Abstract
The continuing spread of drug-resistant tuberculosis (TB) is one of the most urgent and difficult challenges facing global TB control. Patients who are infected with strains resistant to isoniazid and rifampicin, called multidrug-resistant (MDR) TB, are practically incurable by standard first-line treatment. In 2012, there were approximately 450,000 new cases and 170,000 deaths because of MDR-TB. Extensively drug-resistant (XDR) TB refers to MDR-TB strains that are resistant to fluoroquinolones and second-line injectable drugs. The main causes of the spread of resistant TB are weak medical systems, amplification of resistance patterns through incorrect treatment, and transmission in communities and facilities. Although patients harboring MDR and XDR strains present a formidable challenge for treatment, cure is often possible with early identification of resistance and use of a properly designed regimen. Community-based programs can improve treatment outcomes by allowing patients to be treated in their homes and addressing socioeconomic barriers to adherence.
Some strains of tuberculosis (TB) are resistant to first- or second-line drug treatments. Their continuing spread is one of the most urgent challenges facing global TB control.
EPIDEMIOLOGY
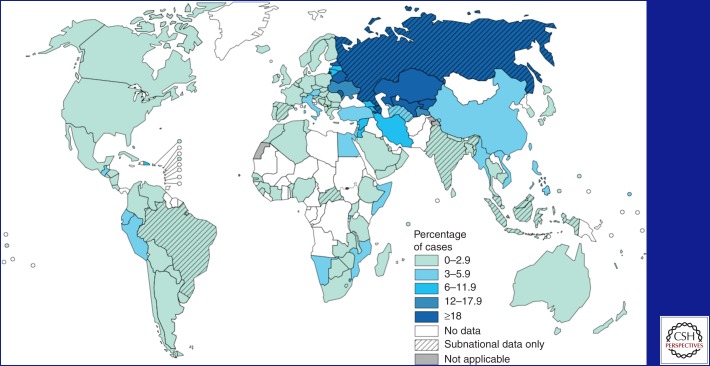
Even though tuberculosis (TB) is a treatable infectious disease, an estimated 1.3 million people died from TB in 2012 (WHO 2013a). One of the major reasons is that TB continues to evolve resistance to drugs. For patients with drug-susceptible TB, standard treatment based on isoniazid and rifampicin, the two most powerful drugs, results in excellent cure rates. Patients who are infected with strains resistant to isoniazid and rifampicin, called multidrug-resistant (MDR) TB, are practically incurable by standard first-line treatment (Fig. 1).
Figure 1.
Percentage of new TB cases with MDR-TB (WHO 2013a).
Today, the continuing spread of MDR-TB is one of the most urgent and difficult challenges facing global TB control. In 2012, there were approximately 450,000 new cases of MDR-TB and 170,000 deaths. Globally, MDR-TB is present in 3.8% of new TB patients and 20% of patients who have a history of previous treatment. The highest MDR rates are found in countries of Eastern Europe and central Asia, where MDR strains threaten to become as common as pan-susceptible strains. In some countries, MDR strains account for up to 20% of new TB cases and well over 50% of patients with a history of previous TB treatment. In 2011, Minsk, Belarus reported that 35% of new patients had MDR-TB, as did 75% of those who had been treated previously for TB (Skrahina et al. 2012).
Equally worrisome rates have emerged from China and India, which have the highest and second-highest number of MDR-TB patients in the world. In 2012, the China Centers for Disease Control and Prevention reported that 10% of China’s 1.4 million TB patients had MDR-TB, and the great majority of MDR-TB patients had never been treated for TB—evidence of unfettered human-to-human transmission (Zhao et al. 2012). MDR-TB is also a growing problem in South Africa, where high rates of HIV (human immunodeficiency virus) have exacerbated both the spread and deadliness of MDR-TB, raising the specter of a “perfect storm” of MDR-TB/HIV coinfection (Wells et al. 2007). In clinical practice today, the possibility of unsuspected drug resistance must always be considered when evaluating a TB patient in any country (Box 1).
BOX 1. TYPES OF DRUG-RESISTANT TB (WHO 2013b) (TYPES ARE NOT MUTUALLY EXCLUSIVE).
Monoresistance: Resistance to one first-line anti-TB drug only.
Polydrug resistance: Resistance to more than one first-line anti-TB drug, other than both isoniazid and rifampicin.
Multidrug resistance (MDR): Resistance to at least both isoniazid and rifampicin.
Rifampicin resistance (RR): Resistance to rifampicin detected using phenotypic or genotypic methods, with or without resistance to other anti-TB drugs. It includes any resistance to rifampicin, whether monoresistance, multidrug resistance, polydrug resistance, or extensive drug resistance.
Extensive drug resistance (XDR): Resistance to any fluoroquinolone, and at least one of three second-line injectable drugs (capreomycin, kanamycin, and amikacin), in addition to multidrug resistance.
In 2006, the term extensively drug-resistant TB (XDR-TB) was coined to describe strains of MDR-TB resistant to fluoroquinolones and second-line injectable drugs. It is estimated that 9.6% of MDR-TB cases worldwide have XDR-TB (WHO 2013a). Because access to second-line drug susceptibility testing (DST) is poor in many areas of the world, XDR-TB often goes unrecognized. Although patients harboring MDR and XDR strains present a formidable challenge for treatment, cure is often possible with early identification of resistance and use of a properly designed regimen.
CAUSES OF DRUG RESISTANCE
Drug resistance is a biological phenomenon that has been observed in Mycobacterium tuberculosis since the discovery of the first anti-TB drug, streptomycin. Many patients who were injected with streptomycin were brought from the brink of death and their sputum became temporarily clear of M. tuberculosis. But despite continuing to receive treatment, they soon began to excrete bacilli that were resistant to streptomycin in the laboratory (Pyle 1947).
With the advent of new drugs—thioacetazone and para-aminosalicylic acid in 1948 and isoniazid in 1952—it became clear that combination chemotherapy was the key to preventing the development of resistance. Initial combination regimens required 18 mo of treatment, but the invention of rifampicin in 1957, the most powerfully sterilizing anti-TB drug, paved the way for development of the shorter and more effective isoniazid- and rifampicin-containing regimens known as short-course chemotherapy. As part of the global TB control strategy called DOTS (directly observed treatment, short-course), these regimens became the standard of care even in resource-limited settings starting in 1993.
Outbreaks of MDR-TB were initially thought to be driven by nosocomial transmission, particularly among HIV-positive patients. One of the largest and best-documented outbreaks occurred in New York in the late 1980s and early 1990s (Frieden et al. 1993; Frieden et al. 1995). As DST laboratory capacity improved in resource-limited settings and global drug-resistant TB surveillance efforts grew, it became clear that MDR-TB was increasingly common throughout the world and a growing threat to the general public health. The causes of the global spread of MDR-TB include the following:
Chaotic treatment. Before the late 1980s, many countries were not using standard protocols for the treatment of TB and did not have systems in place to support patients. Furthermore, in many settings, TB treatment was not provided for free, contributing to poor adherence. Even today, drug-resistant TB can be created very quickly during times of socioeconomic instability if there are stockouts of anti-TB drugs or other structural weaknesses in the health care system.
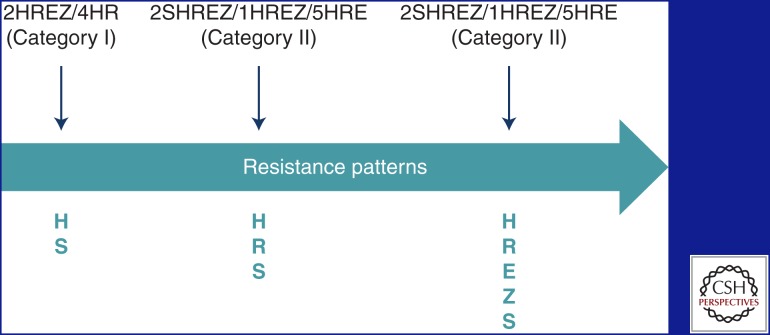
Amplifier effect of short-course chemotherapy. Once drug resistance has been created, the DOTS strategy can paradoxically exacerbate the problem. In Figure 2, the initial strain has polydrug resistance, but, as a result of repeated use of short-course chemotherapy, it becomes resistant to all first-line anti-TB drugs (Seung et al. 2004). Amplification of drug resistance patterns through repeated courses of DOTS short-course chemotherapy continues to be a major driving force of the epidemic in many parts of the world that do not have the resources to diagnose or treat drug-resistant TB correctly (Keshavjee and Farmer 2012).
Community transmission. In the early 2000s, it was believed that resistance mutations conferred a loss of fitness, so the transmission of resistant strains would be self-limited (Dye et al. 2002; Cegielski 2010). This has not turned out to be the case. Current models indicate that in most countries, the majority of MDR-TB patients were infected initially with an MDR-TB strain, rather than slowly acquiring resistance caused by inadequate or irregular treatment (Lin et al. 2011).
Facility-based transmission. Nosocomial transmission in busy, crowded hospitals and health centers is likely an important driver of the epidemic, especially in high HIV prevalence settings. This can result in the spread of drug-resistant strains among patients receiving therapy for drug-susceptible TB as well as to the health workers (Gelmanova et al. 2007).
Figure 2.
Amplifier effect of short-course chemotherapy. H, isoniazid; R, rifampicin; E, ethambutol; Z, pyrazinamide; S, streptomycin.
DIAGNOSIS OF MDR-TB AND XDR-TB
DST is required for the definitive diagnosis of MDR-TB or XDR-TB. The traditional way to do this is through phenotypic (or culture-based) methods. M. tuberculosis is isolated from patient sputum and then tested for growth in the presence of anti-TB drugs. Culture-based methods can take weeks to months. They are also expensive and difficult to master, making them mostly unavailable in resource-limited settings.
Genotypic (or molecular) methods have revolutionized the diagnosis of MDR-TB. These methods generally use polymerase chain reaction techniques to detect the genetic mutations that are known to confer resistance to drugs. Commercially available systems include GeneXpert System (Xpert MTB/RIF, Cepheid, USA), GenoType MTBDRplus and MTBDRsl assays (Hain Lifescience GmbH, Germany), and INNO-LiPA Rif.TB line probe assay (Innogenetics Inc., Belgium), but there are other systems in development. Molecular methods of DST give results much faster than culture-based methods. Some commercially available systems are almost fully automated and require little training. For these reasons, these systems are increasingly the method of choice for DST in resource-limited settings.
There is no “gold standard” for the diagnosis of drug resistance. Molecular testing may detect mutations that confer low levels of resistance that are not detected by culture-based testing but are still clinically significant. Molecular testing also cannot detect all of the mutations that are known to confer resistance to a drug. Nevertheless, it is not necessary to routinely confirm the results of molecular DST with culture-based DST, even though clinicians may choose to do so if the clinical picture warrants it. Specifically, a positive molecular test for rifampicin resistance can be considered diagnostic for MDR-TB, because in most countries, greater than 90% of rifampicin-resistant strains are also resistant to isoniazid.
A public health strategy of “universal DST,” meaning testing all patients with active TB disease for drug resistance at the start of therapy, is certainly feasible in the near future because of the increasing availability of molecular DST. A WHO analysis determined that this would be a lifesaving and cost-effective strategy for any country with greater than 1% MDR-TB in new patients (WHO 2011a).
At the current time, however, DST is not widely available in many countries so patients with risk factors for MDR-TB are prioritized for testing. Empiric treatment for MDR-TB can be considered if there is clear bacteriological evidence of failure to respond to treatment, such as persistently positive sputum smears after 4 mo of regular treatment with a first-line DOTS regimen (Chavez Pachas et al. 2004; Satti et al. 2013). Household contacts of MDR-TB patients should also be started empirically on treatment if any delay in DST is anticipated (Box 2).
BOX 2. RISK FACTORS FOR MDR-TB.
Failure to respond to a first-line DOTS regimen (WHO Category I or II)
Relapse after a full course of treatment with a first-line regimen
Treatment after defaulting from treatment with a first-line regimen
Exposure to a known case of MDR-TB
Exposure to TB in institutions with high prevalence of MDR-TB, such as a prison or hospital
Living in areas or countries with high prevalence of MDR-TB
HIV coinfection
All patients diagnosed with MDR-TB should be tested for XDR-TB. This includes testing for resistance to the three second-line injectable drugs (kanamycin, amikacin, and capreomycin) and at least one fluoroquinolone. Laboratories with a capacity for second-line DST, however, are even less common than those with capacity for first-line DST. Patients who should be prioritized for second-line DST include those who have failed to respond to an MDR-TB treatment regimen containing an injectable and fluoroquinolone, and close contacts with an individual with documented XDR-TB or with an individual for whom treatment with a regimen including second-line drugs is failing or has failed.
The GenoType MTBDRsl assays (Hain Lifescience GmbH, Germany) test for mutations in the gyrA gene, which confers resistance to fluoroquinolones, and in the rrs gene, which confers resistance to injectable drugs. This assay can be considered a “rule-in” test for second-line drug resistance, although it cannot reliably rule out XDR-TB when no genetic mutations are detected. Because the sensitivity and specificity of this assay are not well characterized, culture-based DST should be used as a confirmatory test.
TREATMENT
MDR-TB treatment is difficult because the second-line TB drugs are mostly weak and toxic. Most of these drugs were developed decades ago but hardly ever used because of poor side effect profiles. Because of the weak sterilizing activity of the second-line TB drugs, MDR-TB treatment generally takes 18–24 mo. In the best treatment programs, which address socioeconomic barriers and aggressively manage side effects, cure rates of 60%–80% have been reported (Mitnick et al. 2003; Shin et al. 2006). Globally, however, the cure rate for MDR-TB is much lower. In 2013, the WHO reported that only 48% of MDR-TB patients were cured. The global cure rate for XDR-TB is even lower: Only 20% are cured, and 44% die (WHO 2013a).
Anti-TB drugs have traditionally been divided into first- and second-line anti-TB drugs with isoniazid, rifampicin, pyrazinamide, ethambutol, and streptomycin being the primary first-line anti-TB drugs. In this review, we use the WHO system, which classifies the drugs into five different groups based on efficacy, experience of use, safety, and drug class. Drugs in the same group do not always come from the same drug class nor do they have the same safety profile or efficacy. Table 1 provides detailed information on each drug including the WHO group, dosage, side effects, and monitoring requirements.
Table 1.
Anti-TB drugs and their side effects
| Drug name (abbreviation) | Description and adult dose | Side effects | Monitoring requirements and comments |
|---|---|---|---|
| Group 1: First-line oral drugs | |||
| Isoniazid (H) |
Description: Bactericidal; inhibits mycolic acid synthesis most effectively in dividing cells; hepatically metabolized. Dose: 300 mg daily or 900 mg twice or thrice weekly. |
Common: Hepatitis (10%–20% have elevated transaminases), peripheral neuropathy (dose-related; increased risk with malnutrition, alcoholism, diabetes, concurrent use of aminoglycosides, or ethionamide). Less common: Gynecomastia, rash, psychosis, seizure. |
Monitoring: Consider baseline and monthly liver enzymes, especially if age >50 yr. Comments: Give with pyridoxine 50 mg/d if using large dose or if patient is at risk for peripheral neuropathy (diabetes, alcoholism, HIV, etc.). |
|
Rifamycins Rifampicin (R) Rifabutin (Rfb) Rifapentine (Rpt) |
Description: Bactericidal; inhibits protein synthesis by blocking mRNA transcription and synthesis; hepatically metabolized. Dose: rifampicin 600 mg/d; rifabutin 300 mg/d. |
Common: Orange-colored bodily secretions, transient transaminitis, hepatitis, gastrointestinal distress. Less common: Cholestatic jaundice. |
Monitoring: Consider baseline liver enzymes, repeat if symptoms (jaundice, fatigue, anorexia, weakness, or nausea and vomiting) appear. |
| Pyrazinamide (Z) |
Description: Bactericidal; mechanism unclear; effective in acidic milieu (e.g., cavitary disease, intracellular organisms); hepatically metabolized, renally excreted. Dose: 15–40 mg/kg daily. |
Common: Arthritis/arthralgias, hepatotoxicity, hyperuricemia, abdominal distress. Less common: Impaired diabetic control, rash. |
Monitoring: Baseline liver enzymes; uric acid can be measured if arthralgias, arthritis, or symptoms of gout are present. Comments: Usually given once daily, but can split dose initially to improve tolerance. |
| Ethambutol (E) |
Description: Bacteriostatic at conventional dosing (15 mg/kg); inhibits lipid and cell wall metabolism; renally excreted. Dose: 15–25 mg/kg. |
Common: Generally well-tolerated. Less common: Optic neuritis, gastrointestinal distress, arthritis/arthralgia. |
Monitoring: Baseline and monthly visual acuity and red/green color vision test when dosed at greater than 15 mg/kg daily (>10% loss is considered significant); regularly question patient about visual symptoms. |
| Group 2: Injectable drugs | |||
|
Aminoglycosides Amikacin (Amk) Kanamycin (Km) Streptomycin (S) Polypeptides Capreomycin (Cm) |
Description: Bactericidal; aminoglycosides inhibit protein synthesis through disruption of ribosomal function; less effective in acidic, intracellular environments; polypeptides appear to inhibit translocation of the peptidyl-tRNA and the initiation of protein synthesis; renally excreted. Dose: 15–20 mg/kg daily. |
Common: Pain at injection site; proteinuria; electrolyte wasting (more common with capreomycin); cochlear ototoxicity (hearing loss, dose-related to cumulative and peak concentrations, increased risk with renal insufficiency, may be irreversible). Less common: Nephrotoxicity (dose-related to cumulative and peak concentrations, increased risk with renal insufficiency, often irreversible); peripheral neuropathy; rash; vestibular toxicity (nausea, vomiting, vertigo, ataxia, nystagmus); eosinophilia; ototoxicity potentiated by certain diuretics, especially loop diuretics. |
Monitoring: Baseline and then monthly creatinine, urea, and serum potassium; more frequently in high-risk patients; if potassium is low, check magnesium and calcium; baseline audiometry and monthly monitoring in high-risk patients (high-risk patients: elderly, diabetic, or HIV-positive patients, or patients with renal insufficiency). Comments: Increase dosing interval or reduce dose and monitor serum drug concentrations as needed to control side effects. |
| Group 3: Fluoroquinolones | |||
|
Levofloxacin (Lfx) Moxifloxacin (Mfx) |
Description: Bactericidal; DNA-gyrase inhibitor; renally excreted. Dose: levofloxacin 750–1000 mg/d; moxifloxacin 400 mg/d. |
Common: Generally well-tolerated, well-absorbed. Less common: Diarrhea, dizziness, gastrointestinal distress, headache, insomnia, photosensitivity, rash, vaginitis, tendonitis, psychosis, seizure (CNS effects seen almost exclusively in elderly). |
Monitoring: No laboratory monitoring requirements. Comments: Do not administer with antacids, sucralfate, iron, zinc, calcium, or oral potassium and magnesium replacements; levofloxacin, moxifloxacin have the most activity against M. tuberculosis. |
| Group 4: Oral bacteriostatic drugs | |||
| Cycloserine (Cs) |
Description: Bacteriostatic; alanine analog; interferes with cell-wall proteoglycan synthesis; renally excreted. Dose: 500–1000 mg/d. |
Common: Neurologic and psychiatric disturbances, including headaches, irritability, sleep disturbances, aggression, and tremors. Less common: Psychosis, peripheral neuropathy, seizures (increased risk of CNS effects with concurrent use of ethanol, isoniazid, ethionamide, or other centrally acting medications), hypersensitivity. |
Monitoring: Consider serum drug monitoring to establish optimal dosing. Comments: Give 50 mg of pyridoxine for every 250 mg of cycloserine (to lessen neurologic adverse effects). |
|
Thiamides Ethionamide (Eto) Prothionamide (Pto) |
Description: May be bactericidal or bacteriostatic depending on susceptibility and concentrations attained at the infection site; the carbothioamide group, also found on thiacetazone, and the pyridine ring, also found on isoniazid, appear essential for activity; hepatically metabolized, renally excreted. Dose: 500–1000 mg/d. |
Common: Gastrointestinal distress (nausea, vomiting, diarrhea, abdominal pain, loss of appetite); dysgeusia (metallic taste); hypothyroidism (especially when taken with PAS). Less common: Arthralgias, dermatitis, gynecomastia, hepatitis, impotence, peripheral neuropathy, photosensitivity. |
Monitoring: Consider baseline liver enzymes. Comments: May split dose or give at bedtime to improve tolerability; ethionamide and prothionamide efficacies are considered similar; prothionamide may cause fewer gastrointestinal adverse effects. |
| Para-aminosalicylic acid (PAS) |
Description: Bacteriostatic; disrupts folic acid metabolism (thought to inhibit the biosynthesis of coenzyme F in the folic acid pathway); hepatic acetylation, renally excreted. Dose: Depends on specific formulation. |
Common: Gastrointestinal distress (nausea, vomiting, diarrhea); hypersensitivity; hypothyroidism (especially when taken with ethionamide). Less common: Hepatitis, electrolyte abnormalities. Drug interactions: Decreased isoniazid acetylation; decreased rifampicin absorption in nongranular preparation; decreased vitamin B12 uptake. |
Monitoring: No laboratory monitoring requirements. Comments: PASER consists of enteric coated granules that need to be administered with an acidic food or beverage (e.g., yogurt or acidic juice); PASER is stable for up to 8 wk at 40°C and 75% humidity, and therefore can be distributed to the patient on a monthly basis in most environments with no cold chain; if storage of >8 wk is needed, refrigeration below 15°C is required. |
| Group 5: Drugs with limited data on efficacy or long-term safety | |||
| Bedaquiline (Bdq) |
Description: A diarylquinoline antimycobacterial drug that inhibits ATP synthesis. Dose: 400 mg once daily for 2 wk, followed by 200 mg three times per week for 22 wk with food; the drug has a 5.5-mo half-life. |
Common: Gastrointestinal distress (nausea, vomiting, abdominal pain, loss of appetite); joint pain; headache. Less common: QT prolongation, hyperuricemia, phospholipidosis (the accumulation of phospholipids in the body's tissues), elevated aminotransferases, chest pain, hemoptysis (coughing up blood). Possibly an increased risk of pancreatitis. Drug Interactions: All CYP3A4 inhibitors or inducers. Rifampicin (a CYP3A4 inducer) reduces bedaquiline in blood by half; drugs that prolong the QT interval (e.g., clofazimine, moxifloxacin, antifungals, and many others) may result in additive cardiac toxicity—their use is only indicated when there are no other alternatives; more frequent ECG monitoring is required. |
Monitoring: Monitor QT interval with ECG at baseline, 2, 12, and 24 wk (more often if risk of QT prolongation is present); discontinue if significant ventricular arrhythmia or a QTcF interval >500 msec develops; monitor liver enzymes every month. Comments: A significant imbalance in fatalities was noted in Trial C208 Stage 2, with a higher number of deaths in the bedaquiline group (10 vs. 2 in the placebo group; RR = 5.1; P = 0.017). There was no sudden death reported in the study. There was no discernible pattern for cause of deaths, and the reason for the imbalance in deaths is not clear. |
| Linezolid (Lzd) | Description: Oxazolidinone; inhibits protein synthesis; increasingly used for treatment of XDR-TB.Dose: 600 mg/d (reduce to 300 mg/d if serious side effects or intolerance develops). |
Common: Diarrhea and nausea. Less common: Myelosuppression (decreased level of white blood cells, and/or anemia); lactic acidosis; optic and peripheral neuropathy (may be irreversible, and linezolid should be considered for suspension weighed against the risk of permanent blindness or disabling permanent neuropathy). |
Monitoring: Monitor for peripheral neuropathy and optic neuritis. Monitor with a complete blood count (CBC) weekly during the initial period, then monthly. If symptoms of lactic acidosis develop, a medical evaluation including a lactic acid blood test should be performed. Comments: All patients should receive pyridoxine while receiving linezolid (child: 5–10 mg/d; adult: 50 mg/d); do not use with patients taking serotonergic drugs, such as monoamine oxidase inhibitors (MAOIs), selective serotonin reuptake inhibitors (SSRIs, e.g., fluoxetine, sertraline, paroxetine), lithium, etc., as it may cause serotonin syndrome; avoid or monitor closely with tricyclic antidepressants. |
| Clofazimine (Cfz) |
Description: Riminophenazine; has activity in vitro but limited clinical evidence of efficacy. Dose: 100 to 200 mg/d (oral) has been used. A regimen of 200 mg/d for 2 mo, followed by 100 mg/d has been used. |
Common: Orange/red discoloration of skin, conjunctiva, cornea, and body fluids; dry skin, pruritus, rash, ichthyosis, xerosis; gastrointestinal intolerance; photosensitivity. Less common: Retinopathy, severe abdominal symptoms, bleeding, and bowel obstruction; QT prolongation. |
Monitoring: Symptomatic monitoring only. Comments: Discolors skin and body secretions orange, red, or brownish-black; this should go away after stopping the medicine, but may take a long time; avoid sun; use strong sunscreens. |
| Amoxicillin/clavulanic acid (Amx/Clv) |
Description: Penicillin/β-lactam inhibitor; very limited clinical evidence of efficacy. Dose: 80 mg/kg daily in two divided doses. |
Common: Diarrhea and abdominal discomfort are most common; nausea and vomiting. Less common: Hypersensitivity and rash; rare side effects have been reported in other organ systems. |
Monitoring: Symptomatic monitoring only. Comments: Best tolerated and well absorbed when taken at the start of a standard meal. |
| Imipenem/cilastatin (Imp/Cln) |
Description: β-lactam/carbapenem (related to the penicillin/cephalosporin family of antibiotics but classified as belonging to the carbapenem class); very limited clinical experience. Given that imipenem is rapidly degraded by renal proximal tubule dipeptidases, it is used in combination with the dipeptidase inhibitor cilastatin. Dose: 1000 mg IV every 12 h. |
Common: Diarrhea, nausea, or vomiting. Less common: Seizure (noted with CNS infection), palpitations, pseudomembranous colitis. |
Monitoring: Symptomatic monitoring only. Comments: Meropenem is preferred in children as fewer seizures have been associated with it; consider adding clavulanate (available as Amx/Clv) 125 mg every 8–12 h. |
| Meropenem (Mpm) |
Description: β-lactam/carbapenem (related to the penicillin/cephalosporin family of antibiotics but classified as belonging to the carbapenem class); very limited clinical experience. Dose: 1000 mg IV every 8 h. |
Common: Diarrhea, nausea, or vomiting. Less common: Seizure (but less is seen than with imipenem), palpitations, pseudomembranous colitis. |
Monitoring: Symptomatic monitoring only. Comments: Consider adding clavulanate (available as amoxicillin/clavulanate) 500/125 mg every 8–12 h. |
|
High-dose isoniazid (High-dose H) |
Description: May be bactericidal or bacteriostatic depending on susceptibility and concentrations attained at the infection site; the carbothioamide group, also found on Thz, and the pyridine ring, also found on H, appear essential for activity; hepatically metabolized, renally excreted. Dose: 500–1000 mg/d. |
Common: Gastrointestinal distress (nausea, vomiting, diarrhea, abdominal pain, loss of appetite); dysgeusia (metallic taste); hypothyroidism (especially when taken with PAS). Less common: Arthralgias, dermatitis, gynecomastia, hepatitis, impotence, peripheral neuropathy, photosensitivity. |
Monitoring: Consider baseline and monthly liver enzymes, especially if age >50 yr. Comments: Give with pyridoxine 50 mg/d. |
| Clarithromycin (Clr) |
Description: More active against nontuberculous mycobacteria, especially MAC, but some isolates of TB are susceptible in vitro; does not have proven value for the treatment of TB in humans, and in vitro data are not particularly encouraging (M. tuberculosis is intrinsically resistant to macrolides, a characteristic associated with expression of the ermB gene). Dose: 500 mg twice daily. |
Common: Diarrhea, nausea, abnormal taste, dyspepsia, abdominal pain/discomfort, headache, rare allergic skin reactions, liver toxicity, QT prolongation, pseudomembranous colitis, hearing loss. |
Monitoring: No routine laboratory monitoring is indicated. Comments: This medication may be taken with or without food; contraindicated in patients taking cisapride, pimozide, astemizole, terfenadine, ergotamine, or dihydroergotamine. |
| Thioacetazone (T) |
Description: Known to be active against TB (by inhibiting cyclopropanation of cell wall mycolic acids in mycobacteria), but its role in MDR-TB treatment is not well-established; cross-resistance with some of the other anti-TB drugs (isoniazid, ethionamide, PAS) and overall is a weakly bacteriostatic drug; prevents the emergence of resistance when used with other first-line drugs. Dose: 150 mg once daily. |
Common: Nausea, vomiting, diarrhea, loss of appetite, skin rashes, aching joints and muscles, neuropathy. Rare: Severe cutaneous hypersensitivity (including Stevens–Johnson syndrome), seizures, mood changes, hepatitis, bone marrow suppression. |
Monitoring: No routine laboratory monitoring is indicated. Comments: Contraindicated in HIV-infected individuals because of a risk of serious adverse reactions (Stevens–Johnson syndrome and death); persons of Asian descent also have a higher incidence of Stevens–Johnson syndrome; rarely used in MDR-TB treatment. |
From Seung and Rich 2010; Curry International Tuberculosis Center & California Department of Public Health 2012; Partners In Health 2013.
CNS, central nervous system; ECG, electrocardiogram; RR, relative risk; IV, intravenously; MAC, Mycobacterium avium complex.
In recent years, there has been considerable research devoted to developing new anti-TB drugs, with the goal of improving TB treatment. There are now two new purpose-built anti-TB drugs, the first in over 40 yr. Bedaquiline was conditionally approved by the U.S. FDA for the treatment of MDR-TB in December 2012. Delamanid was conditionally approved by the European Medicines Agency in November 2013. At least three additional new TB drugs are in late-phase clinical testing. These new drugs are likely to revolutionize the treatment of MDR-TB and XDR-TB.
REGIMEN DESIGN
One of the most important steps in designing an MDR-TB regimen is taking a detailed history of past TB treatment. This is particularly important in patients who have received multiple treatment courses with first- or second-line anti-TB drugs, as there may be omissions or errors in the written medical record. As a general rule, any drug that has been used in a regimen that did not cure the patient should be considered unlikely to still be effective, even if a recent DST indicates that the patient’s strain is still susceptible. For example, if the patient previously used ethambutol or pyrazinamide as part of a failed first-line regimen, neither of these drugs should be considered likely to be effective.
All DST results should be compared carefully with the clinical history; DST can be incorrect like any laboratory test. Only DST to first-line anti-TB drugs, injectables, and fluoroquinolones is considered reliable. Laboratory resistance to pyrazinamide, ethionamide, or PAS, combined with a history of use in a failing regimen, however, strongly suggests the drug is ineffective.
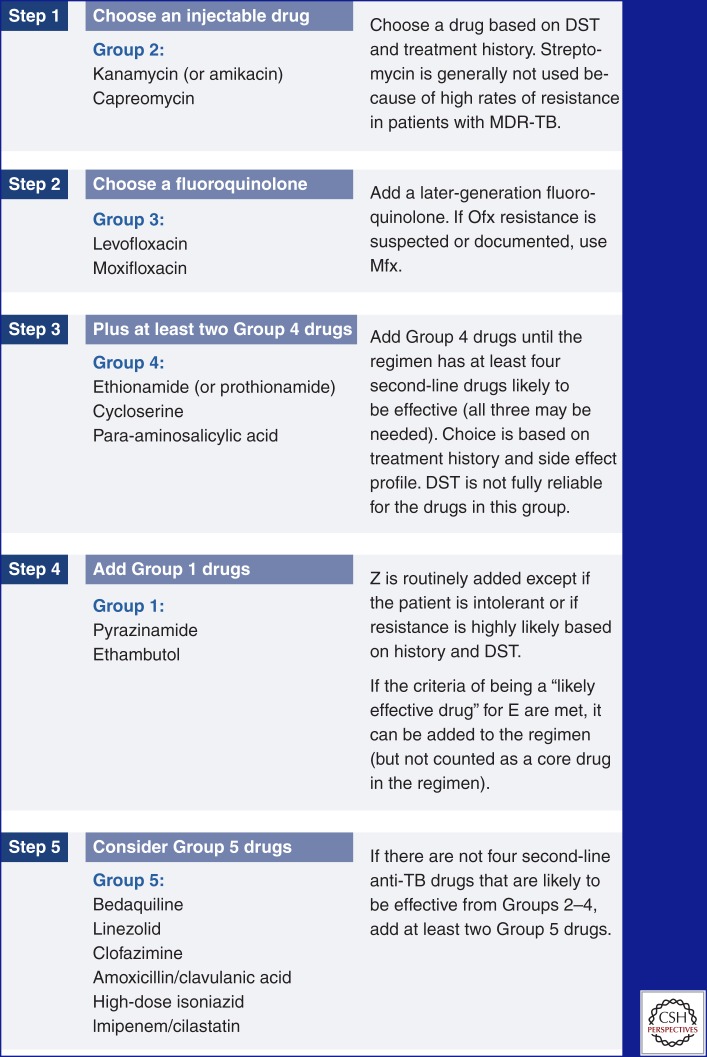
At least four second-line anti-TB drugs likely to be effective should be included in the MDR regimen. All regimens should include a later-generation fluoroquinolone such as levofloxacin or moxifloxacin (Johnson et al. 2006; Peloquin et al. 2008), a second-line injectable drug, and other oral second-line drugs as shown in Figure 3 (Mukherjee et al. 2004). Pyrazinamide may also be included unless there is a contraindication (such a history of a drug allergy) or evidence that it is not likely to be effective against the patient’s strain (a clear history of failing to respond to a pyrazinamide-including regimen and DST indicating resistance) (Mitnick et al. 2003). Pyrazinamide is often routinely included in MDR regimens in resource-limited settings because DST to pyrazinamide is often not available. These recommendations are supported by a large meta-analysis of individual data from more than 9000 patients (Ahuja et al. 2012).
Figure 3.
Designing an MDR-TB regimen. (Created from data in Varaine and Rich 2013.)
Dosing of anti-TB drugs is based on the weight of the patient. For simplicity, commonly used dosing tables use only a few weight bands. When adults gain weight or move into a higher weight band, drug doses should be adjusted. Even though anti-TB drugs are generally administered once a day to improve peak-dependent killing, many of the second-line anti-TB drugs have severe side effects that can be reduced by twice-daily divided dosing. For example, ethionamide and cycloserine are traditionally given in two divided doses to reduce side effects, but once-daily dosing is acceptable if tolerated.
In the case of XDR-TB, the same approach for designing a regimen may be used, with some important caveats. In chronic patients with a history of multiple failed courses of treatment, a minimum of four likely effective second-line anti-TB drugs may not be available. The XDR regimen may then include drugs that are resistant on DST but have never been used or drugs that have been used but are still susceptible on DST. Many clinicians will use a later-generation fluoroquinolone such as moxifloxacin despite documented resistance to an early generation fluoroquinolone such as ofloxacin.
If there is an injectable drug that is still likely to be effective, many clinicians will try to use it for a longer duration, such as 12 mo or even the entire duration of treatment. New drugs with demonstrated efficacy such as bedaquiline or delamanid should be strongly considered, as well as resective surgery in patients with localized disease.
According to provisional guidance from the United States Centers for Disease Control and Prevention (CDC) and WHO, bedaquiline may be used for MDR-TB patients in whom treatment options are limited (CDC 2013; WHO 2013c). Bedaquiline should be added to a conventional MDR or XDR regimen designed as above. There are still many uncertainties about the risks and benefits of bedaquiline (Table 1). Bedaquiline may be used to treat strains with XDR or fluoroquinolone resistance. Bedaquiline may also have a role when the injectable drug is causing toxicity (such as ototoxicity or nephrotoxicity) that requires its suspension or when there is resistance to all second-line injectable drugs. Bedaquiline should be administered within a program that has the capacity to closely monitor for side effects. Delamanid has no international recommendations or published drug insert at the time of this writing.
DURATION AND MONITORING OF TREATMENT
The injectable should be continued for at least 8 mo and at least 4 mo after the patient becomes culture-negative, whichever is longer. Clinicians may use an individualized approach that reviews the cultures, smears, X-rays, and clinical status to decide how long to continue the injectable. With respect to the duration of the injectable, however, the limiting factor is often toxicity. One option is intermittent dosing of the injectable. Many patients have less nephrotoxicity or ototoxicity if the injectable is given three times a week (e.g., Monday, Wednesday, and Friday) compared with daily. For this reason, some clinicians routinely choose to switch to an intermittent schedule after the patient becomes culture-negative even if there is no toxicity.
MDR-TB treatment should continue for a minimum of 20 mo and at least 18 mo after the patient becomes culture-negative, whichever is longer. Chronic patients with extensive pulmonary disease may require MDR-TB treatment for 24 mo or longer, but there is limited evidence about the optimal length of treatment for patients with XDR-TB.
The previous recommendations about the duration of the injectable and of treatment are supported by the meta-analysis of individual data from more than 9000 patients, but there is controversy about the optimal length of treatment. One study showed that a 9-mo treatment regimen was sufficient to affect cure in a small cohort of patients in Bangladesh (Van Deun et al. 2010). This particular regimen is currently the subject of a multisite clinical trial.
Sputum cultures should be performed monthly during treatment. Other symptoms and signs are important, but they cannot be relied on to determine treatment failure or cure. Programs with very limited culture capacity may consider doing smears monthly and cultures every other month after the injectable has been discontinued, but this may delay identification of treatment failure.
MANAGEMENT OF SIDE EFFECTS
Second-line anti-TB drugs have many more side effects than first-line anti-TB drugs. Side effects are expected as part of the normal course of treatment, and it is the responsibility of the clinician to diagnose and manage them. Mismanagement of side effects is the major reason why patients do not adhere or continue to take MDR-TB treatment.
Even before starting treatment, the patient should receive education regarding potential side effects. During treatment, patients should be evaluated regularly by a clinician. Community health workers can be trained to screen for side effects between clinical evaluations. Laboratory testing can be helpful in screening for some side effects; a typical monitoring schedule is shown in Table 2.
Table 2.
Follow-up schedule for uncomplicated MDR-TB patients
| Month | Clinical consult | Weight | Smear | Culture | DST | Chest X-ray | LFT | Cr, K | TSH |
|---|---|---|---|---|---|---|---|---|---|
| 0 (baseline) | √ | √ | √ | √ | √ | √ | √ | √ | |
| 1 | Every 2 wk | √ | √ | √ | √ | ||||
| 2 | Every 2 wk | √ | √ | √ | √ | ||||
| 3 | Every 2 wk | √ | √ | √ | √ | √ | |||
| 4 | √ | √ | √ | √ | If culture pos | √ | |||
| 5 | √ | √ | √ | √ | √ | ||||
| 6 | √ | √ | √ | √ | If culture pos | √ | √ | ||
| 7 | √ | √ | √ | √ | √ | ||||
| 8 | √ | √ | √ | √ | If culture pos | √ | |||
| 9 | √ | √ | √ | √ | If on inj. | ||||
| 10 | √ | √ | √ | √ | If culture pos | If on inj. | |||
| 11 | √ | √ | √ | √ | If on inj. | ||||
| 12 | √ | √ | √ | √ | If culture pos | If on inj. | √ | ||
| Until completion | √ | Monthly | Monthly | Monthly | If culture pos | If on inj. | Every 6 mo |
From Partners in Health 2013.
DST, drug susceptibility testing; pos, positive; LFT, liver function test; Cr, creatinine, K, potassium; TSH, thyroid-stimulating hormone; inj., injectable.
Mild side effects are common, and can be managed symptomatically with ancillary drugs without altering the treatment regimen. Side effects often diminish or disappear with time, allowing patients to finish their treatment without further problems. A number of second-line anti-TB drugs have highly dose-dependent side effects. With cycloserine and ethionamide, for example, a patient may be completely intolerant at one dose and completely tolerant at a slightly lower dose. Unfortunately, given the narrow therapeutic margins of these drugs, lowering the dose may also affect efficacy. Reducing the dose of these drugs should be performed only in cases in which the reduced dose is still expected to produce adequate serum levels and not compromise the regimen.
A poorly tolerated drug may be temporarily suspended. In patients with highly resistant TB, however, a satisfactory replacement drug may not be available. In such cases, permanent discontinuation of the drug should be avoided if possible. The decision to suspend any drug should be made by weighing the risk of continued side effects against the chances of curing a deadly disease.
Gastrointestinal distress is a common side effect of MDR-TB treatment, caused by PAS and ethionamide. Nausea and vomiting are common in early weeks of therapy but usually improve over time and with supportive therapy.
Nephrotoxicity is a known complication of the aminoglycosides and capreomycin. Because symptoms of acute renal failure can be nonspecific, serum creatinine monitoring is recommended. Patients with advanced age or a history of renal disease (including comorbidities such as HIV and diabetes) should be monitored more closely, particularly at the start of treatment.
Electrolyte wasting with similar characteristics to Fanconi’s Syndrome can be induced by all of the injectable drugs. It is reversible once the injectable is suspended, but it may take weeks or months to be resolved completely. Because electrolyte wasting is generally managed with electrolyte replacement therapy, serum potassium should be checked at least monthly in all patients during the initial injectable phase.
Hypothyroidism can be induced by prolonged exposure to PAS or ethionamide/prothionamide. The exact incidence of hypothyroidism during MDR-TB treatment is unknown, but rates of up to 80% have been reported in some patient populations. Because symptoms are nonspecific, all patients should be screened for hypothyroidism starting after the third month of MDR-TB treatment.
Neurotoxic effects such as psychosis or depression can be caused by cycloserine. Clinicians should screen patients for abnormal behavior and symptoms of depression, anxiety, and agitation on a regular basis.
Ototoxicity can be caused by the injectables, which can cause damage to cranial nerve VIII. This may result in hearing loss, tinnitus (ringing in the ear), or other vestibular symptoms such as nystagmus, ataxia, and disequilibrium. Hearing loss is generally not reversible on discontinuation of therapy. Hearing and balance should be assessed at least monthly while the patient is receiving the injectable. Where available, audiometry should be performed monthly and is an excellent way of detecting early hearing loss.
RESECTIVE SURGERY
Lung resection was once a mainstay of TB treatment but disappeared almost completely once effective chemotherapy was discovered. There has been a resurgence of interest in resective surgery as an adjunct to MDR-TB chemotherapy, which is much less effective. For patients with localized disease, resection of a lobe or a lung can significantly improve outcome (Pomerantz et al. 2001; Somocurcio et al. 2007). Experienced thoracic surgeons, stringent infection control measures, and excellent pre- and postoperative care are necessary; resective surgery for TB is often more complicated than for other diseases.
The most important indication for resective surgery is lack of a sustained bacteriological response to chemotherapy. Computerized tomography (CT) is the best way to assess if there is a localized lesion that is amenable to resection. Patients with bilateral cavitary disease, for example, would not be good surgical candidates. Pulmonary function testing with predicted postoperative forced expiratory volume in one second (FEV1) can be helpful to evaluate if the patient has sufficient pulmonary reserve postresection.
Another type of surgical candidate is those who have experienced culture conversion through chemotherapy, but who are thought to have a high probability of future failure or relapse caused by a high level of resistance. Surgical resection can also be considered for complications of MDR-TB, such as massive hemoptysis, empyema, or aspergilloma.
In patients who are smear- or culture-positive at the time of surgery, treatment is continued for minimum of 18 mo of negative sputum cultures, and generally includes an extended period of injectable. In patients who are smear- and culture-negative at the time of surgery, treatment should be continued for a minimum of 18 mo after culture conversion and no less than 6 mo after surgery.
SPECIAL SITUATIONS
Extrapulmonary MDR-TB
There is limited evidence about treatment of extrapulmonary MDR-TB. In general, every effort should be made to obtain tissue or fluid samples to confirm the diagnosis of MDR-TB, as the clinical or radiological picture may be deceptive. The length of therapy has not been clearly defined but should likely be at least as long as treatment for pulmonary MDR-TB.
MDR-TB lymphadenitis. Lymph node aspiration followed by culture-based or molecular DST is a simple way to confirm the diagnosis and can be useful in guiding therapy.
MDR-TB osteomyelitis and spondylitis. Bone biopsy or sampling of paravertebral fluid collections should be attempted to obtain material for DST. Persistent or increasing fluid collections on CT despite treatment with first-line anti-TB drugs may be sufficient evidence for empiric MDR-TB treatment in some patients with other bacteriological evidence of TB. Operative intervention, either through open debridement or by percutaneous drainage of fluid collections, is often required in combination with drug therapy.
MDR-TB meningitis. Diagnosis of MDR-TB meningitis may be difficult because mycobacterial concentration in cerebrospinal fluid can be very low. Treatment of a patient with MDR-TB meningitis is complicated because many second-line drugs do not have good penetration into the cerebrospinal fluid.
Children
Most children with MDR-TB have been infected by someone in the same household (Becerra et al. 2013). For this reason, careful investigation of family members is crucial. If it is difficult to collect a specimen from the child for laboratory analysis for whatever reason, the regimens can be designed based on the DST of the index case in the same household. Otherwise, the basic principles of regimen design for children with MDR-TB are no different than those for adults. Children generally tolerate second-line anti-TB drugs well. Pediatric formulations, however, do not exist for most drugs, and it is often necessary prepare adult formulations, for example, by splitting tablets (The Sentinel Project 2012; Garcia-Prats et al. 2013; Varaine and Rich 2013).
Pregnant Women
Some second-line anti-TB drugs are known to cause birth defects. For this reason, all women of childbearing age should be strongly advised to use a reliable method of contraception during MDR-TB treatment. For pregnant women who are diagnosed with MDR-TB, the benefit of treating MDR-TB in pregnancy in most circumstances outweighs the risks. Most patients should start treatment as soon as the diagnosis is made. Aminoglycosides can be particularly toxic to the developing fetal ear. Capreomycin may also carry a risk of ototoxicity but may be used if an injectable is absolutely necessary. Ethionamide is also usually avoided as it can increase the risk of nausea and vomiting associated with pregnancy, and teratogenic effects have been observed in animal studies. MDR regimens for pregnant women may be designed with three or four oral second-line anti-TB drugs plus pyrazinamide. The regimen can then be reinforced with an injectable and other drugs immediately postpartum. The risk of birth defects in MDR-TB treatment is highest in the first trimester of pregnancy, so the gestational age of the fetus should be carefully confirmed, preferably by dating using ultrasound. In rare cases, in which the mother does not accept the risks of the treatment and is clinically stable, treatment can be delayed until the second trimester.
HIV Coinfection
People living with HIV are vulnerable to MDR-TB infection and are at high risk of developing active MDR-TB once infected. HIV-positive patients often die while waiting for laboratory confirmation of MDR-TB and before starting effective therapy. This was best illustrated by the rapid and deadly spread of XDR-TB among HIV-positive patients in South Africa (Gandhi et al. 2006). The WHO currently recommends Xpert MTB/RIF as the initial diagnostic test in settings with high prevalence of HIV-associated TB or MDR-TB (WHO 2011b).
Early identification and prompt initiation of appropriate treatment can reduce mortality among HIV-infected patients infected with MDR-TB. ART improves survival in MDR-TB patients infected with HIV and should be started as soon as possible, as early as the first week after starting MDR-TB treatment. Stavudine and tenofovir are often avoided because of overlapping toxicities (neuropathy and nephrotoxicity, respectively) with second-line anti-TB drugs; nonetheless, cotreatment with ART is still challenging in most patients because of side effects and the high pill burden. HIV-positive patients seem to experience a higher incidence of side effects with second-line anti-TB drugs (Seung et al. 2009).
COMMUNITY-BASED CARE FOR MDR-TB
MDR-TB may be hospital-, clinic-, or community-based. These approaches may not be mutually exclusive, and in fact, they may all be used within the same program. In some settings, patients are hospitalized at the beginning of treatment to allow for more intensive monitoring of side effects, especially for patients who are physically debilitated. But in many countries, the lack of MDR-TB hospital beds becomes a bottleneck to scale-up. Mandatory hospitalization may also exacerbate nosocomial transmission of MDR-TB because in many settings, infection control measures are not adequate.
Many countries have moved to decentralize MDR-TB treatment to increase access. One common method is to provide DOT at a primary care clinic. This strategy can be quite physically and economically burdensome to the patient because it requires daily travel to the clinic, sometimes twice daily. This is especially true in rural areas where primary care systems are weak.
Community-based MDR-TB treatment allows patients to be treated in their homes. By providing a flexible and convenient solution, it promotes patient adherence and reintegration into family, social, and work life. It can be fully supervised by a nurse or community health worker during daily home visits. Community-based care reduces cost in the health system and can be more cost-effective than hospital care, but in many ways, it is more challenging to implement. Each dose is administered under DOT, often through a system of a compensated, trained, and well-supervised nurse or community health workers.
Another important aspect of MDR-TB treatment is addressing socioeconomic determinants of health. Socioeconomic problems, including hunger, homelessness, and unemployment, are common among MDR-TB patients. These problems have been successfully tackled through socioeconomic interventions that include the use of provisions in the form of “incentives” and “enablers.” Incentives are rewards that encourage patients to adhere to treatment, such as food packages, whereas enablers are goods or services that make it easier for patients to adhere to treatment, such as transportation vouchers. Addressing socioeconomic barriers to adherence is a crucial part of successful MDR-TB treatment (USAID 2011).
CONCLUSION
The continuing spread of MDR-TB is one of the most urgent and difficult challenges facing global TB control. The main causes of the increasing spread of resistant TB strains are weak medical systems, amplification of resistance through incorrect treatment, and ongoing transmission in communities and facilities. New molecular methods of DST have revolutionized the diagnosis of MDR-TB, but they are still not widely available in resource-limited settings. Although patients harboring MDR and XDR strains present a formidable challenge for treatment, cure is often possible with early identification of resistance and use of a properly designed regimen. Community-based programs can improve treatment outcomes by allowing patients to be treated in their homes and addressing socioeconomic barriers to adherence.
Footnotes
Editors: Stefan H.E. Kaufmann, Eric J. Rubin, and Alimuddin Zumla
Additional Perspectives on Tuberculosis available at www.perspectivesinmedicine.org
REFERENCES
- Ahuja SD, Ashkin D, Avendano M, Banerjee R, Bauer M, Bayona J, Becerra M, Benedetti A, Burgos M, Centis R, et al. 2012. Multidrug resistant pulmonary tuberculosis treatment regimens and patient outcomes: An individual patient data meta-analysis of 9,153 patients. PLoS Med 9: e1001300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra M, Franke MF, Appleton SC, Joseph JK, Bayona J, Atwood SS, Mitnick CD. 2013. Tuberculosis in children exposed at home to multidrug-resistant tuberculosis. Pediatr Infect Dis J 32: 115–119. [DOI] [PubMed] [Google Scholar]
- Cegielski JP. 2010. Extensively drug-resistant tuberculosis: “There must be some kind of way out of here”. Clin Infect Dis 50: S195–S200. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC). 2013. Provisional CDC guidelines for the use and safety monitoring of bedaquiline fumarate (Sirturo) for the treatment of multidrug-resistant tuberculosis. MMWR Recomm Rep 62: 1–12. [PubMed] [Google Scholar]
- Chavez Pachas AM, Blank R, Fawzi Smith MC, Bayona J, Becerra M, Mitnick CD. 2004. Identifying early treatment failure on category I therapy for pulmonary tuberculosis in Lima Ciudad, Peru. Int J Tuberc Lung Dis 8: 52–58. [PubMed] [Google Scholar]
- Curry International Tuberculosis Center, California Department of Public Health. 2012. Tuberculosis Drug Information Guide, 2nd ed. Curry International Tuberculosis Center, San Francisco, California. [Google Scholar]
- Dye C, Williams BG, Espinal MA, Raviglione MC. 2002. Erasing the world’s slow stain: Strategies to beat multidrug-resistant tuberculosis. Science 295: 2042–2046. [DOI] [PubMed] [Google Scholar]
- Frieden TR, Sterling T, Pablos-Mendez A, Kilburn JO, Cauthen GM, Dooley SW. 1993. The emergence of drug-resistant tuberculosis in New York City. New Engl J Med 328: 521–526. [DOI] [PubMed] [Google Scholar]
- Frieden TR, Fujiwara PI, Washko RM, Hamburg MA. 1995. Tuberculosis in New York City—Turning the tide. New Engl J Med 333: 229–233. [DOI] [PubMed] [Google Scholar]
- Gandhi NR, Moll A, Sturm AW, Pawinski R, Govender T, Lalloo U, Zeller K, Andrews J, Friedland G. 2006. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet 368: 1575–1580. [DOI] [PubMed] [Google Scholar]
- Garcia-Prats AJ, Donald PR, Hesseling AC, Schaaf HS. 2013. Second-Line antituberculosis drugs in children: A commissioned review for the World Health Organization 19th Expert Committee on the Selection and Use of Essential Medicines. World Health Organization, Geneva. [Google Scholar]
- Gelmanova IY, Keshavjee S, Golubchikova VT, Berezina VI, Strelis AK, Yanova GV, Atwood S, Murray M. 2007. Barriers to successful tuberculosis treatment in Tomsk, Russian Federation: Non-adherence, default and the acquisition of multidrug resistance. Bull World Health Organ 85: 703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JL, Hadad DJ, Boom WH, Daley CL, Peloquin CA, Eisenach KD, Jankus DD, Debanne SM, Charlebois ED, Maciel E, et al. 2006. Early and extended early bactericidal activity of levofloxacin, gatifloxacin and moxifloxacin in pulmonary tuberculosis. Int J Tuberc Lung Dis 10: 605–612. [PubMed] [Google Scholar]
- Keshavjee S, Farmer PE. 2012. Tuberculosis, drug resistance, and the history of modern medicine. New Engl J Med 367: 931–936. [DOI] [PubMed] [Google Scholar]
- Lin H, Shin S, Blaya JA, Zhang Z, Cegielski P, Contreras C, Asencios L, Bonilla C, Bayona J, Paciore CJ, et al. 2011. Assessing spatiotemporal patterns of multidrug-resistant and drug-sensitive tuberculosis in a South American setting. Epidemiol Infect 139: 1784–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitnick C, Bayona J, Palacios E, Shin S, Furin J, Alcántara F, Sánchez E, Sarria M, Becerra M, Fawzi MC, et al. 2003. Community-based therapy for multidrug-resistant tuberculosis in Lima, Peru. New Engl J Med 348: 119–128. [DOI] [PubMed] [Google Scholar]
- Mukherjee JS, Rich ML, Socci AR, Joseph JK, Virú FA, Shin SS, Furin JJ, Becerra MC, Barry DJ, Kim JY, et al. 2004. Programmes and principles in treatment of multidrug-resistant tuberculosis. Lancet 363: 474–481. [DOI] [PubMed] [Google Scholar]
- Partners In Health. 2013. The PIH guide to the medical management of multidrug-resistant tuberculosis, 2nd ed Partners In Health, Boston. [Google Scholar]
- Peloquin CA, Hadad DJ, Molino LP, Palaci M, Boom WH, Dietze R, Johnson JL. 2008. Population pharmacokinetics of levofloxacin, gatifloxacin, and moxifloxacin in adults with pulmonary tuberculosis. Antimicrob Agents Chemother 52: 852–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerantz BJ, Cleveland JC, Olson JK, Pomerantz M. 2001. Pulmonary resection for multi-drug resistant tuberculosis. J Thorac Cardiovasc Surg 121: 448–453. [DOI] [PubMed] [Google Scholar]
- Pyle M. 1947. Relative number of resistant tubercle bacilli in sputa of patients before and during treatment with streptomycin. In Proceedings of the staff meetings Mayo Clinic 22: 465–473. [PubMed] [Google Scholar]
- Satti H, McLaughlin MM, Seung KJ, Becerra MC, Keshavjee S. 2013. High risk of drug-resistant tuberculosis when first-line therapy fails in a high HIV prevalence setting. Int J Tuberc Lung Dis 17: 100–106. [DOI] [PubMed] [Google Scholar]
- Seung KJ, Rich ML. 2010. Diagnosis and treatment of drug-resistant tuberculosis. In Tuberculosis: The essentials, 4th ed (ed. Raviglione MC), pp. 170–214. Informa Healthcare, New York. [Google Scholar]
- Seung KJ, Gelmanova IE, Peremitin GG, Golubchikova VT, Pavlova VE, Sirotkina OB, Yanova GV, Strelis AK. 2004. The effect of initial drug resistance on treatment response and acquired drug resistance during standardized short-course chemotherapy for tuberculosis. Clin Infect Dis 39: 1321–1328. [DOI] [PubMed] [Google Scholar]
- Seung KJ, Omatayo DB, Keshavjee S, Furin JJ, Farmer PE, Satti H. 2009. Early outcomes of MDR-TB treatment in a high HIV-prevalence setting in Southern Africa. PloS ONE 4: e7186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S, Pasechnikov AD, Gelmanova IY, Peremitin GG, Strelis AK, Mishustin S, Barnashov A, Karpeichik Y, Andreev YG, Golubchikova VT, et al. 2006. Treatment outcomes in an integrated civilian and prison MDR-TB treatment program in Russia. Int J Tuberc Lung Dis 10: 402–408. [PubMed] [Google Scholar]
- Skrahina A, Hurevich H, Zalutskaya A, Sahalchyk E, Astrauko A, van Gemert W, Hoffner S, Rusovich V, Zignol M. 2012. Alarming levels of drug-resistant tuberculosis in Belarus: Results of a survey in Minsk. Eur Respir J 39: 1425–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somocurcio JG, Sotomayor A, Shin S, Portilla S, Valcarcel M, Guerra D, Furin J. 2007. Surgery for patients with drug-resistant tuberculosis: Report of 121 cases receiving community-based treatment in Lima, Peru. Thorax 62: 416–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Sentinel Project for Pediatric Drug-Resistant Tuberculosis. 2012. Management of multidrug-resistant tuberculosis in children: A field guide. The Sentinel Project for Pediatric Drug-Resistant Tuberculosis, Boston. [Google Scholar]
- USAID TB CARE II. 2011. Community-based care for drug-resistant tuberculosis: A guide for implementers. Partners In Health, Boston. [Google Scholar]
- Van Deun A, Maug AK, Salim MA, Das PK, Sarker MR, Daru P, Rieder HL. 2010. Short, highly effective, and inexpensive standardized treatment of multidrug-resistant tuberculosis. Am J Respir Crit Care Med 182: 684–692. [DOI] [PubMed] [Google Scholar]
- Varaine F, Rich ML. 2013. Tuberculosis: Practical guide for clinicians, nurses, laboratory technicians and medical auxiliaries. Médecins San Frontières and Partners in Health, Paris. [Google Scholar]
- Wells CD, Cegielski JP, Nelson LJ, Laserson KF, Holtz TH, Finlay A, Castro KG, Weyer K. 2007. HIV infection and multidrug-resistant tuberculosis: The perfect storm. J Infect Dis 196: S86–S107. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO). 2011a. Guidelines for the programmatic management of drug-resistant tuberculosis, 2011 update. World Health Organization, Geneva. [PubMed] [Google Scholar]
- World Health Organization (WHO). 2011b. Policy Statement: Automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF system. World Health Organization, Geneva. [PubMed] [Google Scholar]
- World Health Organization (WHO). 2013a. Global tuberculosis report 2013. World Health Organization, Geneva. [Google Scholar]
- World Health Organization (WHO). 2013b. Definitions and reporting framework for tuberculosis—2013 revision. World Health Organization, Geneva. [Google Scholar]
- World Health Organization (WHO). 2013c. The use of bedaquiline in the treatment of multidrug-resistant tuberculosis: Interim policy guidance. World Health Organization, Geneva. [PubMed] [Google Scholar]
- Zhao Y, Xu S, Wang L, Chin DP, Wang S, Jiang G, Xia H, Zhou Y, Li Q, Ou X, et al. 2012. National survey of drug-resistant tuberculosis in China. New Engl J Med 366: 2161–2170. [DOI] [PubMed] [Google Scholar]