Abstract
MicroRNAs (miRNAs) represent a class of small non-coding RNAs and have been shown to play important roles in various biological processes including cell growth, differentiation and apoptosis by regulating the target genes. miR-7 has been described not only as a tumour suppressor gene but also as an oncogene in human cancers. The aim of this study was to investigate the functional roles of miR-7 in chemoresistance of SCLC and its underlying mechanism. By using a bioinformatic assay, we found that MRP1/ABCC1 was a potential target gene of miR-7. Expression of miR-7 and MRP1/ABCC1 was examined in 44 SCLC samples by quantitative reverse transcription–polymerase chain reaction and immunohistochemistry methods. Low-level expression of miR-7 was associated significantly with drug responsiveness and overall survival rate of patients with SCLC, but not with gender, age and stage. There was an inverse relationship between miR-7 and MRP1/ABCC1 expression. Downregulation of MRP1/ABCC1 level was revealed after transfection with a miR-7 mimic in H69 AR cells. Transfection of a miR-7 inhibitor into H69 cells restored MRP1/ABCC1 expression. A dual-luciferase reporter assay confirmed that miR-7 targeted predicted sites in the 3′-untranslated region (3′-UTR) of the MRP1/ABCC1 gene. Our data suggested that miR-7 mediated SCLC chemoresistance by repressing MRP1/ABCC1 and may be a prognostic predictor and potential therapeutic target in human SCLC.
Keywords: chemoresistance, miR-7, MRP1/ABCC1, SCLC
Small cell lung cancer (SCLC), accounting for approximately 15% of all human lung cancers, is an aggressive malignant tumour (Govindan et al. 2006). Chemotherapy remains the first treatment approach for patients with SCLC including platinum and etoposide. Although most of the patients with SCLC are responsive to chemotherapy, their 2-year survival rate is <5% because drug resistance emerges and leads to high propensity for relapse (Sandler 2003). Therefore, chemoresistance has become one of the major obstacles in the treatment, and a detailed understanding of the molecular mechanism associated with chemoresistance is crucial for improving current therapeutic strategies for SCLC.
MicroRNAs (miRNAs) are a cluster of endogenous phylogenetically conserved small non-coding RNA molecules, which are 18–24 nucleotides in length. They regulate gene expression via binding to the 3′-untranslated region (3′-UTR) of their target mRNA, leading to negative post-transcriptional translation or to degradation of the target mRNA (Bartel 2004). Many studies have shown that aberrantly expressed miRNAs could affect cell biological activities such as cell proliferation, apoptosis and cancer development. Recently, some miRNAs have been proved to be closely relevant to the drug sensitivity or resistance (Yang et al. 2008; Fornari et al. 2009; Song et al. 2009; Valeri et al. 2010; Rao et al. 2011; Xiao et al. 2014). Our previous miRNA expression profile has shown that miR-7 expression markedly decreased in SCLC multidrug-resistant cells (H69AR) as compared to parental H69 cells by microarray and qRT-PCR (Guo et al. 2010).However, there is still no report available about miR-7 functions in SCLC drug resistance.
Multidrug resistance-associated protein 1 (MRP1), also named as ABCC1, is a member of the ATP-binding cassette transporter superfamily, which includes 49 members distributed in 7 subfamilies from ABCA to ABCG (Mo & Zhang 2009; Yin & Zhang 2011). MRP1/ABCC1, as an atypical ABC transporter, has three membrane-spanning domains and two cytosolic nucleotide-binding domains. It is ubiquitously expressed in almost all human tissues with relatively high expression levels in lung, spleen, testis and kidney and can transport a wide spectrum of substrates such as a wide variety of anticancer drugs (Zaman et al. 1993; Flens et al. 1996; St-Pierre et al. 2000; Chang 2007; Yin & Zhang 2011). Previous studies have reported that MRP1/ABCC1 plays an important role in the clinical drug resistance behaviour of several cancers including SCLC. Overexpression of MRP1/ABCC1 was predictive of poor response to chemotherapy in SCLC (Hsia et al. 2002; Kuo et al. 2003; Triller et al. 2006). We also previously showed that MRP1/ABCC1 is closely related to chemoresistance in SCLC (Guo et al. 2010). It has been demonstrated that some miRNAs can directly target and regulate MRP1/ABCC1 expression (Liang et al. 2010; Fang et al. 2012). In our previous study, we showed that MRP1/ABCC1 is negatively regulated by miR-134 and that downregulation of MRP1/ABCC1 at the protein level largely correlates with elevated levels of miR-134 in H69AR cells. Upregulation of MRP1/ABCC1 is also associated with some other miRNAs such as miR-326, miR-199a, miR-199b and miR-296. By using a bioinformatic assay, we found that the 3′-UTR of human MRP1/ABCC1 contains putative regions that match to the seed sequence of miR-7 and not to the other miRNAs stated above, which suggests a possible modulation of MRP1/ABCC1 by miR-7.
To investigate the functional roles of miR-7 in chemoresistance of SCLC and its underlying mechanism, we detected the expression of miR-7 in SCLC tissues and evaluated the relevance of its expression with clinical prognosis of patients with SCLC. We then further investigated the potential miR-7 role in chemoresistance using the cellular model of a human SCLC-resistant cell line (H69AR). Furthermore, MRP1/ABCC1 was identified as a direct downstream target of miR-7.
Materials and methods
Tissue specimens
Forty-four SCLC patient samples were obtained from Zhujiang (Southern Medical University, Guangzhou, China) and Corps Hospital of Chinese People's Armed Police Forces (Guangzhou Medical University, Guangzhou, China). All patients had been confirmed as SCLC by pathologic examination and been clarified as limited disease (20 cases) and extensive disease (24 cases) according to VA criteria (Veterans Administration Lung Study Group). All patients gave informed consent prior to the collection of specimens according to institutional guidelines. Tissue samples were snap-frozen in the operating room immediately after surgery sent to pathology for diagnosis by a board-certified pathologist. For each patient, a paraffin-embedded tissue specimen had to be available. Under the protocol approved by the Institutional Review Board, informed consents were obtained from the patients or their guardians.
Cell Lines
Human SCLC cell line NCI-H69 and the drug-resistant subline H69AR were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA) and maintained in RPMI 1640 medium containing l-glutamine with 10% and 20% foetal calf serum, respectively, in an incubator at 37°C with 5% CO2.
Cell transfection
Cells were transiently transfected with 100 nmol/l of miR-7 mimics, inhibitors and miRNA negative control (miR-NC) (Bioneer, Daejeon, Korea) using Lipofectamine 2000 and OPTI-MEM I (Invitrogen USA, Carlsbad, California). Infection efficiency was confirmed by fluorescent quantitative PCR and Western blotting.
RNA isolation, reverse transcription and quantitative real-time PCR
Total RNA, including miRNAs, was isolated from cell lines and FFPE tissues using TRIzol (Invitrogen USA, Carlsbad, California), miRNeasy kit (Qiagen, Hilden, Germany) and miRNeasy FFPE Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. cDNA synthesis was carried out according to PrimeScript RT reagent Kit (Takara, Dalian, China). The miRNA sequence-specific reverse transcription–qRT-PCR for miR-7 and endogenous control U6 were performed according to Hairpin-it™ miRNAs qRT-PCR quantization kit and U6 snRNA real-time PCR normalization kit (GenePharma, Shanghai, China). GAPDH or U6 snRNA was used as an endogenous control.
Immunohistochemical analysis
Formalin-fixed, paraffin-embedded tissues of SCLC clinical patient samples were sectioned at 4 μm thickness and analysed for MRP1/ABCC1 (1:200 dilutions, Alomone Labs Ltd., Jerusalem, Israel) expression. Visualization was achieved using the EnVision peroxidase system (Dako, Dusseldorf, Germany). A sample was considered positive if more than 50% of the tumour cells retained cytoplasm staining, and 5 fields were randomly selected according to semiquantitative scales. The intensity of staining was scored manually (high, 3; medium, 2; low, 1; no staining, 0) by two independent experienced pathologists, and only tumour cells were scored. Negatively stained MRP1/ABCC1 was considered positive if <50% of the tumour cells retained cytoplasm staining. Negative controls were performed by replacing the primary antibodies stated above with PBS.
Luciferase reporter assay
Cells were seeded in a 48-well plate and cotransfected with 200 ng of either pcDNA/miR-7 or pcDNA/miR-NC vectors, and 10 ng of pLUC vectors containing firefly luciferase reporter gene, as well as the 3′-UTR of ABCC1 gene. Cells were harvested for luciferase activity assays 48 h after transfection. A luciferase assay kit (Promega, Madison, WI, USA) was used according the manufacturer's protocol. The assay was conducted in five replicate wells for each sample and three parallel experiments were performed.
Statistical analysis
All experiments were run in triplicate. The results were given as mean ± SD. Statistical analyses were performed using either an analysis of variance (anova) or Student's t-test. The relationship between MRP1/ABCC1 and miR-7 expressions was explored by Pearson correlation. The difference was considered statistically significant when the P value was <0.05. All statistical analyses were carried out with SPSS 13.0 (Armonk, New York, USA) software. All assay were conducted in three parallel experiments were performed.
Results
Expression of miR-7 and MRP1/ABCC1 and their correlations with clinicopathological characteristics in SCLC
Expression of miR-7 and MRP1/ABCC1 was analysed, respectively, by qRT-PCR and IHC in 44 samples from patients with SCLC. As shown in Table 1, miR-7 expression was shown to correlate significantly with drug responsiveness. However, there was no significant relationship between miR-7 expression and gender, age or clinical stage. Similar results were also observed in MRP1/ABCC1 expression. MRP1/ABCC1 expression was localized on the membrane of the cancer cells. (Figure 1) The positive rate of MRP1/ABCC1 expression was 20% in drug-sensitive SCLC specimens, which was significantly lower than that (75%) in drug-resistant SCLC specimens. The median value of all 44 SCLC cases was chosen as the cut-off point for separating tumours with low-level expression of miR-7 from high-level expression miR-7 tumours. A higher expression level of miR-7 was found in the drug-sensitive group than in the drug-resistant group. In addition, Kaplan–Meier analysis revealed that low-level expression of miR-7 was significantly associated with shorter overall survival (OS) rate (P = 0.048; Figure 2). However, multivariate Cox regression analysis indicated that miR-7 and ABCC1 are not independent prognostic factors for survival of patients with SCLC (P > 0.05; Table 2).
Table 1.
Relationship between miR-7 and ABCC1 expressions and clinicopathological characteristics in patients with SCLC
| MiR-7 expression |
ABCC1 expression |
||||||
|---|---|---|---|---|---|---|---|
| Characteristics | Total | Low | High* | P† | − | + | P† |
| Gender | |||||||
| Male | 38 | 19 | 19 | 1.000 | 19 | 19 | 1.000 |
| Female | 6 | 3 | 3 | 3 | 3 | ||
| Age | |||||||
| <55 | 22 | 13 | 9 | 0.228 | 13 | 9 | 0.228 |
| ≥55 | 22 | 9 | 13 | 9 | 13 | ||
| Stage | |||||||
| Limited | 20 | 9 | 11 | 0.545 | 8 | 12 | 0.226 |
| Extensive | 24 | 13 | 11 | 14 | 10 | ||
| Drug sensitivity | |||||||
| Sensitive group | 20 | 7 | 13 | 0.027 | 16 | 4 | <0.001 |
| Resistant group | 20 | 14 | 6 | 5 | 15 | ||
−, negative; +, positive.
−, Negative stained MRP1/ABCC1 was considered positive if <50% of the tumour cells retained cytoplasm staining; +, positive stained MRP1/ABCC1 was considered positive if more than 50% of the tumour cells retained cytoplasm staining.
The median expression level was used as the cut-off. Low expression of miR-7 patients was classified as values of 2−▵▵ct below 1.0. High miR-7 expression in patients was classified as values of 2−▵▵ct above 1.0.
For the analysis of correlation between miR-7 and ABCC1 levels and clinical features, chi-square test was used. Results were considered statistically significant at P < 0.05.
Figure 1.
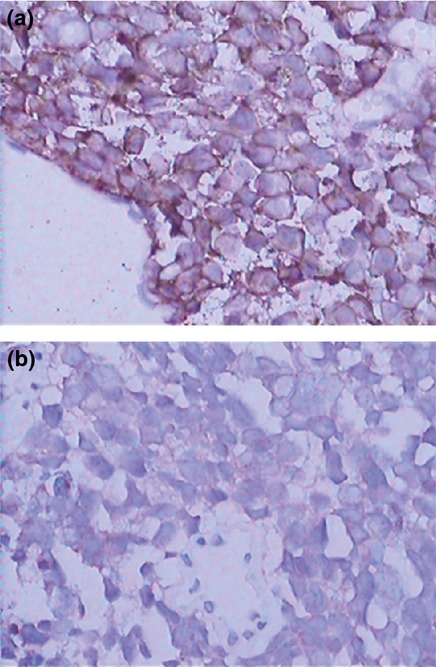
Representative immunohistochemistry staining of MRP1/ABCC1 in SCLC tissue. (a) Drug-resistant and (b) drug-sensitive SCLC tissues were immunohistochemically stained with an anti-MRP1/ABCC1 antibody. Original magnification, ×400.
Figure 2.
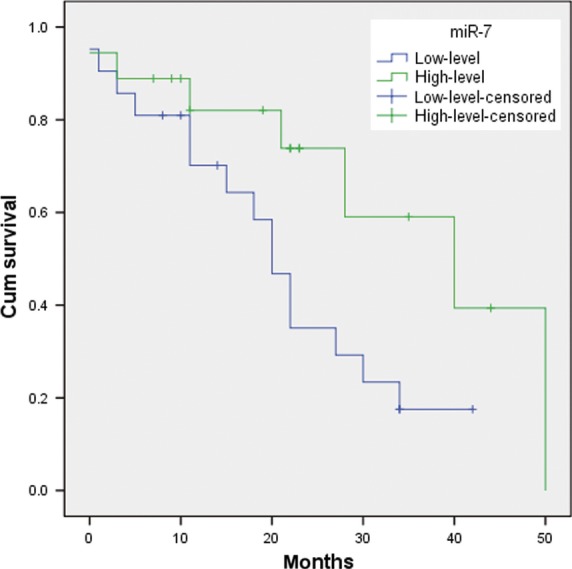
Kaplan–Meier analysis of overall survival of 44 patients with SCLC based on miR-7 expression.
Table 2.
Univariate and multivariate analyses of overall survival with regard to clinicopathological characteristics
| Univariate Analysis |
Multivariate Analysis |
|||
|---|---|---|---|---|
| Characteristics | HR(95% CI) | P | HR(95% CI) | P |
| Gender | 0.705 (0.206–2.414) | 0.578 | 0.339 (0.084–1.375) | 0.339 |
| Age | 0.452 (0.178–1.152) | 0.096 | 0.349 (0.112–1.091) | 0.070 |
| Stage | 1.555 (0.621–3.892) | 0.346 | 2.324 (0.820–6.592) | 0.113 |
| Drug sensitivity | 1.732 (0.724–4.140) | 0.217 | 1.303 (0.401–4.234) | 0.660 |
| MiR-7 | 0.399 (0.153–1.037) | 0.059 | 0.604 (0.161–2.259) | 0.453 |
| ABCC1 | 1.635 (0.677–3.950) | 0.274 | 1.328 (0.336–5.252) | 0.686 |
MRP1/ABCC1 expression is correlated with miR-7 in SCLC tissues
To further evaluate the relationship between MRP1/ABCC1 and miR-7 in SCLC tissues, we measured miR-7 expression level in MRP1/ABCC1-positive SCLC specimens and MRP1/ABCC1-negative specimens which were examined by IHC. The expression of miR-7 decreased significantly in MRP1/ABCC1-positive SCLC samples compared with MRP1/ABCC1-negative tissues (Figure 3a). The results suggest that miR-7 was negatively correlated with MRP1/ABCC1 expression in SCLC tissues (r = −0.844,P < 0.001; Figure 3b).
Figure 3.
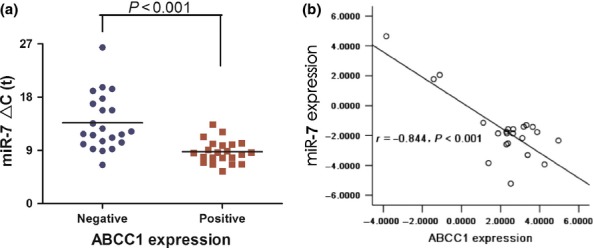
(a) The expression and relationship of miR-7 and MRP1/ABCC1 in SCLC tissues. The expression levels of miR-7 (▵Ct value) in MRP1/ABCC1-negative (n = 22) and MRP1/ABCC1-positive SCLC tissues (n = 22). (b) The correlation of miR-7 and MRP1/ABCC1 expression in tissues.
MiR-7 directly targets MRP1/ABCC1 in SCLC
It is generally accepted that miRNAs exert their functions through activation or repression of the downstream target genes. By searching PicTar, TargetScan and miRBase databases, we found that the 3′UTR of human MRP1/ABCC1 contains putative regions that match to the seed sequence of miR-7. Based on above statistical results and bioinformatic prediction, we hypothesized that MRP1/ABCC1 may be a target of miR-7 in SCLC.
To verify whether MRP1/ABCC1 is a direct target of miR-7, we constructed luciferase reporter vectors with wild-type (psiCHECK2-ABCC1-wt) or mutated (psiCHECK2-ABCC1-mt) 3′UTR of MRP1/ABCC1 gene. Then, either vector was cotransfected with miR-7 agomir or antagomir in H69 cells. The results of dual-luciferase reporter assays showed that miR-7 agomir can suppress luciferase activity when cotransfected with wild-type reporter vector. Moreover, miR-7 antagomir led to an increase in luciferase activity, and the activity of mutated reporter vector was unaffected by a simultaneous transfection with miR-7 agomir or antagomir. (Figure 4) Taken together, all these findings suggested that miR-7 directly targets MRP1/ABCC1 in SCLC. To further evaluate whether miR-7 can affect the endogenous expression of MRP1/ABCC1, we examined MRP1/ABCC1 expression after knockdown or upregulation of miR-7 using miR-7 mimic or inhibitor, respectively. Downregulation of MRP1/ABCC1 level was revealed after transfection with miR-7 mimic in H69AR cells. Transfection of miR-7 inhibitor in H69 cells restored MRP1/ABCC1 expression (Figure 5a–e).
Figure 4.
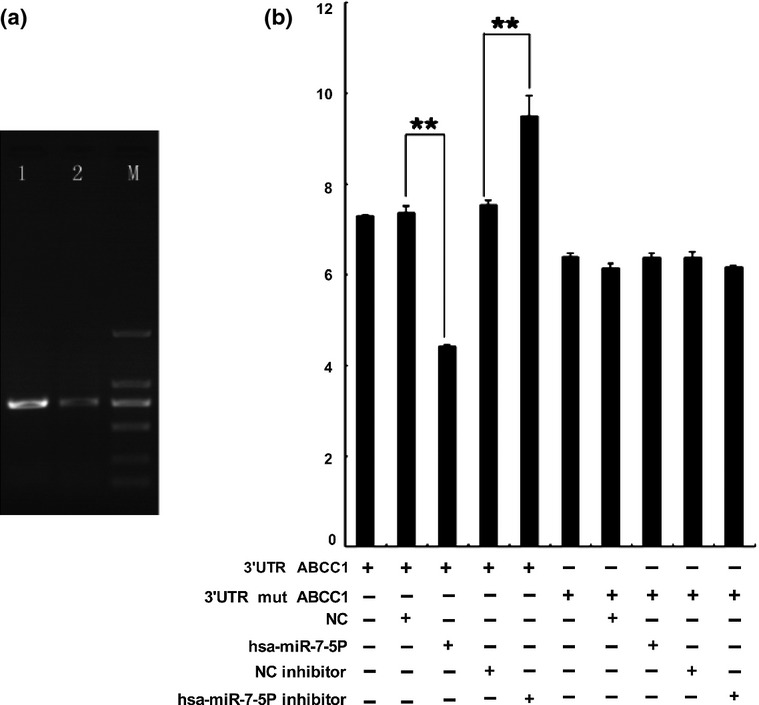
(a) The electrophoresis analyses of PCR product on agarose gels. 1, the PCR product of ABCC1-3′UTR, 779 bp; 2, the PCR product of ABCC1-3′UTR-R, 780 bp; M, DNA marker. (b) Dual-luciferase assay was performed in H69 cells transfected with luciferase construct alone or cotransfected with miR-7 agomir and antagomir. **P < 0.01.
Figure 5.
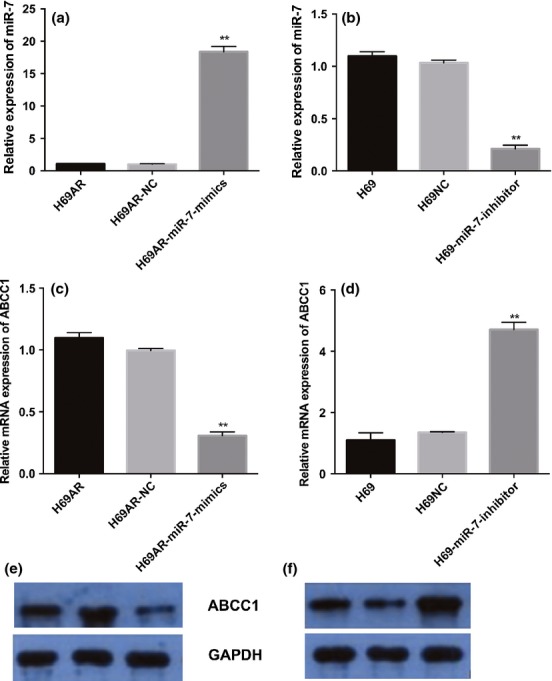
MRP1/ABCC1 is the target of miR-7. Expression of miR-7 was detected after transfection with miR-7 mimics (a) and inhibitor (b) in s H69 cells and H69AR cells by qRT-PCR.; MRP1/ABCC1mRNA level (c) and protein level (e) were assessed 72 h after transfection with miR-7 mimics and negative controls in H69AR cells. SCGN mRNA level (d) and protein level (f) were assessed 72 h after transfection of miR-7 inhibitors and negative controls in H69 cells. **P < 0.001 compared with control.
Discussion
Chemotherapy is still the chief treatment for patients with SCLC, and multidrug resistance (MDR) is one of the most important factors that hinder the outcomes (Liang et al. 2010). Although deregulation of miRNAs has been reported to be associated with MDR in various cancers (Zaman et al. 1993; Flens et al. 1996; St-Pierre et al. 2000; Chang 2007; Yin & Zhang 2011), the molecular mechanisms by which miRNAs modulate drug resistance are still unclear.
miR-7 has been described as a tumour suppressor gene in several human cancers including glioblastoma, hepatocellular carcinoma, lung cancer and gastric tumours (Kefas et al. 2008; Xiong et al. 2011; Fang et al. 2012; Kong et al. 2012; Li et al. 2014), however, also as a oncogene in breast cancer and renal cell carcinoma (Moitra et al. 2012; Yu et al. 2013). miR-7 functions as a tumour suppressor gene by directly targeting or indirectly downregulating central oncogenic factors including epidermal growth factor receptor (EGFR), p21-activated kinase 1 (PAK1) and other genes (Reddy et al. 2008; Saydam et al. 2011; Skalsky & Cullen 2011). However, to our knowledge, the functional roles of miR-7 in chemoresistance have not been reported yet. In this study, we examined miR-7 expression in 44 SCLC samples and its correlation with clinicopathological feature. The results showed that the expression level of miR-7 was downregulated in the drug-resistant group compared with that in the drug-sensitive group, and miR-7 expression was closely correlated with chemotherapy responsiveness, but not with gender, age and clinical stage of patients with SCLC. Similarly to previous researchers, we found low-level expression of miR-7 was associated significantly with shorter OS rate, but multivariate Cox regression analysis indicated that miR-7 is not an independent prognostic factor for survival of patients with SCLC. These finding suggested that miR-7 might be a predictive marker for chemosensitivity in SCLC.
MRP1/ABCC1 is the first gene to be characterized in the ABCC subfamily and was originally identified as being responsible for the multidrug resistance phenotype in a drug-selected human small cell lung cancer cell line H69AR (Cole et al. 1992). Several researchers have indicated that MRP1/ABCC1 has a role in the clinical drug resistance behaviour of SCLC and was predictive of response to chemotherapy (Hsia et al. 2002; Kuo et al. 2003). Correlation analysis between ABC and miRNA expression in individual patients revealed an inverse relationship, confirming an indication for miRNA regulation of ABC genes in HCC 13 cellular miRNAs that target ABCA1, ABCC1, ABCC5, ABCC10 and ABCE1 genes and mediate changes in gene expression (Borel et al. 2012). In breast cancer, elevated levels of miR-326 in the mimic-transfected VP-16-resistant cell line MCF-7/VP downregulated MRP-1/ABCC1 expression and sensitized these cells to VP-16 and doxorubicin (Liang et al. 2010). Small nucleolar RNA-derived microRNA hsa-miR-1291 modulates cellular drug disposition through direct targeting of ABC transporter ABCC1 (Pan et al. 2013). But the molecular mechanism of MRP1/ABCC1 in MDR of SCLC remains unknown. We analysed the expression of MRP1/ABCC1 in 44 cases of human SCLC tissues by IHC staining. Contrary to miR-7, the positive rate of MRP1/ABCC1 expression was significantly lower in drug-sensitive SCLC specimens than that in drug-resistant specimens. In addition, MRP1/ABCC1 expression was closely correlated with chemotherapy response, but not with gender, age and stage of patients with SCLC.
It has been reported that some genes are the direct targets of miR-7. The above data showed MRP1/ABCC1 expression was negatively correlated with miR-7 level in SCLC tissues. To determine whether MRP1/ABCC1 is a direct target of miR-7, we searched potential targets of miR-7 in PicTar, TargetScan and miRBase databases and found that the 3′UTR of human MRP1/ABCC1 contains putative regions that match to the seed sequence of miR-7. Forced expression of miR-7 inhibits MRP1/ABCC1 expression in H69AR cells, and knockdown of miR-7 elevates MRP1/ABCC1 expression in H69 cells. Dual-luciferase assays verified MRP1/ABCC1 as a direct target of miR-7. These findings indicated that miR-7 modulates chemosensitivity of SCLC through the regulation of MRP1/ABCC1.
In summary, our study showed that low-level of miR-7 expression was closely associated with chemotherapy resistance and shorter overall survival. These findings suggest miR-7 may serve as predictive biomarker for chemoresistance and prognostic biomarker for survival in patients with SCLC. Furthermore, we demonstrated that miR-7 mediated chemoresistance by repressing MRP1/ABCC1, which is a novel mechanism of chemoresistance in SCLC, suggesting that miR-7 (MRP1/ABCC1) could be employed as an effective therapeutic target for SCLC.
Acknowledgments
This work was supported in part by the National Natural Science Foundation of China (81041068,30971183).
Conflict of interest
The authors declare no conflict of interest.
References
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Borel F, et al. Adenosine triphosphate-binding cassette transporter genes up-regulation in untreated hepatocellular carcinoma is mediated by cellular microRNAs. Hepatology. 2012;55:821–832. doi: 10.1002/hep.24682. [DOI] [PubMed] [Google Scholar]
- Chang XB. A molecular understanding of ATP-dependent solute transport by multidrug resistance-associated protein MRP1. Cancer Metastasis Rev. 2007;26:15–37. doi: 10.1007/s10555-007-9041-7. [DOI] [PubMed] [Google Scholar]
- Cole SP, et al. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science. 1992;258:1650–1654. doi: 10.1126/science.1360704. [DOI] [PubMed] [Google Scholar]
- Fang Y, et al. MicroRNA-7 inhibits tumor growth and metastasis by targeting the phosphoinositide 3-kinase/Akt pathway in hepatocellular carcinoma. Hepatology. 2012;55:1852–1862. doi: 10.1002/hep.25576. [DOI] [PubMed] [Google Scholar]
- Flens MJ, et al. Tissue distribution of the multidrug resistance protein. Am. J. Pathol. 1996;148:1237–1247. [PMC free article] [PubMed] [Google Scholar]
- Fornari F, et al. MiR-122/cyclin G1 interaction modulates p53 activity and affects doxorubicin sensitivity of human hepatocarcinoma cells. Cancer Res. 2009;69:5761–5767. doi: 10.1158/0008-5472.CAN-08-4797. [DOI] [PubMed] [Google Scholar]
- Govindan R, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J. Clin. Oncol. 2006;24:4539–4544. doi: 10.1200/JCO.2005.04.4859. [DOI] [PubMed] [Google Scholar]
- Guo L, et al. Gene expression profiling of drug-resistant small cell lung cancer cells by combining microRNA and cDNA expression analysis. Eur. J. Cancer. 2010;46:1692–1702. doi: 10.1016/j.ejca.2010.02.043. [DOI] [PubMed] [Google Scholar]
- Hsia TC, et al. Relationship between chemotherapy response of small cell lung cancer and P-glycoprotein or multidrug resistance-related protein expression. Lung. 2002;180:173–179. doi: 10.1007/s004080000091. [DOI] [PubMed] [Google Scholar]
- Kefas B, et al. microRNA-7 inhibits the epidermal growth factor receptor and the Akt pathway and is down-regulated in glioblastoma. Cancer Res. 2008;68:3566–3572. doi: 10.1158/0008-5472.CAN-07-6639. [DOI] [PubMed] [Google Scholar]
- Kong D, et al. Inflammation-induced repression of tumor suppressor miR-7 in gastric tumor cells. Oncogene. 2012;31:3949–3960. doi: 10.1038/onc.2011.558. [DOI] [PubMed] [Google Scholar]
- Kuo TH, et al. To predict response chemotherapy using technetium-99 m tetrofosmin chest images in patients with untreated small cell lung cancer and compare with p-glycoprotein, multidrug resistance related protein-1, and lung resistance-related protein expression. Nucl. Med. Biol. 2003;30:627–632. doi: 10.1016/s0969-8051(03)00058-1. [DOI] [PubMed] [Google Scholar]
- Li P, et al. MicroRNA-137 down-regulates KIT and inhibits small cell lung cancer cell proliferation. Biomed. Pharmacother. 2014;68:7–12. doi: 10.1016/j.biopha.2013.12.002. [DOI] [PubMed] [Google Scholar]
- Liang Z, et al. Involvement of miR-326 in chemotherapy resistance of breast cancer through modulating expression of multidrug resistance-associated protein 1. Biochem. Pharmacol. 2010;79:817–824. doi: 10.1016/j.bcp.2009.10.017. [DOI] [PubMed] [Google Scholar]
- Mo W. Zhang JT. Oligomerization of human ATP-binding cassette transporters and its potential significance in human disease. Expert Opin. Drug Metab. Toxicol. 2009;5:1049–1063. doi: 10.1517/17425250903124371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moitra K, et al. Differential gene and microRNA expression between etoposide resistant andetoposide sensitive MCF7 breast cancer cell lines. PLoS ONE. 2012;7:e45268. doi: 10.1371/journal.pone.0045268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan YZ, et al. Small nucleolar RNA-derived microRNA hsa-miR-1291 modulates cellular drug disposition through direct targeting of ABC transporter ABCC1. Drug Metab. Dispos. 2013;41:1744–1751. doi: 10.1124/dmd.113.052092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao X, et al. MicroRNA-221/222 confers breast cancer fulvestrant resistance by regulating multiple signaling pathways. Oncogene. 2011;30:1082–1097. doi: 10.1038/onc.2010.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy SD, et al. MicroRNA-7, a homeobox D10 target, inhibits p21-activated kinase 1 and regulates its functions. Cancer Res. 2008;68:8195–8200. doi: 10.1158/0008-5472.CAN-08-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler AB. Chemotherapy for small cell lung cancer. Semin. Oncol. 2003;30:9–25. doi: 10.1053/sonc.2003.50012. [DOI] [PubMed] [Google Scholar]
- Saydam O, et al. miRNA-7 attenuation in Schwannoma tumors stimulates growth by upregulating three oncogenic signaling pathways. Cancer Res. 2011;71:852–861. doi: 10.1158/0008-5472.CAN-10-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalsky RL. Cullen BR. Reduced expression of brain-enriched microRNAs in glioblastomas permits targeted regulation of a cell death gene. PLoS ONE. 2011;6:e24248. doi: 10.1371/journal.pone.0024248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song B, et al. Mechanism of chemoresistance mediated by miR-140 in human osteosarcoma and colon cancer cells. Oncogene. 2009;28:4065–4074. doi: 10.1038/onc.2009.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Pierre MV, et al. Expression of members of the multidrug resistance protein family in human term placenta. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000;279:R1495–R1503. doi: 10.1152/ajpregu.2000.279.4.R1495. [DOI] [PubMed] [Google Scholar]
- Triller N, et al. Multidrug resistance in small cell lung cancer: expression of P-glycoprotein, multidrug resistance protein 1 and lung resistance protein in chemo-naive patients and in relapsed disease. Lung Cancer. 2006;54:235–240. doi: 10.1016/j.lungcan.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Valeri N, et al. MicroRNA-21 induces resistance to 5-fluorouracil by down-regulating human DNA MutS homolog 2 (hMSH2) Proc. Natl. Acad. Sci. U S A. 2010;107:21098–21103. doi: 10.1073/pnas.1015541107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F, et al. Downregulation of HOXA1 gene affects small cell lung cancer cell survival and chemoresistance under the regulation of miR-100. Eur. J. Cancer. 2014;50:1541–1554. doi: 10.1016/j.ejca.2014.01.024. [DOI] [PubMed] [Google Scholar]
- Xiong S, et al. MicroRNA-7 inhibits the growth of human non-small cell lung cancer A549 cells through targeting BCL-2. Int. J. Biol. Sci. 2011;7:805–814. doi: 10.7150/ijbs.7.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, et al. MicroRNA expression profiling in human ovarian cancer: miR-214 induces cell survival and cisplatin resistance by targeting PTEN. Cancer Res. 2008;68:425–433. doi: 10.1158/0008-5472.CAN-07-2488. [DOI] [PubMed] [Google Scholar]
- Yin J. Zhang J. Multidrug resistance-associated protein 1 (MRP1/ABCC1) polymorphism: from discovery to clinical application. Zhong. Nan Da Xue Xue Bao Yi Xue Ban. 2011;36:927–938. doi: 10.3969/j.issn.1672-7347.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, et al. Identification of miR-7 as an oncogene in renal cell carcinoma. J. Mol. Histol. 2013;44:669–677. doi: 10.1007/s10735-013-9516-5. [DOI] [PubMed] [Google Scholar]
- Zaman GJ, et al. Analysis of the expression of MRP, the gene for a new putative transmembrane drug transporter, in human multidrug resistant lung cancer cell lines. Cancer Res. 1993;53:1747–1750. [PubMed] [Google Scholar]


