Abstract
Background
Leishmaniasis is caused by the Leishmania parasite, and transmitted by infected phlebotomine sandflies. Of the two distinct clinical syndromes, cutaneous leishmaniasis (CL) affects the skin and mucous membranes, and visceral leishmaniasis (VL) affects internal organs. Approaches to prevent transmission include vector control by reducing human contact with infected sandflies, and reservoir control, by reducing the number of infected animals.
Objectives
To assess the effects of vector and reservoir control interventions for cutaneous and for visceral leishmaniasis.
Search methods
We searched the following databases to 13 January 2015: Cochrane Infectious Diseases Group Specialized Register, CENTRAL, MEDLINE, EMBASE, LILACS and WHOLIS, Web of Science, and RePORTER. We also searched trials registers for ongoing trials.
Selection criteria
Randomized controlled trials (RCTs) evaluating the effects of vector and reservoir control interventions in leishmaniasis‐endemic regions.
Data collection and analysis
Two review authors independently searched for trials and extracted data from included RCTs. We resolved any disagreements by discussion with a third review author. We assessed the quality of the evidence using the GRADE approach.
Main results
We included 14 RCTs that evaluated a range of interventions across different settings. The study methods were generally poorly described, and consequently all included trials were judged to be at high or unclear risk of selection and reporting bias. Only seven trials reported clinical outcome data which limits our ability to make broad generalizations to different epidemiological settings and cultures.
Cutaneous leishmaniasis
One four‐arm RCT from Afghanistan compared indoor residual spraying (IRS), insecticide‐treated bednets (ITNs), and insecticide‐treated bedsheets, with no intervention. Over 15 months follow‐up, all three insecticide‐based interventions had a lower incidence of CL than the control area (IRS: risk ratio (RR) 0.61, 95% confidence interval (CI) 0.38 to 0.97, 2892 participants, moderate quality evidence; ITNs: RR 0.32, 95% CI 0.18 to 0.56, 2954 participants, low quality evidence; ITS: RR 0.34, 95% CI 0.20 to 0.57, 2784 participants, low quality evidence). No difference was detected between the three interventions (low quality evidence). One additional trial of ITNs from Iran was underpowered to show a difference.
Insecticide treated curtains were compared with no intervention in one RCT from Venezuela, where there were no CL episodes in the intervention areas over 12 months follow‐up compared to 142 in control areas (RR 0.00, 95% CI 0.00 to 0.49, one trial, 2938 participants, low quality evidence).
Personal protection using insecticide treated clothing was evaluated by two RCTs in soldiers, but the trials were underpowered to reliably detect effects on the incidence of CL (RR 0.40, 95% CI 0.13 to 1.20, two trials, 558 participants, low quality evidence).
Visceral leishmaniasis
In a single RCT of ITNs versus no intervention from India and Nepal, the incidence of VL was low in both groups and no difference was detected (RR 0.99, 95% CI 0.46 to 2.15, one trial, 19,810 participants, moderate quality evidence).
Two trials from Brazil evaluated the effects of culling infected dogs compared to no intervention or IRS. Although they report a reduction in seroconversion over 18 months follow‐up, they did not measure or report effects on clinical disease.
Authors' conclusions
Using insecticides to reduce phlebotomine sandfly numbers may be effective at reducing the incidence of CL, but there is insufficient evidence from trials to know whether it is better to spray the internal walls of houses or to treat bednets, curtains, bedsheets or clothing.
16 April 2019
Update pending
Studies awaiting assessment
The CIDG is currently examining a new search conducted in April 2019 for potentially relevant studies. These studies have not yet been incorporated into this Cochrane Review. All eligible published studies found in the last search (13 Jan, 2015) were included and one ongoing study was identified (see 'Characteristics of ongoing studies' section).
Plain language summary
Vector and reservoir control for preventing leishmaniasis
This review summarises trials evaluating different measures to prevent leishmaniasis. After searching for relevant trials up to January 2015, we included 14 randomized controlled trials.
What is vector and reservoir control and how might they prevent leishmaniasis?
Leishmaniasis is a group of infectious diseases caused by Leishmania parasites, which are transmitted between humans and animals by the bite of infected phlebotomine sandflies. There are two main clinical diseases: cutaneous leishmaniasis (CL), where parasites infect the skin, and visceral leishmaniasis (VL), where they infect the internal organs.
Leishmaniasis could be prevented by reducing human contact with infected phlebotomine sandflies (the vector), or by reducing the number of infected animals (the reservoir).
What the research says?
Cutaneous leishmaniasis
Using insecticides to reduce the number of sandflies may be effective at reducing the number of new cases of cutaneous leishmaniasis (low quality evidence). However, there is not enough evidence to know whether it is better to use insecticides to spray the internal walls of houses, or use insecticide treated bednets, bedsheets, or curtains.
Personal protection using insecticide treated clothing was also evaluated in two small trials in soldiers, but the trials were too small to know whether this was effective (low quality evidence).
Visceral leishmaniasis
Insecticide treated nets may not be effective at preventing visceral leishmaniasis but this has only been tested in a single trial from India and Nepal (low quality evidence).
Although culling dogs is sometimes discussed as a potential way to reduce visceral leishmaniasis, this has not been tested in trials measuring clinical disease.
Summary of findings
Background
Description of the condition
Leishmaniasis is a group of diseases caused by infection with Leishmania species parasites. Two broad clinical syndromes affect people (Reithinger 2007):
Cutaneous or tegumental leishmaniasis (CL), where Leishmania parasites infect the skin or mucous membranes; and
Visceral leishmaniasis (VL), also known as Kala‐Azar, where Leishmania parasites infect internal organs, such as the spleen, liver, bone marrow and lymph nodes.
The World Health Organization (WHO) considers leishmaniasis to be one of the most serious parasitic diseases in terms of prevalence and geographical distribution. Approximately 350 million, often impoverished, people are at risk of contracting leishmaniasis (Alvar 2006). Worldwide, more than 20 Leishmania species are known to infect humans across 98 countries or territories (Alvar 2012). The WHO estimates that one million to 1.3 million new cases occur each year; one million for CL and 300,000 for VL (WHO 2009).
In the Old World (North Africa, the Mediterranean, the Middle East, Northeast of India, and Central Asia), CL is most commonly caused by Leishmania major, Leishmania tropica and Leishmania aethiopica, and less frequently by Leishmania infantum and Leishmania donovani (Alvar 2012). In the New World (Central and South America), CL may be caused by the Leishmania mexicana species complex (particularly L. mexicana, Leishmania amazonensis and Leishmania venezuelensis) or the Leishmania Viannia sub‐genus (particularly Leishmania (V) braziliensis, Leishmania (V) panamensis,Leishmania (V) guyanensis and Leishmania (V) peruviana). Half of the skin lesions caused by L. mexicana heal in three months, while those due to L. (V) braziliensis,L. (V) panamensis and L. (V) guyanensis persist for much longer and may evolve to mucocutaneous leishmaniasis. VL is caused by L. donovani in the Indian subcontinent and East Africa, and L. infantum in the Middle East, the Mediterranean basin and South America (WHO 2010).
Several drug (topical and systemic), physical and immunological therapeutic modalities have been used for leishmaniasis treatment (Das 2008; González 2008; González 2009; Romero 2010).
The infection is transmitted between humans (anthroponotic leishmaniasis) or from animals to humans (zoonotic leishmaniasis) by the bite of infected phlebotomine sandflies (Desjeux 1996). Sandflies can breed in cracks, in walls or among rocks, animals' burrows, caves, damp leaf litter in forests, holes in the ground, stable floors, poultry houses and termite hills. Both male and female phlebotomine sandflies feed on sugar and plants juices but the females also blood‐feed. Female phlebotomine sandflies usually bite at night; some species feed indoors (endophagic), whilst others feed outdoors (exophagic) (Roberts 2006). In the Old World, the sandfly vectors belong to the genus Phlebotomus, while in the New World they belong to the genus Lutzomyia. Due to a co‐evolution process, there is an association between the Leishmania species, its animal reservoir (host) and the phlebotomine sandfly species involved in the transmission of leishmaniasis (Table 8).
1. Association between the Leishmania species, its animal reservoir and the sandfly species involved in the leishmaniasis transmission.
| CL | |||||
| Epidemiological form | Leishmania species | Sandfly species | Reservoir | Clinical form | Other clinical forms |
| Old World | |||||
| Anthroponotic | L. tropica | P. sergenti | Human | Urban endemic CL | Mucocutaneous, recidivans (chronic) |
| Zoonotic | L. major | P. papatasi,P. duboscqi | Rodents | Rural epidemic CL | Mucocutaneous |
| L. aethiopica | P. longipes, P. pedifer | Hyraxes | CL | Diffuse | |
| L. infantum | P. perniciosus, P. ariasi, P. perfiliewi, P. longiductus, P. chinensis | Dogs | Mucocutaneous | ||
| New World | |||||
| Zoonotic | L. mexicana | Lu. olmeca | Rodents | CL | Disseminated |
| L. amazonensis | Lu. flaviscutellata | Canids, monkeys, rodents, marsupials | Diffuse, disseminated | ||
| L. braziliensis | Lu. intermedia, Lu. gomezi,Lu. wellcomei, Lu. whitmani, Lu. carrerai, Lu. yucumensis, Lu. llanosmartinsi, Lu. spinicrassa,Lu. ovallesi | Edentates, opossums, rodents and dogs | Mucocutaneous, disseminated | ||
| L. panamensis | Lu. rapidoi, Lu. gomezi, Lu. ylephiletor, Lu. panamensis | Sloths, marsupials, rodents | |||
| L. guyanensis | Lu. umbratilis, Lu. whitmani, Lu. anduzei, Lu. longiflocosa | Sloths, edentates, marsupials | Mucocutaneous, disseminated | ||
| Anthroponotic | L. peruviana | Lu. ayacuchensis, Lu. peruensis, Lu. verrucarum | Humans, dogs? | Mucocutaneous (rare) | |
| VL | |||||
| Epidemiological form | Leishmania species | Sandfly species | Reservoir | Clinical form | Possible outcome |
| Old World | |||||
| Anthroponotic | L. donovani | P. argentipes, P. orientalis, P. martini | Human | VL | PKDL |
| Zoonotic | L. infantum | P. perniciosus, P. ariasi, P. perfiliewi, P. neglectus, P. longiductus, P. chinensis and others | Dogs | CL | |
| New World | |||||
| Zoonotic |
L. infantum (= L. chagasi) |
Lu. longipalpis,Lu. evansi | Dogs, marsupials | VL | PKDL (extremely rare) |
Based on WHO 2010. Abbreviations: CL: cutaneous leishmaniasis; VL: visceral leishmaniasis; PKDL: post kala‐azar dermal leishmaniasis.
Description of the intervention
Leishmaniasis could be prevented by reducing the number of infected phlebotomine sandflies (vector control), or by reducing the animal reservoir of Leishmania in areas where the disease in commonly zoonotic (reservoir control). One further possibility is the development of effective human vaccines, but these are evaluated in a separate Cochrane Review (Khanjani 2009).
In general, phlebotomine sandflies are highly sensitive to insecticides although some resistance to DDT has been reported (Dinesh 2010). Insecticide may be sprayed onto the internal walls of houses, also known as indoor residual spraying (IRS), or impregnated into bednets (also known as insecticide treated nets (ITNs)), curtains (insecticide treated curtains (ITCs)), bedsheets (insecticide treated sheets (ITS)) or clothing. IRS is the most widely used intervention for controlling endophagic phlebotomine sandflies but needs to be repeated regularly, which decreases its long‐term sustainability (Davies 2003). ITNs and ITCs also need to be replaced or retreated regularly but usually less frequently than IRS, and therefore may be more sustainable. However, most phlebotomine sandfly activity occurs around sunset, generally before people have retired for the night, which may limit their effects (Roberts 2006). In areas where phlebotomine sandflies are typically exophagic or leishmaniasis represents an occupational hazard, such as for soldiers or hunters, the use of insect repellents or protective clothes may be the only preventive measures available (Alexander 2003), but it is unlikely to be practical or affordable for poor populations living in highly endemic areas.
Alternatively, phlebotomine sandfly numbers could be reduced by removing breeding sites from the environment through activities such as re‐plastering of cracks in walls with mud or lime (Kishore 2006).
The methods used to control the reservoir (host) of zoonotic leishmaniasis depend on which animals act as reservoirs. Dogs play an important role as leishmaniasis reservoirs in some areas, and development of appropriate control measures is necessary (Courtenay 2009; Dogan 2006; Quinell 2009). Other animal reservoirs, such as rodents, have been targeted through poisonous baits (Roberts 2006).
Since disease control efforts are focused on reducing sandfly‐human contact or sandfly populations, other leishmaniasis control strategies on socioeconomic aspects should include (Alvar 2006):
Fight against poverty.
Gender equality and elimination of other sociocultural barriers.
Access to health care (mainly in the case of human reservoirs like anthroponotic VL or post kala‐azar dermal leishmaniasis (PKDL), and asymptomatic infections, including direct non‐medical cost as transport).
House construction and placement of domestic animal enclosures (poor housing conditions are associated with ecological factors that increase the risk of human‐vector contact).
Educational health programmes and community participation.
Why it is important to do this review
A wide range of leishmaniasis preventive options have been used in different parts of the world. This Cochrane Review aims to summarise available research categorised by disease forms, settings and geographical regions.
Objectives
To assess the effects of vector and reservoir control interventions on all forms of leishmaniasis.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs).
Types of participants
People living in leishmaniasis endemic regions.
Types of interventions
Any intervention that aims to reduce leishmaniasis incidence through vector or reservoir control.
Types of outcome measures
Primary outcomes
People developing CL or VL infections.
Secondary outcomes
Estimates of the vector density measured by an appropriate technique (adult sandfly density estimated by counts of vectors either landing on exposed body parts of humans acting as baits or collected resting inside buildings, for example, on walls).
Number of participants with positive immunological or biochemical tests that detect contact with the parasite (for example, leishmanin skin test conversion rates or lymphocyte proliferation rates, or both).
Adverse effects on people.
Adherence to control measures; for example, the extent to which specified intervention components were delivered as prescribed.
Measures of environmental impact (assessment of the possible impact ‐ positive or negative ‐ that the interventions may have on the natural environment) or sustainability (assessment of the ability to change biological and human processes, functions, biodiversity and productivity), or both.
Search methods for identification of studies
We attempted to identify all relevant trials regardless of language or publication status (published, unpublished, in press and ongoing).
Electronic searches
We searched the following databases up to 13 January 2015: Cochrane Infectious Diseases Group (CIDG) Specialized Register, Appendix 1); the Cochrane Central Register of Controlled Trials (CENTRAL) from the Cochrane Library, Issue 12, 2014 (Appendix 2); MEDLINE (PubMed.gov from 1900, Appendix 3); EMBASE (Data Star, from 1947, Appendix 4); LILACS, from 1982 (Appendix 5), WHOLIS (Appendix 6), Web of Science (Science Direct, from 1900, Appendix 7); and RePORT Expenditures and Results (RePORTER) which contains information on controlled trials being funded or supported by the US Department of Health and Human Services http://projectreporter.nih.gov/reporter.cfm, Appendix 8).
Ongoing trials databases
We searched the following ongoing trials registers on 13 January 2015 using the strategies in Appendix 9:
MetaRegister of Controlled trials on www.controlled‐trials.com;
US National Institutes of Health Register on www.clinicaltrials.gov;
Ongoing Skin Trials Register on www.nottingham.ac.uk/ongoingskintrials;
Australian and New Zealand Clinical Trials Registry on www.anzctr.org.au;
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) on www.who.int/trialsearch.
Searching other resources
References from published studies
We looked at the bibliographies of all papers identified by these strategies.
Researchers, organizations and pharmaceutical companies
We contacted researchers in the field to identify additional studies eligible for inclusion.
Adverse events search
We searched for adverse or side effects of interventions using the search strategy in Appendix 10.
Data collection and analysis
Selection of studies
At least two review authors (AF, MP or UG) independently screened the title and abstract of all identified citations for potential eligibility using an eligibility form. We resolved any disagreements by discussion between the review authors, with referral to a third review author if necessary (UG or JA). We removed duplicate publications.
Data extraction and management
At least two review authors (CE and AF; CE and MP; or all three) independently performed data extraction using a pre‐designed data extraction form. We resolved any disagreements by discussion or referral to another review author (UG).
We extracted information regarding the trial characteristics and trial methods, including setting, comparability between sites and outcomes and how these were measured. For dichotomous outcomes, we extracted the number of participants experiencing the event and the number of participants for each treatment group. For continuous outcomes, we extracted the arithmetic mean and standard deviation (SD) for each treatment group, together with the number of participants in each group. However, if the data were reported using geometric means we recorded this information and extracted a SD on the log scale. If median values were used, we extracted medians and ranges. For data on an interval scale, we extracted the number of treatment events and control group and the total person time at risk in each group or the rate ratio and a measure of variance (for example, standard error).
We extracted the number of randomized participants and analysed them in each treatment group and the denominator populations for estimating incidence for each trial and outcome. We checked for co‐interventions and we examined whether both control and intervention arms experienced the same co‐interventions.
For cluster‐RCTs, we extracted information on the number of clusters, average size of the cluster, unit of randomization (such as communities or villages), adjustment for clustering or other covariates in the statistical analysis, and estimates of the intra‐cluster correlation coefficient (ICC) for each outcome. Where results were adjusted for clustering, we extracted the point estimate with 95% confidence intervals (CIs); otherwise we adjusted the unadjusted results before incorporating them into our analyses.
Assessment of risk of bias in included studies
Pairs of review authors (including AF, MP or CE) (AF, MP and CE) independently assessed the risk of bias for each included trial using a 'Risk of bias' assessment form. We resolved any discrepancies between the results of the risk of bias analysis by referral to a third review author (UG). We assigned judgments concerning the risk of bias for each component classified as 'high', 'low' or 'unclear' risk of bias, respectively. We recorded the information in a 'Risk of bias' table and 'Risk of bias' graph.
Measures of treatment effect
For dichotomous outcomes, we presented all results as risk ratios (RR) with 95% CIs. Where trial authors presented results as cluster‐adjusted odds ratio we converted this to a RR using the guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2009). We presented vector density and other outcomes, such as ages of cases, descriptively in tables.
Unit of analysis issues
Where cluster‐RCTs met the inclusion criteria, we assessed whether the trial authors had taken account of clustering in the primary analysis. If trial authors had appropriately adjusted for clustering we extracted the adjusted data for inclusion in our analysis. Where trial authors had not adjusted for clustering, we performed an approximate adjustment using estimates of the ICC derived from similar studies (Table 9).
2. Analysis of cluster‐RCTs reporting clinical outcomes.
| Trial ID | Unit | Mean cluster population | Number of clusters | Cluster adjustment by trial authors | Approximate ICC calculated by review authors1 | Cluster adjustment by review authors2 |
| Costa 2007 BRA | Geographical area | 11 | 34 | "We specify a model that explicitly considered the effect of aggregation of the individual in clusters (cluster effect) and used methods of robust estimation of variance. Data analysis was performed using STATA software." | Unable to calculate because the raw data were not presented. | None necessary. |
| Emami 2009 IRN | Urban sectors | 635 | 12 | None (analysed at the individual level). | ‐ | SE adjusted for clustering using the ICC from Rojas 2006 COL. |
| Kroeger 2002 VEN | City sectors | 210 | 14 | 'We compared data using a paired t test, weighting the data according to the sector size. We also used Wilcoxon's matched pairs test because the small number of pairs made it difficult to assess whether the underlying distribution of the differences was normal'. | Unable to calculate as authors only presented mean difference adjusted for clustering. | RR was calculated from raw data and the SE adjusted for clustering using the ICC from Rojas 2006 COL. |
| Picado 2010a ASIA | Hamlets | 761 | 26 | "Adjusted analyses were carried out in two stages...a standard individual level logistic regression model to calculate expected number of events for each cluster ignoring the intervention...The adjusted intervention effect was calculated with these residuals in a paired t test". | 0.0010 | None necessary. |
| Reyburn 2000 AFG | Household | 5 | 957 | "Because the interventions were allocated at household level, the data were analysed by a random effects logistic regression model to adjust for the possibility that individuals within a household might be more similar with respect to the intervention outcome than individuals from other households". | 0.0321 | Converted from OR to RR using the formula: RR = OR/(1‐ACRx(1‐OR)). |
| Rojas 2006 COL | Village | 182 | 20 | "Once the final model was defined, the generalized estimating equations method was used to estimate the parameters while taking into account the correlation of observations within villages". | 0.0034 | None necessary. |
| Werneck 2014 BRA | City blocks containing ≈ 60 households | 70 | 40 | "using Poisson population‐average models from generalized estimating equations with robust variance, an exchangeable correlation model, and designating each block as the clustering level". | ‐ | None necessary. |
Abbreviations: BRA = Brazil; IRN = Iran; VEN = Venezuela; AFG = Afghanistan; COL= Colombia; ICC = intra‐cluster correlation co‐efficient; SE = standard error; RR = risk ratio; OR = odds ratio. 1We calculated the ICC by comparing the cluster‐adjusted SE with the unadjusted SE to calculate the design effect (DE) and then using the formula: DE = 1+(M‐1)*ICC where M=mean cluster size. 2We chose the ICC value by looking for the trial with the most similar size of clusters and number of clusters.
Dealing with missing data
We reported whether participants or communities were lost to follow‐up during the time period of the trial. We analysed data according to a complete case analysis. We performed sensitivity analyses to asses the effect of missing data and to ensure the robustness of our conclusions.
Assessment of heterogeneity
When we combined trials in a meta‐analysis, we examined forest plots to detect overlapping CIs, and applied the Chi² test (using a P value of 0.10 to indicate statistical heterogeneity), and the I² statistic (using a value of 50% to denote moderate levels of heterogeneity).
Assessment of reporting biases
We searched for citation and multiple publication bias, language bias and outcome reporting bias.
Data synthesis
Three review authors (DS, TE and UG) analysed the data using RevMan 2014 and presented all results with 95% CIs.
In individually RCTs and cluster‐RCTs, we calculated RRs and 95% CIs for dichotomous data. We did not analyse vector densities, but merely presented the results of the individual trials. We could not consider meta‐analysis to calculate a weighted effect across trials regarding participants (different Leishmania spp infections), interventions (reservoir and vector control) and outcome. We aimed to perform an intention‐to‐treat (ITT) analysis when the trial authors accounted for all randomized participants; otherwise we performed a complete‐case analysis.
When we detected no statistically significant heterogeneity, we applied a fixed‐effect model. When we observed statistically significant heterogeneity within groups that could not be explained by subgroup or sensitivity analyses, we applied a random‐effects model to synthesize the data. However, when substantial heterogeneity was determined, we did not carry out meta‐analysis but presented a forest plot with the pooled effect suppressed and reported the I² statistic and P value from a Chi² test.
We described qualitatively the main adverse effects related with insecticides.
Subgroup analysis and investigation of heterogeneity
We anticipated that effects would vary with leishmania species, and the geographic setting of the trial, and grouped studies accordingly.
Sensitivity analysis
We planned to conduct sensitivity analysis examining effects of bias risk but there were too few included trials to do this.
Assessment of quality of evidence
We assessed the quality of evidence using the GRADE approach (GRADE Working Group 2004) and GRADEpro 2015 software.
Results
Description of studies
See: Characteristics of included studies and Characteristics of excluded studies.
Results of the search
We identified 32 trials from our searches, of which we included 14 and excluded 18. We found one ongoing RCT (Characteristics of ongoing studies). We have detailed our search results in a PRISMA flow diagram (Figure 1).
1.
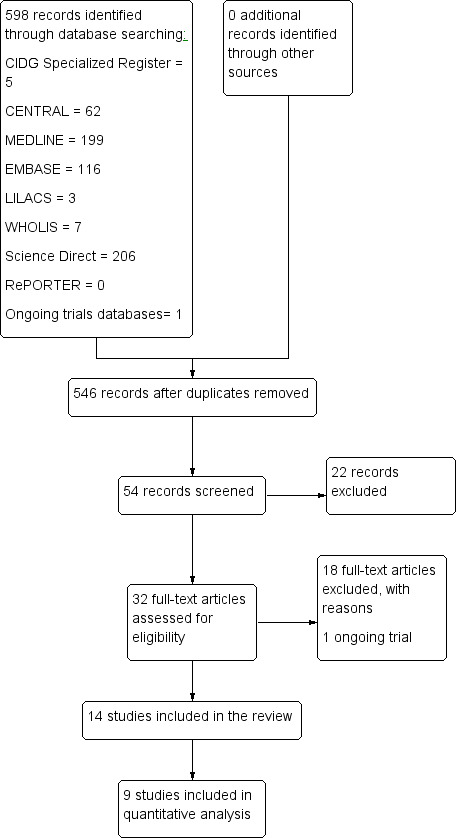
Study flow diagram.
Included studies
We have provided details of the 14 included trials in the Characteristics of included studies tables.
Trial design
Ten trials were cluster‐RCTs that randomized villages (Rojas 2006 COL), urban sectors (Costa 2007 BRA; Emami 2009 IRN; Kroeger 2002 VEN; Werneck 2014 BRA), hamlets or households (Chowdhury 2011 BGD; Joshi 2009 ASIA; Picado 2010a ASIA; Reyburn 2000 AFG) or individual houses (Kelly 1997 BRA). Two were paired RCTs that randomized houses (Dinesh 2008 IND; Feliciangeli 2003 VEN). Two were individually RCTs in soldiers (Asilian 2003a IRN; Soto 1995 COL).
Participants
Seven trials were conducted in Asia: Afghanistan (Reyburn 2000 AFG), Iran (Asilian 2003a IRN; Emami 2009 IRN), India (Dinesh 2008 IND), Bangladesh (Chowdhury 2011 BGD), India and Nepal (Picado 2010a ASIA), India, Bangladesh and Nepal (Joshi 2009 ASIA). Seven trials were conducted in South America: Colombia (Rojas 2006 COL; Soto 1995 COL), Brazil (Costa 2007 BRA; Kelly 1997 BRA; Werneck 2014 BRA) and Venezuela (Feliciangeli 2003 VEN; Kroeger 2002 VEN).
Settings
Most trials mentioned the which Leishmania species were endemic in the area and therefore assumed this species was the causative agent of leishmaniasis. One RCT reported that CL was caused by L. tropica (Emami 2009 IRN), three RCTS by L. chagasi (L. infantum) (Costa 2007 BRA; Kelly 1997 BRA; Werneck 2014 BRA), one trial by L. braziliensis and L. panamensis (Rojas 2006 COL), and one trial by L. braziliensis and L. mexicana (Feliciangeli 2003 VEN). Three RCTs reported that VL was caused by L. donovani (Chowdhury 2011 BGD; Dinesh 2008 IND; Picado 2010a ASIA). Four RCTs failed to mention theLeishmania species involved: one in a VL area (Joshi 2009 ASIA) and three in CL areas (Asilian 2003a IRN; Kroeger 2002 VEN; Soto 1995 COL). One RCT reported that infections in the respective endemic areas were caused by anthroponotic CL (Reyburn 2000 AFG).
Interventions
We found 12 RCTs that evaluated the use of insecticides in vector control. Trials used a variety of different interventions, including IRS (five trials: Chowdhury 2011 BGD; Feliciangeli 2003 VEN; Joshi 2009 ASIA; Kelly 1997 BRA; Reyburn 2000 AFG), ITNs (six trials: Chowdhury 2011 BGD; Emami 2009 IRN; Joshi 2009 ASIA; Picado 2010a ASIA; Reyburn 2000 AFG; Rojas 2006 COL), ITCs (one trial: Kroeger 2002 VEN), ITS (two trials: Kelly 1997 BRA; Reyburn 2000 AFG) or insecticide treated uniforms (two trials: Asilian 2003a IRN; Soto 1995 COL).
Two additional trials evaluated IRS plus reservoir control through spraying houses and animal pens and eliminating infected dogs (Costa 2007 BRA; Werneck 2014 BRA).
Outcomes
Seven trials reported clinical outcomes as the incidence of new CL cases (Asilian 2003a IRN; Emami 2009 IRN; Kroeger 2002 VEN; Reyburn 2000 AFG; Rojas 2006 COL; Soto 1995 COL), or VL (Picado 2010a ASIA). Four trials used immunological or biochemical tests (Costa 2007 BRA; Picado 2010a ASIA; Rojas 2006 COL; Werneck 2014 BRA) for detecting the presence of the Leishmania parasite on participants (for example, leishmanin skin test conversion rates or lymphocyte proliferation rates, or both). Six trials (Costa 2007 BRA; Dinesh 2008 IND; Emami 2009 IRN; Joshi 2009 ASIA; Kelly 1997 BRA; Kroeger 2002 VEN) reported on entomological outcomes (vector density). Only three trials reported adverse effects (Asilian 2003a IRN; Rojas 2006 COL; Soto 1995 COL). Two trials reported acceptability and adherence to control measures from participants (for example, the extent to which specified intervention components were delivered as prescribed) (Picado 2010a ASIA; Reyburn 2000 AFG).
Excluded studies
We excluded 18 RCTs and listed the reasons in the Characteristics of excluded studies table.
Risk of bias in included studies
We have described the risk of bias of each included trial in the Characteristics of included studies tables. We included a 'Risk of bias' summary (Figure 2) and a 'Risk of bias' graph (Figure 3).
2.
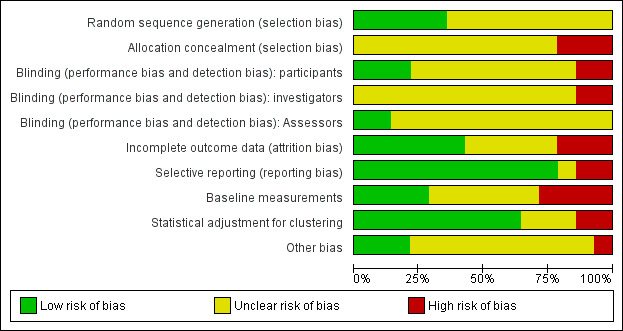
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included trials.
3.
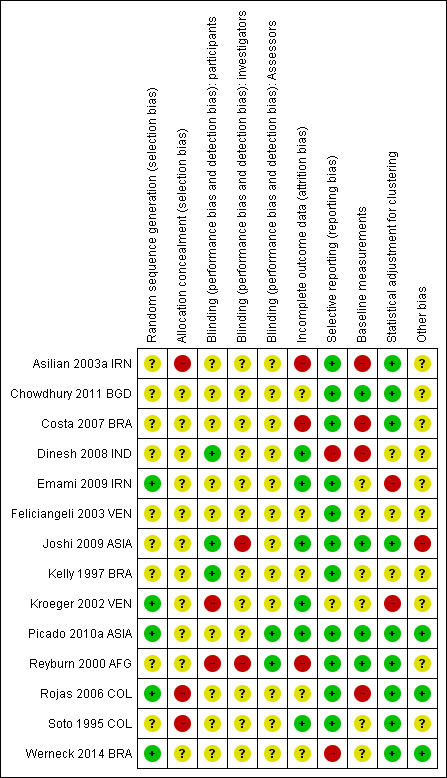
Methodological quality summary: review authors' judgements about each methodological quality item for each included trial.
Allocation
AIl included trials stated or implied that allocation was randomized; however only five trials described the method of sequence generation (Emami 2009 IRN; Kroeger 2002 VEN; Picado 2010a ASIA; Rojas 2006 COL; Werneck 2014 BRA), and no trials described allocation concealment.
Blinding
Two included RCTs were double‐blinded (Asilian 2003a IRN; Soto 1995 COL), two were single‐blinded (Kroeger 2002 VEN; Reyburn 2000 AFG), and ten trials did not use any blinding or did not mention it.
Incomplete outcome data
An individually RCT accounted for losses to follow‐up (Asilian 2003a IRN), and the other individually RCT reported no drop‐outs (Soto 1995 COL). However, Asilian 2003a IRN only assessed participants who completed the use of the preventive measure. We took all participants that were randomized at the beginning of the trial to evaluate the final effect of the intervention. We assumed that missing data were failures. The trial did not specify if they were post randomization or later losses. Overall there was no losses of clusters or the losses were not reported.
Selective reporting
One of the included trials, Dinesh 2008 IND, reported only the results that showed statistically significant differences between intervention groups, instead of all results.
Other potential sources of bias
In nine of the included RCTs the trial authors did not provide a conflict of interest declaration and in five of the included RCTs trial authors declared no competing interests. See risk of bias tables in Characteristics of included studies for more details.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7
Summary of findings for the main comparison. Summary of findings table 1.
| Indoor residual spraying (IRS) versus no intervention for preventing leishmaniasis | ||||||
|
Patient or population: People at risk of cutaneous leishmaniasis (CL) or visceral leishmaniasis (VL) Settings: CL or VL endemic areas Intervention: IRS Comparison: No intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No intervention | IRS | |||||
| Vector density | ‐ | ‐ | Not pooled | (4 trials) | ⊕⊕⊕⊝ moderate1,2,3 | Reductions in sandfly abundance were seen after IRS spraying in all four trials |
|
CL cases > 12 months follow‐up |
52 per 1000 |
32 per 1000 (20 to 50) |
RR 0.61 (0.38 to 0.97) |
2892 (1 trial) |
⊕⊕⊕⊝ moderate1,4,5,6 | ‐ |
|
VL cases > 2 years follow‐up |
‐ | ‐ | ‐ | (0 trials) | ‐ | ‐ |
| *The basis for the assumed risk (for example, the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; CL: cutaneous leishmaniasis; VL: visceral leishmaniasis; IRS: indoor residual spraying. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded by 1 for serious risk of bias: Trials are at high or unclear risk of selection bias and reporting bias. 2No serious inconsistency: Reductions in sandfly abundance were seen after IRS spraying in all four trials (compared to control areas). 3No serious indirectness: The trials used insecticides shown to be effective in the trial area. Trials were from India, Bangladesh, Nepal, Venezuela and Brazil. 4The assumed risk of CL over 12 months follow‐up is taken from the control group in Reyburn 2000 AFG. This trial was conducted in Afghanistan from 1997 to 1998. 5No serious indirectness: This single trial was conducted in urban areas of Afghanistan using lambdacyhalothrin at a target rate of 30 mg/m². Further trials from different settings would increase confidence in this result. 6Downgraded by 1 for serious imprecision: The 95% CI is wide and includes clinically important effects and no real difference.
Summary of findings 2. Summary of findings table 2.
| ITNs versus no intervention for preventing leishmaniasis | ||||||
|
Patient or population: People at risk of CL or VL Settings: CL or VL endemic areas Intervention: ITNs Comparison: No intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No intervention | ITNs | |||||
| Vector density | ‐ | ‐ | Not pooled | (3 trials) | ⊕⊕⊝⊝ low1,2,3,4 | Two trials found a reduction in vector numbers post‐intervention and one did not. |
|
CL cases > 12 months follow‐up |
52 per 1000 |
16 per 1000 (9 to 28) |
RR 0.31 (0.18 to 0.53) |
10,579 (2 trials) |
⊕⊕⊝⊝ low2,5,6,7,11 | ‐ |
|
VL cases > 2 years follow‐up |
4 per 1000 |
4 per 1000 (2 to 9) |
RR 0.99 (0.46 to 2.15) |
19,810 (1 trial) |
⊕⊕⊕⊝ moderate8,9,10 | ‐ |
| *The basis for the assumed risk is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; CL: cutaneous leishmaniasis; VL: visceral leishmaniasis; ITN: insecticide treated bednet. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Three RCTs evaluated vector density, but one did not present before and after data and only stated the difference was statistically significant. 2Downgraded by 1 for serious risk of bias: Trials are at high or unclear risk of selection bias and reporting bias. 3Downgraded by 1 for serious inconsistency: Chowdhury 2011 BGD reports a statistically significant difference in total vector numbers over 12 months follow‐up, Emami 2009 IRN reports statistically significant reduction but did not provide data. Joshi 2009 ASIA found no difference in mean number of vectors per household. 4No serious indirectness: Chowdhury 2011 BGD distributed PermaNet® 2.0 to all households in trial sites in Bangladesh, Emami 2009 IRN distributed Olyset® in Iran, and Joshi 2009 distributed PermaNet® to households in India, Bangladesh and Nepal. 5The assumed risk of CL over 12 months follow‐up is taken from Reyburn 2000 AFG which contributed 99.5% of weight to this analysis. This trial was conducted in Afghanistan from 1997 to 1998. 6No serious indirectness: These two trials were conducted in urban areas of Iran (Olyset® nets), and Afghanistan (family size bednets impregnated with 0.5 g/m² of permethrin). The findings would be expected to apply to other endemic areas. 7No serious inconsistency: The two trials found similar effects. However, once adjusted for clustering the result was not statistically significant in the trial from Iran. 8The assumed risk of VL over 2 years months follow‐up is taken from the control group of Picado 2010a ASIA ‐ a study conducted in India and Nepal in 2006/09. 9No serious indirectness: This single trial was conducted in two areas (India and Nepal) using PermaNet® 2.0. 10Downgraded by 1 for serious imprecision: This trial found no difference between ITNs and control areas. However the 95% CI remains wide and includes the possibility of clinically important effects.
11Downgraded by 1 for serious indirectness: There are single trials from particular geographical areas.
Summary of findings 3. Summary of findings table 3.
| ITCs versus no intervention for preventing leishmaniasis | ||||||
|
Patient or population: People at risk of CL or VL Settings: CL or VL endemic areas Intervention: ITCs Comparison: No intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No intervention | Insecticide treated curtains | |||||
| Vector density | ‐ | ‐ | ‐ | ‐ (1 trial) |
⊕⊕⊝⊝ low1,2,3 | Vector density was substantially lower at 12 months post‐intervention |
|
CL cases > 12 months follow‐up |
52 per 1000 |
0 per 1000 (0 to 25) |
RR 0.00 (0.00 to 0.49) |
2938 (1 trial) |
⊕⊕⊝⊝ low1,2,4,5 | ‐ |
|
VL cases > 2 years follow‐up |
‐ | ‐ | ‐ | (0 trials) | ‐ | ‐ |
| *The basis for the assumed risk is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; CL: cutaneous leishmaniasis; VL: visceral leishmaniasis;ITC: insecticide treated curtains. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded by 1 for serious risk of bias: Trials were at high or unclear risk of selection bias and reporting bias. 2Downgraded by 1 for serious indirectness: There are single trials from particular geographical areas. The result may not be applicable elsewhere. Polyester curtains were impregnated with 12.5 mg/m2 lambdacyhalothrin at baseline and after 6 months. 3No serious imprecision: At 12 months post intervention vector density was substantially lower in the intervention group (P < 0.001) 4The control group risk of CL in Kroeger 2002 VEN was 89 per 1000 people. For consistency with other 'Summary of findings' tables we used an assumed risk of 52 per 1000, which was taken from Reyburn 2000 AFG. 5No serious imprecision: At 12 months post intervention, no CL cases had been reported in the intervention areas, compared to 148 in control areas.
Summary of findings 4. Summary of findings table 4.
| ITS versus no intervention for preventing leishmaniasis | ||||||
|
Patient or population: People at risk of CL or VL Settings: CL or VL endemic areas Intervention: ITS Comparison: No intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No intervention | ITS | |||||
| Vector density | ‐ | ‐ | ‐ | (1 trial) | ⊕⊝⊝⊝ very low1,2,3,4 | No data post‐intervention. |
|
CL cases > 12 months follow‐up |
52 per 1000 | 18 per 1000 (10 to 30) | RR 0.34 (0.20 to 0.57) | 2784 (1 trial) |
⊕⊕⊝⊝ low2,5,6 | ‐ |
|
VL cases > 2 years follow‐up |
‐ | ‐ | ‐ | (0 trials) | ‐ | ‐ |
| *The basis for the assumed risk is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; CL: cutaneous leishmaniasis; VL: visceral leishmaniasis; ITS: insecticide treated bedsheet. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1One trial evaluated the effects of hanging ITS near to a chicken shed. 2Downgraded by 1 for serious risk of bias: Trials are at high or unclear risk of selection bias and reporting bias. 3Downgraded by 2 for very serious indirectness: This is a single trial and does not directly assess the effects of ITS. 4The trial authors state that "the abundance in sheds was approximately 50% below that expected on the first day falling to about 80% at week 12 ‐ the only time the difference was statistically significant". 5The assumed risk of CL over 12 months follow‐up is taken from the control group of Reyburn 2000 AFG. This trial was conducted in Afghanistan from 1997 to 1998. 6Downgraded by 1 for serious indirectness: This trial was conducted in urban areas of Afghanistan using ITS treated with permethrin (1 g/m²). Further trials from different settings would increase confidence in this result.
Summary of findings 5. Summary of findings table 5.
| Insecticide treated uniforms versus no intervention for preventing leishmaniasis | |||||
|
Patient or population: People at risk of CL or VL Settings: CL or VL endemic areas Intervention: Insecticide treated uniforms Comparison: No intervention | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| No intervention | Insecticide treated uniforms | ||||
| Vector density | ‐ | ‐ | ‐ | (0 trials) | ‐ |
|
CL cases > 12 months follow‐up |
52 per 1000 |
21 per 1000 (7 to 62) |
RR 0.40 (0.13 to 1.20) |
558 (2 trials) |
⊕⊕⊝⊝ low1,2,3,4,5 |
|
VL cases > 2 years follow‐up |
‐ | ‐ | (0 trials) | ‐ | |
| *The basis for the assumed risk is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; CL: cutaneous leishmaniasis; VL: visceral leishmaniasis. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1The risk of CL in the control groups was 7% in Iran (Asilian 2003a IRN) and 13% in Colombia (Soto 1995 COL). To be consistent with the other 'Summary of findings' tables, we presented an assumed risk of 5.2%. 2Downgraded by 1 for serious risk of bias: Trials are at high or unclear risk of selection bias and reporting bias. 3No serious inconsistency: Although, one trial reported a statistically significant difference and one does not, this is likely related to the low CL incidence in the trial finding no difference. 4No serious indirectness: In both Iran and Colombia, soldiers were randomized to wear permethrin treated uniforms (concentration of 850 mg/m²) or standard uniforms. 5Downgraded by 1 for serious imprecision: The 95% CI of the overall effect is wide and includes clinically important effects and no difference.
Summary of findings 6. Summary of findings table 6.
| Multifaceted intervention versus no intervention for preventing leishmaniasis | |||||
|
Patient or population: People at risk of CL or VL Settings: CL or VL endemic areas Intervention: Multifaceted intervention Comparison: No intervention | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| No intervention | Multifaceted intervention | ||||
| Vector density | ‐ | ‐ | ‐ | 0 trials | ‐ |
|
CL cases 12 months follow‐up |
13 per 1000 | 6 per 1000 |
RR 0.42 (0.13 to 1.41) |
3631 (1 trial) |
⊕⊝⊝⊝ very low1,2,3 |
|
VL cases > 2 years follow‐up |
‐ | ‐ | ‐ | 0 | ‐ |
| *The basis for the assumed risk (for example, the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: risk ratio; CL: cutaneous leishmaniasis; VL: visceral leishmaniasis; ITNs: insecticide treated bednets. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1Downgraded by 1 for serious indirectness: This trial was conducted in urban areas of Colombia using a multifaceted intervention with ITNs, bars of insect repellent and permethrin painted trunks. Further studies with other combination of interventions and different settings would increase confidence in this result. 2Downgraded by 1 for serious risk of bias: the trial is at high or unclear risk of selection bias and reporting bias. 3Downgraded by 1 for serious imprecision: The 95% CI of the overall effect is wide and includes clinically important effects and no difference.
Summary of findings 7. Summary of findings table 7.
| IRS versus ITNs for preventing leishmaniasis | ||||||
|
Patient or population: People at risk of CL or VL Settings: CL or VL endemic areas Intervention: IRS Comparison: ITNs | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| ITNs | IRS | |||||
| Vector density | ‐ | ‐ | Not pooled | ‐ (2 trials) |
⊕⊕⊝⊝ low1,2,3 | One trial found a reduction in vector numbers post‐intervention and one trial did not. |
|
CL cases > 12 months follow‐up |
15 per 1000 | 30 per 1000 |
RR 1.90 (0.98 to 3.69) |
1655 (1 trial) |
⊕⊕⊝⊝ low1,4,5,6 | ‐ |
|
VL cases > 2 years follow‐up |
‐ | ‐ | ‐ | (0 trials) | ‐ | ‐ |
| *The basis for the assumed risk is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: risk ratio; CL: cutaneous leishmaniasis; VL: visceral leishmaniasis. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded by 1 for serious risk of bias: Trials are at high or unclear risk of selection bias and reporting bias. 2Downgraded by 1 for serious inconsistency: Chowdhury 2011 BGD reports a statistically significant difference in total vector numbers over 12 months follow‐up, Joshi 2009 ASIA found no difference in mean number of vectors per household. 3No serious indirectness: The trials used insecticides shown to be effective in the trial area. Trials were from India, Bangladesh and Nepal.
4Downgraded by 1 for serious indirectness: There is a single trial from a particular geographical area. 5No serious indirectness: This trial was conducted in urban areas of Afghanistan using lambdacyhalothrin at a target rate of 30 mg/m². Further studies from different settings would increase confidence in this result. 6Downgraded by 1 for serious imprecision: The 95% CI is wide and includes clinically important effects and no real difference.
Section A: Intervention versus no intervention
IRS versus no intervention
(See Table 1)
Effect on vector density
Four cluster‐RCTs evaluated the effect of IRS on vector density (Table 10). The insecticide used was deltamethrin (20 mg/m²) in Bangladesh (Chowdhury 2011 BGD), DDT (5%) in India, and alpha‐cypermethrine (0.025 mg/m²) in Nepal (Joshi 2009 ASIA), all against the vector Phlebotomous argentipes; and lambdacyhalothrin (25 mg/m²) in Brazil (Kelly 1997 BRA) and Venezuela (Feliciangeli 2003 VEN), with main vectors: Lu. longipalpis and Lu. ovallesi, respectively. The longest follow‐up was 12 months.
3. Vector density: IRS versus no intervention.
| Trial ID | Unit of randomization | Insecticide | Main vector | Measure (method) | Pre‐intervention | Post‐intervention | Effect measure (95% CI) or P value | ||
| IRS | Control | IRS | Control | ||||||
| Chowdhury 2011 BGD | Cluster of 50 houses | Deltamethrin (20 mg/m²) | P. argentipes | Total sandflies (monthly collections from 40 houses using light traps). |
633 (October 2006) | 683 (October 2006) | 8 (January 2007) 285 (March 2007) |
54 (January 2007) 1219 (March 2007) |
RR 0.38 (0.10 to 1.50) (Jan 2007) RR 0.28 (0.19 to 0.42) (Mar 2007) The benefit with IRS was no longer present at 12 months |
| Joshi 2009 ASIA | Hamlets or neighbourhoods | Deltamethrin ‐ BGD
(20 mg/m²) DDT ‐ IND (1 g/m²) Alpha‐cypermethrin ‐ NPL (0.025 g/m²) |
P. argentipes | Mean number of sandflies per house per night (light traps) | 12.32 (date not stated) |
9.41 (Date not stated) |
6.14 (5 months post intervention) |
12.15 (5 months post intervention) |
Pre‐intervention P = 0.184 Post‐intervention P = 0.035 |
| Kelly 1997 BRA | Chicken sheds | Lambdacyhalothrin (20 mg/m²) |
Lu.longipalpis | Geometric mean sandflies (light traps) | 1132.3* (October 1993) |
404.6* (October 1993) |
Not reported | Not reported | Pre‐intervention P < 0.001 The trial authors state "the abundance of Lu. longipalpis in sprayed sheds fell to approximately 10% of that expected, and remained so up to week 29". |
| Feliciangeli 2003 VEN | House | Lambdacyhalothrin (25 mg/m²) | Lu. ovallesi | Total sandflies over 79 days post‐intervention (daily catches using light traps) |
Not reported | Not reported | 2517 | 2472 | The trial authors state "The estimated catches of males, females, and fed females were significantly lower in sprayed houses immediately after spraying". However, over time the density in the control group also decreased ‐ probably due to seasonality |
| Proportion of blood fed females | Not reported | Not reported | 0.8% | 5.8% | |||||
Abbreviations: VEN = Venezuela; BRA = Brazil; BGD = Bangladesh; IRS = indoor residual spraying; RR = risk ratio).
All four trials reported substantial reductions in vectors at the intervention sites, although the variation in measurement and reporting of these outcomes precludes meta‐analysis. Despite marked seasonal variation in the abundance of flies, large reductions were seen with IRS compared to control areas in the two trials from Asia in areas of VL which randomized clusters of houses (Chowdhury 2011 BGD; Joshi 2009 ASIA). This effect lasted for nine months in Bangladesh but was no longer present at 12 months, and was only measured at a single time‐point of five months in India, Bangladesh and Nepal. The two trials from South America in areas of CL which randomized individual houses or chicken sheds reported short term reductions after the intervention but did not provide data to allow us to quantify the magnitude or duration of this effect (Feliciangeli 2003 VEN; Kelly 1997 BRA).
Effect on disease
CL: One cluster‐RCT from Afghanistan evaluated the effect of IRS on CL incidence (Reyburn 2000 AFG). IRS was applied once using lambdacyhalothrin (30 mg/m²). The cumulative analysis of new cases over 15 months showed a marked reduction in clinical cases with IRS (Intervention 36/1133 (3.2%); control 92/1759 (5.2 %); RR 0.61, 95% CI 0.38 to 0.97, one trial, 2892 participants in approximately 600 clusters, Analysis 1.1). The effect appears to be consistent across age groups (Table 11).
1.1. Analysis.
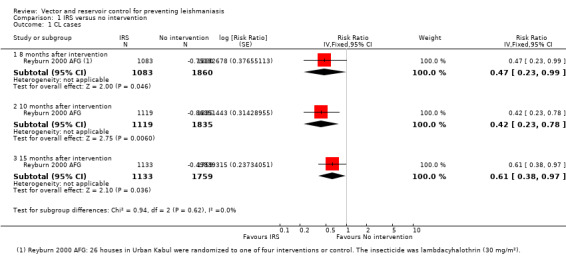
Comparison 1 IRS versus no intervention, Outcome 1 CL cases.
4. Incidence of new CL cases by intervention and age group in a cluster‐RCT from Afghanistan.
| Age group (years) | IRS | ITNs | Insecticide treated chaddar | Control (no intervention) |
| New cases (%) | New cases (%) | New cases (%) | New cases (%) | |
| 0 to 4 | 3 (1.9%) | 1 (0.6%) | 1 (0.8%) | 8 (3.7%) |
| 5 to 9 | 11 (7.9%) | 3 (2%) | 4 (3.5%) | 12 (5.2%) |
| 10 to 19 | 8 (4.5%) | 5 (2.5%) | 4 (2.1%) | 31 (9.1%) |
| ≥ 20 | 14 (4.2%) | 11 (3.3%) | 9 (3.0%) | 41 (8.4%) |
| Total | 36 (4.4%) | 20 (2.4%) | 18 (2.5%) | 92 (7.2%) |
Adapted from Reyburn 2000 AFG. Age distribution of new CL cases among the non‐immune participants at the end of the trial. Acording to trial authors, the age distribution of new cases was not significantly different between the intervention groups (P = 0.48).
VL: No trials evaluated the effects of IRS on VL incidence. However, one trial assessed the effect on seroconversion in a VL endemic area in Brazil (Werneck 2014 BRA) and found no statistically significant difference in seroconversion over 18 months post intervention (Intervention 47/93 (50.5%); control 60/95 (63.2%); RR 0.86, 95% CI 0.63 to 1.17, one trial, 295 participants in 40 clusters, Analysis 1.2).
1.2. Analysis.
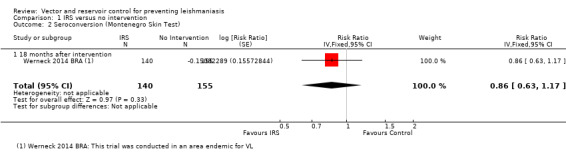
Comparison 1 IRS versus no intervention, Outcome 2 Seroconversion (Montenegro Skin Test).
ITNs versus no intervention or untreated nets
(See Table 2)
Effect on vector density
Three cluster‐RCTs evaluated the effect of ITNs on vector density (Table 12). Two trials in areas of VL from Asia used PermaNet® bednets impregnated with deltamethrin (55 mg/m²) (Chowdhury 2011 BGD; Joshi 2009 ASIA, vector: P. argentipes); and one trial in Iran used Olyset® bednets impregnated with permethrin (2%) (Emami 2009 IRN), main vector: P. sergenti). All three trials randomized clusters of houses (hamlets, neighbourhoods or city sectors).
5. Vector density: ITNs versus no intervention.
| Trial ID | Unit of randomization | Intervention | Main vector | Measure (method) | Pre‐intervention | Post‐intervention | Effect measure (95% CI) or P value | ||
| ITNs | Control | ITNs | Control | ||||||
| Chowdhury 2011 BGD | Cluster of 50 houses | PermaNet® 2.0 (deltamethrin 55mg/m²) distributed to all households in November 2006. | P. argentipes | Total sandflies (monthly collections from 40 houses using light traps). |
724 (October 2006) | 683 (October 2006) | 18 (January 2007) 361 (March 2007) |
54 (January 2007) 1219 (March 2007) |
RR 0.73 (0.23 to 2.25) (Jan 2007) RR 0.31 (0.21 to 0.46) (Mar 2007) The benefit with ITNs was still present at 12 months |
| Emami 2009 IRN | City sector (approx. 3000 houses) | Olyset® (permethrin 2%) distributed to all households in August 2004. | P. sergenti | Total sandflies (monthly collections during transmission season using light traps and sticky traps). |
Not reported | Not reported | Not reported | Not reported | The authors state: 'There were statistically significant differences in the monthly catches of P. sergenti between control and intervention sectors in both cities (P < 0.05)'. |
| Joshi 2009 ASIA | Hamlets or neighnourhoods | PermaNet® (deltamethrin 55mg/m²) distributed to all households (date not stated). | P. argentipes | Mean number of sandflies per per house (light traps) per night at all sites pooled in Nepal, Bangladesh and India. | 9.92 (date not stated) |
9.41 (date not stated) |
8.32 (5 months post intervention) |
12.15 (5 months post intervention) |
Pre‐intervention P = 0.798 Post‐intervention P = 0.16 (The trial authors state the effect was significant in India and Bangladesh but not Nepal) |
Abbreviations: IRN = Iran; BGD = Bangladesh; ITNs = insecticide treated nets; RR = risk ratio).
In Bangladesh, there was a substantial reduction in vector density in the ITN areas for 12 months post intervention (Chowdhury 2011 BGD). In the multicentre trial from Asia, Joshi 2009 ASIA, the overall difference between intervention and control sites was not statistically significant. However the trial authors reported that it was significant at the India and Bangladesh sites but not in Nepal. In Iran, the trial authors reported a statistically significant reduction but did not provide data to enable quantification of the magnitude or duration of effect (Emami 2009 IRN). Variation in measurement and reporting of these outcomes precluded meta‐analysis.
One additional cluster‐RCT in India that randomized clusters of houses compared two different types of ITNs (PermaNet® bednets impregnated with 55 mg/m² deltamethrin and Olyset® bednets impregnated with 2% permethrin) with two control groups of untreated nets (Table 13). The trial authors reported a statistically significant reduction in male P. argentipes in areas with ITNs compared to untreated nets, but no difference in female P. argentipes or other vectors (Dinesh 2008 IND).
6. Vector density: ITNs versus untreated nets.
| Trial ID | Unit of randomization | Intervention | Main vector | Measure (method) | Pre‐intervention | Post‐intervention | Effect measure (95% CI) or P value |
| Dinesh 2008 IND | Two houses |
|
P. argentipes andSergentomyia spp. | Geometric mean sandfly counts per group (CDC light traps) |
Reported graphically | Reported graphically | The trial authors state a statistically significant reduction in male P. argentipides in areas with ITNs compared to untreated nets, but no difference in female P. argentipides or other vectors. |
Abbreviations: IND = India; CDC = Centers for Disease Control and Prevention.
Effect on disease
CL: Two cluster‐RCTs from Afghanistan and Iran evaluated the effect of ITNs on the incidence of CL (Emami 2009 IRN; Reyburn 2000 AFG). In Afghanistan, ITNs impregnated with permethrin (0.5 g/m²) were distributed to all households, and the cumulative analysis of new cases over 15 months showed a marked reduction in CL in areas with ITNs compared to control areas (Intervention 20/1195 (1.7%); control 92/1759 (5.2%); RR 0.32, 95% CI 0.18 to 0.56, one trial, 2954 participants in approximately 600 clusters, Analysis 2.1). In Iran, there again appeared to be a large reduction in CL cases. However, the trial authors did not adjust for the cluster design. Our approximate adjustment for clustering in this trial using the ICC from Rojas 2006 COL suggests this difference may not reach standard levels of statistical significance (intervention 2/3810 (0.05%); control 117/3815 (3.1%); RR 0.02, 95% CI 0.00 to 1.48, one trial, 7625 participants in 12 clusters, Analysis 2.1). In the combined analysis of both trials there was a significant reduction of CL cases.
2.1. Analysis.
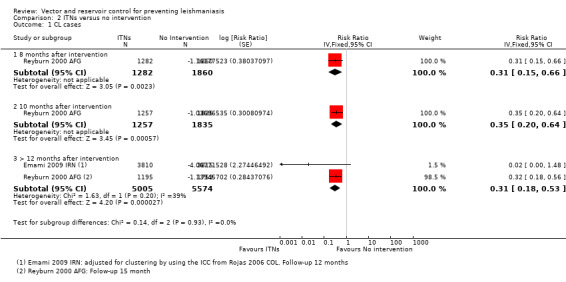
Comparison 2 ITNs versus no intervention, Outcome 1 CL cases.
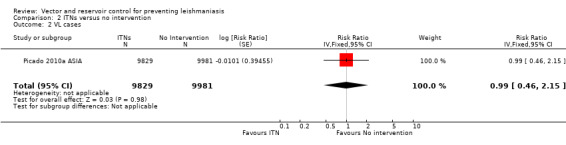
VL: One cluster‐RCT evaluated the effect of PermaNet® ITNs impregnated with deltamethrin (55 mg/m²) on VL in India and Nepal (Picado 2010a ASIA). The overall risk of VL during the 30 months follow‐up was 37/9829 (0.38%) in the intervention group and 40/9981 (0.40%) in the control group (RR 0.99, 95% CI 0.46 to 2.15, one trial, 19,810 participants in 26 clusters, Analysis 2.2). In the same trial, there was also no significant difference in the risk of seroconversion (determined by direct agglutination test) in those who had negative results (titre < 1:1600) at baseline (RR 0.90, 95% CI 0.49 to 1.65, one trial, 19,810 participants, Analysis 2.3).
2.2. Analysis.
Comparison 2 ITNs versus no intervention, Outcome 2 VL cases.
2.3. Analysis.
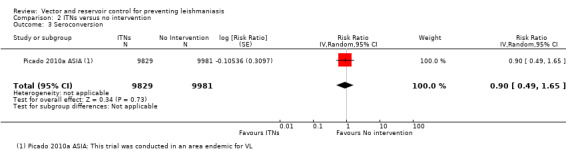
Comparison 2 ITNs versus no intervention, Outcome 3 Seroconversion.
ITCs versus untreated curtains or no curtains
(See Table 3)
Effect on vector density
One cluster‐RCT evaluated the effect of ITCs on vector density (Kroeger 2002 VEN; Table 14). This trial randomized city sectors from urban Venezuela (main vectors: L. youngi and L. ovallesi) and compared ITCs of lambdacyhalothrin (12.5 mg/m²) with unimpregnated curtains or no curtains. There were no significant differences in mean number of phlebotomine sandflies per house per night between the intervention and control groups before the placement of the curtains (averaged over 150 consecutive nights, January to June 2000; P = 0.706), but the mean was substantially lower in the intervention houses three months after the intervention (P < 0.001).
7. Vector density: ITCs versus untreated curtains or no curtains.
| Trial ID | Unit of randomization | Intervention | Main vector | Measure (method) | Pre‐intervention | Post‐intervention | Effect measure (95% CI) or P value | ||
| ITNs | Control | ITNs | Control | ||||||
| Kroeger 2002 VEN | City sectors | Polyester curtains impregnated with lambdacyhalothrin (12.5 mg/m²) at 0 and 6 months. The mesh size of curtains was 0.05 mm. | L. youngi and L. ovallesi | Mean number of sandflies per house (light trap in main room of house for 150 nights). |
15 (January to June 2000) |
16 (January to June 2000) |
2 (August to October 2000) |
17 (August to October 2000) |
Pre‐intervention P = 0.706 Post‐intervention P < 0.001 |
Abbreviations: VEN = Venezuela; ITNs = insecticide treated nets; ITCs = insecticide treated curtains.
Effect on disease
CL: In Kroeger 2002 VEN, over 12 months follow‐up, the incidence of clinical cases of CL was 0/1351 (0%) in the intervention group and 142/1587 (9%) in the control group. The trial authors reported a cluster adjusted mean difference in CL incidence between the intervention and control areas which is statistically significant (MD 8.3, 95% CI 5.0 to 11.7; authors' own figures). For comparison with other interventions we calculated an approximate RR by using a value of 0.5 events in the intervention group and adjusted for clustering using the ICC from Rojas 2006 COL (RR 0.00, 95% CI 0.00 to 0.49, one trial, 2938 participants in 14 clusters, Analysis 3.1).
3.1. Analysis.
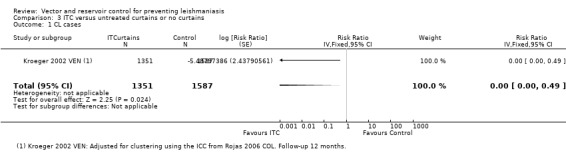
Comparison 3 ITC versus untreated curtains or no curtains, Outcome 1 CL cases.
VL: No trials evaluated the effects of ITCs on VL incidence.
ITS versus no intervention
(Table 4)
Effect on vector density
One cluster‐RCT in areas of Brazil with VL evaluated the effects of treating sheets with lambdacyhalothrin (20 mg/m²) and hanging them near the chicken shed (Kelly 1997 BRA; Table 15). This trial, with main vector Lu. longipalpis, randomized chicken sheds but did not provide data to allow us to quantify the magnitude or duration of this effect. The trial authors reported short term reductions in geometric mean phlebotomine sandflies per trap after the intervention, which only differed statistically from control sheds at week 12 post‐intervention.
8. Vector density: ITS versus no intervention.
| Trial ID | Unit of randomization | Intervention | Main vector | Measure (method) | Pre‐intervention | Post‐intervention | Effect measure (95% CI) or P value | ||
| ITS | Control | ITS | Control | ||||||
| Kelly 1997 BRA | Chicken sheds | Sheets impregnated with lambdacyhalothrin (20 mg/m²) installed 1 meter from the chicken shed. | Lu. longipalpis | Geometric mean sandflies (light traps) | 622.3 (October 1993) |
404.6 (October 1993) |
Not reported | Not reported | The trial authors state "the abundance in sheds was approximately 50% below that expected on the first day falling to about 80% at week 12 ‐ the only time the difference was statistically significant". |
Abbreviations: BRA = Brazil: ITS = insecticide treated sheet.
Effect on disease
CL:Reyburn 2000 AFG, a cluster‐RCT from Afghanistan, evaluated the effect of treating bedsheets with permethrin (1 g/m²) on CL incidence. In the cumulative analysis of new cases over 15 months follow‐up there were substantially fewer in the intervention households (Intervention 18/1025 (1.8%); control 92/1759 (5.2 %); RR 0.34, 95% CI 0.20 to 0.57, one trial, 2784 participants in approximately 600 clusters, Analysis 4.1). The effect appears to be consistent across age groups (Table 11).
4.1. Analysis.
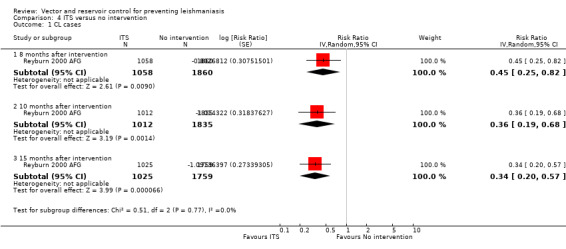
Comparison 4 ITS versus no intervention, Outcome 1 CL cases.
VL: No studies.
Insecticide treated uniforms versus no intervention
(See Table 5)
Effect on disease
CL: Two individually randomized trials evaluated the effect of impregnating soldiers uniforms with permethrin on the incidence of CL (Asilian 2003a IRN; Soto 1995 COL). The trials were small and underpowered to confidently detect or exclude effects. The combined meta‐analysis did not find a statistically significant effect (two trials, 558 participants,Analysis 5.1). However, in Soto 1995 COL the incidence in the control group was 18/143 over 12 weeks (12%), and just 4/143 (3%) in soldiers with impregnated uniforms which did reach standard levels of statistical significance (RR 0.22, 95% CI 0.08 to 0.64). Asilian 2003a IRN reported that no side effects occurred, while Soto 1995 COL reported that two out of 229 soldiers with impregnated uniforms had skin irritation and pruritus that required treatment.
5.1. Analysis.
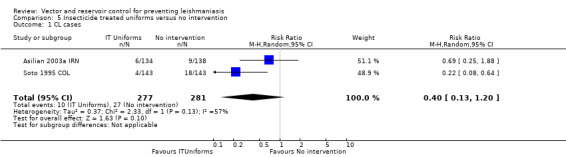
Comparison 5 Insecticide treated uniforms versus no intervention, Outcome 1 CL cases.
VL: No trials evaluated the effects of insecticide treated uniforms on VL incidence.
Reservoir control versus no intervention
Effect on disease
VL: No trials evaluated the effect of reservoir control on clinical disease but one trial from an area endemic with VL in Brazil (Werneck 2014 BRA) found a 38% reduction in seroconversion over 18 months post‐elimination of infected dogs (RR 0.62, 95% CI 0.42 to 0.91, one trial, 376 participants in 20 clusters, Analysis 6.1).
6.1. Analysis.
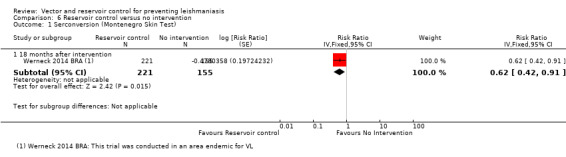
Comparison 6 Reservoir control versus no intervention, Outcome 1 Serconversion (Montenegro Skin Test).
Environmental modification (EVM) versus no intervention
Effect on vector density
VL: The two cluster‐RCTs in areas of Asia with VL evaluated the effect of EVM on vector density (Table 16). Both trials that randomized clusters of houses used trained community mobilizers to promote the filing of cracks in walls and floors with mud or lime (Chowdhury 2011 BGD; Joshi 2009 ASIA). Neither trial found evidence of statistically significant reductions in phlebotomine sandflies compared to no intervention up to 12 months follow‐up. Although the variation in measurement and reporting of these outcomes precludes meta‐analysis.
9. Vector density: EVM versus no intervention.
| Trial ID | Unit of randomization | Intervention | Main vector | Measure (method) | Pre‐intervention | Post‐intervention |
Effect measure (95% CI) or P value |
||
| EVM | Control | EVM | Control | ||||||
| Chowdhury 2011 BGD | Cluster of 50 houses | Community mobilizers conducted weekly home visits and educated household members. The major activity was filling cracks and crevices in the walls and floors of human dwellings, detached kitchens, cattle sheds, and other structures such as cattle troughs with mud plaster. | P. argentipes | Total sandflies (monthly collections from 40 houses using light traps). |
662 (October 2006) |
683 (October 2006) | 43 (January 2007) 954 (March 2007) |
54 (January 2007) 1219 (March 2007) |
RR 0.91 (0.31 to 2.63) (January 2007) RR 0.82 (0.57 to 1.17) (March 2007) The difference was not statistically significant at any time point up to 12 months |
| Joshi 2009 ASIA | Hamlets or neighnourhoods | Community mobilizers promoted filling of cracks and crevices in houses and cattle sheds. In Nepal and India: wall plastering with lime/mud mixture was promoted (lime was provided free of charge). In Bangladesh: wall plastering with mud only (a token incentive was provided) . |
P. argentipes | Mean number of sandflies per per house (light trap) per night at all sites pooled in Nepal, Bangladesh and India. | 13.21 (date not stated) |
9.41 (date not stated) |
10.39 (5 months post intervention) |
12.15 (5 months post intervention) |
Pre‐intervention P = 0.108 Post‐intervention P = 0.503 |
Abbreviations: BGD = Bangladesh; EVM = environmental modification.
Effect on disease
No trials evaluated the effect of EVM on disease.
Multifaceted intervention versus no intervention
(See Table 6)
Effect on disease
CL:Rojas 2006 COL, a cluster‐RCT from Colombia, evaluated a multifaceted intervention combining ITNs (deltamethrin), personal insect repellent (diethyltoluamide 20%), painting of tree trunks around residences with whitewash, and health education. Over one year follow‐up there was no statistically significant difference in new cases of CL between intervention and control villages (Intervention 10/1791 (0.6%); control 23/1840 (1.3%); RR 0.45, 95% CI 0.13 to 1.50, one trial, 3631 participants in 20 clusters, Analysis 7.1), and also no difference in seroconversion (Intervention 82/1066 (7.7%); control 80/1034 (7.7%); RR 0.99, 95% CI 0.51 to 1.95, one trial, 2100 participants in 20 clusters, Analysis 7.2). The trial authors reported adverse events in 2% of those in the intervention groups. The most common adverse effects were headache and itching.
7.1. Analysis.
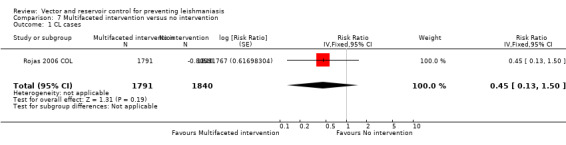
Comparison 7 Multifaceted intervention versus no intervention, Outcome 1 CL cases.
7.2. Analysis.
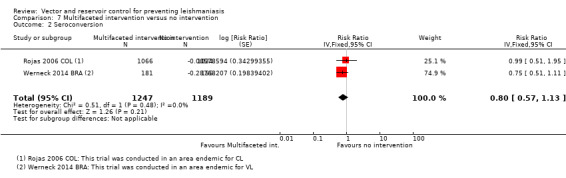
Comparison 7 Multifaceted intervention versus no intervention, Outcome 2 Seroconversion.
VL: One additional trial from an area endemic with VL in Brazil (Werneck 2014 BRA) evaluated IRS plus culling of infected dogs and found no statistically significant difference in seroconversion over 18 months post intervention (Intervention 37/144 (2.6%); control 42/113 (3.7%); RR 0.75, 95% CI 0.51 to 1.11, one trial, 336 participants in 40 clusters, Analysis 7.2).
Section B: Comparisons of different interventions
IRS versus ITNs, ITCs or ITS
(See Table 7)
Effect on vector density
Two cluster‐RCTs in areas of Asia with VL evaluated the comparative effect of IRS and ITNs (55 mg/m² deltamethrin) on vector density (Table 17). In a trial from Bangladesh, India and Nepal, Joshi 2009 ASIA, the pooled data with a follow‐up at five months on trapped phlebotomine sandflies (P. argentipes) in houses showed that IRS was effective with an average sandfly reduction of about 50%, but the ITNs had very little effect. In the other trial from Bangladesh, Chowdhury 2011 BGD, both interventions were associated with an overall decrease in total sandfly (P. argentipes) density at five months. The variation in measurement and reporting of these outcomes precludes meta‐analysis.
10. Vector density: IRS versus ITNs.
| Trial ID | Intervention 1/Intervention 2 | Main vector | Measure (method) | Pre‐intervention | Post‐interventions | P value | ||
| IRS | ITN | IRS | ITN | |||||
| Chowdhury 2011 BGD |
IRS with 20 mg/per m² deltamethrin. versus ITN PermaNet® 2.0 distributed to all households in November 2006 |
P. argentipes | Total sandflies (monthly collections from 40 houses using light traps). |
633 (October 2006) | 724 (October 2006) |
644 (October 2007) |
189 (October 2007) |
Not reported |
| Joshi 2009 ASIA |
IRS Bangladesh: 20 mg/m²deltamethrin. India: 1 g/m² 5% DDT Nepal: 0.025 g/ m² alpha‐cypermethrin versus ITN PermaNet® distributed to all households (date not stated), |
P. argentipes | Mean number of sandflies per per house (light trap) per night at all sites pooled in Nepal, Bangladesh and India. | 12.32 (date not stated) |
9.92 (date not stated) |
6.14 (5 months post intervention) |
8.32 (5 months post intervention) |
Not reported |
Abbreviations: BGD = Bangladesh; IRS = indoor residual spraying; ITNs = insecticide treated nets.
Kelly 1997 BRA, a cluster‐RCT in areas of Brazil with VL, included a comparison of IRS with insecticide‐impregnated (20 mg/m² lambdacyhalothrin) cotton sheets or blankets (focal coverage) (Table 18). Following IRS intervention, Lu.longipalpis abundance fell by only 45% versus 90% after ITS intervention on week 12 post‐intervention.
11. Vector density: IRS versus ITS.
| Trial ID | Intervention 1/Intervention 2 | Main vector | Measure (method) | Pre‐intervention | Post‐intervention | P value | ||
| IRS | ITS | IRS | ITS | |||||
| Kelly 1997 BRA |
IRS with 20 mg/m² of 10% lambdacyhalothrin. versus ITS with 20 mg/m² of lambdacyhalothrin installed c. 1 m from the roost. |
Lu. longipalpis | Ln Odds Ratio (IRS:ITS) | In sheds which were to be sprayed. Geometric mean abundance of
Lu. longipalpis (males + females): 1132.3 (1 to 2 pre‐intervention trapping rounds were conducted from 16 October to 11 November 1993) |
In sheds wich were to receive targets sheets. Geometric mean abundance of
Lu. longipalpis (males + females): 622.3 (1 to 2 pre‐intervention trapping rounds were conducted from 16 October to 11 November 1993) |
0.23 Sheds, time range not reported (x2 = 6.12). 90% reduction in Lu.longipalpis abundance Dining‐huts, not reported Houses, not reported |
Measured as Ln Odds ratio (IRS:ITS) Following blanket intervention, the abundance of Lu. longipalpis in traps fell by 45% (not significant). Catches at untreated dining‐huts actually increased, possibly because the blanket coverage diverted Lu. longipalpis away from major aggregation sites at animal pens. |
< 0.025 |
Abbreviations: BRA = Brazil; IRS = indoor residual spraying; ITS = insecticide treated sheets.
Effect on disease
CL: In the multi‐arm cluster‐RCT from Afghanistan, Reyburn 2000 AFG, the differences in CL incidence between clusters allocated to IRS, ITNs or ITS did not reach standard levels of statistical significant differences among interventions over 15 months (IRS versus ITNs, RR 1.9, 95% CI 0.98 to 3,69 Analysis 8.1; IRS versus ITS, RR 1.83 95% CI 0.92 to 3.64 Analysis 9.1; and ITNs versus ITS, RR 0.96 95% CI 0.45 to 2.08 Analysis 10.1; one trial, 3353 participants in approximately 600 clusters).
8.1. Analysis.
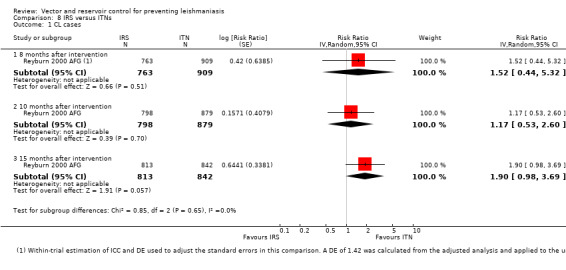
Comparison 8 IRS versus ITNs, Outcome 1 CL cases.
9.1. Analysis.
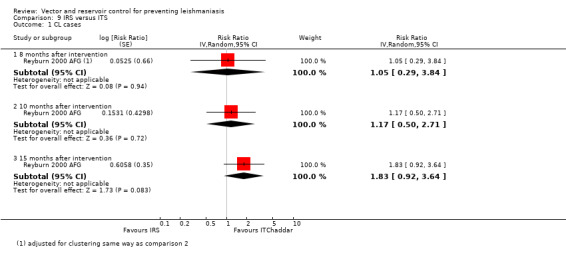
Comparison 9 IRS versus ITS, Outcome 1 CL cases.
10.1. Analysis.
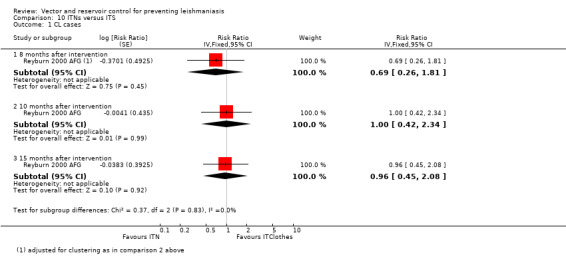
Comparison 10 ITNs versus ITS, Outcome 1 CL cases.
VL: No trials evaluated the effect of this comparison on VL incidence.
IRS versus EVM
Effect on vector density
Two cluster‐RCTs in areas of Asia with VL also evaluated the effect of IRS versus EVM on vector density (Chowdhury 2011 BGD; Joshi 2009 ASIA; Table 19). The pooled data in both trials showed that EVM had no or very little effect on total sandfly (P. argentipes) density at five months but the variation in measurement and reporting of these outcomes precludes meta‐analysis.
12. Vector density: IRS versus EVM.
| Trial ID | Intervention 1/Intervention 2 | Main vector | Measure (method) | Pre‐intervention | Post‐intervention | P value | ||
| IRS | EVM | IRS | EVM | |||||
| Chowdhury 2011 BGD |
IRS with 20 mg/per m² deltamethrin. versus EVM Filling cracks and crevices in the walls and floors of human dwellings, detached kitchens, cattle sheds, and other structures. Promotion of cleaning up debris from the environment using household incentives. |
P. argentipes | Total sandflies (monthly collections from 40 houses using light traps). |
633 (October 2006) |
662 (October 2006) |
644 (October 2007) |
598 (October 2007) |
Not reported |
| Joshi 2009 ASIA |
IRS Bangladesh: 20 mg/m² deltamethrin. India: 1 g/m² 5% DDT Nepal: 0.025 g/m² alpha‐cypermethrin. versus EVM Trained community mobilizers met with each family to discuss the typical resting and breeding sites in and around the houses and the appropriate ways to reducing them. In Nepal and India wall plastering with lime/mud mixture was promoted, the lime being provided free of charge to the households. In Bangladesh plastering was done with mud only. |
P. argentipes | Mean number of sandflies per per house (light trap) per night at all sites pooled in Nepal, Bangladesh and India. | 12.32 (date not stated) |
13.21 (date not stated) |
6.14 (5 months post intervention) |
10.39 (5 months post intervention) |
Not reported |
Abbreviations: IRS = indoor residual spraying; EVM = environmental modification.
Effect on disease
No trials evaluated the effect of this comparison on leishmaniasis.
ITNs vs EVM
Effect on vector density
Two cluster‐RCTs in areas of Asia with VL also compared long‐lasting ITN with EVM (Chowdhury 2011 BGD; Joshi 2009 ASIA; Table 20). Only ITNs had an important effect on the average reduction of phlebotomine sandflies (P. argentipes) at five months. The variation in measurement and reporting of these outcomes precludes meta‐analysis.
13. Vector density: ITNs versus EVM.
| Trial ID | Intervention 1/Intervention 2 | Main vector | Measure (method) | Pre‐intervention | Post‐intervention | P value | ||
| ITNs | EVM | ITNs | EVM | |||||
| Chowdhury 2011 BGD |
ITNs PermaNet® made of polyester containing deltamethrin (55 mg/m²) versus EVM Filling cracks and crevices in the walls and floors of human dwellings, detached kitchens, cattle sheds, and other structures. Promotion of cleaning up debris from the environment using household incentives. |
P. argentipes | Total sandflies (monthly collections from 40 houses using light traps) |
724 (October 2006) | 662 (October 2006) |
18 (January 2007) 361 (March 2007) |
598 (October 2007) |
Not reported |
| Joshi 2009 ASIA |
ITNs PermaNet® made of polyester containing deltamethrin (55 mg/m²). versus EVM Trained community mobilizers met with each family to discuss the typical resting and breeding sites in and around the houses and the appropriate ways to reducing them. In Nepal and India wall plastering with lime/mud mixture was promoted, the lime being provided free of charge to the households. In Bangladesh plastering was done with mud only. |
P. argentipes | Mean number of sandflies per house (light trap) per night at all sites pooled in Nepal, Bangladesh and India. | 9.92 (date not stated) |
13.21 (date not stated) |
8.32 (5 months post intervention) |
10.39 (5 months post intervention) |
Not reported |
Abbreviations: BGD = Bangladesh; ITNs = insecticide treated nets; EVM = environmental modification.
Effect on disease
No trials evaluated the effect of this comparison on leishmaniasis.
Reservoir control versus IRS
Effect on disease
VL:Costa 2007 BRA, a cluster‐RCT based in a VL‐endemic area in Brazil (367 inhabitants; 213 seronegatives), evaluated the effects of insecticide spraying of animal pens, and reservoir control (eliminating infected dogs) on seroconversion, using IRS of houses alone as the control group. Trial authors did not present the total number of participants in each of the four intervention groups.
IRS of houses and elimination of infected dogs appeared to reduce seroconversion compared to IRS alone (RR 0.20, 95% CI 0.05 to 0.85, one trial, number of participants not available, Analysis 11.1). However, this effect was not seen in a similar comparison of IRS of houses and animal pens plus elimination of infected dogs versus IRS alone (RR 0.69, 95% CI 0.27 to 1.76, one trial, number of participants not available, Analysis 11.1).
11.1. Analysis.
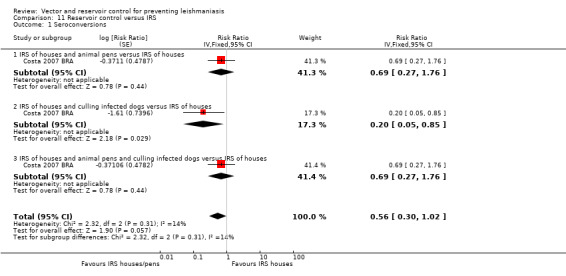
Comparison 11 Reservoir control versus IRS, Outcome 1 Seroconversions.
Discussion
Summary of main results
We included 14 RCTs that evaluated a range of interventions across different settings. All included trials were at high or unclear risk of selection or reporting bias.
In a single trial from Afghanistan (Reyburn 2000 AFG) spraying the internal walls of houses with insecticide reduced CL incidence by about a third (low quality evidence), see Table 1. In two trials from Afganistan and Iran (Reyburn 2000 AFG;Emami 2009 IRN) ITNs reduced the incidence by around two thirds (low quality evidence), see Table 2. However, in direct comparisons between these interventions (Reyburn 2000 AFG), the difference was not statistically significant (low quality evidence), see Table 7. In one additional trial from Venezuela (Kroeger 2002 VEN), ITCs almost completely prevented CL (low quality evidence), see Table 3; and in one trial from Brazil (Kelly 1997 BRA), ITS reduced the incidence by around two thirds (low quality evidence), see Table 4.
Two small trials in soldiers evaluated personal protection for CL by using insecticide treated clothing (Asilian 2003a IRN; Soto 1995 COL). Although there was a statistically significant effect in one trial (Soto 1995 COL), they were both underpowered to reliably evaluate the effects (low quality evidence), see Table 5.
Only ITNs have been evaluated for an effect on VL incidence. A single trial from India and Nepal reported no effect (Picado 2010a ASIA) (moderate quality evidence), see Table 2.
Two trials from Brazil evaluated the effects of culling infected dogs versus no intervention or IRS (Costa 2007 BRA;Werneck 2014 BRA). They reported a reduction in seroconversion over 18 months follow‐up but did not measure or report effects on clinical diseases.
Some included trials evaluated vector density. Four trials (Chowdhury 2011 BGD; Joshi 2009 ASIA; Kelly 1997 BRA; Feliciangeli 2003 VEN) reported reductions in sandfly abundance after spraying (moderate quality evidence). Two trials (Chowdhury 2011 BGD; Emami 2009 IRN) found a reduction in vector density after use of ITNs, while another two (Joshi 2009 ASIA; Dinesh 2008 IND) did not (low quality evidence). In one trial (Kroeger 2002 VEN), vector density was substantially lower after using ITCs (low quality evidence).
Overall completeness and applicability of evidence
In this Cochrane Review, most evidence relates to the use of insecticide to reduce phlebotomine sandfly numbers and prevent CL. When taken as a body of evidence, this appears to be an effective strategy to reduce clinical disease. However, as only one or two trials evaluated each individual intervention (applying insecticide to indoor walls, bednets, bed sheets or curtains), it is unclear which is the best strategy.
Importantly, although insecticide use appears to be effective, we found no evidence from RCTs on the safety or environmental impact of insecticides used in this way. Policy makers should consider evidence from other sources when considering safety in their decisions.
Furthermore, included trials with clinical outcomes were from only a limited number of epidemiological settings (Afghanistan, Iran, India, Nepal, Venezuela, Colombia and Brazil); and this limits our ability to make broad generalizations. The epidemiology of leishmaniasis is extremely complex not only because of the different Leishmania species, vectors and reservoirs, but also because the extreme diversity in human behaviour and settings. For example, annual and seasonal differences in the breeding and resting habits of infected phlebotomine sandflies, coupled with differences in the work and recreational habits of humans are likely to affect the efficacy of preventive measures across settings and cultures. IRS is only considered likely to be effective where infected phlebotomine sandflies are endophilic and the effectiveness of ITNs is considered dependent on the local behaviour of both humans and infected phlebotomine sandflies.
For VL, the evidence is much more limited, due in part to it being a relatively rare disease which would require extremely large trials to demonstrate an effect. Extrapolation of results from CL to VL is likely to be unreliable given the differences in ecological habitats and geographical locations.
This Cochrane Review also highlights that some widely used interventions have very little evidence to support their use. There is only a limited evidence base for the use of insecticide‐treated clothing for protection against CL transmission despite having been used for many years by the military and in recreational activities as personal protection against bites. Although frequently used, cheap and easily available, insect repellents for personal protection against sandfly bites in endemic areas, including chemical agents or local vegetal oils (Dhiman 1994; Kebede 2010), were not assessed in any of the included trials. Very limited evidence is also available on the effect of environmental management and modification aimed to impede phlebotomine sandflies from breeding. The WHO recommends that sandfly control involve more than one method in an integrated vector management approach (WHO 2010) but only one trial with limitations in quality studied a multifaceted intervention combining ITNs, personal insect repellent, painting of tree trunks around residences and health education (Rojas 2006 COL).
The low number of included trials unfortunately prevented us from conducting any subgroup analyses, which would have enabled analysis of the impact of different types of insecticides, resistance to insecticides, the transmission seasons and vector ecology.
Although not all included trials examined the acceptability and compliance of the interventions, low compliance and acceptability can represent potential limitations of the included trials.
Quality of the evidence
We judged the evidence for CL reduction with the individual interventions (ITNs, ITS, ITCs or IRS) to be of moderate or low quality. This means that we have some confidence in these estimates of effect but further research is warranted.
Two main reasons led us to downgrade the evidence. Firstly, descriptions of trial methods was vague for almost all included trials and so the risk of bias was unclear, Secondly, the main evidence was from just three trials (from Afghanistan, Venezuela and Iran), which makes broad generalization to different epidemiological settings and cultures difficult. To have full confidence that these interventions are widely effective requires further well‐conducted trials from different settings.
Only one trial evaluated the protective effect of ITNs against VL and found this intervention to be ineffective (Picado 2010a ASIA) and we judged the evidence to be of moderate quality.
Potential biases in the review process
We did not identify any specific bias in our review process.
Agreements and disagreements with other studies or reviews
A systematic review of RCTs and other controlled studies on preventative methods against human leishmaniasis (Stockdale 2012; Stockdale 2013) was published during the development of this Cochrane Review. The authors' main conclusions also highlight the lack of high quality evidence centred in clinical outcomes and the inability to generalize the findings across different geographic areas and settings. However, a more precise mapping of the best evidence was limited because the inclusion of non‐randomized studies and the lack of a methodological quality assessment of studies.
Romero 2010, a systematic review on VL control in Latin‐America, added that lack of political commitment and the weakness of case management and surveillance systems are important limitations for VL elimination.
Authors' conclusions
Implications for practice.
Using insecticides to reduce phlebotomine sandfly numbers appears to be effective at reducing CL incidence in some settings. However, there is insufficient evidence to know whether it is better to spray the internal walls of houses or to use insecticide impregnated bednets, curtains, bedsheets or clothing. There is currently no evidence that these measures are effective or not in reducing VL incidence.
Policy decisions should consider local sandfly epidemiology and behaviour, as well as the diversity of transmission scenarios (including vector and animal or human reservoirs) when designing and implementing leishmaniasis control programmes.
Implications for research.
Resources are limited for clinical research into neglected diseases, including leishmaniasis. Therefore, there appropriately designed and adequately powered trials are needed. Given the link between a reduction in phlebotomine sandfly populations and a consequent reduction in cases of leishmaniasis is neither guaranteed or proven, future trials of promising interventions should directly assess the effect on reduction in cases of leishmaniasis. The use of standard guidelines, as performed for other leishmaniasis reviews (Gonzalez 2010), may help to resolve these issues. In the case of cluster‐RCTs it is very important to obtain specialist statistical advice throughout the entire process of planning, conducting and analysing the trials (Bowater 2009).
Adequate exploration and reporting of acceptability and compliance of intervention measures is crucial for the correct interpretation of the results assessing preventive measures, otherwise results may not be significant for the main objective of the study.
Given the constraints of IRS, it is worth further exploring the use, effect and impact of insecticide treated materials, particularly long lasting insecticide treated clothes and ITNs. The gap of RCTs in vector control measures in Africa is remarkable. There is also a need for testing the use of different types of insecticide and their impact in different geographical areas. We have found some additional areas of uncertainty that need to be explored in future trials:
Strategies of EVM.
Multifaceted interventions.
Integrated vector management strategies based on understanding the local resources.
Human and animal (domestic and wild) reservoir control (for example, impregnated dog collars or lotions, poisonous baits for rodents eating seeds, removal of plants for rodents which feed on them, vaccines for canine leishmaniasis, destruction of burrows, trapping).
Acknowledgements
The academic editor for this review was Professor Paul Garner.
We acknowledge María Ángeles Mora and Rosa Amill (Roche Farma, Scientific Information) for bibliographical support; and Cristina Martinez and Natalia Mendoza for help in the early development of this Cochrane Review. Also, we thank Paul Garner and CIDG editorial staff for assistance editing the review at various stages. This Cochrane Review is funded by a grant from the Office of Control of Neglected Tropical Diseases (WHO/CDS/NTD/IDM), Communicable Diseases Cluster, WHO. It has been supported in part by the Agencia Española de Cooperación Internacional para el Desarrollo (AECID). The editorial base of the CIDG is funded by the UK Department for International Development (DFID) for the benefit of low‐ and middle‐income countries.
Appendices
Appendix 1. CIDG Specialized Register search strategy
Leshman* AND (prophyla* OR prevent*)
Appendix 2. Cochrane Library search strategy
#1 (prevent*)
#2 (phlebotomus)
#3 (insect*)
#4 (repel*)
#5 (permethrin* or permetrin*)
#6 (sand fly* or sand fli* or sand fly* or sand fli*)
#7 (Lutzom*)
#8 (environment*)
#9 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8)
#10 leishmania*
#11 (#9 AND #10)
Appendix 3. MEDLINE (PubMed) search strategy
prevent*
phlebotomus
insect*
repel*
permethrin* OR permetrin
sand fly* OR sand fli* OR sandfly* OR sanfli*
Lutzom*
environment*
1 OR 2 OR 3 OR 4 OR 5 OR 6 Or 7 OR 8
leishmania*
9 AND 10
randomised controlled trial OR randomized controlled trial
controlled clinical trial
randomi*
placebo
clinical trials as topic
randomly
trial
12 OR 13 OR 14 OR 15 OR 16 OR 17 OR 18
11 AND 19
Appendix 4. EMBASE search strategy
1. PREVENT$
2. PHLEBOTOMUS
3. INSECT$
4. REPEL$
5. PERMETHRIN$ OR PERMETRIN
6. SAND ADJ FLY$ OR SAND ADJ FLI$ OR SANDFLY$ OR SANFLI$
7. LUTZOM$
8. ENVIRONMENT$
9. 1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8
10. LEISHMANIA$
11. 9 AND 10
12. FACTORIAL$
13. RANDOMIZED ADJ CONTROLLED ADJ TRIAL
14. CONTROLLED ADJ CLINICAL ADJ TRIAL
15. RANDOMIZED
16. PLACEBO$
17. CLINICAL‐TRIAL.DE.
18. RANDOM$
19. CROSSOVER$ OR CROSS ADJ OVER$ OR CROSS?OVER$ OR CROSSOVER?PROCEDURE
20. DOUBL$ ADJ BLIND$ OR SINGL$ ADJ BLIND$ OR DOUBLE?BLIND ADJ PROCEDURE OR SINGLE?BLIND ADJ PROCEDURE
21. TRIAL
22. 12 OR 13 OR 14 OR 15 OR 16 OR 17 OR 18 OR 19 OR 20 OR 21
23. 11 AND 22
Appendix 5. LILACS search strategy
((Pt ENSAYO CONTROLADO ALEATORIO OR Pt ENSAYO CLINICO CONTROLADO OR Mh ENSAYOS CONTROLADOS ALEATORIOS OR Mh DISTRIBUCIÓN ALEATORIA OR Mh METODO DOBLE CIEGO OR Mh METODO SIMPLECIEGO OR Pt ESTUDIO MULTICÉNTRICO) or ((tw ensaio or tw ensayo or tw trial) and (tw azar or tw acaso or tw placebo or tw control$ or tw aleat$ or tw random$ or (tw duplo and tw cego) or (tw doble and tw ciego) or (tw double and tw blind)) and tw clinic$)) AND NOT ((Ct ANIMALES OR Mh ANIMALES OR Ct CONEJOS OR Ct RATÓN OR MH Ratas OR MH Primates OR MH Perros OR MH Conejos OR MH Porcinos) AND NOT (Ct HUMANO AND Ct ANIMALES)) [Palabras] and leishmani$ [Palabras] and (preven$ OR phlebotomus OR insect$ OR repel$ OR (permethrin$ OR permetrin$) OR (sand fly$ OR sand fli$ OR sandfly$ OR sandfli$ OR mosc$) OR Lutzom$ OR environment$ OR ambient$) [Palabras]
Appendix 6. WHOLIS search strategy
words or phrase "((randomised controlled trial OR controlled clinical trial OR randomised OR placebo OR clinical trials as topic OR randomly OR trial) NOT (animals NOT humans))" AND words or phrase "leishmania$"
Appendix 7. Science Direct search strategy
prevent*
phlebotomus
insect*
repel*
permethrin* OR permetrin
sand fly* OR sand fli* OR sandfly* OR sanfli*
Lutzom*
environment*
1 OR 2 OR 3 OR 4 OR 5 OR 6 Or 7 OR 8
LEISHMANIA*
9 AND 10
randomised controlled trial OR randomized controlled trial
controlled clinical trial
randomi*
placebo
clinical trials as topic
randomly
trial
12 OR 13 OR 14 OR 15 OR 16 OR 17 OR 18
HUMANS NOT ANIMALS
11 AND 19 AND 20
Appendix 8. RePORTER search strategy
leishmania*
prevent*
1 AND 2
Appendix 9. Ongoing trials search strategies
metaRegister of Controlled trials
leishmania* AND prevent*
US National Institutes of Health Register
leishmania
Ongoing Skin Trials Register
leishmania
Australian and New Zealand Clinical Trials Registry
leishmania
WHO ICTRP
leishmania* and prevent*
Appendix 10. MEDLINE (PubMed) adverse effects
("adverse effects"[Subheading] OR ("adverse"[All Fields] AND "effects"[All Fields]) OR "adverse effects"[All Fields]) AND (("cypermethrine"[Supplementary Concept] OR "cypermethrine"[All Fields] OR "alphacypermethrin"[All Fields]) OR ("ddt"[MeSH Terms] OR "ddt"[All Fields]) OR ("permethrin"[MeSH Terms] OR "permethrin"[All Fields]) OR ("deet"[MeSH Terms] OR "deet"[All Fields]) OR noike[All Fields] OR ("decamethrin"[Supplementary Concept] OR "decamethrin"[All Fields] OR "deltamethrin"[All Fields]) OR ("cyhalothrin"[Supplementary Concept] OR "cyhalothrin"[All Fields] OR "lambdacyhalothrin"[All Fields])) AND (Review[ptyp] AND "2005/01/07"[PDat] : "2015/01/04"[PDat] AND "humans"[MeSH Terms])
Data and analyses
Comparison 1. IRS versus no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 CL cases | 1 | Risk Ratio (Fixed, 95% CI) | Subtotals only | |
| 1.1 8 months after intervention | 1 | 2943 | Risk Ratio (Fixed, 95% CI) | 0.47 [0.23, 0.99] |
| 1.2 10 months after intervention | 1 | 2954 | Risk Ratio (Fixed, 95% CI) | 0.42 [0.23, 0.78] |
| 1.3 15 months after intervention | 1 | 2892 | Risk Ratio (Fixed, 95% CI) | 0.61 [0.38, 0.97] |
| 2 Seroconversion (Montenegro Skin Test) | 1 | 295 | Risk Ratio (Fixed, 95% CI) | 0.86 [0.63, 1.17] |
| 2.1 18 months after intervention | 1 | 295 | Risk Ratio (Fixed, 95% CI) | 0.86 [0.63, 1.17] |
Comparison 2. ITNs versus no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 CL cases | 2 | Risk Ratio (Fixed, 95% CI) | Subtotals only | |
| 1.1 8 months after intervention | 1 | 3142 | Risk Ratio (Fixed, 95% CI) | 0.31 [0.15, 0.66] |
| 1.2 10 months after intervention | 1 | 3092 | Risk Ratio (Fixed, 95% CI) | 0.35 [0.20, 0.64] |
| 1.3 > 12 months after intervention | 2 | 10579 | Risk Ratio (Fixed, 95% CI) | 0.31 [0.18, 0.53] |
| 2 VL cases | 1 | 19810 | Risk Ratio (Fixed, 95% CI) | 0.99 [0.46, 2.15] |
| 3 Seroconversion | 1 | 19810 | Risk Ratio (Random, 95% CI) | 0.90 [0.49, 1.65] |
Comparison 3. ITC versus untreated curtains or no curtains.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 CL cases | 1 | 2938 | Risk Ratio (Fixed, 95% CI) | 0.00 [0.00, 0.49] |
Comparison 4. ITS versus no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 CL cases | 1 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 1.1 8 months after intervention | 1 | 2918 | Risk Ratio (Random, 95% CI) | 0.45 [0.25, 0.82] |
| 1.2 10 months after intervention | 1 | 2847 | Risk Ratio (Random, 95% CI) | 0.36 [0.19, 0.68] |
| 1.3 15 months after intervention | 1 | 2784 | Risk Ratio (Random, 95% CI) | 0.34 [0.20, 0.57] |
Comparison 5. Insecticide treated uniforms versus no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 CL cases | 2 | 558 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.13, 1.20] |
Comparison 6. Reservoir control versus no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serconversion (Montenegro Skin Test) | 1 | Risk Ratio (Fixed, 95% CI) | Subtotals only | |
| 1.1 18 months after intervention | 1 | 376 | Risk Ratio (Fixed, 95% CI) | 0.62 [0.42, 0.91] |
Comparison 7. Multifaceted intervention versus no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 CL cases | 1 | 3631 | Risk Ratio (Fixed, 95% CI) | 0.45 [0.13, 1.50] |
| 2 Seroconversion | 2 | 2436 | Risk Ratio (Fixed, 95% CI) | 0.80 [0.57, 1.13] |
Comparison 8. IRS versus ITNs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 CL cases | 1 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 1.1 8 months after intervention | 1 | 1672 | Risk Ratio (Random, 95% CI) | 1.52 [0.44, 5.32] |
| 1.2 10 months after intervention | 1 | 1677 | Risk Ratio (Random, 95% CI) | 1.17 [0.53, 2.60] |
| 1.3 15 months after intervention | 1 | 1655 | Risk Ratio (Random, 95% CI) | 1.90 [0.98, 3.69] |
Comparison 9. IRS versus ITS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 CL cases | 1 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 1.1 8 months after intervention | 1 | Risk Ratio (Random, 95% CI) | 1.05 [0.29, 3.84] | |
| 1.2 10 months after intervention | 1 | Risk Ratio (Random, 95% CI) | 1.17 [0.50, 2.71] | |
| 1.3 15 months after intervention | 1 | Risk Ratio (Random, 95% CI) | 1.83 [0.92, 3.64] |
Comparison 10. ITNs versus ITS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 CL cases | 1 | Risk Ratio (Fixed, 95% CI) | Subtotals only | |
| 1.1 8 months after intervention | 1 | Risk Ratio (Fixed, 95% CI) | 0.69 [0.26, 1.81] | |
| 1.2 10 months after intervention | 1 | Risk Ratio (Fixed, 95% CI) | 1.00 [0.42, 2.34] | |
| 1.3 15 months after intervention | 1 | Risk Ratio (Fixed, 95% CI) | 0.96 [0.45, 2.08] |
Comparison 11. Reservoir control versus IRS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Seroconversions | 1 | Risk Ratio (Fixed, 95% CI) | 0.56 [0.30, 1.02] | |
| 1.1 IRS of houses and animal pens versus IRS of houses | 1 | Risk Ratio (Fixed, 95% CI) | 0.69 [0.27, 1.76] | |
| 1.2 IRS of houses and culling infected dogs versus IRS of houses | 1 | Risk Ratio (Fixed, 95% CI) | 0.20 [0.05, 0.85] | |
| 1.3 IRS of houses and animal pens and culling infected dogs versus IRS of houses | 1 | Risk Ratio (Fixed, 95% CI) | 0.69 [0.27, 1.76] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Asilian 2003a IRN.
| Methods | Trial design: Doubled‐blind, placebo‐controlled RCT. Unit of randomization: A soldier. Number of participants: 324 male soldiers (162 each group). Entomological data collection: Not performed. Clinical data collection: All soldiers were visited monthly. CL diagnosis was confirmed in every suspected lesion parasitologically using Giemsa‐stained direct smears. If amastigotes were not seen, the lesion was biopsied. Follow‐up: 6 months. Analysis: Analysed at individual level. |
|
| Participants | Male soldiers, aged 19 to 21 years, with no history of leishmaniasis or any evidence of active CL. Endemic disease: CL (no mention of theLeishmania species involved). |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Country: Iran (area of Isfahan). Trial dates: June 2001 to September 2001. Trial sponsor: Not reported. Sample size calculation: Not calculated. Compliance assessment: Done. Soldiers were instructed not to use insect repellents and other protective measures, and adherence to these instructions was monitored. The uniforms covered the whole body except for the head, neck, hands and feet. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient detail was reported about the method used to generate the allocation sequence. "A total of 324 soldiers were randomly divided into two groups". |
| Allocation concealment (selection bias) | High risk | Not reported. |
| Blinding (performance bias and detection bias) participants | Unclear risk | Participants were blinded but with no detail of the method used for it. "The uniforms were distributed in such a way that neither the soldier nor the researcher knew as to which uniform were permethrin‐impregnated". |
| Blinding (performance bias and detection bias) investigators | Unclear risk | Investigators were blinded but with no detail of the method used for it. "The uniforms were distributed in such a way that neither the soldier nor the researcher knew as to which uniform were permethrin‐impregnated". |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT analysis was not used. Intervention: 28 dropouts (the reasons for dropouts were not reported). Control: 24 dropouts (the reasons for dropouts were not reported). |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the methods were reported in the results. |
| Baseline measurements | High risk | No baseline characteristics by group. |
| Statistical adjustment for clustering | Low risk | Not applicable as this trial was individually randomized. |
| Other bias | Unclear risk | Trial authors did not provide a conflict of interest declaration. They did not take into account the activities of soldiers during day and night, or where they slept. |
Chowdhury 2011 BGD.
| Methods | Trial design: Cluster‐RCT. Unit of randomization: 5 households. Number of clusters: 6. Entomological data collection: Adult sandfly density was determined in households sampled monthly by counts of vectors either landing rates on exposed body parts of humans acting as baits or collected resting inside buildings (for example, walls). Clinical data collection: Not done. Length of follow‐up: 12 months. Analysis: Analysed at household level. |
|
| Participants | Four villages were divided into six geographical areas with high, intermediate or low density of phlebotomine sandflies. Five households were selected from each of the density areas by simple random sampling, yielding a subset of (24 X 5) 120 households that participated in the trial. The assignment to intervention arms was stratified by the average vector density to provide comparable vector density distribution in each arm. Endemic disease: VL caused by L. donovani. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Country: Bangladesh (Fulbaria subdistrict, Mymensingh district). Trial dates: October 2006 to September 2007. Unclear timing and duration of interventions. Trial sponsor: Funded by a grant from the Centers for Disease Control and Prevention (CDC) Emerging Infections Initiative and by the Special Programme for Research and Training in Tropical Diseases, WHO. Sample size: Calculated. Compliance assessment: Done. Houses were visited monthly to encourage compliance. This trial is 1 of 4 parallel trials in India, Nepal, and Bangladesh that used similar methods and design (Joshi 2009 ASIA). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient detail was reported about the method used to generate the allocation sequence. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) participants | Unclear risk | People not assessed in this trial. |
| Blinding (performance bias and detection bias) investigators | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information on loss of clusters. Individual participants were not followed up. |
| Selective reporting (reporting bias) | Low risk | All outcome mentioned in the methods were reported in the results. |
| Baseline measurements | Low risk | Assignment to intervention arms were stratified by vector density. |
| Statistical adjustment for clustering | Low risk | The outcome was rates of phlebotomine sandflies trapped, and the statistical model used a random effect which accounted for clustering within households. |
| Other bias | Unclear risk | Trial authors did not provide a conflict of interest declaration. |
Costa 2007 BRA.
| Methods | Trial design: Cluster‐RCT. Unit of randomization: Geographic area. Number of clusters: 34 geographic areas. Entomological data collection: Not done. Clinical data collection: Immunological tests by ELISA in blood samples to detect antigen from Leishmania chagasi, at one year. Length of follow‐up: 6 to 12 months. Analysis: Analysed at cluster level. |
|
| Participants | The central area of Teresina (Brazil) was divided in 34 geographic areas (blocks) randomly allocated to the 4 types of interventions (367 inhabitants; 213 seronegatives/154 seropositives at the beginning). Endemic disease: VL caused by L. infantum (L. chagasi). |
|
| Interventions |
Description of spraying: Pyrethroid insecticide in internal walls (all of 3 m height walls were sprayed) of houses (household spraying) and outdoors close to the houses. The elimination of infected dogs was decided if indirect immunofluorescence test was more or equalled 1:40. |
|
| Outcomes |
|
|
| Notes | Country: Brazil (Teresina, Itararé quarter). Trial dates: 1995 to 1996. Trial sponsor: No source of funding reported. Sample size: Not calculated. Compliance assessment: Not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient detail was reported about the method used to generate the allocation sequence. "Os lotes foram alocados aleatoriamente a 4 tipos de intervenção". |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) participants | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) investigators | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No information on loss of clusters. There were 44% of lost of participants to follow‐up (93/213) although the authors did not specify to which group these people belonged. |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the methods were reported in the results. |
| Baseline measurements | High risk | The prevalence of seropositivity at baseline were similar in the intervention areas but was significantly lower in the control area (only IRS). Groups were not comparable at baseline. Prevalence of infection was similar within the three treatments, but not between the treatments and the control group (lower prevalence in control group). |
| Statistical adjustment for clustering | Low risk | Cluster adjustment was performed as the model considered the effect of aggregation of individuals in batches and used robust variance estimates. |
| Other bias | Unclear risk | Trial authors did not provide a conflict of interest declaration. |
Dinesh 2008 IND.
| Methods | Trial design: Paired RCT. Unit of randomization: Houses. Number of houses: 48. Entomological data collection: Cross‐sectional surveys using one CDC light trap per house. Collection was one night (6pm to 6am) at baseline (week 0), and then at 3, 6 and 9 weeks after net installation. Clinical data collection: Not done. Length of follow‐up: 9 weeks. Analysis: Analysed at household level. |
|
| Participants | Three hamlets in Bihar, India (Gulmehiya Bagh in Patna district, and Rasoolpur and Majlishpur, both located in Vaishali district) were selected for this trial. In each hamlet, 16 houses were selected: 8 human dwellings without cattle inside the house but with cattle within the compound and 8 mixed dwellings where cattle and humans were sharing the same roof. For both types of houses and in each hamlet, 2 houses were allocated to 1 of the 4 treatments). Endemic disease: VL caused by L. donovani. Main vector and seasonality: P. argentipes has well‐defined seasonal patterns with a peak from March to May, and a second lower peak in November. |
|
| Interventions | Three nets were distributed to each house after the baseline survey:
During the trial period, the 3 hamlets were sprayed with DDT by the Governmental Control Programme at a dosage of 1 g active ingredient/m², between surveys 1 and 2. |
|
| Outcomes |
|
|
| Notes | Country: India. Trial dates: April to June 2006. Trial sponsor: the European Union, the Indian Council of Medical Research, the Government of India Health and Family Welfare, New Delhi. The CDC light traps purchase was sponsored by Mr Guy Deckers (Konhef, Belgium). Sample size: Not calculated. Compliance assessment: Not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient detail was reported about the method used to generate the allocation sequence. "In each hamlet, 16 houses were purposively selected: eight human dwellings without cattle inside the house but with cattle within the compound and eight mixed dwellings where cattle and humans are sharing the same roof. For both the categories and in each hamlet, two houses were randomly allocated to one of the four treatments." |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) participants | Low risk | Individual participants not assessed in this trial. |
| Blinding (performance bias and detection bias) investigators | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All houses were analysed. Individual participants not assessed. |
| Selective reporting (reporting bias) | High risk | Non‐significant results not showed. "The model also includes baseline survey in OT, PT, PC allocated houses when compared with LC ones, CDC light traps vs. aspirator collection, mixed houses vs. human houses, hamlets 2 vs. 1, and hamlets 3 vs. 1 (results not shown)." |
| Baseline measurements | High risk | Significantly higher numbers of P. argentipes males were noted during the baseline survey in PT (IRR: 5.70; P = 0.008) and OT (IRR: 4.63; P = 0.028) allocated houses than in LC houses. Larger numbers of females of Sergentomyia, mainly unfed, were observed in OT allocated houses (IRR: 1.96; P = 0.0480). |
| Statistical adjustment for clustering | Unclear risk | No adjustment was done. However, the outcome was sandfly density and the analysis was conducted at the household level, which is the unit of randomization, thus removing any clustering effects. |
| Other bias | Unclear risk | Trial authors did not provide a conflict of interest declaration. |
Emami 2009 IRN.
| Methods | Trial design: Cluster‐RCT. Unit of randomization: Urban sectors. Number of clusters: 12 (6 pairs) sectors (7636 inhabitants in 3000 households). Entomological data: monthly collection of phlebotomine sandflies from fixed indoors sites and from outdoors courtyards using 30 sticky traps and 20 (unspecified) light traps, assessed at one year. Clinical data collection: Follow‐up questionnaires and examinations were conducted every month between August 2004 and July 2005. All members of the participating households were examined. The presence or absence of CL ulcers was indicated on the forms. Length of follow‐up: 12 months. Analysis: Analysed at cluster level. |
|
| Participants | In each city, 6 urban sectors were selected based on the pre‐intervention epidemiological survey of disease in the area so that all sectors had a similar size and distribution of disease. Each sector in a pair was at least 2 km away from the other. Endemic disease: CL caused by L. tropica. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Country: Iran (cities of Sedeh and Shiraz). Trial dates: April 2004 to July 2005. Trial sponsor: This investigation received technical and financial support from the WHO Eastern Mediterranean Region (EMR), Division of Communicable Diseases (DCD) and the WHO Special Programme for Research and Training in Tropical Diseases (TDR): EMRO/DCD/TDR Small Grants Scheme for Operational Research in Tropical and Communicable Diseases. Sample size: Not calculated. Compliance assessment: Done. Health educational messages were disseminated to ensure participants' compliance with the proper use of ITNs and that they did not use other methods of preventing phlebotomine sandflies. To ensure correct use of ITNs, 59 training sessions for families in the intervention group were carried out in schools and mosques. Pre‐intervention: Inhabitants of areas which most active cases of CL were recorded by health centres, were examined and forms were completed for each household during house‐to‐house visits. The interviewers examined scars and ulcers, recording cases that occurred during the 9 months before the interview. Students in all elementary schools were examined and questioned in the 2 cities. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "For each of the 6 pairs we used computer‐generated random numbers to allocate 1 sector to receive Olyset ITNs (intervention group) and the other sector to receive no nets (control group). |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) participants | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) investigators | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No information on loss of clusters. Loss to follow‐up of 11 participants (8/3818 in the intervention group and 3/3818 in the control group). |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the methods were reported in the results. |
| Baseline measurements | Unclear risk | No baseline information. Questionnaire done, but not provided. |
| Statistical adjustment for clustering | High risk | No statistical adjustment for clustering was made in the primary analysis of this cluster‐RCT. |
| Other bias | Unclear risk | Trial authors did not provide a conflict of interest declaration. |
Feliciangeli 2003 VEN.
| Methods | Trial design: Paired RCT. Unit of randomization: Houses. Number of houses: 40. Entomological data collection: Sandflies were collected by CDC miniature light traps that were suspended from the ceiling at about 2 m from the ground floor and left overnight in the bedrooms of control and sprayed houses. Clinical data collection: Not done. Length of follow‐up: 79 days. Analysis: Analysed at house level. |
|
| Participants | Included houses were made of mixture of mud and straw supported by a structure of sticks, called "bahareque" in the local colloquial language (24%), concrete blocks (26%) and wood (26%). Endemic disease: CL caused by L. braziliensis and L. mexicana. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Country: Venezuela (El Ingenio). Trial dates: December 1996 to February 1997. Trial sponsor: STD Programme of the Commission of the European Communities (DG: XII: Science, Research and Development) (Contract no. TS3.CT.930247), the Consejo de Desarrollo Cientifico y Humanístico de la Universidad de Carabobo (CDC‐UC, Project FCS‐91‐044), and the Dirección de Malariologia y Saneamiento Ambiental, Ministerio de Salud y Asistencia Social, Maracay, Venezuela. One author of the trial (D. Campbell‐Lendrum) was supported by the Wellcome Trust. Sample size: Not calculated. Compliance assessment: Not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient details were reported about the method used to generate the allocation sequence. "These were paired according to structure and randomly assigned to the control group (n = 20: B = 7, C = 6, and W = 7) or the group to be sprayed (n = 20: B = 7, C = 7, and W = 6)". |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) participants | Unclear risk | Individual participants not assessed in this trial. |
| Blinding (performance bias and detection bias) investigators | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information if all houses were analysed. Individual participants not assessed. |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the methods were reported in the results. |
| Baseline measurements | Unclear risk | No baseline information. Questionnaire done, but information not provided. |
| Statistical adjustment for clustering | Unclear risk | No adjustment was done, however the outcome was sandfly density and the analysis was conducted at the household level, which is the unit of randomization, thus removing any clustering effects. |
| Other bias | Unclear risk | Trial authors did not provide a conflict of interest declaration. |
Joshi 2009 ASIA.
| Methods | Trial design: Cluster‐RCT. Unit of randomization: Hamlets/neighbourhoods with 50 to 100 houses each. Number of clusters: 96, 24 per intervention arm. Entomological data collection: Cross sectional estimates of the density of the vectors using CDC light traps on 2 consecutive nights, in 5 randomly selected households in each intervention and control cluster. Clinical data collection: Not done. Length of follow‐up: 5 to 6 months. Analysis: Analysed at cluster level. |
|
| Participants | Villages with a high reported incidence of VL in the past 3 years were selected. Socio‐economic conditions are described as comparable between sites but are not further described. Endemic disease: VL caused by unknown L. spp. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Country: India, Bangladesh and Nepal. Trial dates: November 2006 to April 2007. Trial sponsor: Special Programme for Research and Training in Tropical Diseases (TDR/WHO). The DDT for IRS in India was donated by the Hindustan Insecticide Limited and the LLINs (PermaNet®) for Bangladesh were donated by the Vestergaard‐Frandsen Company. The European Union FP6 INCODEV ‐ funded KALANET project supported the LLIN trial in India and Nepal‐BPKIHS. Sample size: Calculated. Compliance assessment: "A spray field worker applied the insecticide to the interior (in Bangladesh also to the exterior) walls of the house and cattle sheds ... Quality control was done by the research team." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The selection of clusters to include in the trial, the allocation of clusters to intervention arms, and the selection of households for entomological assessment are all described as 'random' but no further details are given. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) participants | Low risk | Individual participants not assessed in this trial. |
| Blinding (performance bias and detection bias) investigators | High risk | Investigators were not blinded. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No clusters were lost to follow‐up. Individual participants not assessed. |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the methods were reported in the results. |
| Baseline measurements | Low risk | "Climatic conditions in the study areas were fairly uniform, with a low vector season from December to March due to lower temperatures. Socio‐economic conditions (including age structure, the number of people per household and the illiteracy rate) and disease awareness was comparable in each of the study sites". Baseline measurements of mean phlebotomine sandflies per household were not significantly different at baseline. |
| Statistical adjustment for clustering | Low risk | "Multilevel modelling with sample clusters (hamlet/neighbourhood) as the second level of clustering was applied. The Poisson‐regression procedure in STATA 10.1, with a robust sandwich estimator for clustering, was used in the analysis." |
| Other bias | High risk | Trial authors declared no competing interests. The role of the founder is not clarified. |
Kelly 1997 BRA.
| Methods | Trial design: Cluster‐RCT. Unit of randomization: Homestead with a single chicken shed. Number of clusters: 30 houses randomized to three arms. Entomological data collection: 5 CDC light traps in each cluster (3 in the house, 1 in the chicken shed and 1 in the dining hut) set from 18.00 to 06.15. Nine rounds of collections: 2 pre‐intervention and 7 post‐intervention; approximately 2 weeks apart. Clinical data collection: Not done. Length of follow‐up: 7 months. Analysis: Analysed at cluster level. |
|
| Participants | 30 homesteads with chicken sheds were selected for the trial. After two pre intervention phlebotomine sandflies trapping rounds (4 weeks), each chicken shed of a group was randomly assigned to one of three treatments: spray, target or control (no insecticide). Endemic disease: VL caused by L. chagasi (L. infantum). |
|
| Interventions |
(One homestead received all the interventions but we excluded it as it was not a randomized comparison). |
|
| Outcomes |
|
|
| Notes | Country: Brazil (conducted in 7 villages: Campinas, Pingo d'Agua, Estrada, Vila Ceará, Vila da França, Vila Nova and Bacabau). Trial dates: November 1993 to June 1994. Trial sponsor: A research studentship from the Medical Research Council and a Chadwick Trust Travelling Fellowship, the Brazilian Fundaçao Nacional de Saude. Insecticide for the project was donated by Zeneca Saude Pública, Brasil. Facilities at the Instituto Evandro Chagas through BelBm Research Projects. Field expenses from the Brazilian Fundaçao Nacional de Saúde. Sample size: Not calculated. Compliance assessment: Not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient detail was reported about the method used to generate the allocation sequence. "each chicken shed of a group was randomly assigned to one of three treatments: spray, target or control (no insecticide)." |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) participants | Low risk | Individual participants not assessed in this trial. |
| Blinding (performance bias and detection bias) investigators | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information on loss of clusters. Individual participants not assessed. |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the methods were reported in the results. |
| Baseline measurements | Unclear risk | No baseline information. |
| Statistical adjustment for clustering | Unclear risk | No adjustment was done, however, the outcome was sandfly density and the analysis was conducted at the household level, which is the unit of randomization, thus removing any clustering effects. |
| Other bias | Unclear risk | Trial authors did not provide a conflict of interest declaration. The role of the founder is not clarified. |
Kroeger 2002 VEN.
| Methods | Trial design: Cluster‐RCT. Unit of randomization: City sectors. Number of clusters: 14. Entomological data collection: Cross sectional estimates of the density of the vectors using light traps in the main room of 565 houses for 150 nights at baseline (pre‐intervention) and during the three months after the intervention (post‐intervention). Clinical data collection: Cross sectional questionnaire survey of 569 houses with 2913 inhabitants plus examination for past or current CL (pre‐intervention) at baseline and repeated at 12 months post‐intervention. Length of follow‐up: 12 months. Analysis: Analysed at individual (population) and cluster level (sector/houses). |
|
| Participants | Baseline data on 2913 people living in 569 houses, follow‐up data on similar number. (The original sample size was 578 but 1.6% did not respond). The population was described as having moderate levels of poverty, 31% < 15 years old, 9% > 60 years old, average of 5 people per household, 21% were engaged in domestic activities, 21% were students, 13% were manual workers, self employed artisans, or secretaries, 7% were unemployed, 7% had an academic profession, and only 2% were farmers. Estimated annual incidence of leishmaniasis: 0.5% or above. Endemic disease: CL caused by unknown Leishmania spp (main vector: Lu. youngi and Lu. ovallesi). |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Country: Venezuela (Trujillo). Trial dates: January 2000 to August 2001. Trial sponsor: Funded by the European Commission (contract Alfa Programme 600119 and INCODEV IC18CT 980339). The insecticide was donated by Syngenta. Sample size: Not calculated. Compliance assessment: Not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "For each of the seven matched pairs we randomly allocated one sector (using computer created random numbers) to the intervention group and the other to the control group". |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) participants | High risk | "the population being “blind” towards the group allocation". One sector did not receive unimpregnated curtains so would be aware of their allocation. |
| Blinding (performance bias and detection bias) investigators | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | This was not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No clusters were lost. The final number of participants were increased in 25 persons (see below). ITT analyses and dropouts per group and reasons described. |
| Selective reporting (reporting bias) | Unclear risk | Not reported. |
| Baseline measurements | Unclear risk | Only number cases (%) of CL and mean number of phlebotomine sandflies per traps captured, at baseline (other info not provided by groups). |
| Statistical adjustment for clustering | High risk | No statistical adjustment for clustering was performed in the primary trial. However, sensitivity analysis was done at a range of ICCs in this review and it was concluded that if the ICC had been as high as 0.05, the CIs would have crossed over 1. Statistical analysis: The trial authors used EpiInfo, SPSS, and Stata v6 for analysis. Before the main analysis: Fisher's exact tests to compare cumulative incidence between intervention and control sectors for each pair. They used cumulative incidence rates of CL and the average number of flies per trap (house) for each sector as the units of analysis. They compared data at baseline and then at follow‐up between the intervention and control groups using a paired t test, weighting the data according to the sector size. They also used Wilcoxon's matched pairs test because the small number of pairs made it difficult to assess whether the underlying distribution of the differences was normal (necessary for the validity of the t test), and the Wilcoxon test does not require this assumption. Differences rather than ratios are presented as the estimates of effect because zeroes for the main outcome, CL, precluded the use of ratios. |
| Other bias | Unclear risk | Trial authors declared no competing interests. The founders had no role in the trial. |
Picado 2010a ASIA.
| Methods | Trial design: Cluster‐RCT. Unit of randomization: Hamlets. Number of clusters: 26 (13 intervention and 13 control clusters; 12,691 people). Entomological data collection: Done in Picado 2010b, an excluded non‐randomized entomological study based in this trial. Clinical data collection:
Length of follow‐up: 30 months (from November 2006 to May 2009) for cases of VL and 12 to 24 months after the intervention for seroconversions. Analysis: Analysed at cluster level. |
|
| Participants | Clusters were paired on the basis of incidence of VL between 2003 and 2005. Eligibility criteria: In May 2006, they selected and included in the trial 26 (16 in India, 10 in Nepal) high incidence clusters out of 34 clusters with a high number of reported cases of VL (22 in India, 12 in Nepal) based on the following criteria:
The 26 clusters were stratified by country (16 in India and 10 in Nepal) and population size (6 and 4, respectively, having over 710 residents) and then paired by previous average incidence rate of VL. Clusters in each pair were randomly allocated to group 1 or 2. The random selection of clusters into groups was undertaken in Excel (Microsoft), and the difference in the total number of cases of VL reported in the past three years between group 1 and 2 had to be less than 10%. All individuals living for at least six months a year in the clusters were eligible, but blood sampling was restricted to individuals aged over 2 years. Endemic disease: VL caused by L. donovani. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Country: India (Muzaffarpur district) and Nepal (Sunsari, and Morang districts). Trial dates: November 2006 to May 2009. Trial sponsor: Funded by the European Union under its 6th Framework Program (INCODEV/Project 015374). Contract no INCO‐CT 2005‐01537, KALANET project. Sample size: Calculated. Compliance assessment: Done. "In intervention clusters, 8920/9829 (91%) of the individuals slept regularly (that is, over 80% of the nights) under a treated net. Those observations were confirmed by an additional acceptability survey (V Vanlerberghe, personal communication, January 2010). The use of untreated nets in the control group was variable; 7012/9981 (70%) used a bed net at least once during the trial but only 2978/9981 (30%) used it regularly throughout the year as most of the households did not have enough nets for all their members." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The intervention was then randomly allocated to one of the groups by tossing a coin in the presence of observers." |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) participants | Unclear risk | This was not stated. |
| Blinding (performance bias and detection bias) investigators | Unclear risk | This was not stated. |
| Blinding (performance bias and detection bias) Assessors | Low risk | "All clinically suspected cases detected during the trial were classified as probable or certain visceral leishmaniasis by a clinician who was blinded to the status of the cluster". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No clusters were lost to follow‐up. Analyses and dropouts per group and reasons described. The proportion of people lost to follow‐up (not present or with one or no blood sample) was slightly higher in the control group (21%, (644 +1466)/9981) than in the intervention group (19%, (545+1347)/9829). But the characteristics of the participants lost to follow‐up in both groups were similar (mean age 22 v 23, males 62% v 63%, mean socioeconomic status 2.0 v 2.2, in intervention and control groups respectively). |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the methods were reported in the results. |
| Baseline measurements | Low risk | Yes (table). Intervention and control groups were well balanced at individual and cluster levels, but the prevalence of positive results on the direct agglutination test at baseline in India was almost twice as high as in Nepal, despite the previous annual incidence of VL being similar. |
| Statistical adjustment for clustering | Low risk | Data were analysed at the cluster level. No adjustment for clustering needed as analysis was done at the cluster level. |
| Other bias | Low risk | Trial authors declared no competing interests. The trial founder had no role in the trial design, data collection and analysis, interpretation or reporting of this work, or the decision to submit the work for publication. Competing interests: All authors completed the Unified Competing Interest form and declared: "no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years, no other relationships or activities that could appear to have influenced the submitted work". |
Reyburn 2000 AFG.
| Methods | Trial design: Cluster‐RCT. Unit of randomization: Blocks of 10 houses. Number of clusters: 957. Entomological data collection: Not done. Clinical data collection: Cross‐sectional questionnaire survey of all houses and examination for current or past CL pre‐intervention and at 8, 10 and 15 months post‐intervention. Length of follow‐up: 15 months. Analysis: Analysed at cluster level. |
|
| Participants | The population is described as being 'previously lower middle‐class' in a suburb of Kabul, with houses mostly made from mud or brick. There was no evidence of prior bednet use in the area. The mean age, sex distribution, and prevalence of old and current CL was similar between groups at baseline. Endemic disease: Anthroponotic CL caused by L. tropica. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Country: Afghanistan (Karte‐Naw area of Kabul). Trial dates: May 1997 to August 1998. Trial sponsor: Norwegian Church Aid, the European Commission (ECHO), WHO/UNDP/WB Special Programme for Research and Training in Tropical Diseases (project no. 960662), the Department for International Development (UK), and HealthNet International. Sample size: Calculated. Compliance assessment: Done. "All the trial houses were re‐visited and the household head (mother or father) was asked 3 questions: 'have you noticed less biting by insects this year?', 'are you generally satisfied with the (intervention)?' and 'would you be willing to pay for this service in the future?'. A simple yes‐no response was recorded. Direct observation of bednet compliance or sleeping habits was not socially acceptable". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient detail was reported about the method used to generate the allocation sequence. "interventions were randomly allocated to houses within each block". |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) participants | High risk | Participants were not blinded. |
| Blinding (performance bias and detection bias) investigators | High risk | Investigators were not blinded. |
| Blinding (performance bias and detection bias) Assessors | Low risk | Outcome assessors were blinded. "Survey workers were blinded as to the intervention received by households, having been provided with a survey form that was blank except for the address, and were instructed to ask respondents not to reveal the type of intervention during the interview." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No information on loss of clusters. ITT analysis was not used. Loss to follow‐up of 45% of participants, 7565 persons in total. although they did not specify the group this people belong. |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the methods were reported in the results. |
| Baseline measurements | Low risk | Baseline information: participants mean of age, % of male and female, % of people with CL active or past, and location of lesions. |
| Statistical adjustment for clustering | Low risk | Data were cluster adjusted using a random‐effects logistic regression model. |
| Other bias | Unclear risk | Trial authors did not provide a conflict of interest declaration. |
Rojas 2006 COL.
| Methods | Trial design: Cluster‐RCT. Unit of randomization: Villages. Number of clusters: 20 villages (3631 people). Entomological data collection: Not performed. Clinical data collection:
Length of follow‐up: 12 months. Analysis: Analysed at cluster level. |
|
| Participants | Villages were paired according to prevalence of leishmanin skin test positive in children < 5 years old, number of inhabitants, and community participation score. One village in each pair was randomly assigned to receive the intervention; the other remained as a control. Endemic disease: CL caused by L. braziliensis and L. panamensis. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Country: Colombia (Tumaco, Nariño department). Trial dates: October 1994 to June 1997. Trial sponsor: WHO Research Training Grant and supported by the International Development Research Centre of Canada, IDRC file 92‐0223‐01. It included an educational programme designed and implemented by the Centro de Investigaciones Multidisciplinarias en Desarrollo (CIMDER) that included information about American CL, its mode of transmission and how to use the different preventive measures accompanied the preventive measures. Sample size: Not calculated. Compliance assessment: "Frequency of bednet use was high and consistent during the study. Among the participants who were interviewed during the first and second monitoring visits, 93% and 96% respectively reported sleeping under the bednet every night. This was confirmed during the two unannounced visits to the residences, where approximately 85% of the bednets were in use by the participants. Because there was not enough variation we could not evaluate dose effect for bednet use. Four of the intervention villages only had three impregnation sessions due to logistical constraints. Complete adherence to the impregnation schedule, defined as the percentage of bednets that received all the impregnations (4 or 3 depending on the village), varied among villages (17%‐100%) (data not presented). Very few participants abstained from washing their bednets between two impregnations. Seventythree percent of the participants reported they washed the bednets three or more times during that period (approximately 3 months)." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was performed using a lottery system." |
| Allocation concealment (selection bias) | High risk | "Randomization...was carried out with the participation of delegates from the 20 villages." |
| Blinding (performance bias and detection bias) participants | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) investigators | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No clear information on loss of clusters ("four of the intervention villages only had three impregnation sessions due to logistical constrains"). ITT analysis was not used. There were losses to follow‐up, but the drop‐outs were excluded from the beginning of the trial to analyse the results. Control group: thirteen persons excluded because they moved to an intervention village during the follow‐up period. No movements in the opposite direction were documented. Absence from the village on the days of the post‐intervention exam was somewhat more common in the intervention group (no numbers). |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the methods were reported in the results. |
| Baseline measurements | High risk | In general, the trial groups were comparable in the distribution of behavioral and occupational risk factors, but differed in the distribution of those factors related with the residence and the village. Residences in the control group were more likely to be located at the periphery, close to the forest, have roof made of thatch, have incomplete external walls and have more animals. Also, control villages had lower community participation scores. On the other hand, villages in the intervention group had a greater prevalence of infection in children < 5 years old, had a larger number of inhabitants and had a slightly higher number of males. Characteristics of the residence (distance to the forest < 50 m, and roof made of thatch) and the village (prevalence of infection in children < 5 years old, and community participation score < 50) were strongly associated with American CL in this setting. Several behavioural and occupational activities were moderately associated with infection, as were characteristics of the residence (roof made of thatch and walls made of bamboo) and the village (prevalence of infection in children < 5 years old, and community participation score < 50). |
| Statistical adjustment for clustering | Low risk | Generalised estimating equations were used to adjust for clustering within villages using an exchangeable correlation matrix. |
| Other bias | Low risk | Trial authors declared no competing interests. |
Soto 1995 COL.
| Methods | Trial design: Double‐blind, placebo‐controlled RCT. Unit of randomization: A soldier. Number of participants: 286 soldiers (143 in each group). Entomological data collection: Not done. Clinical data collection: Medical examination. Definitive diagnosis was made by staining a lesion smear with Giemsa and with antileishmanial monoclonal antibodies for detecting amastigotes. If not seen the lesion was biopsied and stained for amastigotes and cultured for promastigotes. If promastigotes detected, they were identified to the species level with the use of isoenzyme electrophoresis. Length of follow‐up: 12 weeks. Analysis: Analysed at individual level. |
|
| Participants | Members of the Colombian army scheduled for patrol in the leishmaniasis‐endemic area of Magdalena Medio with no history of having leishmaniasis and no current signs of infection. Endemic disease: CL caused by L. panamensis. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Country: Colombia. Trial dates: Unknown. Trial sponsor: AB Foundation, Chevy Chase, Maryland, USA, and Rousel Uclaf/Sova de Colombia S.A., Santafe de Bogota, Colombia. Sample size: Not calculated. Compliance assessment: Not done. Adherence to instructions (how to use and wash uniforms) was not monitored. "Because the purpose of the study was to determine the efficacy of permethrin impregnation under conditions of normal duty, adherence to these instructions was not monitored" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient detail was reported about the method used to generate the allocation sequence. "All troops were randomised". |
| Allocation concealment (selection bias) | High risk | Not reported. |
| Blinding (performance bias and detection bias) participants | Unclear risk | Participants were blinded. "The uniforms were distributed in such a way that the participants (soldiers)...did not know which uniforms had been treated with permethrin". |
| Blinding (performance bias and detection bias) investigators | Unclear risk | Investigators were blinded. "The uniforms were distributed in such a way that...the medical attendants did not know which uniforms had been treated with permethrin". |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | This was not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis was used. No dropouts. |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the methods were reported in the results. |
| Baseline measurements | Unclear risk | Baseline information: participants were male soldiers. |
| Statistical adjustment for clustering | Low risk | Not applicable as this trial was individually randomized. |
| Other bias | Unclear risk | Trial authors did not provide a conflict of interest declaration. |
Werneck 2014 BRA.
| Methods | Trial design: Cluster‐RCT. Unit of randomization: Geographic area. Number of clusters: 40 geographic areas. Entomological data collection: Not done. Clinical data collection: Conversion of the Montenegro skin test (MST) at 18 months of follow‐up. Length of follow‐up: 18 months. Analysis: Analysed at cluster level. |
|
| Participants | Ten localities in 7 neighbourhoods of the city of Teresina (Brazil) were divided into blocks, each containing an average of 60 residences. For each locality, 4 blocks were selected to minimize the risk of cross‐contamination of interventions. Eligible participants were residents of selected blocks aged 1 year or above with no history of VL. The 40 geographic areas (blocks) randomly allocated to the 4 types of interventions (697 subjects MST‐). Endemic disease: VL caused by L. chagasi (L. infantum). |
|
| Interventions |
Description of spraying: performed according to the routine of the VL Control Program of the Zoonosis Control Center of the Teresina City Health Department. Interventions were delivered in the selected blocks every 6 months, for three times, beginning just after each household visit. The elimination of infected dogs was decided if indirect immunofluorescence test was more or equalled 1:40. |
|
| Outcomes |
|
|
| Notes | Country: Brazil (Teresina, Itararé quarter). Trial dates: January 2004 to December 2006. Trial sponsor: Funded by Health Surveillance Unit from the Brazilian Ministry of Health. One author was partially funded by the Brazilian Research Council (CNPq 306267/2010‐1 and 202088/2012‐0). The founders had no role in trial design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors have declared that no competing interests exist. Sample size: Calculated. Compliance assessment: Not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Allocation was performed as follows: (a) for each locality, a number was assigned to each block, (b) the intervention schemes were ordered as described above, and (c) using the command "sample" in Stata, the first block sampled was allocated to intervention (i), the second to intervention (ii) and so on. At the end, each intervention scheme was allocated to a total of ten blocks throughout the ten selected localities." |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) participants | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) investigators | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information on loss of clusters. "Losses to follow‐up varied from 35.7% to 40.7% between intervention groups, but no statistically significant difference was found comparing each intervention group with the control group (all P values >0.3)." |
| Selective reporting (reporting bias) | High risk | The trial authors' original plan was to use IFAT test at 6 and 12 months, but due to operational problems, data on IFAT results were not considered valid for the analysis, and serology was not used as a marker of infection in the trial. Problems with serology were poor sensitivity and reproducibility ("For instance, among the 951 subjects for which an IFAT result was available at baseline, only 16 (1.68%) were positive"). The authors decided not to use IFAT results in the trial and relied on conversion of the MST at 18 months of follow‐up as the only outcome measure, since no clinical cases of VL were detected among the studied population. |
| Baseline measurements | Unclear risk | A table shows the distribution of selected baseline socio‐demographic and environmental characteristics for each intervention group. The dog culling groups showed "higher mean years of living in the residence and a smaller percentage of households with a chicken shed in the peri‐domestic environment as compared to the control group (P < 0.015 and P < 0.046, respectively). No other statistically significant difference with any variables or groups was detected." |
| Statistical adjustment for clustering | Low risk | "Using Poisson population‐average models from generalized estimating equations with robust variance, an exchangeable correlation model, and designating each block as the clustering level". |
| Other bias | Low risk | Trial authors declared no competing interests. The trial was funded by Health Surveillance Unit from the Brazilian Ministry of Health. GLW was partially funded by the Brazilian Research Council (CNPq 306267/2010‐1 and 202088/2012‐0). The founders had no role in trial design, data collection and analysis, decision to publish, or preparation of the manuscript. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alexander 1995a | Laboratory and field‐exposure (with pair of volunteers) experiments (no assessment in natural conditions). |
| Alexander 1995c | Cluster quasi‐RCT: assignment to each treatment to the houses was made randomly on the first night and then rotated sequentially from house to house. |
| Asilian 2003b | Duplicate of Asilian 2003a IRN. |
| Boulware 2005 | This study focused on general mosquito bites, not leishmaniasis. |
| Das 2007 | Only preferences between two different kinds of LLIN. |
| Das 2014 | Entomological study of cows placed under different nets in cattle sheds. |
| Davies 2000 | Some houses within each village were allocated on the basis of pre‐intervention sandfly or epidemiological data. |
| Gavgani 2002 | Cluster quasi‐RCT: villages were randomly assigned to the intervention or control group; subsequent pairs were then assigned alternately to either the intervention or control. |
| Jalouk 2007 | Cluster quasi‐RCT: villages were randomly assigned to the intervention or control group; subsequent pairs were then assigned alternately to either the intervention or control. |
| Kumar 1995 | Cluster quasi‐RCT: authors randomly selected 10 houses from a village for the intervention group, but for the control group they used 5 houses separated from the intervention houses by approximately 450 m. |
| Mondal 2008 | Only an assessment about prevention methods used against leishmaniasis in 9 kala‐azar endemic districts. |
| Moosa‐Kazemi 2007 | Treatments were randomly performed in the corresponding districts but all households enrolled in district Shaghayegh received ITNs and ITCs; Households in district Honar received non‐impregnated bed nets and curtains and district Vakilabad was the control area. |
| Nadim 1995 | Only one cluster in each group. |
| Nieves 2008 | Evaluation about knowledge and practices against leishmaniasis. |
| Picado 2010b | Based on an included paired cluster‐RCT (Picado 2010a ASIA) were each group were randomly allocated to ITNs or control, in this excluded trial the design was not random as mentioned in the paper. "Out of the 26 KALANET clusters, 3 intervention and 3 control clusters in each country were selected for the entomological trial on the basis of year round accessibility and VL incidence rates. 13 clusters were initially assessed (6 in India and 7 in Nepal) and one was finally excluded in Nepal. Being a subset of the KALANET clusters, the 12 selected clusters for the entomological trial were not necessarily paired." |
| Rodríguez‐Villamizar 2006 | It is not as trial. It is an assessment on the impact of a basic health plan for preventing CL in rural areas of Colombia. |
| Tayeh 1997 | Allocation not randomized, "the villages were randomly assigned as intervention or control villages based on the prevalence and size of the villages. H and SN were considered an intervention villages, TS and KS as control villages." |
| Yaghoobi‐Ershadi 2006 | Unclear trial design. "Three villages (called Komshecheh, Aliabad‐Mollaali, and Habibabad) were selected randomly in the rural district of Borkhar, Isfahan province, central Iran. Then, in each village, 168 households near each other with similar prevalence (2.1‐ 2.7% for lesions and 70.4‐81.2% for scars) were recruited to the study. Treatments were randomly performed in corresponding villages. All households enrolled in Habibabad received impregnated bed nets and curtains (IBs and ICs); Aliabad‐Mollaali, non‐impregnated bed nets and curtains (NIBs and NICs) and Komshecheh was decided to be the control area." |
Characteristics of ongoing studies [ordered by study ID]
NCT01644682.
| Trial name or title | Replacement of Insecticides to Control Visceral Leishmaniasis |
| Methods | Allocation: randomized, endpoint classification: efficacy study, intervention model: factorial assignment, masking: open label, primary purpose: prevention |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Interventions A: IWFPL: Indoor house walls and floors will be plastered with lime (a traditional method known in the study areas) including treatment of outdoor breeding places with lime and bleaching powder to inhibit sandfly breeding; B: IDWL: Install durable wall lining containing deltamethrin to kill immature stage and as well as adult phlebotomine sandflies; C: ITN: Impregnation of existing bednets available in the community with slow release insecticide, deltamethrin. Control intervention D: Control group, no intervention. |
| Outcomes |
Primary outcome
Secondary outcome
For all outcomes, assessments were at 12 months. |
| Starting date | May 2012 |
| Contact information | Dinesh Mondal, MBBS, MD, PhD Telephone: +8801712027091 Email: din63d@icddrb.org |
| Notes |
Differences between protocol and review
We changed the primary outcome from "Reduction (%) of cases (incidence) of leishmaniasis" in González 2010 to "cases of leishmaniasis".
Contributions of authors
UG was a link with the editorial base and coordinated contributions from review co‐authors. AF and MP searched for trials (including developed a search strategy, obtained papers, contacted authors, investigators or drug companies). CE, AF, UG, MP and IV selected trials for inclusion and extracted data from included trials. MP, UG and DS entered data into RevMan 2014. TE, DS and UG performed analyses. AF, UG, MT, CE, DS, JA interpreted the data. All review authors drafted the final review. JA and IV, the expert representatives, focused on relevance and applicability of the Cochrane Review.
Sources of support
Internal sources
Liverpool School of Tropical Medicine, UK.
External sources
Office of Control of Neglected Tropical Diseases (WHO/CDS/NTD/IDM), Communicable Disease Cluster, World Health Organization, Switzerland.
Agencia Española de Cooperación Internacional para el Desarrollo (AECID), Spain.
Department for International Development (DFID), United States Minor Outlying Islands.
Declarations of interest
None known (All).
Unchanged
References
References to studies included in this review
Asilian 2003a IRN {published data only}
- Asilian A, Sadeghinia A, Shariati F, Imam Jome M, Ghoddusi A. Efficacy of permethrin‐impregnated uniforms in the prevention of cutaneous leishmaniasis in Iranian soldiers. Journal of Clinical Pharmacy and Therapeutics 2003;28(3):175‐8. [DOI] [PubMed] [Google Scholar]
Chowdhury 2011 BGD {published data only}
- Chowdhury R, Dotson E, Blackstock AJ, McClintock S, Maheswary NP, Faria S, et al. Comparison of insecticide‐treated nets and indoor residual spraying to control the vector of visceral leishmaniasis in Mymensingh District, Bangladesh. American Journal of Tropical Medicine and Hygiene 2011;84(5):662‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Costa 2007 BRA {published data only}
- Costa CH, Tapety CM, Werneck GL. Control of visceral leishmaniasis in urban areas: randomized factorial intervention trial [Controle da leishmaniose visceral em meio urbano: estudo de intervençao randomizado fatorial]. Revista da Sociedade Brasileira de Medicina Tropical 2007;40(4):415‐9. [DOI] [PubMed] [Google Scholar]
Dinesh 2008 IND {published data only}
- Dinesh DS, Das P, Picado A, Davies C, Speybroeck N, Ostyn B, et al. Long‐lasting insecticidal nets fail at household level to reduce abundance of sandfly vector Phlebotomus argentipes in treated houses in Bihar (India). Tropical Medicine and International Health 2008;13(7):953‐8. [DOI] [PubMed] [Google Scholar]
Emami 2009 IRN {published data only}
- Emami MM, Yazdi M, Guillet P. Efficacy of Olyset long‐lasting bednets to control transmission of cutaneous leishmaniasis in Iran. Eastern Mediterranean Health Journal 2009;15(5):1075‐83. [PubMed] [Google Scholar]
Feliciangeli 2003 VEN {published data only}
- Feliciangeli MD, Mazzarri MB, Campbell‐Lendrum D, Maroli M, Maingon R. Cutaneous leishmaniasis vector control perspectives using lambdacyhelothrin residual house spraying in El Ingenio, Miranda State, Venezuela. Transaction of the Royal Society of Tropical Medicine and Hygiene 2003;97(6):641‐6. [DOI] [PubMed] [Google Scholar]
Joshi 2009 ASIA {published data only}
- Das ML, Banjara M, Chowdhury R, Kumar V, Rijal S, Joshi A, et al. Visceral leishmaniasis on the Indian sub‐continent: a multi‐centre study of the costs of three interventions for the control of the sandfly vector, Phlebotomus argentipes. Annals of Tropical Medicine and Parasitology 2008;102(8):729‐41. [DOI] [PubMed] [Google Scholar]
- Joshi AB, Das ML, Akhter S, Chowdhury R, Mondal D, Kumar V, et al. Chemical and environmental vector control as a contribution to the elimination of visceral leishmaniasis on the Indian subcontinent: cluster randomized controlled trials in Bangladesh, India and Nepal. BCM Medicine 2009;7:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kelly 1997 BRA {published data only}
- Kelly DW, Mustafa Z, Dye C. Differential application of lambda‐cyhalothrin to control the sandfly Lutzomyia longipalpis. Medical and Veterinary Entomology 1997;11(1):13‐24. [DOI] [PubMed] [Google Scholar]
Kroeger 2002 VEN {published data only}
- Kroeger A, Avila EV, Morison L. Insecticide impregnated curtains to control domestic transmission of cutaneous leishmaniasis in Venezuela: cluster randomised trial. BMJ 2002;325(7368):810‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Picado 2010a ASIA {published data only}
- Picado A, Singh SP, Rijal S, Sundar S, Ostyn B, Chappuis F, et al. Longlasting insecticidal nets for prevention of Leishmania donovani in India and Nepal: paired cluster randomised trial. BMJ 2010;341:c6760. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reyburn 2000 AFG {published data only}
- Reyburn H, Ashford R, Mohsen M, Hewitt S, Rowland M. A randomized controlled trial of insecticide‐treated bednets and chaddars or top sheets, and residual spraying of interior rooms for the prevention of cutaneous leishmaniasis in Kabul, Afghanistan. Transactions of the Royal Society of Tropical Medicine and Hygiene 2000;94(4):361‐6. [DOI] [PubMed] [Google Scholar]
Rojas 2006 COL {published data only}
- Rojas CA, Weigle KA, Tovar R, Morales AL, Alexander B. A multifaceted intervention to prevent American cutaneous leishmaniasis in Colombia: results of a group‐randomized trial. Biomedica 2006;26(Suppl 1):152‐66. [PubMed] [Google Scholar]
Soto 1995 COL {published data only}
- Soto J, Medina F, Dember N, Berman J. Efficacy of permethrin‐impregnated uniforms in the prevention of malaria and leishmaniasis in Colombian soldiers. Clinical Infectious Diseases 1995;21(3):599‐602. [DOI] [PubMed] [Google Scholar]
Werneck 2014 BRA {published data only}
- Werneck GL, Costa CHN, Carvalho FAA, Pires e Cruz Mdo S, Maguire JH, Castro MC. Effectiveness of insecticide spraying and culling of dogs on the incidence of Leishmania infantum infection in humans: a cluster randomized trial in Teresina, Brazil. PLoS Neglected Tropical Diseases 2014;8(10):e3172. [DOI: 10.1371/journal.pntd.0003172] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Alexander 1995a {published data only}
- Alexander B, Cadena H, Usma MC, Rojas CA. Laboratory and field evaluations of a repellent soap containing diethyl toluamide (DEET) and permethrin against phlebotomine sand flies (Diptera: Psychodidae) in Valle del Cauca, Colombia. American Journal of Tropical Medicine and Hygiene 1995;52(2):169‐73. [DOI] [PubMed] [Google Scholar]
Alexander 1995c {published data only}
- Alexander B, Usma MC, Cadena H, Quesada BL, Solarte Y, Roa W, et al. Evaluation of deltamethrin‐impregnated bednets and curtains against phlebotomine sandflies in Valle del Cauca, Colombia. Medical and Veterinary Entomology 1995;9(3):279‐83. [DOI] [PubMed] [Google Scholar]
Asilian 2003b {published data only}
- Asilian A, Sadeghinia A, Shariati FA, Emam Jome M, Ghoddusi AR. Efficacy of permethrin‐impregnated clothes in prevention of cutaneous leishmaniasis: A randomized, double‐blind clinical trial [Persian]. Iranian Journal of Dermatology 2003;6(2):25‐9. [Google Scholar]
Boulware 2005 {published data only}
- Boulware DR, Beisang AA 3rd. Passive prophylaxis with permethrin‐treated tents reduces mosquito bites among North American summer campers. Wildnerness and Environmental Medicine 2005;16(1):9‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Das 2007 {published data only}
- Das ML, Singh SP, Vanlerberghe V, Rijal S, Rai M, Karki P, et al. Population preference of net texture prior to bed net trial in Kala‐Azar‐endemic areas. PloS Neglected Tropical Diseases 2007;1(3):e100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Das 2014 {published data only}
- Das ML, Rowland M, Austin JW, Lazzari E, Picado A. Do size and insecticide treatment matter? Evaluation of different nets against Phlebotomus argentipes, the vector of visceral leishmaniasis in Nepal. PLoS One 2014;9(12):e114915. [DOI: 10.1371/journal.pone.0114915] [DOI] [PMC free article] [PubMed] [Google Scholar]
Davies 2000 {published data only}
- Davies CR, Llanos‐Cuentas EA, Campos P, Monge J, Leon E, Canales J. Spraying houses in the Peruvian Andes with lambda‐cyhalothrin protects residents against cutaneous leishmaniasis. Transactions of the Royal Society of Tropical Medicine and Hygiene 2000;94(6):631‐6. [DOI] [PubMed] [Google Scholar]
Gavgani 2002 {published data only}
- Gavgani AS, Hodjati MH, Mohite H, Davies CR. Effect of insecticide‐impregnated dog collars on incidence of zoonotic visceral leishmaniasis in Iranian children: a matched‐cluster randomised trial. Lancet 2002;360(9330):374‐9. [DOI] [PubMed] [Google Scholar]
Jalouk 2007 {published data only}
- Jalouk L, Al Ahmed M, Gradoni L, Maroli M. Insecticide‐treated bednets to prevent anthroponotic cutaneous leishmaniasis in Aleppo Governorate, Syria: results from two trials. Transactions of the Royal Society of Tropical Medicine and Hygiene 2007;101(4):360‐7. [DOI] [PubMed] [Google Scholar]
Kumar 1995 {published data only}
- Kumar V, Kesari SK, Sinha NK, Palit A, Ranjan A, Kishore K, et al. Field trial of an ecological approach for the control of Phlebotomus argentipes using mud & lime plaster. Indian Journal of Medical Research 1995;101:154‐6. [PubMed] [Google Scholar]
Mondal 2008 {published data only}
- Mondal D, Alam MS, Karim Z, Haque R, Boelaert M, Kroeger A. Present situation of vector‐control management in Bangladesh: a wake up call. Health Policy 2008;87(3):369‐76. [DOI] [PubMed] [Google Scholar]
Moosa‐Kazemi 2007 {published data only}
- Moosa‐Kazemi SH, Yaghoobi‐Ershadir MR, Akhavan AA, Abdoli H, Zahraei‐Ramazani AR, Jafari R, et al. Deltamethrin‐impregnated bed nets and curtains in an anthroponotic cutaneous leishmaniasis control program in northeastern Iran. Annals of Saudi Medicine 2007;27(1):6‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nadim 1995 {published data only}
- Nadim A, Motabar M, Houshmand B, Keyghobadi K, Aflatonian MR, WHO Division of Control of Tropical Diseases. Evaluation of pyrethroid impregnated bednets for control of anthroponotic cutaneous leishmaniasis in Bam (Islamic Republic of Iran). WHO/LEISH/ 95.37. https://extranet.who.int/iris/restricted/bitstream/10665/61138/1/WHO_LEISH_95.37.pdf (accessed dd Month 1995):1‐21.
Nieves 2008 {published data only}
- Nieves E, Villarreal N, Rondón M, Sánchez M, Carrero J. Evaluation of knowledge and practice on tegumentary leishmaniasis in an endemic area of Venezuela. Biomédica 2008;28(3):347‐53. [PubMed] [Google Scholar]
Picado 2010b {published data only}
- Picado A, Das ML, Kumar V, Kesari S, Dinesh DS, Roy L, et al. Effect of village‐wide use of long‐lasting insecticidal nets on visceral Leishmaniasis vectors in India and Nepal: a cluster randomized trial. PLoS Neglected Tropical Diseases 2010;4(1):e587. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rodríguez‐Villamizar 2006 {published data only}
- Rodríguez‐Villamizar LA, Orozco‐Vargas LC, Muñoz‐Mantilla G. The basic health plan's impact on preventing cutaneous leishmaniasis in rural areas of Santander, Colombia [Spanish]. Revista de Salud Pública 2006;8(Suppl 1):116‐28. [PubMed] [Google Scholar]
Tayeh 1997 {published data only}
- Tayeh A, Jalouk L, Al‐Khiami AM. A cutaneous leishmaniasis control trial using pyrethroid‐impregnated bednets in villages near Aleppo, Syria. WHO/LEISH/97.41. http://apps.who.int/iris/bitstream/10665/63792/1/WHO_LEISH_97.41.pdf?ua=1 1997:1‐43.
Yaghoobi‐Ershadi 2006 {published data only}
- Yaghoobi‐Ershadi MR, Moosa‐Kazemi SH, Zahraei‐Ramazani AR, Jalai‐Zand AR, Akhavan AA, Arandian MH, et al. Evaluation of deltamethrin‐impregnated bed nets and curtains for control of zoonotic cutaneous leishmaniasis in a hyperendemic area of Iran. Bulletin de la Société de Pathologie Exotique 2006;99(1):43‐8. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT01644682 {published data only}
- Replacement of Insecticides to Control Visceral Leishmaniasis. Ongoing study May 2012.
Additional references
Alexander 2003
- Alexander B, Maroli M. Control of phlebotomine sandflies. Medical and Veterinary Entomology 2003;17(1):1‐18. [DOI] [PubMed] [Google Scholar]
Alvar 2006
- Alvar J, Yactayo S, Bern C. Leishmaniasis and poverty. Trends in Parasitology 2006;22(12):552‐7. [DOI] [PubMed] [Google Scholar]
Alvar 2012
- Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, et al. Leishmaniasis Worldwide and Global Estimates of Its Incidence. PLoS One 2012;7(5):e35671. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bowater 2009
- Bowater RJ, Abdelmalik SM, Lilford RJ. The methodological quality of cluster randomised controlled trials for managing tropical parasitic disease: a review of trials published from 1998 to 2007. Transactions of the Royal Society of Tropical Medicine and Hygiene 2009;103(5):429‐36. [DOI] [PubMed] [Google Scholar]
Courtenay 2009
- Courtenay O, Kovacic V, Gomes PA, Garcez LM, Quinnell RJ. A long‐lasting topical deltamethrin treatment to protect dogs against visceral leishmaniasis. Medical and Veterinary Entomology 2009;23(3):245‐56. [DOI] [PubMed] [Google Scholar]
Das 2008
- Das M, Banjara MR, Chowdhury R, Kumar V, Rijal S, Joshi A, et al. Visceral leishmaniasis on the Indian sub‐continent: a multi‐centre study of the costs of three interventions for the control of the sand fly vector, Phlebotomus argentipes.. Annals of Tropical Medicine and Parasitology 2008;102:729‐41. [DOI] [PubMed] [Google Scholar]
Davies 2003
- Davies CR, Kaye P, Croft SL, Sundar S. Leishmaniasis: new approaches to disease control. British Medical Journal 2003;326(7385):377‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Desjeux 1996
- Desjeux P. Leishmaniasis. Public health aspects and control. Clinical Dermatology 1996;14:417‐23. [DOI] [PubMed] [Google Scholar]
Dhiman 1994
- Dhiman RC, Sharma VP. Evaluation of neem oil as sandfly, Phlebotomus papatasi (Scopoli) repellent in an Oriental sore endemic area in Rajasthan. Southeast Asian Journal of Tropical Medicine and Public Health 1994;25(3):608‐10. [PubMed] [Google Scholar]
Dinesh 2010
- Dinesh DS, Das ML, Picado A, Roy L, Rijal S, et al. Insecticide susceptibility of Phlebotomus argentipes in visceral leishmaniasis endemic districts in India and Nepal. PLoS Neglected Tropical Diseases 2010;26(4(10)):e859. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dogan 2006
- Dogan N, Ozbel Y, Toz SO, Dinleyici EC, Bor O. Sero‐epidemological survey on canine visceral leishmaniasis and the distribution of sandfly vectors in northwestern Turkey: prevention strategies for childhood visceral leishmaniasis. Journal of Tropical Pediatrics 2006;52(3):212‐7. [DOI] [PubMed] [Google Scholar]
Gonzalez 2010
- Gonzalez U, Pinart M, Reveiz L, Rengifo‐Pardo M, Tweed J, Macaya A, et al. Designing and Reporting Clinical Trials on Treatments for Cutaneous Leishmaniasis. Clinical Infectious Diseases 2010;51(4):409‐19. [DOI] [PubMed] [Google Scholar]
González 2008
- González U, Pinart M, Reveiz L, Alvar J. Interventions for Old World cutaneous leishmaniasis. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD005067.pub3] [DOI] [PubMed] [Google Scholar]
González 2009
- González U, Pinart M, Rengifo‐Pardo M, Macaya A, Alvar J, Tweed JA. Interventions for American cutaneous and mucocutaneous leishmaniasis. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD004834.pub2] [DOI] [PubMed] [Google Scholar]
GRADE Working Group 2004
- GRADE Working Group 2004. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro 2015 [Computer program]
- McMaster University. GRADEpro. McMaster University, 2015.
Higgins 2009
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kebede 2010
- Kebede Y, Gebre‐Michael T, Balkew M. Laboratory and field evaluation of neem (Azadirachta indica A. Juss) and Chinaberry (Melia azedarach L.) oils as repellents against Phlebotomus orientalis and P. bergeroti (Diptera: Psychodidae) in Ethiopia. Acta Tropica 2010;113(2):145‐50. [DOI] [PubMed] [Google Scholar]
Khanjani 2009
- Khanjani N, González U, Leonardi‐Bee J, Mohebali M, Saffari M, Khamesipour A. Vaccines for preventing cutaneous leishmaniasis. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD007634] [DOI] [Google Scholar]
Kishore 2006
- Kishore K, Kumar V, Kesari S, . Dinesh DS, Kumar AJ, Das P, Bhattacharya SK. Vector control in leishmaniasis. Indian Journal of Medical Research 2006;123:467‐72. [PubMed] [Google Scholar]
Quinell 2009
- Quinnell RJ, Courtenay O. Transmission, reservoir hosts and control of zoonotic visceral leishmaniasis. Parasitology 2009;136(14):1915‐34. [DOI] [PubMed] [Google Scholar]
Reithinger 2007
- Reithinger R, Dujardin JC, Louzir H, Pirmez C, Alexander B, Brooker S. Cutaneous leishmaniasis. Lancet Infectious Diseases 2007;7(9):581‐96. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roberts 2006
- Roberts MTM. Current understandings on the immunology of leishmaniasis and recent developments in prevention and treatment. British Medical Bulletin 2006;75, 76:115‐30. [DOI] [PubMed] [Google Scholar]
Romero 2010
- Romero GA, Boelaert M. Control of visceral leishmaniasis in Latin America‐a systematic review. PLoS Neglected Tropical Diseases 2010;4(1):e584. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stockdale 2012
- Stockdale L. Preventative Methods Against Human Leishmaniasis Infection. Thesis. University of York. Department of Health Sciences. September 2012:1‐80.
Stockdale 2013
- Stockdale L, Newton R. A review of preventative methods against human leishmaniasis infection. PLoS Neglected Tropical Diseases 2013;7(6):e2278. [DOI: 10.1371/journal.pntd.0002278] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weigle 1993
- Weigle KA, Santrich C, Martinez F, Valderrama L, Saravia N. Epidemiology of cutaneous leishmaniasis in Colombia: A longitudinal study of the natural history, prevalence and incidence of infection and clinical manifestations. Journal of Infectious Diseases 1993;168:699‐708. [DOI] [PubMed] [Google Scholar]
WHO 2009
- World Health Organization. WHO. Leishmaniasis: background information.The disease and its epidemiology. Burden of disease. Magnitude of the problem.. http://www.who.int/leishmaniasis/burden/magnitude/burden_magnitude/en/index.html 2009, issue date last accessed: 18/3/2009.
WHO 2010
- WHO Expert Committee on the Control of Leishmaniasis, Geneva. Control of the Leishmaniasis. WHO Technical Report Series. Vol. 949, World Health Organization, 2010. [Google Scholar]
References to other published versions of this review
González 2010
- González U, Pinar M, Firooz A, Enk C, Mendoza N, Vélez ID, et al. Preventive measures for leishmaniasis. Cochrane Database of Systematic Reviews 2010, Issue 10. [DOI: 10.1002/14651858.CD008736] [DOI] [Google Scholar]


