Abstract
N-myc downstream-regulated gene 1 (NDRG1) has been implicated in tumorigenesis and metastasis in different cancers. However, its role in nasopharyngeal carcinoma remains unknown. We found that NDRG1 expression level was high in nasopharyngeal cancer 5–8F cells but low in 5–8F-LN cells with lymphatic metastasis potential. Knockdown of NDRG1 by shRNA promoted 5–8F cell proliferation, migration, and invasion in vitro and its tumorigenesis in vivo. Moreover, NDRG1 deficiency induced an epithelial-mesenchymal transition (EMT) of 5–8F cells as shown by an attenuation of E-cadherin and an induction of N-cadherin and vimentin expression. NDRG1 knockdown also enhanced Smad2 expression and phosphorylation. Smad2 signaling was attenuated in 5–8F cells but was significantly activated in 5–8F-LN cells. Knockdown of Smad2 restored E-cadherin but attenuated N-cadherin expression in NDRG1-deficient 5–8F cells, suggesting a reduction of EMT. Consistently, blockade of Smad2 in 5–8F-LN cells increased E-cadherin while diminishing N-cadherin and vimentin expression. These data indicate that Smad2 mediates the NDRG1 deficiency-induced EMT of 5–8F cells. In tumors derived from NDRG1-deficient 5–8F cells, E-cadherin expression was inhibited while vimentin and Smad2 were increased in a large number of cancer cells. Most importantly, NDRG1 expression was attenuated in human nasopharyngeal carcinoma tissues, resulted in a lower survival rate in patients. The NDRG1 was further decreased in the detached nasopharyngeal cancer cells, which was associated with a further reduced survival rate in patients with lymphatic metastasis. Taken together, these results demonstrated that NDRG1 prevents nasopharyngeal tumorigenesis and metastasis via inhibiting Smad2-mediated EMT of nasopharyngeal cells.
Keywords: N-myc downstream-regulated gene 1, Nasopharyngeal carcinoma, proliferation, Metastasis, epithelial-mesenchymal transition
Introduction
Nasopharyngeal carcinoma (NPC) is one type of head and neck cancers with very different epidemiology, pathology, clinical features, treatment and outcome compared with other head and neck cancers. The local recurrences and metastasis of NPC are common though it has a relatively high sensitivity to radiation therapy [1]. The vulnerable populations of NPC are aged people predominantly between 40 and 60 years old [2]. Although NPC is very prevalent among the Cantonese in Southern China where the prevalence rate is approximately 30–80/100000 population/per year, the incidence rate is around 1/100,000 in America and Europe [3]. The main histological appearances of NPC is the poorly differentiated or undifferentiated pathological alteration. Due to the histological characteristic and the abundant lymphatic network in nasopharynx, NPC exhibits highly metastatic potential than other squamous cell carcinoma of head and neck [4]. The carcinogenesis of NPC is a complicated process involving multiple genetic and epigenetic events. Therefore, it is important to examine the genes/proteins altered during the nasopharyngeal carcinogenesis in order to identify potential targets for develop novel treatments against this disease.
Human N-myc downstream-regulated gene 1 (NDRG1) is a member of the N-myc down-regulated gene family which belongs to the alpha/beta hydrolase superfamily [5–7]. The 43 kDa protein encoded by this gene is a highly conserved cytoplasmic protein involved in cell growth arrest and proliferation, cell differentiation, DNA damage response, heavy metal response, the hypoxia response, tumorigenesis, and metastasis [8–10]. Previous studies have shown that NDRG1 plays different roles in different types of tumors. NDRG1 appears to serve as a putative tumor suppressor and is down-regulated in colorectal [11,12], gastric [13], cervical [14], ovarian [15] and prostate [16] cancers.
However, a number of studies have also shown that NDRG1 positively regulates cancer cell proliferation, differentiation, and metastasis. For example, NDRG1 promotes portal vein invasion and intrahepatic metastasis in hepatocellular carcinomas [17]. In vitro, NDRG1 is up-regulated in A549 lung cancer cell after exposing to hypoxia mimics, which results in more cell proliferation and less apoptosis [10]. Furthermore, NDRG1 expression level correlates with the degree of differentiation of several cancer tissues. NDRG1 expression in poorly differentiated carcinoma is significantly higher than that in well-differentiated carcinoma of the colon [12] and liver [18]. However, the correlation between NDRG1 and Nasopharyngeal carcinoma has not been established. Therefore, the biological functions of NDRG1 in the pathogenesis of NPC remain unknown. We hypothesize that NDRG1 affects the differentiation, proliferation, invasion, and metastasis of NPC cells. Indeed, we found that NDRG1 is differentially expressed in NPC cell line 5–8F and 5–8F-LN. NDRG1 appears to be an important factor that maintains NPC homeostasis and thus inhibits the development of NPC and its metastasis.
Materials and Methods
Cell culture
Human nasopharyngeal carcinoma 5–8F cells (5–8F) and 5–8F-LN cells with lymphatic metastasis potential (5–8F-LN) [19] were cultured in RPMI 1640 medium supplemented with 10% FBS (Hyclone). The cells were maintained in standard conditions (5% CO2 and 95% atmosphere, 37 °C).
2-dimension gel electrophoresis (2DE) and mass spectrometry analysis
Proteins were extracted from NPC 5–8F cell and 5–8F-LN cells. Cell lysates were cleared by centrifugation at 12,000 rpm at 4 °C for 1 h, and the protein concentration of the supernatants was determined by the modified Bradford method. 2DE analysis was performed as described by the manufacturer (GE HealthCare, USA). The gels were stained with Coomassie brilliant blue (CBB), and protein spots of interest were cut from the gels. Then, proteins were digested with trypsin, and the peptide mixtures were dried at ambient temperature and then analyzed by MALDI-TOF-TOF (ABI4700) mass spectrometry. The spectrometry was calibrated internally using the Monoisotopic [M+H] + ions of the peptide standards of trypsin. Protein spots were identified by the MASCOT searching program in IPI_human_v3.49 database.
Adenoviral vector construction
NDRG1 adenoviral vector were constructed and the viruses were purified as described previously [20]. NDRG1 short hairpin RNA (shRNA) target sequence was ACC CCG GCA ACC TGC ACC TGT TCA TCA. Double-stranded DNAs coding NDRG1 shRNA was cloned into pRNAT-H1.1 Adeno shuttle vector containing a cGFP marker (Genscript). Adenovirus was packaged in Ad-293 cells (Agilent) and purified by CsCl2 gradient ultracentrifugation. Viral particle titer was determined by plaque assay. For adenoviral transduction, 5–8F cell was transduced with 100 multiplicity of infection of adenoviral control or shRNA for 24–48 h.
Transfection of siRNAs against Smad2 and Smad3
SiRNAs were transfected into cells using lipofectamine 2000 transfection reagent (Life Technologies) by following the manufacturer’s instructions. Two different siRNAs against Smad2 (siSmad2) or Smad3 (siSmad3) were synthesized by Shanghai GenePharma. Sense sequences for Smad2 siRNAs are CAG GCU GUA AUC UGA AGA UTT (siSmad2-1) and GAA GAG GAG UGC GCU UAU ATT (siSmad2-2). Sense sequences for Smad3 siRNAs are GCG UGA AUC CCU ACC ACU ATT (siSmad3-1) and GCC AUC CAU GAC UGU GGA UTT (siSmad3-2). SiRNA knockdown efficiency was verified by western blotting detection of Smad2 and Smad3 protein expression.
Reverse transcriptase-polymerase chain reaction (PCR) and real-time reverse transcriptase-polymerase chain reaction (qPCR)
Total RNAs were isolated from the cells using TRIzol reagents (Takara, Cat # 9108). 10 µg of RNA from each sample was added to 20 µl reaction mixture, and cDNA was synthesized using PrimeScript™ RT reagent Kit with genomic DNA Eraser (Takara, Cat# RR047A). qPCR was performed using SYBR® Premix Ex Taq™ (Tli RNaseH Plus) (Takara, Cat# RR420A) to detect NDRG1 mRNA expression. Glyceraldehyde-3 phosphate dehydrogenase (GAPDH) was used as an internal control. The primers for human NDRG1 were 5’-ACA ACC CTG AGA TGG TGG AG-3’ (forward) and 5’-TGT GGA CCA CTT CCA CGT TA-3’ (reverse).The primers for human TGF-β1 were 5’-AAG GAC CTC GGC TGG AAG TG-3’ (forward) and 5’-CCC GGG TTA TGC TGG TTG TA-3’ (reverse). The primers for human GAPDH were 5’-ACA GTC AGC CGC ATC TTC TT-3’ (forward) and 5’-GAC AAG CTT CCC GTT CTC AG-3’ (reverse). The NDRG1 and TGF-β expression was normalized to GAPDH [21,22].
Western blotting
Cell lysates were prepared by a SDS lysis solution. Protein concentration was measured using a BCA protein assay kit. Equal amount of protein was separated by electrophoresis on a 10% SDS-polyacrylamide gel. The proteins were electrotransferred from the gel to nitrocellulose membrane. The membrane was blocked with 5% non-fat milk solution for 1 h, and then was incubated with primary monoclonal antibody against NDRG1 (Abcam), E-cadherin (Cell signaling), vimentin (Cell Signaling), N-cadherin (Cell Signaling), Smad2 (Cell Signaling), p-Smad2 (CST), Smad3 (Epitomics), p-Smad3 (Epitomics) at 4oC overnight. α-Tubulin was used as an internal control. After washing with TBS-T, the membrane was incubated with secondary antibodies against goat or mouse immunoglobulin G. The membrane was washed and detected by the enhanced chemiluminescence (ECL) detection system (Thermo) according to the manufacturer’s instructions.
Immunohistochemistry and immunofluorescence
Following antigen retrieval, 5 µm-thick tissue sections were incubated with NDRG1 (Abcam), E-Cadherin, vimentin, Smad2, or ki67 antibody (Santa Cruz) at 4°C overnight. For negative control, the primary antibody was replaced with normal nonimmune serum. Cytoplasmic staining was regarded as a positive signal. The degree of staining in the sections was observed and scored independently by 2 pathologists. The percentage of NDRG1 positive cells varied from 0% to 100%, which was recorded on the following 4-point scale: 1 (0–25%), 2 (26–50%), 3 (51–75%), and 4 (76–100%). The intensity of cytoplasmic staining varied from weak to strong. The cells at each intensity of staining were recorded on the following 4-point scale: 0 (no staining), 1 (weak staining, light yellow), 2 (moderate staining, yellowish brown), and 3 (strong staining, brown). Tumor tissues with an intensity score ≥2 in which ≥50% of malignant cells were stained positive for NDRG1 were classified as tumors with high expression (or overexpression), and tumor tissues with an intensity score <2 or of which <50% of malignant cells were stained positive for NDRG1 were classified as tumors with low expression [23].
For immunofluorescence staining of cultured cells, cells seeded on confocal dish were transfected with adenoviral vectors. 48 hours later, cells were fixed with 4% paraformaldehyde for 30 min and permeabilized with 0.5% Triton X-100 for 10 min at room temperature. The cells were then incubated with primary antibodies at 4°C overnight followed by washes with PBS and incubation with fluorescent secondary antibody in dark at room temperature for 1 h. After final washes with PBS, the confocal dish were mounted using an anti-fade mounting solution containing 4, 6-diamidino-2-phenylindole (DAPI). The staining was examined, and images were captured using an Olympus Confocal laser scanning microscopy FV1200.
In vitro cell growth assay
Cells were seeded in 96-well plates at 1×103/well and incubated for 1, 2, 3, 4 or 5 days. Cell proliferation was evaluated using Cell Counting Kit-8 (CCK-8, Dojindo, USA) according to manufacturer's instructions. Briefly, 10 µl of CCK-8 solution was added to culture medium, and incubated for 2 h. The absorbance at 450 nm wavelength was measured with a reference wavelength of 650 nm. All experiments were repeated for three times.
Colony formation assay
About 2×102 cells were added to each well in a 6-well culture plate, and each group contained three wells. After incubation at 37°C for two weeks, the cells were washed twice with PBS and stained with hematoxylin solution. The number of colonies containing ≥50 cells was counted under a microscope.
Scratch wound-healing assay
Cells were seeded in 24-well plates at a density of 1×105 cells/well and cultured in standard conditions until 80–90% confluence and treated with mitomycin C (10 µg/ml) during the wound healing assay. The cell migration was assessed by measuring the movement of cells into the acellular area created by a sterile insert. The wound closure was observed after 48 h.
Transwell in vitro migration assays
5–8F cells in serum-free medium (200 µl containing 1×105 cells) were added to the top chamber of Transwell chambers (Corning Star, Cambridge, Massachusetts, USA) of pore size 8 mm. The bottom chamber contained medium with 10% FBS as a chemoattractant. The cells were incubated at 37°C for 36 h to allow migration. Cells that had migrated through the membrane and attached to the lower surface of the membrane were stained using a fixative/staining solution containing 0.1% crystal violet, 1% formalin and 20% ethanol for visualization and quantified under a microscope. The experiments were repeated for three times.
Animals and in vivo tumor growth assay
Nude mice were maintained in a barrier facility in racks filtered with high-efficiency particulate air filter. The animals were fed with an autoclaved laboratory rodent diet. The mice in this study were purchased from the Experimental Animal Centre of Southern Medical University, which is certified by the Guangdong Provincial Bureau of Science, and the Permission number is SCXK2011-0015. All animal experiments involving ethical and humane treatment were under a license from the Guangdong Provincial Bureau of Science and all procedures were approved by the Institutional Animal Care and Use Committee of the Southern Medical University.
For in vivo tumor growth assay, 5–8F cells were harvested by trypsinization, washed twice with cold serum-free medium, and re-suspended with serum-free medium. To evaluate cancer growth in vivo, control or NDRG1 shRNA-transduced 5–8F cells (2×106) were subcutaneously injected into the left and right flank of 8 nude mice. The tumors were removed 22 days later to analyze the tumor growth by measuring the tumor sizes and observing the histology of the tumor tissues.
Human NPC tissues
Eighty-three paraffin-embedded human NPC samples were obtained from patients who were diagnosed with NPC between June 1996 and June 1999 in the Department of Pathology, Southern Medical University-affiliated Hospital. The histology diagnosis of the disease was determined according to the criteria of the World Health Organization [24]. Pathologic staging was determined according to the current International Union Against Cancer tumor-lymph node-metastasis classification [25]. The studies were approved by the University Ethical Committee of Southern Medical University.
Statistical analysis
All statistical analyses were performed using the SPSS 16.0 statistical software. The significance of correlation between the expression of NDRG1 and histopathological factors was determined using Pearson ×2 test. Survival curves were plotted by Kaplan–Meier method and compared by Log-rank test. Comparisons between groups were performed with a 2-tailed paired Student’s t test. P values < 0.05 was considered statistically significant.
Results
NDRG1 protein was differentially expressed in NPC 5–8F from 5–8F-LN cells
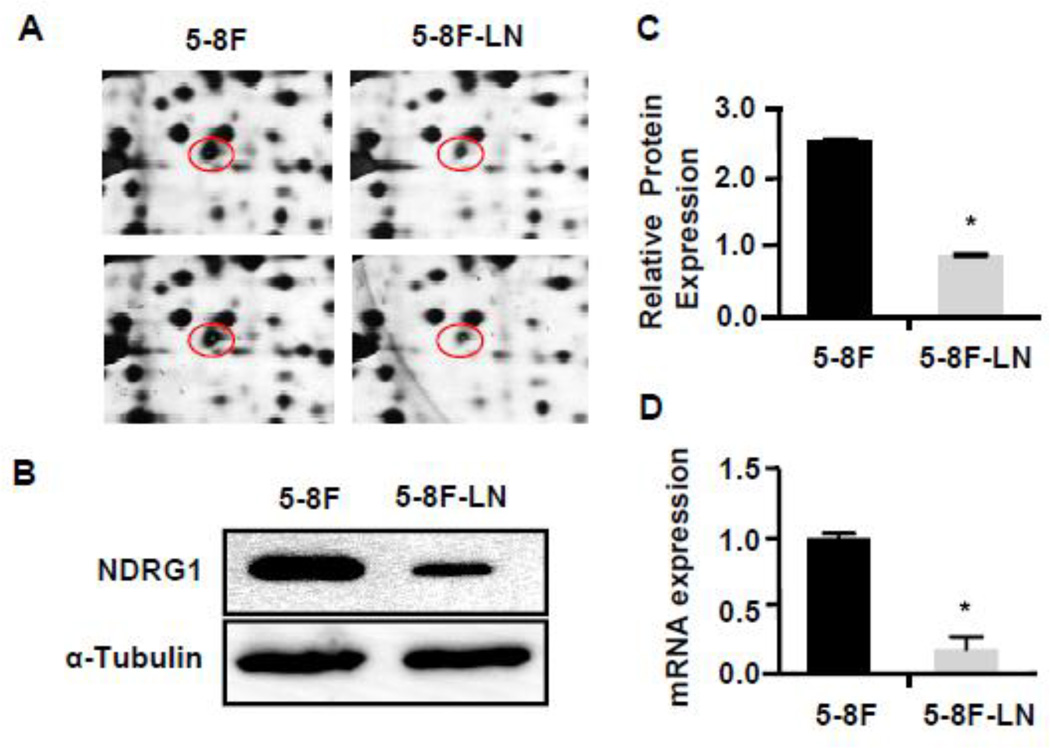
To identify proteins differentially expressed in NPC 5–8F and 5–8F-LN cells with stable lymphatic metastasis potential [19]. 2DE was performed, and differential protein spots were successfully identified by MALDI-TOF-TOF. We identified 42 different protein spots. Mass spectrum identification was performed in 11 differential protein spots (Supplemental Table S1). We found that NDRG1 protein was expressed very differently in 5–8F compared to 5–8F LN cells (Table S1 and Fig. 1A). The NDRG1 levels were much higher in 5–8F than in 5–8F-LN. To confirm its differential expression, we extracted the proteins from both 5–8F and 58F-LN and detected NDRG1 protein and mRNA levels in these two cells. As shown in Fig. 1B–1D, 5–8F expressed a much higher level of NDRG1 compared to 5–8F-LN.
Figure 1. NDRG1 was differentially expressed in NPC 5–8F and 5–8F-LN cells.
(A) Differential NDRG1 protein spots were identified in 2-D gel in 5–8F and 5–8F-LN cells. Two 2-D gel samples were shown. (B) The differential expression of NDRG1 protein in 5–8F and 5–8F-LN cells was confirmed by Western blot. (C) Quantification of NDRG1 protein levels shown in B by normalized to α-Tubulin. *P<0.001 compared to 5–8F cells, n=3. (D) NDRG1 mRNA expression in 5–8F and 5–8F-LN cells were detected by qRCR. *P<0.001 compared to 5–8F cells, n=3.
NDRG1 inhibited NPC 5–8F cell proliferation, migration, and invasion
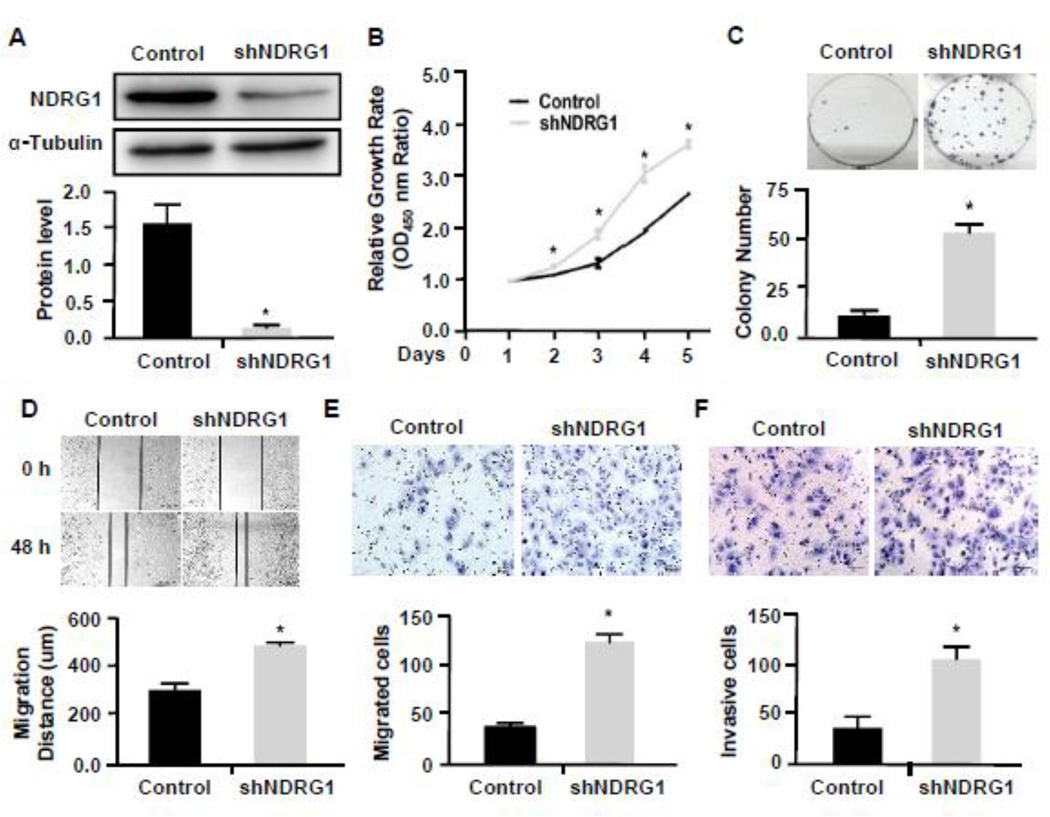
NDRG1 has been found to either promote or inhibit the proliferation of different cancer cells [10][13][26]. To investigate the potential role of NDRG1 in the development of NPC, we used shRNA to knock down NDRG1 in 5–8F cells (Fig. 2A) and performed CCK8 proliferation assay and colony formation assay to detect NPC proliferation. As shown in Fig. 2B–2C, knockdown of NDRG1 promoted NPC growth and colony formation. These data indicate that NDRG1 may play a role in inhibiting the growth of NPC tumor.
Figure 2. Knockdown of NDRG1 promoted the proliferation, migration and invasion of 5–8F cells.
(A) Western blot analysis of NDRG1 protein expression in cells treated with scramble (Control) or NDRG1 shRNA (shNDRG1). The protein levels were normalized to α-Tubulin. *P<0.001 compared to the control group, n=3. (B–C) Knockdown of NDRG1 increased cell proliferation as measured by CCK8 assay (B) and colony formation assay (C). Data were normalized against the OD450 value on day 1 of each treatment. *P<0.05 compared to the controls in each corresponding time point (B) or the control group. n=3. (D–E) Cell migration was measured by both the wound-healing assay (D) and transwell migration assay (E). Knockdown of NDRG1 markedly enhanced the 5–8F cell migration. *P<0.01 compared to scramble shRNA-treated group (Control) in each assay, n=3. (F) Knockdown of NDRG1 promoted 5–8F invasion. The invasive capability was determined using matrigel invasion chambers. *P<0.001 compared to the control group, n=3.
Since NDRG1 was expressed in a greater level in 5–8F than 5–8F-LN, and 5–8F-LN cells has a lymphatic metastasis potential, we speculated that the reduction of NDRG1 in 5–8F-LN may be involved in NPC metastasis. Thus, we tested if NDRG1 affects NPC migration and invasion by knocking down NDRG1 in 5–8F cells. As shown in Fig. 2D–2F, blockade of NDRG1 indeed increased NPC cell migration (Fig. 2D–2E) and invasion (Fig. 2F), suggesting that NDRG1 may play a role NPC metastasis.
Down-regulation of NDRG1 promoted the growth of 5–8F-derived NPC tumor in vivo
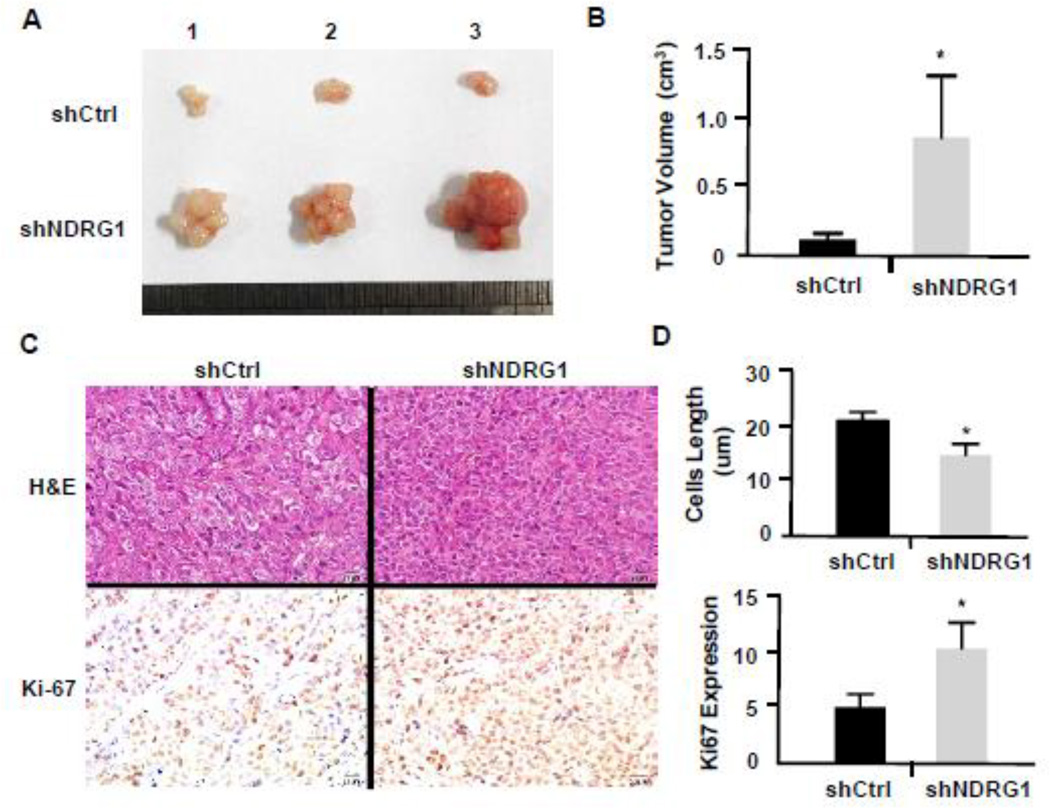
Since knockdown of NDRG1 promoted NPC 5–8F cell proliferation in vitro, we sought to determine if NDRG1 is important for NPC tumor growth in vivo. Thus, we subcutaneously injected 5–8F cells transduced with control or NDRG1 shRNA adenoviral vector into 16 nude mice (8 mice/group). Tumors were removed 22 days after the cell implantation. As shown in Fig. 3A, knockdown of NDRG1 inhibited the overall growth of tumors derived from 5–8F cells. Statistical analysis showed a significant difference in the mean tumor sizes between the control and NDRG1-blocked 5–8F-derived tumors (Fig. 3B). To test if NDRG1 indeed inhibited 5–8F cell growth in vivo, we detected the cell proliferation marker Ki-67 expression in the NPC tumors. As shown in Fig. 3C, knockdown of NDRG1 significantly increased the number of tumor cells expressing Ki-67, suggesting that NDRG1 attenuates NPC cell proliferation in vivo. In addition, we found that knockdown of NDRG1 changed the tumor cell morphology in vivo. The NDRG1-shRNA-transduced cells-derived tumor cells appeared to be smaller compared to the control tumor cells, suggesting that knockdown of NDRG1 may cause an alteration of tumor cell morphology (Fig. 3C, H&E staining and Fig. 3D, upper panel), consistent with the lymphatic metastasis potential of NDRG1-downregulated 5–8F-LN cells.
Figure 3. Knockdown of NDRG1 promoted tumor growth in vivo.
5–8F cells transduced with scramble (shCtrl) or NDGR shRNA (shNDGR1) were injected subcutaneously into nude mice. (A) Tumors were removed 22 days after injection and imaged. Shown was three representative samples. (B) Quantification of tumor sizes. Tumor derived from NDRG1-attenuated 5–8F grew significantly larger than that from the shCtrl-treated cells. *P<0.05 compared to the control group in each corresponding time point, n=8. (C) Representative photographs of H&E and Ki-67 immunohistochemistry staining of the primary growing cancer tissues. Most cancer cells with shNDGR1 treatment were smaller with an elongated morphology compared to the cells treated with shCtrl. The Ki-67 index in shNDRG1-treated cells was significantly higher than shCtrl-treated cells. (D) Quantification of cancer cell size and Ki-67 expression. *P<0.05 compared to the shCtrl-treated group, n=8.
NDRG1 expression reversely correlated with human NPC development, metastasis and patient survival rate
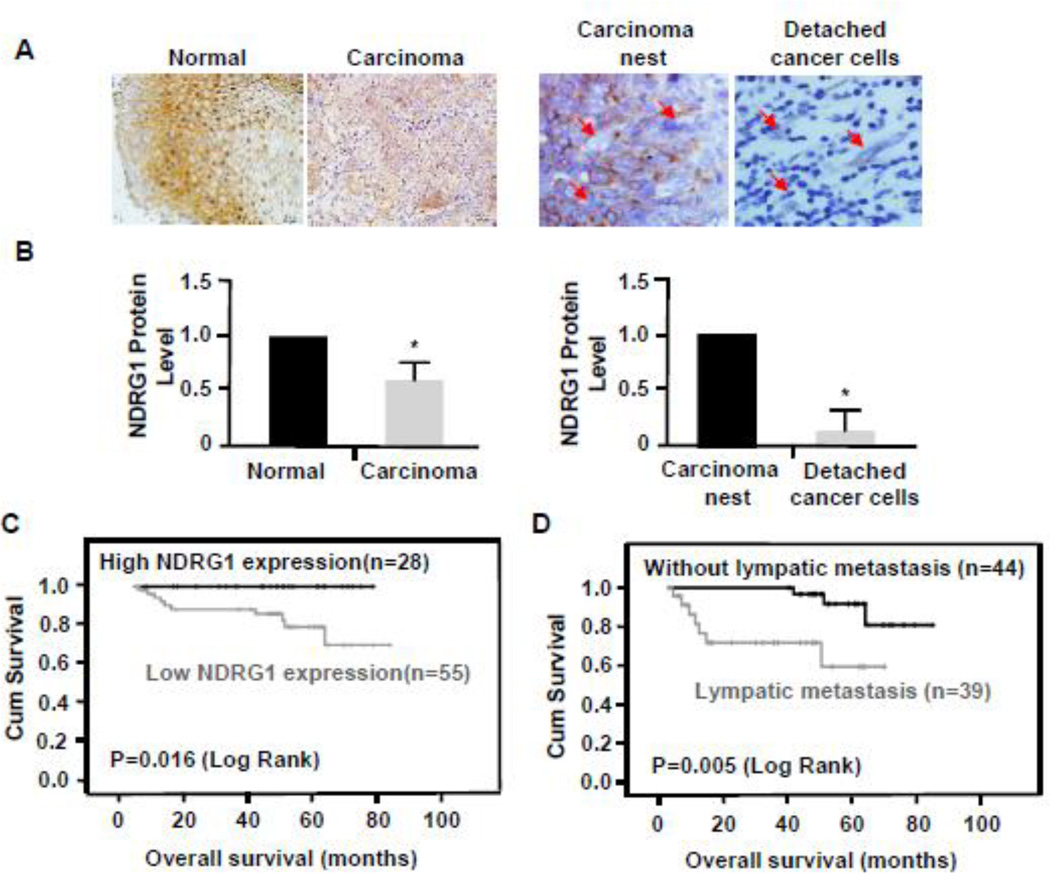
To explore if there is a correlation between NDRG1 level and NPC development in human, we analyzed 83 human patient NPC samples and found that 37 out of 83 paraffin-embedded NPC samples had normal squamous epithelium adjacent to NPC, which allowed us to compare the NDRG1 expression between the normal and NPC tissues. 35 of these samples exhibited a much higher NDRG1 expression level in normal tissues than the corresponding NPC tissues (Fig. 4A–4B, left panels), suggesting that NDRG1 level may be important for maintaining nasopharyngeal cell homeostasis.
Figure 4. NDRG1 expression correlated with the survival rates of patients with nasopharyngeal carcinoma.
(A) NDRG1 protein expression in normal or carcinoma tissue was detected by immunohistochemistry (IHC) staining using NDRG1 antibody. NDRG1 was highly expressed in normal squamous epithelium of nasopharynx but was markedly attenuated in poorly differentiated nasopharyngeal carcinoma. Low level of NDRG1 was observed in nasopharyngeal carcinoma nests, but the expression was not detected in detached cancer cells. Arrows indicate detached cancer cells. (Magnification: 400×). (B) Quantification of NDGR1 expression in normal and carcinoma tissues shown in A by measuring the IHC staining intensity and normalized to the expression in normal tissue or the carcinoma nest. *P<0.01 compared to normal tissue or carcinoma nest, n=35. (C) Overall survival rate of patients who had nasopharyngeal carcinoma with a low level of NDRG1 expression in tumors (n=55) was much lower compared to those who had a high level of NDRG1 expression (n=28). Kaplan-Meier curves with univariate analyses (log-rank) were shown. P=0.016. (D) Overall survival rate of nasopharyngeal carcinoma patients with lymphatic metastasis (n=39) was significantly lower than the patients without lympatic metastasis (n=44). P=0.005. P values were calculated by using log-rank tests.
Since knockdown of NDRG1 caused increased NPC migration and invasion in vitro, we examined the correlation of NDRG1 level with NPC metastasis. Morphometric analyses identified some sporadic detached cancer cells with an elongated morphology in the NPC carcinoma nest. These detached cells are likely to be the migrating cells causing metastasis. They expressed a lower level of NDRG1 compared to other cancer cells in the carcinoma nest (Fig. 4A–4B, right panels), suggesting that NDRG1 may play a role in hindering NPC metastasis. Moreover, by analyzing all the 83 human NPC samples, we found that the NDRG1 expression level was not associated with the patient's age and Dukes staging (Table 1). However, comparing with those who had no lymph node metastasis of NPC, patients with NPC lymphatic metastasis had significantly lower expression of NDRG1 in NPC tissues. The NDRG1 level appeared to be strongly correlated with the lymphatic metastasis (P<0.001) (Table 1).
Table 1.
Expression of NDRG1 in patients with nasopharyngeal carcinoma.
| Characteristic | NDRG1 Expression: No. of Patients (%) | P | ||
|---|---|---|---|---|
| High | Low | |||
| Age (y) | ≥55 | 8 (28.6) | 20 (71.4) | 0.385 |
| <55 | 19 (34.5) | 36 (65.5) | ||
| Dukes | 1 and 2 | 14 (38.9) | 22 (61.1) | 0.262 |
| 3 and 4 | 14 (29.8) | 33 (70.2) | ||
| Lymphatic metastasis | Yes | 5 (12.8) | 34 (87.2) | 0.001 |
| No | 23 (52.3) | 21 (47.7) | ||
| Distant metastasis | Yes | 0 (0) | 9 (100) | 0.027 |
| No | 26 (34.1) | 48 (64.9) | ||
To test if the altered NDRG1 level in NPC is related to patient’s prognosis, we performed Kaplan-Meier survival analyses to explore the correlation of NDRG1 expression with patient survival rate and length of survival times. We found that patients with low NDRG1 expression level had a shorter survival time compared to those with high NDRG1 expression level (P=0.016, Fig. 4C). Moreover, patients with NPC lymph node metastasis and lower NDRG1 expression levels had a shorter survival time than those without lymph node metastasis but with higher NDRG1 expression levels (P=0.005, Fig. 4D). These data suggest that the down-regulation of NDRG1 is closely associated with the NPC growth and metastasis as well as the patient’s survival.
Blockade of NDRG1 initiated an epithelial-to-mesenchymal transition (EMT) and enhanced TGF-β signaling in NPC cells
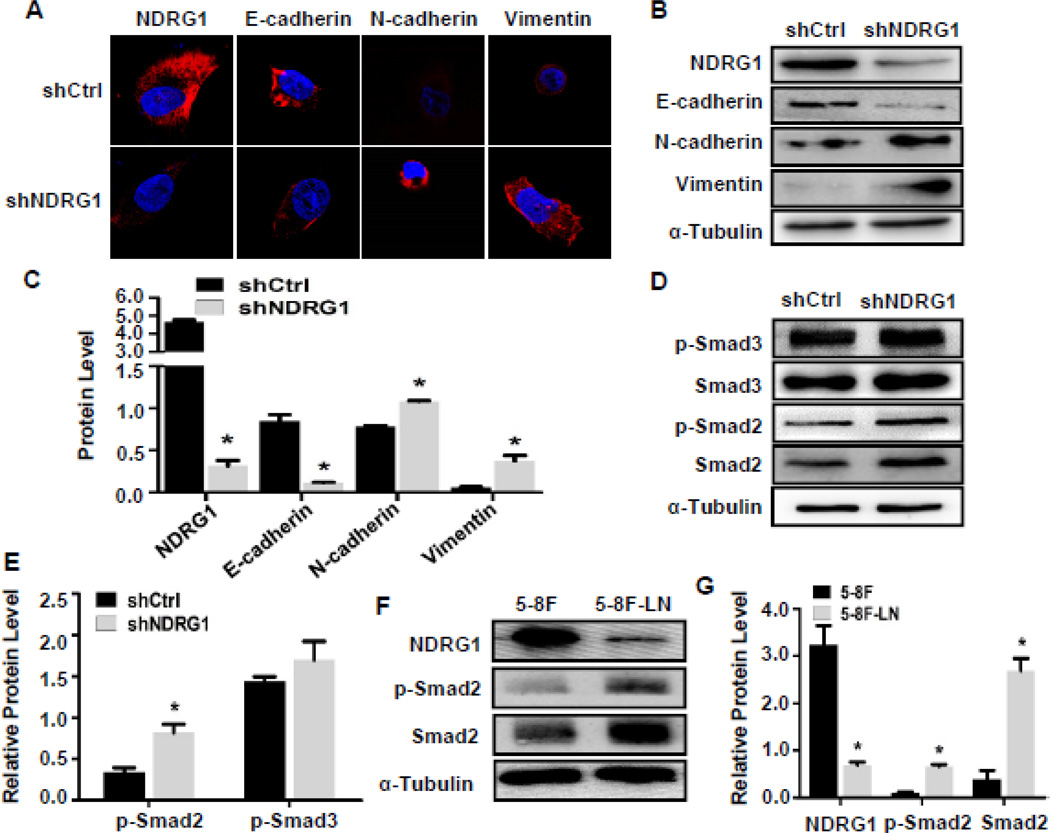
Since knockdown of NDRG1 altered NPC cell morphology both in vivo and in vitro (Fig. 3C–3D and Supplemental Fig. S1), and low levels of NDRG1 correlated with NPC metastasis in human (Fig. 4D and Table 1), we hypothesized that down-regulation of NDRG1 may be involved in the EMT of NPC cells, a process implicated in metastasis of a large number of tumors [27–29]. The key characteristic of EMT are the reduction of membrane E-cadherin along with an increased expression of neuronal cadherin (N-cadherin) and other mesenchymal markers such as vimentin [30–32]. We found that knockdown of NDRG1 in 5–8F cells significantly decreased the expression of E-cadherin while increased N-cadherin and vimentin protein expression (Fig. 5A–5C), indicating that down-regulation of NDRG1 induced an EMT of NPC.
Figure 5. Knockdown of NDRG1 induced epithelial-to-mesenchymal transition (EMT) and enhanced Smad2 signaling in 5–8F cells.
(A) Immunostaining of NDRG1 and EMT markers. Magnification: 1800×. (B) Western blotting analysis of NDRG1 and EMT marker expression. NDRG1 knock-down increased N-cadherin and vimentin expression and decreased E-cadherin expression. (C) Quantification of protein expression shown in B by normalized to α-Tubulin. *P<0.05 compared to the shCtrl group for each individual protein, n=3. (D–G) Smad2 signaling was activated in NDRG1-blocked NPC 5–8F and 5–8F-LN cells. (D) Western blotting analysis of Smad2 and Smad3 expression and phosphorylation. NDRG1 knock-down increased Smad2 signaling. (E) Smad2 or Smad3 phosphorylation was normalized to total protein levels, which was normalized to α-Tubulin. *P<0.05 compared to control shRNA (shCtrl)-treated group, n=3. (F) Smad2 expression and phosphorylation in 5–8F and 5–8F-LN cells were detected by western blot. (G) Smad2 expression and phosphorylation were significantly higher in 5–8F-LN cells compared to 5–8F cells. Smad2 total levels were quantified by normalized to α-Tubulin. Smad2 phosphorylation was normalized to Smad2 level. *P<0.01 compared to 5–8F cells, n=3.
It is known that TGF-β signaling plays a very important role in EMT [33]. Thus, we tested if knockdown of NDRG1 affects Smad2 or Smad3 expression and activation. As shown in Fig. 5D, blockade of NDRG1 expression in 5–8F cells increased Smad2 expression and its phosphorylation. Knockdown of NDRG1 slightly increased Smad3 expression but not the Smad3 phosphorylation (Fig. 5D–5E), suggesting that NDRG1 may regulate NPC EMT by modulating Smad2 signaling. Since 5–8F-LN cells had a much lower level of NDRG1 compared to 5–8F cells (Fig. 1B–1D), we compared the Smad2 signaling in these two cells. As shown in Fig. 5F–5G, 5–8F-LN expressed a much higher level of Smad2 and exhibited a stronger Smad2 activation as compared to that in 5–8F cells, consistent with the effect of NDRG1 knockdown on Smad2 phosphorylation in 5–8F cells (Fig. 5D). Interestingly, a higher level of TGF-β1 expression was also observed in 5–8F-LN as compared to 5–8F (Fig. S2A), suggesting that the higher p-Smad2 level in 5–8F-LN cells may be due to a presence of autocrine TGF-β loop. Importantly, TGF-β1 stimulation attenuated NDGR1 expression while inducing the EMT of 5–8F cells as shown by the decreased E-cadherin and increased N-cadherin and vimentin expression (Fig. S2, B–C), further indicating that NDRG1 downregulation-induced EMT of NPC cells may be mediated by the TGF-β-activated Smad2 signaling.
Smad2 signaling mediated NDRG1 knockdown-induced EMT of NPC
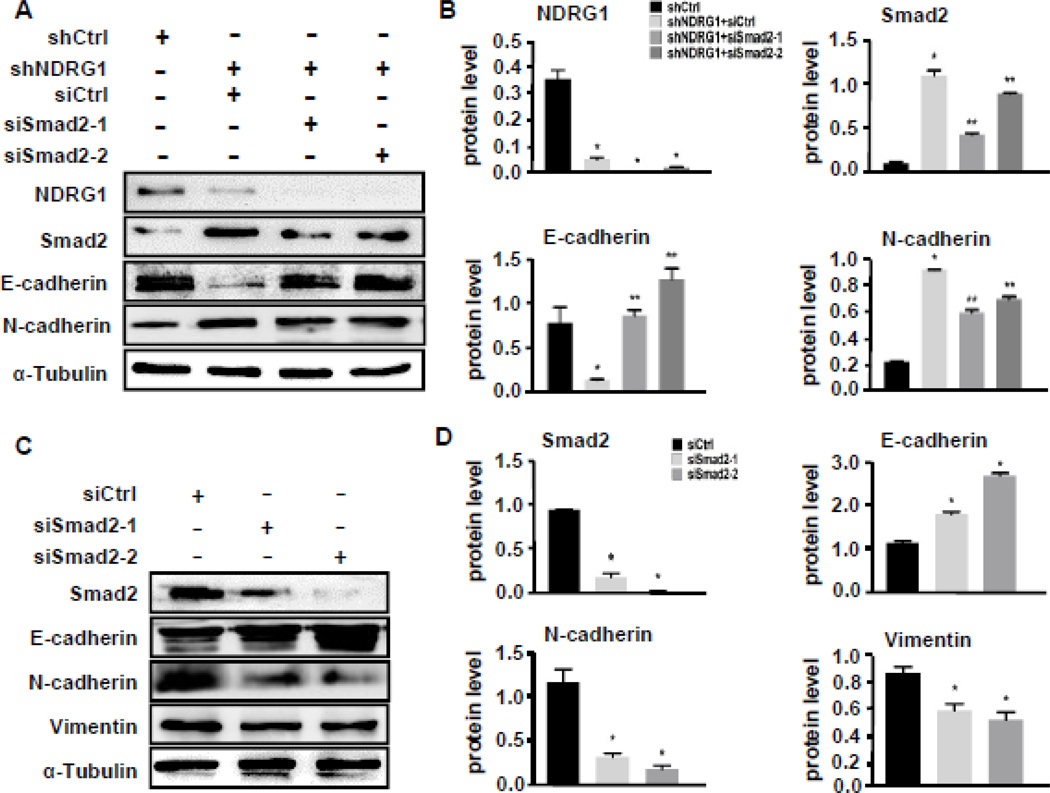
To determine if NDRG1 knockdown-induced EMT of NPC cells indeed via Smad2 signaling, we blocked Smad2 and Smad3 expression in NDRG1-deficient 5–8F cells using two different siRNAs against Smad2 and Smad3, respectively. We found that blockade of Smad2 expression increased E-cadherin expression, while decreasing N-cadherin expression (Fig. 6A–6B), indicating a blockade of the EMT. However, blockade of Smad3 expression had no significant effect on E-cadherin and N-cadherin expression (Supplemental Fig. S3, A–B). To further confirm the role of Smad2 in NPC EMT, we also knocked down Smad2 and Smad3 expression in 5–8F-LN cells. Consistent with the effect in NDRG1-deficient 5–8F cells, blockade of Smad2 enhanced E-cadherin expression while inhibited N-cadherin and vimentin expression (Fig. 6C–6D). On the contrary, blockade of Smad3 did not show significant effect on E-cadherin, N-cadherin, or vimentin expression (Supplemental Fig. S3, C–D). These data demonstrated that Smad2, but not Smad3, played an essential role in NDRG1 deficiency-induced EMT of NPC.
Figure 6. Smad2 signaling was essential for NDRG1 knockdown-induced EMT of NPC 5–8F cells.
(A) Knockdown of Smad2 in NDRG1-deficient 5–8Fcells restored E-Cadherin expression while decreasing N-cadherin expression. (B) Quantification of protein levels shown in A by normalized to α-Tubulin. *P<0.05 compared to NDRG1 control shRNA (shCtrl)-treated groups; **P<0.05 compared to Smad2 control siRNA (siCtrl)-treated groups for each individual protein, n=3. (C) Knockdown of Smad2 in 5–8F-LN cells increased E-Cadherin while decreasing N-cadherin and vimentin expression. (D) Quantification of protein levels shown in C by normalized to α-Tubulin. *P<0.05 compared to Smad2 control siRNA (siCtrl)-treated groups for each individual protein, n=3.
Knockdown of NDRG1 induced EMT of NPC in vivo
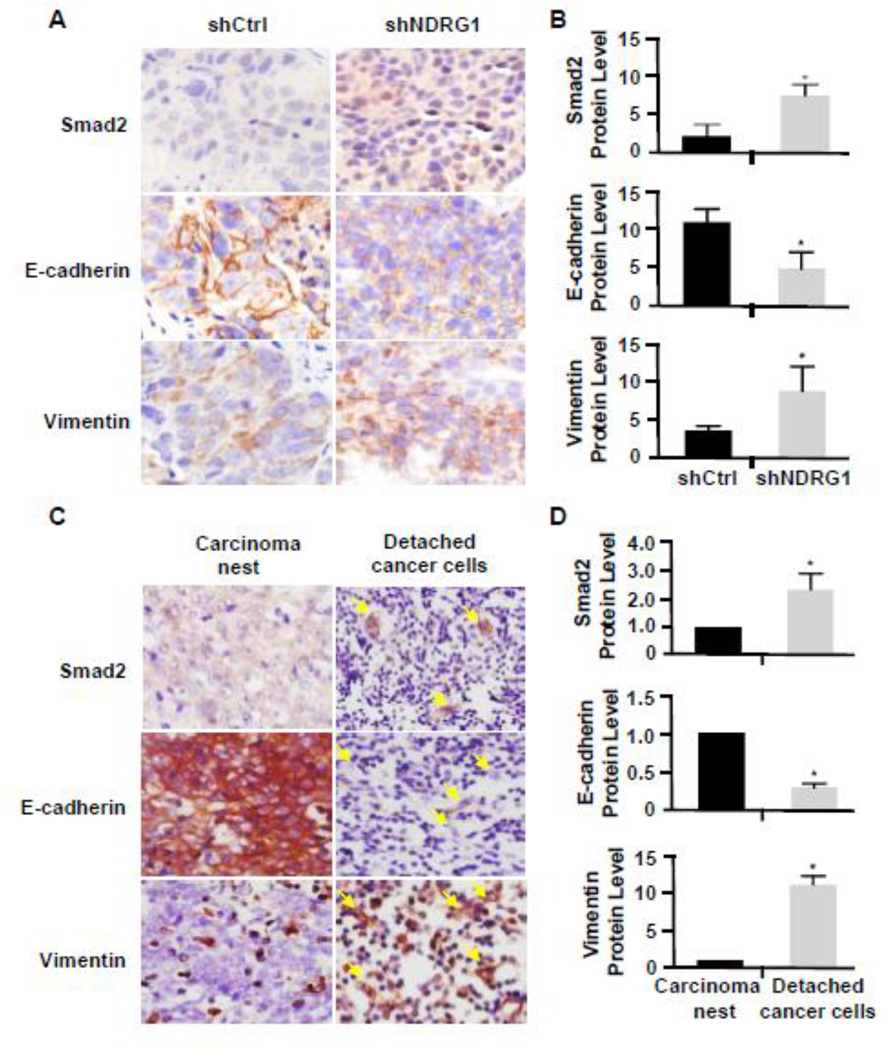
Since we observed a cell morphology alteration in tumor cells derived from NDRG1 deficient 5–8F cells, we speculated that there was an EMT in these cells. Indeed, these cells exhibited a reduction of E-cadherin expression with an increase of vimentin expression (Fig. 7A–7B). Importantly, Smad2 expression was also increased (Fig. 7A–7B), consistent with its role in the EMT in vitro. Moreover, we also observed a high level expression of Smad2 and vimentin with an attenuation of E-cadherin in the detached cancer cells in human NPC tissues (Fig. 7C–7D). These data indicated that Smad2 indeed mediated the EMT of NPC in mice or human patients, which contributed, at least partially, to the NPC metastasis.
Figure 7. Smad2 expression was correlated with the EMT of shNDRG1-deficient 5–8F cells and human NPC in vivo.
(A) Smad2 and EMT-related protein expression in shNDRG1-deficient 5–8F cell-derived tumor. IHC staining was performed to detect Smad2, E-Cadherin, and vimentin as indicated. (B) Quantification of protein expression levels shown in A, *P<0.05 compared to the shCtrl-treated group, n=8. (C) Smad2, E-Cadherin, and vimentin expression in human NPC undergoing metastasis. Arrow indicates the detached cancer cells. (D) Quantification of protein expression levels in nasopharyngeal carcinoma nests and detached cancer cells shown in C by measuring the IHC staining intensity and normalized to the expression in the carcinoma nest. *P<0.05 compared to carcinoma nest group for each corresponding protein, n=12.
Discussion
NDRG1 is involved in cellular differentiation, cell cycle arrest, and apoptosis, and regulates tumor growth and metastasis in various carcinomas [34–39]. Interestingly, depending on the types of tumors NDRG1 can be a metastasis suppressor or a facilitator [11–14][40,41]. So it is important to determine its role in NPC. NDRG1 is strongly expressed in normal nasopharyngeal tissue but significantly reduced in NPC tumors (Fig. 4A–4B, left panels). Functionally, NDRG1 appears to be important for maintaining nasopharyngeal cell homeostasis because reduction of NDRG1 level caused an increased NPC proliferation in vitro (Fig. 2B–2C) and NPC tumor growth in vivo (Fig. 3A–3B). Importantly, NDRG1 reduction is correlated with lymphatic metastasis potential, which is supported by the reduced expression of NDRG1 in 5–8F-LN cells versus 5–8F cells (Fig. 1A–1D), the lower level of NDRG1 in detached human NPC cells comparing to the stable carcinoma cells (Fig. 4A–4B, right panels), and the low expression in human NPC with lymphatic metastasis (Fig. 4D). Most importantly, NDRG1 level is reversely associated with NPC patient prognosis or survival (Fig. 4C), consistent with previous reports on other carcinomas showing that NDRG1 expression has a significant inverse correlation with tumor stromal invasion and lymphatic metastasis [42,43]. Therefore, NDRG1 may serve as a valuable biomarker to monitor NPC development in humans.
Interestingly, instead of its suppression role in NPC and several other carcinoma, NDRG1 promotes the development or metastasis of lung cancer [10], colon cancer [12], and hepatocellular carcinomas [17]. One possible explanation for these opposite observations is that NDRG1 may use different signaling pathways in different tumor cells to regulate the tumor cell growth, migration and invasive ability. It is known that NDRG1 can interact with several different oncogenic signaling pathways such as PI3K/AKT, nuclear factor-kappaB (NF-kB), mammalian target of rapamycin (mTOR), and MAPK signaling pathways [44]. Future investigation is required to identify signaling pathways by which NDRG1 regulates the proliferation, migration and invasion of NPC cells.
Different mechanisms have been implicated in tumor metastasis. Our data provide a link between NDRG1 and EMT of NPC, which is likely to be one of the mechanisms regulating NPC metastasis. Knockdown of NDRG1 blocks E-cadherin expression while increasing vimentin expression both in vitro (Fig. 5A–5C) and in vivo (Fig. 7A–7B). The EMT is also observed in human NPC tissues with lymph node metastasis (Fig. 7C–7D). These observations support that NDRG1 is a metastasis suppressor rather than a metastasis promoter for NPC. EMT can be mediated by many different proteins, but TGF-β-related factors have emerged as major inducers of the transdifferentiation process in development and cancer. Among these factors, Smad3 is considered to be the primary signaling molecule in mediating EMT of many tumors [45–47]. However, EMT of several cancer cells has been shown to depend on Smad2 signaling [48,49]. Indeed, expression of activated Smad2 promotes spindle tumor cell invasion, and a dominant negative form of Smad2 inhibits it, suggesting that Smad2 may promote EMT in vivo [48]. NDRG1 downregulation-induced EMT of NPC cells is primarily mediated by Smad2 (Fig. 5D–5G and Fig. 6A–6D), but not Smad3 signaling (Supplemental Fig. S3, A–D). The possible explanation is that NDRG1 down-regulation causes an increased Smad2 expression and Smad2 phosphorylation without impacting Smad3 expression/phosphorylation. The activated Smad2 may induce the expression of downstream EMT regulators, leading to EMT of NPC cells. Smad2 may also interact with other signaling pathways to control the gene reprogramming during EMT of NPC cells because it is increasingly apparent that signaling pathways cooperate in the execution of EMT [33], which will be an interesting subject for future study. The NDRG1-Smad2 pathway is likely to be dependent of TGF-β because TGF-β induces EMT of NPC cells while inhibiting NDRG1 expression (Supplemental Fig. S2, B–C), and TGF-β expression is increased in the NPC cells with lymph node metastasis potential (Supplemental Fig. S2, A).
The role of NDRG1 in EMT of cancer cells is also supported by other studies [30]. It has been shown that TGF-β induces the EMT of prostate and colon cancer cells in vitro via down-regulation of NDRG1 although NDRG1 appears to affect both Smad2 and Smad3 signaling in these cells [30]. Importantly, since Iron chelators Dp44mT and DFO are found to inhibit TGF-β-induced EMT of prostate and colon cancer cells via increasing NDRG1 expression [30], these chelators may also inhibit the EMT of NPC cells via altering NDRG1 expression and thus may be used as potential therapeutic agents for treating human patients. Extensive preclinical and clinical studies are required to test the efficacy and side effects of Dp44mT and DFO because several iron chelators are known to generate cytotoxic radicals in addition to its antitumor activities [50]. In view of the unique location of NPC, a local application of these chelators may avoid a systematic cytotoxicity.
In summary, we have identified NDRG1 as a novel protein factor that inhibits the growth and metastasis of NPC. NDRG1 appears to prevent NPC EMT by attenuating Smad2 signaling. Moreover, since down-regulation of NDRG1 is correlated with the lymphatic metastasis of NPC and poor patient survival/prognosis, NDRG1 may be used as a biomarker for surveillance of NPC progression and therapeutic target in human patients.
Supplementary Material
Highlights.
NDRG1 is down-regulated in NPC cells with lymphatic metastasis potential.
NDRG1 inhibits NPC cell proliferation in vitro and tumorigenesis in mice.
NDRG1 deficiency induces epithelial-mesenchymal transition (EMT) of NPC cells.
NDRG1 deficiency causes the EMT of NPC via activating Smad2 signaling.
NDRG1 inversely correlates with NPC metastasis and survival rate of human patients.
Acknowledgments
The authors are grateful to Professor Yanqin Ding and the researchers at the Key Laboratory of Molecular Tumor Pathology, located in Guangdong, China for providing assistance in our experiments. We thank Ms Wei Shuang for assistance with paraffin-embedded NPC samples and Dr. Tengfei Liu for providing 5–8F and 5–8F-LN cell lines.
Funding This work was supported by the National Natural Science Foundation of China (NNSF81172054, NNSF81370227, NNSF81201664), Pearl River Science & Technology New Star Foundation of GuangZhou City (Grant No. 2012J2200044), and National Institutes of Health (HL123302, HL119053, HL107526).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: None declared.
References
- 1.Abbasi AN, Zahid S, Bhurgri Y, Ali N, Karsan F. Nasopharyngeal carcinomas: an update of Treatment and Acute Radiation Induced Reactions from a Tertiary-Care Hospital in Pakistan. Asian Pac J Cancer Prev. 2011;12(3):735–738. [PubMed] [Google Scholar]
- 2.Ma LJ, Lee SW, Lin LC, Chen TJ, Chang IW, Hsu HP, Chang KY, Huang HY, Li CF. Fibronectin overexpression is associated with latent membrane protein 1 expression and has independent prognostic value for nasopharyngeal carcinoma. Tumour Biol. 2014;35(2):1703–1712. doi: 10.1007/s13277-013-1235-8. [DOI] [PubMed] [Google Scholar]
- 3.Song LB, Liao WT, Mai HQ, Zhang HZ, Zhang L, Li MZ, Hou JH, Fu LW, Huang WL, Zeng YX, Zeng MS. The clinical significance of twist expression in nasopharyngeal carcinoma. Cancer Lett. 2006;242(2):258–265. doi: 10.1016/j.canlet.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 4.Xia WX, Ye YF, Lu X, Wang L, Ke LR, Zhang HB, Roycik MD, Yang J, Shi JL, Cao KJ, Guo X, Xiang YQ. The Impact of Baseline Serum C-Reactive Protein and C-Reactive Protein Kinetics on the Prognosis of Metastatic Nasopharyngeal Carcinoma Patients Treated with Palliative Chemotherapy. PLoS One. 2013;8(10):e76958. doi: 10.1371/journal.pone.0076958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kovacevic Z1, Richardson DR. The metastasis suppressor, Ndrg-1: a new ally in the fight against cancer. Carcinogenesis. 2006;27(12):2355–2366. doi: 10.1093/carcin/bgl146. [DOI] [PubMed] [Google Scholar]
- 6.Kurdistani SK, Arizti P, Reimer CL, Sugrue MM, Aaronson SA, Lee SW. Inhibition of tumor cell growth by RTP/rit42 and its responsiveness to p53 and DNA Damage. Cancer Res. 1998;58(19):4439–4444. [PubMed] [Google Scholar]
- 7.Zhou D, Salnikow K, Costa M. Cap43, a novel gene specifically induced by Ni2+ compounds. Cancer Res. 1998;58(10):2182–2189. [PubMed] [Google Scholar]
- 8.Murakami Y, Hosoi F, Izumi H, Maruyama Y, Ureshino H, Watari K, Kohno K, Kuwano M, Ono M. Identification of sites subjected to serine/threonine phosphorylation by SGK1 affecting N-myc downstream-regulated gene 1 (NDRG1)/Cap43-dependent suppression of angiogenic CXC chemokine expression in human pancreatic cancer cells. Biochem Biophys Res Commun. 2010;396(2):376–381. doi: 10.1016/j.bbrc.2010.04.100. [DOI] [PubMed] [Google Scholar]
- 9.Han LL, Hou L, Zhou MJ, Ma ZL, Lin DL, Wu L, Ge YL. Aberrant NDRG1 methylation associated with its decreased expression and clinicopathological significance in breast cancer. J Biomed Sci. 2013;20(0):52. doi: 10.1186/1423-0127-20-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Q, Li LH, Gao GD, Wang G, Qu L, Li JG, Wang CM. HIF-1a up-regulates NDRG1 expression through binding to NDRG1 promoter, leading to proliferation of lung cancer A549 cells. Mol Biol Rep. 2013;40(5):3723–3729. doi: 10.1007/s11033-012-2448-4. [DOI] [PubMed] [Google Scholar]
- 11.Song Y, Lv L, Du J, Yue L, Cao L. Correlation of N-myc downstream-regulated gene 1 subcellular localization and lymph node metastases of colorectal neoplasms. Biochem Biophys Res Commun. 2013;439(2):241–246. doi: 10.1016/j.bbrc.2013.08.049. [DOI] [PubMed] [Google Scholar]
- 12.Mao Z, Sun J, Feng B, Ma J, Zang L, Dong F, Zhang D, Zheng M. The metastasis suppressor, N-myc downregulated gene 1 (NDRG1), is a prognostic biomarker for human colorectal cancer. PLoS One. 2013;8(7):e68206. doi: 10.1371/journal.pone.0068206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang X, Xu X, Ma J, Xue X, Li Z, Deng P, Zhang S, Zhi Y, Chen J, Dai D. NDRG1 expression is related to the progression and prognosis of gastric cancer patients through modulating proliferation, invasion and cell cycle of gastric cancer cells. Mol Biol Rep. 2014;41(9):6215–6223. doi: 10.1007/s11033-014-3501-2. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Cai J, Li Z, Hu S, Yu L, Xiao L, Wang Z. Expression and biological function of N-myc down-regulated gene 1 in human cervical cancer. J Huazhong Univ Sci Technolog Med Sci. 2010;30(6):771–776. doi: 10.1007/s11596-010-0656-5. [DOI] [PubMed] [Google Scholar]
- 15.Wang B, Li J, Ye Z, Li Z, Wu X. N-myc downstream regulated gene 1 acts as a tumor suppressor in ovarian cancer. Oncol Rep. 2014;31(5):2279–2285. doi: 10.3892/or.2014.3072. [DOI] [PubMed] [Google Scholar]
- 16.Jin R, Liu W, Menezes S, Yue F, Zheng M, Kovacevic Z, Richardson DR. The metastasis suppressor NDRG1 modulates the phosphorylation and nuclear translocation of β-catenin through mechanisms involving FRAT1 and PAK4. J Cell Sci. 2014;127(Pt 14):3116–3130. doi: 10.1242/jcs.147835. [DOI] [PubMed] [Google Scholar]
- 17.Akiba J, Ogasawara S, Kawahara A, Nishida N, Sanada S, Moriya F, Kuwano M, Nakashima O, Yano H. N-myc downstream regulated gene 1 (NDRG1)/Cap43 enhances portal vein invasion and intrahepatic metastasis in human hepatocellular carcinoma. Oncol Rep. 2008;20(6):1329–1335. [PubMed] [Google Scholar]
- 18.Chua MS, Sun H, Cheung ST, Mason V, Higgins J, Ross DT, Fan ST, So S. Overexpression of NDRG1 is an indicator of poor prognosis in hepatocellular carcinoma. Mod Pathoi. 2007;20(1):76–83. doi: 10.1038/modpathol.3800711. [DOI] [PubMed] [Google Scholar]
- 19.Leng L, Liu TF, Huang ZX, Xie WB, Yao KT. Establishment of a nude mouse model of nasopharyngeal carcinoma lymph node metastasis and screening of the metastasis-related signature genes. Nan Fang Yi Ke Da Xue Xue Bao. 2008;28(9):1519–1522. [PubMed] [Google Scholar]
- 20.Wang JN, Shi N, Xie WB, Guo X, Chen SY. Response gene to complement 32 promotes vascular lesion formation through stimulation of smooth muscle cell proliferation and migration. Arterioscler Thromb Vasc Biol. 2011;31(8):e19–e26. doi: 10.1161/ATVBAHA.111.230706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(Delta Delta C(T)) method. Methods. 2001;25(0):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Liao WT, Wang X, Xu LH, Kong QL, Yu CP, Li MZ, Shi L, Zeng MS, Song LB. Centromere Protein H Is a Novel Prognostic Marker for Human Nonsmall Cell Lung Cancer Progression and Overall Patient Survival. Cancer. 2009;115(7):1507–1517. doi: 10.1002/cncr.24128. [DOI] [PubMed] [Google Scholar]
- 23.Zhao L, Wang H, Liu C, Liu Y, Wang X, Wang S, Sun X, Li J, Deng Y, Jiang Y, Ding Y. Promotion of colorectal cancer growth and metastasis by the LIM and SH3 domain protein. Gut. 2010;59(9):1226–1235. doi: 10.1136/gut.2009.202739. [DOI] [PubMed] [Google Scholar]
- 24.Jacques J, Hill DP, Shier KJ, Jindani A, Miller AB. Appraisal of the World Health Organization classification of lung tumours. Can Med Assoc J. 1980;122(8):897–901. [PMC free article] [PubMed] [Google Scholar]
- 25.Mountain CF. Revisions in the international system for staging lung cancer. Chest. 1997;111(6):1710–1717. doi: 10.1378/chest.111.6.1710. [DOI] [PubMed] [Google Scholar]
- 26.Lee JC, Chung LC, Chen YJ, Feng TH, Juang HH. N-myc downstream-regulated gene 1 downregulates cell proliferation, invasiveness, and tumorigenesis in human oral squamous cell carcinoma. Cancer Lett. 2014;355(2):242–252. doi: 10.1016/j.canlet.2014.08.035. [DOI] [PubMed] [Google Scholar]
- 27.Montemayor-Garcia C, Hardin H, Guo Z, Larrain C, Buehler D, Asioli S, Chen H, Lloyd RV. The role of epithelial mesenchymal transition markers in thyroid carcinoma progression. Endocr Pathol. 2013;24(4):206–212. doi: 10.1007/s12022-013-9272-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watanabe T, Takahashi A, Suzuki K, Kurusu-Kanno M, Yamaguchi K, Fujiki H, Suganuma M. Epithelial-mesenchymal transition in human gastric cancer cell lines induced by TNF-α-inducing protein of Helicobacter pylori. Int J Cancer. 2014;134(10):2373–2382. doi: 10.1002/ijc.28582. [DOI] [PubMed] [Google Scholar]
- 29.Hugo H, Ackland ML. Epithelial-mesenchymal and mesenchymal-epithelial transitions in carcinoma progression. J Cell Physiol. 2007;213(2):374–383. doi: 10.1002/jcp.21223. [DOI] [PubMed] [Google Scholar]
- 30.Chen Z, Zhang D, Yue F, Zheng M, Kovacevic Z, Richardson DR. The iron chelators Dp44mT and DFO inhibit TGF-β-induced epithelial-mesenchymal transition via up-regulation of N-Myc downstream-regulated gene 1 (NDRG1) J Biol Chem. 2012;287(21):17016–17028. doi: 10.1074/jbc.M112.350470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hartsock A, Nelson WJ. Adherens and tight junctions:structure, function, and connections to the actin cytoskeleton. Biochim Biophys Acta. 2008;1778(3):660–669. doi: 10.1016/j.bbamem.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu L, Jiang Y, Zheng J, Xie G, Li J, Shi L, Fan S. Aberrant expression of β-catenin and E-cadherin is correlated with poor prognosis of nasopharyngeal cancer. Hum Pathol. 2013;44(7):1357–1364. doi: 10.1016/j.humpath.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 33.Derynck R, Muthusamy BP, Saeteurn KY. Signaling pathway cooperation in TGF-β-induced epithelial-mesenchymal transition. Curr Opin Cell Biol. 2014;31(0):56–66. doi: 10.1016/j.ceb.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao G, Chen J, Deng Y, Gao F, Zhu J, Feng Z, Lv X, Zhao Z. Identification of NDRG1-regulated genes associated with invasive potential in cervical and ovarian cancer cells. Biochem Biophys Res Commun. 2011;408(1):154–159. doi: 10.1016/j.bbrc.2011.03.140. [DOI] [PubMed] [Google Scholar]
- 35.Lai LC, Su YY, Chen KC, Tsai MH, Sher YP, Lu TP, Lee CY, Chuang EY. Down-Regulation of NDRG1 Promotes Migration of Cancer Cells during Reoxygenation. PLoS One. 2011;6(8):e24375. doi: 10.1371/journal.pone.0024375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hickok JR, Sahni S, Mikhed Y, Bonini MG, Thomas DD. Nitric Oxide Suppresses Tumor Cell Migration through N-Myc Downstream-regulated Gene-1 (NDRG1) Expression. J Biol Chem. 2011;286(48):41413–41424. doi: 10.1074/jbc.M111.287052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akiba J, Murakami Y, Noda M, Watari K, Ogasawara S, Yoshida T, Kawahara A, Sanada S, Yasumoto M, Yamaguchi R, Kage M, Kuwano M, Ono M, Yano H. N-myc downstream regulated gene1/Cap43 overexpression suppresses tumor growth by hepatic cancer cells through cell cycle arrest at the G0/G1 phase. Cancer Lett. 310(1):25–34. doi: 10.1016/j.canlet.2011.05.034. (201) [DOI] [PubMed] [Google Scholar]
- 38.Hosoi F, Izumi H, Kawahara A, Murakami Y, Kinoshita H, Kage M, Nishio K, Kohno K, Kuwano M, Ono M. N-myc downstream regulated gene 1/Cap43 suppresses tumor growth and angiogenesis of pancreatic cancer through attenuation of inhibitor of kappaB kinase beta expression. Cancer Res. 2009;69(12):4983–4991. doi: 10.1158/0008-5472.CAN-08-4882. [DOI] [PubMed] [Google Scholar]
- 39.Maruyama Y, Ono M, Kawahara A, Yokoyama T, Basaki Y, Kage M, Aoyagi S, Kinoshita H, Kuwano M. Tumor growth suppression in pancreatic cancer by a putative metastasis suppressor gene Cap43/NDRG1/Drg-1 through modulation of angiogenesis. Cancer Res. 2006;66(12):6233–6242. doi: 10.1158/0008-5472.CAN-06-0183. [DOI] [PubMed] [Google Scholar]
- 40.Ureshino H, Murakami Y, Watari K, Izumi H, Kawahara A, Kage M, Arao T, Nishio K, Yanagihara K, Kinoshita H, Kuwano M, Ono M. N-myc Downstream Regulated Gene 1 (NDRG1) Promotes Metastasis of Human Scirrhous Gastric Cancer Cells through Epithelial Mesenchymal Transition. PLoS One. 2012;7(7):e41312. doi: 10.1371/journal.pone.0041312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheng J, Xie HY, Xu X, Wu J, Wei X, Su R, Zhang W, Lv Z, Zheng S, Zhou L. NDRG1 as a biomarker for metastasis, recurrence and of poor prognosis in hepatocellular carcinoma. Cancer Lett. 2011;310(1):35–45. doi: 10.1016/j.canlet.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 42.Ando T, Ishiguro H, Kimura M, Mitsui A, Kurehara H, Sugito N, Tomoda K, Mori R, Takashima N, Ogawa R, Fujii Y, Kuwabara Y. Decreased expression of NDRG1 is correlated with tumor progression and poor prognosis in patients with esophageal squamous cell carcinoma. Dis Esophagus. 2006;19(6):454–458. doi: 10.1111/j.1442-2050.2006.00618.x. [DOI] [PubMed] [Google Scholar]
- 43.Jiang K, Shen Z, Ye Y, Yang X, Wang S. A novel molecular marker for early detection and evaluating prognosis of gastric cancer: N-myc downstream regulated gene-1 (NDRG1) Scand J Gastroenterol. 2010;45(7–8):898–908. doi: 10.3109/00365520903242580. [DOI] [PubMed] [Google Scholar]
- 44.Sun J, Zhang D, Bae DH, Sahni S, Jansson P, Zheng Y, Zhao Q, Yue F, Zheng M, Kovacevic Z, Richardson DR. Metastasis suppressor, NDRG1, mediates its activity through signaling pathways and molecular motors. Carcinogenesis. 2013;34(9):1943–1954. doi: 10.1093/carcin/bgt163. [DOI] [PubMed] [Google Scholar]
- 45.Risolino M, Mandia N, Iavarone F, Dardaei L, Longobardi E, Fernandez S, Talotta F, Bianchi F, Pisati F, Spaggiari L, Harter PN, Mittelbronn M, Schulte D, Incoronato M, Di Fiore PP, Blasi F, Verde P. Transcription factor PREP1 induces EMT and metastasis by controlling the TGF-β-SMAD3 pathway in non-small cell lung adenocarcinoma. Proc Natl Acad Sci U S A. 2014;111(36):E3775–E3784. doi: 10.1073/pnas.1407074111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamazaki K, Masugi Y, Effendi K, Tsujikawa H, Hiraoka N, Kitago M, Shinoda M, Itano O, Tanabe M, Kitagawa Y, Sakamoto M. Upregulated SMAD3 promotes epithelial-mesenchymal transition and predicts poor prognosis in pancreatic ductal adenocarcinoma. Lab Invest. 2014;94(6):683–691. doi: 10.1038/labinvest.2014.53. [DOI] [PubMed] [Google Scholar]
- 47.Boudreau HE, Casterline BW, Rada B, Korzeniowska A, Leto TL. Nox4 involvement in TGF-beta and SMAD3-driven induction of the epithelial-to-mesenchymal transition and migration of breast epithelial cells. Free Radic Biol Med. 2012;53(7):1489–1499. doi: 10.1016/j.freeradbiomed.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oft M, Akhurst RJ, Balmain A. Metastasis is driven by sequential elevation of H-ras and Smad2 levels. Nat Cell Biol. 2002;4(7):487–494. doi: 10.1038/ncb807. [DOI] [PubMed] [Google Scholar]
- 49.Lv ZD, Kong B, Li JG, Qu HL, Wang XG, Cao WH, Liu XY, Wang Y, Yang ZC, Xu HM, Wang HB. Transforming growth factor-β 1 enhances the invasiveness of breast cancer cells by inducing a Smad2-dependent epithelial-to-mesenchymal transition. Oncol Rep. 2013;29(1):219–225. doi: 10.3892/or.2012.2111. [DOI] [PubMed] [Google Scholar]
- 50.Merlot AM, Kalinowski DS, Richardson DR. Novel chelators for cancer treatment: where are we now? Antioxid Redox Signal. 2013;18(8):973–1006. doi: 10.1089/ars.2012.4540. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.