Abstract
Objectives
We investigate urinary nerve growth factor as a novel urinary biomarker for high-grade prostate cancer.
Methods and Materials
After IRB approval for a prospective pilot study, we enrolled men at the preoperative visit prior to robotic assisted radical prostatectomy. Demographics, urinary flow parameters, and urine samples were collected. Urinary nerve growth factor (NGF) and urinary creatinine were obtained in the translational science laboratory. Pathologic and post-operative demographics were collected after surgery. NGF is the primary outcome variable (dependent variable). The pathologic Gleason score (ordinal variable ≤6, 7, and 8≤) served as an independent grouping variable. Multivariate analysis using a general linear model was conducted to investigate associations between independent variables and NGF (dependent variable) after adjusting for urinary concentration and volume.
Results
We enrolled and analyzed urine samples and pathologic data from 115 subjects. Patient pathology included 24% (n=28) Gleason 6 or less, 68% (n=78) Gleason 7, and 8% (n=9) Gleason 8 or greater. Perineural invasion was more prevalent in higher-grade disease (p<0.001). The median NGF was 24.1 pg/mL (range 0.16 pg/mL – 270.5 pg/mL) and was transformed to the Log base 10 scale. Total bladder volume, urinary creatinine, PSA, and diabetes were correlated with the Log NGF. In a general linear model, adjusting for bladder volume and urinary creatinine, increasing Log10 NGF was associated with higher Gleason group (Gleason category ≤6, 7, and ≥8; p=0.003).
Conclusions
Urinary nerve growth factor may be a biomarker for higher-grade prostate cancer. Our pilot study suggests further investigation is warranted to determine if urinary NGF could provide unique additional information in a prostate cancer patients.
Keywords: Prostate Cancer, Biomarker, Neurogenesis, Gleason Score
1. Introduction
Prostate cancer (PCa) is considered a neurotropic cancer, a commonality with other epithelial tumors such as pancreatic, bile duct, head/neck cancers [1]. PCa “neurogenesis” is an evolving field of research and considered a dominant pathway of local invasion [2]. Perineural invasion on prostate biopsy specimen may predict adverse outcomes after radical prostatectomy or radiation therapy; however, a consistent consensus has been hindered by variability in pathologic reporting and research study design [3–5].
In vitro studies show the perineural space provides an enhanced environment for PCa survival and growth through cell-cell interactions involving growth factors [3, 6, 7]. Subsequent, animal models involving hypogastric denervation have prevented prostate cancer cell growth attributed to sympathetic and parasympathetic nerve alterations confirmed by increased nerve density in human PCa [8]. Moreover, a recent population based study showed β-blocker use may be associated with a reduction in PCa specific mortality suggesting that β-adrenergic receptor down-regulation may be the mechanism of action [9].
Nerve Growth Factor (NGF) is overexpressed in the prostate and alterations in its receptors may cause PCa proliferation and metastasis.[10, 11] Herein, we investigate the potential utility of urinary NGF as a urinary biomarker for prostate cancer aggressiveness based on pathologic Gleason score.
2. Materials and Methods
2.1 Study Population and Design
After internal review board approval, we prospectively recruited patients at the pre-operative appointment prior to robotic assisted radical prostatectomy. Urine samples are collected when the participants had a strong desire to void. Subjects voided volume was added to the post void residual to calculate total bladder volume (TV) in order to control for volume concentration. The post-void residual volume was obtained by bladder scan ultrasound (Verathon BVI 3000 Bladderscan, Bothell, WA, USA) in all patients. In order to account for bladder volume and concentration the total urinary volume was calculated at the time of the void as well as a urinary creatinine, which has been used with NGF in previous studies [12].
2.2 Sample Collection
Voided urine was placed on ice immediately and transferred to the laboratory for preparation for NGF measurement. The urine samples were centrifuged at 3000g for 10 min at 4°C. The supernatant was separated into aliquots in 1.5 mL tubes and preserved in a freezer at −80°C. At the same time, 3 mL of urine was taken to measure the urinary creatinine (Cr) level.
2.3 Urinary Nerve Growth Factor and Creatinine
Urinary NGF concentration was determined using an immunoassay system (Emax®, Promega, Madison, WI, USA) with a specific and highly sensitive ELISA kit, which had a minimum sensitivity of 7.8 pg/mL. Assays were conducted according to the manufacturer’s instructions. The detailed procedure was described previously. Generally, urine samples were not diluted in the ELISA assay. When the urinary NGF concentration was higher than the upper detection limit (250 pg/mL) the urine samples were diluted to fit the detection limit. For urine samples with NGF concentrations lower than the detectable limit but above zero, they were concentrated using a column-protein concentrate kit (Amicon Ultra-15, Millipore, USA) before measuring the NGF value. All samples were run in triplicate, and urinary NGF levels without a consistent value in three measures were repeated and the values were averaged. The criterion for defining consistent values was that the coefficient of variation (SD/mean) of the three absorbance values was 0.10. If the coefficient of variation of the samples was 0.10, data of the first run were discarded and the samples were re-run in a triplicate. The concentration of urinary creatinine (mg/dL) assay kit (Cayman Chemical Company, Ann Arbor, MI, USA) was obtained and performed as per manufactures directions.
2.4 Study Outcomes
The primary outcome was NGF in patients with higher-grade prostate cancer compared to lower-grade prostate cancer found on final pathology after prostatectomy. Other preoperative, pathological and postoperative outcomes were also collected prospectively.
Pathological data consisted of pathologic Gleason as is grouped into three categories (Gleason 6 or less, Gleason 7, or Gleason 8 or greater). Tumor volume was determined as an overall percent of tumor in the radical prostatectomy specimen. If a range of percentages were given, the average percent tumor volume as calculated and used at the outcome. Additionally we documented positive surgical margins (PSM) and upstaging defined as any increase of Gleason pattern from biopsy to pathology (includes 3+4 to 4+3). The pathologist as per standard at our institution documented perineural invasion; however, no further quantification was performed. Final pathologic staging as per American Joint Committee on Cancer 2009 (AJCC) was used for analysis.
Postoperative oncologic follow-Up consisted of 3 month PSA and any PSA we could obtain thereafter for a goal of 12-month follow up. Biochemical recurrence was defined as a PSA >0.2 ng/mL with a confirmatory PSA or any patient who received adjuvant therapy.
2.5 Statistical Analysis
We performed the statistical analysis with SPSSv.21 statistical program (IBM). Urinary nerve growth factor (NGF) is not normally distributed; therefore, we transformed the variable with the log base 10 (Log NGF). Subsequently, we performed ANOVA and Chi-Squared tests for associations of variables compared to pathologic Gleason score (three groups: Gleason 6 or less, Gleason 7, or Gleason 8 or greater). Other variables are tested with the Students T test or Chi-Square test. We then identified other independent variables associated with NGF, which could confound the association between pathologic grade and NGF using the Spearman correlations and ANOVA. Multivariate analysis using a general linear model for regression was performed including all variables found to be significantly associated with the dependent variable of interest log NGF. Only variables that were significant in the multivariate analysis were retained in the final model.
3. Results
After institutional review board approval, 120 consecutive men were recruited to participate in the study. Three men were excluded from analysis after enrollment due to having previous radiation therapy. The first subject had an undetectable NGF level though all other subjects had some level detected; therefore, this was attributed to error and the patient was excluded. One patient had a urinary tract infection and one patient was an outlier (NGF >1000 pg/mL) and is excluded from final analysis. Therefore, a total of 115 patients had all data available for analysis.
Previous studies noted an association of NGF with overactive bladder, anticholinergic medications, and prostatitis/chronic pelvic pain.[13, 14] We noted patients with moderate (AUAss >8, n= 48) and severe (AUAss >20, n=7) lower urinary tract symptoms (LUTS) had higher urinary LogNGF (1.34 pg/mL vs. 1.30 pg/mL; p=0.715 and 1.64 pg/mL vs. 1.30 pg/mL; p=0.289, respectively) but did not reach statistical significance. LogNGF levels were no different in men taking anti-cholinergic medications (20%, 24/116) than those who did not (1.22 pg/mL vs. 1.34 pg/mL; p=0.457). There was no correlation between post void residual and LogNGF (Pearson −0.48, p=0.608). However, men documented with inflammation on the radical prostatectomy prostate specimen (n=11) did have elevated LogNGF (1.86 pg/mL vs. 1.26 pg/mL; p=0.004).
Patient pathology included 24% (n=28) Gleason 6 or less, 68% (n=78) Gleason 7, and 8% (n=9) Gleason 8 or greater. Demographics are displayed in Table 1 by pathologic Gleason group. Perineural invasion (PNI) was more common among aggressive cancer (Table 1); however, LogNGF was not statistically significantly higher (1.37 pg/mL vs. 1.13 pg/mL; p=0.108) in the presence of PNI. Tumor volume did not correlate with LogNGF (Pearson −0.023; p=0.814). Additionally, no difference was noted in mean percent tumor in each LogNGF quartile (ANOVA p=0.322). Five subjects were lost to follow up and 111 were followed for a median of 15 (1–24) months. During this time, 10 (9%) patients had either biochemical recurrence or adjuvant therapy and were considered a recurrence. Ten patients had a biochemical recurrence or adjuvant therapy and noted no difference in LogNGF values (1.40 pg/mL vs. 1.31 pg/mL; p=0.669)
Table 1.
Demographics by Gleason Group
| Pathologic Gleason ≤6 N= 28 mean (SD) |
Pathologic Gleason 7 N=78 mean (SD) |
Pathologic Gleason ≥8 N=9 mean (SD) |
P Value ANOVA |
|
|---|---|---|---|---|
| Demographics | ||||
| Age | 60.8 (6.2) | 61.6 (7.2) | 64.8 (8.8) | 0.333 |
| Body Mass Index (BMI) | 27.9 (3.1) | 26.9 (3.0) | 27.1 (3.0) | 0.621 |
| AUA Symptom Score | 9.9 (7.1) | 6.8 (5.0) | 8.9 (8.5) | 0.213 |
| Sexual Health Inventory for Men (SHIM) | 20.9 (6.4) | 21.7 (5.2) | 16.6 (10.0) | 0.072 |
| Post Void Residual (PVR) | 90.6 (66.1) | 86.6 (79.2) | 80.0 (102.0) | 0.062 |
| Peak Flow Rate | 14.2 (7.0) | 16.3 (7.2) | 19.3 (15.2) | 0.25 |
| Prostate Weight (gms) | 59.9 (15.9) | 51.8 (18.7) | 59.2 (22.9) | 0.109 |
| Follow-up (months) | 13.6 (6.7) | 13.9 (5.8) | 13.9 (6.0) | 0.962 |
| Biomarker Related | ||||
| Urinary NGF (pg/mL) | 28.8 (52.8) | 47.2 (58.8) | 49.3 (54) | 0.335 |
| Urinary Log10NGF (pg/mL) | 0.98 (0.75) | 1.37 (0.57) | 1.41 (0.63) | 0.018 |
| Urinary NGF (pg/mL) / Urinary Creatinine (mg/dL) | 1.84 (5.01) | 2.33 (5.29) | 2.62 (4.05) | 0.894 |
| Urinary Creatinine (mg/dL) | 36.9 (31.8) | 40.1 (34.5) | 32.3 (24.7) | 0.816 |
| Total Bladder Volume (mL) | 414.2 (190.5) | 436 (245.7) | 358.6 (182.2) | 0.632 |
| Prostate Specific Antigen (PSA; ng/mL) | 5.4 (2.4) | 6.4 (5.3) | 7.1 (3.2) | 0.505 |
| PSA Density (PSA/TRUS volume) | 0.15 (0.10) | 0.19 (0.14) | 0.19 (0.14) | 0.454 |
| N (%) | N (%) | N (%) | Chi-Squared | |
|---|---|---|---|---|
| Ethnicity (white) | 26 (96.3) | 75 (96.2) | 9 (100) | 0.770 |
| Asprin | 7 (25.9) | 20 (26.3) | 3 (33.3) | 0.907 |
| β-Blocker | 1 (3.7) | 8 (10.5) | 0 (0) | 0.364 |
| Statins | 11 (40.7) | 31 (39.7) | 4 (55.6) | 0.666 |
| Diabetes | 0 (0) | 4 (5.1) | 0 (0) | 0.309 |
| Smoker | 3 (11.1) | 3 (3.8) | 0 (0) | 0.251 |
| Clinical Stage | <0.001 | |||
| cT1c | 27 (100) | 0 (0) | 0 (0) | |
| cT2 | 52 (66.7) | 26 (33.3) | 0 (0) | |
| cT3 | 0 (0) | 0 (0) | 9 (100) | |
| D’Amico Risk Category | <0.001 | |||
| Low | 4 (14.8) | 0 (0) | 0 (0) | |
| Intermediate | 22 (81.5) | 56 (71.8) | 0 (0) | |
| High | 1 (3.7) | 22 (28.2) | 9 (100) | |
| Pathologic | ||||
| Pathologic Stage (AJCC) | <0.001 | |||
| pT2 | 26 (96.3) | 57 (73.1) | 1 (11.1) | |
| pT3 | 1 (3.7) | 21 (26.9) | 6 (66.7) | |
| pT4 | 0 (0) | 0 (0) | 2 (22.2) | |
| Upgrading | 0 (0) | 39 (50) | 4 (44.4) | <0.001 |
| Positive Surgical Margin | 2 (7.4) | 7 (9.0) | 4 (44.4) | 0.004 |
| Perineural Invasion | 10 (37) | 70 (89.7) | 8 (87.2) | <0.001 |
| Biochemical recurrence | 0 (0) | 2 (2.6) | 4 (44.4) | <0.001 |
| Tertiary Gleason 5 | 0 (0) | 12 (15.4) | 0 (0) | <0.001 |
| Any Gleason 5 | 0 (0) | 12 (15.4) | 9 (100) | 0.049 |
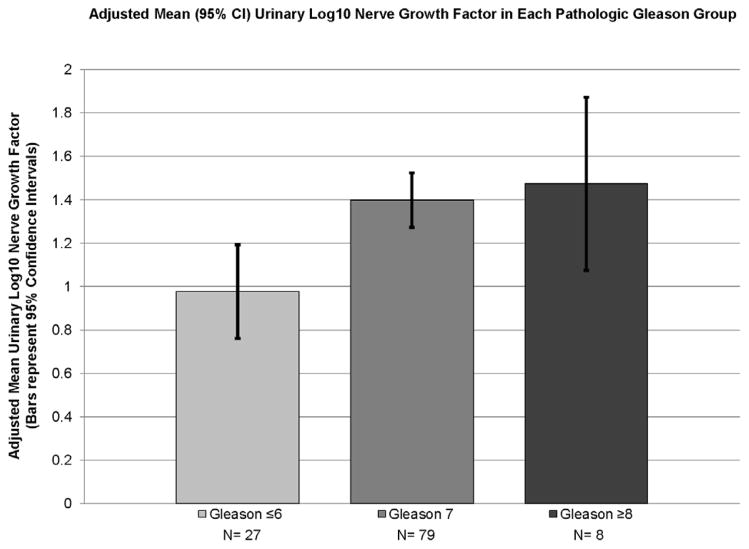
The median NGF was 24.1 pg/mL (range 0.16 – 270.5). Median NGF by Gleason grade was 10.5 pg/mL for grade ≤6 (range: 0.2 to 270.2), 25.8 pg/mL for grade 7 (range: 0.4 to 923.8) and 34 pg/mL for grade ≥8 (range: 1.54 to 176.7) (Kruskal-Wallis; p=0.032). Demographic information by pathologic Gleason group (6 or less, 7, and 8 or greater) is displayed in Table 1. The ANOVA test shows a significantly higher Log NGF with increasing pathologic Gleason score (p=0.018). Specifically, perineural invasion was noted to be more prevalent in higher-grade disease (p<0.001) (Figure 1a).
Figure 1.
Adjusted Log NGF Means by Gleason Group
Log NGF was significantly correlated with measurements of urine volume and concentration measured as total bladder volume (TV) (r=0.425, p<0.001) and urinary creatinine (r=0.451, p<0.001), respectively. Additionally PSA (r=0.284, p=0.002) and the presence of diabetes (p=0.012) were significantly associated with Log10 NGF. (Table 2) There were no significant associations between Log10 NGF and other pathologic factors such as perineural invasion (p=0.118), positive margin (p=0.397), prostate weight (0.080) or pathologic stage (p=0.761). (Table 2)
Table 2.
Univariate and Multivariate associations with Log NGF
| Univariant | Spearman Correlation Coefficient | Spearman (P value) |
|---|---|---|
| Age | 0.117 | 0.211 |
| Body Mass Index | 0.169 | 0.070 |
| AUA symptom score | 0.029 | 0.759 |
| Sexual Health Inventory for Men (SHIM) | −0.094 | 0.326 |
| Prostate Weight (gm) | 0.164 | 0.078 |
| Prostate Specific Antigen (PSA) ng/mL | 0.284 | 0.002 |
| Total urinary volume (ml) | 0.425 | <0.001 |
| Urinary Creatinine (ng/dL) | 0.451 | <0.001 |
| ANOVA (P value) | |
|---|---|
| Smoker | 0.462 |
| Beta Blocker | 0.290 |
| Aspirin | 0.374 |
| Statin | 0.035 |
| Diabetes | 0.012 |
| Pathologic Gleason Sum (Ordinal 6-, 7, 8+) | 0.005 |
| Multivariate | General Linear Model* |
|---|---|
| Pathologic Gleason Sum (Ordinal 6-, 7, 8+) | 0.004 |
| Urinary creatinine (ng/dL) | 0.002 |
| Total Bladder Volume (mL) | 0.001 |
PSA (p=0.147), Statin (p=0.181) and Diabetes (p=0.741) in the model were no longer significant and then removed to arrive at the the final model.
Gleason group, urinary creatinine, total bladder volume, PSA, and diabetes were included as independent variables in a general linear model. (Table 2) PSA and diabetes were no longer significant in the model and were removed from the final model. In the final model, Log10 NGF adjusted for total bladder volume and urinary creatinine was significantly associated with Gleason group (Gleason 6 or less, Gleason 7, or Gleason 8 or greater; (p=0.003). The adjusted Log10 NGF means are 0.98, 1.39, and 1.51, respectively. (Figure 1)
4. Discussion
Among men presenting for robotic assisted radical prostatectomy, urinary nerve growth factor is higher in men with high-grade prostate cancer. We were not able to find significant differences in PSA or PSA density by Gleason grade in this cohort suggesting NGF may be a unique protein in prostate cancer aggressiveness. Previous studies have eluded that neurogenesis can be a mechanism of prostate cancer progression described alterations in nerve growth factor and its receptors.[8, 11] Our study is the first to explore this nerve growth factor as a potential urinary biomarker in prostate cancer.
Previous studies investigating neurogenesis in prostate cancer have stemmed from the common finding of perineural invasion in prostate cancer biopsy or prostatectomy specimens, including >75% of patients in our study [15, 16]. Unfortunately, the utility perineural invasion remains controversial.[4] Recent studies have invigorated the neurogenesis discussion by described changes in nerve density surrounding prostate cancer in radical prostatectomy specimens suggesting the cancer or surrounding tissue may be producing substances to induce nerve growth [8, 17, 18]. In vitro studies with prostate cancer cell lines have described relationships between PCa and nerve growth, specifically the interaction with secreted nerve growth factor [7, 19–21].
NGF can stimulate low-grade PCa cell lines (LNCAP) to metastasize and high-grade cancer PCa cell lines (DU145 and PC3) can secrete NGF to recruit the nervous system similar to angiogenesis mechanisms [22]. Moreover, animal studies have shown that denervation of the hypogastric plexus of animals causes distinct morphologic and functional changes to the glandular epithelium independent of hormonal manipulation and can prevent prostate tumor development [8, 23]. If prostate cancer uses NGF to recruit nervous tissue as a component of progression, NGF pathway might be used as a biomarker or a therapeutic target.
A recent population based study of over 3,000 Norwegians suggested that β-blocker use is associated with reduced PCa specific mortality [9]. The β-blocker is postulated to inhibit cancer by reducing adrenergic stimulation thought to cause cancer progression and epithelial to mesenchymal phenotype switch [24, 25]. The concept of β-blocker use and potential modulation of neurogenesis to reduce progression have been shown across other tumors such as breast, ovarian and melanoma [26–28]. However, to our knowledge, urinary nerve growth factor has not been tested in a prospective observational clinical study for prostate cancer.
NGF has been found in prostatic fluid after prostate message, indicating that NGF is located in the prostate.[29] The previous studies were performed on prostatitis patients and we did confirm that NGF is elevated in men with inflammation on their prostatectomy specimens. We are unsure of the significance of this finding regarding prostate cancer, but there has been interest in prostatic inflammation and prostate cancer progression. [30] Another source of NGF could be the urothelium as described in patients with overactive bladder and bladder outlet obstruction. [12, 14] We documented the uroflometry, post void residual, and AUA symptom scores and noted that anticholinergic medication, elevated post void residual, and lower urinary tract symptoms were not associated with significant differences in LogNGF.
Although NGF has been detected in human prostate cancer tissue and studies supported that NGF may play a role in cancer invasion, there are very little studies about a possible role of NGF in the spread of prostate cancer.[10] Therefore, further studies are in progress to determine whether elevated urine NGF levels in patients with high-grade prostate cancer are associated with the expression of NGF in prostate cancer tissues or with circulating levels of NGF in prostate cancer patients due to cancer metastasis.
Limitations in this study stem from the pilot study design. We acknowledge the absence of control (non-cancer) patients from the study population. However, we were targeting the application in patients with a diagnosis of prostate cancer such as an active surveillance cohort. The origin of NGF is beyond the scope of this manuscript; however, other studies have shown the expression of NGF and its receptors in prostatic tissue.[29] Other organs and diseases that may also increase NGF that are not necessarily known and could change the results of the test. Moreover, we did not perform post-operative urine collection to confirm a reduction in NGF; however, it could be falsely elevated during the healing process. With limited resources, we chose to increase the sample size to assess the primary outcome of pathologic Gleason score. Due to these finding we are currently performing confirmatory testing of urinary NGF as a biomarker. Additionally, we collected urine at the preoperative appointment after prostate biopsy had occurred and acknowledge that the time from biopsy could change results of the test.
Future directions include incorporation of urinary biomarkers into clinical trials targeting neurogenesis, investigation of existing urine in biorepositories, and adjunctive testing in active surveillance cohorts.
5. Conclusions
In our pilot study, urinary nerve growth factor was able to distinguish high-grade prostate cancer prior to radical prostatectomy. Nerve growth factor may be a biomarker for higher-grade prostate cancer and provide unique information for prostate cancer patients pending further confirmatory studies.
Acknowledgments
The project described was supported by Grant Number UL1 RR031985 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and the NIH Roadmap for Medical Research.
Funding: Institute for Clinical and Translational Science, University of California-Irvine
Footnotes
Conflicts of Interest: Dr. Ahlering is a consultant for Astellas and Phillips Healthcare.
Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Reengineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Batsakis JG. Nerves and neurotropic carcinomas. The Annals of otology, rhinology, and laryngology. 1985;94:426–7. [PubMed] [Google Scholar]
- 2.Villers A, McNeal JE, Redwine EA, Freiha FS, Stamey TA. The role of perineural space invasion in the local spread of prostatic adenocarcinoma. The Journal of urology. 1989;142:763–8. doi: 10.1016/s0022-5347(17)38881-x. [DOI] [PubMed] [Google Scholar]
- 3.Liebig C, Ayala G, Wilks JA, Berger DH, Albo D. Perineural invasion in cancer: a review of the literature. Cancer. 2009;115:3379–91. doi: 10.1002/cncr.24396. [DOI] [PubMed] [Google Scholar]
- 4.Harnden P, Shelley MD, Clements H, et al. The prognostic significance of perineural invasion in prostatic cancer biopsies: a systematic review. Cancer. 2007;109:13–24. doi: 10.1002/cncr.22388. [DOI] [PubMed] [Google Scholar]
- 5.Lee IH, Roberts R, Shah RB, Wojno KJ, Wei JT, Sandler HM. Perineural invasion is a marker for pathologically advanced disease in localized prostate cancer. International journal of radiation oncology, biology, physics. 2007;68:1059–64. doi: 10.1016/j.ijrobp.2007.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ayala GE, Dai H, Ittmann M, et al. Growth and survival mechanisms associated with perineural invasion in prostate cancer. Cancer research. 2004;64:6082–90. doi: 10.1158/0008-5472.CAN-04-0838. [DOI] [PubMed] [Google Scholar]
- 7.Montano X, Djamgoz MB. Epidermal growth factor, neurotrophins and the metastatic cascade in prostate cancer. FEBS letters. 2004;571:1–8. doi: 10.1016/j.febslet.2004.06.088. [DOI] [PubMed] [Google Scholar]
- 8.Magnon C, Hall SJ, Lin J, et al. Autonomic nerve development contributes to prostate cancer progression. Science. 2013;341:1236361. doi: 10.1126/science.1236361. [DOI] [PubMed] [Google Scholar]
- 9.Grytli HH, Fagerland MW, Fossa SD, Tasken KA. Association Between Use of beta-Blockers and Prostate Cancer-Specific Survival: A Cohort Study of 3561 Prostate Cancer Patients with High-Risk or Metastatic Disease. European urology. 2013 doi: 10.1016/j.eururo.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 10.DeSchryver-Kecskemeti K, Balogh K, Neet KE. Nerve growth factor and the concept of neural-epithelial interactions. Immunohistochemical observations in two cases of vasitis nodosa and six cases of prostatic adenocarcinoma. Archives of pathology & laboratory medicine. 1987;111:833–5. [PubMed] [Google Scholar]
- 11.Molloy NH, Read DE, Gorman AM. Nerve growth factor in cancer cell death and survival. Cancers. 2011;3:510–30. doi: 10.3390/cancers3010510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu HT, Kuo HC. Urinary nerve growth factor levels are elevated in patients with overactive bladder and do not significantly increase with bladder distention. Neurourology and urodynamics. 2009;28:78–81. doi: 10.1002/nau.20599. [DOI] [PubMed] [Google Scholar]
- 13.Liu HT, Chancellor MB, Kuo HC. Decrease of urinary nerve growth factor levels after antimuscarinic therapy in patients with overactive bladder. BJU international. 2009;103:1668–72. doi: 10.1111/j.1464-410X.2009.08380.x. [DOI] [PubMed] [Google Scholar]
- 14.Liu HT, Chancellor MB, Kuo HC. Urinary nerve growth factor levels are elevated in patients with detrusor overactivity and decreased in responders to detrusor botulinum toxin-A injection. European urology. 2009;56:700–6. doi: 10.1016/j.eururo.2008.04.037. [DOI] [PubMed] [Google Scholar]
- 15.Quinn DI, Henshall SM, Brenner PC, et al. Prognostic significance of preoperative factors in localized prostate carcinoma treated with radical prostatectomy: importance of percentage of biopsies that contain tumor and the presence of biopsy perineural invasion. Cancer. 2003;97:1884–93. doi: 10.1002/cncr.11263. [DOI] [PubMed] [Google Scholar]
- 16.Feng FY, Qian Y, Stenmark MH, et al. Perineural invasion predicts increased recurrence, metastasis, and death from prostate cancer following treatment with dose-escalated radiation therapy. International journal of radiation oncology, biology, physics. 2011;81:e361–7. doi: 10.1016/j.ijrobp.2011.04.048. [DOI] [PubMed] [Google Scholar]
- 17.Brundl J, Schneider S, Weber F, Zeman F, Wieland WF, Ganzer R. Computerized Quantification and Planimetry of Prostatic Capsular Nerves in Relation to Adjacent Prostate Cancer Foci. European urology. 2013 doi: 10.1016/j.eururo.2013.04.043. [DOI] [PubMed] [Google Scholar]
- 18.Ayala GE, Dai H, Powell M, et al. Cancer-related axonogenesis and neurogenesis in prostate cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14:7593–603. doi: 10.1158/1078-0432.CCR-08-1164. [DOI] [PubMed] [Google Scholar]
- 19.Ayala GE, Wheeler TM, Shine HD, et al. In vitro dorsal root ganglia and human prostate cell line interaction: redefining perineural invasion in prostate cancer. The Prostate. 2001;49:213–23. doi: 10.1002/pros.1137. [DOI] [PubMed] [Google Scholar]
- 20.Pflug BR, Dionne C, Kaplan DR, Lynch J, Djakiew D. Expression of a Trk high affinity nerve growth factor receptor in the human prostate. Endocrinology. 1995;136:262–8. doi: 10.1210/endo.136.1.7828539. [DOI] [PubMed] [Google Scholar]
- 21.MacGrogan D, Saint-Andre JP, Dicou E. Expression of nerve growth factor and nerve growth factor receptor genes in human tissues and in prostatic adenocarcinoma cell lines. Journal of neurochemistry. 1992;59:1381–91. doi: 10.1111/j.1471-4159.1992.tb08451.x. [DOI] [PubMed] [Google Scholar]
- 22.Delsite R, Djakiew D. Characterization of nerve growth factor precursor protein expression by human prostate stromal cells: a role in selective neurotrophin stimulation of prostate epithelial cell growth. The Prostate. 1999;41:39–48. doi: 10.1002/(sici)1097-0045(19990915)41:1<39::aid-pros6>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 23.Martinez-Pineiro L, Dahiya R, Nunes LL, Tanagho EA, Schmidt RA. Pelvic plexus denervation in rats causes morphologic and functional changes of the prostate. The Journal of urology. 1993;150:215–8. doi: 10.1016/s0022-5347(17)35449-6. [DOI] [PubMed] [Google Scholar]
- 24.Palm D, Lang K, Niggemann B, et al. The norepinephrine-driven metastasis development of PC-3 human prostate cancer cells in BALB/c nude mice is inhibited by beta-blockers. International journal of cancer Journal international du cancer. 2006;118:2744–9. doi: 10.1002/ijc.21723. [DOI] [PubMed] [Google Scholar]
- 25.Sloan EK, Priceman SJ, Cox BF, et al. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer research. 2010;70:7042–52. doi: 10.1158/0008-5472.CAN-10-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barron TI, Connolly RM, Sharp L, Bennett K, Visvanathan K. Beta blockers and breast cancer mortality: a population- based study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:2635–44. doi: 10.1200/JCO.2010.33.5422. [DOI] [PubMed] [Google Scholar]
- 27.De Giorgi V, Grazzini M, Gandini S, et al. Treatment with beta-blockers and reduced disease progression in patients with thick melanoma. Archives of internal medicine. 2011;171:779–81. doi: 10.1001/archinternmed.2011.131. [DOI] [PubMed] [Google Scholar]
- 28.Diaz ES, Karlan BY, Li AJ. Impact of beta blockers on epithelial ovarian cancer survival. Gynecologic oncology. 2012;127:375–8. doi: 10.1016/j.ygyno.2012.07.102. [DOI] [PubMed] [Google Scholar]
- 29.Watanabe T, Inoue M, Sasaki K, et al. Nerve growth factor level in the prostatic fluid of patients with chronic prostatitis/chronic pelvic pain syndrome is correlated with symptom severity and response to treatment. BJU international. 2011;108:248–51. doi: 10.1111/j.1464-410X.2010.09716.x. [DOI] [PubMed] [Google Scholar]
- 30.Kwon OJ, Zhang L, Ittmann MM, Xin L. Prostatic inflammation enhances basal-to-luminal differentiation and accelerates initiation of prostate cancer with a basal cell origin. Proceedings of the National Academy of Sciences of the United States of America; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]