Abstract
Background:
In Phase 3 double-blind trials (MS-F203 and MS-F204), dalfampridine extended release tablets 10 mg twice daily (dalfampridine-ER; prolonged-release fampridine in Europe; fampridine modified or sustained release elsewhere) improved walking speed relative to placebo in patients with multiple sclerosis (MS).
Objectives:
Evaluation of long-term safety and efficacy of dalfampridine-ER in open-label extensions (MS-F203EXT, MS-F204EXT).
Methods:
Patients received dalfampridine-ER 10 mg twice daily; and had Timed 25-Foot Walk (T25FW) assessments at 2, 14 and 26 weeks, and then every 6 months. Subjects were categorized as dalfampridine-ER responders or non-responders, based on their treatment response in the double-blind parent trials that assessed T25FW.
Results:
We had 269 patients enter MS-F203EXT and 154 patients complete it; for a maximum exposure of 5 years. We had 214 patients enter MS-F204EXT and 146 complete it; for a maximum exposure of 3.3 years. No new safety signals emerged and dalfampridine-ER tolerability was consistent with the double-blind phase. Improvements in walking speed were lost after dalfampridine-ER was discontinued in the parent trial, but returned by the 2-week assessment after re-initiation of the drug. Throughout the extensions, mean improvement in walking speed declined, but remained improved, among the double-blind responders as compared with non-responders.
Conclusions:
The dalfamipridine-ER safety profile was consistent with the parent trials. Although walking speed decreased over time, dalfampridine-ER responders continued to show improved walking speed, which was sustained compared with non-responders.
Keywords: Clinical trial, dalfampridine, efficacy, long-term effects, multiple sclerosis, safety, sustained release, Timed 25-Foot Walk, tolerability, walking speed
Introduction
Walking impairment commonly contributes to the substantial burden of multiple sclerosis (MS). This impairment, which may be present even in individuals in the early stages of the disease,1–3 limits the ability to perform activities of daily living, reduces quality of life, and adversely affects productivity and socioeconomic status.4–7
Dalfampridine is a voltage-dependent potassium-channel blocker that has demonstrated the ability to restore action-potential conduction in demyelinated axons, in animal models.8 It is available in the US to improve walking, as demonstrated by an increase in walking speed in people with MS in an extended-release 10 mg tablet, to be taken twice daily, and is known as dalfampridine extended release (dalfampridine-ER). In Europe, it is known as prolonged-release fampridine, and as fampridine modified or sustained release, elsewhere.
Improvement in walking was demonstrated in two Phase 3, placebo-controlled trials, MS-F2039 and MS-F204,10 by a consistently faster walking speed in the Timed 25-Foot Walk (T25FW) among patients treated with dalfampridine-ER, relative to placebo. Both trials used a responder analysis, in which the responders to the drug were defined by a consistently faster T25FW during on-treatment assessments, relative to off-treatment assessments. In both trials, the proportion of responders was significantly higher with dalfampridine-ER, relative to placebo; 35% versus 8% (p < 0.0001), and 43% versus 9% (p < 0.0001) in the MS-F203 and MS-F204, respectively. A pooled analysis of the two trials demonstrated the robustness of the results; and it showed that treatment response was independent of demographic and disease characteristics, including gender, age, race, body mass index (BMI), clinical course of MS, disease duration, and baseline Expanded Disability Status Scale (EDSS) score and walking speed.11
To evaluate the long-term safety and efficacy of dalfampridine-ER, we conducted open-label extension studies for each of the Phase 3 trials, following the double-blind phase. This paper presents the final results of both extension studies.
Patients and methods
Study population
All patients who completed MS-F203 and MS-F204, regardless of treatment allocation (dalfampridine-ER or placebo) or treatment response, were eligible to enter the respective open-label dalfampridine-ER extension trials (MS-F203EXT and MS-F204EXT). The key inclusion and exclusion criteria for these trials were previously published, as was the definition of the responder criterion used as the primary efficacy outcome in MS-F2039 and MS-F204.10
Study design
Both long-term extension studies (LTEs) were of similar design and they both received approval from the appropriate Institutional Review Boards (IRBs). All patients provided written informed consent, prior to participation. Study MS-F203EXT was conducted between January 2005 and January 2011 (ClinicalTrials.gov identifier: NCT00648908); and study MS-F204EXT was conducted between August 2007 and January 2011(ClinicalTrials.gov identifier: NCT00649792). Screening for the LTE was performed at the time of, or subsequent to the final safety follow-up visit of the parent study, which was 4 weeks (MS-F203) or 2 weeks (MS-F204) after the conclusion of double-blind treatment. Therefore, treatment had been discontinued for at least 2 weeks prior to entry into the LTE; and upon starting the LTE, all patients were initiated on dalfampridine-ER, 10 mg twice daily.
Use of immunomodulatory therapies, including interferon ß-1a, interferon ß-1b, glatiramer acetate and natalizumab was allowed during the LTE, if treatment had started at least 90 days prior to the screening visit and the treatment regimen had been stable for a minimum of 30 days; however, for the purpose of maintaining stable symptoms of MS, scheduled administration of corticosteroids, cyclophosphamide and mitoxantrone were not permitted for the duration of the study.
Assessments
Safety and efficacy assessments were conducted at the screening and regularly scheduled visits; the first post-treatment assessment visit was held 2 weeks after screening, with subsequent on-treatment assessments at 2, 14 and 26 weeks, plus every 6 months thereafter. A safety follow-up was conducted 4 weeks after the final on-treatment visit.
Safety assessments included adverse event (AE) monitoring, laboratory testing, vital signs and electrocardiography. Efficacy was assessed as the average % change from baseline in walking speed, based on the T25FW, with the patient baseline defined as the average walking speed of four pre-treatment visits in those parent double-blind studies.
Results
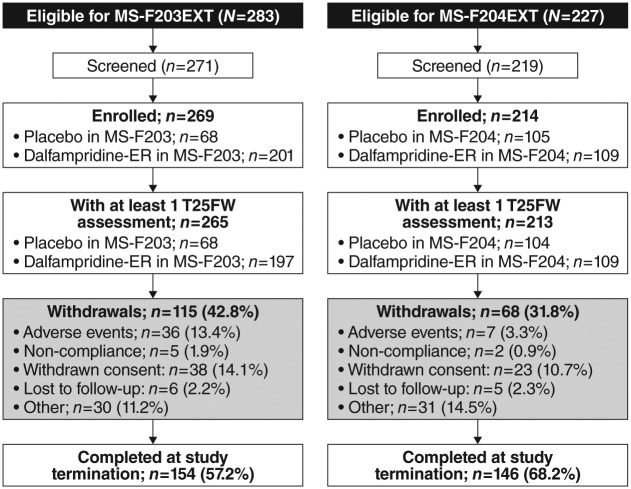
Patient disposition and demographics
Figure 1 shows the enrollment and disposition of patients in the extension phase of the two studies. Of the 269 patients who enrolled in MS-F203EXT, 154 (57.2%) were considered study completers; and 146 (68.2%) of the 214 enrolled patients were study completers in MS-F204EXT. Completers are defined as patients whom were still on therapy at the time of study termination; withdrawal of consent was the main reason for discontinuation in both LTEs, and was 14.1% and 10.7%, respectively. Mean (± SD) treatment exposure in MS-F203EXT was 39.0 (± 18.73) months, with a median of 49.3 months and a range of 0.3–59.9 months. In MS-F204EXT, the mean treatment exposure was 26.3 (± 10.1) months, with a median of 30 months and a range of 0.3–40 months.
Figure 1.
Patient disposition in long-term extension studies of MS-F203 (MS-F203EXT) and MS-F204 (MS-F204EXT) participants.
MS: multiple sclerosis; T25FW: Timed 25-Foot Walk.
Demographic and clinical characteristics of the study populations were similar in both LTEs (Table 1) and reflected the parent studies. Patients at LTE baseline were primarily female (67.5%), white (93.6%), and had a secondary progressive course of MS (51.6%); their mean EDSS score was 5.7 (median of 6.0 in both LTEs; range: 1.5–7.0 in MS-F203EXT and 2.0–7.0 in MS-F204EXT). A post-hoc analysis showed that the demographic characteristics were similar among those who completed the studies and those who discontinued except for a difference in gender distribution in MS-F204EXT; significantly more completers were female relative to those who discontinued (73.3% vs 54.4%; p = 0.0078) (Table 2).
Table 1.
Demographic and disease characteristics of the MS population in the safety extension studies.
| Variable | MS-F203EXT (n = 269) | MS-F204EXT (n = 214) |
|---|---|---|
| Age, mean ± SD (years) | 52.1 ± 8.8 | 52.0 ± 9.6 |
| Gender, n (%) | ||
| Female | 182 (67.7) | 144 (67.3) |
| Male | 87 (32.3) | 70 (32.7) |
| Race, n (%) | ||
| White | 251 (93.3) | 201 (93.9) |
| Black or African American | 11 (4.1) | 8 (3.7) |
| Hispanic | 3 (1.1) | 0 |
| Other | 4 (1.5) | 1 (0.5) |
| Missing | 0 | 4 (1.9) |
| MS type, n (%) | ||
| Relapsing–remitting | 76 (28.3) | 74 (34.6) |
| Primary progressive | 39 (14.5) | 24 (11.2) |
| Secondary progressive | 142 (52.8) | 107 (50.0) |
| Progressive relapsing | 10 (3.7) | 9 (4.2) |
| Disease duration, mean ± SD (months)a | 170.0 ± 101.0 | 170.4 ± 110.4 |
| EDSS score | ||
| Mean ± SDb | 5.8 ± 1.1 | 5.6 ± 1.1 |
| Median (range) | 6.0 (1.5–7.0) | 6.0 (2.0–7.0) |
For MS-F203EXT, n = 267.
For MS-F203EXT, n = 268; for MS-F204EXT, n = 213.
EDSS: Expanded Disability Status Scale; MS: multiple sclerosis; SD: standard deviation.
Table 2.
Demographic and disease characteristics of the safety extension study populations, stratified by patients whom completed or discontinued the extension studies.
| Variable | MS-F203EXT |
MS-F204EXT |
||
|---|---|---|---|---|
| Completed (n = 154) | Discontinued (n = 115) | Completed (n = 146) | Discontinued (n = 68) | |
| Age, mean ± SD (years) | 51.6 ± 8.5 | 52.8 ± 9.1 | 52.6 ± 9.4 | 50.6 ± 10.0 |
| Gender, n (%) | ||||
| Female | 102 (66.2) | 80 (69.6) | 107 (73.3)a | 37 (54.4) |
| Male | 52 (33.8) | 35 (30.4) | 39 (26.7) | 31 (45.6) |
| Race, n (%) | ||||
| White | 144 (93.5) | 107 (93.0) | 140 (95.9) | 61 (89.7) |
| Black or African American | 5 (3.3) | 6 (5.2) | 3 (2.1) | 5 (7.4) |
| Hispanic | 2 (1.3) | 1 (0.9) | 0 | 0 |
| Other | 3 (2.0) | 1 (0.9) | 1 (0.7) | 0 |
| Missing | 0 | 0 | 2 (1.4) | 2 (2.9) |
| MS type, n (%) | ||||
| Relapsing–remitting | 48 (31.2) | 28 (24.4) | 50 (34.3) | 24 (35.3) |
| Primary progressive | 23 (14.9) | 16 (13.9) | 15 (10.3) | 9 (13.2) |
| Secondary progressive | 77 (50.0) | 65 (56.5) | 72 (49.3) | 35 (51.5) |
| Progressive relapsing | 4 (2.6) | 6 (5.2) | 9 (6.2) | 0 |
| Disease duration, mean ± SD (months) | 173.5 ± 103.1 | 165.3 ± 98.4 | 172.2 ± 110.4 | 168.2 ± 112.3 |
| EDSS score | ||||
| Mean ± SD | 5.7 ± 1.2 | 5.9 ± 0.9 | 5.6 ± 1.1 | 5.6 ± 1.2 |
| Median (range) | 6.0 (1.5–7.0) | 6.0 (2.0–6.5) | 6.0 (2.5–7.0) | 6.0 (2.0–7.0) |
Gender distribution was significantly different between those whom completed and those whom discontinued; p = 0.0078 (Fisher’s exact test).
EDSS: Expanded Disability Status Scale; MS: multiple sclerosis; SD: standard deviation.
Long-term safety
At study termination, the total cumulative exposure to dalfampridine-ER was 932.6 person-years in Study MS-F203 and 488.8 person-years in Study MS-F204. Treatment-emergent AEs were reported in 98.1% of patients during MS-F203EXT and 95.8% of patients during MS-F204EXT (Table 3). Serious AEs occurred in 34.9% of patients in MS-F203EXT and in 18.2% of patients in MS-F204EXT; only three serious events were judged by the investigators to be related to treatment. These were seizures, all of which occurred in MS-F203EXT.
Table 3.
TEAEs occurring in ≥10% of MS patients during the extension studies.
| Category/preferred term | Incidence of TEAEs, n (%) |
|
|---|---|---|
| MS-F203EXT (n = 269) | MS-F204EXT (n = 214) | |
| Any AE | 264 (98.1) | 205 (95.8) |
| AEs by severity | ||
| Mild | 16 (5.9) | 29 (13.6) |
| Moderate | 137 (50.9) | 120 (56.1) |
| Severe | 111 (41.3) | 56 (26.2) |
| Any serious AE | 94 (34.9) | 39 (18.2) |
| Deaths | 4 (1.5) | 1 (0.5) |
| Discontinuation due to AEs | 37 (13.8) | 7 (3.3) |
| Urinary tract infection | 112 (41.6) | 75 (35.0) |
| Fall | 107 (39.8) | 88 (41.1) |
| MS relapse | 87 (32.3) | 61 (28.5) |
| Arthralgia | 66 (24.5) | 33 (15.4) |
| Edema, peripheral | 53 (19.7) | 37 (17.3) |
| Back pain | 45 (16.7) | 23 (10.7) |
| Asthenia | 41 (15.2) | — |
| Pain in extremity | 53 (19.7) | 29 (13.6) |
| Insomnia | 40 (14.9) | — |
| Upper respiratory tract infection | 47 (17.5) | 31 (14.5) |
| Fatigue | 44 (16.4) | 37 (17.3) |
| Contusion | 31 (11.5) | 30 (14.0) |
| Dizziness | 28 (10.4) | 24 (11.2) |
| Muscular weakness | 35 (13.0) | 38 (17.8) |
| Cystitis | 28 (10.4) | — |
| Nausea | 40 (14.9) | 27 (12.6) |
| Muscle spasms | 34 (12.6) | — |
| Muscle spasticity | 42 (15.6) | 29 (13.6) |
| Nasopharyngitis | 37 (13.8) | — |
| Diarrhea | 28 (10.4) | — |
| Depression | 38 (14.1) | — |
| Headache | 28 (10.4) | — |
| Constipation | 27 (10.0) | — |
| Balance disorder | — | 23 (10.7) |
AEs: adverse events; MS: multiple sclerosis; TEAEs: treatment-emergent adverse events.
Discontinuations due to AEs were 13.8% and 3.3% in MS-F203EXT and MS-F204EXT, respectively (Table 3). The AEs that most often resulted in discontinuation in MS-F203EXT were seizure and myocardial infarction, each occurring in three (1.1%) patients. In the MS-F204EXT, none of the AEs leading to discontinuation occurred in more than one patient. There were five deaths in the two studies, none of which was considered to be related to study medication. These deaths included two suicides (one in each study), and one each of myocardial infarction, intracranial hemorrhage resulting from a brain aneurysm, and by an unknown cause: All were in MS-F203EXT. Across the LTEs, the most common AEs were urinary tract infections, falls, MS relapses, arthralgia and peripheral edema (Table 3).
A total of four seizure-related AEs were reported during the LTEs (0.8%), all among patients whom had been treated with dalfampridine-ER in the parent studies. None of the placebo-treated patients in the parent studies whom were subsequently initiated on open-label dalfampridine-ER experienced a seizure during the LTE.
Three events were convulsions and one was a complex partial seizure. Two of the convulsions and the complex partial seizure were considered treatment related; the other convulsion occurred in a patient 3 weeks after discontinuation that was due to another serious AE (acute neurological syndrome, after being on treatment for approximately 4 months), and this was not considered treatment related. A fifth event involved a patient whom experienced a grand mal convulsion, approximately 1 month after stopping the study drug. This seizure was not considered a treatment-emergent AE.
No new safety signals emerged and no patterns were observed, with regard to any abnormalities of laboratory findings, vital signs or electrocardiography.
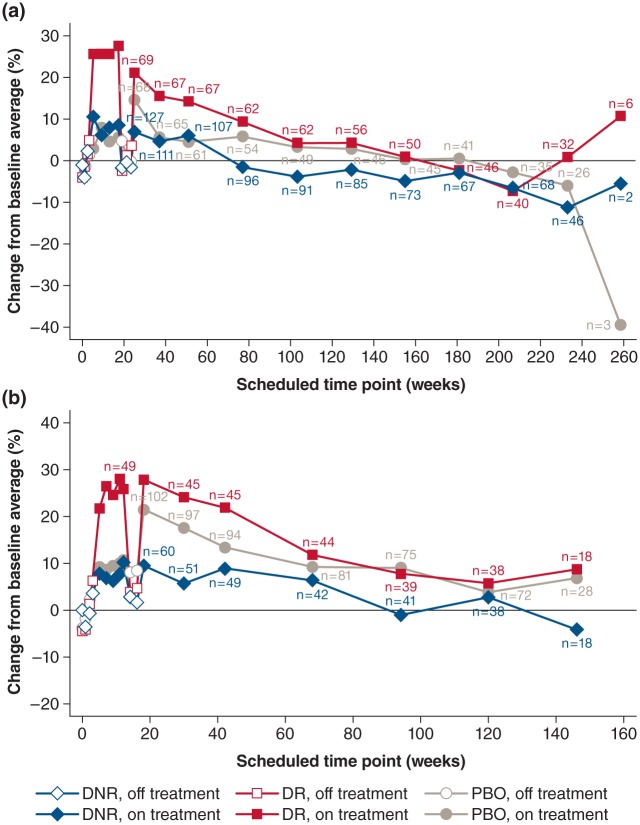
Long-term efficacy
Figure 2 shows the average percent change in walking speed for the double-blind and LTE phases of MS-F203/MS-F203EXT (Figure 2(a)) and MS-F204/MS-F204EXT (Figure 2(b)), stratified by placebo group and the dalfampridine-ER responder status in the parent trials. In general, the patterns of change in walking speed over time across the double-blind and LTE phases were similar between the two studies, at comparable scheduled assessment points.
Figure 2.
Percent change from baseline in walking speed at each scheduled visit in the double-blind phase and open-label extension, by treatment allocation and dalfampridine-ER responder status in the parent study. (a) Study MS-F203EXT; (b) study MS-F204EXT. Time 0 represents the ‘Screening visit’ of the parent study; after which the double-blind phases lasted 21 weeks and 14 weeks in the two parent studies, respectively. The off-treatment periods after 14 and 21 weeks in (a) and (b), respectively, reflect the safety follow-up to the parent trials, prior to initiation of the long-term extensions.
DNR: dalfampridine-ER non-responder; DR: dalfampridine-ER responder; ER: extended release; EXT: extension study; MS: multiple sclerosis; PBO: placebo.
The approximately 25% mean improvement from baseline in walking speed observed during MS-F203 and MS-F204 in patients characterized as dalfampridine-ER responders was lost after treatment discontinuation, in both parent trials. Upon subsequent re-initiation of treatment in the open-label LTEs, the dalfampridine-ER responders again had improvements in walking speed at the initial 2-week assessment, up to levels similar to those observed at the end of the double-blind treatment.
Baseline walking speeds for the overall study populations were 2.11 ft/sec in MS-F203EXT and 2.33 ft/sec in MS-F204EXT. Although by 8 weeks, walking speed improved by 0.24 ft/sec and 0.27 ft/sec in these two studies, respectively, the gain in speed gradually diminished, so by the end of the studies, the mean walking speed was similar to or slightly below the baseline level; however, in MS-F203EXT, more than 70% of the enrolled patients reached the 2-year period with a mean walking speed that remained above the baseline mean.
The pattern of walking speed changes observed among dalfampridine-ER non-responders was similar to that of the responders, although the percent improvement in walking speed was substantially lower than for responders. The 6–8% improvement in walking speed seen at the end of the double-blind phase was lost after treatment discontinuation, but returned upon re-initiation of open-label dalfampridine-ER. Although patient retention in the LTEs was generally greater among the dalfampridine-ER responders of the parent studies, relative to non-responders, the retention rates were high even among the non-responders: 62.0% and 53.9% in MS-F203EXT, and 73.5% and 68.3% in MS-F204EXT for responders and non-responders, respectively. For the placebo-treated patients in the parent studies, the LTE retention rates were 58.8% and 65.7% in MS-F203EXT and MS-F204EXT, respectively.
When patients from the placebo group in the double-blind phase were initiated on dalfampridine-ER in the LTE, the average percent change in walking speed at the 2-week assessment was 14.7% in MS-F203EXT and 21.7% in MS-F204EXT. This change was substantially higher than during the double-blind period, which had been 5.2% and 9.7% in each parent study, respectively. The magnitude of these observed improvements was between that of the responders (21.2% in MS-F203EXT and 28.0% in MS-F204EXT) and non-responders (6.9% and 9.5% in MS-F203EXT and MS-F204EXT, respectively). Relative to the dalfampridine-ER non-responders during the double-blind phase, improvements in walking speed over the duration of the LTEs were generally sustained among dalfampridine-ER responders, as well as the patients treated with placebo in the parent trials.
Changes over time in group mean data could be significantly influenced by missing data from patients whom discontinued the study. Therefore, Figure 3 presents walking speed data only for those patients with continuous participation, i.e. whom had a walking speed assessment at approximately 2 years after initiation of the parent study. In both MS-F203EXT (Figure 3(a)) and MS-F204EXT (Figure 3(b)), walking speed remained higher among the patients classified as dalfampridine-ER responders, compared with non-responders. The placebo group in the double-blind studies, consisting of both responders and non-responders, showed mean improvements in walking speed during the LTE that consistently remained greater than those in the non-responders whom were in the dalfampridine-ER group in the parent studies.
Figure 3.
Percent change from baseline in walking speed at each scheduled visit, among patients with continuous participation at approximately 2 years, by treatment allocation and dalfampridine-ER responder status in the parent study. (a) Study MS-F203EXT and (b) study MS-F204EXT. Time 0 is the ‘Screening visit’ of the parent study; the double-blind phases lasted 21 weeks and 14 weeks in these two parent studies, respectively.
DNR: dalfampridine-ER non-responder; DR: dalfampridine-ER responder; ER: extended release; EXT: extension study; MS: multiple sclerosis; PBO: placebo
Discussion
Results from these open-label LTE studies provided evidence for the long-term safety and tolerability of dalfampridine-ER, suggesting extended efficacy in the subset of patients whom responded to treatment in the double-blind trials.
The overall safety profile in the LTEs was consistent with that observed in the parent trials.9,10 Both LTEs showed that oral administration of dalfampridine-ER for up to 5 years was generally well tolerated by patients with MS, and no new safety signals emerged with long-term drug exposure. Although seizures were observed in four patients, one of whom was not on treatment at the time, the 0.8% seizure rate for the combined LTEs was similar to what was seen in the actively-treated and placebo groups in the parent trials; and so it was within the range expected from the epidemiology of seizures in the MS population.12,13
Dalfampridine-ER efficacy results were consistent with the parent studies in that walking speed improvements among the timed walk responders returned upon re-initiation of treatment; and remained greater among those individuals whom were previously double-blind responders, relative to those whom were non-responders. The return of improvement as early as 2 weeks after re-initiation of treatment in the LTEs suggested that these effects have a relatively rapid onset after treatment initiation.
Of note, placebo-treated patients in the parent study would be expected to represent a mixture of responders and non-responders, when treated with dalfampridine-ER in the LTE studies. These patients showed changes from baseline in walking speed with initial treatment in the LTE studies that were intermediate between the changes seen in the parent study dalfampridine-ER timed walk responder and non-responder groups. Mean changes from baseline became more similar between the original placebo patients and timed walk responders, over the course of 2 years of treatment; and the difference from the original non-responder group, while still present, was somewhat less. This pattern may indicate that responder status may change in either direction over longer periods of time. This possibility could only be investigated effectively with a long-term placebo-controlled study.
The overall decline in walking speed that was observed over the course of the LTEs is consistent with the natural history of MS: These studies showed a rate of decline similar to that previously observed over 2 years, in a longitudinal study of disease progression.14 Since the pattern of decline was similar in the dalfampridine-ER responders and non-responders, it is unlikely that this reflects a decline in the response to the medication.
Although the group who were dalfampridine-ER responders in the double-blind trials had a higher rate of retention in the extension phase, patient retention in both LTE studies was higher than expected, based on the proportion of patients whom were dalfampridine-ER responders in the parent trials. While the reasons for patients who were not responsive on walking measures to remain in the trial are not known, we suggest that other factors not readily captured by objective assessment may have also contributed to retention. Such factors may include: Patients’ perceptions of improvement, improvements in domains not measured in the trials, or variables generic to participation in clinical trials and not associated with any specific drug effect. Of note, the demographic and clinical characteristics were similar between those whom completed the studies and those whom discontinued, except for a significantly higher proportion of female subjects whom completed MS-F204 EXT, which is not likely to be clinically relevant.
These results should be interpreted within the context of the studies’ limitations, which are those generally associated with open-label LTEs: Lack of a placebo group, enrollment of patients from clinical trials that have specific inclusion and exclusion criteria, and the fact that patients completing the study generally represent a self-selected population of those whom tolerate the drug and may be more likely to have perceived a benefit. On the other hand, patients may stay in a long-term study of this kind for reasons unrelated to specific treatment, including altruism and interest in supporting the efforts of their medical practitioners; thus, the results of these LTEs may not be generalizable to the treatment response nor treatment adherence in a real-world setting.
In summary, long-term safety and tolerability of dalfampridine-ER for up to 5 years in open-label extensions of two Phase 3 clinical trials were consistent with observations in the parent trials: No new safety signals were detected. Efficacy with regard to improvement in walking speed was maintained in the subset of patients whom were dalfampridine-ER responders in the parent studies, relative to the dalfampridine-ER non-responders. An overall decline in walking speed was observed over the duration of the open-label extension studies, but this decrease likely represented natural MS disease progression.15
Acknowledgments
The authors thank EJ Bienen of The Curry Rockefeller Group in Tarrytown, NY, for editorial assistance. The authors would also like to thank all the investigators from the MS-F203 and MS-F204 for their valuable contribution to the studies.
Co-investigators for clinical trial MS-F203:
M Agius, BGW Arnason, FA Bethoux, CT Bever Jr, JD Bowen, TR Brown, DW Dietrich, K Edwards, MS Freedman, M Freedman, NJ Kachuck, MD Kaufman, M Keilson, O Khan, LB Krupp, TP Leist, JW Lindsey, FD Lublin, MK Mass, D Mattson, D McGowan, R Naismith, C O’Connell, JJ Oger, H Panitch, MA Picone, KW Rammohan, RT Schapiro, SR Schwid, T Scott, C Short, BW Thrower and TL Vollmer.
Co-investigators for clinical trial MS-F204:
M Agius, BGW Arnason, FA Bethoux, CT Bever Jr, TR Brown, A Camac, JA Cooper, WF Chumley, A Cross, DW Dietrich, RT Dunnigan, K Edwards, M Freedman, JS Gitt, M Hillen, DR Jeffrey, NJ Kachuck, BO Khatri, MD Kaufman, O Khan, K Kresa-Reahl, LB Krupp, TP Leist, FD Lublin, MK Mass, D Mattson, D McGowan, S Moon, C O’Connell, JJ Oger, H Panitch, MA Picone, J Preiningerova, KW Rammohan, RT Schapiro, SR Schwid, C Short, BW Thrower, M Tullman, B Weinstock-Guttman, DR Wynn and TL Vollmer.
Footnotes
Conflict of interest: Goodman has received consultancy fees from Acorda Therapeutics, Biogen Idec, EMD Serono, Genzyme, GW Pharma, Mylan, Novartis, Teva and Vaccinex; and has received research support from Acorda Therapeutics, Avanir, Biogen Idec, EMD Serono, Genzyme, Novartis, Ono, Roche, Sun Pharma and Teva. Bethoux has received research support from the National MS Society, the Consortium of MS Centers, Innovative Neurotronics, Merz Pharmaceuticals, Medtronic, and Acorda Therapeutics; has served on the speakers’ bureaus of Allergan and Acorda Therapeutics; and has received consultancy fees from or served on the advisory board for Acorda Therapeutics, Merz Pharmaceuticals, GW Pharmaceuticals and Concert Pharmaceuticals. Brown has received consultancy fees from Acorda Therapeutics, Bayer, Biogen, Genzyme, Pfizer and Teva; has received honoraria from Acorda Therapeutics, Biogen, Genzyme, Pfizer and Teva; and has received research funding/grants from Acorda Therapeutics, Biogen, Galen Pharmaceuticals, Lilly and Teva. Schapiro has received consultancy fees from Acorda Therapeutics and EMD Serono; and has received research funding from EMD Serono, Bayer, Acorda Therapeutics and Teva Neuroscience. Authors Cohen, Marinucci, and Blight are employees and stockholders of Acorda Therapeutics. Henney was an employee and stockholder of Acorda Therapeutics at the time these studies were conducted.
Funding: This research was supported by Acorda Therapeutics, Inc. Editorial assistance was also supported by Acorda Therapeutics.
Contributor Information
Andrew D Goodman, University of Rochester Medical Center, Rochester, NY, USA.
Francois Bethoux, Cleveland Clinic, Cleveland, OH, USA.
Theodore R Brown, Evergreen Neuroscience Institute and Medical Center, Kirkland, WA, USA.
Randall T Schapiro, The Schapiro Multiple Sclerosis Advisory Group, Eagle, CO, USA.
Ron Cohen, Acorda Therapeutics, Ardsley, NY, USA.
Lawrence N Marinucci, Acorda Therapeutics, Ardsley, NY, USA.
Herbert R Henney, III, Acorda Therapeutics, Ardsley, NY, USA.
Andrew R Blight, Acorda Therapeutics, Ardsley, NY, USA.
Collaborators: M Agius, BGW Arnason, FA Bethoux, CT Bever, Jr, JD Bowen, TR Brown, DW Dietrich, K Edwards, MS Freedman, M Freedman, NJ Kachuck, MD Kaufman, M Keilson, O Khan, LB Krupp, TP Leist, JW Lindsey, FD Lublin, MK Mass, D Mattson, D McGowan, R Naismith, C O’Connell, JJ Oger, H Panitch, MA Picone, KW Rammohan, RT Schapiro, SR Schwid, T Scott, C Short, BW Thrower, TL Vollmer, A Camac, JA Cooper, WF Chumley, A Cross, RT Dunnigan, JS Gitt, M Hillen, DR Jeffrey, BO Khatri, K Kresa-Reahl, S Moon, J Preiningerova, M Tullman, B Weinstock-Guttman, and DR Wynn
References
- 1. Gold MR, Siegel JE, Russell LB, Weinstein MC. (eds) Cost-effectiveness in health and medicine. New York: Oxford University Press, 1996. [Google Scholar]
- 2. Goldman MD, Marrie RA, Cohen JA. Evaluation of the Six-Minute Walk in multiple sclerosis subjects and healthy controls. Mult Scler 2008; 14: 383–390. [DOI] [PubMed] [Google Scholar]
- 3. Burschka JM, Keune PM, Menge U, et al. An exploration of impaired walking dynamics and fatigue in multiple sclerosis. BMC Neurol 2012; 12: 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Salter AR, Cutter GR, Tyry T, et al. Impact of loss of mobility on instrumental activities of daily living and socioeconomic status in patients with MS. Curr Med Res Opin 2010; 26: 493–500. [DOI] [PubMed] [Google Scholar]
- 5. Coleman CI, Sidovar M, Roberts MS, et al. Walking speed and health-related quality of life in multiple sclerosis. Int J MS Care 2012; 14: S29. [Google Scholar]
- 6. Coleman CI, Sidovar MF, Roberts MS, et al. Impact of mobility impairment on indirect costs and health-related quality of life in multiple sclerosis. PLoS One 2013; 8: e54756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pike J, Jones E, Rajagopalan K, et al. Social and economic burden of walking and mobility problems in multiple sclerosis. BMC Neurol 2012; 12: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dunn J, Blight A. Dalfampridine: A brief review of its mechanism of action and efficacy as a treatment to improve walking in patients with multiple sclerosis. Curr Med Res Op 2011; 27: 1415–1423. [DOI] [PubMed] [Google Scholar]
- 9. Goodman AD, Brown TR, Krupp L, et al. Sustained-release oral fampridine in multiple sclerosis: A randomised, double-blind, controlled trial. Lancet 2009; 373: 732–738. [DOI] [PubMed] [Google Scholar]
- 10. Goodman AD, Brown TR, Edwards KR, et al. A Phase 3 trial of extended release oral dalfampridine in mulitple sclerosis. Ann Neurol 2010; 68: 494–502. [DOI] [PubMed] [Google Scholar]
- 11. Goodman AD, Brown TR, Schapiro RT, et al. A pooled analysis of two Phase 3 clinical trials of dalfampridine in patients with multiple sclerosis. Int J MS Care 2014; 16: 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Koch M, Uyttenboogaart M, Polman S, et al. Seizures in multiple sclerosis. Epilepsia 2008; 49: 948–953. [DOI] [PubMed] [Google Scholar]
- 13. Kelley BJ, Rodriguez M. Seizures in patients with multiple sclerosis: Epidemiology, pathophysiology and management. CNS Drugs 2009; 23: 805–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cadavid D, Tang Y, O’Neill G. Responsiveness of the Expanded Disability Status Scale (EDSS) to disease progression and therapeutic intervention in progressive forms of multiple sclerosis. Rev Neurol 2010; 51: 321–329. [PubMed] [Google Scholar]
- 15. Cohen JA, Cutter GR, Fischer JS, et al. Benefit of interferon beta-1a on MSFC progression in secondary progressive MS. Neurology 2002; 59: 679–687. [DOI] [PubMed] [Google Scholar]