Abstract
Ample evidence has demonstrated that sex steroid hormones, such as the potent estrogen 17β-estradiol (E2), affect hippocampal morphology, plasticity, and memory in male and female rodents. Yet relatively few investigators who work with male subjects consider the effects of these hormones on learning and memory. This review describes the effects of E2 on hippocampal spinogenesis, neurogenesis, physiology, and memory, with particular attention paid to the effects of E2 in male rodents. The estrogen receptors, cell-signaling pathways, and epigenetic processes necessary for E2 to enhance memory in female rodents are also discussed in detail. Finally, practical considerations for working with female rodents are described for those investigators thinking of adding females to their experimental designs.
Hormones have long been known to play key roles in regulating learning and memory. Many hormones, including epinephrine, glucocorticoids, and insulin influence learning and memory via inverted U-shaped dose–response relationships in which memory is enhanced by moderate, but not low or high, hormone levels (Roozendaal 2000; Korol and Gold 2007; McNay and Recknagel 2011). Research from the past two decades has demonstrated that sex steroid hormones, particularly the potent estrogen 17β-estradiol (E2), also regulate learning and memory in male and female rodents via an inverted U-shaped dose–response function that is influenced by estrogen receptor expression (Packard and Teather 1997a; Packard 1998; Foster 2012). Yet E2 has not gained widespread acceptance as a hormonal modulator of memory in both females and males, perhaps because the majority of this research has been conducted in female rodents. Moreover, the common view of E2 as a “female” hormone may contribute to the misperception that E2 is not relevant for cognitive function in males. However, considerable evidence supports a vital role for E2 in mediating neural function and behavior in male rodents. Therefore, it is important for investigators working with males to understand the ways in which E2 and other sex steroid hormones (e.g., androgens, progestins, and other estrogens) may influence their brain regions and behaviors of interest.
Another reason for investigators to be cognizant of sex steroid hormone-induced regulation of learning and memory is that males and females may respond differently to various treatments and environmental factors. A classic example is that of acute stress, which enhances classical conditioning and increases apical CA1 dendritic spine density in male rats, but impairs classical conditioning and decreases CA1 spine density in female rats (Wood and Shors 1998; Shors et al. 2001). Therefore, investigators hoping to comply with National Institutes of Health policies that encourage the inclusion of females in biomedical research must be aware that adding females to a study is not as simple as adding another group. In some ways, females are fundamentally different from males, the most obvious of which is the presence of reproductive hormone cycling in females. In rodents, this 4–5 d cycle is termed the “estrous” cycle (Fig. 1) because ovulation leads to a state of behavioral estrus that signals sexual receptivity. No clear consensus has emerged regarding the influence of the estrous cycle on learning and memory, and it has been argued recently that the estrous cycle does not lead to more variability in females relative to males (Prendergast et al. 2014). Nevertheless, it is important that investigators consider the possible effects of sex steroid hormones on neural function and behavior in their experimental designs and data interpretation. However, when thinking about sex differences in brain function or behavior, it is important to note whether differences are due to the activational effects of circulating hormones in adulthood or from organizational effects of hormones in early development, as it has been argued that only the latter can be construed as a true sex difference (McCarthy and Konkle 2005).
Figure 1.
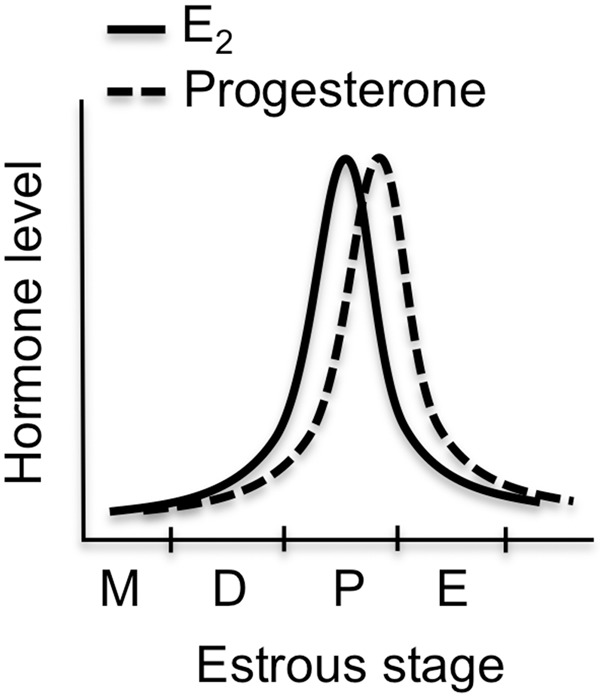
Illustration of serum E2 and progesterone fluctuations during the 4–5 d rodent estrous cycle. Each stage lasts ∼24 h. (M) metestrus (also called diestrus I), (D) diestrus (also called diestrus II), (P) proestrus, (E) estrus.
Numerous recent reviews have discussed the effects of E2 on learning and memory in females (Korol 2002; Foster 2005; Daniel 2006, 2013; Sherwin and Henry 2008; Barha and Galea 2010; Bimonte-Nelson et al. 2010; Gibbs 2010; Kim and Casadesus 2010; Choleris et al. 2012; Foster 2012; Frick 2012; Acosta et al. 2013; Chisolm and Juraska 2013; Ervin et al. 2013; Galea et al. 2013; Hogervorst 2013; Luine and Frankfurt 2013; Maki 2013; Bean et al. 2014; Fortress and Frick 2014; Luine 2014; Frankfurt and Luine 2015; Tuscher et al. 2015). As such, this review will not attempt to provide a comprehensive discussion of all effects of E2 on learning and memory. Rather, the current review highlights effects of E2 on hippocampal function and memory processes in both males and females to provide those working with either sex a sense of how E2 might influence their behaviors of interest. E2 will be the primary focus here because considerably more is known about the effects of E2 on memory than any other sex steroid hormone. Moreover, the discussion below centers largely upon the hippocampus because of the extensive literature on the effects of E2 in this structure. Where appropriate, information about the effects of E2 in other brain regions will be mentioned. The sections below will discuss the localization of estrogen receptors, and the effects of E2 on hippocampal spine density, physiology, biochemistry, and learning and memory in male and female rodents. Finally, to aid those investigators who may seek to incorporate females in their experimental designs, the review will conclude by addressing practical considerations for working with female rodents.
Sex steroid hormones: a primer
To appreciate the complexity inherent to interpreting the biological effects of sex steroid hormones, it is necessary to understand their biosynthesis. Steroid hormones are one of four classes of hormones secreted by endocrine glands. All steroid hormones are synthesized from a cholesterol precursor (Fig. 2) and have a chemical structure that includes three six-carbon rings and one conjugated five-carbon ring (Compagnone and Mellon 2000; see Nelson 2011 for an introduction to steroid biosynthesis). Carbons are cleaved from the 27-carbon cholesterol precursor to generate two functionally distinct groups of steroid hormones, those that mediate the stress response (e.g., cortisol, corticosterone, and aldosterone) and the “sex steroid” hormones that regulate reproductive function (e.g., progestins, androgens, and estrogens). To generate both groups of hormones, six-carbons are cleaved from cholesterol to form the 21-carbon progestins (Nelson 2011). In mammals, these “progestational” hormones, which include pregnenolone and progesterone, are crucial for initiating and terminating mating behavior and for maintaining pregnancy (McCarthy and Becker 2002). These hormones are also essential precursors for all other steroid hormones. Pregnenolone is generated first from cholesterol, and is then converted into progesterone (Compagnone and Mellon 2000). Progesterone can be converted into several other hormones, including corticoids, pregnane neurosteroids, and other sex steroids (although a prohormone for corticoids, progesterone is typically considered a sex steroid hormone because of its importance to reproduction) (Compagnone and Mellon 2000). Because E2 is the subject of this review, the remainder of this section will focus on sex steroid hormones.
Figure 2.
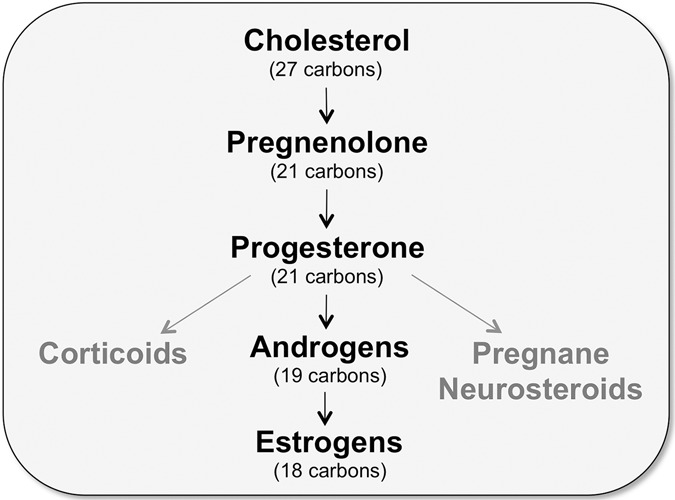
Simplified schematic of steroid hormone biosynthesis. A cholesterol precursor is cleaved to generate the progestins pregnenolone and progesterone. These hormones are essential precursors for all other steroid hormones. Progesterone can then be converted into several other hormones, including corticoids and pregnane neurosteroids (shown in gray). Progesterone can also be further metabolized to produce androgens. The enzyme aromatase cleaves an additional carbon from androgens to yield estrogens.
Within specific tissues (e.g., the gonads, brain, adrenals, and adipose tissue), the enzymes necessary to convert progesterone to androgens (e.g., testosterone, androstenedione, and dihydrotestosterone) cleave two more carbons and an ethyl group from the 21-carbon progesterone structure to yield the 19-carbon androgens (Breedlove and Hampson 2002; Nelson 2011). Androgens derive their name from their ability to generate male (Andros) appearance, behaviors, and brain physiology during development and adulthood. In male mammals, these hormones are necessary for spermatogenesis, maintenance of the genitalia, and the development of secondary sex characteristics and muscle mass after puberty (Baum 2002; Nelson 2011). As such, androgens are involved in numerous behaviors that facilitate reproductive success, including courtship, copulation, and aggression (Baum 2002; Nelson 2011). Although the primary sources of androgens in male mammals are the testes, the brain also generates a substantial amount of sex steroid hormones independent of the testes (see “Hippocampally synthesized E2 and memory”).
Androgens are the precursors to all estrogens (e.g., E2, estrone, and estriol). The enzyme aromatase cleaves a carbon from androgenic precursors to form the 18-carbon estrogens in a process called aromatization (McEwen and Krey 1984; Breedlove and Hampson 2002; Nelson 2011). In female mammals, estrogens are essential for many functions including copulatory and reproductive functions (e.g., stimulating ovulation), development of secondary sex characteristics, calcium metabolism, and water retention (McCarthy and Becker 2002; Nelson 2011). The primary sources of estrogens in females are the ovaries, which synthesize and release estrogens in response to signals from gonadotropin hormones released by the pituitary (Terasawa and Ojeda 2009). Estrogens are “estrous generating,” in that they stimulate female sexual (estrous) behavior and regulate ovulation (McCarthy and Becker 2002; Nelson 2011). However, estrogens are also synthesized in the brain (see “Hippocampally synthesized E2 and memory”), adipose tissue, and adrenals.
Finally, it is important to note that it is erroneous to think of androgens as “male” hormones, and estrogens and progestins as “female” hormones (Becker and Breedlove 2002; Nelson 2011). Both males and females synthesize considerable quantities of progestins, androgens, and estrogens (Terasawa and Ojeda 2009), but differ in the quantity of enzymes on hand to metabolize each hormone. For example, although both male and female gonads contain high levels of androgenic enzymes, ovaries also contain an abundance of aromatase for rapidly converting androgens to estrogens. Similarly, males produce copious quantities of progestins to make androgens, which can then be metabolized into estrogens and other metabolites. As such, any of these sex steroid hormones may affect learning and memory in either sex. However, interpretation of hormone effects is complicated by the fact that the catabolism of one sex steroid leads to the synthesis of another. Therefore, it can be difficult to determine whether a given sex steroid influences biological processes as itself (e.g., by binding to its receptors) or via conversion to a metabolite. This issue can be addressed pharmacologically in several ways. To use progesterone as an example, the role of progesterone receptors in memory may be examined using progesterone receptor agonists (e.g., R5020) and antagonists (e.g., RU486). Effects mediated via metabolism may be assessed by comparing the effects of progesterone with those of a metabolite (e.g., allopregnanolone) or by co-infusing progesterone with an inhibitor that prevents its catabolism. Because the enzyme 5α-reductase converts progesterone into allopregnanolone, co-infusion of progesterone and a 5α-reductase inhibitor (e.g., finasteride) may be used to determine if allopregnanolone mediates the mnemonic effects of progesterone. Similarly, progesterone's catabolism into cortisol or corticosterone can be blocked an 11β-hydroxylase inhibitor (e.g., metyrapone) and its eventual catabolism into estradiol can be blocked by aromatase inhibitors (e.g., letrozole, fadrozole, or anastrozole). Although such experiments may seem excessive, they are important to determine the precise mechanisms through which a specific steroid hormone regulates memory.
Estrogen receptor localization and mechanism of action
ERα and ERβ as mediators of classical estrogen responses
Sex steroid hormones have traditionally been thought to affect cellular function via intracellular receptors that act as nuclear transcription factors. Because steroid hormones are lipids, they easily move through lipid bilayer plasma membranes to gain entry to the intracellular space. In the traditional model (typically called “classical” or “genomic”), the binding of sex steroid hormones to intracellular hormone receptors causes hormone-receptor complexes to translocate into the nucleus where they bind to hormone response elements on the DNA and initiate gene transcription (Fig. 3). Two intracellular estrogen receptors, ERα and ERβ, have been identified. Both ERs can be found throughout the brain, including the prefrontal cortex, hippocampus, entorhinal cortex, perirhinal cortex, basal forebrain, amygdala, thalamus, and cerebellum (Shughrue et al. 1997b, 2000; Osterlund et al. 2000; Shughrue and Merchenthaler 2000). In the medial prefrontal cortex of ovariectomized rats, ERα and ERβ have been observed in axons, terminals, dendrites, and dendritic spines of neurons, as well as in glia (Almey et al. 2014). Axons and dendrites exhibit a greater percentage of ERα-positive profiles, whereas terminals, spines, and glia exhibit a greater percentage of ERβ-positive profiles (Almey et al. 2014). Within the basal forebrain of ovariectomized rats, ERα colocalizes with choline acetyltransferase-positive cholinergic neurons (Shughrue et al. 2000). Because cholinergic neurons in the basal forebrain project to numerous brain regions, including the hippocampus and neocortex (Frotscher and Léránth 1985; Martinez-Murillo et al. 1990; Wainer et al. 1993), the localization of E2 within basal forebrain cholinergic neurons may provide a mechanism for E2 to regulate subcortical input to these structures. In many brain regions, including the infralimbic prefrontal cortex, entorhinal cortex, perirhinal cortex, amygdala, and basal forebrain, ERβ can also be found within parvalbumin-positive inhibitory interneurons (Blurton-Jones and Tuszynski 2002), suggesting that ERβ may regulate inhibitory tone in addition to pyramidal neuron excitability.
Figure 3.
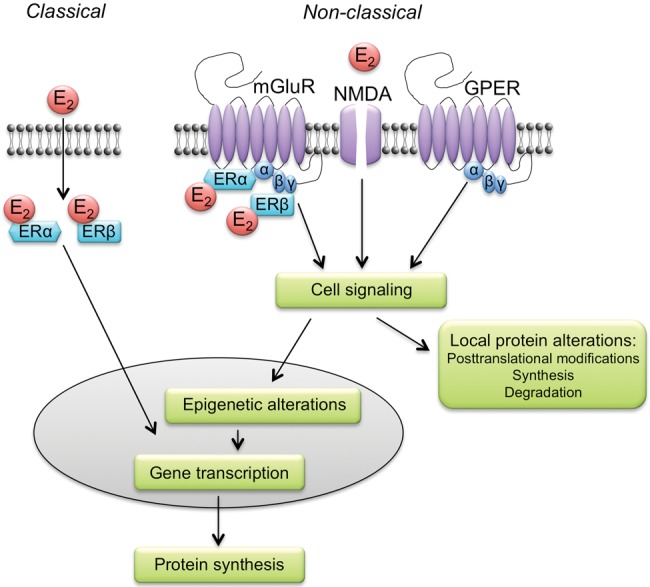
Classical and nonclassical mechanisms of E2 action. In the classical mechanism (left), E2 binds to ERα and ERβ in the cytoplasm, and then the E2–ER complex translocates into the nucleus and binds to an estrogen response element on the DNA. Together with histone acetyltransferases and other co-regulators, the E2–ER complex binds to the estrogen response element on DNA to facilitate gene transcription and protein synthesis. Nonclassical mechanisms (right) involve action at or near the plasma membrane that activates cell-signaling cascades. Within the dorsal hippocampus, cell-signaling enzymes associated with estrogenic regulation of hippocampal memory are phosphorylated by several membrane receptors including: (1) metabotropic glutamate receptor 1a (mGluR) and its interactions with ERα and ERβ, (2) NMDA receptors, and (3) G-protein-coupled estrogen receptor (GPER). The resulting activation of cell-signaling pathways (e.g., ERK) triggers epigenetic alterations (e.g., histone acetylation) that regulate gene transcription and protein synthesis. Cell-signaling alterations may also influence local protein levels in cellular compartments such as dendritic spines. (Adapted from Frick 2015 with permission from Elsevier © 2015.)
ERα and ERβ are distributed throughout the male and female rodent hippocampus (Shughrue et al. 1997a,b; Shughrue and Merchenthaler 2000; Mitra et al. 2003; Mitterling et al. 2010). In adult male and female rats and mice, ERα and ERβ are found in glia and within the nucleus, dendrites, dendritic spines, axons, and terminals of pyramidal neurons (Milner et al. 2001, 2005; Mitterling et al. 2010; Waters et al. 2011a). Findings that ERα-labeled dendritic spine profiles in the rat dentate gyrus were more numerous in proestrus females than in diestrus females and in males suggest the possibility of estrous cycle and sex differences in ER levels among hippocampal subregions (Romeo et al. 2005). In embryonic hippocampal neuron cultures, ERα is also found in vesicle clusters within GABAergic interneurons in CA1, where it mediates an E2-induced decrease in hippocampal GABAergic neurotransmission that is thought to disinhibit pyramidal neurons (Murphy et al. 1998; Hart et al. 2007; Huang and Woolley 2012). Other work has localized ERα to glutamatergic and GABAergic synaptic vesicles in female rat hippocampal synaptosomes (Tabatadze et al. 2013). Thus, similar to ERβ in other brain regions, ERα in the hippocampus appears to regulate both inhibitory and excitatory tone.
A role for nonclassical mechanisms in the effects of E2
The positioning of hippocampal ERs in distal extranuclear cellular compartments like dendritic spines and terminals suggests possible nonclassical mechanisms of action for ERs at or within the plasma membrane (Fig. 3). Although nonclassical and classical mechanisms may ultimately lead to a similar cellular result (e.g., gene transcription), nonclassical mechanisms are typically thought to depend on activation of cell-signaling pathways and/or epigenetic alterations, rather than nuclear binding to an estrogen response element on DNA. Systemic E2 treatment increases the distribution of ERβ in dendritic spines and shafts in the adult female rat hippocampus (Waters et al. 2011b), which may position ERβ to interact with plasma membrane receptors in the dendrite to trigger cell signaling. In support of the notion that intracellular ERs act at the membrane are findings showing that E2 causes ERβ to translocate to the plasma membrane in hippocampal-derived cell lines and rat primary cortical neurons (Sheldahl et al. 2008). At the membrane, ERα and ERβ in neonatal female rats interact with metabotropic glutamate receptor 1 (mGluR1) to rapidly activate hippocampal extracellular signal-regulated kinase (ERK) signaling and promote phosphorylation of cAMP response element binding protein (CREB) (Boulware et al. 2005, 2013). Interestingly, E2 does not interact with mGluRs to increase ERK-dependent CREB phosphorylation in hippocampal cultures from neonatal male rats (Boulware et al. 2005), suggesting potentially important sex differences in estrogenic regulation of rapid cell signaling. In female rat hippocampal cultures, the ability of the ERs to associate with mGluRs and phosphorylate CREB is dependent on S-palmitoylation (Meitzen et al. 2013), a post-translational modification associated with intracellular protein trafficking (Fukata and Fukata 2010). Although the ability of ERs to associate with mGluRs and phosphorylate CREB through an S-palmitoylation process has not yet been tested in males, this finding may help to explain how ERα and ERβ can be shuttled to the plasma membrane to trigger rapid cell-signaling processes in females.
Another way in which E2 may signal at the plasma membrane is by binding to ERs within the membrane itself. The identity of these ERs has been a source of much debate, as the physical structures of ERα and ERβ are not generally consistent with those of integral membrane proteins, and several other proposed membrane ERs (e.g., ER-X and Gq-ER) have proven difficult to clone (Levin 1999, 2011; Woolley 2007; Kelly and Rønnekleiv 2008). Evidence supporting the existence of membrane ERs comes from studies using bovine serum albumin-conjugated E2 (BSA-E2), which is too large to penetrate the plasma membrane. In vitro studies of primary rat hippocampal neurons or hippocampal cell lines demonstrate that BSA-E2 localizes to the plasma membrane, where it rapidly induces calcium signaling, increases ERK phosphorylation and translocation into the nucleus, and increases CREB phosphorylation (Wade and Dorsa 2003; Boulware et al. 2005; Yang et al. 2010; Wu et al. 2011). In vivo, infusion of BSA-E2 into the dorsal hippocampus or cerebral ventricles of ovariectomized rats or mice activates ERK, Akt, and CREB within 5–10 min (Fernandez et al. 2008; Yang et al. 2010). Moreover, dorsal hippocampal infusion of BSA-E2 in adult ovariectomized mice induces a similar ERK-dependent enhancement of object recognition memory consolidation to that observed after dorsal hippocampal infusion of free E2 (Fernandez et al. 2008), suggesting that membrane ERs mediate the memory-enhancing effect of E2 by activating ERK signaling. Data such as these have led to a general acceptance that a membrane-associated ER facilitates the rapid nonclassical effects of E2 (e.g., Micevych and Dominguez 2009) and have prompted some investigators to suggest that BSA-E2 binds to ERα and ERβ localized within the membrane (Meitzen and Mermelstein 2011). Although one provocative report indicates that ERα in the hypothalamus has an extracellular domain and can be internalized by E2 and mGluR1a ligands (Bondar et al. 2009), it is not yet widely accepted that ERα is an integral membrane protein.
However, BSA-E2 may bind to other putative membrane ERs, such as G-protein-coupled estrogen receptor (GPER) (Filardo et al. 2000; Thomas et al. 2005; Funakoshi et al. 2006; Prossnitz et al. 2007). This former orphan G-protein-coupled receptor was previously called GPR30. Evidence that GPER strongly binds E2 in peripheral tissues led recently to its official designation as an ER, despite an ongoing controversy about whether GPER is a true ER in the nervous system (Levin 2009; Langer et al. 2010; Maggiolini and Picard 2010; Barton 2012). GPER can be found throughout the brain in male and female rodents, including the hippocampus, prefrontal cortex, and striatum (Brailoiu et al. 2007). GPER is also found in the female rat basal forebrain, where it colocalizes with cholinergic neurons and, to a lesser extent, with GABAergic neurons (Hammond et al. 2011). Within the male and female mouse hippocampus, GPER has been observed in all lamina and exclusively at extranuclear sites within pyramidal neurons, interneurons, and glia (Waters et al. 2015). GPER has been localized to dendrites, dendritic spines, axons, terminals, and cell bodies, where it can generally be found at or near the plasma membrane in association with postsynaptic scaffolding proteins (Akama et al. 2013; Waters et al. 2015). Interestingly, GPER immunoreactivity in dendrites, spines, terminals, and axons differs in CA1, CA3, and the dentate gyrus during estrus relative to proestrus (Waters et al. 2015), suggesting that GPER expression may be regulated by estrogens and/or progestins. Sex differences have also been observed in relation to the cycle, such that estrus females exhibited fewer GPER-labeled axons in CA1 than males, whereas proestrus females exhibited more GPER-labeled glia in the dentate gyrus than males (Waters et al. 2015). Knockdown of GPER attenuates the neuroprotective effects of BSA-E2 in the hippocampus of ovariectomized rats, indicating a role for GPER in E2-induced neuroprotection (Tang et al. 2014). GPER activation also increases potassium-evoked acetylcholine release in the ovariectomized rat hippocampus in a manner similar to E2 treatment (Gibbs et al. 2014). Importantly, systemic injections of GPER-specific compounds indicate that GPER activation facilitates spatial working memory in ovariectomized rats (Hammond et al. 2009, 2012), suggesting that GPER could mediate the mnemonic effects of E2.
Estrogenic regulation of hippocampal morphology and physiology
Spine density
Interest in the effects of E2 on nonreproductive regions of the brain intensified with the discovery in the early 1990s that both exogenous and endogenous estradiol increased the density of dendritic spines on CA1 pyramidal neurons in adult female rats (Gould et al. 1990; Woolley et al. 1990; Woolley and McEwen 1992, 1993). During the estrous cycle, spine density is highest during the proestrus phase (Woolley and McEwen 1992), which is characterized by high levels of estrogens and progesterone (Fig. 1). Spine density plunges ∼30% within 24 h to reach its nadir during the estrus phase of the cycle (Woolley and McEwen 1992; Kato et al. 2013), which is characterized by low levels of estrogens and progesterone (Fig. 1). After bilateral ovariectomy, spine density decreases gradually and is significantly reduced 6 d after surgery (Gould et al. 1990; Woolley and McEwen 1993). Two systemic injections of estradiol benzoate given 24 h apart fully reversed this decrease within 48 h (although a significant increase was seen within 24 h), and an injection of progesterone 48 h later augmented the effects of estradiol on spines within the first 10 h of treatment (Woolley and McEwen 1993). Two systemic injections of the ERα agonist propyl pyrazole triol (PPT) or the ERβ agonist diarylpropionitrile (DPN) (Stauffer et al. 2000; Meyers et al. 2001) also significantly increase expression of the synaptic proteins PSD-95 and GluR1 in hippocampal CA1 (Waters et al. 2009), suggesting that E2-induced spine increases in adult females could be mediated by either receptor. Additional support for a role of ERα comes from work with hippocampal cultures from neonatal female rats, in which 7 d of treatment with PPT significantly increased CA1 spine density (Zhou et al. 2014). However, DPN significantly decreased CA1 spine density in these cultures (Zhou et al. 2014), suggesting that ERβ may suppress spinogenesis in early development. Some data support the conclusion that input from the basal forebrain is necessary for estradiol to enhance spines in adult female rats (Leranth et al. 2000). However, bath-applied E2 can increase spines in male hippocampal slices (Mukai et al. 2007; Murakami et al. 2014; Hasegawa et al. 2015) and cultured embryonic hippocampal neurons (Murphy and Segal 1996), suggesting that subcortical input may not be essential, at least in an ex vivo system. Other work shows that two injections of estradiol benzoate spaced 24 h apart failed to increase CA1 spine synapse density in ovariectomized rats trained in the Morris water maze (Frick et al. 2004), indicating that stressful behavioral training may interfere with the effects of estradiol on CA1 spinogenesis.
It is important to note potential species differences in the effects of E2 on CA1 dendritic spine density. One early report from ovariectomized mice found that 5 d of systemic estradiol benzoate injection did not increase the total number of spines, but rather increased the number of mushroom spines specifically (Li et al. 2004). However, other more recent studies report an increase in total CA1 spine density in mice after acute systemic treatment with E2 or agonists of ERα and ERβ (Liu et al. 2008; Phan et al. 2011, 2012). Although the reasons for this discrepancy are unclear, these latter findings suggest parallels between the effects of E2 on spine density in female rats and mice.
In male rats, bilateral gonadectomy significantly reduced CA1 spine synapse density, but this effect was not reversed by 2 d of systemic E2 injection as observed in ovariectomized females (Leranth et al. 2003). Rather, the gonadectomy-induced decrease was reversed by 2 d of systemic testosterone or the nonaromatizable androgen dihydrotestosterone (DHT) (Leranth et al. 2003). The effects of androgens on spines are supported by data from male hippocampal slices showing that bath application of testosterone or the nonaromatizable DHT also increased CA1 spine density (Ooishi et al. 2012; Hatanaka et al. 2014). In contrast to the in vivo data, however, studies using hippocampal slices from adult male rats report that the density of dendritic spines in CA1 was increased within 2 h of bath application of E2 (Murakami et al. 2006, 2014; Mukai et al. 2007; Ogiue-Ikeda et al. 2008; Ooishi et al. 2012). This increase was blocked by an inhibitor of ERK phosphorylation (Mukai et al. 2007; Murakami et al. 2014), linking ERK signaling to E2-induced CA1 spinogenesis in males. The spinogenesis in male hippocampal slices appears to be mediated by ERα rather than ERβ, as illustrated by findings showing that CA1 spines were increased by bath application of PPT, but not DPN (Mukai et al. 2007). The role of ERα in CA1 spinogenesis among males is also supported by data from ER knockout mice demonstrating that bath-applied E2 increased CA1 spine density in ERβ knockouts, but not in ERα knockouts (Murakami et al. 2014). Although the effects of E2 on CA1 spines in male hippocampal slices have been observed in multiple studies, discrepancies between these effects and the aforementioned lack of effect of systemic E2 on spines (Leranth et al. 2003) could be due to several factors, including different experimental systems (in vivo versus ex vivo) and timing. The increases induced by E2, testosterone, and DHT in slice preparations were observed 2 h after treatment, whereas spines were assessed in the in vivo work 2 d after treatment. Thus, E2-induced spine changes in males may be relatively transient and dissipate in vivo within 2 d.
One particularly intriguing aspect of the ex vivo spine data is the observation that significant changes could be observed within 2 h of treatment. This effect is not an artifact of the slice preparation, as several in vivo studies of ovariectomized rats have found that E2 increased spine density in both CA1 and prefrontal cortex layer II/III within 30 min of a systemic injection (MacLusky et al. 2005; Inagaki et al. 2012). Moreover, agonists for ERα and ERβ increased CA1 dendritic spine density in ovariectomized mice within 40 min of a single systemic injection (Phan et al. 2011), indicating a role for both ERα or ERβ in rapid E2-induced spinogenesis. At the present time, it is unclear how E2 might facilitate CA1 spine formation. However, several mechanisms are possible, including inducing post-translational modifications of existing dendritic proteins, altering protein degradation, increasing constitutive protein synthesis, or triggering new protein synthesis, as all of these mechanisms have been implicated in hippocampal memory and/or synaptic plasticity (Holahan and Routtenberg 2007; Klann and Sweatt 2008; Routtenberg 2008; Abbas 2013; Jarome and Helmstetter 2014).
One potential mechanism through which new local protein synthesis may occur is via activation of mammalian target of rapamycin (mTOR) signaling, which phosphorylates several key components of the protein synthesis machinery (Hoeffer and Klann 2010). mTOR is necessary for the consolidation of several types of memories (Dash et al. 2006; Parsons et al. 2006; Bekinschtein et al. 2007; Myskiw et al. 2008) and, as will be discussed later, for E2 to enhance consolidation of object recognition memory in ovariectomized mice (Fortress et al. 2013). mTOR signaling can be activated by a host of upstream kinases including phosphatidylinositol 3-kinase (PI3K), Akt, and ERK (Richter and Klann 2009; Hoeffer and Klann 2010; Laplante and Sabatini 2012), and phosphorylation of many of these kinases has been implicated in the ability of E2 to increase CA1 spine density in hippocampal slices from male rats. For example, the aforementioned E2-induced spine increase in male hippocampal slices was blocked by inhibitors of ERK, PI3K, protein kinase A (PKA), protein kinase C (PKC), and CaMKII (Hasegawa et al. 2015). Inhibitors of these signaling cascades also prevented E2 from augmenting θ-bust-stimulated long-term potentiation (LTP) in male hippocampal slices (Hasegawa et al. 2015), suggesting a key role for activation of these cell-signaling cascades in E2-induced synaptic potentiation. Similarly, an E2-induced increase in cortical dendritic spine density observed 30 min after bath application in embryonic rat cultures was blocked by pharmacological inhibition of Rap/AF-6/ERK signaling (Srivastava et al. 2008). As with E2, the spine increase induced by testosterone and DHT in males was blocked by inhibitors of ERK, PKA, PKC, LIM kinase (LIMK), and calcineurin (Ooishi et al. 2012; Hatanaka et al. 2014). As will be discussed below (see “Molecular mechanisms underlying E2’s effects on memory consolidation”), many of these same signaling cascades are involved in estrogenic regulation of hippocampal memory consolidation in ovariectomized mice, potentially linking E2-induced alterations in CA1 spine density and LTP with memory formation.
Synaptic plasticity
Because dendritic spines are found on excitatory pyramidal neurons, increased spine density is thought to lead to enhanced synaptic plasticity. E2 has numerous effects on neuronal excitability and plasticity in the brain. For example, acute E2 regulates intrinsic plasticity in brain regions including the amygdala, striatum, cerebellum, and hippocampus (Nabekura et al. 1986; Smith et al. 1988; Mermelstein et al. 1996; Kumar and Foster 2002). In hippocampal slices from ovariectomized rats, bath-applied E2 has been shown to increase spontaneous firing and suppress the afterhyperpolarization (Kumar and Foster 2002; Carrer et al. 2003). Bath-applied E2 also increased kainate-induced currents in dissociated male and female CA1 neurons, an effect that was blocked by inhibition of PKA activation and appeared to be independent of intracellular ERα and ERβ (Gu and Moss 1996; Gu et al. 1999).
Perhaps, the most notable effect of E2 on hippocampal physiology is its ability to potentiate NMDA-dependent LTP in female CA3-CA1 synapses (for reviews, see Foy 2001; Woolley 2007; Smith et al. 2009). LTP results in a persistent increase in synaptic excitability thought to underlie memory formation. E2 increases NMDA receptor binding in the female rat hippocampus, and the E2-induced increase in CA1 dendritic spine density is correlated with synaptic input mediated by NMDA receptors (Weiland 1992; Woolley et al. 1997). Within the rat estrous cycle, the greatest degree of LTP in CA1 is observed on the afternoon of proestrus, at which point estrogen and progesterone levels peak (Warren et al. 1995). Similarly, exogenous E2 increases baseline EPSP amplitude, reduces the threshold for LTP, and increases LTP amplitude in the hippocampus of male and female rodents (Teyler et al. 1980; Cordoba-Montoya and Carrer 1997; Foy et al. 1999, 2008; Bi et al. 2000; Fugger et al. 2001; Sharrow et al. 2002; Smith and McMahon 2005, 2006; Kramár et al. 2009; Smejkalova and Woolley 2010; Tanaka and Sokabe 2013; Kumar et al. 2015). Bath-applied E2 and PPT facilitated NMDA-mediated transmission and LTP in the dentate gyrus of slices from juvenile males, whereas DPN suppressed both forms of plasticity (Tanaka and Sokabe 2012, 2013). These data suggest that ERα promotes, whereas ERβ represses, hippocampal synaptic plasticity in juvenile males. However, ERβ appears to facilitate, rather than suppress, plasticity in the adult hippocampus. In adult male hippocampal slices, the effects of E2 on synaptic potentiation were mimicked by the ERβ agonist WAY200070, but not by PPT (Kramár et al. 2009), suggesting a selective role of ERβ in mediating the effects of E2 on LTP in adult males. WAY200070 also enhanced LTP in hippocampal slices from adult wild-type females, but not slices from ERβ knockout mice (Liu et al. 2008), supporting an essential role for ERβ in regulating LTP in adult females as well.
Consistent with the localization of ERα to inhibitory interneurons in the hippocampus (Murphy et al. 1998; Hart et al. 2007; Tabatadze et al. 2013), bath-applied E2 suppresses synaptic inhibition in hippocampal slices from ovariectomized rats (Huang and Woolley 2012). Interestingly, this effect was not observed in slices from male rats (Huang and Woolley 2012), which may stem from sex differences in levels of extranuclear ERα found in the hippocampus (Mitterling et al. 2010). The role of ionotropic glutamate receptors in estrogenic regulation of LTP may also be affected by sex, as illustrated by data showing that E2 affected AMPA, but not NMDA, responses in adult male slices, whereas the effects of E2 in adult female slices depended on NR2B-containing NMDA receptors (Smith and McMahon 2005, 2006; Kramár et al. 2009). However, other reports find that E2 enhances NMDA responses in males (Foy et al. 1999; Tanaka and Sokabe 2013), suggesting similar effects of E2 on NMDA receptors in both sexes. In females, the E2-induced increase in NMDA-mediated excitatory postsynaptic currents was not due to an increase in NMDA receptor subunits or phosphorylation of NR2B (Snyder et al. 2011), so likely results from the recruitment of existing NMDA receptors to synaptic sites (Jelks et al. 2007; Snyder et al. 2011). Whether the E2-induced alterations in hippocampal synaptic potentiation have direct functional consequences for the estrogenic regulation of learning and memory in either sex is unknown. However, an E2-induced enhancement of object recognition in ovariectomized rats was found to require an increase in NR2B-containing NMDA receptors, linking E2-induced alterations in LTP and memory in females (Vedder et al. 2013).
Actin polymerization is necessary in rats and mice for the formation of stable LTP (Krucker et al. 2000; Kramár et al. 2006). In both males and females, E2 may facilitate hippocampal LTP through actin polymerization. In hippocampal slices from male rats, bath application of E2 increased filamentous actin levels and actin polymerization in dendritic spines by activating the RhoA > RhoA kinase (ROCK) > LIMK > cofilin pathway, a key modulator of actin polymerization and stabilization (Kramár et al. 2009). The importance of actin dynamics in E2-induced plasticity was demonstrated by studies showing that E2’s effects on LTP in male slices were completely blocked by latrunculin, a toxin that disrupts actin filament assembly (Kramár et al. 2009). In female rat slices, E2 reversed ovariectomy-induced decreases in RhoA levels and actin polymerization (Kramár et al. 2009), suggesting that the RhoA > ROCK > LIMK > cofilin pathway may also be involved in E2-induced plasticity in females.
Interestingly, recent work has shown that the ability of E2 to increase LTP in ovariectomized rats is influenced by the duration of ovarian hormone deprivation prior to treatment. In these studies, two injections of E2 given 24 h apart were unable to increase LTP in rats ovariectomized for 19 mo prior to treatment (Smith et al. 2010). Importantly, this loss was not due to aging, as rats of the same age ovariectomized just 1 mo prior to E2 treatment exhibited increased LTP in response to E2 (Smith et al. 2010). This research group also found that treatment with both acute and chronic E2 was unable to enhance object recognition memory in rats ovariectomized for 19 mo prior to treatment (Vedder et al. 2014), suggesting important parallels between the synaptic and mnemonic responsiveness to E2 in females. Other investigators have reported similarly detrimental effects of long-term ovariectomy on the ability of chronic E2 to enhance spatial working memory (Daniel et al. 2006), supporting the idea that long-term hormone deprivation impairs E2’s capacity to facilitate hippocampal synaptic plasticity and memory. The reasons for this decreased responsiveness are not yet clear, but could be due to ubiquitination and degradation of estrogen receptors as observed in the CA1 of long-term ovariectomized rats (Zhang et al. 2011).
Although the effects of E2 on synaptic plasticity are typically attributed to a postsynaptic mechanism of action, other data indicate that E2 can potentiate glutamate transmission via a presynaptic mechanism. In hippocampal slices prepared from ovariectomized E2-treated rats, bath-applied E2 potentiated EPSCs by increasing the probability of glutamate release from specific inputs with an initially low probability of release (Smejkalova and Woolley 2010). This effect was mediated by ERβ, but not by ERα (Smejkalova and Woolley 2010). Although such presynaptic effects of E2 would be expected based on the localization of ERs to axon terminals (Milner et al. 2001, 2005; Mitterling et al. 2010; Waters et al. 2011a), this novel study was the first to suggest presynaptic facilitation of excitatory transmission by E2.
Finally, E2 also affects hippocampal long-term depression (LTD), yet this has been far less studied than LTP. One series of studies showed that induction of LTD in CA1 by patterned low-frequency stimulation is impaired in ovariectomized rats relative to gonadally intact females and is restored by two injections of estradiol benzoate given 48 h before tissue collection (Desmond et al. 2000; Zamani et al. 2000; Day and Good 2005). The effects of E2 on LTD in ovariectomized females required NMDA receptors and depended on conditioning frequency, as 2- or 4-Hz paired-pulse conditioning facilitated LTD induction, whereas 10-Hz conditioning blocked LTD induction (Zamani et al. 2000). These data have been interpreted to suggest that E2 decreases the threshold for inducing LTD in adult females by activating group I mGlu receptors (Zamani et al. 2000; Shiroma et al. 2005). Similarly, 30 min of E2 pretreatment enhanced NMDA-induced LTD in CA1, CA3, and the dentate gyrus of adult male rats (Mukai et al. 2007; Murakami et al. 2014). In contrast to the important role of ERβ in mediating LTP, this effect was mimicked by the ERα agonist PPT, but not by the ERβ agonist DPN, indicating a specific involvement of ERα in mediating LTD in the male hippocampus (Mukai et al. 2007). However, not all studies find that E2 facilitates LTD in male and female rodents. In hippocampal slices pretreated with E2 for at least 30 min, the induction of LTD by patterned stimulation was unaltered in gonadally intact male rats (Vouimba et al. 2000) and blocked by E2 in young ovariectomized rats (Sharrow et al. 2002). It has been suggested that the inconsistency between these studies and those showing that E2 enhances LTP may result from differential influences of discrepant E2 treatments on intracellular calcium or on activation of classical and nonclassical ER mechanisms (Sharrow et al. 2002; Shiroma et al. 2005; Foy et al. 2008).
In contrast to young adult rats, data from aged rats suggest that E2 suppresses LTD in the aged hippocampus. For example, chronic E2 treatment blocked the induction of LTD induced by patterned stimulation in hippocampal slices from aged ovariectomized rats that had undergone extensive behavioral testing (Foster et al. 2003). Similarly, 30 min of E2 suppressed LTD induced by patterned stimulation in hippocampal slices from aged males (Vouimba et al. 2000; Foy et al. 2008). Enhanced LTD in aged rats is thought to play a role in age-related memory decline (Norris et al. 1996; Foster 1999), and E2 is an important trophic factor that protects against such decline (Frick 2009). As such, suppression of LTD in aged subjects may be one mechanism through which E2 reduces memory loss during aging.
Neurogenesis
Neurogenesis is another major morphological alteration in the hippocampus influenced by E2 (for reviews, see Galea 2008; Pawluski et al. 2009; Galea et al. 2013). Among gonadally intact rats, proestrus females transiently exhibit more cell proliferation than males, such that higher numbers of bromodeoxyuridine (BrdU)-labeled cells are observed in females 2 d, but not 14 d, after BrdU injection (Tanapat et al. 1999). Cell proliferation is also substantially higher during proestrus than during estrus and diestrus, suggesting that elevated levels of estrogens and/or progestins enhance neurogenesis in females (Tanapat et al. 1999). This notion is supported by the observation that ovariectomy significantly reduces cell proliferation and increases numbers of degenerating pyknotic cells (Tanapat et al. 1999, 2005). Exogenous E2 modulates neurogenesis in the dentate gyrus by regulating cell proliferation, although effects vary based on factors such as dose, sex, age, duration of ovariectomy, and timing of injection relative to BrdU labeling. In general, a single brief exposure to E2 transiently increases cell proliferation in the dentate gyrus of ovariectomized rats (Tanapat et al. 1999, 2005; Banasr et al. 2001; Barha et al. 2009). Interestingly, an injection of progesterone given 48 h after E2 treatment reverses the E2-induced increase in cell proliferation (Tanapat et al. 2005), suggesting that the increased neurogenesis during proestrus results from elevated E2 rather than progesterone. In addition to cell proliferation, a few reports indicate that acute or chronic E2 increases cell survival and decreases pyknotic cells in ovariectomized rats (Tanapat et al. 1999; McClure et al. 2013). Both ERα and ERβ appear to mediate the effects of E2 on hippocampal cell proliferation, as a single systemic injection of ERα or ERβ agonists increased dentate cell proliferation in ovariectomized rats (Mazzucco et al. 2006). Acute treatment with other forms of estrogens, including estradiol benzoate, 17α-estradiol, and estrone, also increases cell proliferation in ovariectomized rats in a dose- and time-dependent manner (Ormerod et al. 2003; Mazzucco et al. 2006; Nagy et al. 2006; Barker and Galea 2008; Barha et al. 2009). These data suggest that the ability to increase cell proliferation in female rats is a general feature of estrogens and not an effect specific to E2.
In contrast to the effects of acute E2 on hippocampal neurogenesis, chronic E2 treatment (1–3 wk) administered via silastic capsules or daily injections has minimal effects on cell proliferation in ovariectomized rats (Perez-Martin et al. 2003; Tanapat et al. 2005; Barker and Galea 2008; McClure et al. 2013). The reasons for this lack of effect remain unclear, but the data indicate that chronically elevated levels of E2 are not conducive to cell proliferation. During the rat estrous cycle, E2 levels are elevated only during proestrus, so such chronically elevated E2 levels are not characteristic of the natural hormonal milieu in females. As such, fundamental changes may occur in the brain during chronic treatment (e.g., downregulation of ERs) that do not occur after acute treatment. On the other end of the hormonal spectrum, long-term ovariectomy decreased hippocampal neurogenesis and prevented acute E2 from increasing cell proliferation (Tanapat et al. 2005), which is consistent with the detrimental effects of long-term ovariectomy on LTP and memory discussed above (Daniel et al. 2006; Smith et al. 2010; Vedder et al. 2014). Collectively, these data suggest that either chronically elevated E2 levels or long-term hormone deprivation may permanently alter the hippocampus to reduce its responsiveness to E2.
Contrary to the beneficial effects of acute estrogens on neurogenesis in female rats, acute estrogen treatment does not increase cell proliferation in male rats. Castration of male rats significantly reduces 30-d cell survival without affecting cell proliferation (Spritzer and Galea 2007). Unlike in ovariectomized rats, neither E2 nor estradiol benzoate increased cell proliferation or cell survival in gonadectomized male rats or mice (Spritzer and Galea 2007; Barker and Galea 2008; Zhang et al. 2010). However, acute or chronic testosterone or DHT increased cell survival in gonadectomized rats in an androgen receptor-dependent manner (Spritzer and Galea 2007; Hamson et al. 2013), indicating that androgens promote hippocampal neurogenesis in male rats by increasing cell survival. Interestingly, progesterone, but not testosterone, increased cell survival in a progesterone receptor-dependent manner in male mice (Zhang et al. 2010), suggesting that new cells in the male mouse hippocampus are more sensitive to progesterone than androgens. Moreover, the effects of progesterone were blocked by inhibitors of ERK and PI3K (Zhang et al. 2010), demonstrating a potentially important role for rapid cell signaling in progesterone-induced neurogenesis in male mice. Together, these findings demonstrate an important role for androgens and progesterone, but not estrogens, in hippocampal neurogenesis in male rodents.
Estrogenic modulation of learning and memory
The effects of E2 on learning and memory in female rats and mice have been discussed at length in numerous comprehensive reviews (Korol 2002; Foster 2005; Daniel 2006, 2013; Sherwin and Henry 2008; Barha and Galea 2010; Bimonte-Nelson et al. 2010; Gibbs 2010; Kim and Casadesus 2010; Choleris et al. 2012; Foster 2012; Frick 2012; Acosta et al. 2013; Chisolm and Juraska 2013; Ervin et al. 2013; Galea et al. 2013; Hogervorst 2013; Luine and Frankfurt 2013; Maki 2013; Bean et al. 2014; Fortress and Frick 2014; Luine 2014; Frankfurt and Luine 2015; Tuscher et al. 2015). Therefore, we direct readers to these resources for a more detailed description of this literature than will be provided here. The sections below will provide a broad overview of the effects of the estrous cycle on memory, as well as the effects of gonadectomy and exogenous E2 treatment on memory in female and male rodents.
Memory and the estrous cycle
If hippocampal dendritic spine density, LTP, and cell proliferation are increased during proestrus relative to other cycle stages, then one might expect forms of learning and memory in which the hippocampus is involved to also be enhanced during proestrus. Some evidence does support this idea. For example, several studies report that spatial memory tested in the Morris water maze or object placement tasks was enhanced during proestrus relative to estrus and/or diestrus in mice and rats (Frick and Berger-Sweeney 2001; Frye et al. 2007; Paris and Frye 2008; Pompili et al. 2010). Consistent with these findings, rats in proestrus are more likely than those in estrus to use a spatial learning strategy (Korol et al. 2004). Rats tested during proestrus also exhibit facilitated eyeblink conditioning (Shors et al. 1998). However, the reported proestrus advantage in spatial tasks is inconsistent with other data in rats showing enhanced spatial reference memory in the Morris water maze during estrus relative to proestrus (Frye 1995; Warren and Juraska 1997; Sutcliffe et al. 2007) or no effect of the cycle on spatial reference memory in the water maze or radial arm maze, spatial working memory in the radial arm maze, or spatial novelty in a T-maze (Berry et al. 1997; Stackman et al. 1997; Conrad et al. 2004; Pompili et al. 2010). In tasks modeling selective attention, rats trained during proestrus failed to show latent inhibition (Arad and Weiner 2008; Quinlan et al. 2010). In object recognition tasks, rats and mice tested in proestrus have been reported to outperform those in diestrus and estrus, but other findings show intact object recognition memory in all phases of the cycle (Walf et al. 2006, 2009; Sutcliffe et al. 2007; Paris and Frye 2008). Conflicting effects of the cycle have also been reported in social recognition tasks, where one study reported intact social recognition in proestrus (Sánchez-Andrade and Kendrick 2011), but not estrus, whereas another found intact social recognition in both stages (Markham and Juraska 2007).
It is important to note that discrepancies among estrous findings may be amplified in this literature because so few studies have examined potential effects of the cycle on learning and memory. Thus, methodological differences between this handful of studies may create the false impression of discrepant findings. For example, water temperature in the Morris water maze has been shown to affect the observance of estrous cycle effects in rats, with proestrous rats outperforming estrus rats in warm (33°C) water and estrus rats outperforming proestrus rats in cold (19°C) water (Rubinow et al. 2004). Moreover, because ovarian hormone levels fluctuate rapidly, assessing learning and memory within a single phase of the cycle can be challenging. This is particularly so during the 24 h of proestrus, in which hormone levels rise throughout the day and peak in the evening. As such, studies in which subjects were tested early on the day of proestrus may find fewer effects of the cycle than those in which subjects were tested late in the day. Estrous cycle differences reported in various tests of learning and memory may not lead to substantial sex differences in learning and memory, but could contribute to increased variability with gonadally intact female groups. For mice, however, a recent meta-analysis indicated that the estrous cycle does not lead to greater variability in females relative to males among traits including learning and memory, attention, neuronal morphology, LTP, hormonal and immune function, and epigenetic processes (Prendergast et al. 2014). Given how few studies have examined the effects of the estrous cycle on various forms of memory, this area is particularly ripe for further investigation. See the section “Practical considerations for working with females” below for additional discussion about the use of cycling females in experimental designs.
Effects of exogenous E2 on memory in females
Because bilateral ovariectomy provides better experimental control of circulating ovarian hormone levels than intact gonads, the vast majority of studies on estrogenic regulation of memory in rodents have been conducted using females that have had their ovaries removed. This approach eliminates the primary source of circulating estrogens and progestins, so it is important to realize that levels of several hormones will drop as a result. Ovariectomy itself impairs some forms of memory including spatial working memory in the radial arm maze, spatial reference memory in the Morris water maze, object recognition memory, and memory in a two-way active avoidance task (Singh et al. 1994; Daniel et al. 1999; Wallace et al. 2006; Gibbs and Johnson 2008; Monteiro et al. 2008). However, other studies report no effect or a memory-enhancing effect of ovariectomy in these tasks (Singh et al. 1994; Daniel et al. 1999; Bimonte-Nelson et al. 2003). In vivo, exogenous E2 can be administered in numerous ways, the most common of which are systemic injection, silastic capsule implantation, slow-release pellet implantation, and intracranial infusion. Injections and infusions are most often used for acute treatments, whereas silastic capsules and pellets are typically used for chronic treatments.
In rats and mice, the effects of exogenous E2 have been tested in a variety of tasks that tap into many forms of memory. In general, exogenous E2 improves memory in adult ovariectomized rats and mice. For example, systemic E2 given acutely or chronically improves spatial working memory in the radial arm maze, the Morris water maze, and alternation or delayed nonmatch to position tasks in the T-maze (O'Neal et al. 1996; Daniel et al. 1997; Fader et al. 1998, 1999; Luine et al. 1998; Bimonte and Denenberg 1999; Gibbs 1999; Daniel and Dohanich 2001; Sandstrom and Williams 2001, 2004; Bowman et al. 2002; Heikkinen et al. 2002; Holmes et al. 2002; Garza-Meilandt et al. 2006; Bohacek and Daniel 2007; Hammond et al. 2009). Nonspatial working memory in the T-maze is also improved by systemic E2 (Wide et al. 2004), as is spatial reference memory in the radial arm and Morris water mazes (Packard and Teather 1997a; Heikkinen et al. 2002; Gresack and Frick 2006), recognition memory for the location and identity of objects (Vaucher et al. 2002; Luine et al. 2003; Walf et al. 2006; Frye et al. 2007; Fernandez et al. 2008; Inagaki et al. 2010; Zhao et al. 2010; Phan et al. 2012; Boulware et al. 2013), social recognition memory (Phan et al. 2012), inhibitory avoidance (Singh et al. 1994; Frye and Rhodes 2002, but see Foster et al. 2003), and trace eyeblink conditioning (Leuner et al. 2004). However, not all studies find that E2 benefits memory in adult females under all conditions, as improvements often depend on methodological variables such as dose (Packard and Teather 1997a; Holmes et al. 2002; Leuner et al. 2004; Wide et al. 2004; Gresack and Frick 2006; Foster 2012; Phan et al. 2012), age at treatment (Savonenko and Markowska 2003; Gresack et al. 2007; Markham and Juraska 2007), duration of treatment (Luine et al. 1998; Markowska and Savonenko 2002), prior E2 priming (Markowska and Savonenko 2002), route of administration (Garza-Meilandt et al. 2006), progesterone co-administration (Chesler and Juraska 2000; Bimonte-Nelson et al. 2006; Harburger et al. 2009), duration of daily handling (Bohacek and Daniel 2007), the cognitive demands of the task (Bimonte and Denenberg 1999), and duration of ovariectomy prior to treatment (Gibbs 2000; Daniel et al. 2006; Vedder et al. 2014). Understanding the extent to which these variables influence the response to E2 has potential translational relevance. For example, findings that long-term ovariectomy substantially reduces the mnemonic response to E2 in rodents support the critical period hypothesis developed to explain why menopausal hormone treatment benefits cognition more in younger menopausal women than in older post-menopausal women (Sherwin 2007). As such, duration of ovariectomy in rodents could be used as an experimental variable to develop treatments that lengthen this critical period in women. For more extensive discussion of these variables, see recent reviews that discuss the influence of estrogens on cognitive aging (Sherwin and Henry 2008; Frick 2009; Conrad and Bimonte-Nelson 2010; Foster 2012; Chisolm and Juraska 2013; Maki 2013; Daniel et al. 2015).
To better understand the molecular mechanisms through which E2 regulates memory formation, numerous investigators have administered acute E2 immediately after training in a variety of tasks. When used with a water-soluble cyclodextrin-encapsulated form of E2 that is metabolized within 24 h (Pitha and Pitha 1985; Pitha et al. 1986), post-training treatments allow both training and testing to occur in the absence of circulating E2, which permits the memory-enhancing effects of E2 to be isolated to the memory consolidation phase of memory processing (Frick et al. 2010). Cyclodextrin encapsulation also allows E2 to be more easily infused into the brain, as E2 must otherwise be dissolved in oil. The memory-enhancing effects of post-training cyclodextrin-encapsulated E2 were first observed in gonadally intact male rats. In this initial work, bilateral intrahippocampal infusion of E2 administered immediately, but not 2 h, after eight spatial Morris water maze training trials improved retention of the platform location 24 h later (Packard et al. 1996). Subsequent work in ovariectomized rats and mice showed that post-training systemic injection or intrahippocampal infusion of cyclodextrin-encapsulated E2 enhanced spatial reference memory consolidation in the Morris water maze (Fig. 4A), spatial memory consolidation in an object location task, and object recognition memory consolidation (Fig. 4B; Packard and Teather 1997a,b; Fernandez et al. 2008; Lewis et al. 2008; Zhao et al. 2010, 2012; Boulware et al. 2013; Fortress et al. 2013; Pereira et al. 2014). Post-training systemic injection of noncyclodextrin-encapsulated E2 or estradiol benzoate also enhanced spatial and object recognition memory in object-based tasks in both rats and mice (Luine et al. 2003; Walf et al. 2006, 2008; Frye et al. 2007; Inagaki et al. 2010). Similar to the original male study (Packard et al. 1996), delayed infusion of E2 1–3 h after training had no effect on memory consolidation in female rodents (Walf et al. 2006; Frye et al. 2007; Fernandez et al. 2008), suggesting that the effects of E2 on memory consolidation occur during a discrete time window after training. The cell-signaling, epigenetic, and receptor mechanisms that may underlie the beneficial effects of E2 on object recognition memory consolidation in ovariectomized females will be discussed further in “Molecular mechanisms underlying E2’s effects on memory consolidation.”
Figure 4.
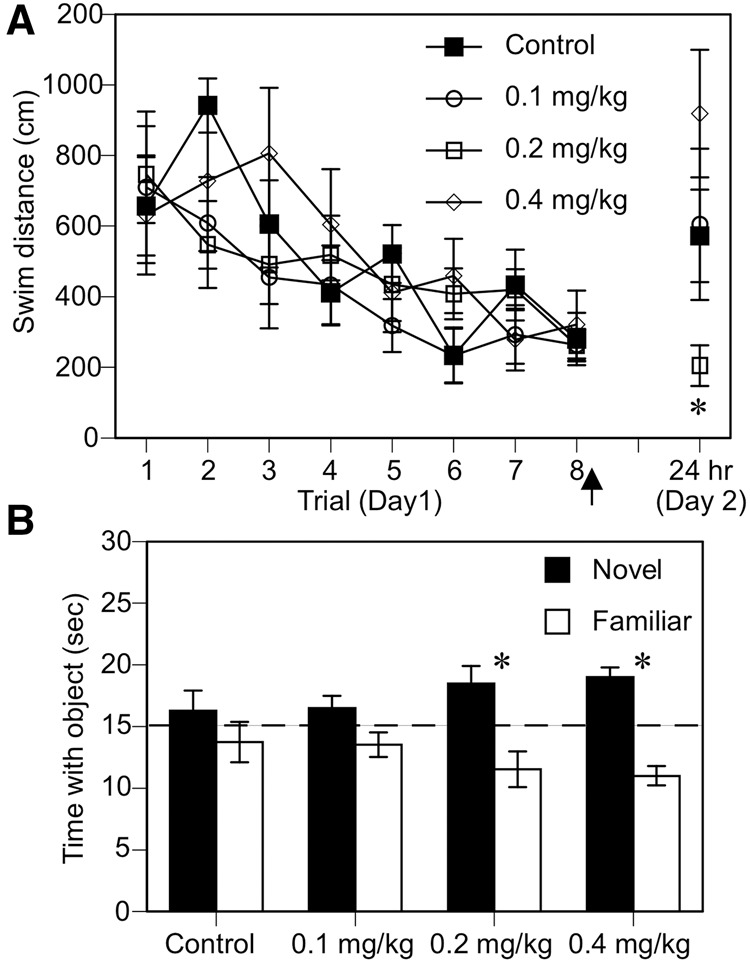
Post-training systemic E2 injection enhances spatial memory consolidation in the Morris water maze and object recognition memory consolidation in ovariectomized mice. (A) Ovariectomized mice received eight hidden-platform training trials prior to E2 administration. Immediately after the final training trial (arrow), mice were injected with cyclodextrin vehicle (Control) or one of three doses (0.1, 0.2, or 0.4 mg/kg) of cyclodextrin-encapsulated E2. When memory for the platform location was tested 24 h later, only mice injected with 0.2 mg/kg E2 remembered the platform location as indicated by the fact that mice in all other groups swam significantly longer distances on day 2 compared with mice in the 0.2 mg/kg group (*P < 0.05). (B) Ovariectomized mice accumulated 30 sec exploring two identical objects and then were immediately injected with cyclodextrin vehicle (Control) or one of three doses (0.1, 0.2, or 0.4 mg/kg) of cyclodextrin-encapsulated E2. During testing 48 h later, mice receiving 0.2 or 0.4 mg/kg E2 spent significantly more time with the novel object than chance (dashed line at 15 sec), indicating that these doses enhanced object recognition memory consolidation (*P < 0.05 relative to chance). In contrast, the control and 0.1 mg/kg groups did not spend more time than chance with the novel object. Error bars in both panels represent the mean ± SEM. (Adapted from Gresack and Frick 2006 with permission from Elsevier © 2015.)
Effects of exogenous E2 on memory in males
Compared with females, far fewer studies have examined the effects of E2 on memory in male rodents. Thus, broad conclusions are more difficult to draw. In general, the bulk of evidence suggests that gonadectomy impairs memory in males tested in the radial arm maze, T-maze, Barnes Maze, and object recognition tasks, as well as several operant tasks that test prefrontal cortex function (Ceccarelli et al. 2001; Kritzer et al. 2001, 2007; Daniel et al. 2003; Sandstrom et al. 2006; Aubele et al. 2008; Gibbs and Johnson 2008; Spritzer et al. 2008; Locklear and Kritzer 2014). However, the deleterious effect of gonadectomy does not extend to all types of memory, particularly spatial reference memory tested in the Morris water maze (Gibbs 2005; Sandstrom et al. 2006; Spritzer et al. 2008).
A handful of studies have administered E2 or testosterone to gonadally intact males. One study found that chronic E2 administered systemically improved spatial reference and working memory in the radial arm maze and T-maze in male mice (Heikkinen et al. 2002). Similarly, acute treatment with estradiol valerate (a synthetic form of E2) improved one-trial passive avoidance in male rats (Vázquez-Pereyra et al. 1995). However, data from the Morris water maze are somewhat inconsistent. As mentioned above, a single post-training intrahippocampal infusion of E2 enhanced 24-h retention of the learned platform position in male rats (Packard et al. 1996). However, another study that administered pretraining intrahippocampal infusions of estradiol valerate or testosterone found no beneficial effect of either hormone (Moradpour et al. 2006). Rather, the data suggested a dose-dependent effect of estradiol and testosterone, with higher doses of each hormone impairing spatial reference memory on certain days of testing, and all other doses having no effect (Moradpour et al. 2006). Numerous factors may have contributed to the discrepant outcomes, including differences in the type of estradiol used (natural versus synthetic), timing of infusion relative to testing (pre- versus post-training), and length of testing (single day versus multiple days).
Among studies of gonadectomized male rats, chronic treatment with systemic E2, but not testosterone, enhanced spatial working memory in the radial arm maze and acquisition of a delayed-match-to-position task in the T-maze (Luine and Rodriguez 1994; Gibbs 2005; Gibbs and Johnson 2008). However, chronic systemic administration of either E2 or testosterone reversed gonadectomy-induced impairments in a different spatial memory task, the Barnes maze (Locklear and Kritzer 2014). The discrepant effects of testosterone in these spatial tasks may result from differential sensitivity of spatial working and reference memory to testosterone in males. E2 can also enhance memory in nonspatial tasks in males. For example, chronic systemic E2 treatment accelerated extinction of conditioned taste aversion in both male and female rats (Yuan and Chambers 1999). In a series of prefrontal-dependent tasks in an operant chamber, systemic E2 administered chronically via pellets was found to enhance response withholding in male rats, but did not reverse gonadectomy-induced deficits in spatial alternation, light–dark discrimination, or progressive ratio responding (Kritzer et al. 2007). In contrast, testosterone tended to reverse these deficits (Kritzer et al. 2007). It should be noted that not all studies have reported a beneficial effect of E2 on memory in castrated males. For example, chronic treatment with testosterone, but not E2, reversed gonadectomy-induced deficits in object recognition (Aubele et al. 2008). Thus, as with spatial memory, some types of nonspatial memory in males may be more sensitive to the effects of E2 or testosterone than others.
Although the effects of E2 and testosterone on memory in male rodents are not always consistent with each other, the studies conducted thus far do show that gonadal hormones regulate learning and memory in males. On balance, most studies find a beneficial effect of E2 and/or testosterone on memory in gonadally intact and gonadectomized male rodents. However, as noted above, some types of memory may be more amenable to modulation by one hormone or the other. Because estradiol is a metabolite of testosterone, it is important to determine if the effects of testosterone on learning and memory are due to testosterone acting on androgen receptors or rather due to its conversion to estradiol or another metabolite. Numerous methods can assist with this determination, including the use of nonaromatizable androgens like DHT, aromatase inhibitors (e.g., letrozole and fadrozole), and androgen or estrogen receptor knockout mice. Although somewhat laborious, isolating the role of androgens and estrogens in mediating memory is important to understanding the mechanisms through which these hormones influence learning and memory in both sexes.
Hippocampally synthesized E2 and memory
Finally, it is important to remember that the gonads are but one source of estrogens in the body. Traditional views on the role of sex steroids in mediating memory have attributed estrogen effects to gonadally derived estrogens. However, the enzymes for synthesizing progestins, androgens, and estrogens are present within the adult male and female rodent hippocampus (Hojo et al. 2004), and aromatase activity in the hippocampus regulates hippocampal E2 synthesis (Kretz et al. 2004). Surprisingly, E2 levels are substantially higher in the hippocampus than in plasma in adult male and female rats (Hojo et al. 2009; Kato et al. 2013). In gonadally intact males, for instance, E2 levels have been reported at 8.4 ± 1.5 nM in the hippocampus and only 0.014 ± 0.003 nM in plasma (Hojo et al. 2009). Castration decreased hippocampal E2 levels by ∼18%, but levels remained quite high at 6.9 ± 0.8 nM (Hojo et al. 2009). Thus, it would appear that gonadally derived E2 makes a fairly minimal contribution to hippocampal E2 levels in males. Interestingly, E2 levels in the hippocampus of male rats were found to be substantially higher than those of gonadally intact female rats, even during proestrus (Kato et al. 2013). During proestrus, hippocampal E2 levels were 4.3 ± 1.0 nM, compared with values of 1.0 nM and lower during other phases of the cycle (Kato et al. 2013). Relative to the nonproestrus phases of the cycle, ovariectomy did not significantly decrease hippocampal E2 levels (Kato et al. 2013), suggesting that the hippocampus continues to synthesize E2 even in the absence of ovaries. Moreover, as in males, hippocampal levels of E2 were substantially higher than in plasma among intact and ovariectomized females (Kato et al. 2013), supporting the notion that the hippocampus is a significant source of E2 in females as well. These findings suggest that the primary endogenous source of E2 for hippocampal neurons in both males and females may be hippocampal neurons or glia (Garcia-Segura et al. 1999; Azcoitia et al. 2003), rather than the gonads.
Emerging data suggest that this hippocampally synthesized E2 may be important for hippocampal synaptic plasticity and learning in males and females. In neonatal hippocampal slice cultures, aromatase inhibition decreased expression of synaptic proteins, dendritic spine density, and presynaptic boutons (Kretz et al. 2004; Prange-Kiel et al. 2006). A recent in vivo study found that systemic injections of the aromatase inhibitor letrozole were associated with impaired LTP and transient dephosphorylation of cofilin in gonadally intact male and female rats, as well as in ovariectomized rats (Vierk et al. 2012). However, the deficits were considerably more striking for females than for males (Vierk et al. 2012), perhaps indicating a greater reliance on hippocampally synthesized E2 for LTP and spine alterations in females. The finding that mature spines, thin spines, and spine synapses were reduced by letrozole in females, whereas only thin spines were reduced by letrozole in males may support this conclusion (Vierk et al. 2012). Given that aromatase inhibition disrupts various aspects of hippocampal function in males and females, one might expect learning and memory to be disrupted as well. Such an effect has been observed in male zebra finches, where hippocampal infusion of the aromatase inhibitor fadrozole impaired spatial memory in a food-finding task (Bailey et al. 2013). Although not specific to the hippocampus, systemic injection of fadrozole either 30 min prior to or immediately after extinction training significantly impaired fear recall during testing in male rats (Graham and Milad 2014). In ovariectomized mice, we recently infused different doses of letrozole into the dorsal hippocampus and our preliminary data indicate that letrozole prevents memory consolidation in both the object recognition and object placement tasks (Tuscher et al. 2013). Collectively, these few studies suggest the intriguing possibility that hippocampal E2 synthesis is necessary for hippocampal memory formation in both males and females.
Molecular mechanisms underlying E2’s effects on memory consolidation
The past few decades of research have revealed a great deal about the molecular mechanisms underlying memory consolidation. Numerous molecules are involved, including neurotransmitter receptors (e.g., NMDA and AMPA), cell-signaling kinases (e.g., ERK, PI3K, PKA, CaMKII, and mTOR), transcription factors, genes, and the enzymes and co-factors that regulate histone acetylation and DNA methylation (e.g., Silva et al. 1992; Guzowski and McGaugh 1997; Atkins et al. 1998; Impey et al. 1998a, b; Schafe et al. 1999; Selcher et al. 1999; Adams and Sweatt 2002; Wood et al. 2005; Horwood et al. 2006; Fischer et al. 2007; Ploski et al. 2008; Guan et al. 2009; Lee and Silva 2009; Sweatt 2009; Day and Sweatt 2010; Hoeffer and Klann 2010; Incontro et al. 2014; Jarome and Helmstetter 2014; Schoch and Abel 2014; Yiu et al. 2014). To examine the roles of these molecules in estrogenic memory modulation, investigators have borrowed a common approach used in neurobiology of learning and memory research in which intracranial infusions of E2 and inhibitor drugs are combined with post-training treatments in one-trial learning tasks. Although this work has barely scratched the surface, quite a bit has already been learned about the molecular underpinnings of estrogenic memory regulation (for recent reviews, see Frick et al. 2010; Frick 2012; Fortress and Frick 2014; Frick 2015). The findings to date will be summarized in this section (see also Fig. 5).
Figure 5.
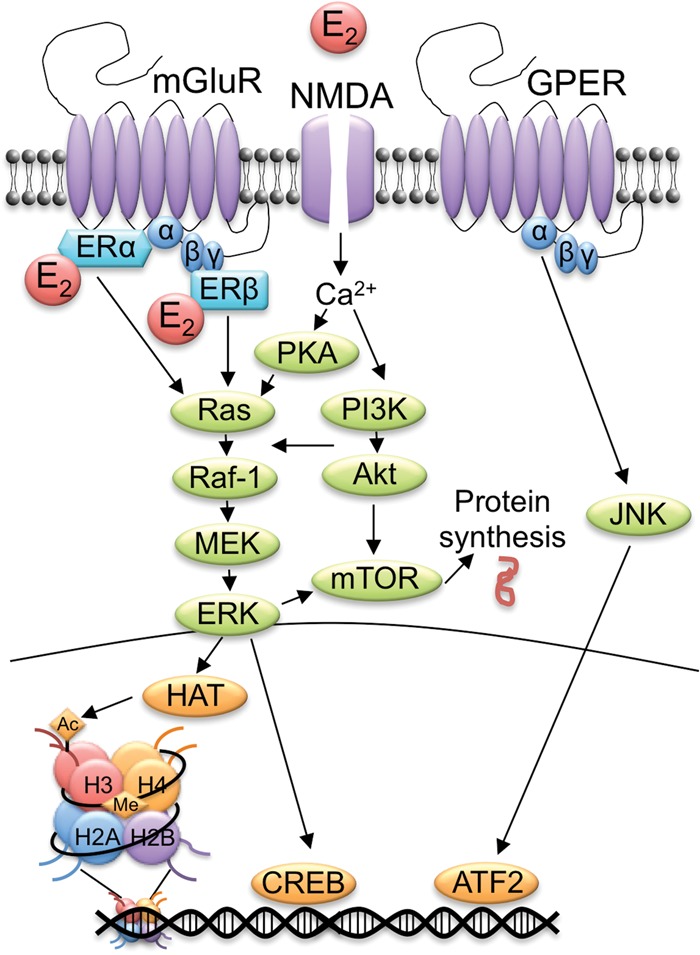
Schematic illustration of the molecular mechanisms required for E2 and ERs to enhance hippocampal memory consolidation. Phosphorylation of the p42 isoform of ERK is necessary for E2 to enhance object recognition memory consolidation. This phosphorylation is triggered by numerous upstream events including interactions between mGluR1a and the canonical ERs (ERα and ERβ), and activation of NMDA receptors, protein kinase A (PKA), and phosphatidylinositol-3-kinase (PI3K). E2-induced phosphorylation of ERK, PI3K, and Akt elicits mTOR signaling, promoting local protein synthesis. E2-activated ERK also transduces into the nucleus to phosphorylate the transcription factor CREB. Activation of ERK and histone acetyltransferases (HAT) is also necessary for E2 to increase histone H3 acetylation (Ac); E2 increases H3 acetylation at the pII and pIV promoters of the Bdnf gene. DNA methylation is also essential for E2 to enhance memory consolidation, although the specific cytosine residues methylated are unknown. Finally, GPER enhances memory consolidation by activating c-Jun N-terminal kinase (JNK), which facilitates gene expression via transcription factors such as ATF2. (Reprinted from Frick 2015 with permission from Elsevier © 2015.)
Cell signaling
As mentioned earlier, E2 can rapidly phosphorylate several cell-signaling cascades, and this phosphorylation is associated with the ability of E2 to increase CA1 dendritic spine density and LTP (Hasegawa et al. 2015). Accordingly, many of these same cascades are necessary for E2 to enhance memory consolidation. In our laboratory's studies on this topic, we infused E2 into the dorsal hippocampus (5 μg/hemisphere) or dorsal third ventricle (10 μg) of ovariectomized mice immediately after training in an object recognition or object placement task. The object placement task is similar to object recognition except that one training object is moved to a new location in the arena during testing rather than being replaced with a novel object. A single post-training infusion of E2 into the either brain region reliably enhances object recognition and object placement memory consolidation in young and middle-aged ovariectomized mice (Fernandez et al. 2008; Fan et al. 2009; Zhao et al. 2010, 2012; Boulware et al. 2013; Fortress et al. 2013, 2014; Pereira et al. 2014). If E2 infusion is delayed until 3 h after training, then no memory enhancement is observed (Fernandez et al. 2008), which is consistent with other studies indicating a specific effect of E2 on memory consolidation within 1–2 h of treatment (Packard et al. 1996; Walf et al. 2006; Frye et al. 2007).
In studies of potential cell-signaling mechanisms involved, the ERK, PI3K/Akt, PKA, and mTOR signaling pathways were of interest based on data showing that E2 rapidly phosphorylates these signaling molecules in a variety of cell types, including hippocampal neurons (Watters et al. 1997; Wade et al. 2001; Wade and Dorsa 2003; Yokomaku et al. 2003; Manella and Brinton 2006). In ovariectomized mice, infusion of E2 increased phosphorylation of p42 ERK, PI3K, Akt, and the mTOR effector proteins p70 ribosomal S6 kinase (S6K) and eukaryotic initiation factor 4E-binding protein 1 (4E-BP1) in the dorsal hippocampus within 5 min of infusion into the dorsal hippocampus or dorsal third ventricle (Fernandez et al. 2008; Fan et al. 2009; Boulware et al. 2013; Fortress et al. 2013). Intrahippocampal infusion of inhibitors of ERK (U0126), PI3K (LY298002), PKA (Rp-cAMP), or mTOR (rapamycin) prevented E2 from phosphorylating these kinases and enhancing object recognition memory consolidation (Fig. 6; Fernandez et al. 2008; Lewis et al. 2008; Fan et al. 2009; Fortress et al. 2013), suggesting that activation of these signaling pathways is critical for E2 to enhance object recognition memory consolidation. A pair of studies showed that E2 first activated PI3K, followed by ERK and then mTOR signaling (Fan et al. 2010; Fortress et al. 2013), which is consistent previous studies showing that PI3K and ERK activate the mTOR pathway (Richter and Klann 2009; Laplante and Sabatini 2012). mTOR signaling is essential for local protein synthesis within neurons (Hoeffer and Klann 2010), and hippocampal infusions of rapamycin impair consolidation of object recognition, contextual fear, and spatial memories (Dash et al. 2006; Parsons et al. 2006; Bekinschtein et al. 2007; Myskiw et al. 2008). As such, the regulation of mTOR signaling suggests a way in which E2 may rapidly regulate protein synthesis and LTP in the hippocampus (Hasegawa et al. 2015).
Figure 6.
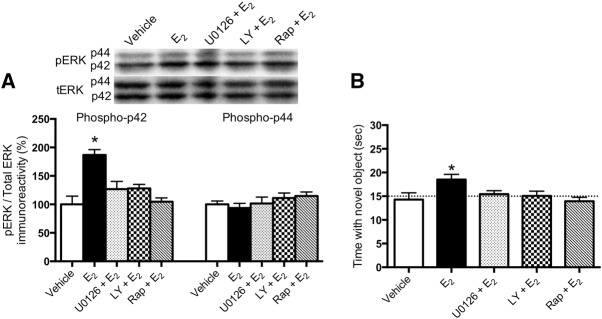
Dorsal hippocampal ERK, PI3K, and mTOR activation are involved in E2-induced enhancement of object recognition memory consolidation in ovariectomized mice. (A) The phosphorylation of p42 ERK was significantly increased in young ovariectomized mice 5 min after bilateral dorsal hippocampal infusion of 5 μg/hemisphere E2 (*P < 0.05 relative to vehicle). This effect was blocked by the ERK inhibitor U0126 (0.5 μg/hemisphere), the PI3K inhibitor LY298002 (0.005 μg/hemisphere), or the mTOR inhibitor rapamycin (0.25 μg/hemisphere). (B) All three inhibitors also prevented E2 from enhancing object recognition memory consolidation, as indicated by the fact that only mice infused with E2 + vehicle spent more time than chance (15 sec) with the novel object (*P < 0.05). Error bars in both panels represent the mean ± SEM. Phosphorylated ERK levels were normalized to total ERK. Insets are representative Western blots of phosphorylated and total protein.
Receptors
Cell-signaling pathways are triggered by activation of plasma membrane receptors, and thus, it is of interest to determine which receptors may stimulate the cell-signaling changes involved in E2-induced memory enhancement. One way in which to investigate the role of plasma receptors is with the use of the membrane-impermeable BSA-E2. Infusion of BSA-E2 into the dorsal hippocampus or dorsal third ventricle significantly increased the phosphorylation of p42 ERK in the dorsal hippocampus within 5 min and mimicked the memory-enhancing effects of E2 (Fernandez et al. 2008). Interestingly, the effects of BSA-E2 on p42 ERK and object recognition were not entirely blocked by the intracellular ER antagonist ICI 182,780 (Fernandez et al. 2008), suggesting that activation of plasma membrane receptors was sufficient to mediate the effects of E2 on memory. But what are these plasma membrane receptors?
This issue remains unresolved, but two studies suggest an important role for glutamate receptors that may interact with ERα and ERβ at the membrane. In one study, dorsal hippocampal infusion of the NMDA receptor antagonist APV prevented systemically injected E2 from enhancing object recognition, indicating a key role for NMDA receptors (Lewis et al. 2008). Nevertheless, it is not yet known how NMDA receptors may interact with E2 or ERs. However, more recent work demonstrates that interactions among ERα, ERβ, and mGluR1a at the membrane are essential for E2 to enhance both object recognition and object placement memory consolidation in ovariectomized mice (Boulware et al. 2013). C57BL/6 mice receiving dorsal hippocampal or dorsal third ventricle infusions of E2, the ERα agonist PPT, or the ERβ agonist DPN (Stauffer et al. 2000; Meyers et al. 2001) immediately after object recognition and object placement training showed enhanced memory in both paradigms (Boulware et al. 2013). Interestingly, intrahippocampal infusion of DPN did not enhance object recognition memory in Swiss mice using the same infusion and testing parameters (Pereira et al. 2014), suggesting potentially important strain differences in the role of specific ERs in modulating memory in mice. In C57BL/6 mice, the ability of E2 to increase p42 ERK and enhance memory was blocked by the mGluR1a antagonist LY367385 (Boulware et al. 2013), demonstrating an important contribution of mGluR1a to the memory-enhancing effects of E2. Moreover, the effects of both agonists on memory was blocked by LY367385 or the ERK inhibitor U0126 (Fig. 7; Boulware et al. 2013), demonstrating that ERα and ERβ can mediate the effects of E2 on ERK signaling and memory consolidation, and indicating an interaction between the ERs and mGluR1a. This interaction was further supported by co-immunoprecipitation experiments showing physical interactions among ERα, ERβ, mGluR1a, and the plasma membrane protein caveolin-1 (Boulware et al. 2013). Although it is not yet clear whether ERα and ERβ are physically located within membrane or just near the membrane, the data demonstrate that interactions between the classical ERs and mGluR1a at the membrane are necessary for E2 to enhance object memory consolidation.
Figure 7.
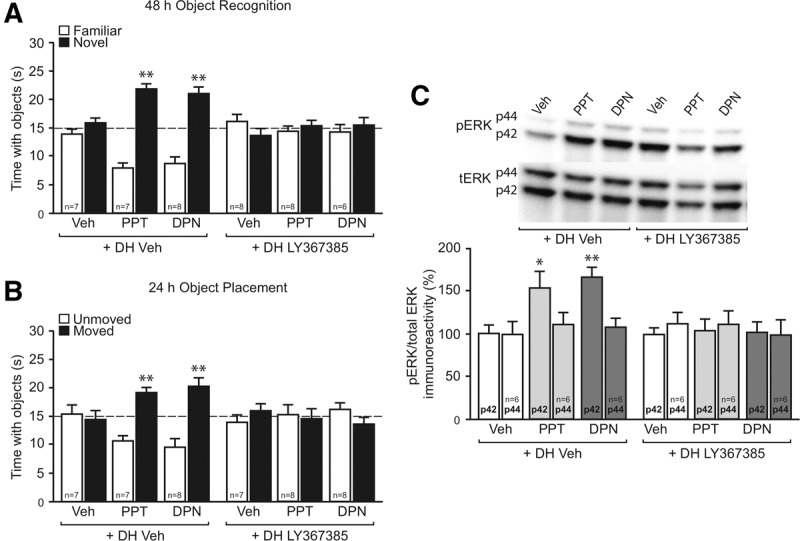
Dorsal hippocampal mGluR1a activation is essential in ovariectomized mice for ERα and ERβ to enhance object recognition and object placement memory consolidation, and to increase dorsal hippocampal ERK phosphorylation. (A,B) Immediate post-training infusion of PPT (0.2 pg) or DPN (20 pg) into the dorsal third ventricle significantly increased the time spent with the novel object (A) and moved object (B) relative to chance (dashed line at 15 sec, **P < 0.01); these effects were blocked by dorsal hippocampal infusion of the mGluR1a antagonist LY367385 (10 pg/hemisphere). Bars represent the mean ± SEM time spent with each object. (C) Five min after dorsal third ventricle infusion, PPT and DPN significantly increased phospho-p42 ERK levels (*P < 0.05; **P < 0.01 relative to vehicle); this effect was completely abolished by dorsal hippocampal infusion of LY367385. Bars represent mean ± SEM % change from vehicle. Phosphorylated ERK levels were normalized to total ERK. Insets are representative Western blots of phosphorylated and total protein. (Reprinted from Boulware et al. 2013).
As discussed earlier, the putative membrane ER called GPER may also mediate the mnemonic effects of E2. This possibility is suggested by studies in ovariectomized rats in which systemic injections of the GPER agonist G-1 agonist enhanced, whereas the GPER antagonist G-15 impaired, spatial working memory (Hammond et al. 2009, 2012). Consistent with these findings, our own preliminary data indicate that dorsal hippocampal infusion of G-1 enhanced, whereas G-15 impaired, object recognition and object placement memory in ovariectomized mice (Kim et al. 2013). Interestingly, however, G-1 did not activate ERK, PI3K, or Akt in the dorsal hippocampus, but instead phosphorylated the p46 and p54 isoforms of c-Jun N-terminal kinase (JNK) (Kim et al. 2013). The JNK inhibitor SP600125 prevented G-1, but not E2, from enhancing object recognition and object placement memory (Kim et al. 2013), suggesting that GPER and E2 may affect memory via different cell-signaling mechanisms. Interestingly, infusion of G-15 into the dorsal hippocampus did not prevent E2 infused into the dorsal third ventricle from enhancing object recognition or object placement memory, indicating that GPER in the dorsal hippocampus may not regulate memory by acting as an ER. This interpretation is supported by a recent study in which a single systemic injection of E2 or G-15 significantly increased, whereas G-1 significantly decreased, hippocampal cell proliferation in ovariectomized female rats (Duarte-Guterman et al. 2015). These findings indicate opposing effects of E2 and GPER activation on hippocampal cell proliferation, and the authors interpret the data to suggest an estrogen-independent role for GPER in the adult hippocampus (Duarte-Guterman et al. 2015). However, this conclusion is complicated by two other recent reports that suggest a role for GPER in the modulatory effects of E2 in the dorsal hippocampus. In one study, repeated systemic administration of G-1 had no effect on either ERK isoform in the dorsal hippocampus of gonadectomized male mice, but increased dorsal hippocampal ERK1/2 activation in gonadectomized female mice (Hart et al. 2014). Another recent study found that bath-applied G-15 prevented E2 from increasing postsynaptic strength and phosphorylation of ERK1/2 in hippocampal slices from ovariectomized mice, suggesting a key role of GPER in mediating the effects of E2 (Kumar et al. 2015). Although these findings contradict the aforementioned data suggesting independent roles of E2 and GPER, it is difficult to directly compare the results because the experimental approaches used in the four studies were considerably different. As such, the nature of the potential interaction between GPER and E2 in the dorsal hippocampus remains unclear, but is a topic of considerable importance for future study.
Finally, it should be noted that the role of ERs in mediating the effects of E2 on memory might differ in males and females. As mentioned earlier, data from hippocampal cultures indicate that the E2-induced regulation of CREB via mGluR1a signaling occurs in neonatal females, but not males (Boulware et al. 2005). Thus, E2-induced memory enhancement in males may not depend on mGluR1-mediated activation of ERK as in females, although this potential sex difference has yet to be tested. Some evidence suggests sex differences in the role of specific ERs in memory. In one study, E2 impaired spatial memory in the Morris water maze among female mice, but not males (Fugger et al. 1998). This E2-induced impairment was not observed in female ERα knockout mice, suggesting a role for ERα in mediating the effects of E2 on spatial memory in females but not males (Fugger et al. 1998). Interestingly, these investigators later reported no sex differences in the role of ERα in hippocampal synaptic responses (Fugger et al. 2001), indicating that other mechanisms likely account for the observed sex difference in spatial memory. More recently, deletion of ERα in mice impaired social recognition memory among females, but not males (Sánchez-Andrade and Kendrick 2011), suggesting sex differences in the role of ERα in multiple forms of memory. No studies to date have examined sex differences in the role of GPER in memory, but one recent study found that systemic administration of G-1 reduced anxiety in gonadectomized male, but not female, mice (Hart et al. 2014). As noted above, this study also found that G-1 increased dorsal hippocampal ERK1/2 activation in females, but not in males (Hart et al. 2014). In models of ischemic or hypoxic brain injury, GPER activation appears to be more beneficial for females than for males (Murata et al. 2013; Broughton et al. 2014). In a particularly dramatic example, G-1 increased neurological deficits and infarct volume after ischemic stroke in gonadally intact male rats, but reduced these parameters in ovariectomized rats (Broughton et al. 2014). Collectively, this work suggests the potential for sex differences in the roles of ERs in mediating memory formation. Thus far, the scant data reported thus far suggests a greater role for ERα and GPER in regulating spatial memory and anxiety, respectively, in female mice than in males.
Epigenetics
Cell-signaling cascades can regulate gene expression in multiple ways, for example, by phosphorylating transcription factors like CREB that promote gene transcription. Cell-signaling pathways can also trigger epigenetic alterations that alter the accessibility of DNA to transcription factors. For example, ERK phosphorylation appears to regulate histone acetylation (Levenson et al. 2004). Over the past decade, it has become clear that epigenetic processes such as histone acetylation and DNA methylation are important regulators of learning and memory mediated by numerous brain regions including the hippocampus (for recent reviews, see Sweatt 2009; Day and Sweatt 2010, 2011; Fischer et al. 2010; Gräff and Tsai 2013; Peixoto and Abel 2013; Jarome and Lubin 2014). Given the importance of ERK activation to estrogenic regulation of memory consolidation, our laboratory conducted a series of studies to examine whether histone acetylation and DNA methylation were involved in the effects of E2 on memory consolidation in ovariectomized mice. Object recognition was used to address this issue because inhibitors of histone deacetylation enhanced memory in this task (Stefanko et al. 2009; Zhao et al. 2010).
DNA is wrapped tightly around a core of four histone proteins (H2A, H2B, H3, and H4) whose bond with DNA can be altered by post-translational modifications including acetylation, phosphorylation, methylation, ubiquitination, and SUMOylation. Histone acetyltransferase (HAT) enzymes add acetyl groups to histones, thereby relaxing the bond between histones and DNA, and allowing transcription factors access to the DNA for gene transcription. Histone deacetylase (HDAC) enzymes remove acetyl groups from histones, thereby tightening the bond between histones and DNA, and decreasing gene transcription. Infusion of the HAT inhibitor garcinol into the dorsal hippocampus of ovariectomized mice blocked object recognition memory consolidation (Zhao et al. 2012), indicating that histone acetylation is necessary for object recognition memory formation. Acetylation of histone H3 in the hippocampus is increased by contextual fear conditioning or ERK activation, the latter of which is necessary for other protein kinases to increase hippocampal H3 acetylation (Levenson et al. 2004). Thus, this work provides a possible link between E2-induced regulation of ERK and H3 acetylation. Indeed, dorsal hippocampal infusion of E2 in young and middle-aged ovariectomized mice selectively increased acetylation of H3 (Fig. 8A), but not H2A, H2B, or H4, in the dorsal hippocampus 30 min later (Zhao et al. 2010, 2012; Fortress et al. 2014). The E2-induced increase in H3 acetylation was dependent on ERK activation, as infusion of the ERK inhibitor U0126 blocked the increase (Fig. 8A; Zhao et al. 2010). These data suggest that E2-induced activation of ERK in the hippocampus mediates acetylation of H3, thereby likely regulating gene expression. Possible gene targets include Bdnf, as indicated by a recent study in which E2 increased H3 acetylation at promoters II and IV of the Bdnf gene in both young and middle-aged ovariectomized mice (Fig. 8D). The regulation of Bdnf gene expression by E2-induced H3 acetylation is supported by data showing that protein levels of BDNF and Pro-BDNF were increased in middle-aged females receiving dorsal hippocampus infusion of E2 (Fortress et al. 2014).
Figure 8.
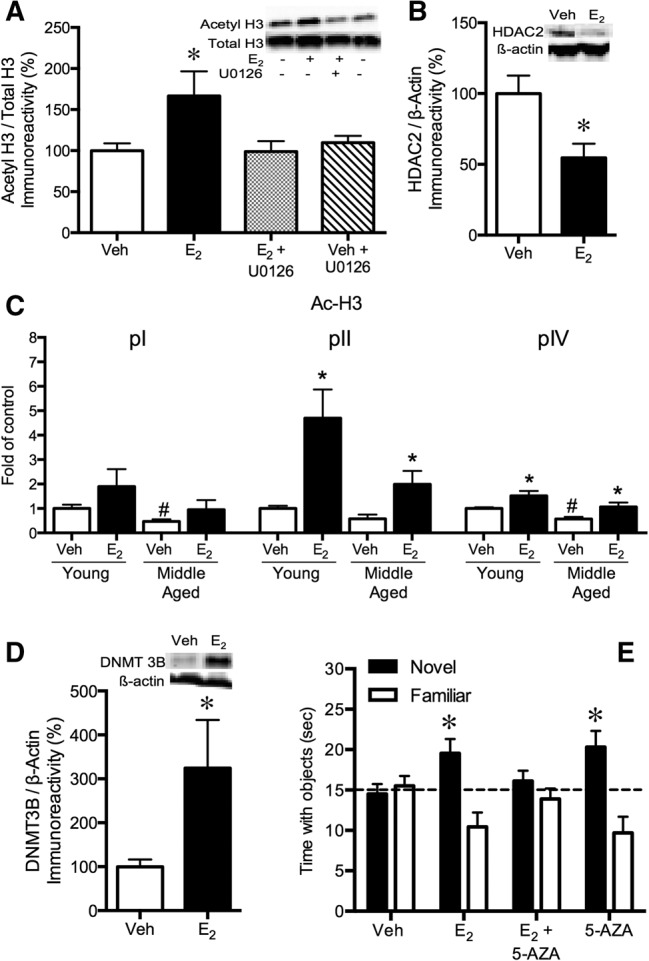
Histone acetylation and DNA methylation are critical for E2 to enhance object recognition memory consolidation in ovariectomized mice. (A) Dorsal hippocampal infusion of 5 μg/hemisphere E2 significantly increased acetyl H3 protein levels in the dorsal hippocampus within 30 min (*P < 0.05 relative to vehicle). This increase was blocked by the ERK activation inhibitor U0126, suggesting that the E2-induced increase in H3 acetylation is dependent on ERK phosphorylation. (Adapted from Zhao et al. 2012.) (B) Dorsal hippocampal infusion of 5 μg/hemisphere E2 significantly decreased HDAC2 protein levels in the dorsal hippocampus within 4 h relative to vehicle (*P < 0.05). (Adapted from Zhao et al. 2012.) (C) Chromatin immunoprecipitation analysis showed that dorsal hippocampal E2 infusion increased acetylation of Bdnf promoters pII and pIV in both young and middle-aged ovariectomized mice (*P < 0.05 relative to age-matched controls). Among vehicle-infused controls, acetylation of pI and pIV was significantly lower in middle-aged females than in young females (#P < 0.05 relative to young vehicle-infused controls), indicating an age-related reduction in acetylation of these promoters. Data were normalized to LINE1 for each sample and then normalized to young vehicle-infused mice for each promoter region and represented as fold of control. (D) Dorsal hippocampal infusion of 5 μg/hemisphere E2 significantly increased DNMT3B protein levels in the dorsal hippocampus of young ovariectomized mice within 4 h relative to vehicle (*P < 0.05). (Adapted from Zhao et al. 2010.) (E) Dorsal hippocampal infusion of 5-AZA prevented E2 from enhancing object recognition memory consolidation. E2 or 5-AZA alone enhanced memory consolidation (*P < 0.05 relative to chance). (Adapted from Zhao et al. 2010.) For all panels, each bar represents the mean ± SEM. Acetyl H3 was normalized to total H3. HDAC2 and DNMT3B were normalized to β-actin. Insets are representative Western blots of phosphorylated and total protein.
E2 also selectively decreased protein levels of HDAC2 (Fig. 8B) and HDAC3, without affecting levels of HDAC1 protein (Zhao et al. 2010, 2012; Fortress et al. 2014). HDAC2 and HDAC3 are potent negative regulators of hippocampal plasticity and memory (Guan et al. 2009; McQuown et al. 2011), so the E2-induced reduction in these proteins supports the notion that E2 regulates memory by altering histone acetylation. Importantly, the HAT inhibitor garcinol prevented E2 from increasing H3 acetylation, decreasing HDAC2 protein, and enhancing object recognition (Fig. 8C) in ovariectomized mice (Zhao et al. 2012), demonstrating an essential role for histone acetylation in estrogenic regulation of object recognition memory consolidation.
Because histone acetylation and DNA methylation are interactive processes (Miller et al. 2008; Zhao et al. 2012), we also examined the role of DNA methylation in estrogenic regulation of memory. DNA methylation involves the addition of methyl groups to cytosine nucleotides located adjacent to guanine nucleotides (CpG islands), which typically serves to silence gene transcription. Three DNA methyltransferase (DNMT) enzymes regulate DNA methylation. DNMT1 is a maintenance methyltransferase that transfers existing methyl marks during DNA replication. DNMT3A and DNMT3B are de novo methyltransferases that add methyl marks to previously unmethylated cytosines. Hippocampal learning selectively increases levels of DNMT3A and DNMT3B (Miller and Sweatt 2007), and dorsal hippocampal infusion of the DNMT inhibitor 5-azacytidine (5-AZA) blocks long-term contextual fear memory (Miller and Sweatt 2007; Miller et al. 2008). Together, these findings indicate an important role for de novo DNA methylation in hippocampal memory formation. Accordingly, dorsal hippocampal infusion of E2 increased mRNA for DNMT3A and DNMT3B in the dorsal hippocampus of young ovariectomized mice within 45 min, whereas DNMT1 mRNA was unchanged up to 180 min after infusion (Zhao et al. 2010). DNMT3B protein levels were also increased 4 h after dorsal hippocampal E2 infusion (Fig. 8D; Zhao et al. 2010), indicating that E2 regulates the expression of this de novo methyltransferase. Moreover, infusion of 5-AZA immediately (Fig. 8E), but not 3 h, after training significantly enhanced memory consolidation (Zhao et al. 2010), suggesting that increasing methylation of certain genes (e.g., memory suppressor genes) enhances object recognition. However, infusion of 5-AZA blocked the memory-enhancing effects of E2 (Fig. 8E; Zhao et al. 2010), indicating that DNA methylation is essential for E2 to facilitate object recognition memory consolidation. At this point, it is not clear which genes are methylated by E2, so additional studies using more sophisticated analyses (e.g., bisulfite sequencing) will be necessary to address this issue.
Practical considerations for working with females
The National Institutes of Health have issued new guidelines for inclusion of more females in biomedical research, which could lead to many more investigators using females in studies of learning and memory. For those investigators considering working with female rodents, there are many practical issues to consider. This final section will discuss briefly some of these issues.
An investigator's first decision is whether to ovariectomize female subjects. Gonadally intact females allow the effects of hormones to be observed in their natural state, which provides a more apt comparison with the vast learning and memory literature on gonadally intact males. However, the use of gonadally intact females is complicated by the estrous cycle, which could influence numerous aspects of learning, memory, and factors such as motivation or sensorimotor abilities that influence performance in learning and memory tasks. One way to address possible estrous-induced fluctuations in learning and memory is to limit testing to one particular phase of the cycle. The fact that each phase lasts ∼24 h would limit the choice of tasks to rapidly learned one-trial learning tasks. This approach also requires regular monitoring of the cycle via a technique such as vaginal lavage (Long and Evans 1922). Alternatively, females could be tested in multi-day tasks (e.g., Morris water maze and radial arm maze) with daily cycle monitoring. Data could then be retrospectively analyzed by cycle phase, but interpretation would be complicated by the fact the same animals would be represented in multiple phase groups and not all subjects would begin and end testing in the same phase. A final option would be to ignore the cycle entirely. As mentioned earlier, effects of the cycle on memory are somewhat subtle and inconsistent, so they may wash out when averaging across multiple females. Thus, ignoring the cycle might be appropriate under certain circumstances. Indeed, it has been argued that the use of females in neuroscience research does not require monitoring of the cycle because females are not intrinsically more variable than males (Prendergast et al. 2014). However, it may be most prudent to first rule out possible effects of the cycle to ensure that effects are not limited to a specific phase.
Experiments using ovariectomized mice typically involve some form of E2, progesterone, or E2 plus progesterone replacement. As noted earlier, there are several options for chronic treatment depending on the scientific question, including silastic capsules, osmotic pumps, or hormone-secreting pellets. Such treatments have been widely used in female rodents and, as illustrated above, generally improve memory (Daniel et al. 1997, 2006; Gibbs 2000; Heikkinen et al. 2004). E2 administered in the drinking water has also been shown to enhance spatial memory in middle-aged female mice (Fernandez and Frick 2004), but dosing is difficult to control. For acute treatments, systemic injections and intracranial infusions are excellent choices that allow for temporal control and observation of rapid effects. As such, these treatments are well suited for studies investigating the molecular and cellular mechanisms underlying hormonal regulation of memory (e.g., Fernandez et al. 2008; Boulware et al. 2013; Fortress et al. 2013; Pereira et al. 2014).
Finally, investigators studying age-related memory decline must keep in mind the impact of reproductive senescence on learning and memory. Female rodents undergo reproductive aging that is similar in many respects (although not identical) to menopause (LeFevre and McClintock 1988; Nelson et al. 1995; Wise 2000). This process is sometimes referred to as estropause. Reproductive function deteriorates gradually in both rats and mice, with the process starting at 9–12 mo of age in female rats and 13–14 mo of age in female mice (Nelson et al. 1981, 1982, 1995; Finch et al. 1984). With respect to spatial memory tested in the Morris water maze, impairments are evident at an earlier age in females than in males. In rats, spatial memory impairments are observed by 12 mo of age in females but not until 18 mo of age in males (Markowska 1999). In mice, the onset of spatial memory deficits is evident by 17 mo in females and by 25 mo in males (Frick et al. 2000). It is thought that the loss of estrogens due to age-related ovarian failure contributes to the premature spatial memory decline in female rodents, and studies showing that E2 replacement can improve some types of memory in aging females support this idea (for reviews, see Sherwin and Henry 2008; Frick 2009; Conrad and Bimonte-Nelson 2010; Foster 2012; Chisolm and Juraska 2013; Maki 2013; Daniel et al. 2015). As such, investigators wishing to study learning and memory in middle-aged or aged rodents should be aware that females may exhibit a different pattern of age-related memory decline than males.
Conclusions
As we hope this review has illustrated, sex steroid hormones such as E2 are crucial regulators of hippocampal morphology, plasticity, and memory in both male and female rodents. Given that receptors for these hormones are located throughout the brain, these hormones may modulate many types of memory mediated by several brain regions. Because sex steroid hormones affect learning and memory in both male and female rodents, it is important to understand how these hormones may regulate the forms of learning and memory in which your laboratory is interested. With E2 as the example highlighted here, we hope this review has provided sufficient inspiration and guidance for other laboratories that do not traditionally work with hormones to begin examining the mechanisms through which E2 and other hormones mediate memory in males and females.
Acknowledgments
The University of Wisconsin-Milwaukee and R01DA038042 supported the writing of this manuscript. Empirical work from our laboratory described herein was supported by R01AG022525, R03MH065460, the American Federation for Aging Research, a University of Wisconsin-Milwaukee Research Growth Initiative Award, the University of Wisconsin-Milwaukee, and Yale University.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.037267.114.
References
- Abbas AK. 2013. Evidence for constitutive protein synthesis in hippocampal LTP stabilization. Neuroscience 246: 301–311. [DOI] [PubMed] [Google Scholar]
- Acosta JI, Hiroi R, Camp BW, Talboom JS, Bimonte-Nelson HA. 2013. An update on the cognitive impact of clinically-used hormone therapies in the female rat: models, mazes, and mechanisms. Brain Res 1514: 18–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JP, Sweatt JD. 2002. Molecular psychology: roles for the ERK MAP kinase cascade in memory. Annu Rev Pharmacol Toxicol 42: 135–163. [DOI] [PubMed] [Google Scholar]
- Akama KT, Thompson LI, Milner TA, McEwen BS. 2013. Post-synaptic density-95 (PSD-95) binding capacity of G-protein-coupled Receptor 30 (GPR30), an estrogen receptor that can be identified in hippocampal dendritic spines. J Biol Chem 288: 6438–6450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almey A, Cannell E, Bertram K, Filardo E, Milner TA, Brake WG. 2014. Medial prefrontal cortical estradiol rapidly alters memory system bias in female rats: ultrastructural analysis reveals membrane-associated estrogen receptors as potential mediators. Endocrinology 155: 4422–4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arad M, Weiner I. 2008. Fluctuation of latent inhibition along the estrous cycle in the rat: modeling the cyclicity of symptoms in schizophrenic women? Psychoneuroendocrinology 33: 1401–1410. [DOI] [PubMed] [Google Scholar]
- Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. 1998. The MAPK cascade is required for mammalian associative learning. Nat Neurosci 1: 602–609. [DOI] [PubMed] [Google Scholar]
- Aubele T, Kaufman R, Montalmant F, Kritzer MF. 2008. Effects of gonadectomy and hormone replacement on a spontaneous novel object recognition task in adult male rats. Horm Behav 54: 244–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azcoitia I, Sierra A, Veiga S, Garcia-Segura LM. 2003. Aromatase expression by reactive astroglia is neuroprotective. Ann N Y Acad Sci 1007: 298–305. [DOI] [PubMed] [Google Scholar]
- Bailey DJ, Ma C, Soma KK, Saldanha CJ. 2013. Inhibition of hippocampal aromatization impairs spatial memory performance in a male songbird. Endocrinology 154: 4707–4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banasr M, Hery M, Brezun JM, Daszuta A. 2001. Serotonin mediates oestrogen stimulation of cell proliferation in the adult dentate gyrus. Eur J Neurosci 14: 1417–1424. [DOI] [PubMed] [Google Scholar]
- Barha CK, Galea LA. 2010. Influence of different estrogens on neuroplasticity and cognition in the hippocampus. Biochim Biophys Acta 1800: 1056–1067. [DOI] [PubMed] [Google Scholar]
- Barha CK, Lieblich SE, Galea LA. 2009. Different forms of oestrogen rapidly upregulate cell proliferation in the dentate gyrus of adult female rats. J Neuroendocrinol 21: 155–166. [DOI] [PubMed] [Google Scholar]
- Barker JM, Galea LA. 2008. Repeated estradiol administration alters different aspects of neurogenesis and cell death in the hippocampus of female, but not male, rats. Neuroscience 152: 888–902. [DOI] [PubMed] [Google Scholar]
- Barton M. 2012. Position paper: the membrane estrogen receptor GPER—Clues and questions. Steroids 77: 935–942. [DOI] [PubMed] [Google Scholar]
- Baum MJ. 2002. Neuroendocrinology of sexual behavior in the male. In Behavioral endocrinology (ed. Becker JB, Breedlove SM, Crews D, McCarthy MM), pp. 153–203. The MIT Press, Cambridge, MA. [Google Scholar]
- Bean LA, Ianov L, Foster TC. 2014. Estrogen receptors, the hippocampus, and memory. Neuroscientist 20: 534–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Breedlove SM. 2002. Introduction to behavioral endocrinology. In Behavioral endocrinology, 2nd ed (ed. Becker JB, Breedlove SM, Crews D, McCarthy MM), pp. 3–38. The MIT Press, Cambridge, MA. [Google Scholar]
- Bekinschtein P, Katche C, Slipczuk LN, Igaz LM, Cammarota M, Izquierdo I, Medina JH. 2007. mTOR signaling in the hippocampus is necessary for memory formation. Neurobiol Learn Mem 87: 303–307. [DOI] [PubMed] [Google Scholar]
- Berry B, McMahan R, Gallagher M. 1997. Spatial learning and memory at defined points of the estrous cycle: effects on performance of a hippocampal-dependent task. Behav Neurosci 111: 267–274. [DOI] [PubMed] [Google Scholar]
- Bi R, Broutman G, Foy MR, Thompson RF, Baudry M. 2000. The tyrosine kinase and mitogen-activated protein kinase pathways mediate multiple effects of estrogen in hippocampus. Proc Natl Acad Sci 97: 3602–3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bimonte HA, Denenberg VH. 1999. Estradiol facilitates performance as working memory load increases. Psychoneuroendocrinology 24: 161–173. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Singleton RS, Hunter CL, Price KL, Moore AB, Granholm AC. 2003. Ovarian hormones and cognition in the aged female rat: I. Long-term, but not short-term, ovariectomy enhances spatial performance. Behav Neurosci 117: 1395–1406. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Francis KR, Umphlet CD, Granholm AC. 2006. Progesterone reverses the spatial memory enhancements initiated by tonic and cyclic oestrogen therapy in middle-aged ovariectomized female rats. Eur J Neurosci 24: 229–242. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Acosta JI, Talboom JS. 2010. Neuroscientists as cartographers: mapping the crossroads of gonadal hormones, memory and age using animal models. Molecules 15: 6050–6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blurton-Jones M, Tuszynski MH. 2002. Estrogen receptor-β colocalizes extensively with parvalbumin-labeled inhibitory neurons in the cortex, amygdala, basal forebrain, and hippocampal formation of intact and ovariectomized adult rats. J Comp Neurol 452: 276–287. [DOI] [PubMed] [Google Scholar]
- Bohacek J, Daniel JM. 2007. Increased daily handling of ovariectomized rats enhances performance on a radial-maze task and obscures effects of estradiol replacement. Horm Behav 52: 237–243. [DOI] [PubMed] [Google Scholar]
- Bondar G, Kuo J, Hamid N, Micevych P. 2009. Estradiol-induced estrogen receptor-α trafficking. J Neurosci 29: 15323–15330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG. 2005. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J Neurosci 25: 5066–5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware MI, Heisler JD, Frick KM. 2013. The memory-enhancing effects of hippocampal estrogen receptor activation involve metabotropic glutamate receptor signaling. J Neurosci 33: 15184–15194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman RE, Ferguson D, Luine VN. 2002. Effects of chronic restraint stress and estradiol on open field activity, spatial memory, and monoaminergic neurotransmitters in ovariectomized rats. Neuroscience 113: 401–410. [DOI] [PubMed] [Google Scholar]
- Brailoiu E, Dun SL, Brailoiu GC, Mizuo K, Sklar LA, Oprea TI, Prossnitz ER, Dun NJ. 2007. Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J Endocrinol 193: 311–321. [DOI] [PubMed] [Google Scholar]
- Breedlove SM, Hampson E. 2002. Sexual differentiation of the brain and behavior. In Behavioral endocrinology (ed. Becker JB, Breedlove SM, Crews D, McCarthy MM), pp. 75–116. The MIT Press, Cambridge, MA. [Google Scholar]
- Broughton BR, Brait VH, Kim HA, Lee S, Chu HX, Gardiner-Mann CV, Guida E, Evans MA, Miller AA, Arumugam TV, et al. 2014. Sex-dependent effects of G protein-coupled estrogen receptor activity on outcome after ischemic stroke. Stroke 45: 835–841. [DOI] [PubMed] [Google Scholar]
- Carrer HF, Araque A, Buño W. 2003. Estradiol regulates the slow Ca2+-activated K+ current in hippocampal pyramidal neurons. J Neurosci 23: 6338–6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarelli I, Scaramuzzino A, Aloisi AM. 2001. Effects of gonadal hormones and persistent pain on non-spatial working memory in male and female rats. Behav Brain Res 123: 65–76. [DOI] [PubMed] [Google Scholar]
- Chesler EJ, Juraska JM. 2000. Acute administration of estrogen and progesterone impairs the acquisition of the spatial Morris water maze in ovariectomized rats. Horm Behav 38: 234–242. [DOI] [PubMed] [Google Scholar]
- Chisolm NC, Juraska JM. 2013. Factors influencing the cognitive and neural effects of hormone treatment during aging in a rodent model. Brain Res 1514: 40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choleris E, Clipperton-Allen AE, Phan A, Valsecchi P, Kavaliers M. 2012. Estrogenic involvement in social learning, social recognition and pathogen avoidance. Front Neuroendocrinol 33: 140–159. [DOI] [PubMed] [Google Scholar]
- Compagnone NA, Mellon SH. 2000. Neurosteroids: biosynthesis and function of these novel neuromodulators. Front Neuroendocrinol 21: 1–56. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Bimonte-Nelson HA. 2010. Impact of the hypothalamic-pituitary-adrenal/gonadal axes on trajectory of age-related cognitive decline. Prog Brain Res 182: 31–76. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Jackson JL, Wieczorek L, Baran SE, Harman JS, Wright RL, Korol DL. 2004. Acute stress impairs spatial memory in male but not female rats: influence of estrous cycle. Pharmacol Biochem Behav 78: 569–579. [DOI] [PubMed] [Google Scholar]
- Cordoba-Montoya DA, Carrer HF. 1997. Estrogen facilitates induction of longterm potentiation in the hippocampus of awake rats. Brain Res 778: 430–438. [DOI] [PubMed] [Google Scholar]
- Daniel JM. 2006. Effects of oestrogen on cognition: what have we learned from basic research? J Neuroendocrinol 18: 787–795. [DOI] [PubMed] [Google Scholar]
- Daniel JM. 2013. Estrogens, estrogen receptors, and female cognitive aging: the impact of timing. Horm Behav 63: 231–237. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Dohanich GP. 2001. Acetylcholine mediates the estrogen-induced increase in NMDA receptor binding in CA1 of the hippocampus and the associated improvement in working memory. J Neurosci 21: 6949–6956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel JM, Fader AJ, Spencer AL, Dohanich GP. 1997. Estrogen enhances performance of female rats during acquisition of a radial arm maze. Horm Behav 32: 217–225. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Roberts SL, Dohanich GP. 1999. Effects of ovarian hormones and environment on radial maze and water maze performance of female rats. Physiol Behav 66: 11–20. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Winsauer PJ, Moerschbaecher JM. 2003. Castration in rats impairs performance during acquisition of a working memory task and exacerbates deficits in working memory produced by scopolamine and mecamylamine. Psychopharmacology (Berl) 170: 294–300. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Hulst JL, Berbling JL. 2006. Estradiol replacement enhances working memory in middle-aged rats when initiated immediately after ovariectomy but not after a long-term period of ovarian hormone deprivation. Endocrinology 147: 607–614. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Witty CF, Rodgers SP. 2015. Long-term consequences of estrogens administered in midlife on female cognitive aging. Horm Behav 10.1016/j.yhbeh.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash PK, Orsi SA, Moore AN. 2006. Spatial memory formation and memory-enhancing effect of glucose involves activation of the tuberous sclerosis complex-mammalian target of rapamycin pathway. J Neurosci 26: 8048–8056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day M, Good M. 2005. Ovariectomy-induced disruption of long-term synaptic depression in the hippocampal CA1 region in vivo is attenuated with chronic estrogen replacement. Neurobiol Learn Mem 83: 13–21. [DOI] [PubMed] [Google Scholar]
- Day JJ, Sweatt JD. 2010. DNA methylation and memory formation. Nat Neurosci 11: 1319–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JJ, Sweatt JD. 2011. Cognitive neuroepigenetics: a role for epigenetic mechanisms in learning and memory. Neurobiol Learn Mem 96: 2–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmond NL, Zhang DX, Levy WB. 2000. Estradiol enhances the induction of homosynaptic long-term depression in the CA1 region of the adult, ovariectomized rat. Neurobiol Learn Mem 73: 180–187. [DOI] [PubMed] [Google Scholar]
- Duarte-Guterman P, Lieblich SE, Chow C, Galea LA. 2015. Estradiol and GPER activation differentially affect cell proliferation but not GPER expression in the hippocampus of adult female rats. PLoS One 10: e0129880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ervin KS, Phan A, Gabor CS, Choleris E. 2013. Rapid oestrogenic regulation of social and nonsocial learning. J Neuroendocrinol 25: 1116–1132. [DOI] [PubMed] [Google Scholar]
- Fader AJ, Hendricson AW, Dohanich GP. 1998. Estrogen improves performance of reinforced T-maze alternation and prevents the amnestic effects of scopolamine administered systemically or intrahippocampally. Neurobiol Learn Mem 69: 225–240. [DOI] [PubMed] [Google Scholar]
- Fader AJ, Johnson PE, Dohanich GP. 1999. Estrogen improves working but not reference memory and prevents amnestic effects of scopolamine of a radial-arm maze. Pharmacol Biochem Behav 62: 711–717. [DOI] [PubMed] [Google Scholar]
- Fan F, Zou Y, Ma A, Yue Y, Mao W, Ma X. 2009. Hormonal changes and somatopsychologic manifestations in the first trimester of pregnancy and post partum. Int J Gynaecol Obstet 105: 46–49. [DOI] [PubMed] [Google Scholar]
- Fan L, Zhao Z, Orr PT, Chambers CH, Lewis MC, Frick KM. 2010. Estradiol-induced object memory consolidation in middle-aged female mice requires dorsal hippocampal extracellular signal-regulated kinase and phosphatidylinositol 3-kinase activation. J Neurosci 30: 4390–4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez SM, Frick KM. 2004. Chronic oral estrogen affects memory and neurochemistry in middle-aged female mice. Behav Neurosci 118: 1340–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez SM, Lewis MC, Pechenino AS, Harburger LL, Orr PT, Gresack JE, Schafe GE, Frick KM. 2008. Estradiol-induced enhancement of object memory consolidation involves hippocampal extracellular signal-regulated kinase activation and membrane-bound estrogen receptors. J Neurosci 28: 8660–8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filardo EJ, Quinn JA, Bland KI, Frackelton ARJ. 2000. Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol 14: 1649–1660. [DOI] [PubMed] [Google Scholar]
- Finch CE, Felicio LS, Mobbs CV, Nelson JF. 1984. Ovarian and steroidal influences on neuroendocrine aging processes in female rodents. Endocr Rev 5: 467–497. [DOI] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Wang X, Dobbin M, Tsai LH. 2007. Recovery of learning and memory is associated with chromatin remodelling. Nature 447: 178–182. [DOI] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Mungenast A, Tsai LH. 2010. Targeting the correct HDAC(s) to treat cognitive disorders. Trends Pharmacol Sci 31: 605–617. [DOI] [PubMed] [Google Scholar]
- Fortress AM, Frick KM. 2014. Epigenetic regulation of estrogen-dependent memory. Front Neuroendocrinol 35: 530–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortress AM, Fan L, Orr PT, Zhao Z, Frick KM. 2013. Estradiol-induced object recognition memory consolidation is dependent on activation of mTOR signaling in the dorsal hippocampus. Learn Mem 20: 147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortress AM, Kim J, Poole RL, Gould TJ, Frick KM. 2014. 17β-Estradiol regulates histone alterations associated with memory consolidation and increases Bdnf promoter acetylation in middle-aged female mice. Learn Mem 21: 457–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TC. 1999. Involvement of hippocampal synaptic plasticity in age-related memory decline. Brain Res Brain Res Rev 30: 236–249. [DOI] [PubMed] [Google Scholar]
- Foster TC. 2005. Interaction of rapid signal transduction cascades and gene expression in mediating estrogen effects on memory over the life span. Front Neuroendocrinol 26: 51–64. [DOI] [PubMed] [Google Scholar]
- Foster TC. 2012. Role of estrogen receptor α and β expression and signaling on cognitive function during aging. Hippocampus 22: 656–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TC, Sharrow KM, Kumar A, Masse J. 2003. Interaction of age and chronic estradiol replacement on memory and markers of brain aging. Neurobiol Aging 24: 839–852. [DOI] [PubMed] [Google Scholar]
- Foy MR. 2001. 17β-estradiol: effect on CA1 hippocampal synaptic plasticity. Neurobiol Learn Mem 76: 239–252. [DOI] [PubMed] [Google Scholar]
- Foy MR, Xu J, Xie X, Brinton RD, Thompson RF, Berger TW. 1999. 17β-estradiol enhances NMDA receptor-mediated EPSPs and long-term potentiation. J Neurophysiol 81: 925–929. [DOI] [PubMed] [Google Scholar]
- Foy MR, Foy JG, Baudry M, Thompson RF. 2008. 17β-estradiol modifies stress-induced and age-related changes in hippocampal synaptic plasticity. Behav Neurosci 122: 301–309. [DOI] [PubMed] [Google Scholar]
- Frankfurt M, Luine V. 2015. The evolving role of dendritic spines and memory: interaction(s) with estradiol. Horm Behav 10.1016/j.yhbeh.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM. 2009. Estrogens and age-related memory decline in rodents: what have we learned and where do we go from here? Horm Behav 55: 2–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM. 2012. Building a better hormone therapy?: How understanding the rapid effects of sex steroid hormones could lead to new therapeutics for age-related memory decline. Behav Neurosci 126: 29–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM. 2015. Molecular mechanisms underlying the memory-enhancing effects of estradiol. Horm Behav 10.1016/j.yhbeh.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Berger-Sweeney J. 2001. Spatial reference memory and neocortical neurochemistry vary with the estrous cycle in C57BL/6 mice. Behav Neurosci 115: 229–237. [DOI] [PubMed] [Google Scholar]
- Frick KM, Burlingame LA, Arters JA, Berger-Sweeney J. 2000. Reference memory, anxiety and estrous cyclicity in C57BL/6NIA mice are affected by age and sex. Neuroscience 95: 293–307. [DOI] [PubMed] [Google Scholar]
- Frick KM, Fernandez SM, Bennett JC, Prange-Kiel J, MacLusky NJ, Leranth C. 2004. Behavioral training interferes with the ability of gonadal hormones to increase CA1 spine synapse density in ovariectomized female rats. Eur J Neurosci 19: 3026–3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Fernandez SM, Harburger LL. 2010. A new approach to understanding the molecular mechanisms through which estrogens affect cognition. Biochim Biophys Acta 1800: 1045–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frotscher M, Léránth C. 1985. Cholinergic innervation of the rat hippocampus as revealed by choline acetyltransferase immunocytochemistry: a combined light and electron microscopic study. J Comp Neurol 239: 237–246. [DOI] [PubMed] [Google Scholar]
- Frye CA. 1995. Estrus-associated decrements in a water maze task are limited to acquisition. Physiol Behav 57: 5–14. [DOI] [PubMed] [Google Scholar]
- Frye CA, Rhodes ME. 2002. Enhancing effects of estrogen on inhibitory avoidance performance may be in part independent of intracellular estrogen receptors in the hippocampus. Brain Res 956: 285–293. [DOI] [PubMed] [Google Scholar]
- Frye CA, Duffy CK, Walf AA. 2007. Estrogens and progestins enhance spatial learning of intact and ovariectomized rats in the object placement task. Neurobiol Learn Mem 88: 208–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fugger HN, Cunningham SG, Rissman EF, Foster TC. 1998. Sex differences in the activational effect of ERα on spatial learning. Horm Behav 34: 163–170. [DOI] [PubMed] [Google Scholar]
- Fugger HN, Kumar A, Lubahn DB, Korach KS, Foster TC. 2001. Examination of estradiol effects on the rapid estradiol mediated increase in hippocampal synaptic transmission in estrogen receptor α knockout mice. Neurosci Lett 309: 207–209. [DOI] [PubMed] [Google Scholar]
- Fukata Y, Fukata M. 2010. Protein palmitoylation in neuronal development and synaptic plasticity. Nat Rev Neurosci 11: 161–175. [DOI] [PubMed] [Google Scholar]
- Funakoshi T, Yanai A, Shinoda K, Kawano MM, Mizukami Y. 2006. G protein-coupled receptor 30 is an estrogen receptor in the plasma membrane. Biochem Biophys Res Commun 346: 904–910. [DOI] [PubMed] [Google Scholar]
- Galea LA. 2008. Gonadal hormone modulation of neurogenesis in the dentate gyrus of adult male and female rodents. Brain Res Rev 57: 332–341. [DOI] [PubMed] [Google Scholar]
- Galea LA, Wainwright SR, Roes MM, Duarte-Guterman P, Chow C, Hamson DK. 2013. Sex, hormones and neurogenesis in the hippocampus: hormonal modulation of neurogenesis and potential functional implications. J Neuroendocrinol 25: 1039–1061. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Wozniak A, Azcoitia I, Rodriguez JR, Hutchison RE, Hutchison JB. 1999. Aromatase expression by astrocytes after brain injury: implications for local estrogen formation in brain repair. Neuroscience 89: 567–578. [DOI] [PubMed] [Google Scholar]
- Garza-Meilandt A, Cantu RE, Claiborne BJ. 2006. Estradiol's effects on learning and neuronal morphology vary with route of administration. Behav Neurosci 120: 905–916. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. 1999. Estrogen replacement enhances acquisition of a spatial memory task and reduces deficits associated with hippocampal muscarinic receptor inhibition. Horm Behav 36: 222–233. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. 2000. Long-term treatment with estrogen and progesterone enhances acquisition of a spatial memory task by ovariectomized aged rats. Neurobiol Aging 21: 107–116. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. 2005. Testosterone and estradiol produce different effects on cognitive performance in male rats. Horm Behav 48: 268–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB. 2010. Estrogen therapy and cognition: a review of the cholinergic hypothesis. Endocr Rev 31: 224–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB, Johnson DA. 2008. Sex-specific effects of gonadectomy and hormone treatment on acquisition of a 12-arm radial maze task by Sprague Dawley rats. Endocrinology 149: 3176–3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB, Nelson D, Hammond R. 2014. Role of GPR30 in mediating estradiol effects on acetylcholine release in the hippocampus. Horm Behav 66: 339–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Frankfurt M, McEwen BS. 1990. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci 10: 1286–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gräff J, Tsai LH. 2013. Histone acetylation: molecular mnemonics on the chromatin. Nat Rev Neurosci 14: 97–111. [DOI] [PubMed] [Google Scholar]
- Graham BM, Milad MR. 2014. Inhibition of estradiol synthesis impairs fear extinction in male rats. Learn Mem 21: 347–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresack JE, Frick KM. 2006. Post-training estrogen enhances spatial and object memory consolidation in female mice. Pharmacol Biochem Behav 84: 112–119. [DOI] [PubMed] [Google Scholar]
- Gresack JE, Kerr KM, Frick KM. 2007. Life-long environmental enrichment differentially affects the mnemonic response to estrogen in young, middle-aged, and aged female mice. Neurobiol Learn Mem 88: 393–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Q, Moss RL. 1996. 17β-estradiol potentiates kainate-induced currents via activation of the cAMP cascade. J Neurosci 16: 3620–3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Q, Korach KS, Moss RL. 1999. Rapid action of 17β-estradiol on kainate-induced currents in hippocampal neurons lacking intracellular estrogen receptors. Endocrinology 140: 660–666. [DOI] [PubMed] [Google Scholar]
- Guan JS, Haggarty SJ, Giacometti E, Dannenberg JH, Joseph N, Gao J, Nieland TJ, Zhou Y, Wang X, Mazitschek R, et al. 2009. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature 459: 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, McGaugh JL. 1997. Antisense oligodeoxynucleotide-mediated disruption of hippocampal cAMP response element binding protein levels impairs consolidation of memory for water maze training. Proc Natl Acad Sci 94: 2693–2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond R, Mauk R, Ninaci D, Nelson D, Gibbs RB. 2009. Chronic treatment with estrogen receptor agonists restores acquisition of a spatial learning task in young ovariectomized rats. Horm Behav 56: 309–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond R, Nelson D, Gibbs RB. 2011. GPR30 co-localizes with cholinergic neurons in the basal forebrain and enhances potassium-stimulated acetylcholine release in the hippocampus. Psychoneuroendocrinology 36: 182–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond R, Nelson D, Kline E, Gibbs RB. 2012. Chronic treatment with a GPR30 antagonist impairs acquisition of a spatial learning task in young female rats. Horm Behav 62: 367–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamson DK, Wainwright SR, Taylor JR, Jones BA, Watson NV, Galea LA. 2013. Androgens increase survival of adult-born neurons in the dentate gyrus by an androgen receptor-dependent mechanism in male rats. Endocrinology 154: 3294–3304. [DOI] [PubMed] [Google Scholar]
- Harburger LL, Saadi A, Frick KM. 2009. Dose-dependent effects of post-training estradiol plus progesterone treatment on object memory consolidation and hippocampal extracellular signal-regulated kinase activation in young ovariectomized mice. Neuroscience 160: 6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart SA, Snyder MA, Smejkalova T, Woolley CS. 2007. Estrogen mobilizes a subset of estrogen receptor-α-immunoreactive vesicles in inhibitory presynaptic boutons in hippocampal CA1. J Neurosci 27: 2102–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart D, Nilges M, Pollard K, Lynn T, Patsos O, Shiel C, Clark SM, Vasudevan N. 2014. Activation of the G-protein coupled receptor 30 (GPR30) has different effects on anxiety in male and female mice. Steroids 81: 49–56. [DOI] [PubMed] [Google Scholar]
- Hasegawa Y, Hojo Y, Kojima H, Ikeda M, Hotta K, Sato R, Ooishi Y, Yoshiya M, Chung BC, Yamazaki T, et al. 2015. Estradiol rapidly modulates synaptic plasticity of hippocampal neurons: involvement of kinase networks. Brain Res 10.1016/j.brainres.2014.12.056. [DOI] [PubMed] [Google Scholar]
- Hatanaka Y, Hojo Y, Mukai H, Murakami G, Komatsuzaki Y, Kim J, Ikeda M, Hiragushi A, Kimoto T, Kawato S. 2014. Rapid increase of spines by dihydrotestosterone and testosterone in hippocampal neurons: dependence on synaptic androgen receptor and kinase networks. Brain Res 10.1016/j.brainres.2014.12.011. [DOI] [PubMed] [Google Scholar]
- Heikkinen T, Puoliväli J, Liu L, Rissanen A, Tanila H. 2002. Effects of ovariectomy and estrogen treatment on learning and hippocampal neurotransmitters in mice. Horm Behav 41: 22–32. [DOI] [PubMed] [Google Scholar]
- Heikkinen T, Puoliväli J, Tanila H. 2004. Effects of long-term ovariectomy and estrogen treatment on maze learning in aged mice. Exp Gerontol 39: 1277–1283. [DOI] [PubMed] [Google Scholar]
- Hoeffer CA, Klann E. 2010. mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci 33: 67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogervorst E. 2013. Effects of gonadal hormones on cognitive behaviour in elderly men and women. J Neuroendocrinol 25: 1182–1195. [DOI] [PubMed] [Google Scholar]
- Hojo Y, Hattori TA, Enami T, Furukawa A, Suzuki K, Ishii HT, Mukai H, Morrison JH, Janssen WG, Kominami S, et al. 2004. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017α and P450 aromatase localized in neurons. Proc Natl Acad Sci 101: 865–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojo Y, Higo S, Ishii H, Ooishi Y, Mukai H, Murakami G, Kominami T, Kimoto T, Honma S, Poirier D, et al. 2009. Comparison between hippocampus-synthesized and circulation-derived sex steroids in the hippocampus. Endocrinology 150: 5106–5112. [DOI] [PubMed] [Google Scholar]
- Holahan MR, Routtenberg A. 2007. Post-translational synaptic protein modification as substrate for long-lasting, remote memory: an initial test. Hippocampus 17: 93–97. [DOI] [PubMed] [Google Scholar]
- Holmes MM, Wide JK, Galea LA. 2002. Low levels of estradiol facilitate, whereas high levels of estradiol impair, working memory performance on the radial arm maze. Behav Neurosci 116: 928–934. [DOI] [PubMed] [Google Scholar]
- Horwood JM, Dufour F, Laroche S, Davis S. 2006. Signalling mechanisms mediated by the phosphoinositide 3-kinase/Akt cascade in synaptic plasticity and memory in the rat. Eur J Neurosci 23: 3375–3384. [DOI] [PubMed] [Google Scholar]
- Huang GZ, Woolley CS. 2012. Estradiol acutely suppresses inhibition in the hippocampus through a sex-specific endocannabinoid and mGluR-dependent mechanism. Neuron 74: 801–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Impey S, Smith DM, Obrietan K, Donahue R, Wade C, Storm DR. 1998a. Stimulation of cAMP response element (CRE)-mediated transcription during contextual learning. Nat Neurosci 1: 595–601. [DOI] [PubMed] [Google Scholar]
- Impey S, Obrietan K, Wong ST, Poser S, Yano S, Wayman G, Deloulme JC, Chan G, Storm DR. 1998b. Cross talk between ERK and PKA is required for Ca2+ stimulation of CREB-dependent transcription and ERK nuclear translocation. Neuron 21: 869–883. [DOI] [PubMed] [Google Scholar]
- Inagaki T, Gautreaux C, Luine V. 2010. Acute estrogen treatment facilitates recognition memory consolidation and alters monoamine levels in memory-related brain areas. Horm Behav 58: 415–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki T, Frankfurt M, Luine V. 2012. Estrogen-induced memory enhancements are blocked by acute bisphenol A in adult female rats: role of dendritic spines. Endocrinology 153: 3357–3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Incontro S, Asensio CS, Edwards RH, Nicoll RA. 2014. Efficient, complete deletion of synaptic proteins using CRISPR. Neuron 83: 1051–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarome TJ, Helmstetter FJ. 2014. Protein degradation and protein synthesis in long-term memory formation. Front Mol Neurosci 7: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarome TJ, Lubin FD. 2014. Epigenetic mechanisms of memory formation and reconsolidation. Neurobiol Learn Mem 115: 116–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelks KB, Wylie R, Floyd CL, McAllister AK, Wise P. 2007. Estradiol targets synaptic proteins to induce glutamatergic synapse formation in cultured hippocampal neurons: critical role of estrogen receptor-α. J Neurosci 27: 6903–6913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato A, Hojo Y, Higo S, Komatsuzaki Y, Murakami G, Yoshino H, Uebayashi M, Kawato S. 2013. Female hippocampal estrogens have a significant correlation with cyclic fluctuation of hippocampal spines. Front Neural Circuits 7: 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MJ, Rønnekleiv OK. 2008. Membrane-initiated estrogen signaling in hypothalamic neurons. Mol Cell Endocrinol 290: 14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Casadesus G. 2010. Estrogen-mediated effects on cognition and synaptic plasticity: what do estrogen receptor knockout models tell us? Biochim Biophys Acta 1800: 1090–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Boulware MI, Frick KM. 2013. Role of G-protein-coupled estrogen receptor (GPER/GPR30) in hippocampal memory and cell signaling in female mice. Soc Neurosci Abstr Poster 376.305. [DOI] [PMC free article] [PubMed]
- Klann E, Sweatt JD. 2008. Altered protein synthesis is a trigger for long-term memory formation. Neurobiol Learn Mem 89: 247–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korol DL. 2002. Enhancing cognitive function across the life span. Ann N Y Acad Sci 959: 167–179. [DOI] [PubMed] [Google Scholar]
- Korol DL, Gold PE. 2007. Modulation of learning and memory by adrenal and ovarian hormones. In Neurobiology of learning and memory, 2nd ed (ed. Kesner RP, Martinez JL), pp. 243–270. Elsevier Science, Burlington, MA. [Google Scholar]
- Korol DL, Malin EL, Borden KA, Busby RA, Couper-Leo J. 2004. Shifts in preferred learning strategy across the estrous cycle in female rats. Horm Behav 45: 330–338. [DOI] [PubMed] [Google Scholar]
- Kramár EA, Lin B, Rex CS, Gall CM, Lynch G. 2006. Integrin-driven actin polymerization consolidates long-term potentiation. Proc Natl Acad Sci 103: 5579–5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramár EA, Chen LY, Brandon NJ, Rex CS, Liu F, Gall CM, Lynch G. 2009. Cytoskeletal changes underlie estrogen's acute effects on synaptic transmission and plasticity. J Neurosci 29: 12982–12993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretz O, Fester L, Wehrenberg U, Zhou L, Brauckmann S, Zhao S, Prange-Kiel J, Naumann T, Jarry H, Frotscher M, et al. 2004. Hippocampal synapses depend on hippocampal estrogen synthesis. J Neurosci 24: 5913–5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritzer MF, McLaughlin PJ, Smirlis T, Robinson JK. 2001. Gonadectomy impairs T-maze acquisition in adult male rats. Horm Behav 39: 167–174. [DOI] [PubMed] [Google Scholar]
- Kritzer MF, Brewer A, Montalmant F, Davenport M, Robinson JK. 2007. Effects of gonadectomy on performance in operant tasks measuring prefrontal cortical function in adult male rats. Horm Behav 51: 183–194. [DOI] [PubMed] [Google Scholar]
- Krucker T, Siggins GR, Halpain S. 2000. Dynamic actin filaments are required for stable long-term potentiation (LTP) in area CA1 of the hippocampus. Proc Natl Acad Sci 97: 6856–6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Foster TC. 2002. 17β-estradiol benzoate decreases the AHP amplitude in CA1 pyramidal neurons. J Neurophysiol 88: 621–626. [DOI] [PubMed] [Google Scholar]
- Kumar A, Bean LA, Rani A, Jackson T, Foster TC. 2015. Contribution of estrogen receptor subtypes, ERα, ERβ, and GPER1 in rapid estradiol-mediated enhancement of hippocampal synaptic transmission in mice. Hippocampus 10.1002/hipo.22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer G, Bader B, Meoli L, Isensee J, Delbeck M, Noppinger PR, Otto C. 2010. A critical review of fundamental controversies in the field of GPR30 research. Steroids 75: 603–610. [DOI] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM. 2012. mTOR signaling in growth control and disease. Cell 149: 274–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Silva AJ. 2009. The molecular and cellular biology of enhanced cognition. Nat Rev Neurosci 10: 126–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeFevre J, McClintock MK. 1988. Reproductive senescence in female rats: a longitudinal study of individual differences in estrous cycles and behavior. Biol Reprod 38: 780–789. [DOI] [PubMed] [Google Scholar]
- Leranth C, Shanabrough M, Horvath TL. 2000. Hormonal regulation of hippocampal spine synapse density involves subcortical mediation. Neuroscience 101: 349–356. [DOI] [PubMed] [Google Scholar]
- Leranth C, Petnehazy O, MacLusky NJ. 2003. Gonadal hormones affect spine synaptic density in the CA1 hippocampal subfield of male rats. J Neurosci 23: 1588–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Mendolia-Loffredo S, Shors TJ. 2004. High levels of estrogen enhance associative memory formation in ovariectomized females. Psychoneuroendocrinology 29: 883–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson JM, O'Riordan KJ, Brown KD, Trinh MA, Molfese DL, Sweatt JD. 2004. Regulation of histone acetylation during memory formation in the hippocampus. J Biol Chem 279: 40545–40559. [DOI] [PubMed] [Google Scholar]
- Levin ER. 1999. Cellular functions of plasma membrane estrogen receptor. Trends Endocrinol Metab 10: 374–377. [DOI] [PubMed] [Google Scholar]
- Levin ER. 2009. G protein-coupled receptor 30: estrogen receptor or collaborator? Endocrinology 150: 1563–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ER. 2011. Minireview: extranuclear steroid receptors: roles in modulation of cell functions. Mol Endocrinol 25: 377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MC, Kerr KM, Orr PT, Frick KM. 2008. Estradiol-induced enhancement of object memory consolidation involves NMDA receptors and protein kinase A in the dorsal hippocampus of female C57BL/6 mice. Behav Neurosci 122: 716–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Brake WG, Romeo RD, Dunlop JC, Gordon M, Buzescu R, Magarinos AM, Allen PB, Greengard P, Luine V, et al. 2004. Estrogen alters hippocampal dendritic spine shape and enhances synaptic protein immunoreactivity and spatial memory in female mice. Proc Natl Acad Sci 101: 2185–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Day M, Muñiz LC, Bitran D, Arias R, Revilla-Sanchez R, Grauer S, Zhang G, Kelley C, Pulito V, et al. 2008. Activation of estrogen receptor-β regulates hippocampal synaptic plasticity and improves memory. Nat Neurosci 11: 334–343. [DOI] [PubMed] [Google Scholar]
- Locklear MN, Kritzer MF. 2014. Assessment of the effects of sex and sex hormones on spatial cognition in adult rats using the Barnes maze. Horm Behav 66: 298–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JA, Evans HM. 1922. The oestrous cycle in the rat and its associated phenomena. Mem Univ Calif 6: 1–148. [Google Scholar]
- Luine VN. 2014. Estradiol and cognitive function: past, present and future. Horm Behav 66: 602–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine V, Frankfurt M. 2013. Interactions between estradiol, BDNF and dendritic spines in promoting memory. Neuroscience 239: 34–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine V, Rodriguez M. 1994. Effects of estradiol on radial arm maze performance of young and aged rats. Behav Neural Biol 62: 230–236. [DOI] [PubMed] [Google Scholar]
- Luine VN, Richards ST, Wu VY, Beck KD. 1998. Estradiol enhances learning and memory in a spatial memory task and effects levels of monoaminergic neurotransmitters. Horm Behav 34: 149–162. [DOI] [PubMed] [Google Scholar]
- Luine VN, Jacome LF, MacLusky NJ. 2003. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology 144: 2836–2844. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Luine VN, Hajszan T, Leranth C. 2005. The 17α and 17β isomers of estradiol both induce rapid spine synapse formation in the CA1 hippocampal subfield of ovariectomized female rats. Endocrinology 146: 287–293. [DOI] [PubMed] [Google Scholar]
- Maggiolini M, Picard D. 2010. The unfolding stories of GPR30, a new membrane-bound estrogen receptor. J Endocrinol 204: 105–114. [DOI] [PubMed] [Google Scholar]
- Maki PM. 2013. Critical window hypothesis of hormone therapy and cognition: a scientific update on clinical studies. Menopause 20: 695–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manella P, Brinton RD. 2006. Estrogen receptor protein interaction with phosphatidylinositol 3-kinase leads to activation of phosphorylated Akt and extracellular signal-regulated kinase 1/2 in the same population of cortical neurons: a unified mechanism of estrogen action. J Neurosci 26: 9437–9447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham JA, Juraska JM. 2007. Social recognition memory: influence of age, sex, and ovarian hormonal status. Physiol Behav 92: 881–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowska AL. 1999. Sex dimorphisms in the rate of age-related decline in spatial memory: relevance to alterations in the estrous cycle. J Neurosci 19: 8122–8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowska AL, Savonenko AV. 2002. Effectiveness of estrogen replacement in restoration of cognitive function after long-term estrogen withdrawal in aging rats. J Neurosci 22: 10985–10995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Murillo R, Villalba RM, Rodrigo J. 1990. Immunocytochemical localization of cholinergic terminals in the region of the nucleus basalis magnocellularis of the rat: a correlated light and electron microscopic study. Neuroscience 36: 361–376. [DOI] [PubMed] [Google Scholar]
- Mazzucco CA, Lieblich SE, Bingham BI, Williamson MA, Viau V, Galea LA. 2006. Both estrogen receptor α and estrogen receptor β agonists enhance cell proliferation in the dentate gyrus of adult female rats. Neuroscience 141: 1793–1800. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Becker JB. 2002. Neuroendocrinology of sexual behavior in the female. In Behavioral endocrinology (ed. Becker JB, Breedlove SM, Crews D, McCarthy MM), pp. 117–151. The MIT Press, Cambridge, MA. [Google Scholar]
- McCarthy MM, Konkle AT. 2005. When is a sex difference not a sex difference? Front Neuroendocrinol 26: 85–102. [DOI] [PubMed] [Google Scholar]
- McClure RE, Barha CK, Galea LA. 2013. 17β-Estradiol, but not estrone, increases the survival and activation of new neurons in the hippocampus in response to spatial memory in adult female rats. Horm Behav 63: 144–157. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Krey LC. 1984. Properties of estrogen sensitive neurons: aromatization, progesterone receptor induction and neuroendocrine effects. In Metabolism of hormonal steroids in the neuroendocrine structures (ed. Celotti F, Naftolin F, Martini L). Raven Press, New York. [Google Scholar]
- McNay EC, Recknagel AK. 2011. Brain insulin signaling: a key component of cognitive processes and a potential basis for cognitive impairment in type 2 diabetes. Neurobiol Learn Mem 96: 432–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuown SC, Barrett RM, Matheos DP, Post RJ, Rogge GA, Alenghat T, Mullican SE, Jones S, Rusche JR, Lazar MA, et al. 2011. HDAC3 is a critical negative regulator of long-term memory formation. J Neurosci 31: 764–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meitzen J, Mermelstein PG. 2011. Estrogen receptors stimulate brain region specific metabotropic glutamate receptors to rapidly initiate signal transduction pathways. J Chem Neuroanat 42: 236–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meitzen J, Luoma JI, Boulware MI, Hedges VL, Peterson BM, Tuomela K, Britson KA, Mermelstein PG. 2013. Palmitoylation of estrogen receptors is essential for neuronal membrane signaling. Endocrinology 154: 4293–4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mermelstein PG, Becker JB, Surmeier DJ. 1996. Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. J Neurosci 16: 595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers MJ, Sun J, Carlson KE, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA. 2001. Estrogen receptor-β potency-selective ligands: structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J Med Chem 44: 4230–4251. [DOI] [PubMed] [Google Scholar]
- Micevych P, Dominguez R. 2009. Membrane estradiol signaling in the brain. Front Neuroendocrinol 30: 315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Sweatt JD. 2007. Covalent modification of DNA regulates memory formation. Neuron 53: 857–869. [DOI] [PubMed] [Google Scholar]
- Miller CA, Campbell SL, Sweatt JD. 2008. DNA methylation and histone acetylation work in concert to regulate memory formation and synaptic plasticity. Neurobiol Learn Mem 89: 599–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE. 2001. Ultrastructural evidence that hippocampal α estrogen receptors are located at extranuclear sites. J Comp Neurol 429: 355–371. [PubMed] [Google Scholar]
- Milner TA, Ayoola K, Drake CT, Herrick SP, Tabori NE, McEwen BS, Warrier S, Alves SE. 2005. Ultrastructural localization of estrogen receptor β immunoreactivity in the rat hippocampal formation. J Comp Neurol 491: 81–95. [DOI] [PubMed] [Google Scholar]
- Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, et al. 2003. Immunolocalization of estrogen receptor β in the mouse brain: comparison with estrogen receptor α. Endocrinology 144: 2055–2067. [DOI] [PubMed] [Google Scholar]
- Mitterling KL, Spencer JL, Dziedzic N, Shenoy S, McCarthy K, Waters EM, McEwen BS, Milner TA. 2010. Cellular and subcellular localization of estrogen and progestin receptor immunoreactivities in the mouse hippocampus. J Comp Neurol 518: 2729–2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro SC, de Mattos CB, Ben J, Netto CA, Wyse AT. 2008. Ovariectomy impairs spatial memory: prevention and reversal by a soy isoflavone diet. Metab Brain Dis 23: 243–253. [DOI] [PubMed] [Google Scholar]
- Moradpour F, Naghdi N, Fathollahi Y. 2006. Anastrozole improved testosterone-induced impairment acquisition of spatial learning and memory in the hippocampal CA1 region in adult male rats. Behav Brain Res 175: 223–232. [DOI] [PubMed] [Google Scholar]
- Mukai H, Tsurugizawa T, Murakami G, Kominami S, Ishii H, Ogiue-Ikeda M, Takata N, Tanabe N, Furukawa A, Hojo Y, et al. 2007. Rapid modulation of long-term depression and spinogenesis via synaptic estrogen receptors in hippocampal principal neurons. J Neurochem 100: 950–967. [DOI] [PubMed] [Google Scholar]
- Murakami G, Tsurugizawa T, Hatanaka Y, Komatsuzaki Y, Tanabe N, Mukai H, Hojo Y, Kominami S, Yamazaki T, Kimoto T, et al. 2006. Comparison between basal and apical dendritic spines in estrogen-induced rapid spinogenesis of CA1 principal neurons in the adult hippocampus. Biochem Biophys Res Commun 351: 553–558. [DOI] [PubMed] [Google Scholar]
- Murakami G, Hojo Y, Ogiue-Ikeda M, Mukai H, Chambon P, Nakajima K, Ooishi Y, Kimoto T, Kawato S. 2014. Estrogen receptor KO mice study on rapid modulation of spines and long-term depression in the hippocampus. Brain Res 10.1016/j.brainres.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Murata T, Dietrich HH, Xiang C, Dacey RG Jr. 2013. G protein-coupled estrogen receptor agonist improves cerebral microvascular function after hypoxia/reoxygenation injury in male and female rats. Stroke 44: 779–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DD, Segal M. 1996. Regulation of dendritic spine density in cultured rat hippocampal neurons by steroid hormones. J Neurosci 16: 4059–4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DD, Cole NB, Greenberger V, Segal M. 1998. Estradiol increases dendritic spine density by reducing GABA neurotransmission in hippocampal neurons. J Neurosci 18: 2550–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myskiw JC, Rossato JI, Bevilaqua LR, Medina JH, Izquierdo I, Cammarota M. 2008. On the participation of mTOR in recognition memory. Neurobiol Learn Mem 89: 338–351. [DOI] [PubMed] [Google Scholar]
- Nabekura J, Oomura Y, Minami T, Mizuno Y, Fukuda A. 1986. Mechanism of the rapid effect of 17 β-estradiol on medial amygdala neurons. Science 233: 226–228. [DOI] [PubMed] [Google Scholar]
- Nagy AI, Ormerod BK, Mazzucco CA, Galea LA. 2006. Estradiol-induced enhancement in cell proliferation is mediated through estrogen receptors in the dentate gyrus of adult female rats. Drug Dev Res 66: 142–149. [Google Scholar]
- Nelson RJ. 2011. An introduction to behavioral endocrinology, 4th ed Sinauer Associates, Inc, Sunderland, MA. [Google Scholar]
- Nelson JF, Felicio LS, Osterburg HH, Finch CE. 1981. Altered profiles of estradiol and progesterone associated with prolonged estrous cycles and persistent vaginal cornification in aging C57BL/6J mice. Biol Reprod 24: 784–794. [DOI] [PubMed] [Google Scholar]
- Nelson JF, Felicio LS, Randall PK, Sims C, Finch CE. 1982. A longitudinal study of estrous cyclicity in aging C57BL/6J mice: I. Cycle frequency, length and vaginal cytology. Biol Reprod 27: 327–339. [DOI] [PubMed] [Google Scholar]
- Nelson JF, Karelus K, Bergman MD, Felicio LS. 1995. Neuroendocrine involvement in aging: evidence from studies of reproductive aging and caloric restriction. Neurobiol Aging 16: 837–843. [DOI] [PubMed] [Google Scholar]
- Norris CM, Korol DL, Foster TC. 1996. Increased susceptibility to induction of long-term depression and long-term potentiation reversal during aging. J Neurosci 16: 5382–5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogiue-Ikeda M, Tanabe N, Mukai H, Hojo Y, Murakami G, Tsurugizawa T, Takata N, Kimoto T, Kawato S. 2008. Rapid modulation of synaptic plasticity by estrogens as well as endocrine disrupters in hippocampal neurons. Brain Res Rev 57: 363–375. [DOI] [PubMed] [Google Scholar]
- O'Neal MF, Means LW, Poole MC, Hamm RJ. 1996. Estrogen affects performance of ovariectomized rats in a two-choice water-escape working memory task. Psychoneuroendocrinology 21: 51–65. [DOI] [PubMed] [Google Scholar]
- Ooishi Y, Kawato S, Hojo Y, Hatanaka Y, Higo S, Murakami G, Komatsuzaki Y, Ogiue-Ikeda M, Kimoto T, Mukai H. 2012. Modulation of synaptic plasticity in the hippocampus by hippocampus-derived estrogen and androgen. J Steroid Biochem Mol Biol 131: 37–51. [DOI] [PubMed] [Google Scholar]
- Ormerod BK, Lee TT, Galea LA. 2003. Estradiol initially enhances but subsequently suppresses (via adrenal steroids) granule cell proliferation in the dentate gyrus of adult female rats. J Neurobiol 55: 247–260. [DOI] [PubMed] [Google Scholar]
- Osterlund MK, Keller E, Hurd YL. 2000. The human forebrain has discrete estrogen receptor α messenger RNA expression: high levels in the amygdaloid complex. Neuroscience 95: 333–342. [DOI] [PubMed] [Google Scholar]
- Packard MG. 1998. Posttraining estrogen and memory modulation. Horm Behav 34: 126–139. [DOI] [PubMed] [Google Scholar]
- Packard MG, Teather LA. 1997a. Posttraining estradiol injections enhance memory in ovariectomized rats: cholinergic blockade and synergism. Neurobiol Learn Mem 68: 172–188. [DOI] [PubMed] [Google Scholar]
- Packard MG, Teather LA. 1997b. Intra-hippocampal estradiol infusion enhances memory in ovariectomized rats. NeuroReport 8: 3009–3013. [DOI] [PubMed] [Google Scholar]
- Packard MG, Kohlmaier JR, Alexander GM. 1996. Posttraining intrahippocampal estradiol injections enhance spatial memory in male rats: interaction with cholinergic systems. Behav Neurosci 110: 626–632. [DOI] [PubMed] [Google Scholar]
- Paris JJ, Frye CA. 2008. Estrous cycle, pregnancy, and parity enhance performance of rats in object recognition or object placement tasks. Reproduction 136: 105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons RG, Gafford GM, Helmstetter FJ. 2006. Translational control via the mammalian target of rapamycin pathway is critical for the formation and stability of long-term fear memory in amygdala neurons. J Neurosci 26: 12977–12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawluski JL, Brummelte S, Barha CK, Crozier TM, Galea LA. 2009. Effects of steroid hormones on neurogenesis in the hippocampus of the adult female rodent during the estrous cycle, pregnancy, lactation and aging. Front Neuroendocrinol 30: 343–357. [DOI] [PubMed] [Google Scholar]
- Peixoto L, Abel T. 2013. The role of histone acetylation in memory formation and cognitive impairments. Neuropsychopharmacology 38: 62–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira LM, Bastos CP, de Souza JM, Ribeiro FM, Pereira GS. 2014. Estradiol enhances object recognition memory in Swiss female mice by activating hippocampal estrogen receptor α. Neurobiol Learn Mem 114: 1–9. [DOI] [PubMed] [Google Scholar]
- Perez-Martin M, Azcoitia I, Trejo JL, Sierra A, Garcia-Segura LM. 2003. An antagonist of estrogen receptors blocks the induction of adult neurogenesis by insulin-like growth factor-I in the dentate gyrus of adult female rat. Eur J Neurosci 18: 923–930. [DOI] [PubMed] [Google Scholar]
- Phan A, Lancaster KE, Armstrong JN, MacLusky NJ, Choleris E. 2011. Rapid effects of estrogen receptor α and β selective agonists on learning and dendritic spines in female mice. Endocrinology 152: 1492–1502. [DOI] [PubMed] [Google Scholar]
- Phan A, Gabor CS, Favaro KJ, Kaschack S, Armstrong JN, MacLusky NJ, Choleris E. 2012. Low doses of 17β-estradiol rapidly improve learning and increase hippocampal dendritic spines. Neuropsychopharmacology 37: 2299–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitha J, Pitha J. 1985. Amorphous water-soluble derivatives of cyclodextrins: nontoxic dissolution enhancing excipients. J Pharm Sci 74: 987–990. [DOI] [PubMed] [Google Scholar]
- Pitha J, Harman SM, Michel ME. 1986. Hydrophilic cyclodextrin derivatives enable effective oral administration of steroidal hormones. J Pharm Sci 75: 165–167. [DOI] [PubMed] [Google Scholar]
- Ploski JE, Pierre VJ, Smucny J, Park K, Monsey MS, Overeem KA, Schafe GE. 2008. The activity-regulated cytoskeletal-associated protein (Arc/Arg3.1) is required for memory consolidation of Pavlovian fear conditioning in the lateral amygdala. J Neurosci 28: 12383–12395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompili A, Tomaz C, Arnone B, Tavares MC, Gasbarri A. 2010. Working and reference memory across the estrous cycle of rat: a long-term study in gonadally intact females. Behav Brain Res 213: 10–18. [DOI] [PubMed] [Google Scholar]
- Prange-Kiel J, Fester L, Zhou L, Lauke H, Carrétero J, Rune GM. 2006. Inhibition of hippocampal estrogen synthesis causes region-specific downregulation of synaptic protein expression in hippocampal neurons. Hippocampus 16: 464–471. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ, Onishi KG, Zucker I. 2014. Female mice liberated for inclusion in neuroscience and biomedical research. Neurosci Biobehav Rev 40: 1–5. [DOI] [PubMed] [Google Scholar]
- Prossnitz ER, Arterburn JB, Sklar LA. 2007. GPR30: a G protein-coupled receptor for estrogen. Mol Cell Endocrinol 265–266: 138–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan MG, Duncan A, Loiselle C, Graffe N, Brake WG. 2010. Latent inhibition is affected by phase of estrous cycle in female rats. Brain Cogn 74: 244–248. [DOI] [PubMed] [Google Scholar]
- Richter JD, Klann E. 2009. Making synaptic plasticity and memory last: mechanisms of translational regulation. Genes Dev 23: 1–11. [DOI] [PubMed] [Google Scholar]
- Romeo RD, McCarthy JB, Wang A, Milner TA, McEwen BS. 2005. Sex differences in hippocampal estradiol-induced N-methyl-D-aspartic acid binding and ultrastructural localization of estrogen receptor-α. Neuroendocrinology 81: 391–399. [DOI] [PubMed] [Google Scholar]
- Roozendaal B. 2000. Glucocorticoids and the regulation of memory consolidation. Psychoneuroendocrinology 25: 213–238. [DOI] [PubMed] [Google Scholar]
- Routtenberg A. 2008. The substrate for long-lasting memory: if not protein synthesis, then what? Neurobiol Learn Mem 89: 225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinow MJ, Arseneau LM, Beverly JL, Juraska JM. 2004. Effect of the estrous cycle on water maze acquisition depends on the temperature of the water. Behav Neurosci 118: 863–868. [DOI] [PubMed] [Google Scholar]
- Sánchez-Andrade G, Kendrick KM. 2011. Roles of α- and β-estrogen receptors in mouse social recognition memory: effects of gender and the estrous cycle. Horm Behav 59: 114–122. [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Williams CL. 2001. Memory retention is modulated by acute estradiol and progesterone replacement. Behav Neurosci 115: 384–393. [PubMed] [Google Scholar]
- Sandstrom NJ, Williams CL. 2004. Spatial memory retention is enhanced by acute and continuous estradiol replacement. Horm Behav 45: 128–135. [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Kim JH, Wasserman MA. 2006. Testosterone modulates performance on a spatial working memory task in male rats. Horm Behav 50: 18–26. [DOI] [PubMed] [Google Scholar]
- Savonenko AV, Markowska AL. 2003. The cognitive effects of ovariectomy and estrogen replacement are modulated by aging. Neuroscience 119: 821–830. [DOI] [PubMed] [Google Scholar]
- Schafe GE, Nadel NV, Sullivan GM, Harris A, LeDoux JE. 1999. Memory consolidation for contextual and auditory fear conditioning is dependent on protein synthesis, PKA, and MAP kinase. Learn Mem 6: 97–110. [PMC free article] [PubMed] [Google Scholar]
- Schoch H, Abel T. 2014. Transcriptional co-repressors and memory storage. Neuropharmacology 80: 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selcher JC, Atkins CM, Trzaskos JM, Paylor R, Sweatt JD. 1999. A necessity for MAP kinase activation in mammalian spatial learning. Learn Mem 6: 478–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharrow KM, Kumar A, Foster TC. 2002. Calcineurin as a potential contributor in estradiol regulation of hippocampal synaptic function. Neuroscience 113: 89–97. [DOI] [PubMed] [Google Scholar]
- Sheldahl LC, Shapiro RA, Bryant DN, Koerner IP, Dorsa DM. 2008. Estrogen induces rapid translocation of estrogen receptor β, but not estrogen receptor α, to the neuronal plasma membrane. Neuroscience 153: 751–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin BB. 2007. The critical period hypothesis: can it explain discrepancies in the oestrogen-cognition literature? J Neuroendocrinol 19: 77–81. [DOI] [PubMed] [Google Scholar]
- Sherwin BB, Henry JF. 2008. Brain aging modulates the neuroprotective effects of estrogen on selective aspects of cognition in women: a critical review. Front Neuroendocrinol 29: 88–113. [DOI] [PubMed] [Google Scholar]
- Shiroma S, Yamaguchi T, Kometani K. 2005. Effects of 17β-estradiol on chemically induced long-term depression. Neuropharmacology 49: 97–102. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Lewczyk C, Pacynski M, Mathew PR, Pickett J. 1998. Stages of estrous mediate the stress-induced impairment of associative learning in the female rat. NeuroReport 9: 419–423. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Chua C, Falduto J. 2001. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J Neurosci 21: 6292–6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shughrue PJ, Merchenthaler I. 2000. Evidence for novel estrogen binding sites in the rat hippocampus. Neuroscience 99: 605–612. [DOI] [PubMed] [Google Scholar]
- Shughrue P, Scrimo P, Lane M, Askew R, Merchenthaler I. 1997a. The distribution of estrogen receptor-ß mRNA in forebrain regions of the estrogen receptor-α knockout mouse. Endocrinology 138: 5649–5652. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. 1997b. Comparative distribution of estrogen receptor-α and -ß mRNA in the rat central nervous system. J Comp Neurol 388: 507–525. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Scrimo PJ, Merchenthaler I. 2000. Estrogen binding and estrogen receptor characterization (ERα and ERβ) in the cholinergic neurons of the rat basal forebrain. Neuroscience 96: 41–49. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Paylor R, Wehner JM, Tonegawa S. 1992. Impaired spatial learning in α-calcium-calmodulin kinase II mutant mice. Science 257: 206–211. [DOI] [PubMed] [Google Scholar]
- Singh M, Meyer EM, Millard WJ, Simpkins JW. 1994. Ovarian steroid deprivation results in a reversible learning impairment and compromised cholinergic function in female Sprague-Dawley rats. Brain Res 644: 305–312. [DOI] [PubMed] [Google Scholar]
- Smejkalova T, Woolley CS. 2010. Estradiol acutely potentiates hippocampal excitatory synaptic transmission through a presynaptic mechanism. J Neurosci 30: 16137–16148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CC, McMahon LL. 2005. Estrogen-induced increase in the magnitude of long-term potentiation occurs only when the ratio of NMDA transmission to AMPA transmission is increased. J Neurosci 25: 7780–7791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CC, McMahon LL. 2006. Estradiol-induced increase in the magnitude of long-term potentiation is prevented by blocking NR2B-containing receptors. J Neurosci 26: 8517–8522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Waterhouse BD, Woodward DJ. 1988. Locally applied estrogens potentiate glutamate-evoked excitation of cerebellar Purkinje cells. Brain Res 475: 272–282. [DOI] [PubMed] [Google Scholar]
- Smith CC, Vedder LC, McMahon LL. 2009. Estradiol and the relationship between dendritic spines, NR2B containing NMDA receptors, and the magnitude of long-term potentiation at hippocampal CA3-CA1 synapses. Psychoneuroendocrinology 34Suppl 1: S130–S142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CC, Vedder LC, Nelson AR, Bredemann TM, McMahon LL. 2010. Duration of estrogen deprivation, not chronological age, prevents estrogen's ability to enhance hippocampal synaptic physiology. Proc Natl Acad Sci 107: 19543–19548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder MA, Cooke BM, Woolley CS. 2011. Estradiol potentiation of NR2B-dependent EPSCs is not due to changes in NR2B protein expression or phosphorylation. Hippocampus 21: 398–408. [DOI] [PubMed] [Google Scholar]
- Spritzer MD, Galea LA. 2007. Testosterone and dihydrotestosterone, but not estradiol, enhance survival of new hippocampal neurons in adult male rats. Dev Neurobiol 67: 1321–1333. [DOI] [PubMed] [Google Scholar]
- Spritzer MD, Gill M, Weinberg A, Galea LA. 2008. Castration differentially affects spatial working and reference memory in male rats. Arch Sex Behav 37: 19–29. [DOI] [PubMed] [Google Scholar]
- Srivastava DP, Woolfrey KM, Jones KA, Shum CY, Lash LL, Swanson GT, Penzes P. 2008. Rapid enhancement of two-step wiring plasticity by estrogen and NMDA receptor activity. Proc Natl Acad Sci 105: 14650–14655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackman RW, Blasberg ME, Langan CJ, Clark AS. 1997. Stability of spatial working memory across the estrous cycle of Long-Evans rats. Neurobiol Learn Mem 67: 167–171. [DOI] [PubMed] [Google Scholar]
- Stauffer SR, Coletta CJ, Tedesco R, Nishiguchi G, Carlson K, Sun J, Katzenellenbogen BS, Katzenellenbogen JA. 2000. Pyrazole ligands: structure-affinity/activity relationships and estrogen receptor-α-selective agonists. J Med Chem 43: 4934–4947. [DOI] [PubMed] [Google Scholar]
- Stefanko DP, Barrett RM, Ly AR, Reolon GK, Wood MA. 2009. Modulation of long-term memory for object recognition via HDAC inhibition. Proc Natl Acad Sci 106: 9447–9452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe JS, Marshall KM, Neill JC. 2007. Influence of gender on working and spatial memory in the novel object recognition task in the rat. Behav Brain Res 177: 117–125. [DOI] [PubMed] [Google Scholar]
- Sweatt JD. 2009. Experience-dependent epigenetic modifications in the central nervous system. Biol Psychiatry 65: 191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabatadze N, Smejkalova T, Woolley CS. 2013. Distribution and posttranslational modification of synaptic ERα in the adult female rat hippocampus. Endocrinology 154: 819–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Sokabe M. 2012. Continuous de novo synthesis of neurosteroids is required for normal synaptic transmission and plasticity in the dentate gyrus of the rat hippocampus. Neuropharmacology 62: 2373–2387. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Sokabe M. 2013. Bidirectional modulatory effect of 17β-estradiol on NMDA receptors via ERα and ERβ in the dentate gyrus of juvenile male rats. Neuropharmacology 75: 262–273. [DOI] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Reeves AJ, Gould E. 1999. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci 19: 5792–5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Gould E. 2005. Ovarian steroids influence cell proliferation in the dentate gyrus of the adult female rat in a dose- and time-dependent manner. J Comp Neurol 481: 252–265. [DOI] [PubMed] [Google Scholar]
- Tang H, Zhang Q, Yang L, Dong Y, Khan M, Yang F, Brann DW, Wang R. 2014. GPR30 mediates estrogen rapid signaling and neuroprotection. Mol Cell Endocrinol 387: 52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasawa E, Ojeda SR. 2009. Neuroendocrine regulation of puberty. In Molecular mechanisms of hormone actions on behavior (ed. Etgen AM, Pfaff DW), pp. 603–680. Academic Press, San Diego, CA. [Google Scholar]
- Teyler TJ, Vardaris RM, Lewis D, Rawitch AB. 1980. Gonadal steroids: effects on excitability of hippocampal pyramidal cells. Science 209: 1017–1019. [DOI] [PubMed] [Google Scholar]
- Thomas P, Pang Y, Filardo EJ, Dong J. 2005. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology 146: 624–632. [DOI] [PubMed] [Google Scholar]
- Tuscher JJ, Szinte JS, Starrett JR, Krentzel AA, Remage-Healey L, Frick KM. 2013. Hippocampally-synthesized estrogens are essential for spatial memory consolidation in female mice. Soc Neurosci Abstr Poster 376.307. [Google Scholar]
- Tuscher JJ, Fortress AM, Kim J, Frick KM. 2015. Regulation of object recognition and object placement by ovarian sex steroid hormones. Behav Brain Res 285: 140–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaucher E, Reymond I, Najaffe R, Kar S, Quirion R, Miller MM, Franklin KBJ. 2002. Estrogen effects on object memory and cholinergic receptors in young and old female mice. Neurobiol Aging 23: 87–95. [DOI] [PubMed] [Google Scholar]
- Vázquez-Pereyra F, Rivas-Arancibia S, Loaeza-Del Castillo A, Schneider-Rivas S. 1995. Modulation of short term and long term memory by steroid sexual hormones. Life Sci 56: PL255–PL260. [DOI] [PubMed] [Google Scholar]
- Vedder LC, Smith CC, Flannigan AE, McMahon LL. 2013. Estradiol-induced increase in novel object recognition requires hippocampal NR2B-containing NMDA receptors. Hippocampus 23: 108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedder LC, Bredemann TM, McMahon LL. 2014. Estradiol replacement extends the window of opportunity for hippocampal function. Neurobiol Aging 35: 2183–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierk R, Glassmeier G, Zhou L, Brandt N, Fester L, Dudzinski D, Wilkars W, Bender RA, Lewerenz M, Gloger S, et al. 2012. Aromatase inhibition abolishes LTP generation in female but not in male mice. J Neurosci 32: 8116–8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vouimba RM, Foy MR, Foy JG, Thompson RF. 2000. 17β-estradiol suppresses expression of long-term depression in aged rats. Brain Res Bull 53: 783–787. [DOI] [PubMed] [Google Scholar]
- Wade CB, Dorsa DM. 2003. Estrogen activation of cyclic adenosine 5′-monophosphate response element-mediated transcription requires the extracellularly regulated kinase/mitogen-activated protein kinase pathway. Endocrinology 144: 832–838. [DOI] [PubMed] [Google Scholar]
- Wade CB, Robinson S, Shapiro RA, Dorsa DM. 2001. Estrogen receptor (ER)α and ERβ exhibit unique pharmacologic properties when coupled to activation of the mitogen-activated protein kinase pathway. Endocrinology 142: 2336–2342. [DOI] [PubMed] [Google Scholar]
- Wainer BH, Steininger TL, Roback JD, Burke-Watson MA, Mufson EJ, Kordower J. 1993. Ascending cholinergic pathways: functional organization and implications for disease models. Prog Brain Res 98: 9–30. [DOI] [PubMed] [Google Scholar]
- Walf AA, Rhodes ME, Frye CA. 2006. Ovarian steroids enhance object recognition in naturally cycling and ovariectomized, hormone-primed rats. Neurobiol Learn Mem 86: 35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Koonce CJ, Frye CA. 2008. Estradiol or diarylpropionitrile administration to wild type, but not estrogen receptor β knockout, mice enhances performance in the object recognition and object placement tasks. Neurobiol Learn Mem 89: 513–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Koonce C, Manley K, Frye CA. 2009. Proestrous compared to diestrous wildtype, but not estrogen receptor β knockout, mice have better performance in the spontaneous alternation and object recognition tasks and reduced anxiety-like behavior in the elevated plus and mirror maze. Behav Brain Res 196: 254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace M, Luine V, Arellanos A, Frankfurt M. 2006. Ovariectomized rats show decreased recognition memory and spine density in the hippocampus and prefrontal cortex. Brain Res 1126: 176–182. [DOI] [PubMed] [Google Scholar]
- Warren SG, Juraska JM. 1997. Spatial and nonspatial learning across the rat estrous cycle. Behav Neurosci 111: 259–266. [DOI] [PubMed] [Google Scholar]
- Warren SG, Humphreys AG, Juraska JM, Greenough WT. 1995. LTP varies across the estrous cycle: enhanced synaptic plasticity in proestrus rats. Brain Res 703: 26–30. [DOI] [PubMed] [Google Scholar]
- Waters EM, Mitterling K, Spencer JL, Mazid S, McEwen BS, Milner TA. 2009. Estrogen receptor α and β specific agonists regulate expression of synaptic proteins in rat hippocampus. Brain Res 1290: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters EM, Yildirim M, Janssen WG, Lou WY, McEwen BS, Morrison JH, Milner TA. 2011a. Estrogen and aging affect the synaptic distribution of estrogen receptor β-immunoreactivity in the CA1 region of female rat hippocampus. Brain Res 1379: 86–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters EM, Yildirim M, Janssen WGM, Lou WYW, McEwen BS, Morrison JH, Milner TA. 2011b. Estrogens and aging affect the synaptic distribution of estrogen receptor β-immunoreactivity in the CA1 region of female rat hippocampus. Brain Res 1379: 86–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters EM, Thompson LI, Patel P, Gonzales AD, Ye HZ, Filardo EJ, Clegg DJ, Gorecka J, Akama KT, McEwen BS, et al. 2015. G-protein-coupled estrogen receptor 1 is anatomically positioned to modulate synaptic plasticity in the mouse hippocampus. J Neurosci 35: 2384–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watters JJ, Campbell JS, Cunningham MJ, Krebs EG, Dorsa DM. 1997. Rapid membrane effects of steroids in neuroblastoma cells: effects of estrogen on mitogen activated protein kinase signalling cascade and c-fos immediate early gene transcription. Endocrinology 138: 4030–4033. [DOI] [PubMed] [Google Scholar]
- Weiland NG. 1992. Estradiol selectively regulates agonist binding sites on the N-methyl-D-aspartate receptor complex in the CA1 region of the hippocampus. Endocrinology 131: 662–668. [DOI] [PubMed] [Google Scholar]
- Wide JK, Hanratty K, Ting J, Galea LA. 2004. High level estradiol impairs and low level estradiol facilitates non-spatial working memory. Behav Brain Res 155: 45–53. [DOI] [PubMed] [Google Scholar]
- Wise PM. 2000. New understanding of the complexity of the menopause and challenges for the future. In: Proceedings of the International Symposium on the Biology of Menopause (ed. Bellino FL), pp. 1–8. Springer, Norwell, MA. [Google Scholar]
- Wood GE, Shors TJ. 1998. Stress facilitates classical conditioning in males, but impairs classical conditioning in females through activational effects of ovarian hormones. Proc Natl Acad Sci 95: 4066–4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood MA, Kaplan MP, Park A, Blanchard EJ, Oliveira AM, Lombardi TL, Abel T. 2005. Transgenic mice expressing a truncated form of CREB-binding protein (CBP) exhibit deficits in hippocampal synaptic plasticity and memory storage. Learn Mem 12: 111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS. 2007. Acute effects of estrogen on neuronal physiology. Annu Rev Pharmacol Toxicol 47: 657–680. [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. 1992. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci 12: 2549–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. 1993. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol 336: 293–306. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Gould E, Frankfurt M, McEwen BS. 1990. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci 10: 4035–4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, Weiland NG, McEwen BS, Schwartzkroin PA. 1997. Estradiol increases the sensitivity of hippocampal CA1 pyramidal cells to NMDA receptor-mediated synaptic input: correlation with dendritic spine density. J Neurosci 17: 1848–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu TW, Chen S, Brinton RD. 2011. Membrane estrogen receptors mediate calcium signaling and MAP kinase activation in individual hippocampal neurons. Brain Res 1379: 34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang LC, Zhang QG, Zhou CF, Yang F, Zhang YD, Wang RM, Brann DW. 2010. Extranuclear estrogen receptors mediate the neuroprotective effects of estrogen in the rat hippocampus. PLoS One 5: e9851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiu AP, Mercaldo V, Yan C, Richards B, Rashid AJ, Hsiang HL, Pressey J, Mahadevan V, Tran MM, Kushner SA, et al. 2014. Neurons are recruited to a memory trace based on relative neuronal excitability immediately before training. Neuron 83: 722–735. [DOI] [PubMed] [Google Scholar]
- Yokomaku D, Numakawa T, Numakawa Y, Suzuki S, Matsumoto T, Adachi N, Nishio C, Taguchi T, Hatanaka H. 2003. Estrogen enhances depolarization-induced glutamate release through activation of phosphatidylinositol 3-kinase and mitogen-activated protein kinase in cultured hippocampal neurons. Mol Endocrinol 17: 831–844. [DOI] [PubMed] [Google Scholar]
- Yuan DL, Chambers KC. 1999. Estradiol accelerates extinction of a conditioned taste aversion in female and male rats. Horm Behav 36: 1–16. [DOI] [PubMed] [Google Scholar]
- Zamani MR, Desmond NL, Levy WB. 2000. Estradiol modulates long-term synaptic depression in female rat hippocampus. J Neurophysiol 84: 1800–1808. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Yang R, Zhou R, Li L, Sokabe M, Chen L. 2010. Progesterone promotes the survival of newborn neurons in the dentate gyrus of adult male mice. Hippocampus 20: 402–412. [DOI] [PubMed] [Google Scholar]
- Zhang QG, Han D, Wang RM, Dong Y, Yang F, Vadlamudi RK, Brann DW. 2011. C terminus of Hsc70-interacting protein (CHIP)-mediated degradation of hippocampal estrogen receptor-α and the critical period hypothesis of estrogen neuroprotection. Proc Natl Acad Sci 108: E617–E624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Fan L, Frick KM. 2010. Epigenetic alterations regulate estradiol-induced enhancement of memory consolidation. Proc Natl Acad Sci 107: 5605–5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Fan L, Fortress AM, Boulware MI, Frick KM. 2012. Hippocampal histone acetylation regulates object recognition and the estradiol-induced enhancement of object recognition. J Neurosci 32: 2344–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Fester L, Haghshenas S, de Vrese X, von Hacht R, Gloger S, Brandt N, Bader M, Vollmer G, Rune GM. 2014. Oestradiol-induced synapse formation in the female hippocampus: roles of oestrogen receptor subtypes. J Neuroendocrinol 26: 439–447. [DOI] [PubMed] [Google Scholar]


