| Song et al, 2005 (49) | No limitations | Limitationsh | Limitationsb | No limitations | No limitations |
| Nicolasora et al, 2006 (47) | No limitations | Unclear | Limitationsb | No limitations | No limitations |
| Dyar et al, 2012 (50) | Unclear | Unclear | Limitationsb | No limitations | Limitationsi |
| Jones et al, 2011 (52) | No limitations | No limitations | Limitationsb | No limitations | No limitations |
Abbreviations: RCT, randomized controlled trial.
Facilitators unblinded because nature of the intervention made blinding impossible.
Excluded patients with missing data, which could have introduced bias because patients with missing data likely died during the course of the study and were different from those with complete data.
Patients and providers were unblinded, because nature of the intervention made blinding impossible.
Physicians were randomized, not patients, so patients were clustered by physician; individual-level estimates are therefore not completely independent.
Very high attrition likely introduced selection bias.
Researchers administering questionnaires were blinded initially, but some responses given by participants made patient allocation obvious.
Study personnel were unblinded because of nature of the study, but patients were blinded.
Patients and personnel were unblinded after baseline because of nature of the intervention.
Stopped study when positive effect was found.
Table A5:
Risk of Bias among Randomized Controlled Trials for Comparison of Team-Based Discussions and Usual Care or No Discussion
| Author, Year | Allocation Concealment | Blinding | Complete Accounting of Patients and Outcome Events | Selective Reporting Bias | Other Limitations |
|---|---|---|---|---|---|
| Gade et al, 2008 (43) | No limitations | Unclear | Limitationsa | No limitations | No limitations |
| Lautrette et al, 2007 (45) | No limitations | No limitations | Limitationsa | No limitations | No limitations |
| Gilmer et al, 2005 (46) | Unclear | Unclear | Limitationsa | No limitations | No limitations |
Excluded patients with missing data, which could have introduced bias because patients with missing data likely died during the course of the study and were different from those with complete data.
Table A6:
Risk of Bias among Observational Trials for Comparison of Single-Provider Discussions and Usual Care or No Discussion
| Author, Year | Appropriate Eligibility Criteria | Appropriate Measurement of Exposure | Appropriate Measurement of Outcome | Adequate Control for Confounding | Complete Follow-Up |
|---|---|---|---|---|---|
| Observational with Contemporaneous Controls | |||||
| Evangelista et al, 2012 (54) | No limitations | No limitations | No limitations | No limitations | No limitations |
| Jacobsen et al, 2011 (55) | No limitations | No limitations | No limitations | Limitationsa | No limitations |
| Engelhardt et al, 2009 (56) | No limitations | No limitations | No limitations | Limitationsb | No limitations |
| Mack et al, 2012 (58) | No limitations | Limitationsc | No limitations | Unclear | No limitations |
| Mack et al, 2010 (59) | No limitations | Limitationsc | No limitations | No limitations | No limitations |
| Wright et al, 2008 (60) | No limitations | Limitationsc | No limitations | No limitations | No limitations |
| Morrison et al, 2005 (62) | No limitations | No limitations | No limitations | Limitationsd | No limitations |
| Zhang et al, 2009 (53) | No limitations | Limitationse | No limitations | Limitationsd | No limitations |
| Briggs et al, 2004 (63) | No limitations | No limitations | No limitations | Limitationsf | No limitations |
| Observational with Historical Controls | |||||
| Lindner et al, 2007 (66) | No limitations | No limitations | No limitations | No limitations | No limitations |
| Cross-Sectional | |||||
| Leung et al, 2012 (67) | No limitations | Limitationse | Limitationsg | No limitations | No limitations |
| Mori et al, 2013 (68) | No limitations | Limitationse | No limitations | No limitations | No limitations |
| Heyland et al, 2009 (11) | No limitations | Limitationse | Limitationsg | Limitationsf | No limitations |
Considered only differences in age and sex; other demographic factors and potential prognostic factors not accounted for.
Participants in the 2 arms differed on the basis of age and sex, and surrogates in the 2 arms differed on the basis of sex; diagnosis was the only prognostic factor assessed.
Exposure was assessed on basis of patient's medical record, not through observation of the intervention being administered.
Participants in the 2 arms differed on a few prognostic factors, but not on demographic factors.
Exposure was assessed on basis of patient's response to a single question about discussing wishes with the physician.
Participants in the 2 arms differed on prognostic factors and demographic factors.
Used Likert score instead of a validated scale.
Table A7:
Risk of Bias Among Observational Trials for Comparison of Team-Based Discussions and Usual Care or No Discussion
| Author, Year | Appropriate Eligibility Criteria | Appropriate Measurement of Exposure | Appropriate Measurement of Outcome | Adequate Control for Confounding | Complete Follow-Up |
|---|---|---|---|---|---|
| Observational with Contemporaneous Controls | |||||
| Rabow et al, 2004 (57) | No limitations | No limitations | No limitations | No limitations | No limitations |
| Casarett et al, 2008 (61) | No limitations | No limitations | No limitations | Limitationsa | No limitations |
| Observational with Historical Controls | |||||
| Lamba et al, 2012 (64) | No limitations | No limitations | No limitations | Limitationsb | No limitations |
| Norton et al, 2007 (65) | No limitations | No limitations | No limitations | No limitations | No limitations |
Participants in the 2 arms differed on a few demographic and prognostic factors, but were similar overall.
Considered only differences in age and sex; other demographic factors and potential prognostic factors not accounted for.
References
- (1).Lorenz KA, Lynn J, Morton SC, Dy SM, Shugarman LM, Wilkinson A, et al. Methodological approaches for a systematic review of end-of-life care. J Palliat Med. 2005; 8 Suppl 1: S4–11. [DOI] [PubMed] [Google Scholar]
- (2).Statistics Canada. Table 3–6. Ten leading causes of death, by sex and geography, 2009—Ontario. [Ottawa: ]: Statistics Canada; 2012. [cited 2014 Feb. 13]. Available from: http://www.statcan.gc.ca/pub/84-215-x/2012001/tbl/t019-eng.htm. [Google Scholar]
- (3).Hales S, Zimmermann C, Rodin G. The quality of dying and death. Arch Intern Med. 2008; 168(9): 912–8. [DOI] [PubMed] [Google Scholar]
- (4).Ferrell BR. Overview of the domains of variables relevant to end-of-life care. J Palliat Med. 2005; 8 Suppl 1: S22–S29. [DOI] [PubMed] [Google Scholar]
- (5).You JJ, Fowler RA, Heyland DK. Just ask: discussing goals of care with patients in hospital with serious illness. CMAJ. 2014 Apr 1; 186(6): 425–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Gauvin F-P, Lavis JN. Evidence brief: improving end-of-life communication, decision-making and care in Ontario. Hamilton (ON): McMaster Health Forum; 2013 Oct.. 119 p. [Google Scholar]
- (7).Fawole OA, Dy SM, Wilson RF, Lau BD, Martinez KA, Apostol CC, et al. A systematic review of communication quality improvement interventions for patients with advanced and serious illness. J Gen Intern Med. 2012; 28(4): 570–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Scheunemann LP, McDevitt M, Carson SS, Hanson LC. Randomized, controlled trials of interventions to improve communication in intensive care: a systematic review. Chest. 2011; 139(3): 543–54. [DOI] [PubMed] [Google Scholar]
- (9).Robinson L, Dickinson C, Rousseau N, Beyer F, Clark A, Hughes, J. et al. A systematic review of the effectiveness of advance care planning interventions for people with cognitive impairment and dementia. Age Ageing. 2012; 41(2): 263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Heyland DK, Barwich D, Pichora D, Dodek P, Lamontagne F, You JJ, et al. Failure to engage hospitalized elderly patients and their families in advance care planning. JAMA Intern Med. 2013; 173(9): 778–87. [DOI] [PubMed] [Google Scholar]
- (11).Heyland DK, Allan DE, Rocker G, Dodek P, Pichora D, Gafni A, et al. Discussing prognosis with patients and their families near the end of life: impact on satisfaction with end-of-life care. Open Med 2009; 3(2): e101–e110. [PMC free article] [PubMed] [Google Scholar]
- (12).Canadian Institute for Health Information. National Health Expenditure Trends, 1975 to 2013. Ottawa (ON): CIHI; 2013. 161 p. [Google Scholar]
- (13).PubMed. Patient Care Planning. 2013. [cited 2014 Jan. 6]; Available from: URL:http://www.ncbi.nlm.nih.gov/mesh/68010347
- (14).Petticrew M. When are complex interventions “complex”? When are simple interventions “simple”? Eur J Public Health 2011; 21(4): 397–8. [DOI] [PubMed] [Google Scholar]
- (15).Higgins JPT, Green SE. Cochrane handbook for systematic reviews of interventions, version 5.1.0. 2011. [cited 2013 Sept. 20]; Available from: www.cochrane-handbook.org.
- (16).Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011; 64(4): 380–2. [DOI] [PubMed] [Google Scholar]
- (17).Goodman C. Literature searching and evidence interpretation for assessing health care practices. Stockholm, Sweden: Swedish Council on Technology Assessment in Health Care; 1996. 81 p. 119E. [DOI] [PubMed] [Google Scholar]
- (18).Barnes S, Gardiner C, Gott M, Payne S, Chady B, Small N, et al. Enhancing patient-professional communication about end-of-life issues in life-limiting conditions: a critical review of the literature. J Pain Symptom Manage. 2012; 44(6): 866–79. [DOI] [PubMed] [Google Scholar]
- (19).Lorenz KA, Lynn J, Dy SM, Shugarman LR, Wilkinson A, Mularski RA, et al. Evidence for improving palliative care at the end of life: a systematic review. Ann Intern Med. 2008; 148(2): 147–59. [DOI] [PubMed] [Google Scholar]
- (20).Dy SM, Shugarman LR, Lorenz KA, Mularski RA, Lynn J. A systematic review of satisfaction with care at the end of life. J Am Geriatr Soc. 2008; 56(1): 124–9. [DOI] [PubMed] [Google Scholar]
- (21).Frost DW, Cook DJ, Heyland DK, Fowler RA. Patient and healthcare professional factors influencing end-of-life decision-making during critical illness: a systematic review. Crit Care Med. 2011; 39(5): 1174–89. [DOI] [PubMed] [Google Scholar]
- (22).Bravo G, Dubois MF, Wagneur B. Assessing the effectiveness of interventions to promote advance directives among older adults: a systematic review and multi-level analysis. Soc Sci Med 2008; 67(7): 1122–1132. [DOI] [PubMed] [Google Scholar]
- (23).Kryworuchko J, Hill E, Murray MA, Stacey D, Fergusson DA. Interventions for Shared Decision-Making About Life Support in the Intensive Care Unit: A Systematic Review. Worldviews Evid Based Nurs 2013; 10(1): 1–16. [DOI] [PubMed] [Google Scholar]
- (24).Tamayo-Velazquez MI, Simon-Lorda P, Villegas-Portero R, Higueras-Callejon C, Garcia-Gutierrez JF, Martinez-Pecino F. et al. Interventions to promote the use of advance directives: an overview of systematic reviews. Patient Educ Couns 2010; 80(1): 10–20. [DOI] [PubMed] [Google Scholar]
- (25).Lukas L, Foltz C, Paxton H. Hospital outcomes for a home-based palliative medicine consulting service. J Palliat Med 2013; 16(2): 179–184. [DOI] [PubMed] [Google Scholar]
- (26).Zaide GB, Pekmezaris R, Nouryan CN, Mir TP, Sison CP, Liberman T. et al. Ethnicity, race, and advance directives in an inpatient palliative care consultation service. Palliat Support Care 2012; 11(1): 5–11. [DOI] [PubMed] [Google Scholar]
- (27).Quenot JP, Rigaud JP, Prin S, Barbar S, Pavon A, Hamet M. et al. Impact of an intensive communication strategy on end-of-life practices in the intensive care unit. Intensive Care Med 2012; 38(1): 145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Pekmezaris R, Cooper L, Efferen L, Mastrangelo A, Silver A, Eichorn A. et al. Transforming the mortality review conference to assess palliative care in the acute care setting: a feasibility study. Palliat Support Care 2010; 8(4): 421–426. [DOI] [PubMed] [Google Scholar]
- (29).Lopez-Acevedo M, Havrilesky LJ, Broadwater G, Kamal AH, Abernethy AP, Berchuck A. et al. Timing of end-of-life care discussion with performance on end-of-life quality indicators in ovarian cancer. Gynecol Oncol 2013; 130(1): 156–161. [DOI] [PubMed] [Google Scholar]
- (30).Doll KM, Stine JE, Van LL, Moore DT, Bae-Jump V, Brewster WR. et al. Outpatient end of life discussions shorten hospital admissions in gynecologic oncology patients. Gynecol Oncol 2013; 130(1): 152–155. [DOI] [PubMed] [Google Scholar]
- (31).Lustbader D, Pekmezaris R, Frankenthaler M, Walia R, Smith F, Hussain E. et al. Palliative medicine consultation impacts DNR designation and length of stay for terminal medical MICU patients. Palliat Support Care 2011; 9(4): 401–406. [DOI] [PubMed] [Google Scholar]
- (32).Schellinger S, Sidebottom A, Briggs L. Disease specific advance care planning for heart failure patients: Implementation in a large health system. J Palliat Med 2011; 14(11): 1224–1230. [DOI] [PubMed] [Google Scholar]
- (33).Nelson C, Chand P, Sortais J, Oloimooja J, Rembert G. Inpatient palliative care consults and the probability of hospital readmission. Perm J 2011; 15(2): 48–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Levy C, Morris M, Kramer A. Improving end-of-life outcomes in nursing homes by targeting residents at high-risk of mortality for palliative care: Program description and evaluation. J Palliat Med 2008; 11(2): 217–225. [DOI] [PubMed] [Google Scholar]
- (35).Bischoff KE, Sudore R, Miao Y, Boscardin WJ, Smith AK. Advance care planning and the quality of end-of-life care in older adults. J Am Geriatr Soc 2013; 61(2): 209–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Bell CL, Kuriya M, Fischberg D. Hospice referrals and code status: Outcomes of inpatient palliative care consultations among Asian Americans and Pacific Islanders with cancer. J Pain Symptom Manage 2011; 42(4): 557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Sawicki GS, Dill EJ, Asher D, Sellers DE, Robinson WM. Advance care planning in adults with cystic fibrosis. J Palliat Med 2008; 11(8): 1135–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Spettell CM, Rawlins WS, Krakauer R, Fernandes J, Breton ME, Gowdy W. et al. A comprehensive case management program to improve palliative care. J Palliat Med 2009; 12(9): 827–832. [DOI] [PubMed] [Google Scholar]
- (39).Detering KM, Hancock AD, Reade MC, Silvester W. The impact of advance care planning on end of life care in elderly patients: Randomised controlled trial. BMJ (Online) 2010; 340(7751): 847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Kirchhoff KT, Hammes BJ, Kehl KA, Briggs LA, Brown RL. Effect of a disease-specific advance care planning intervention on end-of-life care. J Am Geriatr Soc 2012; 60(5): 946–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Kirchhoff KT, Hammes BJ, Kehl KA, Briggs LA, Brown RL. Effect of a disease-specific planning intervention on surrogate understanding of patient goals for future medical treatment. J Am Geriatr Soc 2010; 58(7): 1233–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Au DH, Udris EM, Engelberg RA, Diehr PH, Bryson CL, Reinke LF. et al. A randomized trial to improve communication about end-of-life care among patients with COPD. Chest 2012; 141(3): 726–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Gade G, Venohr I, Conner D, McGrady K, Beane J, Richardson RH. et al. Impact of an inpatient palliative care team: A randomized control trial. J Palliat Med 2008; 11(2): 180–190. [DOI] [PubMed] [Google Scholar]
- (44).Clayton JM, Butow PN, Tattersall MH, Devine RJ, Simpson JM, Aggarwal G. et al. Randomized controlled trial of a prompt list to help advanced cancer patients and their caregivers to ask questions about prognosis and end-of-life care. J Clin Oncol 2007; 25(6): 715–723. [DOI] [PubMed] [Google Scholar]
- (45).Lautrette A, Darmon M, Megarbane B, Joly LM, Chevret S, Adrie C. et al. A communication strategy and brochure for relatives of patients dying in the ICU. N Engl J Med 2007; 356(5): 469–478. [DOI] [PubMed] [Google Scholar]
- (46).Gilmer T, Schneiderman LJ, Teetzel H, Blustein J, Briggs K, Cohn F. et al. The costs of nonbeneficial treatment in the intensive care setting: ethics consultations can help decide about life-sustaining treatments, which are costly and provide little benefit to dying patients. Health Aff 2005; 24(4): 961–971. [DOI] [PubMed] [Google Scholar]
- (47).Nicolasora N, Pannala R, Mountantonakis S, Shanmugam B, DeGirolamo A, Amoateng-Adjepong Y. et al. If asked, hospitalized patients will choose whether to receive life-sustaining therapies. J Hosp Med 2006; 1(3): 161–167. [DOI] [PubMed] [Google Scholar]
- (48).Casarett D, Karlawish J, Morales K, Crowley R, Mirsch T, Asch DA. Improving the use of hospice services in nursing homes: a randomized controlled trial. JAMA 2005; 294(2): 211–217. [DOI] [PubMed] [Google Scholar]
- (49).Song M-K, Kirchhoff KT, Douglas J, Ward S, Hammes B. A randomized, controlled trial to improve advance care planning among patients undergoing cardiac surgery. Med Care 2005; 43(10): 1049–1053. [DOI] [PubMed] [Google Scholar]
- (50).Dyar S, Lesperance M, Shannon R, Sloan J, Colon-Otero G. A Nurse Practitioner Directed Intervention Improves the Quality of Life of Patients with Metastatic Cancer: Results of a Randomized Pilot Study. J Palliat Med 2012; 15(8): 890–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Sampson EL, Jones L, Thune-Boyle IC, Kukkastenvehmas R, King M, Leurent B. et al. Palliative assessment and advance care planning in severe dementia: An exploratory randomized controlled trial of a complex intervention. Palliat Med 2011; 25(3): 197–209. [DOI] [PubMed] [Google Scholar]
- (52).Jones L, Harrington J, Barlow CA, Tookman A, Drake R, Barnes K. et al. Advance care planning in advanced cancer: can it be achieved? An exploratory randomized patient preference trial of a care planning discussion. Palliat Support Care 2011; 9(1): 3–13. [DOI] [PubMed] [Google Scholar]
- (53).Zhang B, Wright AA, Huskamp HA. Health care costs in the last week of life: Associations with end-of-life conversations. Arch Intern Med 2009; 169(5): 480–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Evangelista LS, Lombardo D, Malik S, Ballard-Hernandez J, Motie M, Liao S. Examining the effects of an outpatient palliative care consultation on symptom burden, depression, and quality of life in patients with symptomatic heart failure. J Card Fail 2012; 18(12): 894–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Jacobsen J, Robinson E, Jackson VA, Meigs JB, Billings JA. Development of a cognitive model for advance care planning discussions: Results from a quality improvement initiative. J Palliat Med 2011; 14(3): 331–336. [DOI] [PubMed] [Google Scholar]
- (56).Engelhardt JB, Rizzo VM, Della Penna RD, Feigenbaum PA, Kirkland KA, Nicholson JS. et al. Effectiveness of care coordination and health counseling in advancing illness. Am J Manage Care 2009; 15(11): 817–825. [PubMed] [Google Scholar]
- (57).Rabow MW, Dibble SL, Pantilat SZ, McPhee SJ. The Comprehensive Care Team: A Controlled Trial of Outpatient Palliative Medicine Consultation. Arch Intern Med 2004; 164(1): 83–91. [DOI] [PubMed] [Google Scholar]
- (58).Mack JW, Cronin A, Keating NL, Taback N, Huskamp HA, Malin JL. et al. Associations between end-of-life discussion characteristics and care received near death: a prospective cohort study. J Clin Oncol 2012; 30(35): 4387–4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Mack JW, Paulk ME, Viswanath K, Prigerson HG. Racial disparities in the outcomes of communication on medical care received near death. Arch Intern Med 2010; 170(17): 1533–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Wright AA, Zhang B, Ray A, Mack JW, Trice E, Balboni T. et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA 2008; 300(14): 1665–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Casarett D, Pickard A, Bailey FA, Ritchie C, Furman C, Rosenfeld K. et al. Do palliative consultations improve patient outcomes? J Am Geriatr Soc 2008; 56(4): 593–599. [DOI] [PubMed] [Google Scholar]
- (62).Morrison RS, Chichin E, Carter J, Burack O, Lantz M, Meier DE. The effect of a social work intervention to enhance advance care planning documentation in the nursing home. J Am Geriatr Soc 2005; 53(2): 290–294. [DOI] [PubMed] [Google Scholar]
- (63).Briggs LA, Kirchhoff KT, Hammes BJ, Song MK, Colvin ER. Patient-centered advance care planning in special patient populations: a pilot study. J Prof Nurs 2004; 20(1): 47–58. [DOI] [PubMed] [Google Scholar]
- (64).Lamba S, Murphy P, McVicker S, Smith JH, Mosenthal AC. Changing end-of-life care practice for liver transplant service patients: Structured palliative care intervention in the surgical intensive care unit. J Pain Symptom Manage 2012; 44(4): 508–519. [DOI] [PubMed] [Google Scholar]
- (65).Norton SA, Hogan LA, Holloway RG, Temkin-Greener H, Buckley MJ, Quill TE. Proactive palliative care in the medical intensive care unit: Effects on length of stay for selected high-risk patients. Crit Care Med 2007; 35(6): 1530–1535. [DOI] [PubMed] [Google Scholar]
- (66).Lindner SA, Davoren JB, Vollmer A, Williams B, Landefeld CS. An electronic medical record intervention increased nursing home advance directive orders and documentation. J Am Geriatr Soc 2007; 55(7): 1001–1006. [DOI] [PubMed] [Google Scholar]
- (67).Leung JM, Udris EM, Uman J, Au DH. The effect of end-of-life discussions on perceived quality of care and health status among patients with COPD. Chest 2012; 142(1): 128–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Mori M, Ellison D, Ashikaga T, McVeigh U, Ramsay A, Ades S. In-advance end-of-life discussions and the quality of inpatient end-of-life care: a pilot study in bereaved primary caregivers of advanced cancer patients. Support Care Cancer 2013; 21(2): 629–636. [DOI] [PubMed] [Google Scholar]