Abstract
Background
In the present study, the phenolic compounds were prepared using ultrasonic-aid extraction from sugar beet molasses (SBM).
Methods
Gallic acid (GA), cyanidin-3-O-glucoside chloride (CGC) and epicatechin (EP) were produced after column chromatography from the extraction, and further detected using NMR, QTOF-MS and ESI-MS/MS.
Results
The three compounds exhibited strong antioxidant activities including DPPH radical scavenging activities, ABTS radical scavenging activities and ORAC values. GA showed the strongest antioxidant activity. Antitumor activities significantly increased in a dose-dependent manner. In particular, the CGC had growth inhibitory activities of 94.86, 87.27 and 67.13 % against the human colon (CACO-2), hepatocellular (HepG2) and breast (MCF-7) carcinoma cell lines, respectively, at the highest concentration of 400 μg/mL of the extracts. These results suggest that the three compounds are key chemical compositions valuable for preparing functional foods in the food industry.
Conclusions
The results suggested that SBM is a natural source of antioxidant and antitumor agents for preparing functional foods.
Keywords: Sugar beet molasses, Phenolic compounds, Antioxidant activities, Antitumor activities
Background
Sugar beet is an important sugar crop cultivated for sugar production [1, 2]. Sugar beet molasses (SBM) is a by-product in sugar beet processing [3]. Early studies found that SBM can be used to produce alcohol and fermentation medium [4, 5]. While recent study reported that SBM showed higher antioxidant activity, anti-inflammatory and anti-proliferative activities [6, 7], the bioactive components of SBM are mainly phenolics, alkaloids, tannins, saponins, terpenoids, steroids, and volatile oil [8], and specific bioactive compounds such as syringic acid, vanillin, ferulic acid, hydroxybenzaldehyde, hydroxybenzoic acid, luteolin/kaempferol, feruloyl-arabinose-arabinose and caffeoyltartaric acid which have been prepared and identified [9].
Phenolic contents have been demonstrated to have a variety of bioactivities such as anti-aging, anti-fatigue, anti-hypoxia, immunological, anti-radiation, anti-inflammatory, anti-proliferative and hypoglycemic effects [10–14]. Meanwhile many investigations revealed that phenolic contents contribute to the antioxidant and antitumor activities of plants [15]. Antioxidants can restrict the deleterious effects of these oxidant reactions and these restrictions can involve scavenging free radicals or preventing radical formation [16]. However, to the best of our knowledge, there were few reports on the antioxidant and antitumor activities of phenolics from sugar beet molasses (SBMP). Given the rich natural resources of SBM, the extraction researches of SBMP will have a highly practical value.
The primary objective of the present study is to separate and purify the phenolic compounds in SBM, identify the structure of prepared phenolic compounds, and study the bioactivity of the prepared phenolic compounds in SBM.
Methods
Materials and reagents
SBM was provided by Xinjiang Green Xiang Sugar Industry Co., Ltd (Tacheng, China). 2,2-diphenyl-1-picryhydrazyl (DPPH), 2,2-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), ascorbic acid (Vc), fluorescein, 2,2′-azobis (2-methylpropionamidine) dihydrochloride (AAPH), trolox [(±)-6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid] and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Dulbecco’s modified eagle medium (DMEM), penicillin-streptomycin were purchased from Gibco Co. (Long Shang Industry Park, Beijing, China). Fetal bovine serum (FBS) was purchased from Zhejiang Tianhang Biological Technology Co. (Zhejiang, China). Methanol (HPLC-grade) was purchased from Merck Co. (Whitehouse Station, NJ, USA). Ultra-pure water was prepared by a Milli-Q system (Millipore, Bedford, USA).
Extraction, isolation and purification
The SBM (2.0 kg) was extracted by 30 mL 70 % (w/v) ethanol using a RK102H ultrasonic (BANDELIN SONOREX, Germany). Extraction conditions were ultrasonic power, 450 W, HCl concentrations 1.6 mol/L, temperature 40 °C and time 60 min. The extract was concentrated at 45 °C in vacuum using a rotary evaporator (RE-52 A, Yarong Co. Ltd., Shanghai, P. R. China) to obtain the total extraction fraction (634.02 g). Then, the total extracted fraction was suspended in distilled water. The resulting solution was successively partitioned with different solvents which yielded petroleum ether fraction, chloroform fraction, ethyl acetate fraction, n-butanol fraction and aqueous fraction. After drying in vacuo, five fractions were obtained. These fractions included the petroleum ether fraction (5.26 g, yield coefficient 0.83 %), chloroform fraction (32.97 g, yield coefficient 5.20 %), ethyl acetate fraction (76.7 g, yield coefficient 12.10 %), n-butanol fraction (58.71 g, yield coefficient 9.26 %), and water fraction (142 g, yield coefficient 22.40 %). In the biological activity screening tests, the ethyl acetate fraction showed stronger antioxidant and antitumor activities than other four fractions. Therefore, the ethyl acetate fraction was chosen for further purification.
The ethyl acetate fraction (70.0 g) was loaded onto a column (3.5 cm × 100 cm) of macroporous resin D101, and the column was stepwise eluted with water, 30, 50, 70, and 95 % ethanol at a flow rate of 10 mL/min to yield five sub-fractions. After HPLC analyses, 30 % ethanol sub-fraction was further purified using a Sephadex LH-20 column chromatography and eluted with methanol, followed by a semi-preparative HPLC eluting with methanol/water, to yield compound 1 (1293 mg), compound 2 (241 mg) and compound 3 (486 mg). The semi-preparative high performance liquid chromatography (HPLC) system consisted of a C18 column (RP18, 10 μm, 250 mm × 20 mm), a Waters 600 pump and a Waters 2998 Diode Array Detector (DAD) (Waters, Milford, MA, USA) analyzed at 35 °C. The mobile phase was 1 % acetic acid aqueous solution (A) and methanol (B) with a gradient program of 0–10 min, linear gradient 5–10 % B; 10–70 min, linear gradient 10–20 % B; 70–90 min, 20 % B isocratic; 90–130 min, linear gradient 20–40 % B; 130–140 min, linear gradient 40–100 % B and 140–180 min 100 % B isocratic elution at a flow rate of 15 mL/min. T 2 mL of samples were injected. Absorption wavelengths were set at 280 and 360 nm. The extraction and separation procedure of SBMP is shown in Fig. 1.
Fig. 1.

The extraction and separation procedure of sugar beet molasses
Identification of purity
Sample purity was analyzed using an HPLC-DAD system, which consisted of a C18 column (XBridge™ Shield, RP18, 5 μm, 250 mm × 4.6 mm), a Waters 600 pump and a Waters 2998 DAD (Waters, Milford, USA), column temperature was set at 35 °C. The mobile phase was 1 % acetic acid aqueous solution (A) and methanol (B) with a gradient program of 0–5 min, linear gradient 5–10 % B; 5–35 min, linear gradient 10–20 % B; 35–45 min, 20 % B isocratic; 45–65 min, linear gradient 20–40 % B; 65–70 min, linear gradient 40–100 % B and 70–90 min 100 % B isocratic elution at a flow rate of 1 mL/min. The injection volume is 20 μL, and the detection wavelengths were set at 280 and 360 nm.
Identification of compounds
The purified compounds from SBM were identified by electrospray ionization-mass spectrometry (ESI-MS), 1H NMR and 13C NMR spectrometry. The ESI-MS was carried out in a LCQ-Fleet mass spectrometer (Thermo Fisher Scientific, Waltham, MA), with an electrospray ionization source using a negative mode (m/z 50–800). 1H NMR spectra and 13C NMR spectra were recorded on a Bruker Avance DMX-500 spectrometer (Bruker Biospin GmbH, Germany), operating at 500 and 125 MHz for 1H and 13C, respectively, using D2O or deuterated dimethyl sulfoxide (DMSO-d6). In D2O, tetramethylsilane (TMS) was used as the internal standard. In DMSO-d6, the residual solvent was used as the internal standard. Chemical shifts were expressed in δ (ppm) downfield from TMS as an internal standard, and coupling constants were reported in hertz.
Assay of total antioxidant activity
The scavenging activity on DPPH free radical (DPPH) was measured according to the reported method with some modifications [17]. 0.5 ml of sample solution was mixed with 2.0 ml DPPH methanol solution (6 × 10−5 mol/L) and the mixture was shaken vigorously and incubated in dark at 30 °C for 30 min. Then, the absorbance was measured at 517 nm. Deionized water and ascorbic acid (VC) were used as the blank and positive control, respectively. The ability to scavenge DPPH was calculated by the eq. 1:
| 1 |
where Abs0 is the absorbance of water instead of sample solution, Abs1 is the absorbance of the sample solution, and Abs2 is the absorbance of methanol instead of DPPH solution.
The ABTS radical scavenging activity was assessed according to the recent reports with some modification [18, 19]. Briefly, ABTS solution was produced by reacting 5 mL of ABTS (7 mM) and 5 mL of potassium persulphate (2.45 mM) for 12–16 h in the dark at room temperature. This mixture was then diluted with absolute ethanol to obtain an absorbance of 0.70 ± 0.02 at 734 nm before use. Then, 0.4 mL of tested sample at different concentrations was mixed with 3.0 mL of ABTS solution. The mixture was incubated for 6 min in he dark and the absorbance was measured at 734 nm against blank. Ascorbic acid (Vc) with the same concentration was used as a positive control. The ABTS scavenging activity was calculated using the eq. 2:
| 2 |
where Ac is the absorbance of control without sample, As is the absorbance of the sample.
The oxygen radical absorbance capacity (ORAC) assay was carried out according to the modified method of Ou et al. [20]. Briefly, sample solution was prepared with 75 mM phosphate buffer (pH 7.4). The 20 μL sample aliquots or Trolox standard (6.25–100 μM) were added in a black 96-well plates (Greiner Bio-one Cellstar, Frickenhausen), and then 200 μL of 95.6 nM fluorescein solution was added to each well. The mixture was incubated for 30 min at 37 °C. Finally, 20 μL of AAPH was automatically injected, and the microplate was shaken for 20 s before each measurement. The fluorescence was measured using a Varioskan Flash Multimode Reader (Thermo Fisher Scientific Inc.) at excitation of 540 nm and emission of 565 nm for 35 cycles every 3.0 min. The buffer was used as blank. The ORAC value (μmol Trolox equivalents (TE)/g) refers to the required Trolox content (μmol) when ORAC of Trolox is equal to 1 g sample.
Determination of antitumor activity
Human colon (CACO-2), hepatocellular (HepG2) and breast (MCF-7) carcinoma cell lines were provided by the Medical College of Sun Yat-Sen University (Guangzhou, China). The cells were cultured in DMEM containing 10 % FBS, 100 μg/mL streptomycin and 100 U/mL penicillin in a humidified incubator with 5 % CO2 at 37 °C.
The antitumor activities of samples were evaluated using the MTT assay with some modifications [21]. Briefly, CACO-2, HepG2 and MCF-7 cells were harvested during the logarithmic growth phase, seeded in a 96-well plate (5 × 104 cells/mL), and incubated at 37 °C in an atmosphere of 5 % CO2. After anchoring to the wells, additional medium (100 μL) containing different concentrations of test samples were added to each well. The cells were then incubated at 37 °C for 48 h in an atmosphere containing 5 % CO2. Then, 20 μL of MTT solution (5 mg/mL) was added to each well, and the incubation was continued for an additional 4 h. Then the supernatant was removed and 150 μL of DMSO was added to each well. The plate was shaken for 10 min to dissolve formazan crystals. The absorbance of the above DMSO solution was measured at 570 nm by a microplate reader. The buffer and 5-fluorouracil were used as the blank and positive control respectively. The inhibitory rate was calculated using the eq. 3:
| 3 |
where A is the cancer cell growth inhibitory rate; Ac is the absorbance of the control; As is the absorbance of sample; and Ab is the absorbance of the blank.
Statistical analysis
Data were analyzed using SPSS (SPSS Inc., Chicago, IL, USA) and presented as mean ± SD with triplicates. Significance was determined at p < 0.05 by analysis of variance (ANOVA) followed by Duncan’s least significant test.
Results and discussion
Structural identification
Three compounds isolated and obtained from SBM were identified as gallic acid (GA), cyanidin-3-O-glucoside chloride (CGC) and epicatechin (EP). Their spectroscopic data were listed below.
Gallic acid (Compound 1): C7H6O5, white powder, purity, 99.2 %. QTOF-MS m/z: 177.0158 [M + Na-H2O]+, ESI-MS/MS m/z: 177, 153, 127. 1H-NMR (500 MHz, CDCl3) δ H: 7.12 (2H, s, H-2, 6). 13C-NMR (125 MHz, CDCl3) δ C: 121.2 (C-1), 109.4 (C-2), 145.3 (C-3), 138.6 (C-4), 145.3 (C-5), 109.4 (C-6), 168.5 (COOH) [22].
Cyanidin-3-O-glucoside chloride (Compound 2): C21H21O11, dark brown crystalline powder, purity, 98.7 %. QTOF-MS m/z:450.1157 [M + H]+, ESI-MS/MS m/z:450, 287. 1H-NMR (CD3COCD3, 500 MHz) δ H: 8.92 (1H, s, H-4), 6.88 (1H, d, J = 2.0 Hz, H-6), 6.91 (1H, s, H-8), 8.10 (1H, d, J = 1.0 Hz, H-2′), 7.02 (1H, d, J = 8.5Hz, H-5′), 8.26 (1H, d, J = 7.5Hz, H-6′), 5.28 (1H, d, J = 4.5Hz, H-1″), 3.13 (1H, t, H-4″), 3.38 (1H, q, H-3″), 3.31 (1H, q, H-2″), 3.48 (1H, m, H-5″), 3.82 (1H, m, H-6a″), 3.71 (1H, m, H-6b″) [23].
Epicatechin (Compound 3): C15H14O6, white crystalline powder, purity, 99.5 %. QTOF-MS m/z:291.0863 [M + H]+, ESI-MS/MS m/z: 291, 273, 165, 139. 1H-NMR (CD3COCD3, 400 MHz) δ H: 7.03 (1H, d, J = 2.0 Hz, H-2′), 6.82 (1H, d, J = 8.0 Hz, H-5′), 6.77 (1H, dd, J = 8.0, 2.0 Hz, H-6′), 5.99 (1H, d, J = 2.0 Hz, H-8), 5.89 (1H, d, J = 2.0 Hz, H-6), 4.86 (1H, s, H-2), 4.11 (1H, m, H-3), 2.85 (1H, dd, J = 16.4, 4.4 Hz, H-4), 2.72 (1H, dd, J = 16.4, 3.2 Hz, H-4). 13C-NMR(CD3COCD3, 100 MHZ,) δ C: 28.83 (C-4), 68.33 (C-3), 82.70 (C-2), 95.41 (C-8), 96.13 (C-6), 100.62 (C-10), 115.23 (C-5′), 115.70 (C-2′), 120.06 (C-6′), 132.14 (C-1′), 145.64 (C-3′), 145.72 (C-4′), 156.89 (C-9), 157.22 (C-5), 157.73 (C-7) [24, 25].
Antioxidant activity
The DPPH radical scavenging activities of extractions, GA, CGC and EP were shown in Fig. 2. All fractions showed remarkable scavenging activity against DPPH radicals in a dose-dependent manner. The scavenging activity of the ethyl acetate fraction was higher than those of other fractions (Fig. 2a). GA showed higher scavenging activities against DPPH radicals than Vc. CGC and EP showed relatively lower scavenging activities (Fig. 2b).
Fig. 2.

DPPH radical scavenging activities of each fraction and compound isolated from SBM. a PEF: petroleum ether fraction; CF: chloroform fraction; EF: ethyl acetate fraction; BF: n-butanol fraction; WF: water fraction; b GA: gallic acid, CGC: cyanidin-3-O-glucoside chloride; EP: epicatechin All results are the means ± SD (n = 3)
The ABTS radical scavenging activities of each fraction, GA, CGC and EP were shown in Fig. 3. All fractions showed remarkable scavenging activity against ABTS radicals in a concentration-dependent manner. The scavenging activity of the ethyl acetate fraction was higher than those of other fractions (Fig. 3a). GA showed higher scavenging activities against ABTS radicals than Vc. CGC and EP showed relatively lower scavenging activities (Fig. 3b).
Fig. 3.
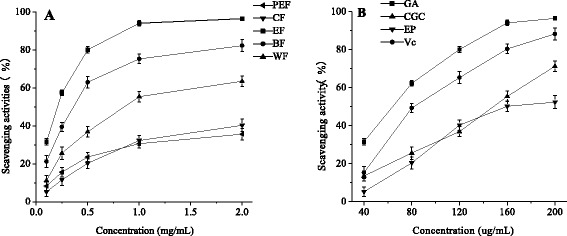
ABTS radical scavenging activities of each fraction and compound isolated from SBM. a PEF: petroleum ether fraction; CF: chloroform fraction; EF: ethyl acetate fraction; BF: n-butanol fraction; WF: water fraction; b GA: gallic acid, CGC: cyanidin-3-O-glucoside chloride; EP: epicatechin. All results are the means ± SD (n = 3)
The oxygen radical absorbance capacities of samples were shown in Fig. 4. As shown in Fig. 4a, all the extraction fractions showed strong oxygen radical absorbance capacity. The ORAC values were in the decreasing order of ethyl acetate fraction > n-butanol fraction > water fraction > chloroform fraction > petroleum ether fraction. The ORAC values of three compounds are shown in Fig. 4b. GA showed stronger oxygen radical absorbance capacity than Vc while those of CGC and EP were relatively low.
Fig. 4.

The ORAC values of each fraction and compound isolated from SBM. a PEF: petroleum ether fraction; CF: chloroform fraction; EF: ethyl acetate fraction; BF: n-butanol fraction; WF: water fraction; b GA: gallic acid, CGC: cyanidin-3-O-glucoside chloride; EP: epicatechin. All results are the means ± SD (n = 3). *p < 0.05, statistically significant in comparison with control
According to the results of DPPH, ABTS radical scavenging and ORAC assays, the ethyl acetate fraction showed the strongest antioxidant activity among five extraction fractions. The three compounds isolated from the ethyl acetate fraction could be explored as natural antioxidants as they showed strong antioxidant activities. GA showed the strongest antioxidant activity, followed by Vc which is greater than those of the CGC while EP showed the least antioxidant activity.
The three compounds, GA, CGC and EP, obtained in our study are three typical phenolic compounds in plants which have been reported to possess strong antioxidant activity [26, 27]. According to reports, free hydroxyl groups in phenolics are mainly responsible for antioxidant activity [28, 29]. GA, CGC and EP, belonging to the phenolic compounds, are rich in multiple phenolic hydroxyl groups, which have been considered to be important antioxidants for a long time, and their antioxidant activities could be attributed to the numerous hydroxyl groups present on their structures. However, according to the literature [29, 30], the number of hydroxyl groups bonded to the aromatic ring and their positions are probably the most important but not the only factors influencing the antioxidant activities of phenolic components. Meanwhile, character of substituents (carboxyl or acetyl group) and their position in relation to the hydroxyl groups seem to influence the antioxidant or anti-radical features.
Antitumor activity
The antitumor activities of five fractions against cancer cells are shown in Fig. 5. Among the five extraction fractions, the ethyl acetate fraction showed the highest inhibitory effect on CACO-2 (Fig. 5a), HepG2 (Fig. 5b) and MCF-7 (Fig. 5c) cell proliferation at the concentrations ranging from 25 to 400 μg/mL. The inhibitory effect was displayed in a dose-dependent manner. As shown in Fig. 6, the inhibitory rates of the ethyl acetate fraction on CACO-2, HepG2 and MCF-7 cells were higher than other fractions. The degree of antitumor activities of the ethyl acetate fractions showed that active compounds may be more concentrated in the ethyl acetate fraction than may be present in other fractions.
Fig. 5.
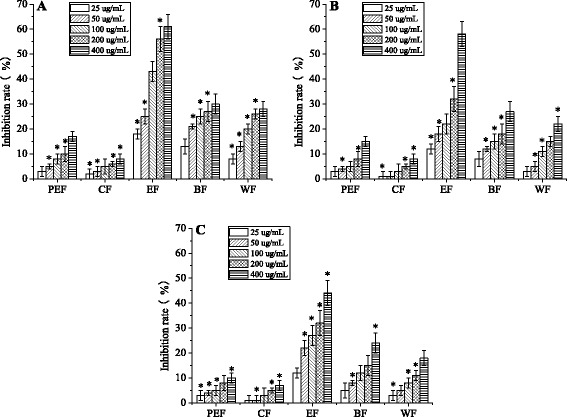
Inhibitory effects in vitro of five fractions against a CACO-2 cells, b HepG2 cells and c MCF-7 cells. PEF: petroleum ether fraction; CF: chloroform fraction; EF: ethyl acetate fraction; BF: n-butanol fraction; WF: water fraction. All results are the means ± SD (n = 3). *p < 0.05, statistically significant in comparison with control
Fig. 6.

Inhibitory effects in vitro of three compounds and 5-fluorouracil (5-Fu) control against a CACO-2 cells, b HepG2 cells and c MCF-7 cells. GA: gallic acid, CGC: cyanidin-3-O-glucoside chloride, EP: epicatechin. All results are the means ± SD (n = 3). *p < 0.05, statistically significant in comparison with control
As shown in Fig. 6, GA, CGC and EP showed significant antitumor activities. CGC exhibited the strongest inhibitory effect on CACO-2 (Fig. 6a), HepG2 (Fig. 6b) and MCF-7 (Fig. 6c) cells. The inhibitory rate of CGC (400 μg/mL) was about 95 %. Additionally, the inhibitory rates of CGC (all concentrations) were much higher than that of 5-fluorouracil. However, GA and EP showed relatively weak inhibitory effects on the CACO-2, HepG2 and MCF-7 cells, and the inhibitory rates increased slowly with the increase in their concentration. From previous studies, compounds with higher antioxidant activities always had higher antitumor activity and prevented the cellular senescence and apoptosis [9, 31]. However, although CGC showed lower antioxidant activity than GA, it had the strongest inhibitory effects on the cancer cell proliferation.
Early studies showed that GA, CGC and EP had anticancer activity. GA has been shown to have antitumor activity in many cancer cells without damaging normal cells [32–36]. Wang et al. [32] reported GA induced apoptosis by triggering the extrinsic or Fas/FasL pathway as well as the intrinsic or mitochondrial pathway in MCF-7 cells. Filipiak et al. [33] reported CGC showed inhibitory activity against gelatinases corresponding to its cytotoxic activity in HT1080 cells. Xu et al. [34] reported CGC attenuated ethanol-induced migration/invasion of breast cancer cells expressing high levels of ErbB2 (BT474, MDA-MB231 and MCF7ErbB2) in a concentration dependent manner. Zhao et al. [35] reported GA significantly decreased human cervical cancer cell proliferation and tube formation in human umbilical vein endothelial cells. Siddique et al. [36] reported EP could cause a decrease in the proliferation, guanosine triphosphate-bound Ras protein, Akt phosphorylation and NF-κB transcriptional activity of premalignant and malignant Krasactivated PDE cells. Though the antitumor activities of these compounds had been previously reported, the mechanism(s) of antitumor activity of the individual compound needs to be investigated. However, the presence of the three compounds may be responsible for the antioxidant and antitumor activities exhibited by SBM and suggested that SBM might be used as an additive in antitumor food.
Conclusions
Three compounds, Gallic acid (GA), cyanidin-3-O-glucoside chloride (CGC) and epicatechin (EP) and the main compounds in SBM with high antioxidant and antitumor activities. GA possessed the strongest antioxidant activity. CGC showed strong antitumor activities against human colon (CACO-2), hepatocellular (HepG2) and breast carcinoma cells. The antitumor activity was higher than positive control (5-fluorouracil). Further studies will be needed to investigate other chemical compounds and bioactivity of SBM. These results suggest that SBM is a potential source of antioxidant and antitumor agents for preparing functional foods.
Acknowledgments
All the authors declare that there are no conflicts of interest. All authors acknowledge the ministry of Science and Technology in Agriculture Science and Technology Achievements Transformation Fund Project (No.2013GB23600669), the Science and Technology Planning Project of Guangzhou Municiple, China (No. 2011Y2-00012), China Postdoctoral Science Foundation funded project (2014 M560664) and this work was supported by the basic research foundation of SCUT (2012ZM0072).
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
MC and SY designed the experiments; MC, HM, and YZ performed the experiments; MC and FC analyzed the data; MC wrote the paper; HM and FC revised the manuscript. All authors read and approved the final manuscript.
References
- 1.McDill BM. Beet Sugar Industry. Ind Eng Chem. 1947;39(5):657–69.
- 2.Balbach JK. The effect of ownership on contract structure, costs, and quality: the case of the U.S. beet sugar industry. In: Royer JS, Rogers RT (eds). The industrialization of agriculture: vertical coordination in the U.S. food system. Aldershot, United Kingdom: Ashgate Publishing Ltd.; 1998. p. 155–184.
- 3.Roukas T. Ethanol production from non-sterilized beet molasses by free and immobilized < i > Saccharomyces cerevisiae</i > cells using fed-batch culture. J Food Eng. 1996;27(1):87–96. doi: 10.1016/0260-8774(94)00076-L. [DOI] [Google Scholar]
- 4.Ahmedna M, Marshall W, Rao R. Production of granular activated carbons from select agricultural by-products and evaluation of their physical, chemical and adsorption properties. Bioresour Technol. 2000;71(2):113–123. doi: 10.1016/S0960-8524(99)00070-X. [DOI] [Google Scholar]
- 5.Paturau JM. By-products of the cane sugar industry. An introduction to their industrial utilization. Amsterdam: Elsevier Science Publishers BV; 1989.
- 6.Filipčev B, Lević L, Bodroža-Solarov M, Mišljenović N, Koprivica G. Quality characteristics and antioxidant properties of breads supplemented with sugar beet molasses-based ingredients. Int J Food Prop. 2010;13(5):1035–1053. doi: 10.1080/10942910902950526. [DOI] [Google Scholar]
- 7.Koprivica G, Mišljenović N, Lević L, Kuljanin T. Influence of the nutrients present in sugar beet molasses and saccharose solutions on the quality of osmodehydrated carrot. Časopis za procesnu tehniku i energetiku u poljoprivredi/PTEP. 2009;13(2):184–187. [Google Scholar]
- 8.Paananen H, Kuisma J. Chromatographic separation of molasses components. Zuckerindustrie. 2000;125(12):978–981. [Google Scholar]
- 9.Valli V, Gómez-Caravaca AM, Di Nunzio M, Danesi F, Caboni MF, Bordoni A. Sugar cane and sugar beet molasses, antioxidant-rich alternatives to refined sugar. J Agric Food Chem. 2012;60(51):12508–12515. doi: 10.1021/jf304416d. [DOI] [PubMed] [Google Scholar]
- 10.Khadem S, Marles RJ. Monocyclic phenolic acids; Hydroxy- and polyhydroxybenzoic acids: Occurrence and recent bioactivity studies. Molecules. 2010;15(11):7985–8005. doi: 10.3390/molecules15117985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu X, Zhao M, Wu K, Chai X, Yu H, Tao Z, Wang J. Immunomodulatory and anticancer activities of phenolics from emblica fruit (Phyllantlius emblica L.) Food Chem. 2012;131(2):685–690. doi: 10.1016/j.foodchem.2011.09.063. [DOI] [Google Scholar]
- 12.Pereira DM, Valentao P, Pereira JA, Andrade PB. Phenolics: From chemistry to biology. Molecules. 2009;14(6):2202–2211. doi: 10.3390/molecules14062202. [DOI] [Google Scholar]
- 13.Vineetha VP, Girija S, Soumya RS, Raghu KG. Polyphenol-rich apple (Malus domestica L.) peel extract attenuates arsenic trioxide induced cardiotoxicity in H9c2 cells via its antioxidant activity. Food Funct. 2014;5(3):502–511. doi: 10.1039/c3fo60470e. [DOI] [PubMed] [Google Scholar]
- 14.Wu L-C, Jou AF-J, Chen S-H, Tien C-Y, Cheng C-F, Fan N-C, Ho J-aA. Antioxidant, anti-inflammatory and anti-browning activities of hot water extracts of oriental herbal teas. Food Funct. 2010;1(2):200–208. doi: 10.1039/c0fo00047g. [DOI] [PubMed] [Google Scholar]
- 15.Ravipati AS, Zhang L, Koyyalamudi SR, Jeong SC, Reddy N, Bartlett J, et al. Antioxidant and anti-inflammatory activities of selected Chinese medicinal plants and their relation with antioxidant content. BMC Complement Altern Med. 2012;12:173. [DOI] [PMC free article] [PubMed]
- 16.Gomaa EZ. In vitro antioxidant, antimicrobial, and antitumor activities of bitter almond and sweet apricot (Prunus armeniaca L.) kernels. Food Sci Biotechnol. 2013;22(2):455–463. doi: 10.1007/s10068-013-0101-1. [DOI] [Google Scholar]
- 17.Luo A, He X, Zhou S, Fan Y, Luo A, Chun Z. Purification, composition analysis and antioxidant activity of the polysaccharides from < i > Dendrobium nobile</i > Lindl. Carbohydr Polym. 2010;79(4):1014–1019. doi: 10.1016/j.carbpol.2009.10.033. [DOI] [Google Scholar]
- 18.Thaipong K, Boonprakob U, Crosby K, Cisneros-Zevallos L, Hawkins Byrne D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J Food Compos Anal. 2006;19(6):669–675. doi: 10.1016/j.jfca.2006.01.003. [DOI] [Google Scholar]
- 19.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26(9):1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 20.Ou B, Huang D, Hampsch-Woodill M, Flanagan JA, Deemer EK. Analysis of antioxidant activities of common vegetables employing oxygen radical absorbance capacity (ORAC) and ferric reducing antioxidant power (FRAP) assays: a comparative study. J Agric Food Chem. 2002;50(11):3122–3128. doi: 10.1021/jf0116606. [DOI] [PubMed] [Google Scholar]
- 21.Zhou J-J, Yue X-F, Han J-X, Yang W-Y. Improved MTT assay for activity of antitumor agents. Chin J Pharm. 1993;24:455–457. [Google Scholar]
- 22.Werner I, Bacher A, Eisenreich W. Retrobiosynthetic NMR studies with C-13-labeled glucose - Formation of gallic acid in plants and fungi. J Biol Chem. 1997;272(41):25474–25482. doi: 10.1074/jbc.272.41.25474. [DOI] [PubMed] [Google Scholar]
- 23.Fernandes A, Ivanova G, Bras NF, Mateus N, Ramos MJ, Rangel M, de Freitas V. Structural characterization of inclusion complexes between cyanidin-3-0-glucoside and beta-cyclodextrin. Carbohydr Polym. 2014;102:269–277. doi: 10.1016/j.carbpol.2013.11.037. [DOI] [PubMed] [Google Scholar]
- 24.Cren-Olive C, Wieruszeski JM, Maes E, Rolando C. Catechin and epicatechin deprotonation followed by C-13 NMR. Tetrahedron Lett. 2002;43(25):4545–4549. doi: 10.1016/S0040-4039(02)00745-1. [DOI] [Google Scholar]
- 25.Berregi I, Santos JI, del Campo G, Miranda JI. Quantitative determination of (−)-epicatechin in cider apple juices by H-1 NMR. Talanta. 2003;61(2):139–145. doi: 10.1016/S0039-9140(03)00236-4. [DOI] [PubMed] [Google Scholar]
- 26.Panyathep A, Chewonarin T, Taneyhill K, Vinitketkumnuen U. Antioxidant and anti-matrix metalloproteinases activities of dried longan (Euphoria longana) seed extract. Scienceasia. 2013;39(1):12–18. doi: 10.2306/scienceasia1513-1874.2013.39.012. [DOI] [PubMed] [Google Scholar]
- 27.Othman A, Jalil AMM, Weng KK, Ismail A, Abd Ghani N, Adenan I. Epicatechin content and antioxidant capacity of cocoa beans from four different countries. Afr J Biotechnol. 2010;9(7):1052–1059. [Google Scholar]
- 28.Feng J-Y, Liu Z-Q. Phenolic and enolic hydroxyl groups in curcumin: Which plays the major role in scavenging radicals? J Agric Food Chem. 2009;57(22):11041–11046. doi: 10.1021/jf902244g. [DOI] [PubMed] [Google Scholar]
- 29.Sroka Z, Cisowski W. Hydrogen peroxide scavenging, antioxidant and anti-radical activity of some phenolic acids. Food Chem Toxicol. 2003;41(6):753–758. doi: 10.1016/S0278-6915(02)00329-0. [DOI] [PubMed] [Google Scholar]
- 30.Vulic JJ, Cebovic TN, Canadanovic VM, Cetkovic GS, Djilas SM, Canadanovic-Brunet JM, Velicanski AS, Cvetkovic DD, Tumbas VT. Antiradical, antimicrobial and cytotoxic activities of commercial beetroot pomace. Food Funct. 2013;4(5):713–721. doi: 10.1039/c3fo30315b. [DOI] [PubMed] [Google Scholar]
- 31.Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 32.Wang K, Zhu X, Zhang K, Zhu L, Zhou F. Investigation of gallic acid induced anticancer effect in human breast carcinoma MCF-7 cells. J Biochem Mol Toxicol. 2014;28(9):387–393. doi: 10.1002/jbt.21575. [DOI] [PubMed] [Google Scholar]
- 33.Filipiak K, Hidalgo M, Manuel Silvan J, Fabre B, Carbajo RJ, Pineda-Lucena A, Ramos A, de Pascual-Teresa B, de Pascual-Teresa S. Dietary gallic acid and anthocyanin cytotoxicity on human fibrosarcoma HT1080 cells. A study on the mode of action. Food Funct. 2014;5(2):381–389. doi: 10.1039/c3fo60465a. [DOI] [PubMed] [Google Scholar]
- 34.Xu M, Bower KA, Wang SY, Frank JA, Chen G, Ding M, Wang SO, Shi XL, Ke ZJ, Luo J. Cyanidin-3-Glucoside inhibits ethanol-induced invasion of breast cancer cells overexpressing ErbB2. Mol Cancer. 2010;9:285. doi: 10.1186/1476-4598-9-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao B, Hu M. Gallic acid reduces cell viability, proliferation, invasion and angiogenesis in human cervical cancer cells. Oncology Letters. 2013;6(6):1749–1755. doi: 10.3892/ol.2013.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siddique HR, Liao DJ, Mishra SK, Schuster T, Wang L, Matter B, Campbell PM, Villalta P, Nanda S, Deng Y, et al. Epicatechin-rich cocoa polyphenol inhibits Kras-activated pancreatic ductal carcinoma cell growth in vitro and in a mouse model. Int J Cancer. 2012;131(7):1720–1731. doi: 10.1002/ijc.27409. [DOI] [PubMed] [Google Scholar]


