Abstract
Rationale
Coronary artery disease (CAD), the direct result of atherosclerosis, is the most common cause of death in Western societies. Vascular smooth muscle cell (VSMC) apoptosis occurs during the progression of atherosclerosis and in advanced lesions, promotes plaque necrosis, a common feature of high-risk/vulnerable atherosclerotic plaques. Akt1, a serine-threonine protein kinase, regulates several key endothelial cell (EC) and VSMC functions including cell growth, migration, survival and vascular tone. While global deficiency of Akt1 results in impaired angiogenesis and massive atherosclerosis, the specific contribution of VSMC Akt1 remains poorly characterized.
Objective
To investigate the contribution of VSMC Akt1 during atherogenesis and in established atherosclerotic plaques.
Methods and Results
We generated two mouse models in which Akt1 expression can be suppressed specifically in VSCMs before (Apoe−/−Akt1fl/flSm22αCRE) and after (Apoe−/−Akt1fl/flSM-MHC-CreERT2E) the formation of atherosclerotic plaques. This approach allows us to interrogate the role of Akt1 during the initial and late steps of atherogenesis. Absence of Akt1 in VSMCs during the progression of atherosclerosis results in larger atherosclerotic plaques characterized by bigger necrotic core areas, enhanced VSMC apoptosis and reduced fibrous cap and collagen content. In contrast, VSMC Akt1 inhibition in established atherosclerotic plaques does not influence lesion size but markedly reduces the relative fibrous cap area in plaques and increases VSMC apoptosis.
Conclusions
Akt1 expression in VSMCs influences early and late stages of atherosclerosis. Absence of Akt1 in VSMCs induces features of plaque vulnerability including fibrous cap thinning and extensive necrotic core areas. These observations suggest that interventions enhancing Akt1 expression specifically in VSMCs may lessen plaque progression.
Keywords: Akt, atherosclerosis, VSMCs, atherogenesis
INTRODUCTION
Atherosclerosis is a chronic inflammatory disease characterized by the accumulation of lipids and fibrous elements in large arteries1–3. This complex disease involves interactions of modified lipoproteins, monocyte-derived macrophages or foam cells, T lymphocytes, endothelial cells (ECs), vascular smooth muscle cells (VSCMs) and fibroblasts. Monocytes and macrophages lead to the inflammatory pathology of atherosclerosis, whereas changes in athermanous plaque thickness and matrix composition are attributed to VSMCs. VSMCs are responsible for promoting plaque stability in advanced lesions. In this regard, studies from Bennett’s laboratory have elegantly demonstrated that VSMC apoptosis alone is sufficient to induce multiple features of atherosclerotic plaque vulnerability4. Interestingly, in vitro studies have indicated that VSMCs isolated from atheromas in humans and mice have downregulated activity of the Akt signaling pathway and are more prone to apoptosis4, 5.
The serine/threonine kinase Akt (also known as protein kinase B, or PKB) is a multifunctional kinase implicated in the regulation of cellular growth, survival and metabolism6, 7. Akt is at the center of a complex signaling network regulating multiple cellular functions, suggesting a possibility that Akt signaling pathways may be involved in the pathogenesis of atherosclerosis. Mammalian cells express three Akt isoforms (Akt1, Akt2 and Akt3) encoded by three separate genes8. Despite their similarity, gene knockout studies have shown that the three Akt isoforms have some non-redundant functions. The relative expression of the three isoforms varies between tissues, with Akt1 being the most abundantly expressed9. Akt1 regulates multiple functions in the cardiovascular system, playing an important role during angiogenesis, apoptosis and regulating cardiac hypertrophy10–12. In vitro studies suggest that Akt1 might play either a pro-atherogenic or anti-atherogenic role, possibly due to compensation by multiple isoforms when a single isoform is deleted. For example nitric oxide (NO), derived from endothelial nitric oxide synthase (eNOS), has physiological properties that can be atheroprotective, including the inhibition of apoptosis, VSMC proliferation, platelet aggregation and adhesion, and leukocyte activation and adhesion13, 14. However PI3K-Akt signaling pathway activation enhances macrophage survival in lesions, and the persistent activation of Akt promotes cellular hypertrophy and hyperplasia, thereby promoting atherogenesis11, 15. Most importantly, absence of global Akt1 in an ApoE-deficient background, leads to severe atherosclerosis due to the enhanced expression of pro-inflammatory genes and endothelial cell and macrophage apoptosis16. Bone marrow transplantation experiments demonstrated that macrophages from ApoE−/−Akt1−/− donors did not confer increased atherogenesis, suggesting that the lesion expansion in those mice may be of vascular origin. However, these studies were performed in whole body Akt1 deficient mice, which do not allow for the specific interrogation of Akt1 in VSMCs during the progression of atherosclerosis. Thus, to assess the role of Akt1 in VSMCs during atherogenesis in vivo, we generated two novel mouse models in which Akt1 expression can be suppressed in VSMCs before (Apoe−/−Akt1fl/flSm22αCRE) and after (Apoe−/−Akt1fl/flSM-MHC-CreERT2) the formation of atherosclerotic plaques. We show that specific Akt1 disruption in VSMCs accelerates atherosclerosis after 5 months on a Western diet (WD). Importantly, Apoe−/−Akt1fl/flSm22αCRE mice develop features of plaque vulnerability, such as thinner fibrous caps, reduced collagen deposition, increased necrotic cores and enhanced VSMC apoptosis. Similarly, inducible genetic silencing of Akt1 in mice with established atherosclerotic plaques results in thinner fibrous caps. Altogether, these findings provide genetic evidence demonstrating the critical role of Akt1 at different stages of atherogenesis and suggest that Akt1 activation in advanced plaques may be beneficial for treating CAD.
METHODS
Because of space limitations, a detailed description of the Materials and Methods is presented in the Online Data Supplement.
RESULTS
Generation and characterization of Apoe−/−Akt1fl/flSm22αCRE mice
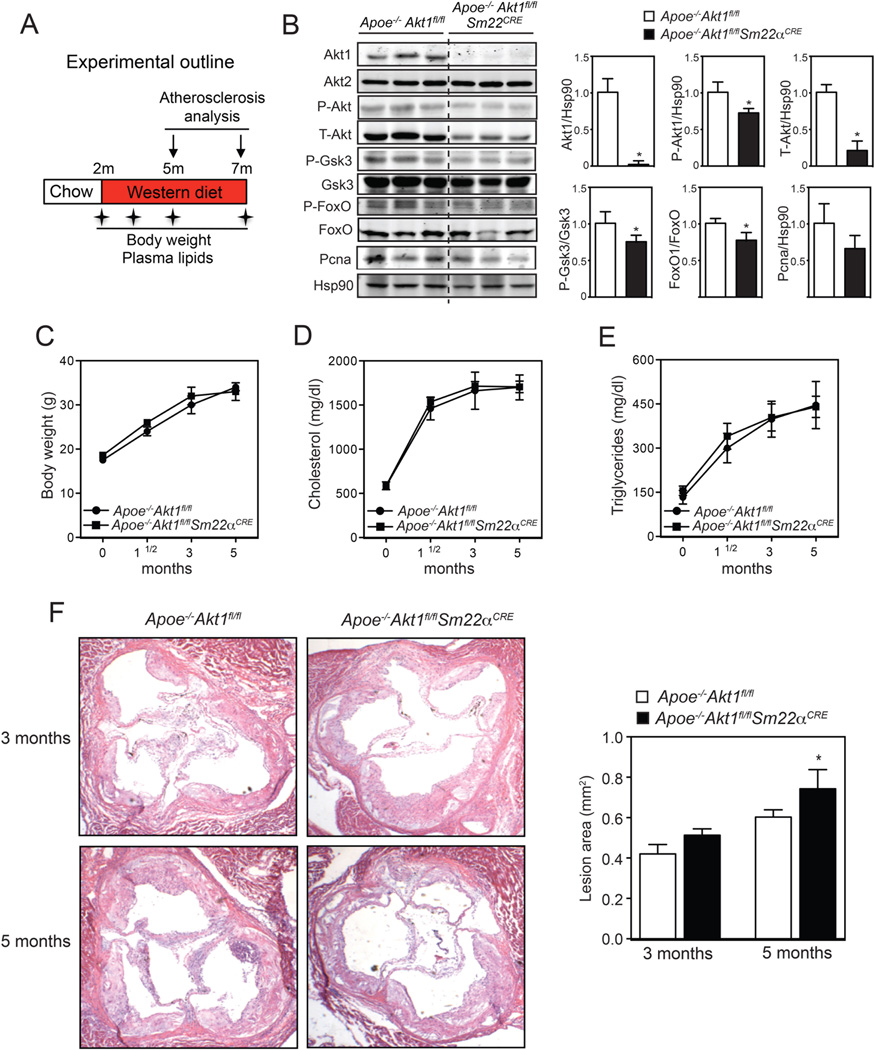
To elucidate the specific role of Akt1 in VSMCs during atherogenesis, we generated 2 mouse models in which Akt1 expression can be inhibited specifically in VSMCs before (Apoe−/−Akt1fl/flSm22αCRE) and after (Apoe−/−Akt1fl/flSM-MHC-CreERT2E) the formation of atherosclerotic lesions. VSMC specific and Tamoxifen-inducible specific deletion of Akt1 was achieved by Cre-Lox recombination. We initially studied the role of Akt1 in VSMCs during the progression of atherosclerosis in Apoe−/−Akt1fl/flSm22αCRE mice fed a WD for 3 and 5 months (experimental outline in Figure 1A). As expected, Akt1 expression was markedly reduced in aortic lysates isolated from Apoe−/−Akt1fl/flSm22αCRE mice compared to those isolated from Apoe−/−Akt1fl/fl mice (Figure 1B). Accordingly, total Akt protein expression and the phosphorylation of well-known downstream Akt targets, including Gsk3 and FoxO1, were significantly decreased in aorta lysates isolated from Apoe−/−Akt1fl/flSm22αCRE mice (Figure 1B, quantified in the right panels). The expression levels of Akt2, the other major Akt isoform in VSMCs, were similar in both groups of mice (Figure 1B). Surprisingly, we found a moderate but significant decrease in p-Akt levels, suggesting that Akt2 could be more activated and help compensate the loss of Akt1 in VSMCs. We did not find differences in proliferating cell nuclear antigen (PCNA) expression between both genotypes (Figure 1B). We next examined multiple metabolic parameters in the mice. As seen in Figures 1C–E, VSMC specific Akt1 genetic deletion did not influence body weight, total cholesterol or triglycerides at various time points during the study.
Figure 1. Prolonged Akt1 silencing in VSMCs results in moderately increased atherosclerosis.
A) Experimental outline of the atherosclerosis study of Apoe−/−Akt1fl/fl and Apoe−/−Akt1fl/flSm22αCRE mice fed a WD during 3 or 5 months. B) Representative Western blot analysis of Akt1, Akt2, total Akt and downstream Akt targets from aortic lysates of male Apoe−/−Akt1fl/fl and Apoe−/−Akt1fl/flSm22αCRE mice fed a WD during 3 months (n=6). Hsp90 was used as a loading control. Band densitometry is shown in the right panels. *Indicates p<p 0.05 compared with Apoe−/−Akt1fl/fl mice. C–E) Body weight (C), total cholesterol (D) and triglyceride levels (E) at the starting point (0 month) and after 1.5, 3 and 5 months on WD. All of the data represent the mean ± SEM; (Apoe−/−Akt1fl/fl n=14 and Apoe−/−Akt1fl/flSm22αCRE n=10). F) Representative histological analysis of cross-sections from the aortic sinus stained with hematoxylin and eosin (H&E). The right panel shows the quantification of the lesion area. All of the data represents the mean ± SEM; (3 months: Apoe−/−Akt1fl/fl n=8, Apoe−/−Akt1fl/flSm22αCRE n=13; 5 months: Apoe−/−Akt1fl/fl n=14, Apoe−/−Akt1fl/flSm22αCRE n=10). *Indicates p< 0.05 compared to Apoe−/−Akt1fl/fl mice.
Prolonged Akt1 inhibition in VSMCs results in accelerated atherosclerosis and increased plaque necrosis
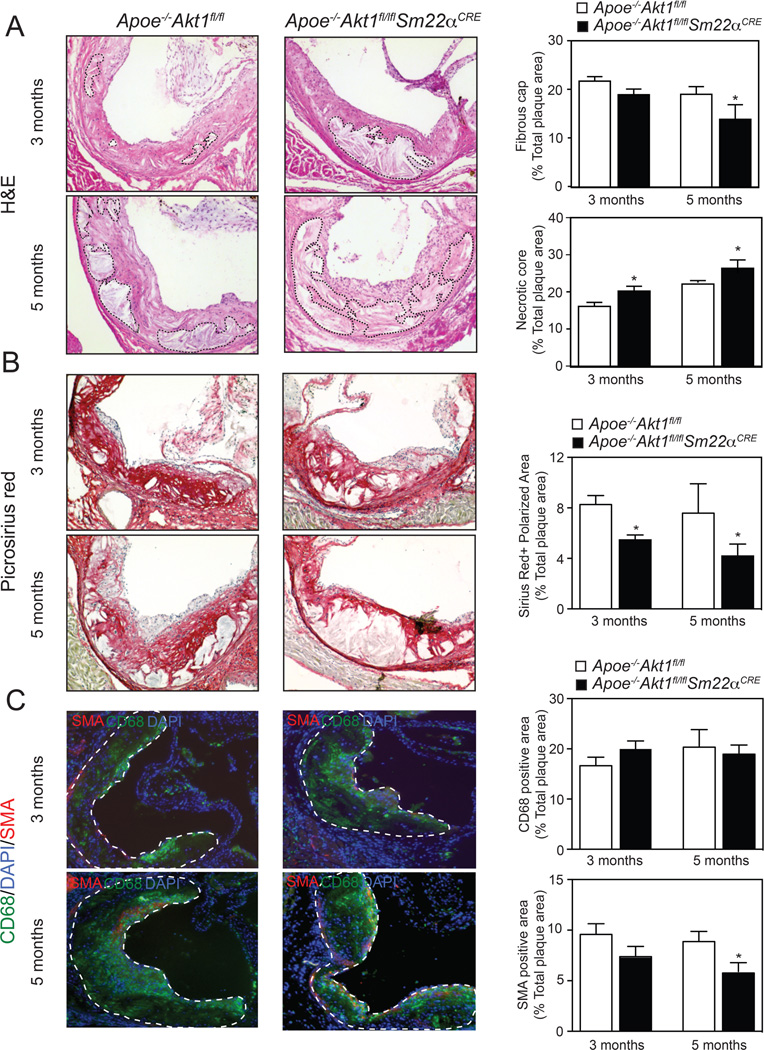
Next, we analyzed the atherosclerotic lesions in Apoe−/−Akt1fl/fl and Apoe−/−Akt1fl/flSm22αCRE mice fed a WD for 3 and 5 months. The aortic root lesion area was similar in both groups of mice fed a WD for 3 months (Figure 1F, quantification in the right panel). However, we observed a modest, but significant, increase in atherosclerotic plaque size in mice lacking Akt1 in VSMCs after 5 months of feeding a WD (Figure 1F, quantification in the right panel). Even though the differences in plaque size were small, we found a marked change in the morphology of atherosclerotic plaques isolated from Apoe−/−Akt1fl/flSm22αCRE mice. In Apoe−/−Akt1fl/fl mice, plaques contained thick fibrous caps with abundant collagen and matrix overlying small necrotic cores (Figure 2A and B, quantified in the right panels). In contrast, fibrous caps were significantly thinner, with reduced collagen content and increased necrotic core areas in atherosclerotic lesions of Apoe−/−Akt1fl/flSm22αCRE mice (Figure 2A and B, quantified in the right panels). Moreover, VSMC content [smooth muscle actin (SMA)-positive cells] in plaques was markedly reduced in lesions isolated from Apoe−/−Akt1fl/flSm22αCRE mice (Figure 2C, quantified in the right panels). The accumulation of monocytes/macrophages in atherosclerotic lesions (CD68-positive cells) was similar between both groups of mice (Figure 2C, quantified in the right panels).
Figure 2. Absence of Akt1 in VSMCs during the progression of atherosclerosis results in larger atherosclerotic plaques characterized by bigger necrotic core areas, reduced VSMCs and collagen content and thinner fibrous caps.
A–C) Representative histological analysis of cross-sections from the aortic sinus stained with hematoxylin and eosin (H&E) (A), Picrosirus red (B), and CD68 (green) and SMA (red) staining (C). Quantification of the fibrous cap, necrotic core, collagen, macrophage and VSMC content are represented in the right panels. All of the data represent the mean ± SEM; (3 months: Apoe−/−Akt1fl/fl n=8, Apoe−/−Akt1fl/flSm22αCRE n=13; 5 months: Apoe−/−Akt1fl/fl n=14, Apoe−/−Akt1fl/flSm22αCRE n=10). The dashed lines demarcate necrotic core areas in panel A and the intima of the atherosclerotic lesions in panel C.
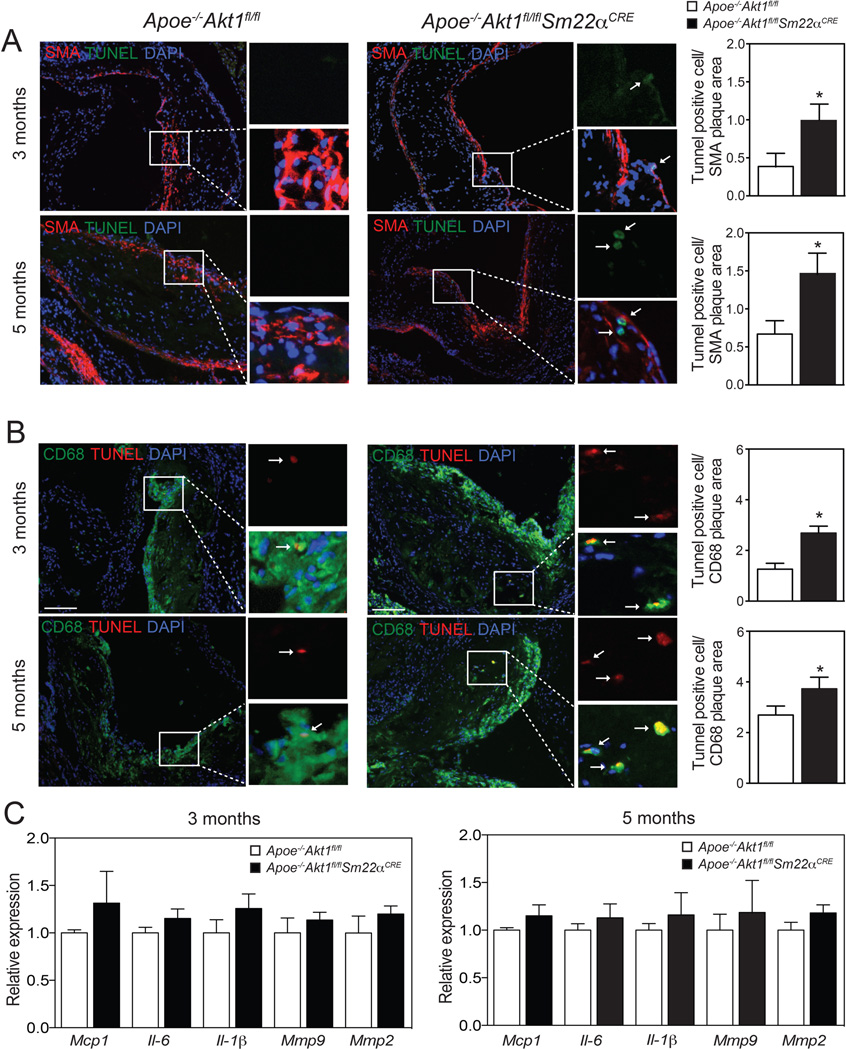
Absence of Akt1 in VSMCs increases apoptosis in atherosclerotic plaques
The increase in plaque necrosis observed in Apoe−/−Akt1fl/flSm22αCRE mice could reflect a primary increase in VSMC apoptosis. Therefore, we examined cellular apoptosis using TUNEL staining in cross-sections of the aortic sinus. We found that apoptosis was markedly increased in SMA-positive cells (Figure 3A, quantified in the right panels). Moreover, we also observed a marked increase in TUNEL CD68-positive cells in lesions of Apoe−/−Akt1fl/flSm22αCRE mice (Figure 3B, quantified in the right panels). These results suggest that loss of Akt1 in VSMCs influences not only VSMC survival but also macrophage viability in atherosclerotic lesions. Another interpretation of these results could be that many TUNEL CD68-positive cells are derived from VSMCs. Indeed, recent reports have shown that about 50% of CD68-positive cells in mouse and human atherosclerotic plaques are derived from VSMCs17, 18. We next analyzed whether the absence of Akt1 in VSMCs influences macrophage and VSMC proliferation in atherosclerotic plaques. To this end, we co-stained aortic root lesions with Ki-67 (a cellular marker of proliferation) and CD68 or SMA. We found that most of Ki-67 positive cells co-stained with the CD68 marker (Online Supplemental Figure IA). Moreover, the number of CD68/Ki-67 and SMA/Ki-67 positive cells in atherosclerotic lesions was similar between both groups of mice (Online Supplemental Figure IB).
Figure 3. Loss of Akt1 in VSMCs results in marked VSMC and macrophage apoptosis in atherosclerotic plaques.
A and B) Representative immunofluorescence analysis of atherosclerotic plaques isolated from Apoe−/−Akt1fl/fl and Apoe−/−Akt1fl/flSm22αCRE mice stained with smooth muscle cell alpha actin (SMA) and TUNEL (A) or with CD68 and TUNEL (B). Quantification is shown in the right panels. Data represent the mean ± SEM; (3 months: Apoe−/−Akt1fl/fl n=8 Apoe−/−Akt1fl/flSm22αCRE n=13; 5 months: Apoe−/−Akt1fl/fl n=14, Apoe−/−Akt1fl/flSm22αCRE n=10). Arrows indicate cells that are positive for SMA or CD68 and TUNEL labeling. C) qRT-PCR analysis of Mcp1, Il-6, Il-1β, Mmp9 and Mmp2 in whole aortic samples isolated from Apoe−/−Akt1fl/fl and Apoe−/−Akt1fl/flSm22αCRE mice fed a WD for 3 and 5 months. Data are expressed as relative expression and correspond to mean ± SEM (n=5 each group).
The size and content of the fibrous cap is also regulated by matrix metalloproteases (MMPs), protease enzymes that degrade and remodel the extracellular matrix, and inflammatory cytokines that influence VSMC, EC and macrophage activation in the artery wall2. Thus, we assessed the mRNA expression of Mmp9, Mmp2, Mcp-1, Il-6 and Il-1β in whole aortas of Apoe−/−Akt1fl/fl and Apoe−/−Akt1fl/flSm22αCRE mice. The results show that loss of Akt1 in VSMCs does not influence the expression of MMPs and inflammatory cytokines in the artery wall of Apoe−/−Akt1fl/fl and Apoe−/−Akt1fl/flSm22αCRE mice fed a WD for 3 and 5 months (Figure 3C). In summary, these findings provide the first in vivo evidence that specific genetic ablation of Akt1 in VSMCs results in pronounced changes in atherosclerotic plaque morphology.
Generation and characterization of Apoe−/−Akt1fl/flSM-MHC-CreERT2
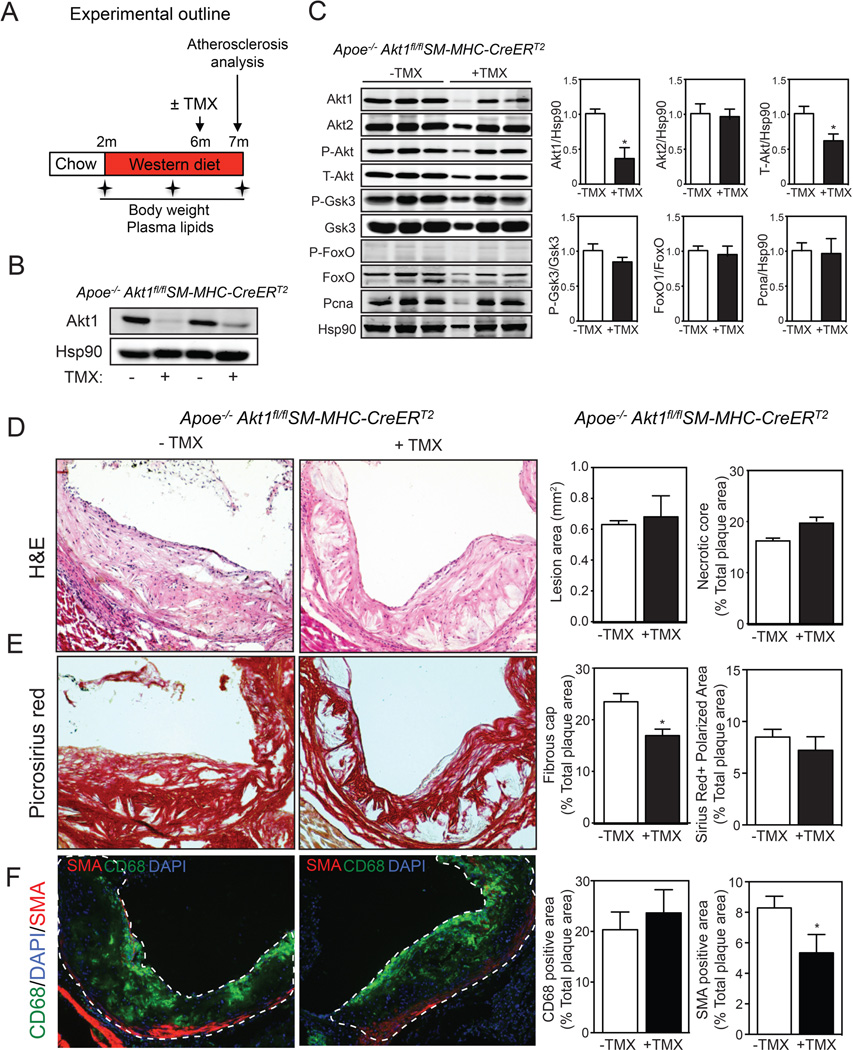
The migration and proliferation of VSMCs plays crucial roles in the development of atherosclerotic lesions. We have previously shown that absence of Akt1 reduces VSMC migration and proliferation19. Thus, the morphological changes observed in atherosclerotic plaques of Apoe−/−Akt1fl/flSm22αCRE mice could be influenced by differences in VSMC migration and proliferation. To directly address the importance of Akt1 in late stages of atherogenesis, we generated an additional mouse model (Apoe−/−Akt1fl/flSM-MHC-CreERT2) that allows us to inhibit VSMC Akt1 expression upon tamoxifen administration in mice with established atherosclerotic plaques. Cre expression in this mouse line has previously been demonstrated to be specific for vascular and visceral SMCs20. Indeed, we observed a significant reduction of Akt1 expression in aortas of Apoe−/−Akt1fl/flSM-MHC-CreERT2 mice treated with tamoxifen for one week (Figure 4A). In order to assess the role of Akt1 in established atherosclerotic plaques, we fed Apoe−/−Akt1fl/flSM-MHC-CreERT2 mice a WD for 4 months and then administered tamoxifen for one week and analyzed atherosclerosis three weeks later (Figure 4B, experimental outline). As expected by our previous results, mice administered tamoxifen expressed significantly reduced levels of Akt1 (Figure 4C, quantified in right panel). These results demonstrate that our mouse model is adequate to study the contribution of Akt1 in regulating VSMC functions in late stages of atherosclerosis.
Figure 4. Silencing Akt1 in VSMCs in established lesions promotes fibrous cap thinning.
A) Representative Western blot analysis of Akt1 expression after Tamoxifen (TMX) treatment from aortic lysates of Apoe−/−Akt1fl/flSM-MHC-CreERT2E mice. Hsp90 was used as a loading control. B) Experimental outline of the atherosclerosis study and Tamoxifen (TMX) administration in Apoe−/−Akt1fl/flSM-MHC-CreERT2 mice fed a WD for 5 months. C) Representative Western blot analysis of Akt1, Akt2, total Akt and downstream Akt targets from aortic lysates of male Apoe−/−Akt1fl/flSM-MHC-CreERT2 mice 3 weeks post-tamoxifen treatment. Hsp90 was used as a loading control. Band densitometry is shown in the right panels. *Indicates p<0.05 compared to non-treated Apoe−/−Akt1fl/flSM-MHC-CreERT2 mice. D–F) Representative histological analysis of cross-sections from the aortic sinus stained with hematoxylin and eosin (H&E) (D), Picrosirus red (E), and CD68 (green) and SMC (red) staining (F). Quantification of the lesion area, necrotic core, fibrous cap, collagen, macrophage and VSMC content are shown in the right panels. All of the data represent the mean ± SEM; (TMX− n=10, TMX+ n=8). *Indicates p< 0.05 compared to control untreated mice. The dashed white lines demarcate the intima of the atherosclerotic lesions.
Inhibition of Akt1 in VSMCs in mice with established atherosclerotic plaques results in a marked decrease in lesion fibrous cap area
We next examined the effects of inhibiting VSMC Akt1 on plaque size and morphology. In contrast to the results obtained in mice lacking Akt1 before atherosclerotic lesions develop, silencing Akt1 during the late stage of atherosclerosis does not influence plaque size (Figure 4D, quantified in right panel). However, we found a significant decrease in the lesion fibrous cap area and enhanced apoptosis (Figure 4E and Online Supplemental Figure IIA and IIB). Similar to the results observed in Apoe−/−Akt1fl/flSm22αCRE mice, most of the apoptotic cells expressed CD68 (Online Supplemental Figure IIA). VSMC content in atherosclerotic lesions was markedly reduced in Apoe−/−Akt1fl/flSM-MHC-CreERT2 mice treated with tamoxifen compared to untreated mice (Figure 4F, quantified in the right panel). In contrast, the accumulation of monocytes/macrophages in atherosclerotic lesions (CD68-positive cells) was similar between both groups of mice (Figure 4F, quantified in the right panels). No differences were observed in collagen content and necrotic core area and cellular proliferation between both groups of mice (Figure 4E and Online Supplemental Figure IIC and IID). However, we cannot rule out that longer inactivation of Akt1 in established atherosclerotic plaques could significantly influence plaque size, necrotic core area and collagen content in the lesions. Thus, further studies will be important to determine if prolonged inhibition (more than one month) of Akt1 in VSMCs results in significant changes in plaque size and morphology. Finally, we measured the expression of Mmp9, Mmp2, Mcp-1, Il-6 and Il-1β in whole aortas of Apoe−/−Akt1fl/flSM-MHC-CreERT2 mice treated or not with tamoxifen. The results show that temporal loss of Akt1 in VSMCs does not influence the expression of MMPs and inflammatory cytokines in the artery (Online Supplemental Figure IIE). Collectively these results strongly support an important role for VSMC Akt1 in all stages of atherogenesis.
DISCUSSION
Our study identifies Akt1 as a major factor for controlling VSMC functions during the progression of atherosclerosis. Using two novel conditional mouse models, we demonstrate that VSMC Akt1 regulates plaque morphology and its absence promotes VSMC apoptosis and plaque necrosis.
Several groups have studied the contribution of different Akt isoforms during the progression of atherosclerosis16, 21–23. Global absence of Akt1 results in massive atherosclerosis and occlusive arterial disease, a rare phenotype observed in few mouse models of atherosclerosis16. In contrast, lack of Akt2 does not influence the progression of atherosclerosis in mice despite the dyslipidemia and glucose intolerance observed in these mice22. Surprisingly, we and others recently demonstrated that Akt2 is required for macrophage migration in response to monocyte chemotactic protein-1 (MCP-1) and its absence results in an alternatively activated, or M2-type phenotype, when stimulated with pro-inflammatory cytokines22, 24. Akt3 also plays a role during atherogenesis by suppressing macrophage foam cell formation, thereby protecting against the progression of atherosclerosis21. These observations demonstrate that Akt2 and Akt3 mostly influence atherogenesis by controlling monocyte/macrophage functions, whereas Akt1 controls the progression of atherosclerosis by regulating vessel wall homeostasis. However the use of Akt1 global-deficient mice limits the ability to study its specific effect in particular cell types in the artery wall (i.e. ECs and VSMCs).
Akt1 regulates important cellular functions in ECs, including eNOS activation and cellular migration and proliferation. Of note, the increased atherosclerosis observed in Akt1-null mice was associated with enhanced apoptosis and reduced eNOS phosphorylation in ECs. Other studies also showed that transgenic expression of an activated form of Akt (myr-Akt) in the endothelium reduces vascular lesion formation in vivo25. Moreover, a recent study has also demonstrated that the genetic disruption of the three genes encoding for the isoforms of the FoxO transcription factors prevents atherosclerosis26. These observations suggest that the enhanced atherosclerosis observed in the Akt1 null mice could be mediated by decreased eNOS activity and increased FoxO transcriptional activation because this family of transcription factors are phosphorylated and inactivated by Akt. Interestingly, loss of the three FoxO isoforms in ECs results in a significant downregulation of Akt expression by an unknown mechanism26. However, FoxO deficient mice have a significant increase in eNOS expression and enhanced eNOS-dependent arterial relaxation26.
Akt1 controls VSMC migration and proliferation and its activity has been associated with plaque remodeling during atherogenesis. In addition to Akt1, Akt2 also regulates VSMC homeostasis. Indeed, absence of Akt2 protects against aortic aneurism formation by preventing FoxO1 association with the MMP9 and TIMP1 promoters27. Moreover, Akt2 deficiency in Ldlr−/− mice markedly reduces collagen content and increases necrotic cores in atherosclerotic lesions28. Thus, both Akt1 and Akt2 appear to regulate VSMC migration and survival during vessel remodeling and atherogenesis.
In the present study, we provide definitive evidence that Akt1 in VSMCs regulates the progression of atherosclerosis and lesion morphology. Specifically, we demonstrate that absence of Akt1 in VSMCs results in profound changes in the morphology of atherosclerotic plaques characterized by larger necrotic core areas and thinner fibrous caps, both features of vulnerable plaques in humans. In agreement with these observations, a recent report has shown that overexpression of Akt1 in VSMCs inhibited VSMC apoptosis during atherogenesis and increased relative fibrous cap area in plaques29. This study also identified FoxO3a-Apaf1 as major Akt substrates that mediate Akt1 functions during the progression of atherosclerosis. Apaf1 induces apoptosis in VSMCs and Akt1 inhibits its activity, thus promoting survival of VSMCs in atherosclerotic lesions. Here, we report that absence of Akt1 in VSMCs significantly reduces Gsk3 phosphorylation, which has previously been associated with reduced survival of VSMCs5. Gsk3 regulates apoptosis via phosphorylation and subsequent ubiquitin-mediated degradation of the Bcl-2 family member, Mcl1, in VSMCs5. Interestingly, treatment of hyperglycemic ApoE-deficient mice with valproic acid, an inhibitor of Gsk3β, had antiatherogenic effects30, providing further evidence that Gsk-3β activation may be playing a direct role in the development of advanced atherosclerosis. Moreover, in diabetic pigs fed a WD, the disinhibiting of Gsk-3β by hypophosphorylated Akt was associated with an increase in cellular apoptosis and developed advanced atherosclerosis31. These observations suggest that the Akt1-Gsk3 signaling pathway may mediate pro-survival effects in VSMCs. In summary, our data genetically demonstrate the key role of Akt1 in VSMCs during atherogenesis and that Akt1 expression in VSMCs regulates vascular remodeling during the progression of atherosclerosis.
Supplementary Material
Novelty and Significance.
What Is Known?
VSMC apoptosis occurs during the progression of atherosclerosis and in advanced lesions and promotes plaque necrosis, a common feature of high-risk/vulnerable atherosclerotic plaques.
Akt1 is essential for VSMC proliferation, migration and protection against oxidative stress-induced apoptosis. However, its specific contribution during the progression of atherosclerosis remains unknown.
What New Information Does This Article Contribute?
Absence of Akt1 in VSMCs during atherogenesis results in larger atherosclerotic lesions characterized by bigger necrotic core areas, enhanced VSMC apoptosis and reduced fibrous cap and collagen content.
Deficiency of Akt1 in the setting of advanced atherosclerosis in mice decreases fibrous caps in the lesions and enhances VSMC apoptosis.
Prolonged absence of Akt1 in VSMCs results in a marked reduction in Gsk3 phosphorylation, which has been associated with reduced VSMC survival.
Prominent areas of lesional apoptosis characterize the types of atherosclerotic plaques that cause acute cardiovascular events in humans. However the specific mechanism that regulates cellular apoptosis and lesion morphology remains to be fully determined. Our study reveals that the expression of Akt1 in VSMCs is critical during the progression and in late stages of atherogenesis. Absence of Akt1 promotes plaque necrosis and enhances VSMC apoptosis. This effect may be mediated by reduced phosphorylation of Gsk3, which has previously been associated with reduced VSMC survival.
Acknowledgments
SOURCES OF FUNDING
We thank Dr. Morris Birnbaum for the Akt1flox/flox mice. This work was supported by grants from the National Institutes of Health, R01HL107953 and R01HL106063 (to CF-H), and R01HL061371 and RO1HL064793 (to W.C.S). N.R is supported by a postdoctoral fellowship from the Spanish Ministry (Programa Nacional de Movilidad de Recursos Humanos del Plan Nacional de I-D+i 2008–2011), and A.G.S and A.C.W by the Capes Foundation, Ministry of Education of Brazil, Brazil.
Nonstandard Abbreviations and Acronyms
- AKT/PKB
Protein kinase B
- Bcl-2
B-lymphoma 2
- EC
Endothelial cell
- ERK
Extracellular signal-regulated kinases
- eNOS
Endothelial nitric oxide synthase
- FOXO
Forkhead box O
- GSK3
Glycogen synthase kinase 3
- MCL1
Myeloid cell leukemia-1
- NO
Nitric oxide
- SM22α
Smooth muscle protein 22-alpha
- TG
Triglycerides
- TMX
Tamoxifen
- TUNEL
Terminal deoxynucleotidyl transferase dUTP end labeling
- VSMC
Vascular smooth muscle cell
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 3.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 4.Clarke MC, Figg N, Maguire JJ, Davenport AP, Goddard M, Littlewood TD, Bennett MR. Apoptosis of vascular smooth muscle cells induces features of plaque vulnerability in atherosclerosis. Nature medicine. 2006;12:1075–1080. doi: 10.1038/nm1459. [DOI] [PubMed] [Google Scholar]
- 5.Allard D, Figg N, Bennett MR, Littlewood TD. Akt regulates the survival of vascular smooth muscle cells via inhibition of FoxO3a and GSK3. J Biol Chem. 2008;283:19739–19747. doi: 10.1074/jbc.M710098200. [DOI] [PubMed] [Google Scholar]
- 6.Whiteman EL, Cho H, Birnbaum MJ. Role of Akt/protein kinase B in metabolism. Trends in endocrinology and metabolism: TEM. 2002;13:444–451. doi: 10.1016/s1043-2760(02)00662-8. [DOI] [PubMed] [Google Scholar]
- 7.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 8.Fayard E, Tintignac LA, Baudry A, Hemmings BA. Protein kinase B/Akt at a glance. J Cell Sci. 2005;118:5675–5678. doi: 10.1242/jcs.02724. [DOI] [PubMed] [Google Scholar]
- 9.Yang ZZ, Tschopp O, Di-Poi N, Bruder E, Baudry A, Dummler B, Wahli W, Hemmings BA. Dosage-dependent effects of Akt1/protein kinase Balpha (PKBalpha) and Akt3/PKBgamma on thymus, skin, and cardiovascular and nervous system development in mice. Mol Cell Biol. 2005;25:10407–10418. doi: 10.1128/MCB.25.23.10407-10418.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ackah E, Yu J, Zoellner S, Iwakiri Y, Skurk C, Shibata R, Ouchi N, Easton RM, Galasso G, Birnbaum MJ, Walsh K, Sessa WC. Akt1/protein kinase Balpha is critical for ischemic and VEGF-mediated angiogenesis. The Journal of clinical investigation. 2005;115:2119–2127. doi: 10.1172/JCI24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shiojima I, Sato K, Izumiya Y, Schiekofer S, Ito M, Liao R, Colucci WS, Walsh K. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. The Journal of clinical investigation. 2005;115:2108–2118. doi: 10.1172/JCI24682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Somanath PR, Razorenova OV, Chen J, Byzova TV. Akt1 in endothelial cell and angiogenesis. Cell Cycle. 2006;5:512–518. doi: 10.4161/cc.5.5.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ignarro LJ, Napoli C. Novel features of nitric oxide, endothelial nitric oxide synthase, and atherosclerosis. Current atherosclerosis reports. 2004;6:281–287. doi: 10.1007/s11883-004-0059-9. [DOI] [PubMed] [Google Scholar]
- 14.Kuhlencordt PJ, Gyurko R, Han F, Scherrer-Crosbie M, Aretz TH, Hajjar R, Picard MH, Huang PL. Accelerated atherosclerosis, aortic aneurysm formation, and ischemic heart disease in apolipoprotein E/endothelial nitric oxide synthase double-knockout mice. Circulation. 2001;104:448–454. doi: 10.1161/hc2901.091399. [DOI] [PubMed] [Google Scholar]
- 15.Dzau VJ, Braun-Dullaeus RC, Sedding DG. Vascular proliferation and atherosclerosis: new perspectives and therapeutic strategies. Nature medicine. 2002;8:1249–1256. doi: 10.1038/nm1102-1249. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez-Hernando C, Ackah E, Yu J, Suarez Y, Murata T, Iwakiri Y, Prendergast J, Miao RQ, Birnbaum MJ, Sessa WC. Loss of Akt1 leads to severe atherosclerosis and occlusive coronary artery disease. Cell metabolism. 2007;6:446–457. doi: 10.1016/j.cmet.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allahverdian S, Chehroudi AC, McManus BM, Abraham T, Francis GA. Contribution of intimal smooth muscle cells to cholesterol accumulation and macrophage-like cells in human atherosclerosis. Circulation. 2014;129:1551–1559. doi: 10.1161/CIRCULATIONAHA.113.005015. [DOI] [PubMed] [Google Scholar]
- 18.Feil S, Fehrenbacher B, Lukowski R, Essmann F, Schulze-Osthoff K, Schaller M, Feil R. Transdifferentiation of vascular smooth muscle cells to macrophage-like cells during atherogenesis. Circulation research. 2014;115:662–667. doi: 10.1161/CIRCRESAHA.115.304634. [DOI] [PubMed] [Google Scholar]
- 19.Fernandez-Hernando C, Jozsef L, Jenkins D, Di Lorenzo A, Sessa WC. Absence of Akt1 reduces vascular smooth muscle cell migration and survival and induces features of plaque vulnerability and cardiac dysfunction during atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 2009;29:2033–2040. doi: 10.1161/ATVBAHA.109.196394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wirth A, Benyo Z, Lukasova M, Leutgeb B, Wettschureck N, Gorbey S, Orsy P, Horvath B, Maser-Gluth C, Greiner E, Lemmer B, Schutz G, Gutkind JS, Offermanns S. G12–G13-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nature medicine. 2008;14:64–68. doi: 10.1038/nm1666. [DOI] [PubMed] [Google Scholar]
- 21.Ding L, Biswas S, Morton RE, Smith JD, Hay N, Byzova TV, Febbraio M, Podrez EA. Akt3 deficiency in macrophages promotes foam cell formation and atherosclerosis in mice. Cell metabolism. 2012;15:861–872. doi: 10.1016/j.cmet.2012.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rotllan N, Chamorro-Jorganes A, Araldi E, Wanschel AC, Aryal B, Aranda JF, Goedeke L, Salerno AG, Ramirez CM, Sessa WC, Suarez Y, Fernandez-Hernando C. Hematopoietic Akt2 deficiency attenuates the progression of atherosclerosis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2014 doi: 10.1096/fj.14-262097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Babaev VR, Hebron KE, Wiese CB, Toth CL, Ding L, Zhang Y, May JM, Fazio S, Vickers KC, Linton MF. Macrophage deficiency of Akt2 reduces atherosclerosis in Ldlr null mice. J Lipid Res. 2014;55:2296–2308. doi: 10.1194/jlr.M050633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arranz A, Doxaki C, Vergadi E, Martinez de la Torre Y, Vaporidi K, Lagoudaki ED, Ieronymaki E, Androulidaki A, Venihaki M, Margioris AN, Stathopoulos EN, Tsichlis PN, Tsatsanis C. Akt1 and Akt2 protein kinases differentially contribute to macrophage polarization. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:9517–9522. doi: 10.1073/pnas.1119038109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mukai Y, Rikitake Y, Shiojima I, Wolfrum S, Satoh M, Takeshita K, Hiroi Y, Salomone S, Kim HH, Benjamin LE, Walsh K, Liao JK. Decreased vascular lesion formation in mice with inducible endothelial-specific expression of protein kinase Akt. J Clin Invest. 2006;116:334–343. doi: 10.1172/JCI26223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsuchiya K, Tanaka J, Shuiqing Y, Welch CL, DePinho RA, Tabas I, Tall AR, Goldberg IJ, Accili D. FoxOs integrate pleiotropic actions of insulin in vascular endothelium to protect mice from atherosclerosis. Cell metabolism. 2012;15:372–381. doi: 10.1016/j.cmet.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen YH, Zhang L, Ren P, Nguyen MT, Zou S, Wu D, Wang XL, Coselli JS, LeMaire SA. AKT2 confers protection against aortic aneurysms and dissections. Circulation research. 2013;112:618–632. doi: 10.1161/CIRCRESAHA.112.300735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rensing KL, de Jager SC, Stroes ES, Vos M, Twickler MT, Dallinga-Thie GM, de Vries CJ, Kuiper J, Bot I, von der Thusen JH. Akt2/LDLr double knockout mice display impaired glucose tolerance and develop more complex atherosclerotic plaques than LDLr knockout mice. Cardiovascular research. 2014;101:277–287. doi: 10.1093/cvr/cvt252. [DOI] [PubMed] [Google Scholar]
- 29.Tucka J, Yu H, Gray K, Figg N, Maguire J, Lam B, Bennett M, Littlewood T. Akt1 Regulates Vascular Smooth Muscle Cell Apoptosis Through FoxO3a and Apaf1 and Protects Against Arterial Remodeling and Atherosclerosis. Arterioscler Thromb Vasc Biol. 2014;34:2421–2428. doi: 10.1161/ATVBAHA.114.304284. [DOI] [PubMed] [Google Scholar]
- 30.Bowes AJ, Khan MI, Shi Y, Robertson L, Werstuck GH. Valproate attenuates accelerated atherosclerosis in hyperglycemic apoE-deficient mice: evidence in support of a role for endoplasmic reticulum stress and glycogen synthase kinase-3 in lesion development and hepatic steatosis. The American journal of pathology. 2009;174:330–342. doi: 10.2353/ajpath.2009.080385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamamdzic D, Fenning RS, Patel D, Mohler ER, 3rd, Orlova KA, Wright AC, Llano R, Keane MG, Shannon RP, Birnbaum MJ, Wilensky RL. Akt pathway is hypoactivated by synergistic actions of diabetes mellitus and hypercholesterolemia resulting in advanced coronary artery disease. American journal of physiology Heart and circulatory physiology. 2010;299:H699–H706. doi: 10.1152/ajpheart.00071.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.