Abstract
BACKGROUND
Recent research has demonstrated that aerobic exercise can attenuate craving for drugs of abuse and reduce escalation and reinstatement of drug-seeking behavior in animal models. The present study examined the effects of aerobic exercise on the development of the incubation of cocaine-seeking behavior or the progressive increase in cocaine seeking over a protracted withdrawal period from cocaine self-administration.
METHODS
Female rats were trained to self-administer cocaine (0.4 mg/kg/inf) during daily 6-h sessions for 10 days. Subsequently, access to cocaine and cocaine-paired cues was discontinued during a 3- or 30-day withdrawal period when rats had access to either a locked or unlocked running wheel. At the end of the withdrawal period, rats were reintroduced to the operant conditioning chamber and reexposed to cocaine-paired cues to examine cocaine-seeking behavior under extinction conditions.
RESULTS
Rats with access to a locked running wheel during 30 days of withdrawal had significantly greater cue-induced cocaine-seeking behavior than rats that had access to an unlocked running wheel for 30 days. Further, there was robust incubation of cocaine seeking in rats with access to a locked running wheel as cocaine seeking was notably elevated at 30 vs. 3 days of withdrawal. However, cocaine-seeking behavior did not differ between rats with access to an unlocked running wheel for 30 vs. 3 days, indicating that incubation of cocaine seeking was suppressed following access to exercise for 30 days.
CONCLUSION
Aerobic exercise during extended withdrawal from cocaine self-administration decreased incubation of cue-induced cocaine-seeking behavior and may reduce vulnerability to relapse.
Keywords: cocaine, exercise, incubation of craving, relapse, wheel running, withdrawal
Introduction
Relapse to drug use is a major barrier to the treatment of addiction, with 80-90% of former users relapsing within 1 year of drug use cessation (Brandon et al. 2007, Kirshenbaum et al. 2009). Relapse is often preceded and accompanied by robust craving elicited by drug-paired stimuli and environments, and reports have demonstrated a progressive increase in drug craving or drug seeking over the withdrawal period in both humans (Gawin and Kleber 1986) and animals (Grimm et al. 2001, Neisewander et al. 2000). In 1986, Gawin and Kleber (1986) sought to characterize drug craving in cocaine-dependent individuals. Their subjects described a period of low craving for cocaine during initial weeks following drug use cessation; however, after a period of abstinence as long as 28 weeks, they experienced the return of intense craving precipitated by cocaine-paired cues. In the laboratory, this phenomenon was reproduced in rats (Grimm et al. 2001, Neisewander et al. 2000) and, subsequently, humans (Bedi et al. 2011, Wang et al. 2013, Li et al. 2014), and it has been referred to as the “incubation” of drug craving or drug seeking (Grimm et al. 2001). Incubation results from the development of time-dependent, withdrawal-induced neuroadaptations (Grimm et al. 2001, Conrad et al. 2008, Pickens et al. 2011, Wolf and Tseng 2012) and may contribute to relapse after protracted withdrawal (Conrad et al. 2008). Laboratory models of incubation typically compare drug-seeking behavior in response to drug-paired cues during early and late withdrawal from drug self-administration (Grimm et al. 2001). Cue-induced drug seeking has been shown to peak around 2-3 months of withdrawal in both rats (Grimm et al. 2001, Lu et al. 2004) and humans (Wang et al. 2013, Li et al. 2014), and greater drug seeking during late vs. early withdrawal was found using a range of reinforcers including cocaine (Gawin and Kleber 1986, Grimm et al. 2003, Shaham 2002), nicotine (Abdolahi et al. 2010, Bedi et al. 2011), methamphetamine (Shepard et al. 2004, Wang et al. 2013), heroin (Shalev et al. 2001), alcohol (Bienkowski et al. 2004, Li et al. 2014), and sucrose (Grimm et al. 2005).
As it may enhance vulnerability to relapse after an extended withdrawal, incubation of drug seeking is an important factor to consider in designing treatment strategies for drug abuse (Lu et al. 2004, Conrad et al. 2008, Pickens et al. 2011, Chauvet et al. 2012). Thus far, however, few investigations have examined treatments to reduce or eliminate the incubation of cue-induced drug seeking. A growing body of research has evaluated the physiological and affective benefits of exercise and physical activity and has begun exploring potential treatment applications for exercise as a behavioral intervention (USDHHS 1996). Results have shown a negative correlation between physical health and fitness and relapse to smoking (Metheny and Weatherman 1998) and a significantly reduced risk of smoking relapse in adults who were physically active (McDermot et al. 2009). Controlled laboratory studies in humans also have demonstrated that moderate intensity aerobic exercise decreased drug cravings (Ussher et al. 2004, Daniel et al. 2004, Buchowski et al. 2011, Prapavessis et al. 2014) and alleviated symptoms of drug withdrawal (Ussher et al. 2001, Daniel et al. 2004, Williams et al. 2011, Prapavessis et al. 2014). Additionally, animal experiments have revealed promising treatment effects of exercise on drug seeking behaviors. Voluntary wheel running decreased reinstatement of cocaine-seeking behavior precipitated by exposure to cocaine (Zlebnik et al. 2010, 2014a; Smith et al. 2012), yohimbine (Zlebnik et al. 2014a), and cocaine-paired cues (Lynch et al. 2010, Smith et al. 2012, Peterson et al. 2014a, Zlebnik et al. 2014a).
While the effects of exercise on the incubation of drug seeking are unknown at present, a recent study by Chauvet et al. (2012) explored the effects of housing in an enriched environment that included a running wheel vs. a standard laboratory environment during withdrawal on subsequent cocaine seeking. Animals housed in the enriched environment had significantly less responding for cocaine-paired cues compared to standard housing controls after both 30 and 60 days vs. 1 day of withdrawal. Although drug seeking was not assessed during early withdrawal, Lynch et al. (2010) found that daily wheel running introduced during a 2-week withdrawal period significantly attenuated reinstatement of cocaine-seeking behavior provoked by exposure to cocaine-paired cues. The treatment outcomes from these studies are promising, suggesting that enriching the environment with opportunities for exercise may have a lasting impact on cue-induced drug seeking and the potential for this drug seeking to increase over time.
In the present study, we examined the effects of aerobic exercise on the incubation of cocaine-seeking behavior. Female rats were studied because they achieve higher wheel running rates than males (Boakes et al. 1999, Cosgrove et al. 2002, Eikelboom and Mills 1988, Lambert and Kinsley 1993) and demonstrate greater receptivity than males to the attenuating effects of wheel running on cocaine-maintained behaviors (Cosgrove et al. 2002, Thanos et al. 2010, Smith et al. 2011). They were given access to a locked or unlocked running wheel during a withdrawal period of 3 or 30 days following cocaine self-administration. Following this period of withdrawal, cocaine-seeking behavior was measured in response to cocaine-paired cues. We hypothesized that unlocked wheel access would attenuate responding for cocaine-paired cues compared to locked wheel conditions and prevent the incubation of cocaine-seeking behavior.
Materials and methods
Animals
Fifty-three female adult Wistar rats were obtained from Harlan Sprague-Dawley, Inc. (Madison, WI, USA) and began behavioral testing around postnatal day 90. After arrival at the laboratory, rats were pair-housed in plastic cages with free access to laboratory chow (Teklad 2018, Harlan Laboratories, Madison, WI, USA) and water for at least 3-days of acclimation. Upon commencement of behavioral testing, rats had free access to water and were fed 16 g of rodent meal outside of behavioral sessions to maintain them at 85% of their free-feeding body weight. Mean body weights throughout the experiment did not differ among the groups throughout the study. Estrous cycle was not monitored to prevent disruption of cocaine- and exercise-maintained behavior by repeated vaginal lavage (Walker et al. 2002). All rodent holding rooms were maintained at 24°C and at 40-50% humidity under a light/dark cycle (12/12-h) with room lights on at 6:00 am. The experimental protocol was approved by the University of Minnesota Institutional Animal Care and Use Committee. The study was conducted in compliance with the Principles of Laboratory Animal Care (National Research Council 2011), and all laboratory facilities were accredited by the American Association for the Accreditation of Laboratory Animal Care.
Apparatus
During wheel training and withdrawal, rats were housed in plastic bins with attached running wheels (ENV-046, MedAssociates, Inc., St. Albans, VT, USA), and during cocaine self-administration, rats were housed and tested in custom-built operant conditioning chambers as previously described (Zlebnik et al. 2010, Zlebnik et al. 2012). Data collection and programming were conducted using PC computers with a Med-PC interface (MedAssociates, Inc.).
Cocaine
Cocaine HCl was provided by the National Institute on Drug Abuse (Research Triangle Institute, Research Triangle Park, NC, USA) and dissolved in 0.9 % NaCl at a concentration of 1.6 mg cocaine HCl/1 ml saline. Heparin (5 USP/ml) was added to the cocaine solution to prevent catheter occlusion from thrombin accumulation. The flow rate of each cocaine infusion was 0.025 ml/sec, and the duration of pump activation (1 sec/100 g of body weight) was adjusted weekly to provide a 0.4 mg/kg cocaine dose throughout self-administration testing.
Catheterization Surgery
Following wheel training, rats were implanted with a chronic indwelling catheter in the right jugular vein by methods previously described (Carroll and Boe 1982, Zlebnik et al. 2010). Antibiotic (enrofloxacin, 10 mg/kg, sc) and analgesic (buprenorphine, 0.05 mg/kg, sc) medications were administered daily during the 3-day recovery period. Each day, catheters were flushed with a solution (0.3 ml, iv) of heparinized saline (20 USP/ml) and cefazolin (10.0 mg/ml) to prevent catheter blockage and infection. Weekly, catheter patency was assessed by injecting a 0.1-ml solution containing ketamine (60 mg/kg), midazolam (3 mg/kg), and saline. If loss of the righting reflex did not manifest from an iv infusion of this solution, a second catheter was implanted in the left jugular vein, and the experiment was resumed following a 3-day recovery period.
Procedure
Cocaine self-administration
Following wheel and self-administration training, the experimental procedure consisted of 3 phases: 1) cocaine self-administration, 2) withdrawal, and 3) test for cue-induced cocaine-seeking behavior. Acquisition of wheel running and training of cocaine self-administration followed methods previously published (Zlebnik et al. 2010, Zlebnik et al. 2012). During self-administration training and maintenance, sessions began with illumination of the house light and extension of the active lever into the operant conditioning chamber. Responses on the active/drug-paired lever started the infusion pump and illuminated the stimulus lights located directly above the lever for the duration of the infusion. Responses on the active lever during the length of the infusion (1 sec/100 g of body wt) and responses on the inactive lever were recorded but had no programmed consequences. Rats were allowed to self-administer unlimited iv cocaine (0.4 mg/kg/infusion) for 6-h sessions (9:00 am – 3:00 pm) over 10 consecutive days.
Withdrawal
Following 10 days of cocaine self-administration, catheters were tied off, and rats were randomly assigned to 1 of 4 treatment groups, receiving either 3 or 30 days of withdrawal and either access to a locked (L) or unlocked (U) running wheel: U3, N = 14; U30, N = 12; L3, N = 15; L30, N = 12. Rats then were moved to housing in plastic bins with attached running wheels (MedAssociates, Inc.) and given 6-h daily access to the running wheel (9:00 am – 3:00 pm) (Peterson et al. 2014a,b).
Cue-induced cocaine-seeking test
Rats were returned to the operant conditioning chambers for a 30-min test (Koya et al. 2009) of cocaine-seeking behavior under extinction conditions after 3 or 30 days of withdrawal. The experimental conditions were the same as during cocaine self-administration except that active lever presses no longer resulted in cocaine reinforcement; the house light was illuminated, and responses on the lever previously associated with cocaine infusions activated the sound of the infusion pump and the stimulus lights directly above the lever. Responses on the inactive lever again had no consequences.
Data analysis
The primary dependent measures were responses and infusions during cocaine self-administration, wheel revolutions during withdrawal, and responses during the cocaine-seeking test. For cocaine self-administration, data were grouped into 2-day blocks to reduce daily variability and the number of post-hoc contrasts. Measures were analyzed with 2-factor mixed analyses of variance (ANOVA) with group as the between-subjects factor and blocks of days as the repeated measure. Wheel revolutions during withdrawal were compared 2-tailed Student’s t-tests, and responses during the cocaine-seeking test were examined with a 2-factor ANOVA (wheel access X incubation length). Results were considered significant if p < 0.05. Planned contrasts were performed with Dunn’s (Bonferroni) procedure. To examine the relationship between wheel revolutions during the withdrawal period and subsequent cocaine-seeking behavior, mean wheel revolutions were correlated with mean active lever responses during the cocaine-seeking test using Pearson’s product-moment correlations. Statistical analyses were performed using GB Stat (Dynamic Microsystems, Inc., Silver Spring, MD, USA).
Results
Cocaine self-administration
Figure 1 displays mean responses (Fig. 1A) and infusions (Fig. 1B) over the 10-day cocaine self-administration period, averaged into five 2-day blocks. Separate 2-factor ANOVA (group X block of days) revealed significant main effects of block of days for responses (F4,196 = 4.89, p < 0.01) and infusions (F4,196 = 5.10, p < 0.01) but no significant effects of group or group X block of days interaction. Additionally, there were no significant trends in inactive responses throughout the self-administration period (data not shown). These results indicate similar, increasing levels of cocaine self-administration over the 10-day period for all wheel access groups.
Figure 1.
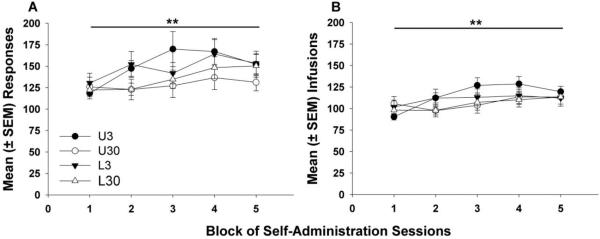
Mean (± SEM) responses (A) for and infusions (B) of cocaine (0.4 mg/kg/inf) averaged into five 2-day blocks over the 10-day cocaine self-administration period. Overall, there were no group differences, and all groups escalated their responding and infusions earned over the self-administration period (main effect of block of days, ** p <0.01).
Withdrawal
Wheel revolutions during the first 3 days of the withdrawal period (Fig. 2A) did not differ between the groups. For the U30 group, wheel revolutions remained stable over the course of the 30-day incubation period with no significant increase in revolutions from Days 1-3 to Days 28-30.
Figure 2.
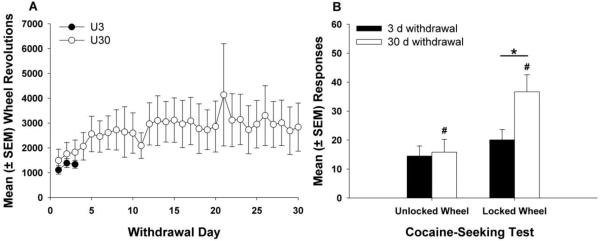
(A) Mean (± SEM) wheel revolutions across the first 3 days of the withdrawal period did not differ among the unlocked wheel access groups. (B) Mean (± SEM) responses on the lever previously paired with cocaine were significantly higher for the L30 group compared to the U30 group (# p < 0.01). Incubation of cocaine seeking was apparent in the animals with access to a locked running wheel, as the L30 group had considerably more responding than the L3 group (** p < 0.05). However, this increase in responding was absent in the animals that had access to an unlocked running wheel during the withdrawal period (U3 vs. U30).
Cue-induced cocaine-seeking test
Fig. 2B depicts the number of responses on the previously active lever following reintroduction to the operant conditioning chamber after 3 or 30 days of withdrawal. Data were analyzed by a 2-factor ANOVA, and results revealed significant main effects of wheel access (F1,49 = 10.49, p < 0.01) and incubation length (F1,49 = 4.84, p < 0.05) but no significant wheel access X incubation length interaction (F1,49 = 3.21, p = 0.0671). Planned comparisons showed a notable increase in cocaine-seeking behavior in the L30 group compared to the U30 group (p < 0.01) but no differences between the L3 and U3 groups. The L30 group also exhibited a greater level of responding compared to the L3 group (p < 0.05), demonstrating incubation of cocaine-seeking behavior in rats that had access to a locked running wheel. However, for groups that had access to an unlocked running wheel during the withdrawal period, this same increase in cocaine seeking was absent, as there were no significant differences between the U3 and U30 groups. Inactive lever responding (not shown) was low and did not differ among any of the groups during the cocaine-seeking test.
Wheel revolutions vs. cocaine-seeking responses
Mean wheel revolutions during the entire 3- or 30-day withdrawal period for both unlocked wheel access groups or during the last 3 days of the withdrawal period for the U30 group were not significantly correlated with total responses on the previously active lever during the cocaine-seeking test (data not shown).
Discussion
The primary goal of the present experiment was to investigate the effects of aerobic exercise during a withdrawal period from cocaine self-administration on subsequent relapse-related behavior. Consistent with prior work, results revealed a robust increase in cue-induced cocaine-seeking behavior during late vs. early withdrawal following only 10 days of long access to cocaine self-administration under control conditions, suggesting that even brief exposure to drugs of abuse can produce long-term vulnerability to relapse. Further, access to an unlocked running wheel during 30 days of cocaine withdrawal significantly attenuated cocaine-seeking behavior compared to locked wheel conditions and prevented increases in cocaine seeking over the 30-day withdrawal period. These findings suggest that aerobic exercise during protracted withdrawal may help to reduce relapse to drug use.
Prior research established reductions in cocaine- (Zlebnik et al. 2010, Smith et al. 2012), yohimbine- (Zlebnik et al. 2014a), and cue- (Lynch et al. 2010, Smith et al. 2012, Zlebnik et al. 2014a) primed reinstatement of cocaine-seeking behavior by wheel running, but the current experiment is noteworthy in its demonstration that wheel running suppressed or prevented the incubation of cocaine seeking over extended withdrawal. In particular, results showed that wheel running reduced cocaine seeking at 30 but not 3 days after discontinuation of cocaine self-administration, indicating that aerobic exercise may not necessarily have a general inhibitory effect on relapse-related behaviors but that its effects may be specific to time-dependent changes in drug seeking that occur over several weeks after cocaine access is terminated. These results are consistent with work showing prevention of the incubation of sucrose seeking following housing in an enriched environment that included opportunities for exercise (Grimm et al. 2008). However, while cocaine self-administration studies have shown that enriched vs. standard environmental conditions attenuated cue-elicited cocaine seeking after an extended withdrawal period (Thiel et al. 2012, Chauvet et al. 2012), enriched conditions did not succeed in fully suppressing the incubation of cocaine-seeking behavior. Typically, enriched laboratory environments involve larger cages, novel objects, social interaction, and running wheels, and many of the beneficial consequences of exposure to enriched environments (i.e., improved learning and memory, neurogenesis, cell proliferation) cannot be disambiguated from exposure to exercise alone (van Praag et al. 2000). However, the level of voluntary wheel running achieved in traditional enriched environments likely does not reach the level achieved in the present study. Therefore, while both environmental enrichment and wheel running alone are effective in reducing drug-motivated behaviors, differential levels of voluntary exercise may account for the discrepancy in the effects of these conditions on time-dependent changes in cocaine seeking.
Additional work will be needed to reveal the time course of development of incubation and the critical period of exposure to aerobic exercise with respect to its ability to suppress incubation of cocaine seeking. For instance, only 3 days of wheel running may not have been sufficient to reduce cocaine-seeking behavior compared to locked wheel conditions. However, while some reports suggest that greater access to daily wheel running (6 vs. 1 h/day for 14 days) has a larger attenuating effect on cocaine-motivated responding (Peterson et al. 2014a,b), Smith and Witte (2012) found that only 4 days of housing with a running wheel was enough to suppress motivation to lever-press for cocaine infusions under a progressive ratio schedule. Based on these results, access to wheel running for 6 h/day for 3 days as in the present experiment likely could have been adequate to have an effect on cocaine-seeking behavior. Another explanation for the lack of attenuation in cocaine seeking after 3 days of unlocked vs. locked wheel conditions may be due to floor effects of cocaine-seeking behavior during early withdrawal. During the initial days following cessation of cocaine self-administration, cocaine seeking has been shown to be low (Grimm et al. 2001) and may have obscured treatment effects of wheel running during early withdrawal. Overall, however, the present results demonstrated that wheel running suppressed cocaine seeking during late compared to early withdrawal and support significant treatment effects of aerobic exercise on relapse-related behavior and its progressive increase over time.
Empirical work with humans has already demonstrated significant reduction of tobacco craving (Ussher et al. 2004, Daniel et al. 2004, Prapavessis et al. 2014) and withdrawal symptoms (Ussher et al. 2001, Daniel et al. 2004, Williams et al. 2011, Prapavessis et al. 2014) following bouts of moderate intensity aerobic exercise, and recent reports indicated that 8 weeks of supervised aerobic exercise in methamphetamine-dependent individuals helped restore D2/3 receptor availability to control levels (Robertson et al. 2013). In addition to notable treatment effects of exercise alone, emerging clinical (Potenza et al. 2011) and preclinical (Zlebnik et al. 2014a, Zlebnik and Carroll 2015) findings suggest that combined behavioral and pharmacological treatments may result in more effective, longer-lasting, and self-maintained treatment outcomes that will reduce relapse to drug use and promote overall better health. Aerobic exercise has been suggested as an adjunctive treatment for the standard treatment for anxiety disorders (Sciolino and Holmes 2012), and evidence supports better treatment success when exercise is combined with cognitive (Martin et al. 1997) and motivational (Weinstock et al. 2008) treatment strategies. While the present results demonstrate a significant treatment effect of exercise during extended withdrawal, there may be an additional role for exercise in augmenting standard addiction treatments and in facilitating the adoption and maintenance of healthier patterns of behavior (Ussher et al. 2012) after prolonged abstinence.
Conclusions
The present results demonstrate a strong role for aerobic exercise in reducing the likelihood of relapse-related behaviors in rats during an extended withdrawal period of 30 days. These findings also indicate that it will be important for future work to not only determine the mechanism of the effects of wheel running on time-dependent increases in cocaine seeking but also examine the efficacy of aerobic exercise to decrease incubation of cocaine craving in humans. This persistence of intense craving during protracted withdrawal from drug use presents a major barrier to the treatment of addiction, and implementation of aerobic exercise regimens during the abstinence period may facilitate more successful treatment outcomes.
Acknowledgements
The authors would like to acknowledge helpful feedback from Drs. Yavin Shaham, Robert Meisel, Valerie Hedges, and Jack Smethells. The authors would also like to thank Vanessa Adamson, Yosef Amrami, Cole Batty, Luke Bushman, Clare Chamberlain, Arit Harvanko, Seth Johnson, Sarah Korthauer, Torie Lepak, Nathan Omdalen, Amy Saykao, Heather Veglahn, and Ashley Xiong for technical assistance and Krista Walkowiak, DVM, for veterinary care. Funding for this study was provided by the National Institute on Drug Abuse (NIDA) grants T32 DA007097 (PI: Thomas W. Molitor), F31 DA036248 (NEZ), R01 DA003240 (MEC), and K05 DA015267 (MEC).
References
- Abdolahi A, Acosta G, Breslin FJ, Hemby SE, Lynch WJ. Incubation of nicotine seeking is associated with enhanced protein kinase A-regulated signaling of dopamine- and cAMP-regulated phosphoprotein of 32 kDa in the insular cortex. Eur J Neurosci. 2010;31(4):733–41. doi: 10.1111/j.1460-9568.2010.07114.x. PMID: 2038481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedi G, Preston KL, Epstein DH, Heishman SJ, Marrone GF, Shaham Y, de Wit H. Incubation of cue-induced cigarette craving during abstinence in human smokers. Biol Psychiatry. 2011;69(7):708–11. doi: 10.1016/j.biopsych.2010.07.014. PMID: 20817135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienkowski P, Rogowski A, Korkosz A, Mierzejewski P, Radwanska K, Kaczmarek L, Bogucka-Bonikowska A, Kostowski W. Time-dependent changes in alcohol-seeking behaviour during abstinence. Eur Neuropsychopharmacol. 2004;14(5):355–60. doi: 10.1016/j.euroneuro.2003.10.005. PMID: 15336295. [DOI] [PubMed] [Google Scholar]
- Boakes RA, Mills KJ, Single JP. Sex differences in the relationship between activity and weight loss in the rat. Behav Neurosci. 1999;113(5):1080–9. PMID: 10571490. [PubMed] [Google Scholar]
- Brandon TH, Vidrine JI, Litvin EB. Relapse and relapse prevention. Annu Rev Clin Psychol. 2007;3:257–284. doi: 10.1146/annurev.clinpsy.3.022806.091455. PMID: 17716056. [DOI] [PubMed] [Google Scholar]
- Buchowski MS, Meade NN, Charboneau E, Park S, Dietrich MS, Cowan RL, Martin PR. Aerobic exercise training reduces cannabis craving and use in non-treatment seeking cannabis-dependent adults. PLoS One. 2011;6(3):e17465. doi: 10.1371/journal.pone.0017465. PMID: 21408154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, Boe IN. Increased intravenous drug self-administration during deprivation of other reinforcers. Pharmacol Biochem Behav. 1982;17(3):563–7. doi: 10.1016/0091-3057(82)90319-7. PMID: 6128744. [DOI] [PubMed] [Google Scholar]
- Chauvet C, Goldberg SR, Jaber M, Solinas M. Effects of environmental enrichment on the incubation of cocaine craving. Neuropharmacology. 2012;63(4):635–41. doi: 10.1016/j.neuropharm.2012.05.014. PMID: 22634364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454(7200):118–21. doi: 10.1038/nature06995. PMID: 18500330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corominas-Roso M, Roncero C, Eiroa-Orosa FJ, Gonzalvo B, Grau-Lopez L, Ribases M, Rodriguez-Cintas L, Sánchez-Mora C, Ramos-Quiroga JA, Casas M. Brain-derived neurotrophic factor serum levels in cocaine-dependent patients during early abstinence. Eur Neuropsychopharmacol. 2013;23(9):1078–84. doi: 10.1016/j.euroneuro.2012.08.016. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Hunter R, Carroll ME. Wheel-running attenuates intravenous cocaine self-administration in rats: sex differences. Pharm Biochem Behav. 2002;73:663–67. doi: 10.1016/s0091-3057(02)00853-5. PMID: 15122955. [DOI] [PubMed] [Google Scholar]
- Daniel J, Cropley M, Ussher M, West R. Acute effects of a short bout of moderate versus light intensity exercise versus inactivity on tobacco withdrawal symptoms in sedentary smokers. Psychopharmacology. 2004;174(3):320–6. doi: 10.1007/s00213-003-1762-x. PMID: 14997270. [DOI] [PubMed] [Google Scholar]
- Eikelboom R, Mills R. A microanalysis of wheel running in male and female rats. Physiol Behav. 1988;43(5):625–30. doi: 10.1016/0031-9384(88)90217-x. PMID: 3200918. [DOI] [PubMed] [Google Scholar]
- Gawin FH, Kleber HD. Abstinence symptomatology and psychiatric diagnosis in cocaine abusers. Clinical observations. Arch. Gen. Psychiatry. 1986;43:107–113. doi: 10.1001/archpsyc.1986.01800020013003. PMID: 3947206. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Fyall AM, Osincup DP. Incubation of sucrose craving: effects of reduced training and sucrose pre-loading. Physiol Behav. 2005;84(1):73–9. doi: 10.1016/j.physbeh.2004.10.011. PMID: 15642609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412(6843):141–2. doi: 10.1038/35084134. PMID: 11449260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Lu L, Hayashi T, Hope BT, Su TP, Shaham Y. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J Neurosci. 2003;23:742–747. doi: 10.1523/JNEUROSCI.23-03-00742.2003. PMID: 12574402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Osincup D, Wells B, Manaois M, Fyall A, Buse C, Harkness JH. Environmental enrichment attenuates cue-induced reinstatement of sucrose seeking in rats. Behav Pharmacol. 2008;19(8):777–85. doi: 10.1097/FBP.0b013e32831c3b18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirshenbaum AP, Olsen DM, Bickel WK. A quantitative review of the ubiquitous relapse curve. J Subst Abuse Treat. 2009;36:8–17. doi: 10.1016/j.jsat.2008.04.001. PMID: 18571890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koya E, Uejima JL, Wihbey KA, Bossert JM, Hope BT, Shaham Y. Role of ventral medial prefrontal cortex in incubation of cocaine craving. Neuropharmacology. 2009;56(Suppl 1):177–85. doi: 10.1016/j.neuropharm.2008.04.022. PMID: 18565549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert KG, Kinsley CH. Sex differences and gonadal hormones influence susceptibility to the activity-stress paradigm. Physiol Behav. 1993;53:1085–90. doi: 10.1016/0031-9384(93)90363-k. PMID: 8346291. [DOI] [PubMed] [Google Scholar]
- Li P, Wu P, Xin X, Fan YL, Wang GB, Wang F, Ma MY, Xue MM, Luo YX, Yang FD, Bao YP, Shi J, Sun HQ, Lu L. Incubation of alcohol craving during abstinence in patients with alcohol dependence. Addict Biol. 2014 doi: 10.1111/adb.12140. doi: 10.1111/adb.12140. [DOI] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Hope BT, Shaham Y. Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology. 2004;47(Suppl 1):214–26. doi: 10.1016/j.neuropharm.2004.06.027. PMID: 15464139. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Piehl KB, Acosta G, Peterson AB, Hemby SE. Aerobic exercise attenuates reinstatement of cocaine-seeking behavior and associated neuroadaptations in the prefrontal cortex. Biol Psychiatry. 2010;68(8):774–7. doi: 10.1016/j.biopsych.2010.06.022. PMID: 20692647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JE, Kalfas KJ, Patten CA. Prospective evaluation of three smoking interventions in 205 recovering alcoholics: one-year results of project SCRAP-Tobacco. Journal of Consulting and Clinical Psychology. 1997;65:190–4. doi: 10.1037//0022-006x.65.1.190. PMID: 9103749. [DOI] [PubMed] [Google Scholar]
- McDermot L, Dobson A, Owen N. Determinants of continuity and change over 10 years in young women’s smoking. Addiction. 2009;104:478–87. doi: 10.1111/j.1360-0443.2008.02452.x. PMID: 19207359. [DOI] [PubMed] [Google Scholar]
- Metheny KB, Weatherman KE. Predictors of smoking cessation and maintenance. Journal of Clinical Psychology. 1998;54:223–35. doi: 10.1002/(sici)1097-4679(199802)54:2<223::aid-jclp12>3.0.co;2-l. PMID: 9467767. [DOI] [PubMed] [Google Scholar]
- National Research Council . Guide for the Care and Use of Animals. 8th The National Academies Press; Washington, DC: 2011. [Google Scholar]
- Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine selfadministration environment. J Neurosci. 2000;20:798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. PMID: 10632609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson AB, Abel JM, Lynch WJ. Dose-dependent effects of wheel running on cocaine-seeking and prefrontal cortex Bdnf exon IV expression in rats. Psychopharmacology. 2014a;231(7):1305–14. doi: 10.1007/s00213-013-3321-4. [DOI] [PubMed] [Google Scholar]
- Peterson AB, Hivick DP, Lynch WJ. Dose-dependent effectiveness of wheel running to attenuate cocaine-seeking: impact of sex and estrous cycle in rats. Psychopharmacology. 2014b doi: 10.1007/s00213-014-3437-1. doi 10.1007/s00213-014-3437-1. [DOI] [PubMed] [Google Scholar]
- Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y. Neurobiology of the incubation of drug craving. Trends Neurosci. 2011;34(8):411–20. doi: 10.1016/j.tins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza MN, Sofuoglu M, Carroll KM, Rounsaville BJ. Neuroscience of behavioral and pharmacological treatments for addictions. Neuron. 2011;69(4):695–712. doi: 10.1016/j.neuron.2011.02.009. PMID: 21338880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prapavessis H, De Jesus S, Harper T, Cramp A, Fitzgeorge L, Mottola MF, Ussher M, Faulkner G, Selby P. The effects of acute exercise on tobacco cravings and withdrawal symptoms in temporary abstinent pregnant smokers. Addict Behav. 2014;39(3):703–8. doi: 10.1016/j.addbeh.2013.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson CL, Chudzynski J, Rawson R, Cooper CB, Mooney L, Brown AK, Mandelkern MA, Ishibashi K, London ED. Dopamine D2/D3 receptor recovery with methamphetamine abstinence and exercise. Society for Neuroscience. 2013 Abstracts 817.05/R9. [Google Scholar]
- Sciolino NR, Holmes PV. Exercise offers anxiolytic potential: a role for stress and brain noradrenergic-galaninergic mechanisms. Neurosci Biobehav Rev. 2012;36(9):1965–84. doi: 10.1016/j.neubiorev.2012.06.005. doi: 10.1016/j.neubiorev.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y. The drug withdrawal period and susceptibility to relapse to heroin and cocaine seeking. Behav Pharmacol. 2002;13:503. [Google Scholar]
- Shalev U, Morales M, Hope B, Yap J, Shaham Y. Time-dependent changes in extinction behavior and stress-induced reinstatement of drug seeking following withdrawal from heroin in rats. Psychopharmacology (Berl) 2001;156(1):98–107. doi: 10.1007/s002130100748. PMID: 11465640. [DOI] [PubMed] [Google Scholar]
- Shepard JD, Bossert JM, Liu SY, Shaham Y. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol Psychiatry. 2004;55(11):1082–9. doi: 10.1016/j.biopsych.2004.02.032. PMID: 15158427. [DOI] [PubMed] [Google Scholar]
- Smith MA, Pennock MM, Walker KL, Lang KC. Access to a running wheel decreases cocaine-primed and cue-induced reinstatement in male and female rats. Drug Alcohol Depend. 2012;121(1-2):54–61. doi: 10.1016/j.drugalcdep.2011.08.006. PMID: 21885215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Walker KL, Cole KT, Lang KC. The effects of aerobic exercise on cocaine self-administration in male and female rats. Psychopharmacology. 2011;218(2):357–69. doi: 10.1007/s00213-011-2321-5. PMID: 21567123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Witte MA. The effects of exercise on cocaine self-administration, food-maintained responding, and locomotor activity in female rats: importance of the temporal relationship between physical activity and initial drug exposure. Exp Clin Psychopharmacol. 2012;20(6):437–46. doi: 10.1037/a0029724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos PK, Tucci A, Stamos J, Robison L, Wang GJ, Anderson BJ, Volkow ND. Chronic forced exercise during adolescence decreases cocaine conditioned place preference in Lewis rats. Behav Brain Res. 2010;215(1):77–82. doi: 10.1016/j.bbr.2010.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel KJ, Painter MR, Pentkowski NS, Mitroi D, Crawford CA, Neisewander JL. Environmental enrichment counters cocaine abstinence-induced stress and brain reactivity to cocaine cues but fails to prevent the incubation effect. Addict Biol. 2012;17(2):365–77. doi: 10.1111/j.1369-1600.2011.00358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USDHHS . Physical activity and health: A report of the surgeon general. Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion; Atlanta, GA: 1996. [Google Scholar]
- Ussher M, Nunziata P, Cropley M, West R. Effect of a short bout of exercise on tobacco withdrawal symptoms and desire to smoke. Psychopharmacology. 2001;158(1):66–72. doi: 10.1007/s002130100846. PMID: 11685385. [DOI] [PubMed] [Google Scholar]
- Ussher M, Sampuran AK, Doshi R, West R, Drummond DC. Acute effect of a brief bout of exercise on alcohol urges. Addiction. 2004;99(12):1542–7. doi: 10.1111/j.1360-0443.2004.00919.x. PMID: 15585045. [DOI] [PubMed] [Google Scholar]
- Ussher MH, Taylor A, Faulkner G. Exercise interventions for smoking cessation. Cochrane Database Syst Rev. 2012 Jan 18;1:CD002295. doi: 10.1002/14651858.CD002295.pub4. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1(3):191–8. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- Walker QD, Nelson CJ, Smith D, Kuhn CM. Vaginal lavage attenuates cocaine-stimulated activity and establishes place preference in rats. Pharmacol Biochem Behav. 2002;73:743–752. doi: 10.1016/s0091-3057(02)00883-3. [DOI] [PubMed] [Google Scholar]
- Wang G, Shi J, Chen N, Xu L, Li J, Li P, Sun Y, Lu L. Effects of length of abstinence on decision-making and craving in methamphetamine abusers. PLoS One. 2013;248(7):e68791. doi: 10.1371/journal.pone.0068791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock J, Barry D, Petry NM. Exercise-related activities are associated with positive outcome in contingency management treatment for substance use disorders. Addict Behav. 2008;33:1072–1075. doi: 10.1016/j.addbeh.2008.03.011. PMID: 18486352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DM, Dunsiger S, Whiteley JA, Ussher MH, Ciccolo JT, Jennings EG. Acute effects of moderate intensity aerobic exercise on affective withdrawal symptoms and cravings among women smokers. Addict Behav. 2011;36(8):894–7. doi: 10.1016/j.addbeh.2011.04.001. PMID: 21543158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME, Tseng KY. Calcium-permeable AMPA receptors in the VTA and nucleus accumbens after cocaine exposure: when, how, and why? Front Mol Neurosci. 2012;5:72. doi: 10.3389/fnmol.2012.00072. PMID: 22754497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlebnik NE, Anker JJ, Carroll ME. Exercise to reduce the escalation of cocaine self-administration in adolescent and adult rats. Psychopharmacology. 2012;224(3):387–400. doi: 10.1007/s00213-012-2760-7. PMID: 22752381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlebnik NE, Anker JJ, Gliddon LA, Carroll ME. Reduction of extinction and reinstatement of cocaine seeking by wheel running in female rats. Psychopharmacology. 2010;209(1):113–25. doi: 10.1007/s00213-010-1776-0. PMID: 20112008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlebnik NE, Carroll ME. Effects of the combination of wheel running and atomoxetine on cue- and cocaine-primed reinstatement in rats selected for high or low impulsivity. Psychopharmacology. 2015;232(6):1049–5. doi: 10.1007/s00213-014-3744-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlebnik NE, Hedges VL, Carroll ME, Meisel RL. Chronic wheel running affects cocaine-induced c-Fos expression in brain reward areas in rats. Behav Brain Res. 2014b;261:71–8. doi: 10.1016/j.bbr.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlebnik NE, Saykao AT, Carroll ME. Effects of combined exercise and progesterone treatments on cocaine seeking in male and female rats. Psychopharmacology. 2014a doi: 10.1007/s00213-014-3513-6. doi 10.1007/s00213-014-3513-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


