Abstract
Several groups have reported the results of clinical trials of gene augmentation therapy for Leber congenital amaurosis (LCA) because of mutations in the RPE65 gene. These studies have used subretinal injection of adeno-associated virus (AAV) vectors to deliver the human RPE65 cDNA to the retinal pigment epithelial (RPE) cells of the treated eyes. In all of the studies reported to date, this approach has been shown to be both safe and effective. The successful clinical trials of gene augmentation therapy for retinal degeneration caused by mutations in the RPE65 gene sets the stage for broad application of gene therapy to treat retinal degenerative disorders.
Multiple clinical trials using gene augmentation therapy for RPE65-associated vision loss have been successful. These results set the stage for broader application of gene therapy to treat inherited retinal degenerative disorders.
Inherited retinal degenerations (IRDs) are important causes of blindness that are characterized by progressive dysfunction and death of rod and cone photoreceptor cells leading to vision loss (Berger et al. 2010). Several types of IRD, such as Leber congenital amaurosis (LCA), cause vision loss in infancy or childhood (Weleber 2002; Michaelides et al. 2006; Chung and Traboulsi 2009). More than 200 different types of IRDs have been identified across all age groups by clinical and genetic studies, making IRDs one of the most genetically diverse groups of inherited disorders (Hsiau et al. 2007; RetNet 2014). This includes 19 genes initially reported to be associated with LCA (RetNet 2014). LCA is usually diagnosed in infancy, based on the presence of poor vision, nystagmus, abnormal electroretinography responses, and pupillary light reflexes (Perrault et al. 1999; Lorenz et al. 2000; Aleman et al. 2004; Simonelli et al. 2007). Most patients with LCA have severe visual impairment throughout childhood; vision deteriorates over time and patients often have total blindness by the third or fourth decade of life (Perrault et al. 1999). Despite the notable progress made in identifying the genetic causes for IRDs, the specific genetic cause remains elusive for up to half of IRD patients (Hartong et al. 2006; Daiger et al. 2007; Stone 2007; den Hollander et al. 2010; Audo et al. 2012; Neveling et al. 2012; Shanks et al. 2013).
Research on gene therapy for IRDs is now in a translational phase, following the reports of the success of Phase I clinical trials of gene therapy for RPE65 LCA, choroideremia, X-linked adrenoleukodystrophy, and hemophilia (Bainbridge et al. 2008; Cideciyan et al. 2008; Maguire et al. 2008, 2009; Cartier et al. 2009; Nathwani et al. 2011; Bennett et al. 2012; Jacobson et al. 2012; MacLaren et al. 2014). As a follow-up to these initial proof-of-concept trials, clinical trials of gene therapy for four other genetic forms of IRD are currently in progress (Table 1). Further, gene therapy studies in animal models have reported efficacy for multiple additional genetic types of IRD (Ali et al. 2000; Min et al. 2005; Alexander et al. 2007; Pang et al. 2008, 2012; Tam et al. 2008; Boye et al. 2010; Cai et al. 2010; Komaromy et al. 2010; Pawlyk et al. 2010; Sun et al. 2010; Mao et al. 2011; Mihelec et al. 2011; Simons et al. 2011; Beltran et al. 2012; Han et al. 2012). There is thus an unprecedented opportunity to translate research progress into sight preserving and/or restoring treatment for patients with IRDs.
Table 1.
Clinical trials that have been initiated for gene therapy of IRDs
| Disease target | Vector name (serotype or envelope) | Surgical administration sitea | Phase | Target enrollment | Approximate start date | ClinicalTrials.gov identifier | Publications |
|---|---|---|---|---|---|---|---|
| LCA2 | AAV2-hRPE65v2 | Consortium of CHOP, UPENN, TIGEM, and SUN | I/II | 12 | October 2007 | NCT00516477 | Maguire et al. 2008, 2009; Simonelli et al. 2010; Testa et al. 2013 |
| LCA2 | AAV2-CBSB-hRPE65 | UFL,UPENN | I | 15 | October 2007 | NCT00481546 | Cideciyan et al. 2008, 2009a,b, 2013; Hauswirth et al. 2008; Jacobson et al. 2012 |
| LCA2 | AAV2-CB-hRPE65 (contained a longer CMV sequence than AAV2-CBSB-hRPE65) | Hadassah Hospital, Jerusalem, Israel | I | 10 | January 2009 | NCT00821340 | Banin et al. 2010 |
| LCA2 | AAV2-CB-hRPE65 | OHSU, UPort, UMass | I/II | 12 | December 2008 | NCT00749957 | |
| LCA2 | rAAV2/4.hRPE65 | Nantes University, France | I/II | 9 | November 2011 | NCT01496040 | |
| LCA2 | rAAV2/2.hRPE65p.hRPE65 | Moorefields, UCL, London, UK | I/II | 12 | 2007 | NCT00643747 | Bainbridge et al. 2008 |
| LCA2 | AAV2-hRPE65v2 | CHOP, UPENN | I/II Follow on | 12 (same subjects as above) | September 2010 | NCT01208389 | Bennett et al. 2012 |
| LCA2 | AAV2-hRPE65v2 | CHOP, UPENN, UIowa | III | 24 | November 2013 | NCT00999609 | |
| Stargardt | StarGen (recombinant lentivirus based in equine infectious anemia virus [EIAV]) | OHSU, UPort, Paris, France | I/IIa | 28 | NCT01367444 | ||
| Usher IB | UshStat (recombinant lentivirus based in equine infectious anemia virus (EIAV) | OHSU, UPort and Paris, France | Ib | 18 | January 2012 | NCT01505062 | |
| RP-MERTK | rAAV2-VMD2-hMERTK | Riyadh, Saudi Arabia | I | 6 | September 2011 | NCT01482195 | |
| CHM | rAAV2.REP1 | Oxford, Southampton, Manchester, UK | I | 9 | October 2011 | NCT01461213 | Maclaren et al. 2014 |
The trials targeting LCA2 used different vectors. Although all trials using AAV2 used a chicken beta enhancer with a CMV promoter, the CHOP group also incorporated a Kozak sequence and a splice donor-acceptor sequence and the proviral plasmid included a long stuffer sequence than prevented reverse packaging (i.e., packaging of empty virus capsids).
The groups at CHOP, UIowa, and Oxford used a surfactant in the excipient to minimize binding of vector to inert surfaces (Maguire et al. 2008, 2009; Maclaren et al. 2014).
aCHOP, Children’s Hospital of Philadelphia; TIGEM, Telethon Institute of Genetics and Medicine; SUN, Second University of Naples; UFL, University of Florida; OHSU, Oregon Health Sciences University; UIowa, University of Iowa, Iowa City, IA; UPort, University of Portland, Portland, OR; UMass, University of Massachusetts, Worcester, MA.
Much of the optimism about the use of gene therapy to treat IRDs comes from the success of the initial trials of gene augmentation therapy for RPE65-associated LCA. The goal of this article is to briefly review the results of the Phase I and Phase II clinical trials of gene therapy for RPE65 LCA and discuss the importance of these reports.
RPE65
Mutations in the RPE65 gene were first identified to cause LCA in 1997 (Gu et al. 1997; Marlhens et al. 1997b). The RPE65 gene encodes the enzyme that catalyzes the isomerization of all-trans retinyl esters into the chromophore 11-cis retinal (Redmond et al. 1998, 2005; Jin et al. 2005; Moiseyev et al. 2006). Lack or reduction of this enzyme activity limits response of photoreceptors and thus vision and subsequently results in degeneration of the retinal pigment epithelial (RPE) cells and neural retina (Jacobson et al. 2005; Travis et al. 2007).
Dogs with retinal degeneration, caused by a mutation in the RPE65 gene, were identified in 1999 (Veske et al. 1999), shortly after the first RPE65 mutations were identified in humans with LCA (Gu et al. 1997; Marlhens et al. 1997a). The phenotype of the RPE65 mutant dog had already been characterized (Narfstrom et al. 1989). The parallels of the animal model to the human counterpart led to tests of adeno-associated virus (AAV) mediated gene augmentation therapy for the RPE65-associated retinal degeneration in these dogs beginning in 2000. Subretinal injection of an adeno-associated virus serotype 2 (AAV2) vector delivering the canine wild-type RPE65 cDNA resulted in rapid development of visual function, which was sustained over many years and more than a decade in a dog followed the longest (Acland et al. 2001, 2005; Cideciyan et al. 2013). Successful restoration of vision in the treated dogs was an important step toward clinical trials of gene augmentation therapy for RPE65-associated disease in humans (Acland et al. 2001; Narfstrom et al. 2003). Further studies in affected dogs compared effects of dose, vector capsid, species of origin of the cDNA, volume, and age of intervention. Optimal results were found in juvenile animals (Acland et al. 2005; Bennicelli et al. 2008). Several groups also reported successful treatment of (young) Rpe65-deficient mice with AAV-mediated gene therapy (Dejneka et al. 2004; Lai et al. 2004; Chen et al. 2006; Pang et al. 2006; Bennicelli et al. 2008).
PHASE I/II CLINICAL TRIALS
Initial Results from Phase I/II Trials
Based on preclinical studies performed in dogs and mice, clinical trials of gene augmentation therapy for RPE65 LCA were initiated in 2007. The initial reports of the results of these trials were published in 2008.
Before the initiation of human clinical trials, safety studies of AAV-RPE65 therapy were performed in affected dogs and unaffected nonhuman primates (NHPs) (Jacobson et al. 2006b). Several of the groups used AAV2 and a cytomegalovirus promoter and chicken beta-actin enhancer (CMV-CBA) to drive expression (Table 1), although the constructs were not identical. Another group used AAV serotype 1 and drove expression with an RPE65 promoter (Table 1). For the preclinical toxicity studies that were published, affected dogs were injected subretinally with AAV2.RPE65 with doses ranging from 1.5E8 to 4.5E12 vector genomes (vg) in 100–150 µL (Jacobson et al. 2006a). Additional affected dogs were injected with 8.25E10 vg AAV2.RPE65 (Bennicelli et al. 2008).
Normal cynomolgus monkeys were injected subretinally with a single dose/volume of AAV-2/2.RPE65. There were no serious adverse effects of vector injection on retinal structure or function, as tested at 3 wk, 3 mo posttreatment. Healed retinotomy sites from subretinal injections were noted, as were subtle abnormalities in foveal architecture in a subset of eyes injected with vector or vehicle (Jacobson et al. 2006b).
The first results of clinical trials of gene augmentation therapy for RPE65-LCA were reported by two groups in 2008 (Table 1). The group from University College London reported treatment of three subjects with an AAV 2/2 vector containing the human RPE65 cDNA, with expression driven by the human RPE65 promoter (ClinicalTrials.gov number NCT00643747). The vector was delivered by subretinal injection using 1 mL volume, with detachment of approximately one-third of the retina, including the macula. There were no serious adverse events. There were also no clinically significant changes in visual acuity or in peripheral visual fields detected in any of the three patients. One of the treated subjects did have significant improvement in visual function on microperimetry and on dark-adapted perimetry. This subject also showed improvement in a subjective test of visual mobility (Bainbridge et al. 2008).
The consortium led by Children’s Hospital of Philadelphia (CHOP) and including the Telethon Institute of Genetics and Medicine (TIGEM), University of Pennsylvania (UPENN), and the Second University of Naples (SUN) also reported the results from treatment of three subjects with AAV2.hRPE65v2 (Table 1) (Maguire et al. 2008). In this study, the vector solution contained a surfactant to prevent the loss of the vector to the surfaces of the syringe and injection tubing. Treatment was also via subretinal injection, in this case with 1.5 × 1010 vg in 150 µL volume. Resolution of the subretinal bleb produced by the injection was noted within 14 h. All three subjects had an acceptable local and systemic adverse event profile after delivery of AAV2.hRPE65v2. In one patient, an asymptomatic macular hole developed, and although the occurrence was considered to be an adverse event, the patient had some return of retinal function. The macular hole was thought to be caused by the presence of an epiretinal membrane, which was not removed before subretinal injection. Pupillary light responses improved notably in the injected eyes of each subject. Consistent with this, all three subjects reported improved vision in dim light starting 2 wk after surgery. Visual acuity also improved significantly, and there was a trend toward enlarged visual field areas (Maguire et al. 2008). These improvements in vision were noted by 6 wk; there was slower improvement in visual function after that time.
Several months later, a group from UPENN and the University of Florida (UFL) also reported similar results in two publications (Table 1) (Cideciyan et al. 2008; Hauswirth et al. 2008). In that study, three subjects were treated with subretinal injections of AAV2-CBSB-hRPE65 (Jacobson et al. 2006b). Injections contained 6 × 1010 vg in 150 µL, and were delivered to regions outside the macula in two of the three subjects; the injection involved the macula and fovea in the third subject. No serious adverse events or evidence of systemic toxicity was reported. Visual acuity did not improve compared with baseline. Dark adapted full field threshold sensitivities increased significantly in all three treated eyes and subjects reported improved visual sensitivity in their treated eye (Hauswirth et al. 2008). In the regions of the retinas treated with gene therapy, cone and rod sensitivities were recorded to be increased significantly. The investigators estimated that treatment was able to restore almost normal light sensitivity in the treated regions of retina, although the recovery time of the rods in the treated portions of retina were slow compared with normal (Cideciyan et al. 2008).
Although the follow-up periods for these three studies were short, and normal vision was not achieved, these studies provided the basis for further gene therapy studies in patients with RPE65-LCA.
Complete Results from Phase I/II Trials
More complete results from the Phase I/II trials were published beginning in 2009. The results from all trials reported showed improvements in vision in treated subjects. In aggregate, the reported results suggest that the response to treatment is at least in part age dependent.
The CHOP/TIGEM/UPENN/SUN group reported the retinal and visual function in 12 patients (aged 8–44 yr) with RPE65-associated LCA treated with a subretinal injection of the AAV2-hRPE65v2 vector (NCT00516477). Doses given were 1.5 × 1010 (low), 4.8 × 1010 (medium), and 1.5 × 1011 (high) vg delivered in 150 or 300 µL. These data included the first three subjects reported on in 2008, and nine additional subjects. The surgical procedure was altered after the first three subjects to include removal of epiretinal membranes when present, and use of perfluorooctane liquid to tamponade the fovea during the subretinal injections (Maguire et al. 2009). The follow-up period ranged from 3 mo to 2 yr. As in the initial report, the AAV2-hRPE65v2 treatment was well tolerated and all patients showed sustained improvement in subjective and objective measurements of vision. For example, all subjects had at least a 2 log unit increase in pupillary light responses and corresponding increases in full-field threshold sensitivities. Seven of the 12 subjects had improvement in visual acuity. The greatest improvements in visual function were noted in children, all of who gained ambulatory vision. One 8-yr-old subject had nearly the same level of light sensitivity as that in age-matched normal-sighted individuals. Four children also had substantial improvement in their ability to navigate a standardized obstacle course, especially in dim light (Maguire et al. 2009).
Additional reports regarding the sustained benefit observed in these subjects have been published, with follow-up periods of up to 3 yr. Analyses show that the maximum improvement in vision was achieved within 6 mo after treatment and the improvements in vision observed in the initial report have been stable (Simonelli et al. 2010; Testa et al. 2013). Further, fMRI (functional MRI) measurements, performed after they already had their initial eye injected, showed restoration of the neural circuitry connecting the retina and the visual cortex in individuals who had been deprived of vision for up to 35 yr (Ashtari et al. 2011). The subjects were able to see dim and low contrast stimuli with their treated eyes—targets that had been invisible to them before treatment. The areas of activation correlated closely with the areas of retina that had been treated. It had been previously thought that the brain would not be able to interpret signal delivered from the retina if retinal function were to be restored beyond early childhood. The data thus suggest that there may be more plasticity in the visual pathways than previously thought (Ashtari et al. 2011). Although not quantified in publications, the changes in visual function in the treated subjects has enabled many of them to do activities which they were not able to perform before treatment. This includes riding a bicycle without assistance, reading books, and being able to see faces during a candlelight dinner. Based on the improvements in vision obtained, some of the treated no longer meet the definition of “legally blind.”
The UPENN/UFL group also reported longer-term results from their clinical trial (Cideciyan et al. 2009a; Jacobson et al. 2012). In these publications, the investigators report their results from treatment of 15 subjects who were 11–30 yr of age with the AAV2-CBSB-hRPE65 vector. Four different dose levels and two injection strategies were evaluated. The first three cohorts received single subretinal injections of 150 or 300 µL of vector. Cohorts 4 and 5 had two injections of 225 µL each, some involving the fovea and some not. Total vector doses ranged from 6 × 1010 to 18 × 1010 vg. The only adverse events reported were related to surgery, with retinal detachment requiring additional surgery in one subject and persistent choroidal effusions in another subject. All study eyes were reported to have recovered completely. There was no evidence of systemic toxicity. Visual function improved in all subjects, although to different degrees. Cone and rod sensitivities improved significantly in the treated regions of retina and the improvements reported initially at 3 mo were sustained through 3 yr. Some improvement in visual acuity was reported, and this was largest in eyes with the lowest entry acuities. It was suggested that some subjects with better foveal structure may have experienced decreases in retinal thickness and acuity following subfoveal injections (Jacobson et al. 2012).
Although more than half a dozen Phase I clinical trials for LCA-RPE65 have been initiated (Table 1), there is only one additional peer-reviewed publication describing results in one subject treated in Israel. This describes the short-term results of one subject who received injection of a vector that was slightly different than that injected in the UPENN/UFL study (Table 1). In that subject, there was an increase in vision present in the treated area as early as 15 d after the intervention (Banin et al. 2010).
PHASE II CLINICAL TRIAL
The success of the unilateral injections in the Phase I/II studies described above begged the question of whether further benefit would result from injection of the second eye. Before evaluating the safety of AAV2-hRPE65v2 in humans, the concern was that the initial injection of AAV might have served as a vaccination. An immune response resulting from exposure of the first eye might not only prevent benefit in the second eye but might also cause damage to the initially injected eye through inflammatory response.
The readministration studies in the human clinical trial subjects was preceded by testing readministration in large animal models (Amado et al. 2010). Sequential subretinal readministration of high dose (1.5E11 vg) AAV2-hRPE65v2 was tested in both Briard (affected) dogs as well as unaffected NHPs that had been previously exposed systemically to AAV (Amado et al. 2010). There were no safety concerns with respect to readministration in either the initially injected eye or the second (contralateral) eye (Amado et al. 2010). An additional preclinical toxicology study examined the effects of readministration in unaffected NHPs of doses that were two to five times higher than the highest dose of the Phase I human trial and at CHOP. Again, there was no indication of ocular toxicity and there were no test article-related clinical signs of systemic toxicity, paving the way to initiate a human readministration clinical trial (Amado et al. 2010).
In the human clinical trial, oldest individuals (least likely to benefit from the intervention) were enrolled first. Subjects were evaluated on a weekly basis and there was a 3-mo stagger between enrollments of each of these individuals (Bennett et al. 2012). The data showed that not only was readministration to the second eye safe, but also it was efficacious. Administration of AAV2-hRPE65v2 to the contralateral eye was well tolerated. There were no cytotoxic T-cell responses to either vector (AAV2) or transgene product (RPE65) in any of the subjects. Neutralizing antibody (NAb) responses to AAV2 and RPE65 protein remained at or close to baseline in the postoperative period (Bennett et al. 2012). Equally important, each of the subjects showed improved pupillary light reflexes and light sensitivity, and two of the three subjects became able to navigate the mobility course (Bennett et al. 2012). Results from fMRI testing showed that the visual cortex responded to the newly treated retina in a manner reflecting the region of retina exposed to vector and the corresponding known neuro-anatomic pathways (Bennett et al. 2012). The remaining eligible subjects have been enrolled and injected and long-term follow-up data will soon be available.
PHASE III CLINICAL TRIAL
Based on the results of the Phase I and II trials described above, a Phase III (“pivotal”) trial for RPE65 gene augmentation therapy is now in progress at CHOP and University of Iowa (NCT00999609). Enrollment is expected to be completed in late 2014, leading to potential registration of this as a therapy in 2015.
The Phase III study involves bilateral subretinal delivery of AAV2.hRPE65v2 at 1.5E11 vg in eligible individuals ages 3 yr and older. To obtain additional natural history data, the study includes a control arm. Subjects are randomized 2:1 to the intervention or control group, respectively. Individuals in the control arm are evaluated at the same time intervals as the intervention subjects for 1 yr, and then they cross to the intervention group. Efforts are taken to assure that the individuals grading the primary endpoints are masked as to the study visit and as to whether the subjects had been assigned to the control or the intervention arm of the study. Every step of the protocol is monitored, starting with the informed consent/assent process. All of the data are processed by an outside data/statistical analysis organization, Westat (Rockville, MD).
Enrollment in the Phase III study is time-consuming for the subjects and their families. After a screening visit, they undergo baseline testing. The testing is noninvasive and is designed such that it is appropriate for individuals in the wide age range enrolled in the study. After baseline testing, randomization to the intervention or the control arm is made by the outside data organization. Subjects assigned to the intervention arm of the study who do not live in the area typically stay near the Center for 3.5 wk at the time of the surgical administration. There are then at least five follow-up visits in year 1 for both intervention and control subjects. Although the number and frequency of follow-up visits tapers after year 1, the follow-up visits will continue for another 14 yr, as the U.S. (FDA) mandates 15 yr of follow-up for gene therapy trial subjects.
True to the reiterative nature of translational research, there will likely be additional studies in the future relating to development of this particular therapeutic. One question is, will it be safe to readminister the reagent to a different part of the previously injected retina? Initial studies in two affected dogs indicated that this approach can be safe and effective (J Bennett, F Mingozzi, AM Maguire, unpubl.), however, the potential for an adverse immune response should be further explored before moving to humans.
CONTROVERSIES IN THE FIELD
Will Gene Augmentation Therapy Prevent Further Degeneration of the Retina?
This is an important question in the neurodegeneration field in general as the ultimate gene therapy would not only restore function, but also prevent any further deterioration. Either effect alone would be meaningful to a patient with neurodegeneration. We know the answer for the retina with respect to the question regarding restoration of function (see above), however, we do not yet know the answer to the question—can we halt the disease in its tracks?
There are several challenges to answering this question: (1) It likely takes ∼5–10 yr to observe progression of retinal degeneration in these slowly progressive IRDs. How reliably will we be able to measure this after only a few years have passed? Loss-of-function may potentially be used to monitor progression of retinal degeneration. So far, these have not been reported. Structural studies can also be used to quantify retinal cell structure and density now that sensitive equipment is available (this was not available when LCA2—LCA caused by RPE65 mutations—trials were initiated in 2007). (2) As in most Phase I (safety) clinical trials in orphan diseases, there are a huge amount of variables to negotiate and only a small number of subjects to study. Variables include type of vector, the dose and volume of the vector, the location of subretinal injection and whether there were one or two injections made in the retina, the region of the retina that is analyzed (i.e., exposed to vector or not exposed to vector; macular vs. peripheral), the age of the subject and the corresponding amount of disease progression, the type of mutation in each subject, whether or not there were complications associated with the surgery, which subjects were included in the measurements, and given that nystagmus is a common finding in LCA2, the fixation/concentration abilities of the subject, and the effects therein on the precision of measurements of retinal thickness and structure (Wojno et al. 2013).
With respect to the first challenge, the slow nature of the disease progression, Cideciyan et al. 2013 used a creative and unconventional statistical modeling approach. They generated their own model of disease progression by taking multiple measures within a small set of samples and then related multiple measurements within their experimental samples to this model. Their answer to this question in studies of patients aged 11–30 yr old was that, although function was recovered, degeneration continues (Wojno et al. 2013). Notably, when Cideciyan et al. 2013 studied an affected dog that had been injected at age 4 mo and then evaluated 11 yr later, they showed that degenerative changes in the injected portion of the retina were minimal. If there was indeed a halting of disease progression after injection in an affected puppy, but not in the humans, is the difference because of the young age at treatment in the dog model, the mutation, the lack of complications, or one of the other variables (see above)? It will be interesting to see, in future studies, whether similar conclusions are drawn using data from subjects injected with similar injection techniques, with the same dose and volume, in similar retinal locations, and in the same age groups.
It may indeed be that treatment beyond a certain age/stage of disease does not prevent degeneration, but it may require longer studies in a larger cohort of (young) subjects treated with the same dose and volume of vector to be sure. What should not be dismissed is that the results of the Phase I interventions achieved the goals of the studies—a safe, stable, and long-term improvement in retinal function (Wojno et al. 2013).
Is it Safe to Deliver Gene Therapy through Subretinal Injection?
Subretinal delivery of AAV2, lentivirus (and the majority of other recombinant and nonviral vectors) is required to transduce photoreceptors and RPE cells efficiently and safely (Fig. 1). Because, normally photoreceptors and RPE cells are tightly apposed, subretinal injection causes a localized disruption of the outer retinal architecture. The retina usually reattaches within hours leaving a minimal amount of histologic damage. Given that primate photoreceptors normally renew their outer segments within a 2 mo time period (Young and Bok 1969) and that after experimental retinal detachment, primate outer segments reform quickly (Guerin et al. 1993), the normal configuration is thought to return close to normal within 1 to 2 mo. This knowledge forms the foundation for use of subretinal surgery techniques, such as those used by retinal surgeons to remove neovascular membranes (Cooper and Thomas 2000). Subretinal surgery in IRDs carries an added risk of physical stress of the injection damaging the fovea, the most vulnerable area of the retina, because the disease can make this already thin region even thinner. The major concern with respect to outcomes is that the fovea is the region which confers best visual acuity. A reason to target the fovea, however, is that this is also the region that, if rescued, could provide the most meaningful visual improvements (Fig. 1). The risk-benefit ratio of subfoveal injection thus can vary depending on the level of baseline function of this region. An accumulation of data relating to the effects of subretinal injection of foveal thickness and function is therefore of great interest. Jacobson et al. (2012) reported that subretinal injections, even outside of the fovea, could cause a decrease in foveal thickness. However, it was not clear whether these changes had any effect on visual acuity as one individual with a foveal injection and decreased foveal thickness showed improved visual acuity (Wojno et al. 2013; Jacobson et al. 2012). More recently, MacLaren et al. (2014) published the first set of results of a gene therapy trial for choroideremia. In that study, the goal was to deliver AAV.hCHM to the central macula, as this was the only viable tissue in those adults with choroideremia. The foveas of five of the six subjects were exposed to the vector. Notably, all six patients recovered their baseline acuity and two of the patients who had received foveal injections showed large gains in acuity, with one patient gaining more than three lines on the eye chart (i.e., significant improvement) (Maclaren et al. 2014). Thus, AAV can be delivered safely to the human fovea through subretinal injection.
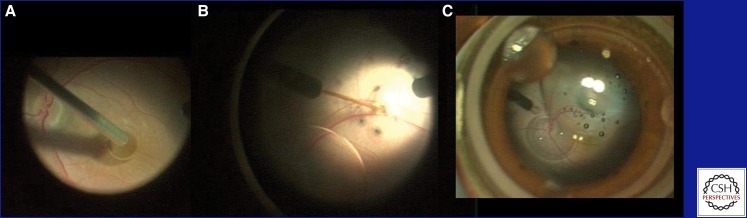
Figure 1.
Frames from an intraoperative video of surgical delivery of AAV2-hRPE65.v2 in a Phase I/II clinical trial at CHOP. (A) A drop of dense Perfluoron liquid is layered onto the fovea to buttress it during the retinal detachment. (B) The subretinal injection cannula is opposed to the neural retina. Injection of the excipient (containing the AAV) causes a separation between the neural retina and the underlying retinal pigment epithelium (i.e., a localized retinal detachment). (C) A view of the detached region of the retina at lower magnification. The optic disk is visible and the detachment includes the superior arcade and extends through the fovea. A few small bubbles, introduced with the AAV, are now visible in the subretinal space.
Nevertheless, there can be complications as with any surgery, and in the case of subretinal delivery, these can include those that have been reported in the various LCA2 clinical trials, including macular hole (Maguire et al. 2008; Simonelli et al. 2010), unresolved retinal detachment requiring surgical repair, choroidal effusions, hypotonia, and retinal tears (Hauswirth et al. 2008; Jacobson et al. 2012) (see above). Further, the ideal anesthesia for subretinal injection is general anesthesia, which carries its own risks and requires an operating room. Therefore, there is a great deal of interest in developing vectors that can target the outer retina after intravitreal injection, which can be performed as an office procedure.
The more “traditional” AAV vectors do not target the outer retina efficiently after intravitreal injection (Bennett et al. 2000; Lebherz et al. 2008; Vandenberghe et al. 2011; Yin et al. 2011). It is thus exciting that experiments using either directed evolution or generating specific capsid mutations have led to vectors that can target the outer retina after intravitreal injection (Dalkara et al. 2013; Kay et al. 2013). Initial proof-of-concept studies using these vectors were performed in rodent models. It will be interesting to see whether the data can be extrapolated to large animal models and using doses that are safe.
What Outcome Measures Should Be Used in Retinal Gene Therapy Trials?
At present, there are only two outcome measures that have been used successfully to approve new drugs for retinal applications and these are based on measures that reflect improvements in day-to-day life: visual acuity (the ability to resolve high contrast visual angles) and, in one case, visual fields using an automated threshold perimeter (see Briefing document: FDA Cellular, Tissue, and Gene Therapies Advisory Committee; CTGTAC Meeting #52; Cellular and Gene Therapies for Retinal Disorders; available at http://www.fda.gov/downloads/advisor…/ucm259087.pdf). For many forms of IRD, these outcome measures are irrelevant. There can be a loss of sensitivity in patients with either end of the visual acuity range (very good or poor visual acuity) that would make it difficult to achieve a 15 letter change. Many patients with IRD have such poor vision or fixation that visual field testing cannot be performed reliably. Thus, there is a great need for development of additional outcome measures. Although it is now possible to obtain reliable imaging data, imaging data can only be supportive as it is not deemed “clinically meaningful.” The current Phase III clinical trial for LCA2 uses a primary outcome measure based on an improvement in the ability to navigate accurately. If these data show improvement, the mobility test endpoint could be used to monitor efficacy in future clinical trials. It will be important to develop additional endpoints that could be used to assess efficacy of gene-based treatments for other IRDs.
CHALLENGES THAT LIE AHEAD
LCA2 is likely one of the easiest targets for gene augmentation therapy. There are both real and theoretical challenges in developing gene therapy for other retinal degenerative conditions. The details that made LCA2 “easy,” include: (1) a slow degenerative component of the disease; (2) the RPE65 transgene cassette fits within the limited confines of the AAV cargo hold; (3) RPE cells are the targets, and they are efficiently transduced by AAV2; (4) because each RPE cell subserves ∼20–40 photoreceptor cells in the primate retina (Snodderly et al. 2002), the effect of intervention is amplified; and (5) the RPE65 defect is enzymatic in nature and it appears that as long as there is a critical amount of enzyme delivered, the disease can be corrected. The presence of excess RPE65 protein does not appear to be harmful.
Some forms of LCA, for example, LCA caused by AIPL1 or CRX mutations, will be challenging to target as there is an early onset degenerative component or even a component which may prevent the retina from developing appropriately. In such forms, a gene augmentation intervention would need to be applied very early in life and perhaps even in utero. In utero or early postnatal delivery would entail both ethical and practical challenges.
For diseases such as autosomal recessive Stargardt disease and LCA caused by CEP290 mutations, the cDNA is too large to fit into AAV. Nonviral vectors are appealing in that there are no size limits, but so far none have been tested in humans with blinding diseases. For use of AAV, there may be complementation mediated by the infected cell after coinfection with virus particles carrying different portions of the gene cassette (Colella et al. 2014; Puppo et al. 2014; Trapani et al. 2014). Other viral or nonviral vectors may also be useful for those conditions (Kong et al. 2008; Puppo et al. 2014). Alternative strategies, such as use of a truncated cDNA, trans-splicing, gene correction, or delivery of antisense nucleic acids may be effective (Lai et al. 2008; Collin et al. 2012; Drivas et al. 2013).
Whereas RPE cells are the target for LCA2, photoreceptors are the primary cell targets for the majority of retinal degenerative diseases. AAV2 transduces photoreceptors, but is not as efficient at photoreceptor transduction as other, more recently identified vectors. Higher doses of AAV2 would have to be used compared with AAV8, for example, to achieve similar levels of transduction (Vandenberghe et al. 2011). The most efficient transduction will be required in photoreceptor diseases because there will be no amplification of effect inherent in the system, such as that mediated by transduction of one RPE cell that then contacts up to fourfold photoreceptor cells (see above). Use of higher doses of vector may increase the risk of toxicity or harmful immune response. In some cases, the selection of vector capsid may not be scientific but may be based on business principles. For example, while AAV2 is in the common domain, there is intellectual property on most other vectors which would require negotiations/licensing fees should there ultimately be a product that is sold.
Finally, in many retinal degenerative conditions, such as diseases involving protein trafficking or cilia function deficits, the cell may only tolerate a particular amount of protein product. An upset in variables affecting protein trafficking parameters could potentially exacerbate disease.
With the great progress in development of proof-of-principle in animal models and in personalized cell models of human retinal disease, we are now at a stage in which strategies aimed to deliver corrective genes/proteins to photoreceptors can be tested directly in humans. Indeed, there are already two different disease targets for which studies testing the safety and efficacy of lentivirus-mediated gene augmentation therapy for primary photoreceptor disease in humans are in progress (Table 1).
SUMMARY AND VISION
The results of multiple clinical trials of gene augmentation therapy for RPE65-associated retinal degeneration show that this approach is both safe and effective. These landmark studies thus provide the basis for testing gene augmentation therapy in other genetic forms of inherited retinal degeneration (IRD). The recently reported results from a clinical trial of gene augmentation therapy for choroideremia support this conclusion, as do reports of successful preclinical studies of gene therapy for multiple other genetic forms of IRD.
Footnotes
Editors: Eric A. Pierce, Richard H. Masland, and Joan W. Miller
Additional Perspectives on Retinal Disorders: Genetic Approaches to Diagnosis and Treatment available at www.perspectivesinmedicine.org
REFERENCES
- Acland GM, Aguirre GD, Ray J, Zhang Q, Aleman TS, Cideciyan AV, Pearce-Kelling SE, Anand V, Zeng Y, Maguire AM, et al. 2001. Gene therapy restores vision in a canine model of childhood blindness. Nat Genet 28: 92–95. [DOI] [PubMed] [Google Scholar]
- Acland GM, Aguirre GD, Bennett J, Aleman TS, Cideciyan AV, Bennicelli J, Dejneka NS, Pearce-Kelling SE, Maguire AM, Palczewski K, et al. 2005. Long-term restoration of rod and cone vision by single dose rAAV-mediated gene transfer to the retina in a canine model of childhood blindness. Mol Ther 12: 1072–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleman TS, Jacobson SG, Chico JD, Scott ML, Cheung AY, Windsor EA, Furushima M, Redmond TM, Bennett J, Palczewski K, et al. 2004. Impairment of the transient pupillary light reflex in Rpe65−/− mice and humans with Leber congenital amaurosis. Invest Ophthalmol Vis Sci 45: 1259–1271. [DOI] [PubMed] [Google Scholar]
- Alexander JJ, Umino Y, Everhart D, Chang B, Min SH, Li Q, Timmers AM, Hawes NL, Pang JJ, Barlow RB, et al. 2007. Restoration of cone vision in a mouse model of achromatopsia. Nat Med 13: 685–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali RR, Sarra GM, Stephens C, Alwis MD, Bainbridge JW, Munro PM, Fauser S, Reichel MB, Kinnon C, Hunt DM, et al. 2000. Restoration of photoreceptor ultrastructure and function in retinal degeneration slow mice by gene therapy. Nat Genet 25: 306–310. [DOI] [PubMed] [Google Scholar]
- Amado D, Mingozzi F, Hui D, Bennicelli J, Wei Z, Chen Y, Bote E, Grant R, Golden J, Narfstrom K, et al. 2010. Safety and efficacy of subretinal readministration of an AAV2 vector in large animal models: Implications for studies in humans. Sci Transl Med 2: 21ra16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashtari M, Cyckowski LL, Monroe JF, Marshall KA, Chung DC, Auricchio A, Simonelli F, Leroy BP, Maguire AM, Shindler KS, et al. 2011. The human visual cortex responds to gene therapy-mediated recovery of retinal function. J Clin Invest 121: 2160–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audo I, Bujakowska KM, Leveillard T, Mohand-Said S, Lancelot ME, Germain A, Antonio A, Michiels C, Saraiva JP, Letexier M, et al. 2012. Development and application of a next-generation-sequencing (NGS) approach to detect known and novel gene defects underlying retinal diseases. Orphanet J Rare Dis 7: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, Viswanathan A, Holder GE, Stockman A, Tyler N, et al. 2008. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N Engl J Med 358: 2231–2239. [DOI] [PubMed] [Google Scholar]
- Banin E, Bandah-Rozenfeld D, Obolensky A, Cideciyan AV, Aleman TS, Marks-Ohana D, Sela M, Boye S, Sumaroka A, Roman AJ, et al. 2010. Molecular anthropology meets genetic medicine to treat blindness in the North African Jewish population: Human gene therapy initiated in Israel. Hum Gene Ther 21: 1749–1757. [DOI] [PubMed] [Google Scholar]
- Beltran WA, Cideciyan AV, Lewin AS, Iwabe S, Khanna H, Sumaroka A, Chiodo VA, Fajardo DS, Roman AJ, Deng WT, et al. 2012. Gene therapy rescues photoreceptor blindness in dogs and paves the way for treating human X-linked retinitis pigmentosa. Proc Natl Acad Sci 109: 2132–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J, Anand V, Acland GM, Maguire AM. 2000. Cross-species comparison of in vivo reporter gene expression after recombinant adeno-associated virus-mediated retinal transduction. Methods Enzymol 316: 777–789. [DOI] [PubMed] [Google Scholar]
- Bennett J, Ashtari M, Wellman J, Marshall KA, Cyckowski LL, Chung DC, McCague S, Pierce EA, Chen Y, Bennicelli JL, et al. 2012. AAV2 gene therapy readministration in three adults with congenital blindness. Sci Transl Med 4: 120ra115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennicelli J, Wright JF, Komaromy A, Jacobs JB, Hauck B, Zelenaia O, Mingozzi F, Hui D, Chung D, Rex TS, et al. 2008. Reversal of blindness in animal models of Leber congenital amaurosis using optimized AAV2-mediated gene transfer. Mol Ther 16: 458–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger W, Kloeckener-Gruissem B, Neidhardt J. 2010. The molecular basis of human retinal and vitreoretinal diseases. Prog Retin Eye Res 29: 335–375. [DOI] [PubMed] [Google Scholar]
- Boye SE, Boye SL, Pang J, Ryals R, Everhart D, Umino Y, Neeley AW, Besharse J, Barlow R, Hauswirth WW. 2010. Functional and behavioral restoration of vision by gene therapy in the guanylate cyclase-1 (GC1) knockout mouse. PLoS ONE 5: e11306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Conley SM, Nash Z, Fliesler SJ, Cooper MJ, Naash MI. 2010. Gene delivery to mitotic and postmitotic photoreceptors via compacted DNA nanoparticles results in improved phenotype in a mouse model of retinitis pigmentosa. FASEB J 24: 1178–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartier N, Hacein-Bey-Abina S, Bartholomae CC, Veres G, Schmidt M, Kutschera I, Vidaud M, Abel U, Dal-Cortivo L, Caccavelli L, et al. 2009. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science 326: 818–823. [DOI] [PubMed] [Google Scholar]
- Chen Y, Moiseyev G, Takahashi Y, Ma JX. 2006. RPE65 gene delivery restores isomerohydrolase activity and prevents early cone loss in Rpe65−/− mice. Invest Ophthalmol Vis Sci 47(3): 1177–1184. [DOI] [PubMed] [Google Scholar]
- Chung DC, Traboulsi EI. 2009. Leber congenital amaurosis: Clinical correlations with genotypes, gene therapy trials update, and future directions. J AAPOS 13: 587–592. [DOI] [PubMed] [Google Scholar]
- Cideciyan AV, Aleman TS, Boye SL, Schwartz SB, Kaushal S, Roman AJ, Pang JJ, Sumaroka A, Windsor EA, Wilson JM, et al. 2008. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc Natl Acad Sci 105: 15112–15117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cideciyan AV, Hauswirth WW, Aleman TS, Kaushal S, Schwartz SB, Boye SL, Windsor EA, Conlon TJ, Sumaroka A, Pang JJ, et al. 2009a. Human RPE65 gene therapy for Leber congenital amaurosis: Persistence of early visual improvements and safety at 1 year. Hum Gene Ther 20: 999–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cideciyan AV, Hauswirth WW, Aleman TS, Kaushal S, Schwartz SB, Boye SL, Windsor EA, Conlon TJ, Sumaroka A, Roman AJ, et al. 2009b. Vision 1 year after gene therapy for Leber’s congenital amaurosis. N Engl J Med 361: 725–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cideciyan AV, Jacobson SG, Beltran WA, Sumaroka A, Swider M, Iwabe S, Roman AJ, Olivares MB, Schwartz SB, Komaromy AM, et al. 2013. Human retinal gene therapy for Leber congenital amaurosis shows advancing retinal degeneration despite enduring visual improvement. Proc Natl Acad Sci 110: E517–E525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colella P, Trapani I, Cesi G, Sommella A, Manfredi A, Puppo A, Iodice C, Rossi S, Simonelli F, Giunti M, et al. 2014. Efficient gene delivery to the cone-enriched pig retina by dual AAV vectors. Gene Ther 21: 450–456. [DOI] [PubMed] [Google Scholar]
- Collin RW, den Hollander AI, van der Velde-Visser SD, Bennicelli J, Bennett J, Cremers FP. 2012. Antisense oligonucleotide (AON)-based therapy for Leber congenital amaurosis caused by a frequent mutation in CEP290. Mol Ther Nucleic Acids 1: e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper BA, Thomas MA. 2000. Submacular surgery to remove choroidal neovascularization associated with central serous chorioretinopathy. Am J Ophthalmol 130: 187–191. [DOI] [PubMed] [Google Scholar]
- Daiger SP, Bowne SJ, Sullivan LS. 2007. Perspective on genes and mutations causing retinitis pigmentosa. Arch Ophthalmol 125: 151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalkara D, Byrne LC, Klimczak RR, Visel M, Yin L, Merigan WH, Flannery JG, Schaffer DV. 2013. In vivo-directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous. Sci Transl Med 5: 189ra176. [DOI] [PubMed] [Google Scholar]
- Dejneka NS, Surace EM, Aleman TS, Cideciyan AV, Lyubarsky A, Savchenko A, Redmond TM, Tang W, Wei Z, Rex TS, et al. 2004. In utero gene therapy rescues vision in a murine model of congenital blindness. Mol Ther 9(2): 182–188. [DOI] [PubMed] [Google Scholar]
- den Hollander AI, Black A, Bennett J, Cremers FP. 2010. Lighting a candle in the dark: Advances in genetics and gene therapy of recessive retinal dystrophies. J Clin Invest 120: 3042–3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drivas TG, Holzbaur ELF, Bennett J. 2013. Disruption of CEP290 microtubule/membrane-binding domains causes retinal degeneration. J Clin Invest 123: 4525–4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu SM, Thompson DA, Srikumari CR, Lorenz B, Finckh U, Nicoletti A, Murthy KR, Rathmann M, Kumaramanickavel G, Denton MJ, et al. 1997. Mutations in RPE65 cause autosomal recessive childhood-onset severe retinal dystrophy. Nat Genet 17: 194–197. [DOI] [PubMed] [Google Scholar]
- Guerin CJ, Lewis GP, Fisher SK, Anderson DH. 1993. Recovery of photoreceptor outer segment length and analysis of membrane assembly rates in regenerating primate photoreceptor outer segments. Invest Ophthalmol Vis Sci 34: 175–183. [PubMed] [Google Scholar]
- Han Z, Conley SM, Makkia RS, Cooper MJ, Naash MI. 2012. DNA nanoparticle-mediated ABCA4 delivery rescues Stargardt dystrophy in mice. J Clin Invest 122: 3221–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartong DT, Berson EL, Dryja TP. 2006. Retinitis pigmentosa. Lancet 368: 1795–1809. [DOI] [PubMed] [Google Scholar]
- Hauswirth WW, Aleman TS, Kaushal S, Cideciyan AV, Schwartz SB, Wang L, Conlon TJ, Boye SL, Flotte TR, Byrne BJ, et al. 2008. Treatment of Leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: Short-term results of a phase I trial. Hum Gene Ther 19: 979–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiau TH, Diaconu C, Myers CA, Lee J, Cepko CL, Corbo JC. 2007. The cis-regulatory logic of the mammalian photoreceptor transcriptional network. PLoS ONE 2: e643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SG, Aleman TS, Cideciyan AV, Sumaroka A, Schwartz SB, Windsor EA, Traboulsi EI, Heon E, Pittler SJ, Milam AH, et al. 2005. Identifying photoreceptors in blind eyes caused by RPE65 mutations: Prerequisite for human gene therapy success. Proc Natl Acad Sci 102: 6177–6182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SG, Acland GM, Aguirre GD, Aleman TS, Schwartz SB, Cideciyan AV, Zeiss CJ, Komaromy AM, Kaushal S, Roman AJ, et al. 2006a. Safety of recombinant adeno-associated virus type 2-RPE65 vector delivered by ocular subretinal injection. Mol Ther 13: 1074–1084. [DOI] [PubMed] [Google Scholar]
- Jacobson SG, Boye SL, Aleman TS, Conlon TJ, Zeiss CJ, Roman AJ, Cideciyan AV, Schwartz SB, Komaromy AM, Doobrajh M, et al. 2006b. Safety in nonhuman primates of ocular AAV2-RPE65, a candidate treatment for blindness in Leber congenital amaurosis. Hum Gene Ther 17: 845–858. [DOI] [PubMed] [Google Scholar]
- Jacobson SG, Cideciyan AV, Ratnakaram R, Heon E, Schwartz SB, Roman AJ, Peden MC, Aleman TS, Boye SL, Sumaroka A, et al. 2012. Gene therapy for Leber congenital amaurosis caused by RPE65 mutations: Safety and efficacy in 15 children and adults followed up to 3 years. Arch Ophthalmol 130: 9–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M, Li S, Moghrabi WN, Sun H, Travis GH. 2005. Rpe65 is the retinoid isomerase in bovine retinal pigment epithelium. Cell 122: 449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay CN, Ryals RC, Aslanidi GV, Min SH, Ruan Q, Sun J, Dyka FM, Kasuga D, Ayala AE, Van Vliet K, et al. 2013. Targeting photoreceptors via intravitreal delivery using novel, capsid-mutated AAV vectors. PLoS ONE 8: e62097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komaromy AM, Alexander JJ, Rowlan JS, Garcia MM, Chiodo VA, Kaya A, Tanaka JC, Acland GM, Hauswirth WW, Aguirre GD. 2010. Gene therapy rescues cone function in congenital achromatopsia. Hum Mol Genet 19: 2581–2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Kim SR, Binley K, Pata I, Doi K, Mannik J, Zernant-Rajang J, Kan O, Iqball S, Naylor S, et al. 2008. Correction of the disease phenotype in the mouse model of Stargardt disease by lentiviral gene therapy. Gene Ther 15: 1311–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CM, Yu MJ, Brankov M, Barnett NL, Zhou X, Redmond TM, Narfstrom K, Rakoczy PE. 2004. Recombinant adeno-associated virus type 2-mediated gene delivery into the Rpe65−/− knockout mouse eye results in limited rescue. Genet Vaccines Ther 2: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y, Li D, Yue Y, Duan D. 2008. Design of trans-splicing adeno-associated viral vectors for Duchenne muscular dystrophy gene therapy. Methods Mol Biol 433: 259–275. [DOI] [PubMed] [Google Scholar]
- Lebherz C, Maguire A, Tang W, Bennett J, Wilson JM. 2008. Novel AAV serotypes for improved ocular gene transfer. J Gene Med 10: 375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz B, Gyurus P, Preising M, Bremser D, Gu S, Andrassi M, Gerth C, Gal A. 2000. Early-onset severe rod-cone dystrophy in young children with RPE65 mutations. Invest Ophthalmol Vis Sci 41: 2735–2742. [PubMed] [Google Scholar]
- MacLaren RE, Groppe M, Barnard AR, Cottriall CL, Tolmachova T, Seymour L, Clark KR, During MJ, Cremers FP, Black GC, et al. 2014. Retinal gene therapy in patients with choroideremia: Initial findings from a phase 1/2 clinical trial. Lancet 383: 1129–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire AM, Simonelli F, Pierce EA, Pugh EN Jr, Mingozzi F, Bennicelli J, Banfi S, Marshall KA, Testa F, Surace EM, et al. 2008. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N Engl J Med 358: 2240–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire AM, High KA, Auricchio A, Wright JF, Pierce EA, Testa F, Mingozzi F, Bennicelli JL, Ying GS, Rossi S, et al. 2009. Age-dependent effects of RPE65 gene therapy for Leber’s congenital amaurosis: A phase 1 dose-escalation trial. Lancet 374: 1597–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao H, James T Jr, Schwein A, Shabashvili AE, Hauswirth WW, Gorbatyuk MS, Lewin AS. 2011. AAV delivery of wild-type rhodopsin preserves retinal function in a mouse model of autosomal dominant retinitis pigmentosa. Hum Gene Ther 22: 567–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlhens F, Bareil C, Friffoin J-M, Zrenner E, Amalric P, Eliaou C, Liu S-Y, Harris E, Redmond TM, Arnaud B, et al. 1997a. Mutations in RPE65 cause Leber’s congenital amaurosis. Nat Genet 17: 139–141. [DOI] [PubMed] [Google Scholar]
- Marlhens F, Bareil C, Griffoin JM, Zrenner E, Amalric P, Eliaou C, Liu SY, Harris E, Redmond TM, Arnaud B, et al. 1997b. Mutations in RPE65 cause Leber’s congenital amaurosis. Nat Genet 17: 139–140. [DOI] [PubMed] [Google Scholar]
- Michaelides M, Hardcastle AJ, Hunt DM, Moore AT. 2006. Progressive cone and cone-rod dystrophies: Phenotypes and underlying molecular genetic basis. Surv Ophthalmol 51: 232–258. [DOI] [PubMed] [Google Scholar]
- Mihelec M, Pearson RA, Robbie SJ, Buch PK, Azam SA, Bainbridge JW, Smith AJ, Ali RR. 2011. Long-term preservation of cones and improvement in visual function following gene therapy in a mouse model of Leber congenital amaurosis caused by guanylate cyclase-1 deficiency. Hum Gene Ther 22: 1179–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min SH, Molday LL, Seeliger MW, Dinculescu A, Timmers AM, Janssen A, Tonagel F, Tanimoto N, Weber BH, Molday RS, et al. 2005. Prolonged recovery of retinal structure/function after gene therapy in an Rs1h-deficient mouse model of X-linked juvenile retinoschisis. Mol Ther 12: 644–651. [DOI] [PubMed] [Google Scholar]
- Moiseyev G, Takahashi Y, Chen Y, Gentleman S, Redmond TM, Crouch RK, Ma JX. 2006. RPE65 is an iron(II)-dependent isomerohydrolase in the retinoid visual cycle. J Biol Chem 281: 2835–2840. [DOI] [PubMed] [Google Scholar]
- Narfstrom K, Wrigstad A, Nilsson S. 1989. The Briard dogs: A new animal model of congenital stationary night blindness. Brit J Ophthalmol 73: 750–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narfstrom K, Katz ML, Bragadottir R, Seeliger M, Boulanger A, Redmond TM, Caro L, Lai CM, Rakoczy PE. 2003. Functional and structural recovery of the retina after gene therapy in the RPE65 null mutation dog. Invest Ophthalmol Vis Sci 44: 1663–1672. [DOI] [PubMed] [Google Scholar]
- Nathwani AC, Tuddenham EG, Rangarajan S, Rosales C, McIntosh J, Linch DC, Chowdary P, Riddell A, Pie AJ, Harrington C, et al. 2011. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med 365: 2357–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neveling K, Collin RW, Gilissen C, van Huet RA, Visser L, Kwint MP, Gijsen SJ, Zonneveld MN, Wieskamp N, de Ligt J, et al. 2012. Next-generation genetic testing for retinitis pigmentosa. Hum Mutat 33: 963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang JJ, Chang B, Kumar A, Nusinowitz S, Noorwez SM, Li J, Rani A, Foster TC, Chiodo VA, Doyle T, et al. 2006. Gene therapy restores vision-dependent behavior as well as retinal structure and function in a mouse model of RPE65 Leber congenital amaurosis. Mol Ther 13(3): 565–572. [DOI] [PubMed] [Google Scholar]
- Pang JJ, Boye SL, Kumar A, Dinculescu A, Deng W, Li J, Li Q, Rani A, Foster TC, Chang B, et al. 2008. AAV-mediated gene therapy for retinal degeneration in the rd10 mouse containing a recessive PDEβ mutation. Invest Ophthalmol Vis Sci 49: 4278–4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang JJ, Deng WT, Dai X, Lei B, Everhart D, Umino Y, Li J, Zhang K, Mao S, Boye SL, et al. 2012. AAV-mediated cone rescue in a naturally occurring mouse model of CNGA3-achromatopsia. PLoS ONE 7: e35250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlyk BS, Bulgakov OV, Liu X, Xu X, Adamian M, Sun X, Khani SC, Berson EL, Sandberg MA, Li T. 2010. Replacement gene therapy with a human RPGRIP1 sequence slows photoreceptor degeneration in a murine model of Leber congenital amaurosis. Hum Gene Ther 21: 993–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrault I, Rozet JM, Gerber S, Ghazi I, Leowski C, Ducroq D, Souied E, DuFier JL, Munnich A, Kaplan J. 1999. Leber congenital amaurosis. Mol Genet Metab 68: 200–208. [DOI] [PubMed] [Google Scholar]
- Puppo A, Cesi G, Marrocco E, Piccolo P, Jacca S, Shayakhmetov DM, Parks RJ, Davidson BL, Colloca S, Brunetti-Pierri N, et al. 2014. Retinal transduction profiles by high-capacity viral vectors. Gene Ther 10.1038/gt.2014.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond TM, Yu S, Lee E, Bok D, Hamasaki D, Chen N, Goletz P, Ma JX, Crouch RK, Pfeifer K. 1998. Rpe65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nat Genet 20: 344–351. [DOI] [PubMed] [Google Scholar]
- Redmond TM, Poliakov E, Yu S, Tsai JY, Lu Z, Gentleman S. 2005. Mutation of key residues of RPE65 abolishes its enzymatic role as isomerohydrolase in the visual cycle. Proc Natl Acad Sci 102: 13658–13663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RetNet. 2014. RetNet web site. http://www.sph.uth.tmc.edu/Retnet/.
- Shanks ME, Downes SM, Copley RR, Lise S, Broxholme J, Hudspith KA, Kwasniewska A, Davies WI, Hankins MW, Packham ER, et al. 2013. Next-generation sequencing (NGS) as a diagnostic tool for retinal degeneration reveals a much higher detection rate in early-onset disease. Eur J Hum Genet 21: 274–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonelli F, Ziviello C, Testa F, Rossi S, Fazzi E, Bianchi PE, Fossarello M, Signorini S, Bertone C, Galantuomo S, et al. 2007. Clinical and molecular genetics of Leber’s congenital amaurosis: A multicenter study of Italian patients. Invest Ophthalmol Vis Sci 48: 4284–4290. [DOI] [PubMed] [Google Scholar]
- Simonelli F, Maguire AM, Testa F, Pierce EA, Mingozzi F, Bennicelli JL, Rossi S, Marshall K, Banfi S, Surace EM, et al. 2010. Gene therapy for Leber’s congenital amaurosis is safe and effective through 1.5 years after vector administration. Mol Ther 18: 643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons DL, Boye SL, Hauswirth WW, Wu SM. 2011. Gene therapy prevents photoreceptor death and preserves retinal function in a Bardet-Biedl syndrome mouse model. Proc Natl Acad Sci 108: 6276–6281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodderly DM, Sandstrom MM, Leung IY, Zucker CL, Neuringer M. 2002. Retinal pigment epithelial cell distribution in central retina of rhesus monkeys. Invest Ophthalmol Vis Sci 43: 2815–2818. [PubMed] [Google Scholar]
- Stone EM. 2007. Genetic testing for inherited eye disease. Arch Ophthalmol 125: 205–212. [DOI] [PubMed] [Google Scholar]
- Sun X, Pawlyk B, Xu X, Liu X, Bulgakov OV, Adamian M, Sandberg MA, Khani SC, Tan MH, Smith AJ, et al. 2010. Gene therapy with a promoter targeting both rods and cones rescues retinal degeneration caused by AIPL1 mutations. Gene Ther 17: 117–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam LC, Kiang AS, Kennan A, Kenna PF, Chadderton N, Ader M, Palfi A, Aherne A, Ayuso C, Campbell M, et al. 2008. Therapeutic benefit derived from RNAi-mediated ablation of IMPDH1 transcripts in a murine model of autosomal dominant retinitis pigmentosa (RP10). Hum Mol Genet 17: 2084–2100. [DOI] [PubMed] [Google Scholar]
- Testa F, Maguire AM, Rossi S, Pierce EA, Melillo P, Marshall K, Banfi S, Surace EM, Sun J, Acerra C, et al. 2013. Three-year follow-up after unilateral subretinal delivery of adeno-associated virus in patients with Leber congenital Amaurosis type 2. Ophthalmology 120: 1283–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapani I, Colella P, Sommella A, Iodice C, Cesi G, de Simone S, Marrocco E, Rossi S, Giunti M, Palfi A, et al. 2014. Effective delivery of large genes to the retina by dual AAV vectors. EMBO Mol Med 6: 194–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis GH, Golczak M, Moise AR, Palczewski K. 2007. Diseases caused by defects in the visual cycle: Retinoids as potential therapeutic agents. Annu Rev Pharmacol Toxicol 47: 469–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe L, Bell P, Maguire A, Cearley C, Xiao R, Calcedo R, Wang L, Castle M, Maguire A, Grant R, et al. 2011. Dosage thresholds for AAV2 and AAV8 photoreceptor gene therapy in monkey. Sci Transl Med 3: 88ra54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veske A, Nilsson SE, Narfstrom K, Gal A. 1999. Retinal dystrophy of Swedish briard/briard–beagle dogs is due to a 4-bp deletion in RPE65. Genomics 57: 57–61. [DOI] [PubMed] [Google Scholar]
- Weleber RG. 2002. Infantile and childhood retinal blindness: A molecular perspective. Ophthalmic Genet 23: 71–97. [DOI] [PubMed] [Google Scholar]
- Wojno AP, Pierce EA, Bennett J. 2013. Seeing the light. Sci Transl Med 5: 175fs178. [DOI] [PubMed] [Google Scholar]
- Yin L, Greenberg K, Hunter JJ, Dalkara D, Kolstad KD, Masella BD, Wolfe R, Visel M, Stone D, Libby RT, et al. 2011. Intravitreal injection of AAV2 transduces macaque inner retina. Invest Ophthalmol Vis Sci 52: 2775–2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RW, Bok D. 1969. Participation of the retinal pigment epithelium in the rod outer segment renewal process. J Cell Biol 42: 392–403. [DOI] [PMC free article] [PubMed] [Google Scholar]